Abstract
In the light of recently disclosed crystallographic studies on migrastatin ketone 2 complex with fascin, the structures of migrastatin ketone 2 and migrastatin ether 4 have been re-evaluated by NMR and X-Ray crystallographic techniques. The results of these studies established the correctness of the previously reported structural assignment and confirm that the “small molecule” co-crystallized in complex with fascin is not the migrastatin ketone, which was provided for the infusion experiment, but rather its stereoisomer.
The devastation and lethality of many cancers are enhanced by the migration of the elements of the tumor from its primary site to distal sites, which then become new launching areas for progression.1 One way to counter the deadly phenomenon of metastasis would be through inhibition of such migration. Accordingly, we took a keen interest following disclosure of the natural product migrastatin 1 (Figure 1) isolated from Streptomyces sp. MK929-43F1. 2 As its name implies, this compound inhibits the migration of cancer cells, albeit with IC50s in the μM range.
Figure 1.
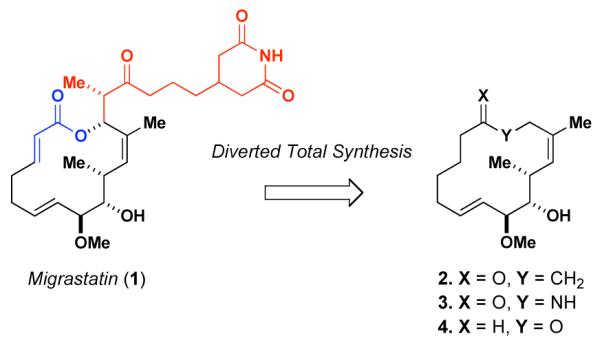
These properties of 1 prompted us to pursue its total synthesis. 3, 4 Having accomplished that goal and having confirmed the activity claimed for migrastatin, we undertook an intensive program of diverted total synthesis.5 Indeed, we succeeded in exploiting the total synthesis route 1, as a platform for preparing structurally simpler analogs including ketone 2, lactam 3 and core ether 4.5 Remarkably, these compounds exhibited far greater potency than migrastatin itself, suppressing the migration of cancer both in vitro and in vivo. For instance, as suggested in the mouse models, compounds 2 and 3 achieve reduction of 4T1 murine breast cancer metastasis5c while migrastatin ether 4 significantly reduces the metastatic spread of human MDA231 and LM2 breast cancers.5d Following our recent and intensive in vivo study with compound 4, it was theorized, though certainly not proven, that its impressive activity might reflect its ability to inhibit fascin-1 dependent migratory tendencies.
In this connection, we took particular note of a disclosure by Chen et al. of studies demonstrating that migrastatin ketone 2 does indeed target fascin.6 These studies were conducted on compounds, which were designed and synthesized in our program and furnished to Huang and coworkers as a part of a mechanism-directed collaboration. Indeed, the Huang paper did herald that the ketone analog, 2, binds to one of the actin bundling sites of fascin. In support of this claim, Chen etal. reported X-ray crystal structures for both fascin, in its native state, as well as in a complex with 2 (Figure 2).7
Figure 2.
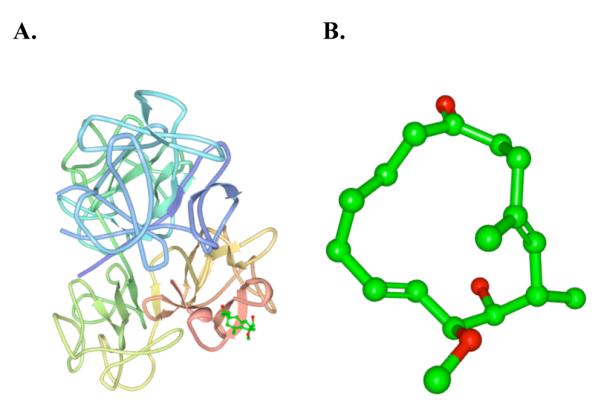
(A) Crystal Structure of Migrastatin Ketone complex with Fascin. (B) Magnified structure of Migrastatin Ketone in complex with Fascin.
a These images were obtained from the Protein Data Bank (PDB) website and visualized and processed with Protein Workshop software.7
Given this context, we were particularly surprised upon closer inspection of the migrastatin ketone (2) complex with fascin, that the “small molecule” binding to the protein emerged not as 2, which was furnished, but rather as a stereoisomer of 2, i.e. compound 5 (Figure 3). This structure is different from the compound we had supplied in both the geometry of the 4,5-double bond (Z in 2; E in 5) and the configuration at C6 (R in 2, S in 5). While we had been quite confident of our structural assignments of 2–4 based on their linkage to our eventual total synthesis of 1 by rational chemistry and supportive characterization, the crystallograpically-based disclosure by Chen et al. depicting structure 5, rather than 2, obliged us to re-visit those assignments. Accordingly, we re-analyzed samples of 2 (and 4) routinely furnished to Huang and coworkers at purity levels of > 98%.
Figure 3.
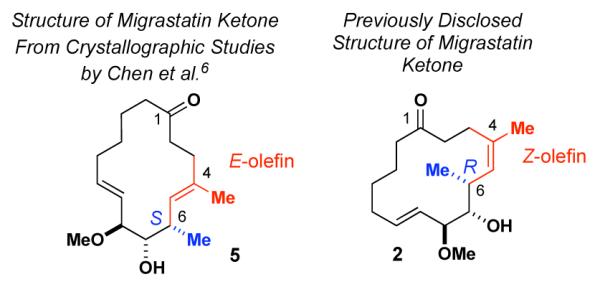
Structural Features of Migrastatin Ketone (2) and Small Molecule Bound to Fascin (5).
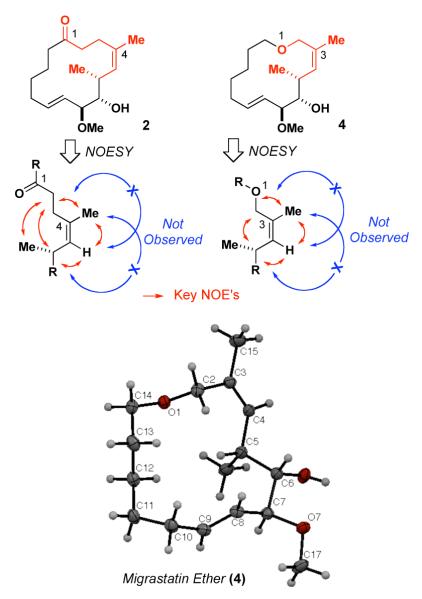
First, we addressed the issue of the olefin geometry by obtaining the corresponding NOESY spectra for 2 and 4 (cf. Figure 4).8 Thus, if the isomerization of the tri-substituted olefin from Z– to E–geometry indeed took place during the elaboration of 10 to 2 or 4, one would observe no NOE signal between the C4 methyl group and C5 hydrogen of 2 (and C3 methyl group and C4 hydrogen of 4). Also, there would not be any NOE correlation between C3 or C2 methylenes and C6 methine of 2 (and C2 methylene and C5 methine of 4). The examination of the NOESY spectra of 2 and 4 did not provide any support for the presence of the E-trisubstituted olefins in these compounds (cf. Figure 4). Quite contrary, the observed NOE correlations are only consistent with the Z-olefin geometry for both 2 and 4, as originally assigned.5 Specifically, for migrastatin ketone 2 there are observed strong NOE correlations between C4 methyl group and C5 hydrogen, and C2, C3 methylenes and C6 methine, and no correlations between C4 methyl group and C6 methine or C5 hydrogen and C2, C3 methylenes. These experiments are further supported by X-ray crystallographic analysis of 4 (Figure 4), which unambiguously confirmed the structure of migrastatin ether originally disclosed by our group.
Figure 4.
Observed NOE’s for the Trisubstituted Olefins of 2 and 4 and X-Ray Structure of 4.
Given the fact that we gained access to 2 and 4 in almost identical fashion from a very well-characterized intermediate, and that the NOESY NMR experiments serve as a reliable instrument in establishing the olefin geometry of 4, we remain confident that isomerization had not occured during our synthesis of 2 and that the “small molecule” bound to fascin as described by Chen et al. is not migrastatin ketone 2, but rather its isomer.
The significance of these serious chemistry level discrepancies on the structural biology based picture of “small molecule”-to-fascin binding remains to be sorted out and is the subject of an ongoing study.
Supplementary Material
Acknowledgments
This work was supported by NIH grant CA103823 (SJD). P.N. thanks the National Institutes of Health for a Ruth L. Kirschstein postdoctoral fellowship (CA125934-02).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1(a).Hedley BD, Winquist E, Chambers AF. Expert Opin. Ther. Targets. 2004;8:527–536. doi: 10.1517/14728222.8.6.527. [DOI] [PubMed] [Google Scholar]; (b) Perez L, Danishefsky SJ. ACS Chem. Biol. 2007;2:159–162. doi: 10.1021/cb7000395. [DOI] [PubMed] [Google Scholar]
- 2(a).Nakae K, Yoshimoto Y, Sawa T, Homma Y, Hamada M, Takeuchi T, Imoto M. J. Antibiot. 2000;53:1130–1136. doi: 10.7164/antibiotics.53.1130. [DOI] [PubMed] [Google Scholar]; (b) Nakae K, Yoshimoto Y, Ueda M, Sawa T, Takahashi Y, Naganawa H, Takeuchi T, Imoto M. J. Antibiot. 2000;53:1228–1230. doi: 10.7164/antibiotics.53.1228. [DOI] [PubMed] [Google Scholar]; (c) Takemoto Y, Nakae K, Kawatani M, Tahahashi Y, Naganawa H, Imoto M. J. Antibiot. 2001;54:1104–1107. doi: 10.7164/antibiotics.54.1104. [DOI] [PubMed] [Google Scholar]; (d) Nakamura H, Takahashi Y, Naganawa H, Nakae K, Imoto M, Shiro M, Matsumura K, Watanabe H, Kitahara T. J. Antibiot. 2002;55:442–444. doi: 10.7164/antibiotics.55.442. [DOI] [PubMed] [Google Scholar]
- 3(a).Gaul C, Danishefsky SJ. Tetrahedron Lett. 2002;43:9039–9042. [Google Scholar]; (b) Gaul C, Njardarson JT, Danishefsky SJ. J. Am. Chem. Soc. 2003;125:6042–6043. doi: 10.1021/ja0349103. [DOI] [PubMed] [Google Scholar]
- 4.For the review of the recent work on the synthesis of migrastatin and its analogs refer to: Reymond S, Cossy J. C. R. Chimie. 2008;11:1447–1462.
- 5(a).Njardarson JT, Gaul C, Shan D, Huang X-Y, Danishefsky SJ. J. Am. Chem. Soc. 2004;126:1038–1040. doi: 10.1021/ja039714a. [DOI] [PubMed] [Google Scholar]; (b) Gaul C, Njardarson JT, Shan D, Dorn DC, Wu K-D, Tong WP, Huang X-Y, Moore MAS, Danishefsky SJ. J. Am. Chem. Soc. 2004;126:11326–11337. doi: 10.1021/ja048779q. [DOI] [PubMed] [Google Scholar]; (c) Shan D, Chen L, Njardarson JT, Gaul C, Ma X, Danishefsky SJ, Huang X-Y. Proc. Natl. Acad. Sci. U.S.A. 2005;102:3772–3776. doi: 10.1073/pnas.0500658102. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Oskarsson T, Nagorny P, Krauss I, Perez L, Mandal M, Yang G, Ouerfelli O, Xiao D, Moore M, Massague J, Danishefsky SJ. J. Am. Chem. Soc. 2010;132:3224–3228. doi: 10.1021/ja9101503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Yang S, Jakoncic J, Zhang JJ, Huang X-Y. Nature. 2010;464:1062–1066. doi: 10.1038/nature08978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. The structures of the migrastatin ketone/fascin complex were accessed through Protein Data Bank: http://www.pdb.org/pdb/explore/explore.do?structureId=3LNA.
- 8.The 1H NMR signal assignment, and 1H, COSY and NOESY NMR spectra of 2 and 4 are provided in the supporting information.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.