Abstract
In the present study, we investigated the role of Toll-like receptors (TLRs) in host responses to the saliva-binding region (SBR) of Streptococcus mutans expressed by a recombinant, attenuated Salmonella vaccine. C57BL/6 wild type (wt), TLR2−/−, TLR4−/− and MyD88−/− mice were immunized by the intranasal route on days 0, 18 and boosted on day 98 with Salmonella typhimurium BRD 509 containing a plasmid encoding SBR. Serum and saliva samples were collected throughout the experiment and assessed for antibody activity by ELISA. Evidence is provided that the induction of a serum IgG2a (Th1-type) anti-SBR antibody response involved TLR2 signaling, whereas the anti-Salmonella response involved signaling through TLR4. The adaptor molecule MyD88 was not essential for the induction of a primary Th1-type response to SBR or Salmonella, but was necessary for a secondary response to SBR. Furthermore, the absence of TLR2, TLR4 or MyD88 resulted in enhanced Th2-type serum IgG1 anti-SBR and anti-Salmonella responses. Mucosal IgA responses to SBR were TLR2-, TLR4- and MyD88-dependent, while IgA responses to Salmonella were TLR4- and MyD88-dependent.
Keywords: TLRs, Streptococcus mutans: Salmonella
1. Introduction
Streptococcus mutans is a principal etiologic agent of human dental caries [1]. Although dental caries is not a life-threatening disease, it is among the most prevalent and costly diseases in both developing and industrialized countries, and the development of a safe and effective vaccine is viewed as a beneficial preventive measure [2]. The tropism of S. mutans for the saliva-coated tooth surfaces depends on the presence of the saliva-binding region (SBR) of antigen (Ag) I/II [3]. SBR is involved in the initial adherence of the bacterium to the tooth surface and is localized at the N-terminal one-third of the AgI/II molecule, which contains the alanine-rich repeat region [2, 4–6]. The postulated involvement of the SBR in S. mutans colonization suggests that it is an important immunogenic component for use in the development of a caries vaccine [2]. In this regard, human secretory immunoglobulin A (IgA) antibodies to the whole AgI/II molecule, as well as rabbit IgG antibodies to an AgI/II segment, which contains the SBR, have been shown to inhibit the adherence of S. mutans to saliva-coated hydroxyapatite [7, 8].
The ability of a live antigen-delivery system to invade the mucosal IgA induction tissues and to persist there while continuing to produce a heterologous antigen are considered to be significant advantages for the development of a mucosal vaccine. This is in contrast to the use of a vaccine consisting of a soluble protein that is usually denatured by low pH in the stomach and degraded by enzymes in the gut when given via the oral route [9]. Furthermore, the use of a vaccine consisting of a live antigen-delivery system eliminates the requirement for purification of the vaccine protein. We have used attenuated Salmonella enterica serovar Typhimurium BRD509, a vaccine strain with aroA aroD mutations causing an inability to produce or obtain essential metabolites in a mammalian host [10], for targeted delivery of the expressed cloned SBR antigen to gut- and nose-associated lymphoid tissues (NALT) in mice [11, 12]. We have reported the induction of high levels of antibodies against the cloned heterologous antigen SBR in serum and mucosal secretions of mice after oral or intranasal (i.n.) immunization [12, 13]. The anti-SBR antibody response induced was protective against S. mutans infection [13, 14]. Furthermore, the immune responses induced to the Salmonella and to SBR persisted for a long time [15].
Toll-like receptors (TLRs) play important roles in the initiation of both innate and adaptive immune responses. TLRs are mainly expressed on antigen-presenting cells (APC) including macrophages, monocytes and dendritic cells [16–18]. TLRs activate innate immune responses to invading microorganisms by recognizing pathogen-associated molecule patterns (PAMPs) [16]. For example, TLR2 is involved in the response to components of gram-positive bacteria [e.g., peptidoglycan (PGN) and lipoproteins/lipopeptides], while TLR4 is required for the recognition of the lipopolysaccharide (LPS) of gram-negative bacteria, such as Salmonella [19–22]. Recognition of microbial products by TLRs expressed on APC can lead to the activation of NF-κB, and the subsequent production of cytokines, as well as an up-regulation in the expression of costimulatory molecules [16, 23–25]. Signal transduction by most of the known TLRs requires the adapter molecule myeloid differentiation factor 88 (MyD88) [16, 23–25]. Thus, MyD88−/− mice have been used as a tool for studying the role of TLRs in innate and adaptive immunity. MyD88−/− animals fail to generate both pro-inflammatory and Th1-type responses when stimulated with most TLR ligands [16, 23–25]. These animals are highly susceptible to infection with a wide variety of pathogens [16, 23–25], indicating a critical role for MyD88 in host resistance to microbial infection.
The purpose of the present study was to determine the role of TLRs in host responses to SBR expressed by the Salmonella vector strain BRD509 under the control of the T7 promoter. Evidence is provided that the induction of serum Th1-type IgG and mucosal IgA antibody responses against SBR is mediated via TLR2 and TLR2 and TLR4 signaling, respectively, while the induction of serum Th1-type IgG and mucosal IgA antibody responses against Salmonella is mediated via TLR4 signaling.
2. Materials and methods
2.1. Preparation of the recombinant, attenuated Salmonella vaccine for immunization
The genetic construction of Salmonella enterica serovar Typhimurium BRD509 expressing the cloned SBR under the control of the T7 promoter used in the present study has been previously described [12, 13]. For intranasal (i.n.) immunization of mice, the recombinant Salmonella was grown and prepared as previously described [12, 13]. Briefly, a freezer stock of Salmonella strain BRD509 pGP1-2/pSBR (T7) was used to inoculate Luria-Bertani (LB) broth (15 ml) supplemented with carbenicillin (50 µg/ml) and kanamycin (50 µg/ml). The culture was grown overnight at 30!C under aerobic condition with shaking to prevent premature induction of protein expression. This culture was re-suspended in 500 ml LB broth with carbenicillin and kanamycin and incubated aerobically at 30!C for 2 h. The culture was harvested at mid-log phase by centrifugation and the cell pellets were suspended in PBS at a concentration of 5 × 1010 colony-forming units (cfu)/ml.
2.2. Immunizations
Groups of female C57BL/6 wild type (wt), TLR2−/−, TLR4−/− and MyD88−/− mice (6 mice per groups, 6–8 weeks of age) were immunized by the i.n. route with the Salmonella vaccine (1 × 109 cfu) on days 0 and 18. Each dose was applied slowly to both nares, in a final volume of 20 µl of PBS. All groups of immunized mice were boosted on day 98, as described for the initial immunization. All animal studies were performed according to the National Institutes of Health guidelines and protocols were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
2.3. Samples collection
Serum and saliva samples were collected on day 0 (pre-immune samples), and on days 15, 27, 42, 55, 70, 82, 97, 104 and 112. The serum samples were obtained from blood collected from the retro-orbital plexus using heparinized micro-hematocrit capillary tubes (Fisher Scientific Co., Pittsburgh, PA). Saliva samples (approximately 100 µl) were collected after the induction of salivary flow by intraperitoneal injection of 5 µg of carbachol (Sigma Chemical Co., St. Louis, MO), as previously described [12, 13, 26]. All samples were stored at −70!C until assayed for antibody activity.
2.4. Evaluation of antibody responses
The levels of IgG-specific antibodies in serum, of IgA-specific antibodies in saliva, and of total salivary IgA were determined by ELISA, as previously described [27]. Briefly, microtiter plates (Nunc, Rosklide, Denmark) were coated overnight at 4!C with purified SBR (2 µg/ml), Salmonella (5 × 108 cfu/ml) or goat anti-mouse IgA (0.25 µg/ml; Southern Biotechnology Associates, Inc., Birmingham, AL). Plates were blocked by incubating for 4 h at room temperature with 0.01 M phosphate buffer (pH 7.2) containing 0.5 M NaCl, 0.15% Tween 20 and 1% bovine serum albumin (BSA). Serial two-fold dilutions of the samples were then added to wells in duplicate and the plates were incubated overnight at 4!C. Samples were developed by the addition of the appropriate HRP-conjugated goat anti-mouse IgG, IgG1, IgG2a or IgA antibody (Southern Biotechnology), followed by o-phenylenediamine substrate (Sigma) with H2O2 [27]. The concentration of antibodies in the samples was determined by interpolation on standard curves generated using a mouse immunoglobulin reference serum (ICN Biomedical, Inc., Costa Mesa, CA) and constructed by a computer program based on four parameter logistic algorithms (Softmax/Molecular Devices Corp., Menlo Park, CA). The levels of IgA antibody activity in saliva samples were expressed as the percent of IgA anti-SBR or anti-Salmonella per total IgA, in order to normalize for variation in total immunoglobulin content in the samples.
2.5. Isolation of CD4+ T cells
CD4+ T cells were purified as previously described [28]. Briefly, mice were sacrificed two weeks after the last immunizations and spleens were surgically removed and placed in ice-cold Hank’s balanced salt solution (HBSS). Using sterile technique, spleen cells were dispersed into a polystyrene Petri dish using a 40-mm cell strainer and a rubber stopper from a sterile 5-ml syringe. The single-cell suspensions were transferred to sterile 15-ml tubes. The tubes were centrifuged at 1,200 rpm for 5 min at 4°C. The supernatant was discarded, and the cell pellet was resuspended in 25 ml ammonium chloride lyses buffer for 5 min at room temperature to lyse erythrocytes. The cell preparations were washed twice with 25 ml HBSS by centrifugation at 1,200 rpm for 5 min at 4°C. The supernatant was discarded and the pellet was resuspended in 25 ml HBSS. CD4+ T cells were purified from the spleen cell suspension by negative selection using a mouse CD4+ subset column, according to the manufacturer's protocol (R&D Systems, Minneapolis, MN). This procedure routinely results in >95% of the cells staining positive for CD4.
2.6. Cytokine and proliferation analysis
In 96-well plates purified splenic CD4+ T cells (2.5 × 106 cells/ml) from immunized C57BL/6 wt, TLR2−/−, TLR4−/−, MyD88−/− and non-immunized C57/BL6 wt mice were co-cultured with irradiated (2,500 rad) splenic feeder cells (5 × 106 cells/ml) in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 50 µM 2-mercaptoethanol, 1 mM sodium pyruvate, 1.5 mg/ml of sodium bicarbonate, 20 mM HEPES, 50 U/ml of penicillin, and 50 µg/ml of streptomycin (complete medium) alone or with SBR (2 µg/ml) or with killed Salmonella (107 equivalent cfu/ml) in a humidified CO2 incubator at 37°C for 4 days. CD4+ T-cell proliferation was determined by adding 0.5 µCi/well of [3H-]thymidine for the last 18 h of 96-h cultures. The amount of [3H]thymidine incorporated into cells was determined using a liquid scintillation counter [28].
2.7. Statistical analysis
The levels of antibody activity were logarithmically transformed to normalize their distribution and homogenize the variances; and statistical analysis (one-way analysis of variance in conjunction with the Tukey multiple-comparisons test) was performed using the InStat program (Graphpad Software, San Diego, CA). The data was finally retransformed and presented as the means ×/÷ standard deviations (SD) for ease of interpretation. Statistical significance of the proliferation responses between groups was evaluated by analysis of variance and the Tukey multiple-comparisons test using the InStat program. Differences between groups were considered significant at the level of P < 0.05.
3. Results
3.1 Role of MyD88 in serum IgG antibody responses
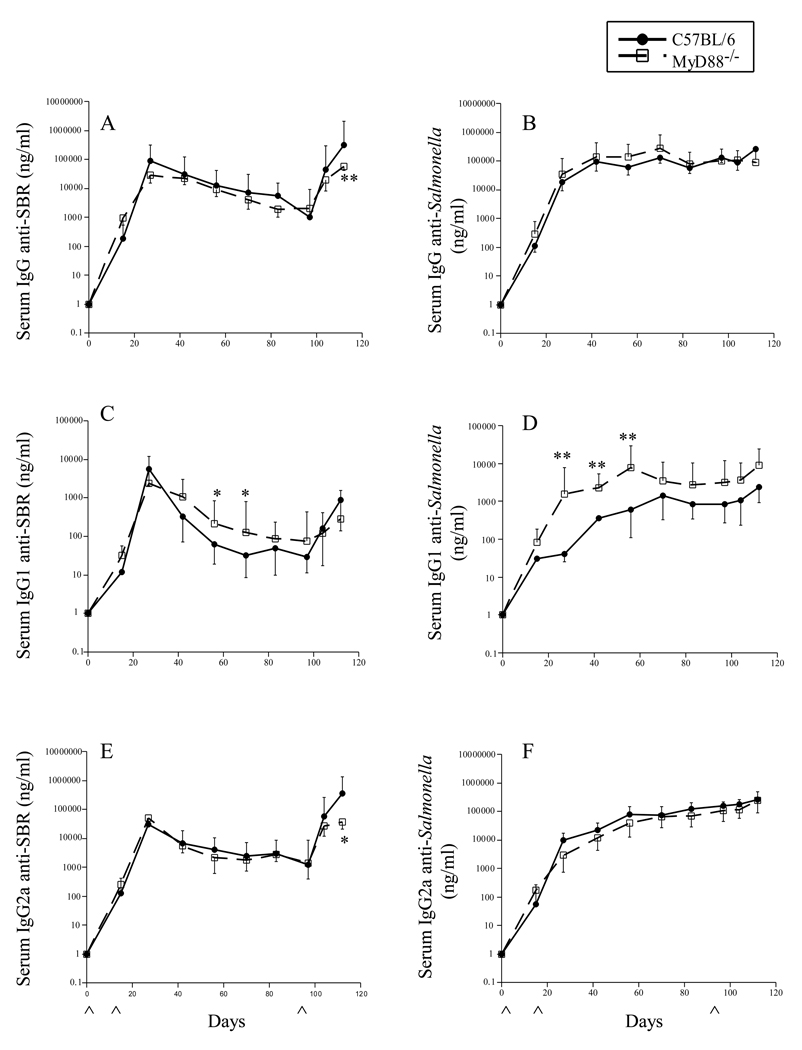
Since MyD88 is an adaptor molecule used by all TLRs except TLR3 [29], we initially assessed the role of MyD88 in adaptive immune response to the expressed cloned surface protein SBR of the gram-positive pathogen S. mutans and to the gram-negative Salmonella expression vector. Groups of wt and MyD88−/− mice were immunized by the i.n. route with the Salmonella vector vaccine and the kinetics of the serum IgG antibody responses to SBR and Salmonella were determined by ELISA. The recombinant, attenuated Salmonella expressing SBR induced serum IgG anti-SBR and anti-Salmonella antibody responses in both wt and MyD88−/− of mice after the initial immunizations (Fig. 1). The serum IgG anti-SBR response peaked on day 27 following the initial immunization, gradually declined, and then an augmented response was seen following the secondary immunization on day 98. The serum IgG antibody response to SBR in MyD88−/− mice was similar to that seen in wt mice following the primary immunization; however, the response in MyD88−/− mice was significantly lower (P < 0.002) than that seen in wt mice after the booster immunization (day 112) (Fig. 1A). These results suggest that signaling through MyD88 may not be necessary for the induction of a primary adaptive immune response to SBR; however, it appears to play a role for the secondary serum IgG anti-SBR response. Since no significant difference was seen in the kinetics of the serum IgG anti-Salmonella response in MyD88−/− and wt mice following the initial and secondary immunization (Fig. 1B), these results suggest that adaptive serum IgG response to Salmonella is independent of MyD88 signaling. These results confirm and extend recent findings demonstrating that MyD88 signaling was not essential for the induction of a serum IgG anti-Salmonella LPS [30] or an anti-Streptococcus pneumoniae surface protein A [31] antibody response following oral immunization of mice with a recombinant attenuated Salmonella Typhimurium vaccine or a recombinant attenuated Salmonella Typhimurium vaccine expressing PspA, respectively.
Fig. 1.
Time course of the serum IgG anti-SBR (A, C, E) and anti-Salmonella (B, D, F) antibody response in mice following i.n. immunization with a Salmonella vaccine clone expressing SBR under the control of the T7 promoter. Both groups of mice were given a single dose of 109 cfu of the Salmonella clone on days 0, 18 and 98. The levels of IgG (A–B), IgG1 (C–D) and IgG2a (E–F) antibody activity to SBR and Salmonella were determined by ELISA. The results are expressed as the mean ×/÷ SD. ^ Indicates time of immunization. Values were significantly different from those of wt mice at P < 0.05 (*) and P < 0.005 (**).
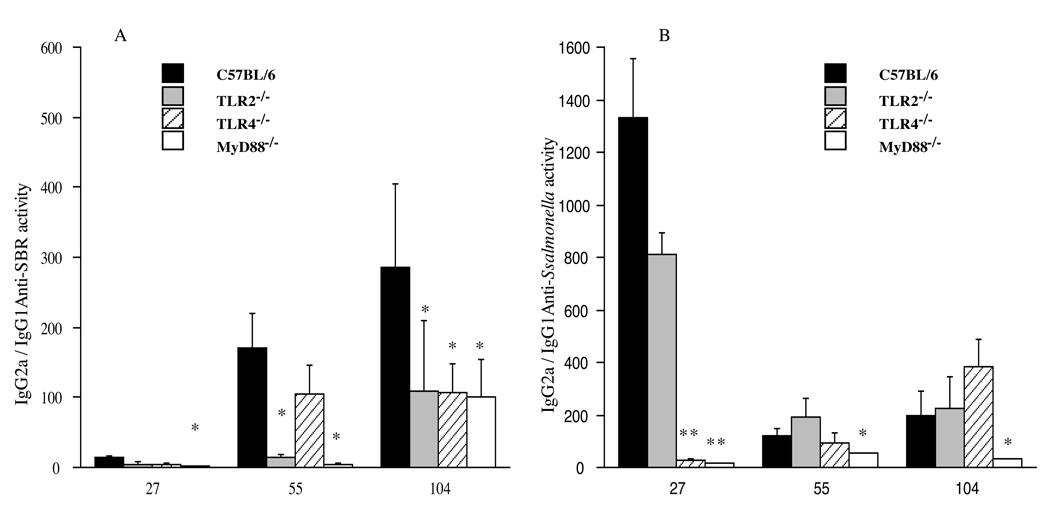
To better understand the nature of the serum IgG response, we next assessed the kinetics of the serum IgG subclass response to SBR and to Salmonella following the initial and booster immunizations (Fig. 1C–1F). The serum IgG1 anti-SBR antibody response in MyD88−/− mice persisted at a higher level than that seen in wt mice following the initial (P < 0.05; days 55 and 70), but not booster immunization (Fig. 1C). In terms of the serum IgG2a anti-SBR response, a similar response was induced in MyD88−/− and wt mice following the initial immunization (Fig. 1E); however, the response in MyD88−/− mice was significantly lower (P < 0.03) than that seen in wt mice following the booster immunization (day 112). Furthermore, the IgG2a to IgG1 ratio of antibodies specific to SBR in MyD88−/− mice was significantly lower compared to that seen in wt mice on days 27 (P < 0.02), 55(P < 0.02), and 104 (P < 0.008) following the initial and booster immunizations (Fig. 3A). These results suggest that the absence of MyD88 results in an enhanced Th2-type IgG antibody response to SBR. However, the results also suggest a role for MyD88 for the secondary Th1-type IgG anti-SBR response.
Fig. 3.
Ratio of serum IgG2a/IgG1 antibodies responses to the cloned antigen SBR (A) and to the Salmonella (B). The results are shown as the mean ×/÷ SD of the IgG2a/IgG1 ratios from pooled serum samples. Values were significantly different from those of wt mice at P <0.05 (*) and P <0.005 (**).
A higher serum IgG1 anti-Salmonella antibody response was observed in MyD88−/− compared to wt mice following the primary and secondary immunization (Fig. 1D). The response in MyD88−/− mice was significantly higher (P < 0.002) on days 27–55. The serum IgG2a anti-Salmonella antibody response in MyD88−/− mice was slightly lower than that seen in wt mice (Fig. 1F). Furthermore, the IgG2a to IgG1 ratio of anti-Salmonella antibody activity in MyD88−/− mice was significantly lower compared to that seen in wt mice on days 27 (P < 0.0001), 55 (P < 0.05), and 104 (P < 0.05) following the initial and booster immunizations (Fig. 3B). Taken together, these results indicate that MyD88 is not essential for the induction of primary Th1-type responses to SBR and Salmonella, whereas the activation of adaptive immune responses and induction of Th1-type effector immune responses to SBR following a secondary mucosal immunization involves MyD88-mediated recognition and signaling. Furthermore, the absence of MyD88 resulted in enhanced Th2-type primary serum IgG immune responses to Salmonella and SBR. These latter results support and extend the recent findings of others demonstrating the induction of a dominant Th2-type response following mucosal immunization of MyD88−/− mice with a recombinant attenuated Salmonella Typhimurium vaccine [30, 31].
3.2 Role of TLR2 and TLR4 in serum IgG antibody responses
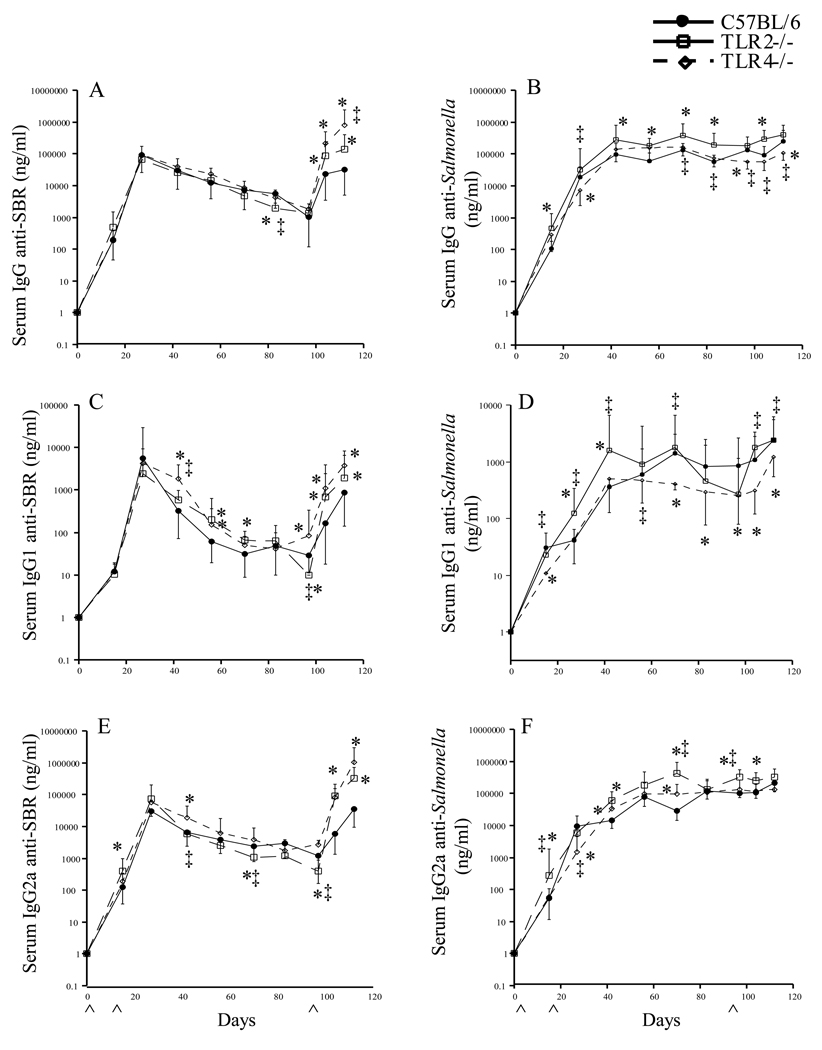
Since TLR2 and TLR4 play a central role in the host’s responses to bacterial components, we next compared the kinetics of the serum IgG responses in TLR2−/− and TLR4−/− mice immunized by the i.n. route with our Salmonella vaccine to that seen in similarly immunized wt mice. A difference in the kinetics of the serum IgG antibody response to SBR and to Salmonella was seen in TLR2−/− and TLR4−/− mice compared to that seen in wt mice (Fig. 2). The serum IgG anti-SBR antibody response diminished more quickly in TLR2−/− compared to wt mice following the initial immunization, and was significantly lower (P < 0.05) on day 97, but significantly higher after booster immunization (Fig. 2A). In terms of the serum IgG anti-Salmonella response pattern, a higher response was seen in TLR2−/− than in wt mice, which was significantly different (P < 0.05) on days 15, 42, 55, 70, 82 and 104 (Fig 2B). The serum IgG anti-SBR response in TLR4−/− mice was also significantly higher (P < 0.05) on days 104 and 112, whereas the anti-Salmonella response was significantly lower (P < 0.05) on days 27, 97 and 112 compared to that seen in TLR2−/− and wt mice following the initial and booster immunizations (Fig. 2A & B). These results indicate that the initial serum IgG anti-SBR response is TLR2-dependent, while recall responses to SBR appear to be TLR2- and TLR4-independent. Furthermore, our results also indicate that the serum IgG anti-Salmonella response is TLR4-dependent and TLR2-independent following the initial and booster immunizations. These results suggest a role for TLR2 for responses to SBR and a role for TLR4 for responses to Salmonella. The elevated antibody response in TLR2−/− and TLR4−/− mice to Salmonella and to SBR, respectively, suggests that these TLRs may act in down-regulating responses. However, further studies will be required to resolve this possibility.
Fig. 2.
Time course of the serum IgG anti-SBR (A, C, E) and anti-Salmonella (B, D, F) antibody response in C57BL/6, TLR2−/− and TLR4−/− mice following i.n. immunization with a Salmonella vaccine clone expressing SBR under the control of the T7 promoter. Immunization procedure is as described in legend to Figure 1. The results are expressed as the mean ×/÷ SD. ^ Indicates time of immunization. Values were significantly different from those of wt mice at P < 0.05 (*) and values were significantly different between TLR2−/− and TLR4−/− at P <0.05 (‡).
TLR2−/− and TLR4−/− mice exhibited a higher serum IgG1 anti-SBR antibody responses compared to wt mice following the initial and booster immunizations, which were significantly higher (P < 0.05) on days 55, 70, 104 and 112 in TLR2−/− mice and on days 42, 55, 104 and 112 in TLR4−/− mice (Fig. 2C). However, following the initial immunization, TLR2−/− mice exhibited a lower serum IgG2a anti-SBR response, which was significantly lower (P < 0.05) on days 70 and 97 compared to that seen in wt mice and significantly lower (P < 0.05) on days 42, 70, and 97 compared to that seen in TLR4−/− mice (Fig. 2 E). The serum IgG2a anti-SBR response in TLR4−/− mice was higher than that seen in wt mice following the initial and booster immunizations. These results suggest a role for TLR2 signaling for Th1-type response to SBR.
In terms of the serum anti-Salmonella response, TLR4−/− mice exhibited lower (P < 0.05) serum IgG1 anti-Salmonella antibody responses than wt mice following the initial (days 15, 70, 97) and booster (days 104, 112) immunization (Fig. 2D). The serum IgG2a anti-Salmonella antibody response in TLR4−/− mice was in general not lower compared to that seen in wt mice, but was significantly lower (P < 0.05) compared to that seen in TLR2−/− mice on days 27, 70 and 97 (Fig. 2F). These results suggest a role for TLR4 for the persistence of the primary and for the secondary serum IgG anti-Salmonella response.
The IgG2a/IgG1 ratio of the anti-SBR antibody response in TLR2−/− mice was significantly lower (P < 0.05) compared to that seen in wt mice on days 55 and 104 following the initial and booster immunizations (Fig. 3A). The IgG2a/IgG1 ratio of anti-SBR antibody activity in TLR4−/− mice was also lower than that seen in wt mice following the initial immunization, but significantly lower (P < 0.01) following the booster immunization (day 104) (Fig. 3A). No significant difference was seen in the IgG2a/IgG1 ratio of the anti-Salmonella antibody responses in TLR2−/− mice compared to that seen in wt mice following the initial and booster immunized with the recombinant, attenuated Salmonella vaccine expressing SBR. However, the IgG2a/IgG1 ratio of the anti-Salmonella antibody responses in TLR4−/− mice was significantly lower compared to that seen in wt mice on days 27 (P < 0.0001) following the initial immunization (Fig. 3B). Taken together, our findings indicate that the serum Th1-type IgG response to SBR is primarily regulated via a TLR2-dependent signaling pathway, while the early serum Th1-type IgG response to Salmonella is mediated via a TLR4-dependent pathway.
3.3. Role of TLR2, TLR4 and MyD88 for mucosal IgA antibody responses to Salmonella and the expressed cloned antigen SBR
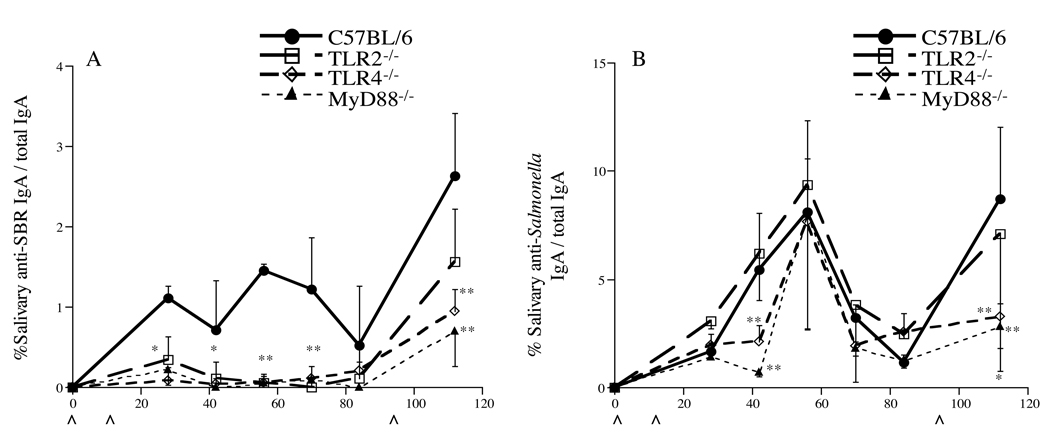
Since mucosal immunity plays a major role in host defense against microbial pathogens at mucosal surfaces (reviewed in [2, 9]), we next determined the role of TLR2, TLR4 and MyD88 for the induction of a mucosal immune response to SBR and Salmonella following i.n. immunization of mice with our recombinant, attenuated Salmonella vaccine. Individual saliva samples were collected throughout the experimental period and assessed for the levels of specific and total salivary IgA activity. Significantly lower salivary IgA anti-SBR responses were observed on days 27– 42 (P < 0.01) and 55–112(P < 0.0001) in TLR2−/−, TLR4−/− and MyD88−/− mice compared to that seen in wt mice (Fig. 4A). However, a significant increase was seen in the % specific/total salivary IgA responses in TLR2−/−, TLR4−/− and wt mice following the booster immunization (day 112) compared to their responses prior to the boost. Only a slightly increased in the salivary anti-SBR response was seen in MyD88−/− mice following the booster immunization. The % salivary IgA anti-Salmonella/total IgA response in TLR4−/− and MyD88−/− mice was significantly lower (P < 0.001) on days 42 and 112 compared to those observed in TLR2−/− and wt mice (Fig. 4B). Taken together, these results indicate that mucosal responses to SBR are mediated via TLR2-, TLR4- and MyD88-dependent signaling pathways, whereas responses to Salmonella are mediated via a TLR4- and MyD88-dependent signaling pathway following mucosal immunization with the recombinant, attenuated Salmonella vaccine expressing SBR.
Fig. 4.
% specific IgA antibody/total IgA antibody level to SBR (A) and to Salmonella (B). Immunization procedure is as described in legend to Figure 1. The results are expressed as the means of the % specific IgA antibody/total IgA antibody ×/÷ SD. Values were significantly different from those of wt mice at P < 0.05 (*) and P < 0.005 (**). ^ Indicates time of immunization.
3.4. Role of TLR2, TLR4 and MyD88 in the response of antigen-specific CD4+ T cells
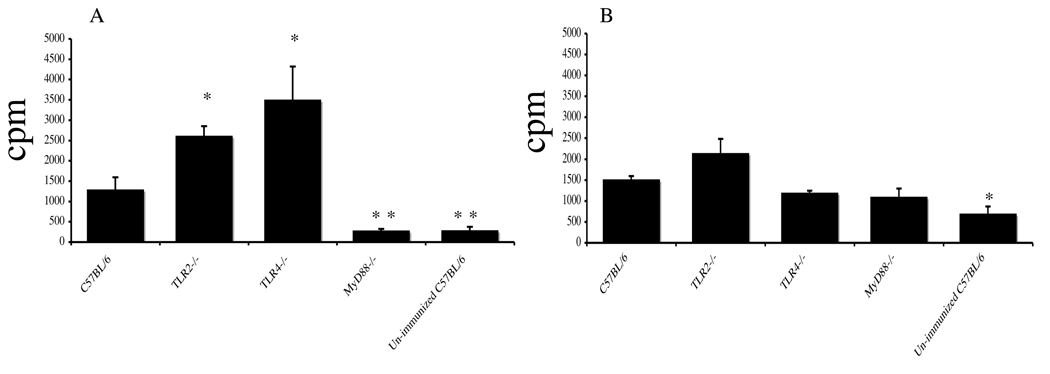
Due to the importance of the TLRs in influencing both qualitative and quantitative aspects of CD4+ T cells responses, we next examined the role of the TLRs on the proliferative response of antigen-specific CD4+ T cells (Fig. 5). Purified splenic CD4+ T cells from immunized wt, TLR2−/−, TLR4−/−, MyD88−/− and non-immunized wt mice were co-cultured with irradiated splenic feeder cells in medium alone or with SBR and/or killed Salmonella. The proliferative response of CD4+ T cells from immunized MyD88−/− mice stimulated with SBR was significantly lower (P < 0.005) compared to that seen with SBR-stimulated CD4+ T cells from immunized wt mice (Fig. 5A). In contrast, the proliferative response of SBR-stimulated CD4+ T cells from immunized TLR2−/− and TLR4−/− mice were significantly higher (P < 0.05) compared to that seen with SBR-stimulated CD4+ T cells from immunized wt mice. The proliferative responses of CD4+ T cells from immunized TLR2−/−, TLR4−/− and MyD88−/− mice stimulated with Salmonella were not significantly different from that seen with Salmonella-stimulated CD4+ T cells from immunized wt mice (Fig. 5B). Taken together, T cells derived from spleens of MyD88−/− mice failed to proliferate in response to SBR, indicating that the SBR-specific priming of T cells were deficient in these mice.
Fig. 5.
T-cell proliferative responses of SBR (A) or Salmonella (B)-specific CD4+ T cells isolated 2 wks after the last immunization of mice. Immunization procedure is as described in legend to Figure 1. CD4+ T cells (2 × 106/ ml) were isolated from the spleens and were co-cultured with irradiated, T cell-depleted splenic feeder cells (5 × 106/ml) in the presence or absence of SBR (2 µg/ml) or of killed Salmonella (107 equivalent cfu/ml). Proliferation of antigen-specific CD4+ T cells was determined following the addition of 0.5 microCi/well of [3H]TdR for the last 18 h of a 96-h culture. The results are expressed as the mean ×/÷ SD. Values were significantly different from those of wt mice at P < 0.05 (*) and P < 0.005 (**).
4. Discussion
We have previously shown that i.n. immunization of mice with a recombinant, attenuated Salmonella vaccine expressing the S. mutans’ adhesin SBR resulted in the induction of salivary and serum antibody responses to SBR and to the attenuated Salmonella [11, 12], and protection against S. mutans infection [13]. However, the functional role of the TLRs and the adaptor protein MyD88 for these immune responses has not been defined. Putative differences in the function of TLR2, TLR4 and MyD88 have been suggested to be potentially due to differences in the patterns of expression, as well as differences in the recognition of different cell wall components [21, 32, 33]. Since differences in the individual roles of TLR2, TLR4 or MyD88 have been observed in vitro and in vivo, it is important to investigate the role of TLRs and their adaptor molecules for responses to specific immunogens. In the present study, we examined the role of TLR2, TLR4 and the adaptor molecule MyD88 in immune responses to SBR expressed by Salmonella under the control of the T7 promoter and to the Salmonella after i.n. immunization of wt, TLR2−/−, TLR4−/− and MyD88−/− mice. Our results demonstrate that the induction of serum Th1-type IgG antibody responses to SBR were at least partially mediated via a TLR2-dependent, MyD88-dependent signaling pathway, whereas early responses to Salmonella were mediated via a TLR4-dependent, MyD88-dependent signaling pathway. The mucosal IgA antibody response to SBR was mediated via TLR2 and TLR4 signaling and a MyD88-dependent pathway, whereas responses to Salmonella were mediated via a TLR4-dependent and MyD88-dependent signaling pathway. Surprisingly, our in vitro findings indicate a role for MyD88, but not TLR2 or TLR4 for the activation of SBR-specific CD4+ T cells, unless these two TLRs can compensate for each other for activation of the T cells. Thus, TLR2, TLR4 and MyD88 all play important roles in the host’s systemic and mucosal immune responses to the SBR of S. mutans when expressed by an attenuated Salmonella vector vaccine and given by the i.n. route.
In our study, the reduced Th1-type IgG subclass response to SBR antigen in TLR2−/− and MyD88−/− mice demonstrated that MyD88 signaling through TLR2 in response to SBR is necessary for the induction of Th1-type immunity, whereas signaling in response to Salmonella is independent of TLR2 for induction of Th1-type immunity. Our observation is consistent with the results of others showing that TLR2-dependent, MyD88-dependent signaling in response to S. pneumoniae is essential for the induction of Th1-type immunity, while TLR2 signaling in response to Salmonella LPS was not required for the induction of Th1-type immunity [21, 32, 33]. We observed elevated levels of IgG1 specific antibody activity to SBR and to Salmonella in MyD88−/− mice, demonstrating that the Th2-type immune responses to SBR and Salmonella is independent of MyD88. The immune system of these groups of mice is skewed toward Th2-type responses, possibly due to lack of TLR2-dependent MyD88 Th1-type polarization, and this could account for their high serum IgG1 antibody response. These results are in line with published reports in other experimental systems showing that Th2-type immune responses are independent of TLR function [25, 34–37]. In the present study, we have shown that TLR4−/− mice had significantly lower early Th1-type IgG anti-Salmonella responses compared to that seen in wt mice following the initial immunization. This finding indicates that TLR4 is involved in mediating an early host defense against Salmonella. This result is in agreement with other recent studies showing a role for TLR4 in early host defense against the bacterial pathogen Salmonella [21] and respiratory syncytial virus infection [38]. Our data also indicates that MyD88 is required for both early and late immune response, while TLR4 is dispensable for the induction of late Th1-type immune response to Salmonella. These finding leave open the possibility that an additional TLR(s) and its ligand(s) might play a role in antibody induction in response to Salmonella.
Cells within the nose-associated lymphoid tissues (NALT) and gut-associated lymphoid tissues (GALT), such as macrophages and dendritic cells, express TLRs and are thought to play a major role in the detection of pathogens within these mucosal inductive site [39]. In the present study, we observed significantly lower salivary IgA anti-SBR and anti-Salmonella responses in MyD88−/− compared to wt mice following the initial and booster immunizations. This finding was not totally unexpected, since previous studies have suggested that MyD88 is essential for class switching of naïve B cells to IgA committed B cells and for differentiation of IgA committed B cells to IgA- producing cells [40]. However, others have reported that MyD88 was not essential for the induction of a mucosal IgA response to a recombinant attenuated Salmonella vaccine [30, 31]. A possible explanation for the difference in findings compared to those reported in the present study is the experimental approach used, such as the immunization regimen and/or sampling times. A significant decrease was also seen in the salivary IgA anti-SBR response in TLR2−/− and TLR4−/− mice. Taken together, these finding demonstrated that mucosal immune responses to SBR are mediated via MyD88-dependent, TLR2 and TLR4 signaling pathways. To our knowledge, this study provides the first description of the activation of the TLR2 and TLR4, MyD88-dependent signaling pathways by SBR. This mucosal result is in partially agreement with other recent studies showing that the mucosal immune response to Shigella ribosomal-based vaccines [41] and lactic acid bacteria [42] is TLR2-, MyD88-dependent and TLR4-independent.
Finally, we investigated whether TLR signaling plays a role in antigen-specific CD4+ T-cell recall responses. In vitro stimulation of CD4+ stimulated T cells two weeks after the booster immunization resulted in SBR-specific CD4+ T-cell proliferative responses in TLR2−/− and TLR4−/− primed mice that were comparable to the responses seen in wt mice. These results indicate that signaling through TLR2 and TLR4 was not required for the recall response. This finding is consistent with those of others demonstrating that TLR signaling is not required for activation of memory T cells [43, 44]. The complete abrogation of CD4+ T-cell proliferation in MyD88−/− mice suggests that the antigen-specific priming of T cells was deficient in MyD88−/− mice. This result is in line with other studies demonstration that the lack of a memory T cell response in MyD88−/− mice is not due to an inability of memory T cells to overcome suppression, but might be due to a defect in commitment, differentiation or survival of memory T cells in the absence of TLR signals [25, 43]. Taken together, the induction of an adaptive T cell response to SBR expressed by our Salmonella vaccine was MyD88-dependent, but TLR2- and TLR4-independent.
In summary, we have demonstrated that the induction of a Th1-type serum response to the SBR of S. mutans was MyD88-independent for the primary response and MyD88-dependent for the secondary response and involved TLR2 signaling, while early responses to Salmonella were mediated via TLR4 signaling through the MyD88-dependent pathway. We have also demonstrated that mucosal immune responses to SBR are mediated via TLR2 and TLR4, and the MyD88-dependent signaling pathway, while mucosal immunity to Salmonella was induced via a TLR4-dependent, MyD88-dependent signaling pathway. Furthermore, the low proliferative response to SBR of primed CD4+ T cells from MyD88−/− mice indicates that the MyD88 adaptor molecule plays a role in the development of an adaptive immune response to SBR.
ACKNOWLEGMENTS
We thank Gregory Harber for excellent technical assistance. This study was supported by U.S. Public Health Service grants DE 09081 and DE 14215 from the National Institute of Dental and Craniofacial Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- 1.Loesche WJ. Role of Streptococcus mutans in human dental decay. Micobiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajishengallis G, Michalek SM. Current status of a mucosal vaccine against dental caries. Oral Microbiol Immunol. 1999;14:1–20. doi: 10.1034/j.1399-302x.1999.140101.x. [DOI] [PubMed] [Google Scholar]
- 3.Russell MW, Lehner T. Characterization of antigens extracted from cells and culture fluids of Streptococcus mutans serotype c. Archs Oral Biol. 1978;23:7–15. doi: 10.1016/0003-9969(78)90047-x. [DOI] [PubMed] [Google Scholar]
- 4.Crowley PJ, Brady LJ, Piacentini DA, Bleiweis AS. Identification of a salivary agglutinin-binding domain within cell surface adhesin P1 of Streptococcus mutans. Infect Immun. 1993;61:1547–1552. doi: 10.1128/iai.61.4.1547-1552.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hajishengallis G, Koga T, Russell MW. Affinity and specificity of the interactions between Streptococcus mutans antigen I/II and salivary components. J Dent Res. 1994;73:1493–1502. doi: 10.1177/00220345940730090301. [DOI] [PubMed] [Google Scholar]
- 6.Moisset A, Schatz N, Lepoivre Y, Amadio S, Wachsmann D, Scholler M, et al. Conservation of salivary glycoprotein-interacting and human immunoglobulin G-cross-reactive domains of antigen I/II in oral streptococci. Infect Immun. 1994;62:184–193. doi: 10.1128/iai.62.1.184-193.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hajishengallis G, Nikolova EB, Russell MW. Inhibition of Streptococcus mutans adherence to saliva-coated hydroxyappatite by human secretory immunoglobulin A antibodies to the cell surface protein antigen I/II: reversal by IgA1 protease cleavage. Infect Immun. 1992;60:5057–5064. doi: 10.1128/iai.60.12.5057-5064.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu H, Nakano Y, Yamashita Y, Oho T, Koga T. Effects of antibodies against cell surface protein antigen PAc-glucosyltransferase fusion proteins on glucan synthesis and cell adhesion of Streptococcus mutans. Infect Immun. 1997;65:2292–2298. doi: 10.1128/iai.65.6.2292-2298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michalek SM, Childers NK. Development and outlook for a caries vaccine. Crit Rev Oral Biol. 1990;1:37–54. doi: 10.1177/10454411900010010401. [DOI] [PubMed] [Google Scholar]
- 10.Strugnell R, Dougan G, Chatfield S, Charles I, Fairweather N, Tite J, et al. Characterization of a Salmonella typhimurium aro vaccine strain expressing the P.69 antigen of Bordetella pertussis. Infect Immun. 1992;60:3994–4002. doi: 10.1128/iai.60.10.3994-4002.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y, Hajishengallis G, Michalek SM. Construction and characterization of a Salmonella enterica serovar Typhimurium clone expressing a salivary adhesin of Streptococcus mutans under control of the anaerobically inducible nirB promoter. Infect Immun. 2000;68:1549–1556. doi: 10.1128/iai.68.3.1549-1556.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harokopakis E, Hajishengallis G, Greenway TE, Russell MW, Michalek SM. Mucosal immunogenicity of a recombinant Salmonella typhimurium-cloned heterologous antigen in the absence or presence of coexpressed cholera toxin A2 and B subunits. Infect Immun. 1997;65:1445–1454. doi: 10.1128/iai.65.4.1445-1454.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Y, Hajishengallis G, Michalek SM. Induction of protective immunity against Streptococcus mutans colonization after mucosal immunzation with attenuated Salmonella enterica serovar Typhimurium expressing an S mutans adhesin under control of in vivo-inducible nirB promoter. Infect Immun. 2001;69:2154–2161. doi: 10.1128/IAI.69.4.2154-2161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hajishengallis G, Russell MW, Michalek SM. Comparison of an adherence domain and a structural region of Streptococcus mutans antigen I/II in protective immunity against dental caries in rats after intranasal immunization. Infect Immun. 1998;66:1740–1743. doi: 10.1128/iai.66.4.1740-1743.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salam MA, Katz J, Zhang P, Hajishengallis G, Michalek SM. Immunogenicity of Salmonella vector vaccines expressing SBR of Streptococcus mutans under the control of a T7-nirB (dual) promoter system. Vaccine. 2006;24:5003–5015. doi: 10.1016/j.vaccine.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 16.Medzhitov R, Preston-Hurlburt P, Janeway CAJ. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 17.Beutler B, Hoebe K, Du X, Ulevitch RJ. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J Leukoc Biol. 2003;74 doi: 10.1189/jlb.0203082. [DOI] [PubMed] [Google Scholar]
- 18.Muzio M, Bosisio D, Polentarutti N, D'amico G, Stoppacciaro A, Mancinelli R, et al. Differential expression and regulation of Toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 19.Bergman MA, Cummings LA, Barrett SL, Smith KD, Lara JC, Aderem A, et al. CD4+ T cells and Toll-like receptors recognize Salmonella antigens expressed in bacterial surface organelles. Infect Immun. 2005;73 doi: 10.1128/IAI.73.3.1350-1356.2005. 1350-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000;165 doi: 10.4049/jimmunol.165.10.5392. 5392-1596. [DOI] [PubMed] [Google Scholar]
- 21.Weiss DS, Raupach B, Takeda K, Akira S, Zychlinsky A. Toll-like receptors are temporally involved in host defense. J Immunol. 2004;172:4463–4469. doi: 10.4049/jimmunol.172.7.4463. [DOI] [PubMed] [Google Scholar]
- 22.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 23.Re F, Strominger JL. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J Biol Chem. 2001;274:37692–37699. doi: 10.1074/jbc.M105927200. [DOI] [PubMed] [Google Scholar]
- 24.Katz J, Zhang P, Martin M, Vogel SN, Michalek SM. Toll-like receptor 2 is required for inflammatory responses to Francisella tularensis LVS. Infect Immun. 2006;74:2809–2816. doi: 10.1128/IAI.74.5.2809-2816.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Tolllike receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 26.Wu HY, Russell MW. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with the cholera toxin B subunit. Infect Immun. 1993;61:314–322. doi: 10.1128/iai.61.1.314-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hajishengallis G, Hollingshead SK, Koga T, Russell MW. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J Immunol. 1995;154:4322–4332. [PubMed] [Google Scholar]
- 28.Martin M, Sharpe AH, Clements JD, Michalek SM. Role of B7 costimulatory molecules in the adjuvant activity of the heat-labile enterotoxin of Escherichia coli. J Immunol. 2002;169:1744–1752. doi: 10.4049/jimmunol.169.4.1744. [DOI] [PubMed] [Google Scholar]
- 29.Miggin SM, O'Neill LA. New insights into the regulation of TLR signaling. J Leuko Biol. 2006;80:220–226. doi: 10.1189/jlb.1105672. [DOI] [PubMed] [Google Scholar]
- 30.Ko HJ, Yang JY, Shim DH, Yang H, Park SM, Curtiss R, et al. Innate immunity mediated by MyD88 signal is not essential for the induction of lipopolysaccharide-specific B cell responses but is indispensable for protection against Salmonella enterica serovar Typhimurium infection. J Immunol. 2009;182:2305–2312. doi: 10.4049/jimmunol.0801980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park SM, Ko HJ, Shim DH, Yang JY, Park YH, Curtiss R, et al. MyD88 signaling is not essential for the induction of antigen-specific B cell responses but is indispensable for protection against Streptococcus pneumoniae infection following oral vaccination with attenuated Salmonella expressing PspA antigen. J Immunol. 2008;181:6447–6455. doi: 10.4049/jimmunol.181.9.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan AQ, Chen Q, Wu ZQ, Paton JC, Snapper CM. Both innate immunity and type 1 humoral immunity to Streptococcus pneumoniae are mediated by MyD88 but differ in their relative levels of dependence on Toll-like receptor 2. Infect Immun. 2005;73:298–307. doi: 10.1128/IAI.73.1.298-307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 34.O'Brien GC, Wang JH, Redmond HP. Bacterial lipoprotein induces resistance to Gram-negative sepsis in TLR4-deficient mice via enhanced bacterial clearance. J Immunol. 2005;174:1020–1026. doi: 10.4049/jimmunol.174.2.1020. [DOI] [PubMed] [Google Scholar]
- 35.Qureshi ST, Medzhitov R. Toll-like receptors and their role in experimental models of microbial infection. Genes Immun. 2003;4:87–94. doi: 10.1038/sj.gene.6363937. [DOI] [PubMed] [Google Scholar]
- 36.McLay J, Leonard E, Petersen S, Shapiro D, Greenspan NS, Schreiber JR. Gamma 3 gene-disrupted mice selectively deficient in the dominant IgG subclass made to bacterial polysaccharides II. Increased susceptibility to fatal pneumococcal sepsis due to absence of anti-polysaccharide IgG3 is corrected by induction of anti-polysaccharide IgG1. J Immunol. 2002;168:3437–3443. doi: 10.4049/jimmunol.168.7.3437. [DOI] [PubMed] [Google Scholar]
- 37.Shao BS, Silver PB, Grajewski RS, Agarwal RK, Tang J, Chan C-C, et al. Essential role of the MyD88 pathway, but nonessential roles of TLRs 2, 4, and 9, in the adjuvant effect promoting Th1-mediated autoimmunity. J Immunol. 2005;175:6303–6310. doi: 10.4049/jimmunol.175.10.6303. [DOI] [PubMed] [Google Scholar]
- 38.Haeberle HA, Takizawa R, Casola A, Brasier AR, Dieterich HJ, Van Rooijen N, et al. Respiratory syncytial virus-induced activation of nuclear factor-kappaB in the lung involves alveolar macrophages and Toll-like receptor 4-dependent pathways. J Infect Dis. 2002;186:1199–1206. doi: 10.1086/344644. [DOI] [PubMed] [Google Scholar]
- 39.Balachandran P, Pugh ND, Ma G, Pasco DS. Toll-like receptor 2-dependent activation of monocytes by Spirulina polysaccharide and its immune enhancing action in mice. Int Immunopharmacol. 2006;6:1808–1814. doi: 10.1016/j.intimp.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Kaneko M, Akiyama Y, Takimoto H, Kumazawa Y. Mechanism of up-regulation of immunoglobulin A production in the intestine of mice unresponsive to lipopolysaccharide. Immunology. 2005;116:64–70. doi: 10.1111/j.1365-2567.2005.02198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shim D-H, Chang SY, Park SM, Jang H, Carbis R, Czerkinsky C, et al. Immunogenicity and protective efficacy offered by a ribosomal-based vaccine from Shigella flexneri 2a. Vaccine. 2007;25:4828–4836. doi: 10.1016/j.vaccine.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 42.Hisbergues M, Magi M, Rigaux P, Steuve J, Garcia L, Goudercourt D, et al. in vivo and in vitro immunomodulation of Der p1 allergen-specific response by Lactobacillus plantarum bacteria. Clinical and Experimental Allergy. 2007;37:1286–1295. doi: 10.1111/j.1365-2222.2007.02792.x. [DOI] [PubMed] [Google Scholar]
- 43.Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21:733–741. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;17:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]