Abstract
Tremendous progress in nanotechnology has lead to the development of nanometer-sized objects as medical implants or devices. Many of these nanodevices have recently been tested in many cancer diagnostic and therapeutic applications, such as leukemia, melanoma, breast tumor, prostate tumor, and brain cancer. Despite the increasing importance of nanotechnology in cancer, the potential of these nanodevices in diagnosing and treating intraocular cancers has not been systematically evaluated. This review summarizes the significant advancements and potential areas for development in the field of nanotechnology-based intraocular drug delivery and imaging.
Keywords: Intraocular cancer, uveal melanoma, retinoblastoma, nanoparticles, liposomes, quantum dots, intravitreal injection, drug delivery, imaging
1. Intraocular Cancers – Overview and detection
Though less common, the risk of complications and metastatic potential of intraocular cancers presents a very dangerous condition, warranting the same vigilant management as other cancers. Due to their proximity to critical ocular structures, early diagnosis and treatment of intraocular cancer are essential to preserve vision. There are two common forms of intraocular tumors which can be characterized based on the typical age of occurrence: ocular melanoma in adults and retinoblastoma in children. Ocular melanoma is the most common malignancy originating in the eye in older patients with a median onset age of 55.1,2 Retinoblastoma is a very common form of ocular malignancy occurring in children.1
Uveal melanoma is the most common type of ocular melanoma and typically presents as a small tumor near critical structures in the eye.1,3 The potential causes and risk factors of uveal melanoma remain undetermined,6 though incidence is slightly higher in men, with rates 150 times higher in Caucasians compared to those with darker skin. This is consistent with the statistically higher incidence seen in patients with light skin, blue eyes, and blonde hair.1 Compared with other intraocular tumors, uveal melanoma has the highest rate of metastasis, with a 40% metastasis rate (median survival 2–7 months) and approximately 50% of mortal cases due to metastasis, most commonly to the liver.2,5 Approximately 30% of patients with successfully treated primary tumors will develop metastasis.5
The most concerning issue with this condition are problems with detection and treatment.5 As such, the rate of metastasis and mortality has remained unchanged over the years. Typically, uveal melanoma is diagnosed by binocular indirect ophthalmicroscopy.6 Indocyanine green angiography is also used to visualize the tumor and tumor margins.6
The most common treatment options include enucleation, local resection, brachytherapy, of which brachytherapy would represent the most common.6 These approaches are plagued by high failure rates and complications with large tumors or those near the optic nerve,4 with other complications including iris neovascularization and neovascular glaucoma.7 External beam radiotherapy has success rates similar to enucleation, providing local control and organ preservation along with unmatched cosmetic results and visual preservation.3 Chemotherapy using drug or drug combinations has also been employed (Table I). Some common active chemotherapeutic drugs used to treat advanced melanoma include dacarbazine (DTIC) and cisplatin.8,9 Single agent response rates with chemotherapy are below 10%.11 Thus, the general trend of chemotherapy is to include multiple drugs/agents simultaneously. For that, the major challenge is how different drugs/agents would be constantly and locally delivered to the tumor site for a prolonged period of time.
Table I.
List of chemotherapy drugs commonly used for the intraocular tumors.
Despite the wide range of available treatments, the problem of metastasis remains, due to our inability to detect the development the tumor at early non-symptomatic period.5 This is complicated by the fact that a recent UK study suggests approximately 45% of patients were asymptomatic at the time of tumor diagnosis.6 Several imaging modalities are under investigation for use in the detection and analysis of treatments in uveal melanoma. Functional MRI has been used to detect uptake of magnetic contrast agents and determine perfusion and blood volume in uveal melanoma.12 Positron emission tomography has been investigated to detect liver metastasis in patients with uveal melanoma.13 However, none of these methods can be used to detect uveal melanoma in its early stages.
Retinoblastoma, often a hereditary condition, occurs in children under 5 years of age and is caused by inactivation of the RB gene. The incidence of this form of intraocular cancer is highest in the developing countries.14 Inactivation of this gene takes off constraints on cell-cycle control leading to unregulated cell proliferation. The cancer presents itself as either an abnormal white discoloration in one or both pupils or as an ocular misalignment due to loss of central vision in the eyes. In advanced cases ocular swelling is seen due to extraocular invasion of the tumor. Large retinal tumors often lead to retinal detachment. Retinoblastoma can spread to the subarachnoid space and from there to the brain and spinal cord. It can also invade the choroid vasculature and spread to the bone and bone marrow.15
Diagnosis of retinoblastoma is usually by examination and imaging of the eye by an ophthalmologist. Detection is by funduscopy, which typically shows a large white to creamy colored tumor with lesions around the retinal and vitreous space.16 The intraocular tumor is identified and analyzed by ultrasonography since CT scan is not recommended for small children.17 Magnetic Resonance Imaging (MRI) of the brain and the orbits is conducted to examine the extraocular extension of the tumor.18
Chemotherapy and enucleation are the common treatment methods for children with advanced retinoblastoma. Typical chemotherapeutic drugs include carboplatin, vincristine, cyclophosphamide and doxorubicin.16,19 Some other treatments have also been used. For example, thermotherapy involving the application of heat in the form of infrared radiation directly on to the tumor or chemothermotherapy with a combination of chemotherapy and thermotherapy.16 The common chemotherapeutic drugs used for treating uveal melanoma, 8,9,16 and retinoblastoma 16 have been summarized in Table I.
Despite the various treatment methods available, almost all current treatments fail to completely eradicate cancer or prevent its recurrence.19 The ineffectiveness of chemotherapy agents may be associated with the inability to deliver large amount of chemotherapy drugs into tumor tissue in vivo.20 Studies have found that most systemically administered drugs are consumed by other organs/tissues prior to accumulating in cancer tissue. Such deficiency can not be resolved by increasing the amounts of administered drugs, since it is well established that certain chemotherapy agents have inherent systemic side-effects, including bone marrow toxicity.21 To maintain a therapeutic dosage of chemotherapeutic drug at the ocular tumor site, it is imperative that anti-cancer drugs be targeted, delivered and released at the cancer site.
To achieve localized release of anti-tumor agents, many different methods have been tested including intra-tumor injection, intra-tumor implantation, and targeted delivery.22,23 Such approaches may not be suitable to combat ocular tumor, since invasive surgical procedures are difficult to carry out in eye and surgical trauma associated with the operation may lead to vision impairment. To avoid such complications, increasing research interests have been placed on the development of nanodevices which can target the tumor for both tumor drug delivery and imaging. Such efforts are summarized as the following.
2. Nanodevices for ocular tumor
Although significant progress has been made in the application of nanotechnology-based cancer diagnostics and therapy for the rest of the body, a very limited number of studies have been done to develop nanodevices for ocular tumor treatment. Some studies, however, have been varied out on the use of nanoparticles for tissue targeted and slow drug release in various structures of the ocular tissue. The outcomes of those works are summarized below.
2.1. Delivery routes for ocular drug delivery
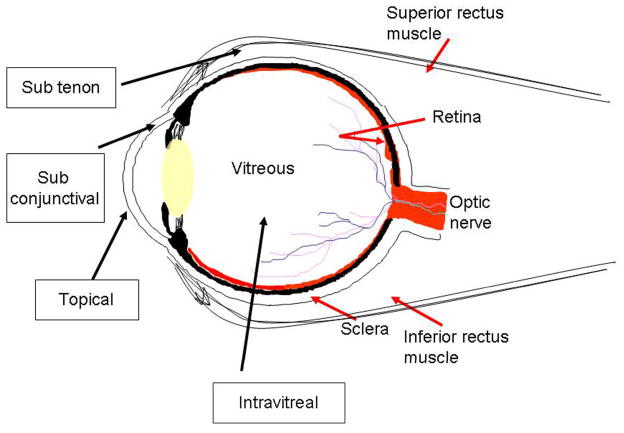
Numerous works have focused on evaluating the efficacy and limitations of different implantation methods on the extent of drug diffusion and retention in ocular tissues.24–29 Some of the ocular drug administration routes are topical treatment, systemic, intravitreal, subconjunctiva, suprachoroidal, juxtascleral, and subtenon injection (Figure 1). Each ocular drug delivery route has unique advantages and disadvantages that are related to where the nanodevices are positioned in the ocular tissues. Systemic administration via intravenous injection is the most popular method to deliver drugs to many parts of the body. However, studies have shown that very small portions of systemically administered drugs/nanodevices are found to accumulate in the ocular tissue.30 The majority (>90%) of ocular drug delivery relies on topical administration via eye drops. The success of such treatment depends on the efficacy of spontaneous diffused drug or drug-eluting carriers through the cornea.28,31–34 Although topical administration has shown some success to treat anterior chamber eye diseases (diseases associated with cornea and iris), such treatment is often found to be ineffective in treating posterior eye diseases, including retina diseases and intraocular tumors.35 It is generally believed that this is caused by the inability of drugs to diffuse through the cornea and lens barriers.35
Figure 1.
Schematic showing the eye with some of the routes of intraocular drug/nanodevice administration.35
Periocular drug delivery through a subconjunctival, suprachoroidal, juxtascleral or subtenon routes offers the advantage of noninvasive approach. However, such treatments often failed to deliver therapeutic dosage of drugs to intraocular tissues for a prolonged period of time.35–38 Therefore, using biocompatible or cell-specific coatings/receptors intensive research efforts have been placed on periocular drug delivery to enhance the intraocular diffusion of various devices.33,39–45 Finally, intravitreal injection of nanodevices is still the most effective method currently available to deliver sufficient amounts of drugs into posterior ocular tissues, including ocular tumor tissues.35,39,46 However, intravitreal injection may lead to damaging side-effects, such as retinal detachment and endophthalmitis. To reduce such complications, intensive research efforts are placed to produce nanodevices to release drugs for prolonged periods of time which indirectly reduce the requirement for repeated treatments. 43,46 Some of these design strategies that could be adopted for intraocular delivery of nanodevices are summarized in Table II.
Table II.
Design strategies for ocular cancer therapy based on administration route and material selection
| Adminstration route | Drug release venues | Carrier Materials | Advantage | Disadvantage |
|---|---|---|---|---|
| Systemic | Injection in to blood stream | Degradable and hydrogel nanoparticles. Eg: N isopropyl acrylamide (NIPAM) nanoparticles.47 | Less invasive to ocular tissue | Blood ocular barrier hinders drug delivery; potential systemic drug toxicity.34 |
| Topical | via corneal diffusion | Nanocapsules, nanoparticles and mucoadhesive polymers Eg: Chitosan nanoparticles.48 | Easy access to iris and ciliary body.33,34 | Poor drug delivery efficiency and unsuitable for posterior eye diseases.28 |
| Sub-conjunctiva | Released from conjunctiva tissue | Polymeric nanoparticles.37 eg:polystyrene nanoparticles various sizes & charge | Prolonged drug release with increased drug delivery to uveal tissue. | Small (<20nm) nanoparticles may be cleared by lymphatic.37,38 |
| Sub-tenon | Released from void space between Tenon’s capsule and sclera | No studies on subtenon injection of nanodevices. Mainly used for injection of drugs.49,50 | Prolonged drug penetration and low clearance from vitreous tissue.35 | Requires skilled surgeon for implantation and retinal pigment epithelium poses a barrier.35 |
| Intravitreal | Direct injection in to the vitreous | PLA/PLGA nanoparticles.43,51,52 | Deliver high molecular weight drugs. Accumulation in the retina for long time periods.51 | May cause retinal detachment & endophthalmitis |
2.2 Nanodevices for ocular drug delivery
The design of ocular drug-delivery systems is based on many therapeutic criteria, including carrier tissue compatibility, tissue targeting, drug therapeutic dosage, stability and release rate.39 Many strategies have been developed to meet such design criteria. These strategies include the modification of drug delivery carrier chemical compositions, physical morphology, degradation rates and tissue affinity. Specifically, carrier chemical composition (material chemical and biological properties) affects drug release and tissue compatibility. The majority of drug release nanodevices are made of polymeric (PLLA, PLGA, polyacrylic acid, or polyamidoamine) or biological materials (oligosaccharides, albumin, or chitosan). Most of these nanodevices are in nanoparticle form with different sizes ranging from 10 nm–1000 nm.35,39 Nanodevice degradation rates are often affected by material molecular weights and overall tissue responses. Generally speaking, high molecular weights slow down material degradation and strong tissue responses expedite material breakdown.53 Tissue affinity is determined by both particle size and particle: cell interactions. Increasing numbers of studies have found that tissue has high affinity to particles between 20 nm– 200 nm.37,38 The specific mechanism(s) governing such size-dependent cellular responses has yet to be determined. It should also be noted that incorporation of cell specific antigen to nanodevices enhances nanodevices’ tissue affinity and drug targeting ability.
The recent findings on ocular drug delivery are summarized in the following paragraph. Liposomes containing cholesterol were used for intravitreal delivery of plasmid DNA.54 Nanoparticles made from chitosan have been used for topical drug administration and found to possess good ocular biocompatibility.48,55,56 Polymeric PLA and PLGA nanoparticles have been used both topically and intravitreally. These FDA approved polymers have very good biocompatibility and have also shown impressive drug delivery capabilities in both the anterior and posterior chamber. 41,42,51 Intravitreally injected albumin nanoparticles have also been shown to accumulate in the vitreous and ciliary body. 57 Studies have shown that most of these nanoparticles are well tolerated by the body with minimal foreign body reaction and have prolonged residence time.58 It should be noted that dendrimers,52,59 and cyclodextrins,60,61 have been used in different forms of nano-devices. In particular, dendrimers are a relatively new in the field of ocular drug delivery and hence more work needs to be done. Currently, they have been used for anterior chamber drug-delivery applications, although there are concerns of blurring of vision.59 The applications and outcomes of these nanodevices in intra-ocular drug delivery are summarized in table III. The results from these works and their pros and cons suggest that polymeric nanoparticles have a great potential to serve as intraocular cancer chemotherapeutic drug carrier.
Table III.
Effect of material composition and drug delivery routes on the efficacy of nano-device drug delivery in eyes
| Nanodevice | Location | Nanodevice Type | Outcome | References |
|---|---|---|---|---|
| Liposome | Anterior chamber | Cationic liposome-drug and peptide delivery | Better drug delivery than topical ointment. | 31,32 |
| Posterior chamber | Sterically stabilized liposome -Oligonucelotide delivery | High delivery efficiency. | 54,62 | |
| Polymeric nanoparticles | Anterior chamber | Chitosan nanoparticles | High conjunctival & corneal penetration than free drug. Good tissue compatibility | 48,55,56 |
| PLA nanoparticles with PEG coating | High drug delivery | 41,42 | ||
| Posterior chamber | PLA nanoparticles injected intravitreous | Migration toward retina and accumulation in retinal pigment epithelial cells up to 4 months. | 42 | |
| PLGA nanoparticles with pigment epithelium derived factor (PEDF) | Possess neuroprotective effects. | 51 | ||
| Albumin nanoparticles | Accumulate inside the vitreous and ciliary body | 57 | ||
| Dendrimers | Anterior chamber | Poly(acrylic) acid & poly(amidoamine) | Enhanced biorecognition with blurring of vision. | 59,63 |
| Cyclodextrins | Anterior chamber | Cyclic oligosaccharides | Well tolerated with enhanced release of drugs. | 60,63 |
| Nanosuspensions | Anterior chamber | Eudragit RS 100® and RL 100 ® polymer resins | High corneal adhesion Well tolerated since no toxic chemicals. | 58,61,63 |
2.3 Nanoparticles for ocular imaging
Many nanodevices have recently been developed to improve imaging quality of conventional imaging modalities. Despite this progress limited research has been done in using nanodevices to improve conventional ocular imaging modalities like MRI, ultrasound imaging and Optical Coherence Tomography (OCT). Despite limited research efforts, many of these nanodevices have shown great promise in improving the imaging and diagnosis of retinal diseases, including intraocular tumors. Quantum dots have been investigated for their ocular imaging capabilities. They have good optical stability and can facilitate multi-modal detection.64–66 A recent study which demonstrated the diffusion of quantum dots in to the lens has proven that they can be applied in the eye as well.67 In addition to quantum dots, nanoshells,68,69 and gold nanoparticles,70,71 have the potential to serve as good contrast agents for imaging. Magnetic nanoparticles which can provide good contrast for MRI have been successful in an in vitro setting so far.24 The limited research done in this area has been summarized in Table IV.
Table IV.
Potential applications of nanodevices for ocular imaging
| Nanodevice | Pros and Cons | Current applications |
|---|---|---|
| Quantum dots | Superior optical stability, suitable for multi-modal detection, designed to target specific tissue, potential cytotoxity (required encapsulation).64–66 | Diffusion in to lens in vitro. 67 |
| Nanoshells | Good contrast agents for OCT imaging.68 | Gold nanoshells to enhance tissue contrast.69 |
| Gold nanoparticles | Good contrast agents, RES uptake.70 | TNF-gold nanoparticles for tumor targeting.71 |
| Magnetic nanoparticles | Good MRI contrast property. | In vitro use only.72 |
3 Challenges faced in using nanodevices for intraocular tumors
One of the major challenges in the use of nanodevices is their availability in the posterior of the eye which in turn is determined by the administration route. Topically applied nanodevices have to overcome the conjunctival and scleral barriers. Hence the amount of drug delivered to the posterior of the eye would be seriously limited.28 Even if the nanodevices were to be delivered in the posterior of the eye by injection there are problems of internal ocular bleeding and retinal damage, often caused by the injection itself. In addition there is also a high risk of infections. Systemic injections lead to clearance of the nanodevice from the circulation and systemic side effects. A possible solution for this is the use of intravitreal sustained release systems like microspheres and liposomes.28,33,43 Subconjunctival and subtenon routes have also shown a lot of promise in delivering drugs and nanodevices to the posterior of the eye.35
As with any foreign material, the safety and compatibility of the nanodevices is a major concern. The earliest studies involving ocular drug delivery in humans used latex nanoparticles and nanoparticles made from pilocarpine salt and a co-polymer of laurylmethacrylate-acrylic acid. However, neither of them was successful; partly due to its inability to release drugs in a sustained manner and also due to poor biodegradability and high toxicity.73 A lot of importance has to be laid on developing nanodevices from materials which have good ocular biocompatibility. In this light, the fact that FDA approved polymers like PLLA and PLGA are well tolerated by the body assumes a lot of significance. In fact, recent research has focused on biodegradable polymers and smart hydrogels.74–76 PLLA and PLGA nanoparticles have been used to deliver high molecular weight drugs and were found to have accumulated in the retina for long time periods.43,51,52,75 It has been suggested that low molecular weight polymers like PLGA, which degrade rapidly, are suitable candidates for use as micro and nanospheres.77 Poly(ortho esters) which are bioerodible have very good ocular biocompatibility. Poly (ε-caprolactone) is well tolerated by retinal tissue for at least 4 weeks.78 These polymers can be used for delivering hydrophobic drugs. For hydrophilic drugs, hydrophilic polymers of natural origin, like chitosan and hyaluronan serve as good carriers. These are non toxic, biocompatible and biodegradable.79,80 Hydrogels like poly(N-isopropyl acrylamide) (PNIPAM) have been grafted with chitosan to form a thermally responsive ophthalmic drug delivery device.81 Coating with chitosan has proven to be advantageous as many corneal and conjunctival cells have high affinity for chitosan.82 Hence coating with naturally occurring proteins can greatly enhance the ocular biocompatibility.
4 Smart drug delivery and imaging nanodevices for intraocular tumor
The array of nanotechnological approaches in the field of cancer imaging and therapeutics for the rest of the body makes it crucial that more efforts be channeled toward applying them in the field of ocular diagnostics and therapeutics as well. Equally important, in addition to the current single-function nanodevices, part of future research efforts should be placed on developing bi-functional nanodevices to diagnose and to treat cancer simultaneously. Such devices would allow concurrent monitoring of the intraocular tumor response to various localized release chemotherapeutic drug in to the eye.
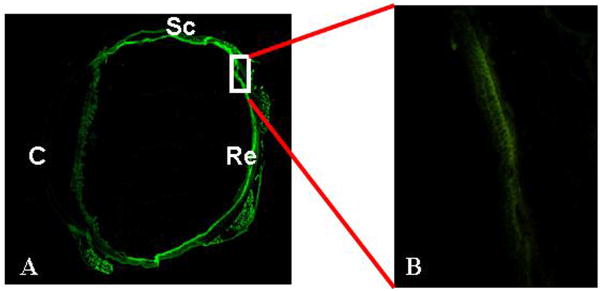
We have recently launched studies toward the development of bi-functional nanodevices with this goal in mind. First, rather unexpectedly, we found that poly (N-isopropylscarylamide) (PNIPAM) nanoparticles injected systemically, accumulated more preferentially in the uveal tissue than microparticles made of the same materials.47 Similarly, intravitreous injection of PNIPAM particles resulted in accumulation in the retina as shown in Figure 2. This could provide many useful clinical options as hydrogel nanoparticles have been shown to have good drug loading and release characteristics.29,83,84 Subsequent studies were carried out to improve the imaging properties of PNIPAM nanoparticles by physically embedding quantum dots in hydrogel nanoparticles.66 Using a subcutaneous melanoma tumor implantation model, we have found that quantum dots encapsulated PNIPAM nanoparticles have superior intratumoral accumulation ability.66 These results support the general concept of bi-functional nanodevices with a quantum dot core and hydrogel shell would aid tumor imaging and drug delivery, respectively. These findings could also be explored for intraocular tumor imaging and treatment.
Figure 2.
PNIPAM nanoparticles conjugated with fluorescein dye were injected intravitreous in rabbit eyes; animals were sacrificed. The tissue sections were then imaged to document the distribution of nanoparticles. Most of the particles accumulated in the retina tissue in 24 hours (A). A closer look at the section under a microscope showed that the particles were preferentially accumulated in the retinal layer (B). Specific ocular tissues are labeled as follows: C: Cornea; Sc: Sclera; Re: Retina
We believe that more efforts should also be placed on engineering intraocular tumor specific nanodevices. Such nanodevices may be produced by placing ligands or antibodies unique to intraocular tumor cell surface markers. In fact, quite a few studies have been done to identify various markers expressed on uveal melanoma tumor cells. For example, cripto-1 is expressed in uveal melanoma cells and this in fact increases as the tumor size increases.85 The expression of osteopontin is an indicator of the metastatic potential of uveal melanoma.86 In addition, our studies have determined the expression of gp 100 and Melan A receptors on more than 95% of the uveal melanoma cells tested. It would be interesting to target these antigens and study the ability of the nanoparticles to be taken up.
5 Conclusion
With the progressive development of nanotechnology in cancer therapy, research efforts are needed to develop cell/tissue-specific nanodevices to meet the challenging demands of intraocular chemotherapy and diagnostics. This review summarizes the overall design criteria and our current understanding on general drug delivery and imaging in eyes. It is our belief that combinatory strategies should be developed to meet different design criteria in order to achieve the ultimate goal of producing “smart nanodevices” against the potent lethal intraocular tumors.
Acknowledgments
This work was supported by a NIH grant RO1 GM074021 and a Texas Higher Education Coordinating Board’s Advanced Technology Program (003594-0003-2006).
References
- 1.Hoppe E, Frankel R. Optometrists as key providers in the prevention and early detection of malignancies. Optometry. 2006;77:397–404. doi: 10.1016/j.optm.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Harbour JW. Eye cancer: unique insights into oncogenesis: the Cogan Lecture. Invest Ophthalmol Vis Sci. 2006;47:1736–1745. doi: 10.1167/iovs.05-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber RW, O’Day S, Rose M, Deck R, Ames P, Good J, Meyer J, Allen R, Trautvetter S, Timmerman M, Cruickshank S, Cook M, Gonzalez R, Spitler LE. Low-dose outpatient chemobiotherapy with temozolomide, granulocyte-macrophage colony stimulating factor, interferon-alpha2b, and recombinant interleukin-2 for the treatment of metastatic melanoma. J Clin Oncol. 2005;23:8992–9000. doi: 10.1200/JCO.2005.02.5791. [DOI] [PubMed] [Google Scholar]
- 4.Triozzi PL, Eng C, Singh AD. Targeted therapy for uveal melanoma. Cancer Treat Rev. 2008;34:247–58. doi: 10.1016/j.ctrv.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Elshaw SR, Sisley K, Cross N, Murray AK, MacNeil SM, Wagner M, Nichols CE, Rennie IG. A comparison of ocular melanocyte and uveal melanoma cell invasion and the implication of alpha1beta1, alpha4beta1 and alpha6beta1 integrins. Br J Ophthalmol. 2001;85:732–738. doi: 10.1136/bjo.85.6.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damato A. Developments in the management of uveal melanoma. Clin Experiment Ophthalmol. 2004;32:639–47. doi: 10.1111/j.1442-9071.2004.00917.x. [DOI] [PubMed] [Google Scholar]
- 7.Boyd SR, Tan D, Bunce C, Gittos A, Neale MH, Hungerford JL, Charnock-Jones S, Cree IA. Vascular endothelial growth factor is elevated in ocular fluids of eyes harbouring uveal melanoma: identification of a potential therapeutic window. Br J Ophthalmol. 2002;86:448–452. doi: 10.1136/bjo.86.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pyrhonen S. The Treatment of Metastatic Uveal Melanoma. Eur J Cancer. 1998;34:S27–S30. doi: 10.1016/s0959-8049(97)10161-7. [DOI] [PubMed] [Google Scholar]
- 9.Atzpodien J, Hanninen E, Kirchner H. Chemoimmunotherapy of advanced malignant melanoma: sequential administration of subcutaneous interleukin-2 and interferon-alpha after intravenous dacarbazine and carboplatin or intravenous dacarbazine, cisplatin, carmustine and tamoxifen. Eur J Cancer. 1995;31A:876–881. doi: 10.1016/0959-8049(94)00459-5. [DOI] [PubMed] [Google Scholar]
- 10.Peters S, Voelter V, Zografos L, Pampallona S, Popescu R, Gillet M, Bosshard W, Fiorentini G, Lotem M, Weitzen R, Keilholz U, Humblet Y, Piperno-Neumann S, Stupp R, Leyvraz S. Intra-arterial hepatic fotemustine for the treatment of liver metastases from uveal melanoma: experience in 101 patients. Ann Oncol. 2006;17:578–583. doi: 10.1093/annonc/mdl009. [DOI] [PubMed] [Google Scholar]
- 11.Mallikarjuna K, Vaijayanthi P, Krishnakumar S. Cripto-1 expression in uveal melanoma: an immunohistochemical study. Exp Eye Res. 2007;84:1060–6. doi: 10.1016/j.exer.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Krause MH, Kwong KK, Gragoudas ES, Young LH. MRI of blood volume with superparamagnetic iron in choroidal melanoma treated with thermotherapy. Magn Reson Imaging. 2004;22:779–787. doi: 10.1016/j.mri.2004.01.052. [DOI] [PubMed] [Google Scholar]
- 13.Francken AB, Fulham MJ, Millward MJ, Thompson JF. Detection of metastatic disease in patients with uveal melanoma using positron emission tomography. Eur J Surg Oncol. 2006;32:780–784. doi: 10.1016/j.ejso.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Leal-Leal A, Flores-Rojo M, Medina-Sanson A, Cerecedo-Diaz F, Sanchez-Felix S, Gonzalez-Ramella O, Perez-Perez F, Gomez-Martinez R, Quero-Hernandez A, Altamirano-Alvarez E, Alejo-Gonzalez F, Figueroa-Carbajal J, Ellis-Irigoyen A, Tejocote-Romero I, Cervantes-Paz R, Pantoja-Guillen F, Vega-Vega L, Carrete-Ramirez F. A multicentre report from the Mexican Retinoblastoma Group. Br J Ophthalmol. 2004;88:1074–7. doi: 10.1136/bjo.2003.035642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karcioglu ZA, al-Mesfer SA, Abboud E, Jabak MH, Mullaney PB. Workup for metastatic retinoblastoma. A review of 261 patients. Ophthalmology. 1997;104:307–312. doi: 10.1016/s0161-6420(97)30319-4. [DOI] [PubMed] [Google Scholar]
- 16.Chintagumpala M, Chevez-Barrios P, Paysse EA, Plon SE, Hurwitz R. Retinoblastoma: review of current management. Oncologist. 2007;12:1237–46. doi: 10.1634/theoncologist.12-10-1237. [DOI] [PubMed] [Google Scholar]
- 17.Smith EV, Gragoudas ES, Kolodny NH, D’Amico DJ. Magnetic resonance imaging: an emerging technique for the diagnosis of ocular disorders. Int Ophthalmol. 1990;14:119–24. doi: 10.1007/BF00154211. [DOI] [PubMed] [Google Scholar]
- 18.Brenner, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001;176:289–96. doi: 10.2214/ajr.176.2.1760289. [DOI] [PubMed] [Google Scholar]
- 19.Coleman K, Baak JP, Van Diest P, Mullaney J, Farrell M, Fenton M. Prognostic factors following enucleation of 111 uveal melanomas. Br J Ophthalmol. 1993;77:688–692. doi: 10.1136/bjo.77.11.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson MW, Czechonska G, Finger PT, Rausen A, Hooper ME, Haik BG. Chemotherapy for eye cancer. Survey of Ophthalmology. 2001;45:416–444. doi: 10.1016/s0039-6257(00)00210-1. [DOI] [PubMed] [Google Scholar]
- 21.Schmid KE, Kornek GV, Scheithauer W, Binder S. Update on ocular complications of systemic cancer chemotherapy. Survey of Ophthalmology. 2006;51:19–40. doi: 10.1016/j.survophthal.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Sutton D, Nasongkla N, Blanco E, Gao J. Functionalized micellar systems for cancer targeted drug delivery. Pharmaceutical Research. 2007;24:1029–1046. doi: 10.1007/s11095-006-9223-y. [DOI] [PubMed] [Google Scholar]
- 23.Micames CG, Gress FG. Local EUS-guided injection of chemotherapeutic agents as adjuvant to systemic treatment: the first steps are made. Gastrointestinal Endoscopy. 2007;65:454–456. doi: 10.1016/j.gie.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Kim H, Lizak MJ, Tansey G, Csaky KG, Robinson MR, Yuan P, Wang NS, Lutz RJ. Study of ocular transport of drugs released from an intravitreal implant using magnetic resonance imaging. Ann Biomed Eng. 2005;33:150–164. doi: 10.1007/s10439-005-8974-7. [DOI] [PubMed] [Google Scholar]
- 25.Bourges JL, Bloquel C, Thomas A, Froussart F, Bochot A, Azan F, Gurny R, BenEzra D, Behar-Cohen F. Intraocular implants for extended drug delivery: therapeutic applications. Adv Drug Del Rev. 2006;58:1182–1202. doi: 10.1016/j.addr.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 26.Booth BA, Vidal Denham L, Bouhanik S, Jacob J, Hill JM. Sustained-release ophthalmic drug delivery systems for treatment of macular disorders: present and future applications. Review article Drugs & Aging. 2007;24:581–602. doi: 10.2165/00002512-200724070-00006. [DOI] [PubMed] [Google Scholar]
- 27.Ali Y, Lehmussaari K. Industrial perspective in ocular drug delivery. Adv Drug Del Rev. 2006;58:1258–1268. doi: 10.1016/j.addr.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006;58:1131–5. doi: 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 29.Sultana Y, Jain R, Aqil M, Ali A. Review of ocular drug delivery. Current Drug Delivery. 2006;3:207–217. doi: 10.2174/156720106776359186. [DOI] [PubMed] [Google Scholar]
- 30.Sakurai E, Ozeki H, Kunou N, Ogura Y. Effect of particle size of polymeric nanospheres on intravitreal kinetics. Ophthalmic Res. 2001;33:31–36. doi: 10.1159/000055638. [DOI] [PubMed] [Google Scholar]
- 31.Cortesi R, Argnani R, Esposito E, Dalpiaz A, Scatturin A, Bortolotti F, Lufino M, Guerrini R, Cavicchioni G, Incorvaia C, Menegatti E, Manservigi R. Cationic liposomes as potential carriers for ocular administration of peptides with anti-herpetic activity. Int J Pharm. 2006;317:90–100. doi: 10.1016/j.ijpharm.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 32.Chetoni P, Rossi S, Burgalassi S, Monti D, Mariotti S, Saettone MF. Comparison of liposome-encapsulated acyclovir with acyclovir ointment: ocular pharmacokinetics in rabbits. J Ocul Pharmacol Ther. 2004;20:169–77. doi: 10.1089/108076804773710849. [DOI] [PubMed] [Google Scholar]
- 33.Mainardes RM, Urban MC, Cinto PO, Khalil NM, Chaud MV, Evangelista RC, Gremiao MP. Colloidal carriers for ophthalmic drug delivery. Curr Drug Targets. 2005;6:363–71. doi: 10.2174/1389450053765914. [DOI] [PubMed] [Google Scholar]
- 34.Hughes PM, Olejnik O, Chang-Lin JE, Wilson CG. Topical and systemic drug delivery to the posterior segments. Adv Drug Deliv Rev. 2005;57:2010–2032. doi: 10.1016/j.addr.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Janoria KG, Gunda S, Boddu SHS, Mitra AK. Novel approaches to retinal drug delivery. Expert Opin Drug Deliv. 2007;4:371–388. doi: 10.1517/17425247.4.4.371. [DOI] [PubMed] [Google Scholar]
- 36.Raghava S, Hammond M, Kompella UB. Periocular routes for retinal drug delivery. Expert Opin Drug Deliv. 2004;1:99–114. doi: 10.1517/17425247.1.1.99. [DOI] [PubMed] [Google Scholar]
- 37.Amrite AC, Kompella UB. Size-dependent disposition of nanoparticles and microparticles following subconjunctival administration. J Pharm Pharmacol. 2005;57:1555–1563. doi: 10.1211/jpp.57.12.0005. [DOI] [PubMed] [Google Scholar]
- 38.Amrite AC, Edelhauser HF, Singh SR, Kompella UB. Effect of circulation on the disposition and ocular tissue distribution of 20 nm nanoparticles after periocular administration. Mol Vis. 2008;14:150–160. [PMC free article] [PubMed] [Google Scholar]
- 39.Vandervoort J, Ludwig A. Ocular drug delivery: nanomedicine applications. Nanomed. 2007;2:11–21. doi: 10.2217/17435889.2.1.11. [DOI] [PubMed] [Google Scholar]
- 40.Afouna MI, Khattab IS, Reddy IK. Preparation and characterization of demeclocycline liposomal formulations and assessment of their intraocular pressure-lowering effects. Cutan Ocul Toxicol. 2005;24:111–24. doi: 10.1081/CUS-200059582. [DOI] [PubMed] [Google Scholar]
- 41.Vega, Egea MA, Valls O, Espina M, Garcia ML. Flurbiprofen loaded biodegradable nanoparticles for ophthalmic administration. J Pharm Sci. 2006;95:2393–405. doi: 10.1002/jps.20685. [DOI] [PubMed] [Google Scholar]
- 42.Bourges JL, Gautier SE, Delie F, Bejjani RA, Jeanny JC, Gurny R, BenEzra D, Behar-Cohen FF. Ocular drug delivery targeting the retina and retinal pigment epithelium using polylactide nanoparticles. Invest Ophthalmol Vis Sci. 2003;44:3562–9. doi: 10.1167/iovs.02-1068. [DOI] [PubMed] [Google Scholar]
- 43.Yasukawa T, Tabata Y, Kimura H, Kunou N, Ogura Y. Development of drug-delivery systems to the posterior segments of the eye. Exp Rev Ophthalmol. 2007;2:197–211. [Google Scholar]
- 44.Bodor N, Buchwald P. Ophthalmic drug design based on the metabolic activity of the eye: soft drugs and chemical delivery systems. The AAPS Journal. 2005;7 doi: 10.1208/aapsj070479. Article 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barbault-Foucher S, Gref R, Russo P, Guechot J, Bochot A. Design of poly-ε-caprolactone nanospheres coated with bioadhesive hyaluronic acid for ocular delivery. J Cont Rel. 2002;83:365–375. doi: 10.1016/s0168-3659(02)00207-9. [DOI] [PubMed] [Google Scholar]
- 46.Hsu J. Drug delivery methods for posterior segment disease. Retinal, vitreous and macular disorders. Curr Opin Ophthalmol. 2007;18:235–239. doi: 10.1097/ICU.0b013e3281108000. [DOI] [PubMed] [Google Scholar]
- 47.Zhang S, Zhou J, Hu Z, Nair A, Tang L. Nanoparticles for uveal melanoma treatment. Proc IEEE Nano2008 Conference; 2008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Campos M, Diebold Y, Carvalho EL, Sanchez A, Alonso MJ. Chitosan nanoparticles as new ocular drug delivery systems: in vitro stability, in vivo fate, and cellular toxicity. Pharm Res. 2004;21:803–10. doi: 10.1023/b:pham.0000026432.75781.cb. [DOI] [PubMed] [Google Scholar]
- 49.Cardillo JA, Melo LAS, Jr, Costa RA, Skaf M, Belfort R, Jr, Souza-Filho AA, Farah ME, Kuppermann BD. Comparison of intravitreal versus posterior sub-tenon’s capsule injection of triamcinolone acetonide for diffuse diabetic macular edema. Opthalmol. 2005;112:1557–1563. doi: 10.1016/j.ophtha.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 50.Thomas ER, Wang J, Edge E, Madsen R, Hainsworth DP. Intravitreal triamcinolone acetonide concentration after subtenon injection. Am J Ophthalmol. 2006;142:860–861. doi: 10.1016/j.ajo.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 51.Li H, Tran VV, Hu Y, Mark Saltzman W, Barnstable CJ, Tombran-Tink J. A PEDF N-terminal peptide protects the retina from ischemic injury when delivered in PLGA nanospheres. Exp Eye Res. 2006;83:824–33. doi: 10.1016/j.exer.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 52.Quintana A, Raczka E, Piehler L, Lee I, Myc A, Majoros I, Patri AK, Thomas T, Mule J, Baker JR., Jr Design and function of a dendrimer-based therapeutic nanodevice targeted to tumor cells through the folate receptor. Pharm Res. 2002;19:1310–6. doi: 10.1023/a:1020398624602. [DOI] [PubMed] [Google Scholar]
- 53.Barbu E, Verestiuc L, Nevell TG, Tsibouklis J. Polymeric materials for ophthalmic drug delivery: trends and perspectives. J Mat Chem. 2006;16:3439–3443. [Google Scholar]
- 54.Kawakami S, Harada A, Sakanaka K, Nishida K, Nakamura J, Sakaeda T, Ichikawa N, Nakashima M, Sasaki H. In vivo gene transfection via intravitreal injection of cationic liposome/plasmid DNA complexes in rabbits. Int J Pharm. 2004;278:255–262. doi: 10.1016/j.ijpharm.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 55.Yuan X, Li H, Yuan Y. Preparation of cholesterol-modified chitosan self-aggregated nanoparticles for delivery of drugs to ocular surface. Carbohydr Polym. 2006;65:337–345. [Google Scholar]
- 56.Enriquez de Salamanca, Diebold Y, Calonge M, Garcia-Vazquez C, Callejo S, Vila A, Alonso MJ. Chitosan nanoparticles as a potential drug delivery system for the ocular surface: toxicity, uptake mechanism and in vivo tolerance. Invest Ophthalmol Vis Sci. 2006;47:1416–25. doi: 10.1167/iovs.05-0495. [DOI] [PubMed] [Google Scholar]
- 57.Merodio M, Irache JM, Valamanesh F, Mirshahi M. Ocular disposition and tolerance of ganciclovir-loaded albumin nanoparticles after intravitreal injection in rats. Biomaterials. 2002;23:1587–1594. doi: 10.1016/s0142-9612(01)00284-8. [DOI] [PubMed] [Google Scholar]
- 58.Bu Z, Gukasyan HJ, Goulet L, Lou XJ, Xiang C, Koudriakova T. Ocular disposition, pharmacokinetics, efficacy and safety of nanoparticle-formulated ophthalmic drugs. Curr Drug Metab. 2007;8:91–107. doi: 10.2174/138920007779815977. [DOI] [PubMed] [Google Scholar]
- 59.Padilla De Jesus L, Ihre HR, Gagne L, Frechet JM, Szoka FC., Jr Polyester dendritic systems for drug delivery applications: in vitro and in vivo evaluation. Bioconjug Chem. 2002;13:453–61. doi: 10.1021/bc010103m. [DOI] [PubMed] [Google Scholar]
- 60.Pignatello R, Bucolo C, Spedalieri G, Maltese A, Puglisi G. Flurbiprofen-loaded acrylate polymer nanosuspensions for ophthalmic application. Biomaterials. 2002;23:3247–55. doi: 10.1016/s0142-9612(02)00080-7. [DOI] [PubMed] [Google Scholar]
- 61.Kawashima Y, Niwa T, Handa T, Takeuchi H, Iwamoto T, Itoh K. Preparation of controlled-release microspheres of ibuprofen with acrylic polymers by a novel quasi-emulsion solvent diffusion method. J Pharm Sci. 1989;78:68–72. doi: 10.1002/jps.2600780118. [DOI] [PubMed] [Google Scholar]
- 62.De Campos M, Sanchez A, Alonso MJ. Chitosan nanoparticles: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin. A Int J Pharm. 2001;224:159–68. doi: 10.1016/s0378-5173(01)00760-8. [DOI] [PubMed] [Google Scholar]
- 63.Sahoo SK, Dilnawaz F, Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov Today. 2008;13:144–51. doi: 10.1016/j.drudis.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 64.Gao X, Yang L, Petros JA, Marshall FF, Simons JW, Nie S. In vivo molecular and cellular imaging with quantum dots. Curr Opin Biotechnol. 2005;16:63–72. doi: 10.1016/j.copbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 65.Dubertret, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 2002;298:1759–62. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 66.Nair A, Shen J, Thevenot P, Cai T, Hu Z, Tang L. Novel quantum dots for enhanced tumor imaging. Proc IEEE Nano2008 Conference; 2008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schachar RA, Chen W, Woo BK, Pierscionek BK, Zhang X, Ma L. Diffusion of nanoparticles in to the capsule and cortex of a crystalline lens. Nanotechnology. 2008;19:1–4. doi: 10.1088/0957-4484/19/02/025102. [DOI] [PubMed] [Google Scholar]
- 68.Loo A, Lowery A, Halas N, West J, Drezek R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Letters. 2005;5:709–11. doi: 10.1021/nl050127s. [DOI] [PubMed] [Google Scholar]
- 69.Faber DJ, van Velthoven MEJ, de Bruin M, Aalders MCG, Verbraak FD, Graf C, van Leeuwen TG. NAOMI: nanoparticles assisted optical molecular imaging. Proc SPIEE. 2006;6079:607905. [Google Scholar]
- 70.Oyewumi O, Yokel RA, Jay M, Coakley T, Mumper RJ. Comparison of cell uptake, biodistribution and tumor retention of folate-coated and PEG-coated gadolinium nanoparticles in tumor-bearing mice. J Cont Rel. 2004;95:613–26. doi: 10.1016/j.jconrel.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 71.Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004;11:169–83. doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- 72.Holligan DL, Gillies GT, Dailey JP. Magnetic guidance of ferrofluidic nanoparticles in an in vitro model of intraocular retinal repair. Nanotechnology. 2003;14:661–666. [Google Scholar]
- 73.Ding S. Recent developments in ophthalmic drug delivery. Pharm Sc Tech Today. 1998;1:328–335. [Google Scholar]
- 74.Huang S-F, Chen J-L, Yeh M-K, Chiang C-H. Physicochemical properties and in vivo assessment of Timolol-loaded poly (D,L-lactide-co-glycolide) films for long-term intraocular pressure lowering effects. J Ocul Pharmacol Ther. 2005;21:445–453. doi: 10.1089/jop.2005.21.445. [DOI] [PubMed] [Google Scholar]
- 75.Andrieu-Soler C, Aubert-pouessel A, Doat M, Picaud S, Halhal M, Simonutti M, Venier-Julienne MC, Benoit JP, Behar-Cohen F. Intravitreous injection of PLGA microspheres encapsulating GDNF promotes the survival of photoreceptors in the rd1/rd1 mouse. Molecular Vision. 2005;11:1002–1011. [PubMed] [Google Scholar]
- 76.De Faria TJ, De Campos AM, Senna EL. Preparation and characterization of poly (d,l-lactide) (PLA) and poly (d,l-lactide)-poly (ethylene glycol) (PLA-PEG) nanocapsules containing antitumoral agent methotrexate. Macromol Symp. 2005;229:228–233. [Google Scholar]
- 77.Yasukawa T, Ogura Y, Sakurai E, Tabata Y, Kimura H. Intraocular sustained drug delivery using implantable polymeric devices. Adv Drug Delivery Rev. 2005;57:2033–2046. doi: 10.1016/j.addr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 78.Beeley NRF, Rossi JV, Mello-Filho PA, Mahmoud MI, Fujii GY, de Juan E, Jr, Varner SE. Fabrication, implantation, elution, and retrieval of a steroid-loaded polycaprolactone subretinal implant. J Biomed Mater Res. 2005;73A:437–444. doi: 10.1002/jbm.a.30294. [DOI] [PubMed] [Google Scholar]
- 79.Prabaharan M, Mano JF. Chitosan-based particles as controlled drug delivery systems. Drug Delivery. 2005;12:41–57. doi: 10.1080/10717540590889781. [DOI] [PubMed] [Google Scholar]
- 80.Liao YH. Hyaluronan: Pharmaceutical characterization and drug delivery. Drug Delivery. 2005;12:327–342. doi: 10.1080/10717540590952555. [DOI] [PubMed] [Google Scholar]
- 81.Cao Y, Zhang C, Shen W, Cheng Z, Yu L, Ping Q. Poly (N-isopropylacrylamide)-chitosan as thermosensitive in situ gel-forming system for ocular drug delivery. J Cont Rel. 2007;120:186–194. doi: 10.1016/j.jconrel.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 82.Yoo MK, Cho KY, Song HH, Choi YJ. Release of ciprofloxacin from chondroitin 6-sulfate-graft-poloxamer hydrogel in vitro for ophthalmic drug delivery. Drug Dev, Ind Pharm. 2005;31:455–463. doi: 10.1080/03639040500214688. [DOI] [PubMed] [Google Scholar]
- 83.Cho KY, Chung TW, Kim BC, Kim MK, Lee JH, Wee WR, Cho CS. Release of ciprofloxacin from poloxamers-graft-hyaluronic acid hydrogels in vitro. Int J Pharm. 2003;260:83–91. doi: 10.1016/s0378-5173(03)00259-x. [DOI] [PubMed] [Google Scholar]
- 84.Venkatesh S, Sizemore SP, Byrne ME. Biomimetic hydrogels for enhanced loading and extended release of ocular therapeutics. Biomaterials. 2007;28:717–724. doi: 10.1016/j.biomaterials.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 85.Reiniger W, Wolf A, Welge-Lussen U, Mueller AJ, Kampik A, Schaller UC. Osteopontin as a serologic marker for metastatic uveal melanoma: results of a pilot study. Am J Ophthalmol. 2007;143:705–7. doi: 10.1016/j.ajo.2006.11.040. [DOI] [PubMed] [Google Scholar]