Abstract
Background
Prostate-specific antigen (PSA) measurements are increasingly used to monitor men with localised prostate cancer (PCa), but there is little consensus about the method to use.
Objective
To apply age-specific predictions of PSA level (developed in men without cancer) to one cohort of men with clinically identified PCa and one cohort of men with PSA-detected PCa. We hypothesise that among men with clinically identified cancer, the annual increase in PSA level would be steeper than in men with PSA-detected cancer.
Design, setting, and participants
The Scandinavian Prostatic Cancer Group 4 (SPCG-4) cohort consisted of 321 men assigned to the watchful waiting arm of the SPCG-4 trial. The UK cohort consisted of 320 men with PSA-detected PCa in the Prostate Testing for Cancer and Treatment (ProtecT) study in nine UK centres between 1999 and 2007 who opted for monitoring rather than treatment. Multilevel models describing changes in PSA level were fitted to the two cohorts, and average PSA level at age 50, change in PSA level with age, and predicted PSA values were derived.
Measurements
PSA level.
Results and limitations
In the SPCG-4 cohort, mean PSA at age 50 was similar to the cancer-free cohort but with a steeper yearly increase in PSA level (16.4% vs 4.0%). In the UK cohort, mean PSA level was higher than that in the cancer-free cohort (due to a PSA biopsy threshold of 3.0 ng/ml) but with a similar yearly increase in PSA level (4.1%). Predictions were less accurate for the SPCG-4 cohort (median observed minus predicted PSA level: −2.0 ng/ml; interquartile range [IQR]: −7.6–0.7 ng/ml) than for the UK cohort (median observed minus predicted PSA level: −0.8 ng/ml; IQR: −2.1–0.1 ng/ml).
Conclusions
In PSA-detected men, yearly change in PSA was similar to that in cancer-free men, whereas in men with symptomatic PCa, the yearly change in PSA level was considerably higher. Our method needs further evaluation but has promise for refining active monitoring protocols.
Keywords: active surveillance, localised prostate cancer, PSA doubling time, PSA velocity, reference ranges
1. Introduction
Prostate cancer (PCa) is one of the most common newly detected cancers in men worldwide, primarily because of the widespread use of prostate-specific antigen (PSA) testing. The suitability of PSA for PCa population screening remains debatable [1,2], and there is controversy over the most appropriate treatment for early PCa. While the Scandinavian Prostatic Cancer Group 4 (SPCG-4) trial showed a survival benefit for radical surgery over watchful waiting in men with clinically detected, localised disease [3,4], it is uncertain whether such benefits would extend to men with earlier, PSA-detected PCa [5].
Most men diagnosed with localised PCa have high survival rates, particularly those with the most common low-risk tumours [6,7]. Hence, there is increasing interest in monitoring men with low-risk tumours to permit delay or avoidance of radical treatments [8–10], but there is no consensus about the best methods for monitoring and/or surveillance or for detecting progression [11]. Serial measures of PSA level are used consistently, but various aspects of PSA kinetics are used to trigger intervention. Little information is available on longitudinal changes in PSA levels in men that could establish ranges for benign PSA change against which PSA measurements for an individual could be compared. A study combining longitudinal studies of PSA levels in controls with men before diagnosis of cancer identified a linear change in log(PSA) for controls, with a change point for men with cancer followed by a rapid rise in PSA level [12]. Cases that metastasised had more rapid increases in PSA levels after this change point [12]. Data from SPCG-4 showed that higher PSA level at baseline and faster yearly increase in PSA level were associated with, although not strongly predictive of, adverse outcome [13].
In this paper, we examine whether age-specific predictions of PSA level (developed from a population of cancer-free men) [14] can be applied to two very different cohorts: (1) men who underwent watchful waiting as part of the SPCG-4 study [3] and (2) men who opted for monitoring following population-based PSA testing in the Prostate Testing for Cancer and Treatment (ProtecT) study in the United Kingdom. We hypothesised that in men with clinically detected localised cancer (from the SPCG-4 cohort), the yearly change in PSA level would be increased.
2. Methods
Age-specific reference ranges for PSA levels in cancer-free men were developed using serial measurements of PSA level from 1462 men aged 50–78 yr (Krimpen cohort) [15]. Men with a raised PSA level (>4 ng/ml) were investigated, and if PCa was found, they were excluded [15]. A multilevel growth curve model was used to relate log(PSA) to age. The model allowed for correlation between the yearly change in PSA level and baseline level of log(PSA) and estimated the influence of individual characteristics on PSA level. Each man has his own pattern of changing PSA level with age, and these patterns are assumed to be normally distributed across a population average. This model allows PSA patterns to differ, even among men with the same baseline characteristics [14]. In this way, each man can be provided with his own reference ranges, accommodating his current PSA level and other baseline characteristics and predicting his future PSA levels. Thus, future PSA pattern for a man can be predicted, conditional on one observed PSA measurement, using the coefficients and random effects from the multilevel model [14]. Such models have been used to predict outcome following stroke [16], with technical aspects described in detail elsewhere [17]. Development of these models requires that men should have at least two PSA measurements, but the model can include men with differing numbers of PSA measurements taken at different ages.
2.1. Scandinavian Prostatic Cancer Group 4 cohort
From 1989 to 1999 the SPCG-4 trial enrolled 695 men from 14 centres in Sweden, Finland, and Iceland [3]. Eligibility criteria included age <75 yr; newly diagnosed, untreated, localised PCa verified by histologic examination, well differentiated or moderately well differentiated, with a tumour stage of T0d (later changed to T1b), T1, or T2 (T1c was included in 1994); health status permitting radical prostatectomy; life expectancy >10 yr; a normal bone scan; and PSA level <50 ng/ml. Most had clinically identified T2 stage cancer, with 12% having T1c tumours, and only 5% had case records showing that the cancer was detected by PSA screening. Some 348 men were randomly assigned to watchful waiting, receiving no initial treatment. This analysis used the 321 men who had at least two PSA measurements, at least 4 mo between first and second PSA measurements, and a first PSA measurement <8 mo after randomisation. The mean length of follow-up was 6.6 yr (standard deviation [SD]: 3.5 yr).
2.2. United Kingdom cohort
Men aged 50–69 yr at randomly selected general practices underwent a PSA test as part of the ProtecT study in nine UK centres between 1999 and 2007 [8]. Men with a PSA level ≥3.0 ng/ml had further diagnostic tests, including a 10-core prostate biopsy. Men with histologically proven PCa and clinical evidence that it was localised were invited to take part in the randomised treatment trial comparing radical surgery, radical conformal radiotherapy, and active monitoring (involving regular PSA tests). Men who refused to participate in the ProtecT treatment trial and opted for monitoring formed the UK cohort for this analysis. There were 320 men with at least two PSA tests, including the baseline test. The study was approved by the Trent multicentre research ethics committee, and all participants gave informed consent. The mean length of follow-up was 2.6 yr (SD: 1.6 yr).
2.3. Monitoring protocols
Participants in the SPGC-4 cohort were seen every 6 mo during the first 2 yr and then annually for clinical examination and blood tests, including a PSA test. A bone scan and chest radiograph were obtained annually until 1997; thereafter, chest radiographs were obtained annually for the first 2 yr. Men in the UK cohort received PSA tests every 3 mo in year 1 and every 6 mo thereafter. Rebiopsy was not routinely undertaken in either cohort. Test results were reviewed annually, and patient and clinician decided whether to continue with monitoring.
2.4. Statistical methods
Due to the skewed distribution of PSA levels, the outcome in all models was log(PSA). Separate multilevel growth-curve models were fitted to log(PSA) for each cohort of men with PCa, and the coefficients (describing the PSA level at age 50 and yearly change in PSA level) were compared with those from cancer-free men (Krimpen model) [18]. These models fit a separate regression line for each man by estimating PSA level at age 50 and yearly change in PSA level as two correlated normal distributions (see section 3.2). A further multilevel model was fitted to each cohort, including baseline Gleason score as an explanatory variable; it treated both distributions as a linear variable and categorised them (2–6, 7, and 8–10). Gleason score was allowed to affect both the intercept (PSA level at age 50) and the yearly change in PSA level. No other variables were considered for inclusion in these models.
The individual pattern of change in PSA level with age for each man in both cohorts was predicted using the coefficients from the respective multilevel models including age only. These predictions were compared with those from the Krimpen model. Predictions were made using a man’s first observed PSA measurement (using the model coefficients and random effects [14]), and then repeated using his first two PSA measures. All statistical analyses were performed using Stata v.9 (StataCorp, College Station, TX, USA) [19].
3. Results
3.1. Baseline data
Table 1 shows baseline PSA values by age group for each cohort. In these cross-sectional data, PSA levels increase with age in the Krimpen and UK cohorts, with no age-related increase in the SPCG-4 cohort. PSA values in the SPCG-4 cohort were highly variable, with a few men having very high values. The Krimpen cohort was larger than either of the PCa cohorts and had a greater proportion of men aged >70 yr. The SPCG-4 study cohort had a wider spread of Gleason scores than the UK cohort and had more evidence of higher PSA levels in men with higher Gleason scores (Table 2).
Table 1.
Distribution of baseline prostate-specific antigen (PSA) level by age at baseline for the Krimpen study, the Scandinavian Prostatic Cancer Group 4 (SPCG-4) cohort, and the UK cohort, showing median and interquartile range (IQR)
| Krimpen study | SPCG-4 cohort | UK cohort | ||||
|---|---|---|---|---|---|---|
| Age group, yr |
PSA median, ng/ml (IQR) |
n (%) | PSA median, ng/ml (IQR) |
n (%) | PSA median, ng/ml (IQR) |
n (%) |
| <50 | 1.1 (0.6–1.6) |
8 ( 0.2) |
– | 0 | 3.8 (1.7–4.1) |
3 ( 0.9) |
| 51–55 | 0.9 (0.6–1.3) |
309 ( 9.5) |
9.7 (4.2–20.6) |
8 ( 2.5) |
4.2 (3.4–6.2) |
39 (12.2) |
| 56–60 | 1.0 (0.6–1.7) |
377 (19.6) |
12.0 (5.8–18.3) |
44 (13.7) |
4.9 (3.5–7.0) |
68 (21.3) |
| 61–65 | 1.1 (0.7–2.1) |
351 (20.0) |
8.9 (5.2–15.5) |
95 (29.6) |
4.9 (3.5–7.0) |
116 (36.3) |
| 66–70 | 1.4 (0.8–2.5) |
266 (15.5) |
8.8 (5.7–16.0) |
117 (36.4) |
5.1 (3.7–6.9) |
93 (29.1) |
| >70 | 1.8 (1.0–3.4) |
151 (35.1) |
9.2 (5.8–16.3) |
57 (17.8) |
4.4 | 1 ( 0.3) |
| Total | 1.1 (0.7–2.0) |
1462 | 9.2 (5.4–16.0) |
321 | 4.8 (3.6–6.9) |
320 |
Table 2.
Distribution of baseline prostate-specific antigen (PSA) level by Gleason score at baseline for the Scandinavian Prostatic Cancer Group 4 (SPCG-4) cohort and the UK cohort, showing median and interquartile range (IQR)
| SPCG-4 cohort* | UK cohort† | |||
|---|---|---|---|---|
| Gleason score | PSA median, ng/ml(IQR) |
n (%) | PSA median, ng/ml (IQR) |
n (%) |
| 3 | 3.4 | 1 (0.3) | – | 0 |
| 4 | 6.7 (3.2–11.0) | 41 (14.0) | 6.1 (4.7–7.5) | 2 (0.6) |
| 5 | 10.0 (5.3–15.0) | 75 (25.6) | 4.4 (3.7–8.7) | 7 (2.2) |
| 6 | 9.2 (6.7–16.0) | 80 (27.3) | 4.7 (3.5–6.5) | 256 (80.3) |
| 7 | 11.0 (7.2–23.0) | 75 (25.6) | 5.4 (3.9–8.1) | 52 (16.3) |
| 8 | 10.5 (4.1–14.1) | 18 (6.1) | 6.4 (3.2–9.6) | 2 (0.6) |
| 9 | 17.8 (1.5–18.0) | 3 (1.0) | – | 0 |
| Total | 9.6 (5.6–16.3) | 293 | 4.8 (3.5–6.9) | 319 |
Twenty-eight men missing Gleason data.
Two men missing Gleason data.
3.2. Model development
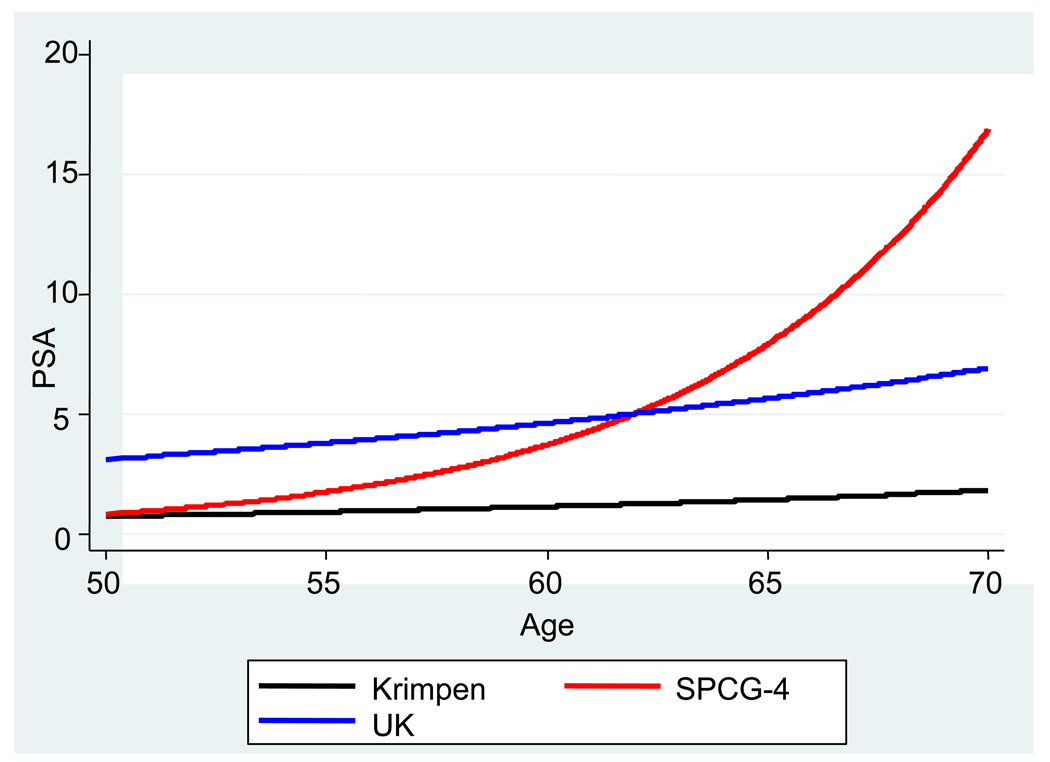
There were between 2 and 22 PSA measurements per participant in the SPCG-4 study (mean: 10.5; SD: 4.5) and between 2 and 25 PSA measurements per participant in the UK cohort (mean: 9.5; SD: 4.7). The coefficients from the cohort-specific multilevel models are shown in Table 3 compared with those from the Krimpen model. For the SPCG-4 cohort, the mean PSA level at age 50 was similar to that from the Krimpen study but with a steeper age-related increase in PSA level. Conversely, for the UK cohort the average PSA level (at age 50) was higher than that from the Krimpen study (because PSA level >3.0 ng/ml was one inclusion criterion for the study) but with a similar age-related change in PSA level. The average predicted patterns of change in PSA level with age (from the coefficients in Table 3) for each of these three models are shown in Figure 1. The following equations were used for the average patterns of PSA level:
Table 3.
Coefficients for the relationship between prostate-specific antigen (PSA) and age for the Krimpen study, the Scandinavian Prostatic Cancer Group 4 (SPCG-4) cohort, and the UK cohort
| Krimpen study | SPCG-4 cohort | UK cohort | |
|---|---|---|---|
| PSA level at age 50, ng/ml (95% CI) | 0.8 (0.7–0.8) |
0.8 (0.6–1.2) | 3.1 (2.7–3.6) |
| Relative increase in PSA per year in age, % (95% CI) |
4.0 (3.7–4.5) |
16.4 (14.0–18.8) |
4.1 (3.1–5.2) |
CI = confidence interval.
Fig. 1.
Average patterns of change of prostate-specific antigen (PSA) level (ng/ml) with age (in years) in men without cancer (Krimpen model) and in men with localised prostate cancer (SPCG-4 cohort and UK cohort).
For each of the cohorts of men with PCa, Gleason score at diagnosis was added to the model (Table 4). Using the SPCG-4 cohort, there was no evidence for an association between Gleason score and average PSA level (Table 4, p for trend = 0.24), but there was strong evidence of a greater yearly increase in PSA level with higher Gleason scores (Table 4; p = 0.001 for trend). Similar results were obtained from the UK cohort, with less variation in Gleason score; there was no evidence that a higher Gleason score was associated with baseline PSA level (Table 4; p = 0.28) and with evidence of a greater yearly increase in PSA level with higher Gleason scores (Table 4; p = 0.06). Thus, men with a higher Gleason score have the same baseline PSA level as those with lower Gleason scores, but their PSA level increases faster with age. The following equations were used for the average patterns of PSA level with Gleason score:
Table 4.
Coefficients for the relationship between prostate-specific antigen (PSA) level and Gleason score for the Scandinavian Prostatic Cancer Group 4 (SPCG-4) cohort and the UK Cohort
| SPCG-4 cohort | UK cohort | |
|---|---|---|
| Increase in PSA per one grade increase in Gleason score, % (95% CI) |
−15.9 (−37.0–12.1) |
−13.0 (−36.8–17.2) |
| Change in yearly increase in PSA per one grade increase, % (95% CI) |
2.8 (1.1–4.6) | 2.1 (−0.1–4.3) |
CI = confidence interval.
A similar pattern was observed in both cohorts when Gleason score was used as a categorical variable with three categories (2–6, 7, and 8–10); we found a lower yearly increase in PSA level for Gleason scores 2–6 than for Gleason score 7, and we found little evidence of a higher yearly increase in PSA for Gleason scores 8–10. There was no strong evidence of a difference in baseline PSA between Gleason categories (2–6, 7, and 8–10) for either cohort.
3.3. Model evaluation
Predictions of PSA level were calculated conditionally on the first and the first two observed PSA measurements for the SPCG-4 cohort and the UK cohort, respectively, using the respective model parameters and those derived from the Krimpen model (Table 5). For the SPCG-4 cohort, both models were unable to predict PSA levels accurately (median difference for Krimpen model conditional on first observation: −2.0; IQR: −7.6–0.7), largely because of a small number of men with very high PSA levels. For the UK cohort, the Krimpen model and the UK model (using the first observation and then using the first two observations) performed well, with small differences between observed PSA level and the predicted PSA level (median difference for Krimpen model using first observation: −0.8; IQR: −2.1–0.1).
Table 5.
Predicted prostate-specific antigen (PSA) level using the first and first two observed PSA measurements from the Krimpen study model parameters and from the parameters of the models developed on each data set
| SPCG-4 cohort | UK cohort | |||
|---|---|---|---|---|
| PSA median level, ng/ml( IQR) |
Predicted- observed median level, ng/ml (IQR) |
PSA median level, ng/ml (IQR) |
Predicted- observed median level, ng/ml (IQR) |
|
| Using first observation |
N (observations) = 3042, n (men) = 321 |
N (observations) = 2726, n (men) = 320 |
||
| Observed PSA level, ng/ml |
11.3 (5.5–23.0) |
– | 5.2 (3.8–7.9) | – |
| Krimpen model | 9.1 (5.3–15.3) | −2.0 (−7.6–0.7) |
4.5 (3.5–6.3) | −0.8 (−2.1–0.1) |
| Study model | 13.2 (7.6–23.1) |
0.9 (−2.8–5.5) | 5.1 (4.0–7.1) | −0.1 (−1.4–0.7) |
| Using first two observations |
N (observations) = 2721, n (men) = 313 |
N (observations) = 2407, n (men) = 320 |
||
| Observed PSA level, ng/ml |
12 (5.7–24) | – | 5.3 (3.8–8.1) | – |
| Krimpen model | 9.9 (5.3–17.4) | −1.4 (−6.4–0.9) |
4.9 (3.8–6.9) | −0.4 (−1.6–0.5) |
| Study model | 12.4 (6.8–22.4) |
0.6 (−2.8–3.8) | 5.1 (4.0–7.2) | −0.2 (−1.4–0.7) |
IQR = interquartile range; SPCG-4 = Scandinavian Prostatic Cancer Group study.
4. Discussion
This is the first time that these longitudinal reference ranges allowing individually calibrated predictions have been applied to PSA measures of men with PCa [14]. We modelled the pattern of change of PSA level in two different large cancer populations and compared the results to those obtained from a model derived from a reference, noncancer population (Krimpen). The model derived from the UK cohort was very similar to the Krimpen model calibrated to that population. The model derived from the SPCG-4 cohort had a greater yearly increase in PSA than the calibrated Krimpen model. Hence, in men with PSA-detected PCa, the yearly PSA change is similar to that in cancer-free men, whereas in men diagnosed with PCa following clinical presentation, the yearly change in PSA level was considerably higher. There is an urgent need to develop protocols that allow safe monitoring and/or surveillance of men with low-risk and localised (T1c, Gleason <7) PCa, the majority of whom will not develop clinically important, let alone advanced, disease in their lifetimes [6,20]. Many monitoring protocols have been developed, but there is little consensus about what should be measured or what constitutes evidence of risk of progression [11]. The validation of such protocols is difficult, primarily because of the time required for clinically relevant outcomes (ie, death or the development of metastases) to occur, and there are few data sets with such outcomes from men with PSA-detected PCa. The method described in this paper attempts to take into account the benign growth of the prostate and consequent changes in PSA level over time. Further research, however, is required to investigate whether patients at the highest risk of progression can be identified when their disease is still curable by local treatment.
The differences found in the PSA patterns between the UK cohort and the SPCG-4 cohort may be due to their different participant characteristics. The UK study population consists of men diagnosed with PCa following communitywide PSA testing based at general practices, whereas SPCG-4 men presented clinically to urologic centres. The UK men were younger than those in the SPCG-4 study and had lower average PSA levels and Gleason scores. Calculations of lead times for PCa suggest that UK men will have been diagnosed some 8–12 yr before SPCG-4 men [21]. It is also important to note that the SPCG-4 cohort had a much longer follow-up than the UK cohort (6.6 yr vs 2.6 yr) and, thus, contained many men who had progressed to locally advanced and metastatic disease. These factors are likely to account for the difference in age-related increase in PSA levels between the studies. Our model suggests a yearly change in PSA level of 15% in the SPCG-4 study versus 4% in the UK study. A study combining results from three longitudinal studies of PSA (up to time of diagnosis with cancer) suggested similar rates of change of 2% per year in men without cancer and 15% per year in men with localised disease [12]. These studies were undertaken before the PSA screening era, so there may be many more men among the controls who had undiagnosed PCa, which could account in part for the similarity between the estimated age-related increases in the PSA-detected men in the UK study and those in cancer-free men from the earlier studies.
It is encouraging that the Krimpen model and the UK model show similar increases in PSA level with increasing age; a higher increase in the SPCG-4 cohort implies an increase in rate of change in PSA level with increased disease severity or time since diagnosis. Thus, as we hypothesised, age-related increase in PSA level seems to be higher for clinically detected symptomatic cancer than for PSA-detected cancers. A potential advantage of the reference-range method for monitoring men with PSA-detected PCa is its ease of use: serial PSA measures can be plotted against the pattern predicted using the first PSA measurement. Additionally, the method avoids concerns raised by day-to-day fluctuations in PSA level and takes into account age and PSA level at the beginning of monitoring rather than just observed PSA level, velocity, or relative increase, as in current protocols. It is unknown whether changes in PSA occur in advance of progression (indicating a role for PSA monitoring) or are an expression of it (so that rapidly increasing PSA could identify those who would benefit from early hormone therapy).
This study has some weaknesses. The original model is based on data from one community-based study in the Krimpen area of the Netherlands. While we anticipate that these findings should be generalisable, they may not be representative beyond Northern Europe. We have no PSA measurements of men both before and after they developed cancer, so we cannot examine a change-point model. Men in the Krimpen study were investigated for PCa if they had PSA >4 ng/ml [15], so some men in the cancer-free Krimpen model may have had undetected disease. This is, however, likely to be the case in any data set of men of this age [22], and when later-detected cases of PCa were removed from the Krimpen data set, it made little difference to the fitted model [14].
Considerable further research needs to be undertaken. These methods (and alternative formulations) must be assessed for their ability to predict progressive disease. This requires data on a large number of men undergoing active monitoring for many years, with information on progression of disease (presence of metastases, death) obtained independently of their PSA status (eg, all men restaged at the end of follow-up, regardless of PSA level).
5. Conclusions
Yearly change in PSA level appears to be steeper in those with longer follow-up and with more severe cancer (symptomatic as opposed to PSA detected). This factor may distinguish men with cancer who are at low risk of progression from those who are at high risk. Our method has promise for use in men undergoing active monitoring and/or surveillance but requires further validation.
Take-home message
In men with localised prostate cancer, yearly change in prostate-specific antigen (PSA) level was considerably higher in those whose cancer was detected through presentation of symptoms than in those whose cancer was detected through PSA screening. Our reference range method needs further evaluation but has promise for refining active monitoring protocols.
Supplementary Material
Acknowledgments
The authors would like to thank Professor Ruud Bosch and the Krimpen study group for the collaboration that led to the development of the original model. Our thanks also go to the study groups of the SPCG-4 and ProtecT for their generosity and encouragement of this research. We thank all the participants of the Krimpen study, the SPCG-4 study, and the ProtecT study.
Funding/Support and role of the sponsor: Health Technology Assessment Programme, Swedish Cancer Society, and US National Institutes of Health provided funding and support for the design and conduct of the study and for the collection of data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Kate Tilling had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Tilling, Metcalfe, Donovan
Acquisition of data: Metcalfe, Tilling, Davis, Garmo, Holmberg, Adami, Johansson, Adami
Analysis and interpretation of data: Metcalfe, Tilling, Garmo, Holmberg
Drafting of the manuscript: Tilling, Metcalfe, Donovan, Holmberg
Critical revision of the manuscript for important intellectual content: Tilling, Garmo, Metcalfe, Holmberg, Hamdy, Neal, Adolfsson, Martin, Davis, Fall, Lane, Adami, Bill-Axelson, Johansson, Donovan
Statistical analysis: Metcalfe, Tilling, Garmo
Obtaining funding: Johansson, Adami (SPCG-4 funding); Hamdy, Donovan, Neal (ProtecT funding)
Administrative, technical, or material support: Davis, Holmberg
Supervision: Donovan
Other (specify): none
Financial disclosures: I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/ affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: The ProtecT study and the observational UK cohort study are funded by the Health Technology Assessment Programme (projects 96/20/06, 96/20/99). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the UK Department of Health. SPCG-4 is funded by the Swedish Cancer Society and the US National Institutes of Health.
References
- 1.Lu-Yao G, Albertsen PC, Barry MJ, et al. Natural experiment examining impact of aggressive screening and treatment on prostate cancer mortality. BMJ. 2002;325:740. doi: 10.1136/bmj.325.7367.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frankel S, Davey Smith G, Donovan J, Neal D. Screening for prostate cancer. Lancet. 2003;361:1122–1128. doi: 10.1016/S0140-6736(03)12890-5. [DOI] [PubMed] [Google Scholar]
- 3.Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352:1977–1984. doi: 10.1056/NEJMoa043739. [DOI] [PubMed] [Google Scholar]
- 4.Bill-Axelson A, Holmberg L, Filén F, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian Prostate Cancer Group-4 randomized trial. J Natl Cancer Inst. 2008;100:1144–1154. doi: 10.1093/jnci/djn255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collin SM, Martin RM, Metcalfe C, et al. Prostate-cancer mortality in the USA and UK in 1975–2004: an ecological study. Lancet Oncol. 2008;9:445–452. doi: 10.1016/S1470-2045(08)70104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293:2095–2101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 7.Albertsen PC, Hanley JA, Penson DF, Barrows G, Fine J. 13-year outcomes following treatment for clinically localized prostate cancer in a population based cohort. J Urol. 2007;177:932–936. doi: 10.1016/j.juro.2006.10.051. [DOI] [PubMed] [Google Scholar]
- 8.Donovan JL, Hamdy FC, Neal DE, et al. Prostate testing for cancer and treatment (ProtecT) feasibility study. Health Technol Assess. 2003;7:1–42. doi: 10.3310/hta7140. [DOI] [PubMed] [Google Scholar]
- 9.Klotz L, Nam R. Active surveillance with selective delayed intervention for favourable risk prostate cancer: clinical experience and a “number needed to treat” analysis. Eur Urol Suppl. 2006;5:479–486. [PubMed] [Google Scholar]
- 10.Parker C. Active surveillance: an individualized approach to early prostate cancer. BJU Int. 2003;92:2. doi: 10.1046/j.1464-410x.2003.04295.x. [DOI] [PubMed] [Google Scholar]
- 11.Martin RM, Gunnell D, Hamdy F, Neal D, Lane A, Donovan J. Continuing controversy over monitoring men with localized prostate cancer: a systematic review of programs in the prostate specific antigen era. J Urol. 2006;176:439–449. doi: 10.1016/j.juro.2006.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoue LY, Etzioni R, Slate EH, Morrell C, Penson DF. Combining longitudinal studies of PSA. Biostatistics. 2004;5:483–500. doi: 10.1093/biostatistics/5.3.483. [DOI] [PubMed] [Google Scholar]
- 13.Fall K, Garmo H, Andrén O, et al. Prostate-specific antigen levels as a predictor of lethal prostate cancer. J Natl Cancer Inst. 2007;99:526–532. doi: 10.1093/jnci/djk110. [DOI] [PubMed] [Google Scholar]
- 14.Bosch JLHR, Tilling K, Bohnen AM, Donovan JL. Establishing normal reference ranges for PSA change with age in a population-based study: the Krimpen study. Prostate. 2006;66:335–343. doi: 10.1002/pros.20293. [DOI] [PubMed] [Google Scholar]
- 15.Blanker MH, Groeneveld FP, Prins A, Bernsen RMD, Bohnen AM, Bosch JLHR. Strong effects of definition and nonresponse bias on prevalence rates of clinical benign prostatic hyperplasia: the Krimpen study of male urogenital tract problems and general health status. BJU Int. 2000;85:665–671. doi: 10.1046/j.1464-410x.2000.00570.x. [DOI] [PubMed] [Google Scholar]
- 16.Tilling K, Sterne JAC, Rudd AG, Glass TA, Wityk RJ, Wolfe CDA. A new method for predicting recovery after stroke. Stroke. 2001;32:2867–2873. doi: 10.1161/hs1201.099413. [DOI] [PubMed] [Google Scholar]
- 17.Tilling K, Sterne JAC, Wolfe CDA. Multilevel growth curve models with covariate effects: application to recovery after stroke. Stat Med. 2001;20:685–704. doi: 10.1002/sim.697. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein H. Kendall’s Library of Statistics 3: Multilevel Statistical Models. 3rd ed. London, UK: Hodder Arnold; 2003. [Google Scholar]
- 19.Stata. Version 9.0. College Station, TX: StataCorp; 2005. [computer programme] [Google Scholar]
- 20.Johansson JE, Andrén O, Andersson SO, et al. Natural history of early, localized prostate cancer. JAMA. 2004;291:2713–2719. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]
- 21.Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomised Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95:868–878. doi: 10.1093/jnci/95.12.868. [DOI] [PubMed] [Google Scholar]
- 22.Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [published correction appears in N Engl J Med 2004;351:1470] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.