Abstract
Nationwide surveillance of invasive pneumococcal disease has been conducted in Germany since 1992. From 1992 to 2008, a total of 12,137 isolates from invasive pneumococcal disease were collected. Data on serotypes were available for 9,394 invasive isolates. The leading serotypes were serotypes 14 (16.5%), 3 (8.0%), 7F (7.6%), 1 (7.3%), and 23F (6.0%). Variations in serotype distribution over the years are particularly extensive, especially concerning serotype 14 (min 7.4%, max 33.5%) with the highest percentages among the isolates serotyped from around 1997 to 2006. Serotypes 1 and 7F increased over the last decade. No increase was observed concerning serotype 19A. Higher pneumococcal conjugate vaccine coverages were observed among children (7v, 57.3%; 10v, 72.8%; 13v, 83.5%) than among adults (7v, 39.9%; 10v, 55.5%; 13v, 73.5%). The temporal variations in serotype distribution have to be kept in mind when interpreting vaccine coverages reported in epidemiological studies.
1. Introduction
Streptococcus pneumoniae is one of the most important pathogens in bacterial pneumonia, sepsis, and meningitis worldwide [1]. Significant temporal [2–4] and regional [5–7] variations among pneumococcal serotypes have been described. While the “epidemic” serogroups (serogroups 1-3 and 5) decreased considerably in the United States during the last century, the serogroups included in the seven-valent pneumococcal conjugate vaccine (PCV7) increased clearly [2]. A similar trend has been described in Spain, where the prevalence of PCV7 serotypes increased significantly (except serotype 4) since the start of the study in the early 1980s but then decreased considerably in the 2000s for all PCV7 serotypes except serotype 23F. Among the “epidemic” serotypes, a significant decrease of serotypes 1 and 5 has been observed in the 1980s followed by a significant increase in the late 1990s, while serotype 3 decreased continuously during the observational period. Furthermore, serotypes 6A, 7F, and 19A increased significantly since the late 1990s [4].
The widespread use of antibiotics and the increasing application of pneumococcal conjugate vaccines (a general recommendation of pneumococcal conjugate vaccination for children <2 years in Germany was issued at the end of July 2006) will have an impact on future changes in serotype distribution.
The NRCS has conducted surveillance for invasive pneumococcal disease in Germany since 1992 [8]. Between 1992 and 1996 surveillance was based on a limited number of laboratories in Germany on a voluntary basis [9]. In 1996, nationwide population-based surveillance of IPD in children was started [10]. This surveillance system was extended to adults in one federal state (North-Rhine Westphalia) in 2001 [11] and successively broadened to nationwide surveillance until 2009.
The aim of this study was to evaluate the serotype distribution of S. pneumoniae among the isolates with invasive pneumococcal disease (IPD) that were sent to the German National Reference Center for Streptococci (NRCS) between 1992 and 2008, analyze temporal trends, and calculate the vaccine coverages for the 7-valent, 10-valent, and 13-valent pneumococcal conjugate vaccines.
2. Materials and Methods
2.1. Study Design
In this study data about invasive disease caused by Streptococcus pneumoniae in children (<16 years) and adults (≥16 years) in Germany were included using the data sources described above. Cases from January 1, 1992 to December 31, 2008 were included in this study. A case of IPD was defined by the isolation of S. pneumoniae from a normally sterile site.
2.2. Microbiological Investigations
Isolates were identified using standard procedures including bile solubility and optochin sensitivity. As a control strain, Streptococcus pneumoniae ATCC 49619 was used. Pneumococcal isolates were serotyped by the Neufeld's Quellung reaction using type- and factor-specific antisera (Statens Serum Institut, Copenhagen, Denmark). Among isolates of adults, high levels of resistance were a main trigger for initiation of serotyping during the early years of this study. Since cross-reactive serotypes were not included in the calculation, coverage information strictly refers to the serotypes included in the vaccines. Serogroup 6 isolates were not tested with respect to serotype 6C.
3. Results
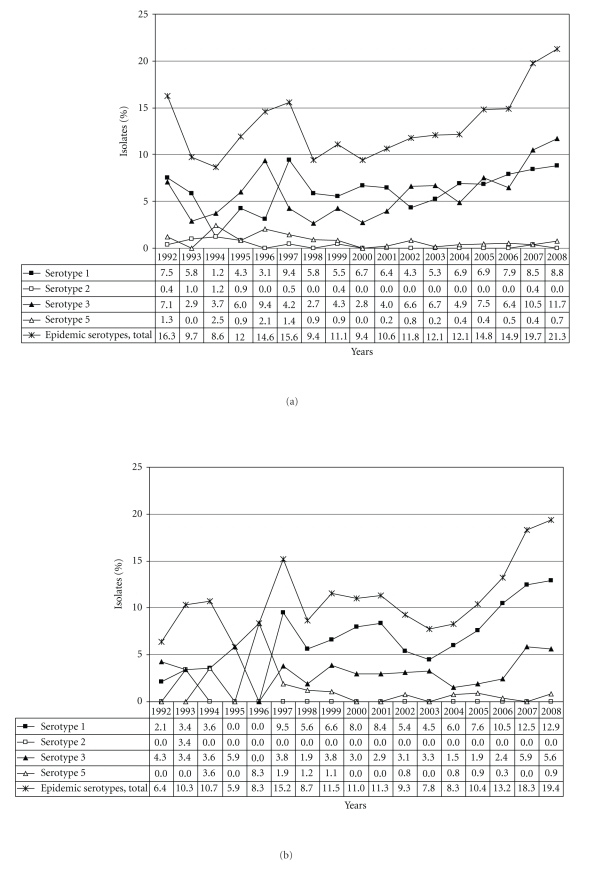
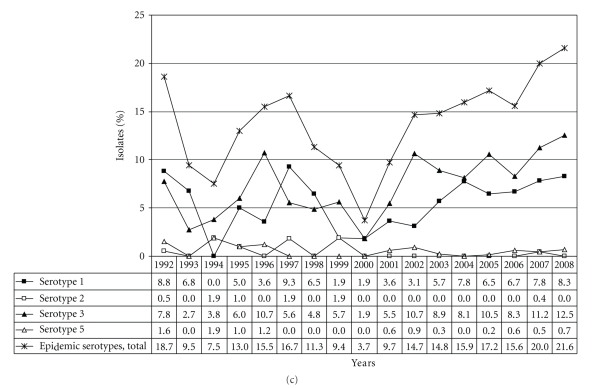
A total of 12,137 isolates from invasive pneumococcal disease were collected between January 1, 1992 and December 31, 2008. The total numbers of cases for each year varied between 297 and 2,037 cases (median: 505 cases). Data on serotypes were available for 9,394 isolates (77.4% of all invasive isolates; 31.4% children, 68.6% adults; 54.9% male, 43.7% female, 1.4% no information on gender). The serotype distribution of the years 1992–2008 is shown in Table 1(a). The leading serotypes were serotypes 14 (16.5%), 3 (8.0%), 7F (7.6%), 1 (7.3%), and 23F (6.0 %). Concerning the epidemic serotypes 1–3 and 5, a slight increase was noticed during the last years, reaching levels around 20% among children and adults in 2008 (Figures 1(a)–1(c)). Variations in serotype distribution over the years are partly extensive, especially concerning serotype 14 (min 7.4%, max 33.5%) (Figure 2(a)). Differences in serotype distribution among children and adults are shown in Tables 1(b) and 1(c). The amount of serotyped isolates among all pneumococcal isolates sent to the NRCS in Germany is shown in Table 2. Over the years the percentage of isolates serotyped increased continuously and in the last four years of the study almost all isolates were serotyped.
Table 1.
(a) Serotype distribution of IPD in Germany (1992–2008, n = 9,394) in all age groups.
| Sero- type |
1992 (%) |
1993 (%) |
1994 (%) |
1995 (%) |
1996 (%) |
1997 (%) |
1998 (%) |
1999 (%) |
2000 (%) |
2001 (%) |
2002 (%) |
2003 (%) |
2004 (%) |
2005 (%) |
2006 (%) |
2007 (%) |
2008 (%) |
Total (%) |
Total (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14 | 8.8 | 12.6 | 7.4 | 8.5 | 13.5 | 16.5 | 17.5 | 29.8 | 33.5 | 25.2 | 21.3 | 22.8 | 23.0 | 18.8 | 19.3 | 13.3 | 8.9 | 16.5 | 1,549 |
| 3 | 7.1 | 2.9 | 3.7 | 6.0 | 9.4 | 4.2 | 2.7 | 4.3 | 2.8 | 4.0 | 6.6 | 6.7 | 4.9 | 7.5 | 6.4 | 10.5 | 11.7 | 8.0 | 755 |
| 7F | 6.7 | 2.9 | 4.9 | 4.3 | 5.2 | 7.5 | 2.7 | 2.1 | 4.3 | 3.7 | 5.8 | 7.3 | 9.0 | 6.4 | 8.0 | 8.7 | 10.4 | 7.6 | 718 |
| 1 | 7.5 | 5.8 | 1.2 | 4.3 | 3.1 | 9.4 | 5.8 | 5.5 | 6.7 | 6.4 | 4.3 | 5.3 | 6.9 | 6.9 | 7.9 | 8.5 | 8.8 | 7.3 | 690 |
| 23F | 8.3 | 9.7 | 6.2 | 8.5 | 9.4 | 6.1 | 8.5 | 8.9 | 6.3 | 7.7 | 8.5 | 5.1 | 4.9 | 6.9 | 6.8 | 4.9 | 4.3 | 6.0 | 559 |
| 4 | 7.5 | 11.7 | 4.9 | 5.1 | 2.1 | 5.2 | 7.2 | 3.4 | 3.9 | 3.5 | 4.3 | 7.5 | 4.9 | 6.0 | 6.2 | 5.6 | 4.6 | 5.4 | 507 |
| 6B | 3.3 | 7.8 | 7.4 | 6.8 | 6.3 | 8.0 | 5.8 | 6.8 | 3.1 | 7.7 | 6.8 | 5.7 | 5.2 | 7.1 | 4.8 | 3.7 | 2.5 | 4.8 | 449 |
| 19F | 6.7 | 1.9 | 9.9 | 6.8 | 3.1 | 5.2 | 4.9 | 7.7 | 7.1 | 6.9 | 5.2 | 3.8 | 5.4 | 5.4 | 4.5 | 4.5 | 3.4 | 4.8 | 447 |
| 9V | 4.2 | 1.0 | 8.6 | 6.0 | 8.3 | 2.8 | 3.1 | 6.4 | 5.5 | 2.2 | 3.3 | 5.3 | 5.6 | 4.8 | 5.1 | 5.1 | 3.6 | 4.5 | 425 |
| 6A | 6.7 | 5.8 | 4.9 | 8.5 | 3.1 | 5.2 | 5.4 | 2.6 | 4.3 | 3.5 | 3.5 | 4.6 | 3.9 | 2.8 | 3.1 | 4.9 | 4.6 | 4.3 | 401 |
| 18C | 4.6 | 4.9 | 6.2 | 1.7 | 1.0 | 6.6 | 6.7 | 3.8 | 7.5 | 4.5 | 4.3 | 2.9 | 3.6 | 3.4 | 3.6 | 2.3 | 3.0 | 3.5 | 326 |
| 19A | 0.8 | 2.9 | 1.2 | 1.7 | 5.2 | 2.4 | 5.8 | 3.4 | 4.3 | 3.0 | 1.4 | 1.6 | 2.6 | 2.9 | 4.3 | 4.0 | 4.2 | 3.4 | 320 |
| 22F | 1.3 | 2.9 | 3.7 | 0.0 | 2.1 | 0.5 | 0.9 | 0.0 | 0.0 | 0.7 | 1.0 | 1.0 | 1.5 | 3.0 | 3.0 | 2.9 | 3.0 | 2.2 | 208 |
| 8 | 2.1 | 1.9 | 0.0 | 0.9 | 2.1 | 0.5 | 0.9 | 0.0 | 0.4 | 1.5 | 2.3 | 2.1 | 2.1 | 2.5 | 2.2 | 2.5 | 3.0 | 2.2 | 208 |
| 10A | 1.7 | 1.0 | 3.7 | 2.6 | 2.1 | 1.4 | 1.3 | 0.9 | 1.6 | 1.7 | 2.5 | 0.6 | 2.2 | 1.5 | 1.9 | 1.9 | 2.2 | 1.8 | 172 |
| 9N | 2.1 | 1.0 | 1.2 | 3.4 | 1.0 | 1.4 | 0.9 | 1.3 | 1.6 | 1.2 | 1.7 | 1.3 | 1.3 | 1.4 | 1.6 | 2.0 | 2.6 | 1.8 | 172 |
| 11A | 2.5 | 1.9 | 1.2 | 1.7 | 4.2 | 1.4 | 0.4 | 0.9 | 0.0 | 0.2 | 0.6 | 1.8 | 1.5 | 1.3 | 1.1 | 1.7 | 2.5 | 1.6 | 149 |
| 12F | 2.5 | 1.9 | 2.5 | 1.7 | 6.3 | 1.4 | 1.3 | 1.3 | 0.0 | 2.2 | 1.0 | 1.8 | 0.7 | 0.4 | 0.4 | 1.0 | 1.7 | 1.3 | 118 |
| 24F | 2.1 | 1.0 | 1.2 | 0.9 | 1.0 | 0.5 | 1.8 | 1.3 | 0.8 | 2.0 | 0.6 | 1.3 | 1.5 | 0.7 | 1.2 | 0.9 | 1.8 | 1.2 | 116 |
| 23A | 0.8 | 1.0 | 1.2 | 0.0 | 0.0 | 0.9 | 1.3 | 0.0 | 0.8 | 0.5 | 0.8 | 0.8 | 0.2 | 0.4 | 0.8 | 1.0 | 1.7 | 0.9 | 88 |
| 15B | 0.8 | 1.9 | 0.0 | 0.0 | 1.0 | 0.0 | 0.9 | 0.9 | 1.2 | 0.0 | 0.6 | 0.3 | 0.9 | 1.1 | 0.9 | 1.0 | 1.2 | 0.9 | 83 |
| 35F | 0.8 | 0.0 | 1.2 | 0.9 | 0.0 | 0.5 | 0.4 | 0.9 | 0.8 | 0.5 | 0.0 | 0.3 | 0.0 | 0.4 | 0.5 | 0.7 | 1.8 | 0.8 | 74 |
| 33F | 1.3 | 1.0 | 1.2 | 0.9 | 0.0 | 0.9 | 1.8 | 0.9 | 0.0 | 0.5 | 0.6 | 1.0 | 0.9 | 0.7 | 0.2 | 0.6 | 0.9 | 0.7 | 68 |
| 38 | 0.4 | 0.0 | 2.5 | 0.0 | 0.0 | 0.9 | 0.4 | 0.0 | 0.4 | 0.5 | 0.6 | 0.6 | 0.7 | 0.2 | 0.3 | 0.4 | 1.4 | 0.6 | 61 |
| 5 | 1.3 | 0.0 | 2.5 | 0.9 | 2.1 | 1.4 | 0.9 | 0.9 | 0.0 | 0.2 | 0.8 | 0.2 | 0.4 | 0.4 | 0.5 | 0.4 | 0.7 | 0.6 | 55 |
| 15C | 1.7 | 0.0 | 0.0 | 1.7 | 0.0 | 3.8 | 0.0 | 0.0 | 0.4 | 0.0 | 0.4 | 0.6 | 0.4 | 0.6 | 0.9 | 0.5 | 0.3 | 0.6 | 53 |
| 15A | 0.0 | 0.0 | 0.0 | 1.7 | 0.0 | 0.0 | 1.8 | 1.7 | 0.0 | 0.7 | 1.2 | 0.8 | 0.7 | 0.6 | 0.5 | 0.1 | 0.4 | 0.5 | 48 |
| 9A | 0.4 | 1.0 | 1.2 | 0.9 | 2.1 | 0.9 | 0.9 | 0.0 | 0.8 | 3.5 | 2.1 | 0.6 | 0.0 | 0.4 | 0.0 | 0.2 | 0.0 | 0.5 | 47 |
| 20 | 0.8 | 1.9 | 2.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.0 | 0.0 | 0.0 | 0.6 | 0.6 | 0.8 | 0.3 | 0.6 | 0.4 | 0.5 | 43 |
| NT | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 | 0.4 | 0.0 | 0.2 | 0.2 | 0.0 | 0.7 | 0.4 | 0.3 | 0.7 | 0.5 | 0.4 | 40 |
| 17F | 0.0 | 1.9 | 0.0 | 0.9 | 0.0 | 0.0 | 0.9 | 0.0 | 0.0 | 0.0 | 1.2 | 0.3 | 0.7 | 0.8 | 0.3 | 0.4 | 0.2 | 0.4 | 39 |
| 16F | 0.0 | 0.0 | 0.0 | 1.7 | 1.0 | 0.5 | 0.0 | 0.4 | 0.0 | 1.0 | 0.6 | 0.5 | 0.4 | 0.2 | 0.4 | 0.1 | 0.4 | 0.4 | 34 |
| 33A | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.8 | 0.8 | 0.5 | 0.2 | 0.3 | 30 |
| 31 | 0.8 | 0.0 | 1.2 | 0.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.4 | 0.5 | 0.0 | 0.1 | 0.3 | 0.1 | 0.5 | 0.3 | 26 |
| 18A | 0.0 | 0.0 | 1.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.4 | 0.6 | 0.2 | 0.2 | 0.1 | 0.3 | 0.2 | 0.2 | 22 |
| 34 | 0.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.0 | 0.0 | 0.0 | 0.4 | 0.3 | 0.2 | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 | 22 |
| rough | 0.0 | 1.0 | 1.2 | 0.9 | 1.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.5 | 1.0 | 1.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 20 |
| 23B | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.4 | 0.0 | 0.0 | 0.5 | 0.0 | 0.2 | 0.4 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 17 |
| 9L | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.0 | 0.0 | 0.7 | 0.8 | 0.3 | 0.2 | 0.0 | 0.2 | 0.2 | 0.0 | 0.2 | 17 |
| 13 | 0.0 | 2.9 | 0.0 | 0.0 | 0.0 | 0.9 | 0.0 | 0.0 | 0.0 | 0.2 | 0.6 | 0.0 | 0.2 | 0.0 | 0.0 | 0.1 | 0.1 | 0.2 | 15 |
| 18F | 0.4 | 1.9 | 0.0 | 0.9 | 0.0 | 0.5 | 0.0 | 0.9 | 0.0 | 0.0 | 0.2 | 0.2 | 0.0 | 0.1 | 0.0 | 0.3 | 0.0 | 0.2 | 15 |
| 2 | 0.4 | 1.0 | 1.2 | 0.9 | 0.0 | 0.5 | 0.0 | 0.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.0 | 0.1 | 13 |
| 12A | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 0.4 | 0.0 | 0.0 | 0.2 | 0.0 | 0.2 | 0.0 | 0.3 | 0.2 | 0.0 | 0.1 | 12 |
| 28A | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 0.0 | 0.4 | 0.0 | 0.4 | 0.2 | 0.0 | 0.1 | 0.1 | 0.2 | 0.0 | 0.1 | 12 |
| 35B | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.2 | 0.0 | 0.0 | 0.2 | 0.2 | 0.1 | 11 |
| 10B | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.2 | 0.0 | 0.2 | 0.1 | 0.1 | 10 |
| Others⋆ | 2.9 | 1.9 | 2.5 | 7.7 | 3.1 | 0.5 | 4.9 | 1.7 | 1.2 | 1.5 | 0.6 | 1.6 | 0.6 | 1.3 | 0.3 | 1.7 | 0.9 | 1.4 | 130 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 9,394 |
|
| |||||||||||||||||||
| Not sero- typed |
52.5 | 77.8 | 76.0 | 67.0 | 67.7 | 40.8 | 40.5 | 43.9 | 38.2 | 39.4 | 29.9 | 21.5 | 19.1 | 1.5 | 0.0 | 0.1 | 0.0 | 22.6 | 2,743 |
NT: nontypeable; n: number of isolates tested.
Others⋆ includes the serotypes (number of isolates): 15F (9), 18B (9), 12B (8), 7C (8), 33B (7), 10F (6), 11B (6), 11F (6), 35A (6), 19C (5), 29 (5), 37 (5), 21 (4), 24A (4), 28F (4), 35C (4), 19B (3), 22A (3), 36 (3), 45 (3), 7A (3), 24B (2), 25F (2), 39 (2), 48 (2), 6 (2), 7B (2), 12 (1), 17A (1), 18 (1), 19 (1), 23 (1), 35 (1), 9 (1).
(b) Serotype distribution of IPD in Germany (1992–2008, n = 2,948) in children (<16 years).
| Sero-type | 1992 (%) |
1993 (%) |
1994 (%) |
1995 (%) |
1996 (%) |
1997 (%) |
1998 (%) |
1999 (%) |
2000 (%) |
2001 (%) |
2002 (%) |
2003 (%) |
2004 (%) |
2005 (%) |
2006 (%) |
2007 (%) |
2008 (%) |
Total (%) |
Total (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14 | 12.8 | 6.9 | 14.3 | 5.9 | 16.7 | 15.2 | 18.0 | 26.9 | 31.5 | 25.5 | 26.7 | 27.0 | 30.6 | 26.3 | 25.4 | 13.9 | 5.6 | 22.5 | 664 |
| 1 | 2.1 | 3.4 | 3.6 | 0.0 | 0.0 | 9.5 | 5.6 | 6.6 | 8.0 | 8.4 | 5.4 | 4.5 | 6.0 | 7.6 | 10.5 | 12.5 | 12.9 | 7.9 | 234 |
| 6B | 6.4 | 13.8 | 0.0 | 11.8 | 8.3 | 8.9 | 6.2 | 7.7 | 3.5 | 7.5 | 8.9 | 7.8 | 7.2 | 10.8 | 6.6 | 7.0 | 4.3 | 7.3 | 216 |
| 19F | 8.5 | 3.4 | 7.1 | 23.5 | 0.0 | 6.3 | 6.8 | 8.8 | 8.0 | 9.6 | 5.4 | 5.7 | 6.8 | 7.3 | 8.0 | 8.4 | 4.7 | 7.2 | 213 |
| 23F | 10.6 | 3.4 | 7.1 | 11.8 | 16.7 | 7.0 | 7.5 | 8.2 | 5.5 | 7.9 | 10.5 | 6.1 | 4.9 | 6.6 | 8.7 | 6.2 | 2.6 | 6.9 | 204 |
| 7F | 2.1 | 3.4 | 0.0 | 5.9 | 0.0 | 7.6 | 3.7 | 2.2 | 5.5 | 4.6 | 5.8 | 8.6 | 7.9 | 6.0 | 7.7 | 9.9 | 13.8 | 6.9 | 204 |
| 18C | 8.5 | 10.3 | 17.9 | 0.0 | 0.0 | 8.9 | 8.7 | 4.9 | 8.0 | 6.3 | 5.8 | 5.7 | 3.8 | 5.4 | 6.6 | 5.1 | 5.6 | 6.2 | 182 |
| 6A | 14.9 | 20.7 | 3.6 | 5.9 | 0.0 | 5.1 | 5.6 | 2.7 | 4.5 | 3.8 | 4.7 | 4.9 | 3.8 | 3.5 | 1.4 | 4.8 | 5.6 | 4.4 | 130 |
| 9V | 4.3 | 3.4 | 14.3 | 11.8 | 8.3 | 2.5 | 2.5 | 7.1 | 4.0 | 2.1 | 2.3 | 4.1 | 4.2 | 5.1 | 3.1 | 3.3 | 1.3 | 3.7 | 108 |
| 4 | 6.4 | 10.3 | 7.1 | 11.8 | 8.3 | 5.1 | 7.5 | 3.8 | 4.0 | 2.1 | 3.5 | 3.7 | 3.0 | 2.8 | 3.1 | 2.2 | 0.4 | 3.5 | 102 |
| 3 | 4.3 | 3.4 | 3.6 | 5.9 | 0.0 | 3.8 | 1.9 | 3.8 | 3.0 | 2.9 | 3.1 | 3.3 | 1.5 | 1.9 | 2.4 | 5.9 | 5.6 | 3.3 | 96 |
| 19A | 0.0 | 3.4 | 0.0 | 0.0 | 8.3 | 1.9 | 6.2 | 2.2 | 3.5 | 2.5 | 1.6 | 2.0 | 3.0 | 2.5 | 3.8 | 4.0 | 4.7 | 3.1 | 90 |
| 10A | 2.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 | 0.0 | 1.1 | 1.5 | 2.1 | 1.6 | 0.4 | 2.3 | 1.9 | 2.8 | 2.2 | 3.4 | 1.7 | 51 |
| 24F | 4.3 | 3.4 | 0.0 | 0.0 | 0.0 | 0.0 | 2.5 | 1.6 | 1.0 | 2.1 | 0.8 | 2.5 | 1.9 | 0.6 | 1.4 | 1.1 | 2.2 | 1.5 | 44 |
| 15B | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 | 1.1 | 1.0 | 0.0 | 0.4 | 0.4 | 0.8 | 1.3 | 0.7 | 2.2 | 2.2 | 0.9 | 26 |
| 15C | 2.1 | 0.0 | 0.0 | 0.0 | 0.0 | 4.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.8 | 0.4 | 1.3 | 1.4 | 0.4 | 2.2 | 0.9 | 26 |
| 9N | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 | 1.1 | 1.5 | 0.8 | 0.4 | 1.6 | 1.5 | 0.3 | 1.0 | 0.4 | 1.7 | 0.9 | 26 |
| 38 | 2.1 | 0.0 | 3.6 | 0.0 | 0.0 | 0.6 | 0.6 | 0.0 | 0.5 | 0.4 | 0.8 | 0.4 | 0.8 | 0.3 | 0.0 | 0.7 | 3.9 | 0.8 | 23 |
| 15A | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.2 | 1.6 | 0.0 | 0.4 | 1.2 | 1.6 | 0.8 | 0.6 | 1.0 | 0.0 | 0.4 | 0.7 | 21 |
| 22F | 0.0 | 0.0 | 7.1 | 0.0 | 0.0 | 0.6 | 0.6 | 0.0 | 0.0 | 0.8 | 1.2 | 1.2 | 0.8 | 0.6 | 0.3 | 0.4 | 1.3 | 0.7 | 21 |
| 9A | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.3 | 0.6 | 0.0 | 1.0 | 3.8 | 1.9 | 0.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.7 | 21 |
| 5 | 0.0 | 0.0 | 3.6 | 0.0 | 8.3 | 1.9 | 1.2 | 1.1 | 0.0 | 0.0 | 0.8 | 0.0 | 0.8 | 0.9 | 0.3 | 0.0 | 0.9 | 0.6 | 19 |
| 8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 | 1.2 | 0.0 | 0.5 | 0.4 | 1.2 | 0.4 | 0.8 | 0.9 | 0.7 | 0.4 | 0.4 | 0.6 | 18 |
| 33F | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.3 | 1.9 | 0.5 | 0.0 | 0.4 | 1.2 | 0.8 | 0.4 | 0.0 | 0.0 | 0.4 | 1.3 | 0.6 | 17 |
| 12F | 2.1 | 0.0 | 3.6 | 0.0 | 0.0 | 0.0 | 0.6 | 1.1 | 0.0 | 1.7 | 0.0 | 0.0 | 0.8 | 0.0 | 0.0 | 0.4 | 1.7 | 0.5 | 16 |
| 11A | 0.0 | 0.0 | 0.0 | 0.0 | 8.3 | 1.9 | 0.0 | 0.5 | 0.0 | 0.0 | 0.4 | 1.2 | 0.0 | 0.0 | 0.3 | 1.1 | 0.9 | 0.5 | 15 |
| 23A | 2.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 | 1.2 | 0.0 | 0.5 | 0.0 | 0.4 | 0.4 | 0.0 | 0.0 | 0.7 | 0.4 | 1.7 | 0.5 | 14 |
| 18A | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.8 | 0.8 | 0.8 | 0.4 | 0.0 | 0.0 | 0.4 | 0.9 | 0.3 | 10 |
| 35F | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 | 0.5 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.3 | 0.4 | 1.3 | 0.3 | 10 |
| NT | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 | 1.5 | 0.3 | 0.0 | 0.7 | 0.9 | 0.3 | 10 |
| 33A | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 1.3 | 0.7 | 0.4 | 0.4 | 0.3 | 9 |
| rough | 0.0 | 3.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.8 | 0.8 | 1.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 9 |
| 17F | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.8 | 0.3 | 0.0 | 0.4 | 0.9 | 0.3 | 8 |
| 16F | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 | 0.0 | 0.0 | 0.0 | 0.8 | 0.4 | 0.0 | 0.0 | 0.3 | 0.0 | 0.0 | 0.9 | 0.2 | 7 |
| 18B | 0.0 | 0.0 | 0.0 | 0.0 | 8.3 | 0.0 | 1.2 | 0.5 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 | 0.0 | 0.0 | 0.0 | 0.2 | 7 |
| 23B | 0.0 | 3.4 | 0.0 | 0.0 | 0.0 | 0.6 | 0.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.4 | 0.0 | 0.0 | 0.0 | 0.4 | 0.2 | 6 |
| 34 | 2.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.3 | 0.0 | 0.7 | 0.0 | 0.2 | 6 |
| 18F | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 | 0.0 | 1.1 | 0.0 | 0.0 | 0.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.0 | 0.2 | 5 |
| 20 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 0.0 | 0.0 | 0.4 | 0.4 | 0.3 | 0.0 | 0.4 | 0.0 | 0.2 | 5 |
| 9L | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.8 | 0.4 | 0.0 | 0.0 | 0.3 | 0.0 | 0.0 | 0.2 | 5 |
| Others⋆ | 2.1 | 3.4 | 3.6 | 5.9 | 8.3 | 2.5 | 2.5 | 1.6 | 1.5 | 0.8 | 0.8 | 0.4 | 1.5 | 1.6 | 0.3 | 3.3 | 3.0 | 1.7 | 50 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 2,948 |
|
| |||||||||||||||||||
| Not sero- typed |
7.8 | 17.1 | 3.4 | 34.6 | 61.3 | 7.1 | 2.4 | 3.2 | 3.8 | 0.0 | 0.4 | 0.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.4 | 72 |
NT: nontypeable; n: number of isolates tested.
Others⋆ includes the serotypes (number of isolates): 28A (4), 11B (3), 12A (3), 12B (3), 13 (3), 33B (3), 35B (3), 15F (2), 19C (2), 21 (2), 24A (2), 28F (2), 29 (2), 35A (2), 37 (2), 7A (2), 10F (1), 19B (1), 2 (1), 31 (1), 35 (1), 35C (1), 36 (1), 39 (1), 6 (1), 7C (1).
(c) Serotype distribution of IPD in Germany (1992–2008, n = 6,446) in adults (≥16 years).
| Sero-type | 1992 (%) |
1993 (%) |
1994 (%) |
1995 (%) |
1996 (%) |
1997 (%) |
1998 (%) |
1999 (%) |
2000 (%) |
2001 (%) |
2002 (%) |
2003 (%) |
2004 (%) |
2005 (%) |
2006 (%) |
2007 (%) |
2008 (%) |
Total (%) |
Total (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14 | 7.8 | 14.9 | 3.8 | 9.0 | 13.1 | 20.4 | 16.1 | 39.6 | 40.7 | 24.8 | 15.1 | 20.1 | 15.6 | 14.8 | 16.6 | 13.2 | 9.4 | 13.7 | 885 |
| 3 | 7.8 | 2.7 | 3.8 | 6.0 | 10.7 | 5.6 | 4.8 | 5.7 | 1.9 | 5.5 | 10.7 | 8.9 | 8.1 | 10.5 | 8.3 | 11.2 | 12.5 | 10.2 | 659 |
| 7F | 7.8 | 2.7 | 7.5 | 4.0 | 6.0 | 7.4 | 0.0 | 1.9 | 0.0 | 2.4 | 5.8 | 6.5 | 10.0 | 6.6 | 8.1 | 8.5 | 9.9 | 8.0 | 514 |
| 1 | 8.8 | 6.8 | 0.0 | 5.0 | 3.6 | 9.3 | 6.5 | 1.9 | 1.9 | 3.6 | 3.1 | 5.7 | 7.8 | 6.5 | 6.7 | 7.8 | 8.3 | 7.1 | 456 |
| 4 | 7.8 | 12.2 | 3.8 | 4.0 | 1.2 | 5.6 | 6.5 | 1.9 | 3.7 | 5.5 | 5.3 | 9.9 | 6.7 | 7.7 | 7.6 | 6.1 | 5.2 | 6.3 | 405 |
| 23F | 7.8 | 12.2 | 5.7 | 8.0 | 8.3 | 3.7 | 11.3 | 11.3 | 9.3 | 7.3 | 6.2 | 4.4 | 4.8 | 7.0 | 5.9 | 4.7 | 4.5 | 5.5 | 355 |
| 9V | 4.1 | 0.0 | 5.7 | 5.0 | 8.3 | 3.7 | 4.8 | 3.8 | 11.1 | 2.4 | 4.4 | 6.0 | 7.0 | 4.6 | 6.1 | 5.4 | 3.9 | 4.9 | 317 |
| 6A | 4.7 | 0.0 | 5.7 | 9.0 | 3.6 | 5.6 | 4.8 | 1.9 | 3.7 | 3.0 | 2.2 | 4.4 | 4.1 | 2.4 | 3.8 | 4.9 | 4.5 | 4.2 | 271 |
| 19F | 6.2 | 1.4 | 11.3 | 4.0 | 3.6 | 1.9 | 0.0 | 3.8 | 3.7 | 3.0 | 4.9 | 2.6 | 4.1 | 4.4 | 2.9 | 3.8 | 3.3 | 3.6 | 234 |
| 6B | 2.6 | 5.4 | 11.3 | 6.0 | 6.0 | 5.6 | 4.8 | 3.8 | 1.9 | 7.9 | 4.4 | 4.4 | 3.3 | 5.1 | 4.0 | 3.2 | 2.3 | 3.6 | 233 |
| 19A | 1.0 | 2.7 | 1.9 | 2.0 | 4.8 | 3.7 | 4.8 | 7.5 | 7.4 | 3.6 | 1.3 | 1.3 | 2.2 | 3.1 | 4.5 | 4.0 | 4.1 | 3.6 | 230 |
| 8 | 2.6 | 2.7 | 0.0 | 1.0 | 2.4 | 0.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.6 | 3.1 | 3.3 | 3.4 | 2.9 | 2.8 | 3.4 | 2.9 | 190 |
| 22F | 1.6 | 4.1 | 1.9 | 0.0 | 2.4 | 0.0 | 1.6 | 0.0 | 0.0 | 0.6 | 0.9 | 0.8 | 2.2 | 4.3 | 4.1 | 3.3 | 3.3 | 2.9 | 187 |
| 9N | 2.6 | 1.4 | 1.9 | 4.0 | 1.2 | 5.6 | 1.6 | 1.9 | 1.9 | 1.8 | 3.1 | 1.0 | 1.1 | 2.0 | 1.9 | 2.3 | 2.7 | 2.3 | 146 |
| 18C | 3.6 | 2.7 | 0.0 | 2.0 | 1.2 | 0.0 | 1.6 | 0.0 | 5.6 | 1.8 | 2.7 | 1.0 | 3.3 | 2.4 | 2.2 | 1.8 | 2.7 | 2.2 | 144 |
| 11A | 3.1 | 2.7 | 1.9 | 2.0 | 3.6 | 0.0 | 1.6 | 1.9 | 0.0 | 0.6 | 0.9 | 2.1 | 3.0 | 2.0 | 1.4 | 1.8 | 2.7 | 2.1 | 134 |
| 10A | 1.6 | 1.4 | 5.7 | 3.0 | 2.4 | 3.7 | 4.8 | 0.0 | 1.9 | 1.2 | 3.6 | 0.8 | 2.2 | 1.4 | 1.4 | 1.8 | 2.0 | 1.9 | 121 |
| 12F | 2.6 | 2.7 | 1.9 | 2.0 | 7.1 | 5.6 | 3.2 | 1.9 | 0.0 | 3.0 | 2.2 | 2.9 | 0.7 | 0.7 | 0.6 | 1.1 | 1.7 | 1.6 | 102 |
| 23A | 0.5 | 1.4 | 1.9 | 0.0 | 0.0 | 1.9 | 1.6 | 0.0 | 1.9 | 1.2 | 1.3 | 1.0 | 0.4 | 0.7 | 0.8 | 1.1 | 1.7 | 1.1 | 74 |
| 24F | 1.6 | 0.0 | 1.9 | 1.0 | 1.2 | 1.9 | 0.0 | 0.0 | 0.0 | 1.8 | 0.4 | 0.5 | 1.1 | 0.7 | 1.1 | 0.8 | 1.7 | 1.1 | 72 |
| 35F | 1.0 | 0.0 | 1.9 | 1.0 | 0.0 | 1.9 | 0.0 | 1.9 | 0.0 | 1.2 | 0.0 | 0.5 | 0.0 | 0.5 | 0.6 | 0.8 | 1.9 | 1.0 | 64 |
| 15B | 1.0 | 2.7 | 0.0 | 0.0 | 1.2 | 0.0 | 1.6 | 0.0 | 1.9 | 0.0 | 0.9 | 0.3 | 1.1 | 1.0 | 1.0 | 0.8 | 1.1 | 0.9 | 57 |
| 33F | 1.6 | 1.4 | 1.9 | 1.0 | 0.0 | 0.0 | 1.6 | 1.9 | 0.0 | 0.6 | 0.0 | 1.0 | 1.5 | 1.0 | 0.3 | 0.6 | 0.9 | 0.8 | 51 |
| 20 | 1.0 | 2.7 | 3.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.8 | 0.7 | 1.0 | 0.5 | 0.6 | 0.4 | 0.6 | 38 |
| 38 | 0.0 | 0.0 | 1.9 | 0.0 | 0.0 | 1.9 | 0.0 | 0.0 | 0.0 | 0.6 | 0.4 | 0.8 | 0.7 | 0.2 | 0.5 | 0.3 | 1.1 | 0.6 | 38 |
| 5 | 1.6 | 0.0 | 1.9 | 1.0 | 1.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 | 0.9 | 0.3 | 0.0 | 0.2 | 0.6 | 0.5 | 0.7 | 0.6 | 36 |
| 17F | 0.0 | 2.7 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.7 | 0.5 | 0.7 | 1.0 | 0.5 | 0.4 | 0.2 | 0.5 | 31 |
| NT | 0.0 | 0.0 | 0.0 | 0.0 | 1.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 | 0.4 | 0.0 | 0.0 | 0.5 | 0.5 | 0.7 | 0.5 | 0.5 | 30 |
| 15A | 0.0 | 0.0 | 0.0 | 2.0 | 0.0 | 0.0 | 3.2 | 1.9 | 0.0 | 1.2 | 1.3 | 0.3 | 0.7 | 0.5 | 0.3 | 0.1 | 0.4 | 0.4 | 27 |
| 15C | 1.6 | 0.0 | 0.0 | 2.0 | 0.0 | 1.9 | 0.0 | 0.0 | 1.9 | 0.0 | 0.4 | 0.5 | 0.4 | 0.2 | 0.6 | 0.5 | 0.1 | 0.4 | 27 |
| 16F | 0.0 | 0.0 | 0.0 | 2.0 | 1.2 | 0.0 | 0.0 | 1.9 | 0.0 | 1.2 | 0.9 | 0.8 | 0.7 | 0.2 | 0.6 | 0.1 | 0.4 | 0.4 | 27 |
| 9A | 0.5 | 1.4 | 1.9 | 1.0 | 2.4 | 0.0 | 1.6 | 0.0 | 0.0 | 3.0 | 2.2 | 0.5 | 0.0 | 0.7 | 0.0 | 0.2 | 0.0 | 0.4 | 26 |
| 31 | 1.0 | 0.0 | 1.9 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.2 | 0.9 | 0.8 | 0.0 | 0.2 | 0.5 | 0.1 | 0.5 | 0.4 | 25 |
| 33A | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.5 | 0.8 | 0.5 | 0.2 | 0.3 | 21 |
| 34 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.9 | 0.5 | 0.0 | 0.2 | 0.3 | 0.2 | 0.2 | 0.2 | 16 |
| 13 | 0.0 | 4.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 | 1.3 | 0.0 | 0.4 | 0.0 | 0.0 | 0.1 | 0.2 | 0.2 | 12 |
| 18A | 0.0 | 0.0 | 1.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 0.3 | 0.2 | 0.2 | 0.1 | 0.2 | 12 |
| 2 | 0.5 | 0.0 | 1.9 | 1.0 | 0.0 | 1.9 | 0.0 | 1.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.0 | 0.2 | 12 |
| 9L | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.6 | 0.0 | 0.0 | 1.2 | 0.9 | 0.3 | 0.4 | 0.0 | 0.2 | 0.2 | 0.0 | 0.2 | 12 |
| 23B | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.2 | 0.0 | 0.0 | 0.4 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | 11 |
| rough | 0.0 | 0.0 | 1.9 | 1.0 | 1.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.3 | 1.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 11 |
| 10B | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.3 | 0.0 | 0.2 | 0.2 | 0.2 | 10 |
| 18F | 0.5 | 2.7 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.2 | 0.0 | 0.2 | 0.0 | 0.2 | 10 |
| 12A | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.0 | 0.0 | 0.0 | 0.5 | 0.2 | 0.1 | 0.1 | 9 |
| 28A | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.9 | 0.3 | 0.0 | 0.0 | 0.2 | 0.2 | 0.0 | 0.1 | 8 |
| 35B | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.2 | 0.1 | 8 |
| 15F | 0.5 | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.9 | 0.0 | 0.0 | 0.4 | 0.3 | 0.4 | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | 7 |
| 7C | 0.5 | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.2 | 0.0 | 0.0 | 0.2 | 0.1 | 7 |
| 11F | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.1 | 6 |
| 10F | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 | 1.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 5 |
| 12B | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.1 | 5 |
| Others⋆ | 1.0 | 2.7 | 1.9 | 6.0 | 1.2 | 0.0 | 8.1 | 0.0 | 0.0 | 1.8 | 0.4 | 1.6 | 0.0 | 0.9 | 0.3 | 0.8 | 0.6 | 0.9 | 58 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 6,446 |
|
| |||||||||||||||||||
| Not sero- typed |
57.5 | 82.8 | 82.8 | 69.6 | 68.4 | 71.3 | 70.5 | 77.1 | 73.4 | 61.4 | 47.7 | 30.7 | 31.8 | 2.3 | 0.0 | 0.1 | 0.0 | 29.3 | 2,671 |
NT: nontypeable; n: number of isolates tested.
Others⋆ includes the serotypes (number of isolates): 33B (4), 35A (4), 11B (3), 19C (3), 22A (3), 29 (3), 35C (3), 37 (3), 45 (3), 18B (2), 19B (2), 21 (2), 24A (2), 24B (2), 25F (2), 28F (2), 36 (2), 48 (2), 7B (2), 12 (1), 17A (1), 18 (1), 19 (1), 23 (1), 39 (1), 6 (1), 7A (1), 9 (1).
Figure 1.
(a) Distribution of the epidemic serotypes 1, 2, 3, and 5 in percent (1992–2008, n = 9,394) in all age groups. (b) Distribution of the epidemic serotypes 1–3 and 5 among children in percent (1992–2008, n = 2,948). (c) Distribution of the epidemic serotypes 1–3 and 5 among adults in percent (1992–2008, n = 6,446).
Figure 2.
(a) Distribution of the 7-valent pneumococcal conjugate vaccine serotypes and the 7-valent vaccine coverage in percent (1992–2008, n = 9,394). (b) Distribution of the 7-valent pneumococcal conjugate vaccine serotypes and the 7-valent vaccine coverage among children in percent (1992–2008, n = 2,948). (c) Distribution of the 7-valent pneumococcal conjugate vaccine serotypes and the 7-valent vaccine coverage among adults in percent (1992–2008, n = 6,446).
Table 2.
Distribution of isolates serotyped among pneumococcal isolates sent to the NRCS in Germany (1992–2008).
| Isolates serotyped |
1992 (%) |
1993 (%) |
1994 (%) |
1995 (%) |
1996 (%) |
1997 (%) |
1998 (%) |
1999 (%) |
2000 (%) |
2001 (%) |
2002 (%) |
2003 (%) |
2004 (%) |
2005 (%) |
2006 (%) |
2007 (%) |
2008 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| children | |||||||||||||||||
| serotyped (%) |
92,2 | 82,9 | 96,6 | 65,4 | 38,7 | 92,9 | 97,6 | 96,8 | 96,2 | 100,0 | 99,6 | 99,2 | 100,0 | 100,0 | 100,0 | 100,0 | 100,0 |
| serotyped (n) |
47 | 29 | 28 | 17 | 12 | 158 | 161 | 182 | 200 | 239 | 258 | 244 | 265 | 316 | 287 | 273 | 232 |
| total | 51 | 35 | 29 | 26 | 31 | 170 | 165 | 188 | 208 | 239 | 259 | 246 | 265 | 316 | 287 | 273 | 232 |
|
| |||||||||||||||||
| adults | |||||||||||||||||
| serotyped (%) |
42,5 | 17,2 | 17,2 | 30,4 | 31,6 | 28,7 | 29,5 | 22,9 | 26,6 | 38,6 | 52,3 | 69,3 | 68,2 | 97,7 | 100,0 | 99,9 | 100,0 |
| serotyped (n) |
193 | 74 | 53 | 100 | 84 | 54 | 62 | 53 | 54 | 165 | 225 | 384 | 270 | 588 | 628 | 1654 | 1805 |
| Total | 454 | 429 | 309 | 329 | 266 | 188 | 210 | 231 | 203 | 428 | 430 | 554 | 396 | 602 | 628 | 1655 | 1805 |
|
| |||||||||||||||||
| overall | |||||||||||||||||
| serotyped (%) |
47,5 | 22,2 | 24,0 | 33,0 | 32,3 | 59,2 | 59,5 | 56,1 | 61,8 | 60,6 | 70,1 | 78,5 | 80,9 | 98,5 | 100,0 | 99,9 | 100,0 |
| serotyped (n) |
240 | 103 | 81 | 117 | 96 | 212 | 223 | 235 | 254 | 404 | 483 | 628 | 535 | 904 | 915 | 1927 | 2037 |
| total | 505 | 464 | 338 | 355 | 297 | 358 | 375 | 419 | 411 | 667 | 689 | 800 | 661 | 918 | 915 | 1928 | 2037 |
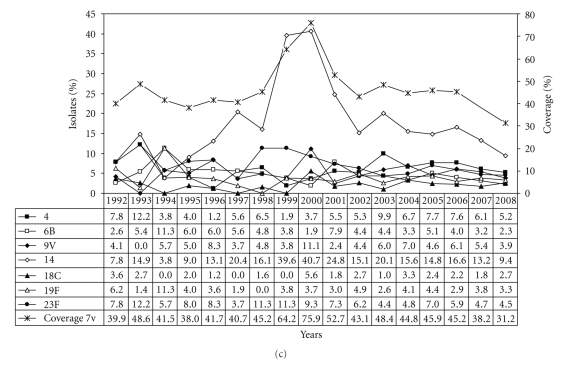
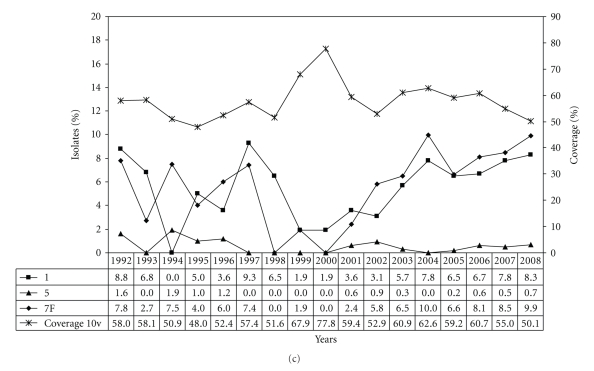
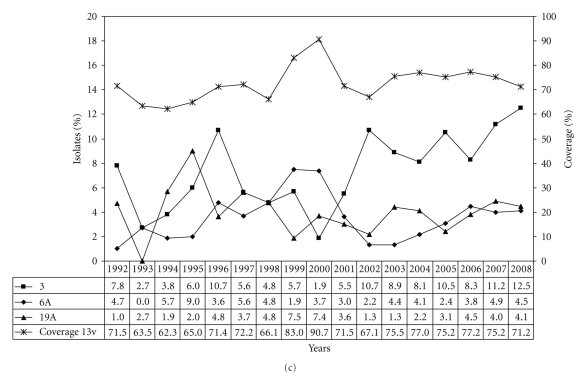
Serotype 14 is considerably more frequent among children (22.5%) than among adults (13.7%). Overall, an increase of serotype 14 can be noticed from around 1997 to 2006 (Figure 2(a)). A high percentage of serotype 14 isolates remain in existence for a longer time among children (1999–2006, Figure 2(b)) than among adults (1999–2001, Figure 2(c)). Serotype 1 and serotype 7F have been clearly increasing over the last approximately 10 years, both among children and among adults. While for children the percentage of serotypes 1 and 7F reach the highest values during this study in 2008, among adults they are high, but still within the range already noted before (Figures 3(a)-3(c)). Serotype 3 is far more common among adults (10.2%) than among children (3.3%) (Tables 1(b)-1(c)). The increase observed during the last years seems to be slightly higher among adults than among children (Figures 4(a)–4(c)). Concerning serotype 19A no clear change in frequency can be observed during the period under study (Figures 4(a)–4(c)).
Figure 3.
(a) Distribution of the additional 10v-pneumococcal conjugate vaccine serotypes 1, 5, and 7F and the 10-valent vaccine coverage in percent (1992–2008, n = 9,394). (b) Distribution of the additional 10v-pneumococcal conjugate vaccine serotypes 1, 5, and 7F and the 10-valent vaccine coverage among children in percent (1992–2008, n = 2,948). (c) Distribution of the additional 10v-pneumococcal conjugate vaccine serotypes 1, 5, and 7F and the 10-valent vaccine coverage among adults in percent (1992–2008, n = 6,446).
Figure 4.
(a) Distribution of the additional 13v-pneumococcal conjugate vaccine serotypes 3, 6A, and 19A and the 13-valent vaccine coverage in percent (1992–2008, n = 9,394). (b) Distribution of the additional 13v-pneumococcal conjugate vaccine serotypes 3, 6A, and 19A and the 13-valent vaccine coverage among children in percent (1992–2008, n = 2,948). (c) Distribution of the additional 13v-pneumococcal conjugate vaccine serotypes 3, 6A, and 19A and the 13-valent vaccine coverage among adults in percent (1992–2008, n = 6,446).
Variations in serotype distribution also affect theoretical vaccine coverages. The overall serotype coverage for the 7-valent conjugate vaccine was 45.4% during 1992–2008. For the 10-valent vaccine and the 13-valent vaccine the overall coverages were 60.9% and 76.6%, respectively. Generally, higher coverages were observed among children (7v, 57.3%; 10v, 72.8%; 13v, 83.5%) than among adults (7v, 39.9%; 10v, 55.5%; 13v, 73.5%). Among children, the coverage of the 7-valent vaccine decreased from 64.2% in 2005 to 24.6% in 2008 (Figure 2(b)), while for adults the coverage declined to a lesser extent (2005, 45.9%; 2008, 31.2%) (Figure 2(c)). Temporal changes of the additional 10-valent and 13-valent pneumococcal vaccine serotypes and the corresponding vaccine coverages are shown in Figures 3(a)–3(c) (10v) and Figures 4(a)–4(c) (13v). Since the general recommendation of pneumococcal conjugate vaccination for children <2 years in Germany at the end of July 2006 a percentage increase among the new (10v) or upcoming (13v) vaccine serotypes was noticed especially for serotypes 1 and 7F in children (Figure 3(b)) and for serotype 3 in adults (Figure 4(c)). In comparison, coverages of the 23-valent polysaccharide vaccine are very similar among children (min, 69.0%; max, 92.3%) and adults (min, 71.0%; max, 92.6%) from 1992 to 2008 (Figure 5).
Figure 5.
Coverage of the 23v-pneumococcal polysaccharide vaccine for children (n = 2,948), adults (n = 6,446), and overall (n = 9,394).
4. Discussion
In this paper we present the results of 17 years of surveillance concerning serotypes of invasive pneumococcal disease in Germany.
In the present study serotype 14 was the most prevalent serotype among children, followed in frequency by the serotypes 1, 6B, 19F, 23F, and 7F, respectively. These data are similar to those published for German children previously [12, 13] and in line with data from England [14], Belgium [15], and Denmark [16]. Among adults, the most frequent serotypes were 14, 3, 7F, 1, 4, and 23F (sorted in descending order). These results are similar to a previous study from the NRCS among adults in North-Rhine Westphalia, Germany, between 2003 and 2006 [17], and are generally in line with results reported from other countries [6, 15, 18–20]; however, they deviate in part from older German data [12, 21].
The variation of serotype 14 over the years is extensive in our study with an increased prevalence from about 1997 to 2006, reaching maximum values in 2000. Although the rate of serotyped isolates among adults was low from 1999 to 2001 (1999, 22.9%; 2000, 26.6%; 2001, 38.6%), the increase seems plausible. First, the increase among adults is paralleled by an increase among children, where nearly all isolates have been serotyped. Second, the rate of isolates serotyped among adults is within the range serotyped in the years before 1999, where a similar increase of serotype 14 was not observed. Furthermore, similar data have been shown in a report from Spain [4]. Also, a small rise in frequency of serotype 14 has been reported from Denmark in 1995–1999 [16], and data from England demonstrated a rise in incidence in 2000 and 2001 [14]. The rise of serotypes 1 and 7F for nearly one decade is similar to results from Spain [4].
Concerning serotype 19A, the highest prevalences were observed in 1996 (8.3%) and 1998 (6.2%) among children, and in 1999 (7.5%) and 2000 (7.4%) among adults. Although there seems to be a slight upward movement during the years 2002 to 2008, the prevalences still are below the maximum values reported. The increase of serotype 19A reported from other countries [4, 22, 23] has not been found in Germany so far.
Although changes in the incidence of different serotypes have been reported, the reasons for this are not fully understood [14]. Potential reasons discussed are changes in socioeconomic conditions, antibiotic use and resistance levels, immunocompromised status of populations, precise age distributions of the populations studied and increased life expectancy, and blood-culturing rates [2, 6, 24]. Since the general recommendation of pneumococcal conjugate vaccination for children <2 years in Germany at end of July 2006 a reduction in the percentage of IPD caused by the 7-valent vaccine serotypes was observed [25]. This effect is more apparent among children, but also present among the adult population. Similar results have been reported from other countries [3, 4, 26–29]. Logically, the percentage decrease in vaccine serotypes is paralleled by a percentage increase in the amount of IPD caused by nonvaccine serotypes, which was most prominent for serotypes 1 and 7F in children and serotype 3 in adults in this study. Serotype coverage for the 7-valent conjugate vaccine in Germany was 45.4% from 1992 to 2008. Calculated coverages for the 10-valent vaccine and 13-valent vaccine are 60.9% and 76.6%, respectively. These data are comparable to those previously reported [5, 14, 30, 31]. Coverages of all three vaccine formulations in our study are approximately 15% (7v, 17.4%; 10v, 17.3%; and 13v, 10.0%) higher among children than among adults. Differences in age distribution among study populations are known to have a major impact on vaccine coverage of the current and proposed vaccines for different age groups and, furthermore, comparable data have been published [14].
Nevertheless, some factors and limitations must be regarded when interpreting the results of this study. First of all, the isolates were sent by the participating laboratories on a voluntary basis, as participation in surveillance is not mandatory in Germany. Furthermore, the systematic sampling of invasive isolates from adults (1992) and children (1997) was taken up at different points of time, and the included population-based studies in three German federal states started in 2001 (North Rhine-Wesphalia) and 2006 (Bavaria and Saxony). Among isolates of adults, high levels of resistance were a main trigger for initiation of serotyping during the early years of this study. Therefore, the isolates may not be fully representative of all IPD in Germany over the last two decades.
Moreover, the structure of the surveillance project has been continousely improved over the 15 years and, in particular, after the general recommendation of pneumococcal conjugate vaccine for children <2 years in Germany at the end of July 2006 an increased disease awareness by both clinical micobiologists and pediatricians was observed [32].
Ongoing nationwide surveillance is necessary to observe further developments of pneumococcal serotype distribution in Germany.
Acknowledgment
The authors thank the microbiological laboratories in Germany for their cooperation and for providing the isolates.
References
- 1.Austrian R. Pneumococcus: the first one hundred years. Reviews of Infectious Diseases. 1981;3(2):183–189. doi: 10.1093/clinids/3.2.183. [DOI] [PubMed] [Google Scholar]
- 2.Feikin DR, Klugman KP. Historical changes in pneumococcal serogroup distribution: implications for the era of pneumococcal conjugate vaccines. Clinical Infectious Diseases. 2002;35(5):547–555. doi: 10.1086/341896. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs MR, Good CE, Beall B, Bajaksouzian S, Windau AR, Whitney CG. Changes in serotypes and antimicrobial susceptibility of invasive Streptococcus pneumoniae strains in Cleveland: a quarter century of experience. Journal of Clinical Microbiology. 2008;46(3):982–990. doi: 10.1128/JCM.02321-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fenoll A, Granizo JJ, Aguilar L, et al. Temporal trends of invasive Streptococcus pneumoniae serotypes and antimicrobial resistance patterns in Spain from 1979 to 2007. Journal of Clinical Microbiology. 2009;47(4):1012–1020. doi: 10.1128/JCM.01454-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinert RR. Pneumococcal conjugate vaccines—a European perspective. International Journal of Medical Microbiology. 2004;294(5):277–294. doi: 10.1016/j.ijmm.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Feikin DR, Klugman KP, Facklam RR, Zell ER, Schuchat A, Whitney CG. Increased prevalence of pediatric pneumococcal serotypes in elderly adults. Clinical Infectious Diseases. 2005;41(4):481–487. doi: 10.1086/432015. [DOI] [PubMed] [Google Scholar]
- 7.Imöhl M, Reinert RR, van der Linden M. Regional differences in serotype distribution, pneumococcal vaccine coverage, and antimicrobial resistance of invasive pneumococcal disease among German federal states. International Journal of Medical Microbiology. 2010;300(4):237–247. doi: 10.1016/j.ijmm.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Reinert RR, Queck A, Kaufhold A, Kresken M, Lütticken R. Antimicrobial resistance and type distribution of Streptococcus pneumoniae isolates causing systemic infections in Germany, 1992–1994. Clinical Infectious Diseases. 1995;21(6):1398–1401. doi: 10.1093/clinids/21.6.1398. [DOI] [PubMed] [Google Scholar]
- 9.Reinert RR, Al-Lahham A, Lemperle M, et al. Emergence of macrolide and penicillin resistance among invasive pneumococcal isolates in Germany. Journal of Antimicrobial Chemotherapy. 2002;49(1):61–68. doi: 10.1093/jac/49.1.61. [DOI] [PubMed] [Google Scholar]
- 10.Von Kries R, Siedler A, Schmitt HJ, Reinert RR. Proportion of invasive pneumococcal infections in German children preventable by pneumococcal conjugate vaccines. Clinical Infectious Diseases. 2000;31(2):482–487. doi: 10.1086/313984. [DOI] [PubMed] [Google Scholar]
- 11.Reinert RR, Haupts S, van der Linden M, et al. Invasive pneumococcal disease in adults in North-Rhine Westphalia, Germany, 2001–2003. Clinical Microbiology and Infection. 2005;11(12):985–991. doi: 10.1111/j.1469-0691.2005.01282.x. [DOI] [PubMed] [Google Scholar]
- 12.Kaufhold A, Lütticken R, Henrichsen J. Capsular types and antibiotic susceptibility of Streptococcus pneumoniae isolated from patients with systemic infections in West Germany. European Journal of Clinical Microbiology. 1987;6(6):696–697. doi: 10.1007/BF02013080. [DOI] [PubMed] [Google Scholar]
- 13.Rückinger S, von Kries R, Reinert RR, van der Linden M, Siedler A. Childhood invasive pneumococcal disease in Germany between 1997 and 2003: variability in incidence and serotype distribution in absence of general pneumococcal conjugate vaccination. Vaccine. 2008;26(32):3984–3986. doi: 10.1016/j.vaccine.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 14.Foster D, Knox K, Walker AS, et al. Invasive pneumococcal disease: epidemiology in children and adults prior to implementation of the conjugate vaccine in the Oxfordshire region, England. Journal of Medical Microbiology. 2008;57(4):480–487. doi: 10.1099/jmm.0.47690-0. [DOI] [PubMed] [Google Scholar]
- 15.Flamaing J, Verhaegen J, Vandeven J, Verbiest N, Peetermans WE. Pneumococcal bacteraemia in Belgium (1994–2004): the pre-conjugate vaccine era. Journal of Antimicrobial Chemotherapy. 2008;61(1):143–149. doi: 10.1093/jac/dkm435. [DOI] [PubMed] [Google Scholar]
- 16.Konradsen HB, Kaltoft MS. Invasive pneumococcal infections in Denmark from 1995 to 1999: epidemiology, serotypes, and resistance. Clinical and Diagnostic Laboratory Immunology. 2002;9(2):358–365. doi: 10.1128/CDLI.9.2.358-365.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imöhl M, Reinert RR, van der Linden M. Adult invasive pneumococcal disease between 2003 and 2006 in North-Rhine Westphalia, Germany: serotype distribution before recommendation for general pneumococcal conjugate vaccination for children <2 years of age. Clinical Microbiology and Infection. 2009;15(11):1008–1012. doi: 10.1111/j.1469-0691.2009.02895.x. [DOI] [PubMed] [Google Scholar]
- 18.de Cunto Brandileone MC, Simonsen DVV, Tadeu Casagrande S, et al. Characteristics of isolates Streptococcus pneumoniae from middle-aged and elderly adults in Brazil: capsular serotypes and antimicrobial sensitivity to invasive infections. Brazilian Journal of Infectious Diseases. 1998;2:90–96. [PubMed] [Google Scholar]
- 19.Plouffe JF, Moore SK, Davis R, Facklam RR. Serotypes of Streptococcus pneumoniae blood culture isolates from adults in Franklin County, Ohio. Journal of Clinical Microbiology. 1994;32(6):1606–1607. doi: 10.1128/jcm.32.6.1606-1607.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitney CG, Farley MM, Hadler J, et al. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. New England Journal of Medicine. 2000;343(26):1917–1924. doi: 10.1056/NEJM200012283432603. [DOI] [PubMed] [Google Scholar]
- 21.Lütticken R, Kaufhold A. Serotypen und Antibiotikaempfindlichkeit von Streptococcus pneumoniae (Pneumokokken) im Raum Köln. Immunitat und Infektion. 1985;13(3):99–107. [PubMed] [Google Scholar]
- 22.Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to non- pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. Journal of Infectious Diseases. 2007;196(9):1346–1354. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- 23.Reinert RR. The antimicrobial resistance profile of Streptococcus pneumoniae . Clinical Microbiology and Infection. 2009;15(supplement 3):7–11. doi: 10.1111/j.1469-0691.2009.02724.x. [DOI] [PubMed] [Google Scholar]
- 24.Hausdorff WP. Invasive pneumococcal disease in children: geographic and temporal variations in incidence and serotype distribution. European Journal of Pediatrics. 2002;161(supplement 2):S135–S139. doi: 10.1007/s00431-002-1066-x. [DOI] [PubMed] [Google Scholar]
- 25.Rückinger S, van der Linden M, Reinert RR, von Kries R, Burckhardt F, Siedler A. Reduction in the incidence of invasive pneumococcal disease after general vaccination with 7-valent pneumococcal conjugate vaccine in Germany. Vaccine. 2009;27(31):4136–4141. doi: 10.1016/j.vaccine.2009.04.057. [DOI] [PubMed] [Google Scholar]
- 26.Aguiar SI, Serrano I, Pinto FR, Melo-Cristino J, Ramirez M. Changes in Streptococcus pneumoniae serotypes causing invasive disease with non-universal vaccination coverage of the seven-valent conjugate vaccine. Clinical Microbiology and Infection. 2008;14(9):835–843. doi: 10.1111/j.1469-0691.2008.02031.x. [DOI] [PubMed] [Google Scholar]
- 27.Dias R, Caniça M. Invasive pneumococcal disease in Portugal prior to and after the introduction of pneumococcal heptavalent conjugate vaccine. FEMS Immunology and Medical Microbiology. 2007;51(1):35–42. doi: 10.1111/j.1574-695X.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 28.Lepoutre A, Varon E, Georges S, Gutmann L, Lévy-Bruhl D. Impact of infant pneumococcal vaccination on invasive pneumococcal diseases in France, 2001–2006. Euro Surveillance. 2008;13(35) doi: 10.2807/ese.13.35.18962-en. [DOI] [PubMed] [Google Scholar]
- 29.Mera R, Miller LA, Fritsche TR, Jones RN. Serotype replacement and multiple resistance in Streptococcus pneumoniae after the introduction of the conjugate pneumococcal vaccine. Microbial Drug Resistance. 2008;14(2):101–107. doi: 10.1089/mdr.2008.0782. [DOI] [PubMed] [Google Scholar]
- 30.Akduman D, Ehret JM, Judson FN. Comparison of secular trends in pneumococcal serotypes causing invasive disease in Denver, Colorado (1971–2004) and serotype coverage by marketed pneumococcal vaccines. Clinical Microbiology and Infection. 2006;12(11):1141–1143. doi: 10.1111/j.1469-0691.2006.01544.x. [DOI] [PubMed] [Google Scholar]
- 31.Hausdorff WP, Bryant J, Paradiso PR, Siber GR. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use—part I. Clinical Infectious Diseases. 2000;30(1):100–121. doi: 10.1086/313608. [DOI] [PubMed] [Google Scholar]
- 32.Ständige Impfkommission (STIKO) Empfehlungen der Ständigen Impfkommission (STIKO) am Robert Koch-Institut (Stand Juli 2006):Begründungen zur allgemeinen Empfehlung der Impfungen gegen Pneumokokken- und Meningokokken im Säuglings- und Kindesalter. Epidemiolgisches Bulletin. 2006;30:255–260. [Google Scholar]