Abstract
In this study, we investigated the role of hyaluronan (HA) in non-small cell lung cancer (NSCLC) since close association between HA level and malignancy has been reported. HA is an abundant extracellular matrix component and its synthesis is regulated by growth factors and cytokines that include epidermal growth factor (EGF) and interleukin-1β (IL-1β). We showed that treatment with recombinant EGF and IL-1β, alone or in combination with TGF-β, was able to stimulate HA production in lung adenocarcinoma cell line A549. TGF-β/IL-1β treatment induced epithelial to mesenchymal-like phenotype transition (EMT), changing cell morphology and expression of vimentin and E-cadherin. We also overexpressed hyaluronan synthase-3 (HAS3) in epithelial lung adenocarcinoma cell line H358, resulting in induced HA expression, EMT phenotype, enhanced MMP9 and MMP2 activities and increased invasion. Furthermore, adding exogenous HA to A549 cells and inducing HA H358 cells resulted in increased resistance to epidermal growth factor receptor (EGFR) inhibitor, Iressa. Together, these results suggest that elevated HA production is able to induce EMT and increase resistance to Iressa in NSCLC. Therefore, regulation of HA level in NSCLC may be a new target for therapeutic intervention.
1. Introduction
Lung cancer is the leading cause of death, both in the United States as well as worldwide. There are two main classifications for lung cancer, namely, non-small cell lung cancer (NSCLC) that accounts for 75%–80% and small cell lung cancer that make up the remaining 20%–25%. Despite extensive research in diagnostic and treatment strategies, the overall 5-year survival rate is only 8%–14% [1]. There is an urgent need to identify potential therapeutic targets for novel therapeutic approaches to manage this disease. One potential target is HA as it has previously been reported that high HA expression in the tumor cells and stroma of patients with lung adenocarcinoma, a subtype of NSCLC, is associated poor tumor differentiation and high recurrence rate [2].
HA is a linear unsulfated glycosaminoglycan composed of repeating disaccharides of D-glucuronic acid and N-acetylglucosamine, whose molecular weight can reach up to 107 dalton [3]. The synthesis of HA is regulated by three mammalian enzymes namely HAS1, HAS2, and HAS3 [4] which are integral plasma membrane proteins with the active sites that are located at the intracellular face of the membrane [5]. Each enzyme synthesizes HA but at different rates and terminates synthesis with polymer chains of differing size [6]. HAS3 is the most active that drives the synthesis of short HA chains (100 to 1000 kDa) and is thought to contribute to the pericellular matrix or may interact with cell surface HA receptors thereby triggering signaling cascades and profound changes in cell behavior. HAS3 is known to contribute to the malignant phenotype in many malignancies [7]. HAS1 is the least active and drives the synthesis of high molecular weight HA (2000 kDa). HAS2 is more catalytically active than HAS1 and it also produces high molecular weight HA (2000 kDa) and is implicated in developmental processes involving tissue expansion and growth. The existence of these three different isoforms implies that HA functions are diversely regulated through the activities and expression of the HAS genes. Various growth factors and cytokines including TFG-β1 [8], EGF [9] and heregulin [10] have been shown to modulate HAS expressions and HA production in tumor cells.
Increased synthesis of HA is often associated with malignancy in many different tumors including lung cancer [2], ovarian cancer [11], and breast cancer [12]. HA production is contributed by the tumor-associated stroma and/or tumor cells [13, 14]. This association between HA and tumorigenesis have been supported by both in vitro and in vivo studies whereby overexpression of various HAS isoforms caused increased growth or metastasis in fibrosarcoma [15], prostate [16], colon [17], and breast [18] cancers. Conversely, inhibition of various HAS genes and thereby downregulation of HA production caused a decreased in tumor growth in prostate carcinoma cells [19] and metastasis in breast cancer [20]. Furthermore, in vitro studies have also demonstrated that exogenous addition of HA to tumor cells was able to promote cell migration [21] in ovarian cancer cell line, induces chemoresistance in NSCLC and meloma cell lines [22, 23], and promotes, cell invasion by stimulating production of metalloproteinases (MMPs) in lymphoma and small lung cancer cell lines [24, 25]. These studies highlight the importance of hyaluronan in the progression of tumorigenesis.
EMT is a process that plays important role in normal development and in cancer progression [26]. EMT involves morphological and biochemical changes resulting in the loss of E-cadherin, an epithelial marker while gaining mesenchymal markers such as vimentin or fibronectin. Besides, EMT has also been reported to induce the production of MMPs resulting in the tumor cells gaining invasive abilities which represents one of the hallmarks of cancer [27]. Downregulation of E-cadherin is associated with poor prognosis in NSCLC [28, 29] and prostate cancer [30, 31] indicating that E-cadherin has a tumor suppressing role.
EGFR is expressed in a variety of human malignancies and EGFR-TKIs have been used in treatment for a number of cancers including NSCLC [32]. It has been reported that EMT is a determinant of sensitivity of NSCLC [33, 34] as well as head and neck cancer [35, 36]. In this context, tumor cells with mesenchymal phenotype were less sensitive to these inhibitors. Overexpression of E-cadherin in in vitro study restored the sensitivity to EGFR-TKIs [37], thus indicating that E-cadherin expression has a role in the mechanism underlying response to these drugs.
It has recently been demonstrated that HA is involved in EMT as defects in HAS2 gene knockout mice failed to undergo EMT that is required for cardiac tissue development [38]. This notion was further supported by HAS2 overexpression study whereby increased in HA production was sufficient to induce EMT and acquisition of transformed properties in normal epithelial cells [39]. In another study, overexpression of E-cadherin negatively regulated the interaction of HA with its cell surface receptor, CD44 and inhibits CD44-mediated tumor invasion and branching morphogenesis [40]. These finding supports the role of HA in EMT and in the acquisition of malignant phenotype. In this paper, we examine the nature of HA interaction with non small lung cancer cell lines relative to EMT.
2. Materials and Methods
2.1. Cell Culture
Lung adenocarcinoma cell lines, A549 and H358 cells were purchased from American Tissue Type Culture Collection (Manassas, VA) and cultured in RPMI-1640 media supplemented with 5% FBS (Hyclone, Logan, UT). HAS3 stable transfectants (HAS3) and vector transfectants (vector-only control) were cultured in RPMI-1640 supplemented with 5% FBS and 125 μg/mL of Zeocin (Invitrogen, Carlsbad, CA). Cells were grown at 37°C and 5% CO2 in a humidified incubator.
2.2. Plasmid Construction and Preparation of Stable Transfectant
HAS3 cDNA was amplified by RT-PCR from H358 mRNA using oligonucleotides that incorporated HindIII and XhoI restriction sites using Platinum Pfx DNA polymerase, sequence was verified by DNA sequencing and ligated into pcDNA3.1 vector. The primer sequences are as follows: HAS3 (HindIII forward primer) 5′-GCGAAGCTTACCATGCCGGTGCAGCTGACGACAGCC-3′ and HAS3 (XhoI reverse primer) 5′-GCGCTCGAGTCACACCTCAGCAAAAGCCAAGCTG-3′. H358 cells were transfected with pcDNA HAS 3 (HAS3) or pcDNA vector (vector-only control) using Fugene 6 (Roche, Indianapolis, IN) following manufacturer's instruction. Stable clones were obtained by 125 μg/mL Zeocin selection and screened for HA synthesizing capacity (see HA Assay). Cloning reagents and DNA polymerase were from Invitrogen (Carlsbad, CA).
2.3. Treatment with Growth Factors and Cytokines
A549 cells (5 × 105/dish) were plated in 35 mm dishes overnight in RPMI-1640 supplemented with 5% FBS. Media was then replaced with RPMI-1640 supplemented with insulin-transferrin-selenium (ITS) for 16 hours before treatment with various growth factors or cytokines (Pepro Tech, Rocky Hill, NJ) for 24 hours. Media was collected for HA quantification (see HA Assay) while cells were harvested for cell lysate using lysis buffer (100 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100 and protease inhibitors) for total protein determination by BCA Protein Assay (Pierce, Rockford, IL).
2.4. RT-PCR Analysis
Total RNA was extracted, from cells seeded at 5 × 105 cells/35 mm dish, with Trizol and reverse transcribed with Superscript III RT using oligo dT priming (Invitrogen, Carlsbad, CA) to generate cDNA templates according to manufacturer's instructions. Accumulation of PCR products was measured in real-time by using SYBR Green PCR Master Mix with 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA). The sequences of primers are listed in Table 1. The PCR reactions conditions consisted of a 10 minutes preincubation at 94°C followed by 40 amplification cycles as follows: 94°C for 15 seconds and annealing and extension at 60°C for 1 minute. The data was normalized to the expression of the house keeping gene, glyceraldehydes-3- phosphate dehydrogenase (GAPDH). Quantification was performed using the comparative cycle threshold (Ct) method using the formula 2−ΔΔCt where ΔΔCt = ΔCtsample − ΔCtreference to calculate relative expression.
Table 1.
Primer sequences for real-time PCR.
| Genes | Primer sequences (forward/reverse) | Size bp | Annealing temperature °C | Accession number |
|---|---|---|---|---|
| HAS1 | GCTCAGCATGGGTTATGCTACC GTTGTACAGCCACTCACGGAAG | 133 | 58 | NM_001523.1 |
| HAS2 | AAGAAAGCTCGCAACACGTAACGCACACCTCCAACCATGGGATCTTCT | 220 | 60 | NM_005328.1 |
| HAS3 | CTTCTTTGTGTGGCGCAGCCACCTGGATGTAGTCCACCGAAT | 199 | 58 | NM_005329.1 |
| MMP9 | ACCTGAGAACCAATCTCACCGACAGGAAGGTTTGGAATCTGCCCAGGTCTGG | 229 | 62 | NM_004994.2 |
| MMP2 | ACAAATATGAGAGCTGCACCAGCGTTGGTGTAGGTGTAAATGGGTGCC | 214 | 59 | NM_004530.2 |
| GAPDH | GTCAACGGATTTGGTCGTATTGGGTGCCATGGGTGGAATCATATTGG | 142 | 58 | NM_002046.3 |
2.5. HA Assay
Cells were grown in RPMI-1640 supplemented with ITS to determine the amount of HA released into the media. Cells were plated at 5 × 105 cells/dish (35 mm dish). Conditioned media was collected at either 24 hours or 48 hours, clarified by centrifugation and analyzed for total amount of HA using DuoSet HA kit (R and D Systems, Minneapolis, MN). Media was diluted between 10- and 100-fold so that the samples were within the range of the standard curve of the DuoSet assay. Plate preparation and assay procedures were performed according to the manufacturer's protocols. The values are normalized to total protein and expressed as nanograms/mL of HA/μg of protein.
2.6. Immunofluorescence Staining
A549 cells were seeded on 4-well chamber slides (Nunc, Rochester, NY) at cells seeded at 1 × 105 cells/well and treated with growth factors and cytokines for 24 hours following overnight incubation in serum-free media. In the case of HAS3 and vector-only control transfectants, these cells were plated in 4-well chamber slides for 48 hours in serum containing media. The cells were then fixed with 4% formaldehyde in phosphate buffered saline (PBS) for 15 minutes at RT, washed with PBS, permeabilized with 100% ice-cold methanol at −20°C for 10 minutes, washed with PBS and blocked with PBS containing 5% of normal goat serum and 0.3% Triton X-100 for 1 hour. They were then incubated with the primary antibody to either E-cadherin (#3195) (1 : 100) or vimentin (#3932) (1 : 50) (Cell Signaling Technology, Danvers, MA) overnight at 4°C, washed with PBS and then counterstained with secondary antibodies conjugated with TRIC (1 : 100, Pierce, Rockford, IL) for 1.5 hours. After washing, the coverslips were attached to glass slides with mounting media containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA) and edges sealed with nail polish. The slides were examined and photographed using an Olympus BX41 microscope (Olympus, Center Valley, PA).
2.7. Particle Exclusion Assay
Pericellular HA matrices were visualized using a particle exclusion assay as described by Knudson [41]. Briefly, 1 × 105 cells cells were treated in the absence or presence of TGF-β1 in combination with IL-1β (10 ng/mL each) for 24 hours followed by treated for 2 hours in the absence or presence of 10 units/mL Streptomyces hyaluronidase (Sigma, St. Louis, MO). In the case of HAS3 and vector-only control transfectants, they were incubated overnight in serum containing media and then treated with or without 10 units/mL Streptomyces hyaluronidase for 2 hours. The media was aspirated and the cells were washed and further incubated with 0.75 mL of formalin fixed horse erythrocytes (108 per mL) in PBS containing 0.1% BSA for 15 minutes. At which time, cells were viewed and photographed with an inverted phase-contrast microscope (Nikon, Melville, NY). The HA matrix was evidenced by halos surrounding the cells from which the fixed erythrocytes were excluded. Cells were photographed at a magnification of 400x.
2.8. Cell Proliferation Assay
Cell proliferation was assessed using the Cell Titer 96 Aqueous One Solution Cell Proliferation Assay (MTS) (Promega, Madison, WI) according to manufacturer's instructions. Cells (A549@ 5000 cells/well; H358@ 10,000 cells/well) were plated in 96-well plate in RPMI-1640 supplemented with 5% FBS and allowed to attached overnight. Next day, media was replaced with RPMI-1640 supplemented with ITS for another 24 hours before it was subjected to treatment with different concentrations of EGFR-TKI, Iressa (AstraZeneca, Wilmington, DE) ranging from 0.5 μM to 10 μM in the absence or presence of either 500 μg/mL of 132 kDa HA (R and D Systems, Minneapolis, MN) or 500 μg/mL HA plus 10 units/mL of Streptomyces hyaluronidase. In the case of H358 HAS3 and vector-only control, cells were cultured in RPMI-1640 supplemented with ITS for 48 hours before treatment with EGFR-TKI was commenced. Following 72 hours treatment, MTS reagent was added, incubated for 1 to 2 hours and absorbance measured at 490 nm in a FLUOstar Optima 96-well plate reader (BMG Labtech, Durham, NC). All experimental data points were set up in six wells. The data was expressed as percentage growth relative to that of the untreated control cells.
2.9. Cell Invasion Assay
The ability of cells to migrate through a Matrigel basement membrane matrix and filter was measured in a Matrigel invasion chamber (BD Biosciences, San Jose, CA) with 8 μm membrane pore. After rehydration for 2 hours, 1 × 105 cells in RPMI-1640 were applied to the upper chamber while the lower chamber contained RPMI-1640 supplemented with 5% FBS that acts as a chemoattractant. After incubation at 37°C for 72 hours, the upper chamber were removed and placed into a clean well with RPMI-1640 containing 8 μM Calcein AM (Sigma, St. Louis, MO) for 20 minutes at 37°C. At which time, media was removed from the upper chamber and cells attached to the lower surface of the filter were isolated by placing the insert in trypsin solution without phenol red for 10 minutes at 37°C. Aliquots of the trypsinate were then assayed at excitation wavelength 480 nm and emission wavelength 520 nm in a FLUOstar Optima 96-well plate reader. To determine the invaded cell number, a fluorescent cell dose curve was generated by plotting a graph with the y-axis representing fluorescence units against the x-axis representing different cell numbers.
2.10. MMP2 and MMP9 Zymography
Conditioned media was obtained from HAS3 and vector-only control cells that were cultured for 48 hours in RPMI-1640 supplemented with ITS. The media was concentrated using Microcon YM-10 spin column (Millipore, Billerica, MA) and protein concentration was determined by BCA Protein Assay (Pierce, Rockford, IL). Equal amount of protein (20 μg) was loaded onto an 8% SDS polyacrylamide gel that contains 0.1% (w/v) gelatin and was run under nonreducing conditions. The gel was then incubated in 2.5% Triton X-100 in 50 mM Tris-HCl for 1 hour and subsequently incubated in a buffer containing 150 mM NaCl, 10 mM CaCl2, 0.02% NaN3, and 50 mM Tris-HCl, pH 7.5 at 37°C for 48–72 hours. The gel was then stained with 0.25% Coomassie Blue and proteolysis was detected as a white band against a blue background and documented using gel imaging system (Alpha Innotech, San Leandro, CA). The zymogram image was reversed with the dark bands indicating MMP2 and MMP9 activities.
2.11. Statistical Analysis
The statistical significance of the data was determined with Student's t-test. Statistical significance was indicated by P < .05.
3. Results
3.1. Cytokines and Growth Factor Increases HA Production in A549 Cells
Present in the extracellular milieu of lung tumors are various cytokines and growth factors that are secreted by tumor cells, fibroblasts and immune cells [42, 43]. We tested various cytokines and growth factor for their ability to enhance HA production in A549 cells, a lung adenocarcinoma to assess its role in NSCLC as HA has been implicated to play a role in numerous types of cancer including breast, prostate, and colon cancers [3].
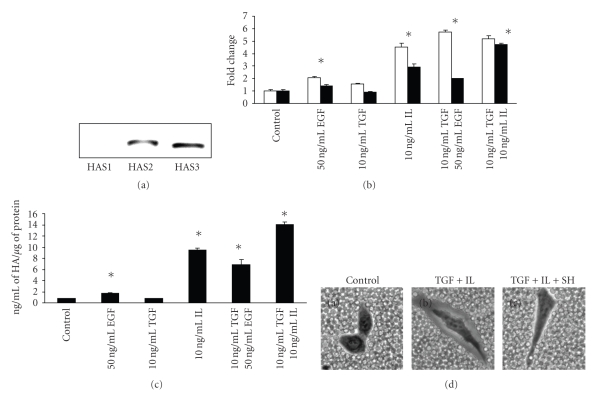
A549 cells do not expressed HAS1 mRNA but transcripts for HAS2 and HAS3 were detected (Figure 1(a)) in unstimulated cells with HAS3 being the more abundant transcript that was approximate 2-fold higher as assessed by quantitative real time RT-PCR (data not shown). Next the effect of specific growth factors and cytokines on HA production was determined. When A549 cells were treated individually with EGF (50 ng/mL) or IL-1β (10 ng/mL), an increased in both mRNA for HAS2 and HAS3 was observed at 24 hours for EGF (2-fold for HAS2, 1.4 fold for HAS3) and IL-1β (4.5-fold for HAS2, 2.9-fold for HAS3) (Figure 1(b)). Treatment with TGF-1β (10 ng/mL) resulted in a modest increase in HAS2 (1.5-fold) with a little or no change in HAS3 (Figure 1(b)). However, combination treatments resulted in a substantial increase in both HAS transcripts for TGF/EGF (10 ng/mL and 50 ng/mL, resp.), (5-fold for HAS2, 2 fold for HAS3), and TGF/IL (10 ng/mL each) (5.2-fold for HAS2 and 4.7-fold for HAS3). In each case, these factors tested had a greater stimulatory effect on HAS2 than HAS3 mRNA while no HAS1 transcript was detected (data not shown). In addition, induction of HAS transcripts paralleled with total increased in HA production resulting from these treatments except for TGF whether no change was observed (Figure 1(c)). Since TGF/IL resulted in the highest HA stimulation at 14.09 ± 0.39 ng/mL of HA/μg of protein, this culture was assessed in the assembly of HA-dependent pericellular matrices as visualized by particle exclusion assay, a frequently used test for HA coat formation and HA synthesis [41]. Control A549 cells do not exhibit these matrices whereby the fixed red blood cells closely abutted the surface of each cell (Figure 1(d), Panel A: Control). However, when treated with TGF/IL, the cells appear to be surrounded by a clear halo as the red blood cells were not able to penetrate the matrices (Figure 1(d), Panel B: TGF+IL) and the matrices disappears following treatment with Streptomyces hyaluronidase (Figure 1(d), Panel C: TGF+IL+SH), an enzyme specific for HA, indicating that the assembly of the matrices is HA-dependent.
Figure 1.
Effect of growth factors and cytokines on HA production. (a) Expression of HAS mRNA in A549 cells as visualized by ethidium bromide staining of agarose gel. A549 cells were treated with or without various growth factors and cytokines for 24 hours in RPMI-1640 supplemented with ITS following overnight serum starvation and assayed for (b) mRNA expression for HAS2 (open bar) and HAS3 (close bar) by quantitative real time RT-PCR, and (c) total HA production using DuoSet HA kit. (d) HA-dependent pericellular matrix was visualized by particle exclusion assay following 24 hours incubation with or without combination treatment with 10 ng/mL each of TGF-β1 and IL-1β: Panel (a), Control; Panel (b), TGF + IL; Panel (c), 2 hours incubation with 10 units/mL of Streptomyces hyaluronidase following treatment with TGF + IL. The experiments were repeated three times and analyzed in triplicate. Representatives are shown for (b) and (c). (Values are mean ± SD, n = 3, *P < .05).
3.2. Combination of TGF and IL Treatment Induces Mesenchymal-Like Phenotype in A549 Cells
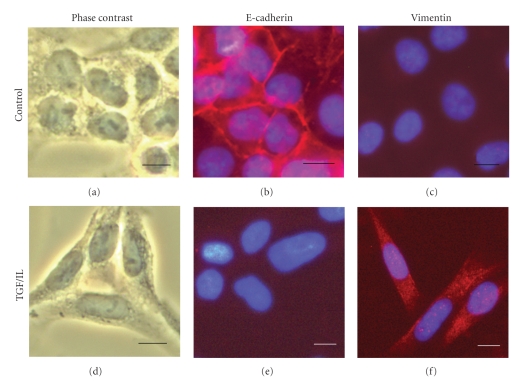
It was also noted that combination treatment with TGF/IL resulted in a change in cell phenotype from epithelial-like that closely adhered to each other to a more fibroblast-like appearance (Figure 2, Panel (a) and (d): Phase contrast). This shift in morphology could represent a transition from epithelial to mesenchymal phenotype. To determine whether TGF/IL treatment of A549 cells promotes this transition, we detect the presence for E-cadherin as well as vimentin that are epithelial and mesenchymal markers, respectively in these cells by immunocytochemistry. Control A549 cells expressed strong staining for E-cadherin (Figure 2, Panel (b)) with no evidence of vimentin expression (Figure 2, Panel (c)) while treatment with TGF/IL cells expressed strong staining for vimentin (Figure 2, Panel (f)) with reduced staining for E-cadherin (Figure 2, Panel (e)) indicating that combination treatment of these cytokines is able to induce EMT-like transition in A549.
Figure 2.
Combination treatment with TGF-β1 and IL-1β of A549 cells induces mesenchymal-like phenoptype. A549 cells following overnight serum starvation were treated with 10 ng/mL each of TGF-β1 and IL-1β for 24 hours. Following which time, the cells were fixed, permeabilized, and stained with antibody against E-cadherin ((b) and (e)) and vimentin ((c) and (f)) and nuclei were counterstained using 4′,6-diamidino-2-phenylindole. Cells in (a) and (d) were photographed in a phase-contrast microscope. Scale bar: 10 μm.
3.3. Overexpression of HA Induces Mesenchymal Characteristics in H358 Cells
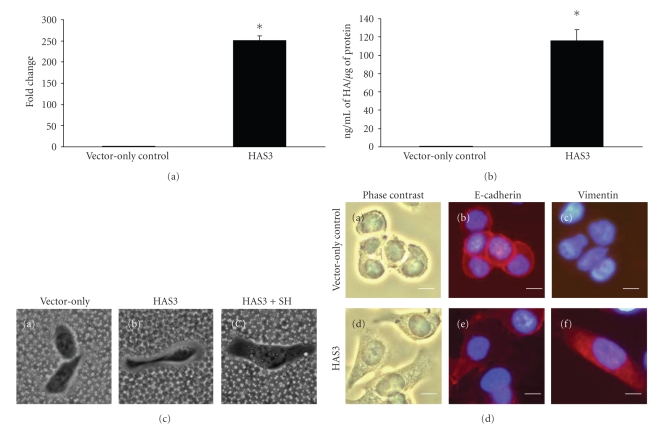
We next asked if an increased in HA production can induce the transition from an epithelial-like to mesenchymal-like phenotype in NSCLC. To address this question, we transfected H358 cells, another lung adenocarcinoma cell line that is more epithelial-like in phenotype as compared to A549 cells [33] with HAS3 gene to generate a stable cell line that overproduce HA. Overexpression of HAS3 gene induced over 200-fold increased in HAS3 transcript as compared to vector-only control (Figure 3(a)) resulting in 115 ± 12.33 ng/mL of HA/μg of protein while the vector-only control produced 0.74 ± 0.085 ng/mL of HA/μg of protein (Figure 3(b)). This observed increased in HA production was also supported by particle cell exclusion assay whereby HAS3 transfectant assembled a pericellular coat (Figure 3(c), Panel (b): HAS3) that was HA-dependent as treatment with Streptomyces hyaluronidase (Figure 3(c), Panel (c): HAS3+SH) resulted in the loss of the matrix while the vector-only control showed an absence of a cell coat (Figure 3(c), Panel (a): Vector-only). We next stained these cells for E-cadherin and vimentin expression to assess whether HA overexpression is involved in EMT-like transition in NSCLC cell line. Vector-only control cells were more compact and adhered to one another (Figure 3(d), Panel (a)) with a strong staining for E-cadherin (Figure 3(d)c, Panel (b)) at the cell surfaces and no staining for vimentin (Figure 3(d), Panel (c)). On the other hand, HAS3 overexpressing cells tend to spread and are more elongated in appearance (Figure 3(d), Panel (d)) with weaker staining for E-cadherin (Figure 3(d), Panel (e)) that is disperse within the cytoplasm while vimentin is strongly expressed in the cytoplasm of these cells (Figure 3(d), Panel (f)). These results support the contribution of HA in inducing morphological and biochemical changes in cells resulting in a mesenchymal phenotype in NSCLC cells.
Figure 3.
Enhanced HA production promotes mesenchymal-like phenotype in H358 cells. H358 cells were stably transfected with either vector only acting as a control or HAS3 gene. Stable transfectants were assessed for (a) HAS3 mRNA expression by quantitative real time RT-PCR; (b) total HA production; (c) HA-dependent matrix visualized by particle exclusion assay; (d) expression of E-cadherin ((b) and (e)) and vimentin ((c) and (f)) by immunocytochemistry using the antibody against E-cadherin and vimentin and counterstained using 4′,6-diamidino-2-phenylindole. Cells in (a) and (d) were taken using a phase-contrast microscope. Scale bar: 10 μm. Three experiments analyzed in triplicate were carried out for (a) and (b). The data shown is a representative. (Values are mean ± SD, n = 3, *P < .05.)
3.4. Increased HA Production Promotes Cell Invasion and Enhanced MMP2 and MMP9 Activities in H358 Cells
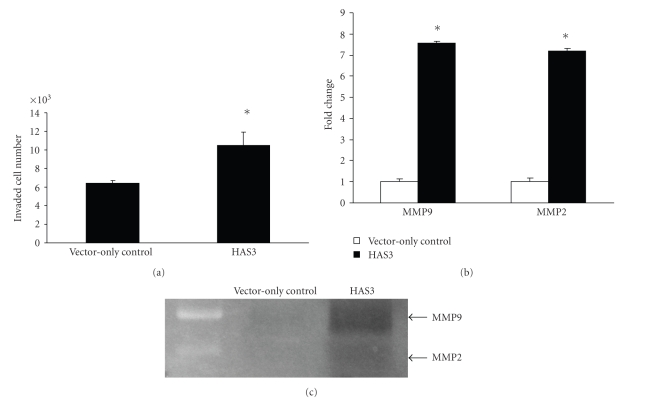
One of the functional characteristics of mesenchymal-like cells as a result of the loss of cell-cell adhesion is the acquisition of invasion ability [27]. To determine whether HAS3 overexpressing cells that produced increased amount of HA were more invasive as compared to the vector-only control, an invasion assay was performed. As shown in Figure 4(a), HAS3 transfectants had more cells that transverse through the Matrigel as compared to the vector-only control, suggesting that overexpression of HA can promote an increased in invasive ability. As MMP2 and MMP9 are involved in invasion [44, 45], we assessed whether an increased in HA production is able to induce the production of these MMPs in NSCLC cell line. Over 7-fold increased in both MMP9 and MMP2 transcripts were observed in HAS3 transfectant as compared to the vector-only control (Figure 4(b)) as determined by quantitative real time RT-PCR. However, MMP2 transcript was approximately 30-fold less than MMP9 transcript in both vector-only control and HAS3 transfectants (data not shown). This result is supported by gelatin zymography that indicated a strong MMP9 activity at 97 kDa with a weaker MMP2 activity at 72 kDa (Figure 4(c)) suggesting that HA is able to induce MMP production to promote cell invasion in lung adenocarcinoma that have undergone EMT-like transition.
Figure 4.
Increased HA production promotes cell invasion and induces enhanced MMP2 and MMP9 activities in H358 cells. (a) Invasive capacity of HAS3 and vector—only control transfectants were determined using invasion chamber assay (BD Bioscience) as outlined in Material and Method. (b) mRNA expression for MMP2 and MMP9 were determined by quantitative real time RT-PCR. (c) Following 48 hours incubation in serum-free media, media was collected and gelatinase activities for MMP2 and MMP9 were assayed by gelatin zymography. The experiments were repeated twice. The data shown for (a) and (b) is a representative. (Values are mean ± SD, n = 3, *P < .05.)
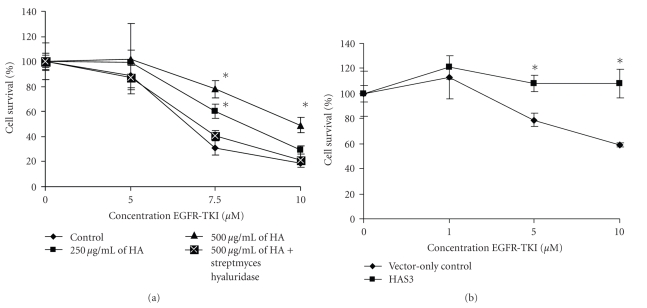
3.5. Increased HA Production Reduces Sensitivity to EGFR-TKI in A549 and H358 Cells
EGFR has been shown to be overexpressed in human cancers including NSCLC and overexpression has been shown to correlate with poor survival [32, 46]. Inhibitors of EGFR have been used to block EGF signaling pathway as a treatment option in NSCLC [32, 47]. As previous studies have shown that HA can promote chemoresistance in different cancer types including lung [23] and myeloma [22] cancers, we wanted to examine whether HA is able to promote cell survival by reducing sensitivity of NSCLC cell lines to EGFR-TKI. We evaluated a range of concentrations of exogenous HA (132 kDa) in A549 cells. This well differentiated adenocarcinoma has an intermediate sensitivity to EGFR-TKI [33] showed that increasing concentration of HA reduced the cells sensitivity to the inhibitor and thereby promoted cell survival (Figure 5(a)). Furthermore, coincubation of HA with Streptomyces hyaluronidase resulted in the loss of this protective effect indicating that HA has a role in promoting resistance to EGFR-TKI (Figure 5(a)). It has previously been observed that EMT is a determinant of sensitivity of NSCLC cell lines to EGFR-TKI [33, 34] and since HA can promote EMT as presented in the current study, we assess whether overproduction of HA could play a role in reduced sensitivity to EGFR-TKI. HAS3 overexpressing cells showed no cell death at the concentrations of EGFR-TKI tested while vector-only control exhibited a steady decline and at 10 μM EGFR-TKI approximately 40% cell death was observed (Figure 5(b)). These data indicates that HA can promote cell survival by reducing sensitivity of the cells to EGFR-TKI. The mechanism by which HA induces resistance to EGFR-TKI remains to be investigated.
Figure 5.
Exogenous HA and overexpression of HAS3 gene reduces sensitivity to EGFR-TKI in A549 and H358 cell lines. (a) A549 cells were seeded in a 96-well flat bottom plates and treated with either 250 μg/mL or 500 μg/mL of 132 kDa HA in the absence or presence of varying concentration of EGFR-TKI for 72 hours. To determine whether this reduced sensitivity is due to HA, 10 unit/mL of Streptomyces hyaluronidase were coincubated with 500 μg/mL HA and treated with varying concentration of EGFR-TKI for 72 hours. The in vitro EGFR-TKI sensitivity of A549 cells was evaluated by cell proliferation assay. (b) HAS3 and vector-only control were cultured for 48 hours before treatment in the absence or presence of varying concentration of EGFR-TKI for 72 hours before assaying for cell growth. The experiments were repeated three times. A representative is shown for (a) and (b). (Values are mean ± SD, n = 6, *P < .05.)
4. Discussion
The close association between high HA level and malignancy has been reported in many different types of cancer including breast, ovarian, and bladder cancers [3]. However, there is only limited information regarding the contribution of HA in the development of NSCLC. Existing information suggests that HA might have a contributing role in this process [2, 23].
The synthesis of HA is a tightly regulated process and is modulated by cytokines and growth factors in a number of different cancer cells [8–10]. Cytokines and growth factors are found in the lung tumor microenvironment and are derived from tumor cells as well as stromal and inflammatory cells [42]. In this paper, we investigated the effect of EGF, IL-1β and TGF-β1 in modulating HA synthesis in A549 cells, a lung adenocarcinoma cell line. These factors were chosen as they have been reported to play a role in the progression of lung cancer as elevated expression of EGF, IL-1β, and TGF-β1 have been reported in patients with lung cancer [48–50]. These factors either separately or in combination was able to stimulate HAS2 transcript more than HAS3 transcript (Figure 1(b)) suggesting that HAS2 mRNA is more responsive to external stimuli which has also been observed in human mesothelial cells [51]. However, induction of HAS isoform expressions resulted in an increase in HA production that mimic the up-regulation of HAS3 isoform, with TGF/IL combination giving the highest stimulation (Figure 1(c)), suggesting a dominant role for HAS3 isoform.
Increased in HA production resulted in a HA-dependent pericellular matrix in TGF/IL cultures (Figure 1(d)) that could participate in biological processes pertaining to migration and proliferation as these HA-rich matrices have been reported in migrating and proliferating cells [52]. In addition, TGF/IL treatment also resulted in morphological and biological changes whereby there is the combined loss of staining for epithelial marker, E-cadherin, and a gain in mesenchymal marker, vimentin (Figure 2) indicating a shift to a mesenchymal-like phenotype. This finding suggests that HA synthesis can be modulated by various cytokines and growth factors in NSCLC. Increase in HA level might play a role in the EMT-like transition in NSCLC as HA has been shown to be involved in EMT in normal cardiac development [38].
To define the role of HA in EMT-like process in NSCLC, we overexpressed HAS3 gene in H358 cells as these cells exhibit a more epithelial phenotype as compared to A549 [33]. Overexpression of HAS3 gene in H358 resulted in a change is morphology from a more compact and cobblestone-like appearance to cells that are elongated in shape with a concomitant appearance of vimentin expression and a reduced staining for E-cadherin, indicating a gain in mesenchymal-like phenotype (Figure 3(c)). Our finding is in agreement with a report that showed HA can induced EMT-like transition and acquisition of transformed characteristics in normal epithelial cells [39]. Moreover, it has been reported that the HA-CD44 axis plays an important role in morphogenesis in mammalian organogenesis. In this context, overexpression of E-cadherin, in cells with low levels of E-cadherin expression, negatively modulates HA interaction with its receptor, CD44, that leads to the inhibition of CD44-mediated cell invasion and branching morphogenesis [40]. Our study indicates that increase in HA production can modulate cell surface expression of E-cadherin and promote a shift to a mesenchymal-like phenotype in NSCLC. Therefore, this result suggests that there is a cross-talk between E-cadherin and HA regulation. Although the mechanism by which HA mediates this effect in NSCLC remains to be studied it may be possible that anomalous overexpression of HA destabilizes the balance in normal epithelial cells with high levels of E-cadherin expression and induces a shift to an EMT differentiation process.
One of the characteristic of cells that have undergone EMT is the acquisition of an invasive phenotype [27]. We observed that overexpression of HAS3 gene in H358 resulted in the cells were more invasive compared to vector-only control cells (Figure 4(a)). MMP2 and MMP9 have been implicated to play a role in cell invasion and metastasis as they are involved in the turnover of extracellular matrix components in the basement membrane [44, 45]. In our system, both MMP9 and MMP2 transcripts were elevated with the MMP9 mRNA expression being more abundant (Figure 4(b)) and this was reflected by an increased in their activities as assessed by the gelatin zymography (Figure 4(c)). Our data supports that increased HA production is able to stimulate the production of MMP2 and MMP9 leading to a more invasive phenotype in NSCLC cell line. This is in line with a recent report that demonstrated that normal epithelial cells that have undergone EMT following overexpression of HAS2 gene also induced elevated expressions of MMP9 and MMP2 [39]. Further, treatment with exogenous HA was also able to stimulate MMP2 secretion in small cell lung cancer [25].
EGFR is overexpressed in human cancer including NSCLC and is correlated with poor prognosis [46]. EGFR-TKIs have been used in the treatment of NSCLC that produced a response rate of 9 to 27% [53–55]. To improve the treatment outcome, extensive investigations have been carried out to identify the underlining causes for the low response rate [56, 57]. One such finding indicated that EMT is a determinant of sensitivity of NSCLC to EGFR inhibition whereby tumor cells exhibiting mesenchymal-like phenotypes were more resistant to EGFR-TKI [33, 34] which could be attributed to kinase switching to alternate autocrine signaling thereby attenuate the dependence of EGFR signaling [58]. Further, a study showed that restoring E-cadherin expression in EGFR-TKI resistance cells increased sensitivity to EGFR inhibition [37] that implies EMT has a contributing role in EGFR-TKI resistance. Additional data to support this hypothesis was from a study that reported that patients with epithelial-like NSCLC that showed strong E-cadherin staining exhibited a significant longer time to progression [34]. Our finding showed that H358 that have been induced to undergo EMT-like transition by overexpression of HAS3 were more resistant to EGFR-TKI treatment, as compared to vector-only control. There was no detectable cell death at 10 μM EGFR-TKI in the HAS3 transfectants while control cells growth rate was reduced by 40% (Figure 5(b)). Similarly, addition of exogenous HA to A549 cells showed more resistance to EGFR-TKI and this resistance was abolished with coincubation with Streptomyces hyaluronidase indicating the role of HA in promoting resistance to EGFR-TKI (Figure 5(a)). Studies have also documented that interaction between HA and its receptor, CD44 induces chemoresistance in NSCLC [23] as well as in myeloma cells [22] and promote survival. Thus, this implies that HA can promote cell survival by inducing drug resistance to both EGFR-TKI and chemotherapeutic drugs.
In conclusion, our result suggests that HA is involved in EMT in NSCLC. Overexpressing HAS3 in H358 cells results in loss of immunoreactivity for E-cadherin and gain of vimentin expression. These cells also showed enhanced MMP2 and MMP9 activities and were more invasive. Finally, these cells were also more resistance to treatment with EGFR-TKI. In addition, we also demonstrated that various cytokines and growth factors can stimulate HA production in a NSCLC cell line that could be a contributing factor in the EMT-like transition observed in the cytokines-treated cultures. Taken together, these results support that HA can regulate epithelial to mesenchymal-like transition and therefore contribute to tumorigenesis. Thus, the control of HA level may be a potential target for therapeutic intervention for managing this disease.
Acknowledgments
This study was supported by institutional funding from Rush University Medical Center. The authors thank AstraZeneca for the kind gift of EGFR-TKI, Iressa.
References
- 1.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA: A Cancer Journal for Clinicians. 2000;50(1):7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Pirinen R, Tammi R, Tammi M, et al. Prognostic value of hyaluronan expression in non-small-cell lung cancer: increased stromal expression indicates unfavorable outcome in patients with adenocarcinoma. International Journal of Cancer. 2001;95(1):12–17. doi: 10.1002/1097-0215(20010120)95:1<12::aid-ijc1002>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 3.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nature Reviews Cancer. 2004;4(7):528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 4.Itano N, Kimata K. Mammalian hyaluronan synthases. IUBMB Life. 2002;54(4):195–199. doi: 10.1080/15216540214929. [DOI] [PubMed] [Google Scholar]
- 5.Weigel PH, Hascall VC, Tammi M. Hyaluronan synthases. Journal of Biological Chemistry. 1997;272(22):13997–14000. doi: 10.1074/jbc.272.22.13997. [DOI] [PubMed] [Google Scholar]
- 6.Itano N, Sawai T, Yoshida M, et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. Journal of Biological Chemistry. 1999;274(35):25085–25092. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- 7.Adamia S, Maxwell CA, Pilarski LM. Hyaluronan and hyaluronan synthases: potential therapeutic targets in cancer. Current Drug Targets—Cardiovascular and Haematological Disorders. 2005;5(1):3–14. doi: 10.2174/1568006053005056. [DOI] [PubMed] [Google Scholar]
- 8.Berdiaki A, Zafiropoulos A, Fthenou E, et al. Regulation of hyaluronan and versican deposition by growth factors in fibrosarcoma cell lines. Biochimica et Biophysica Acta. 2008;1780(2):194–202. doi: 10.1016/j.bbagen.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Tzanakakis GN, Karamanos NK, Hjerpe A. Effects on glycosaminoglycan synthesis in cultured human mesothelioma cells of transforming, epidermal, and fibroblast growth factors and their combinations with platelet-derived growth factor. Experimental Cell Research. 1995;220(1):130–137. doi: 10.1006/excr.1995.1299. [DOI] [PubMed] [Google Scholar]
- 10.Bourguignon LYW, Gilad E, Peyrollier K. Heregulin-mediated ErbB2-ERK signaling activates hyaluronan synthases leading to CD44-dependent ovarian tumor cell growth and migration. Journal of Biological Chemistry. 2007;282(27):19426–19441. doi: 10.1074/jbc.M610054200. [DOI] [PubMed] [Google Scholar]
- 11.Anttila MA, Tammi RH, Tammi MI, Syrjänen KJ, Saarikoski SV, Kosma V-M. High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Research. 2000;60(1):150–155. [PubMed] [Google Scholar]
- 12.Auvinen P, Tammi R, Parkkinen J, et al. Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. American Journal of Pathology. 2000;156(2):529–536. doi: 10.1016/S0002-9440(10)64757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimata K, Honma Y, Okayama M, Oguri K, Hozumi M, Suzuki S. Increased synthesis of hyaluronic acid by mouse mammary carcinoma cell variants with high metastatic potential. Cancer Research. 1983;43(3):1347–1354. [PubMed] [Google Scholar]
- 14.Asplund T, Versnel MA, Laurent TC, Heldin P. Human mesothelioma cells produce factors that stimulate the production of hyaluronan by mesothelial cells and fibroblasts. Cancer Research. 1993;53(2):388–392. [PubMed] [Google Scholar]
- 15.Kosaki R, Watanabe K, Yamaguchi Y. Overproduction of hyaluronan by expression of the hyaluronan synthase Has2 enhances anchorage-independent growth and tumorigenicity. Cancer Research. 1999;59(5):1141–1145. [PubMed] [Google Scholar]
- 16.Liu N, Gao F, Han Z, Xu X, Underhill CB, Zhang L. Hyaluronan synthase 3 overexpression promotes the growth of TSU prostate cancer cells. Cancer Research. 2001;61(13):5207–5214. [PubMed] [Google Scholar]
- 17.Jacobson A, Rahmanian M, Rubin K, Heldin P. Expression of hyaluronan synthase 2 or hyaluronidase 1 differentially affect the growth rate of transplantable colon carcinoma cell tumors. International Journal of Cancer. 2002;102(3):212–219. doi: 10.1002/ijc.10683. [DOI] [PubMed] [Google Scholar]
- 18.Itano N, Sawai T, Miyaishi O, Kimata K. Relationship between hyaluronan production and metastatic potential of mouse mammary carcinoma cells. Cancer Research. 1999;59(10):2499–2504. [PubMed] [Google Scholar]
- 19.Simpson MA, Wilson CM, McCarthy JB. Inhibition of prostate tumor cell hyaluronan synthesis impairs subcutaneous growth and vascularization in immunocompromised mice. American Journal of Pathology. 2002;161(3):849–857. doi: 10.1016/S0002-9440(10)64245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Udabage L, Brownlee GR, Waltham M, et al. Antisense-mediated suppression of hyaluronan synthase 2 inhibits the tumorigenesis and progression of breast cancer. Cancer Research. 2005;65(14):6139–6150. doi: 10.1158/0008-5472.CAN-04-1622. [DOI] [PubMed] [Google Scholar]
- 21.Bourguignon LYW, Peyrollier K, Gilad E, Brightman A. Hyaluronan-CD44 interaction with neural Wiskott-Aldrich syndrome protein (N-WASP) promotes actin polymerization and ErbB2 activation leading to β-catenin nuclear translocation, transcriptional up-regulation, and cell migration in ovarian tumor cells. Journal of Biological Chemistry. 2007;282(2):1265–1280. doi: 10.1074/jbc.M604672200. [DOI] [PubMed] [Google Scholar]
- 22.Ohwada C, Nakaseko C, Koizumi M, et al. CD44 and hyaluronan engagement promotes dexamethasone resistance in human myeloma cells. European Journal of Haematology. 2008;80(3):245–250. doi: 10.1111/j.1600-0609.2007.01014.x. [DOI] [PubMed] [Google Scholar]
- 23.Ohashi R, Takahashi F, Cui R, et al. Interaction between CD44 and hyaluronate induces chemoresistance in non-small cell lung cancer cell. Cancer Letters. 2007;252(2):225–234. doi: 10.1016/j.canlet.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 24.Alaniz L, García M, Cabrera P, et al. Modulation of matrix metalloproteinase-9 activity by hyaluronan is dependent on NF-κB activity in lymphoma cell lines with dissimilar invasive behavior. Biochemical and Biophysical Research Communications. 2004;324(2):736–743. doi: 10.1016/j.bbrc.2004.09.120. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Thant AA, Machida K, et al. Hyaluronan-CD44s signaling regulates matrix metalloproteinase-2 secretion in a human lung carcinoma cell line QG90. Cancer Research. 2002;62(14):3962–3965. [PubMed] [Google Scholar]
- 26.Thiery JP. Epithelial-mesenchymal transitions in tumor progression. Nature Reviews Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 27.Hugo H, Ackland ML, Blick T, et al. Epithelial—mesenchymal and mesenchymal—epithelial transitions in carcinoma progression. Journal of Cellular Physiology. 2007;213(2):374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 28.Bremnes RM, Veve R, Hirsch FR, Franklin WA. The E-cadherin cell-cell adhesion complex and lung cancer invasion, metastasis, and prognosis. Lung Cancer. 2002;36(2):115–124. doi: 10.1016/s0169-5002(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 29.Deeb G, Wang J, Ramnath N, et al. Altered E-cadherin and epidermal growth factor receptor expressions are associated with patient survival in lung cancer: a study utilizing high-density tissue microarray and immunohistochemistry. Modern Pathology. 2004;17(4):430–439. doi: 10.1038/modpathol.3800041. [DOI] [PubMed] [Google Scholar]
- 30.Loric S, Paradis V, Gala J-L, et al. Abnormal E-cadherin expression and prostate cell blood dissemination as markers of biological recurrence in cancer. European Journal of Cancer. 2001;37(12):1475–1481. doi: 10.1016/s0959-8049(01)00143-5. [DOI] [PubMed] [Google Scholar]
- 31.Ray ME, Mehra R, Sandler HM, Daignault S, Shah RB. E-cadherin protein expression predicts prostate cancer salvage radiotherapy outcomes. Journal of Urology. 2006;176(4):1409–1414. doi: 10.1016/j.juro.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Hegymegi-Barakonyi B, Eros D, Szántai-Kis C, et al. Tyrosine kinase inhibitors—small molecular weight compounds Inhibiting EGFR. Current Opinion in Molecular Therapeutics. 2009;11(3):308–321. [PubMed] [Google Scholar]
- 33.Thomson S, Buck E, Petti F, et al. Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Research. 2005;65(20):9455–9462. doi: 10.1158/0008-5472.CAN-05-1058. [DOI] [PubMed] [Google Scholar]
- 34.Yauch RL, Januario T, Eberhard DA, et al. Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clinical Cancer Research. 2005;11(24):8686–8698. doi: 10.1158/1078-0432.CCR-05-1492. [DOI] [PubMed] [Google Scholar]
- 35.Frederick BA, Helfrich BA, Coldren CD, et al. Epithelial to mesenchymal transition predicts gefitinib resistance in cell lines of head and neck squamous cell carcinoma and non-small cell lung carcinoma. Molecular Cancer Therapeutics. 2007;6(6):1683–1691. doi: 10.1158/1535-7163.MCT-07-0138. [DOI] [PubMed] [Google Scholar]
- 36.Haddad Y, Choi W, McConkey DJ. Delta-crystallin enhancer binding factor 1 controls the epithelial to mesenchymal transition phenotype and resistance to the epidermal growth factor receptor inhibitor erlotinib in human head and neck squamous cell carcinoma lines. Clinical Cancer Research. 2009;15(2):532–542. doi: 10.1158/1078-0432.CCR-08-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Witta SE, Gemmill RM, Hirsch FR, et al. Restoring E-cadherin expression increases sensitivity to epidermal growth factor receptor inhibitors in lung cancer cell lines. Cancer Research. 2006;66(2):944–950. doi: 10.1158/0008-5472.CAN-05-1988. [DOI] [PubMed] [Google Scholar]
- 38.Camenisch TD, Spicer AP, Brehm-Gibson T, et al. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. Journal of Clinical Investigation. 2000;106(3):349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zoltan-Jones A, Huang L, Ghatak S, Toole BP. Elevated hyaluronan production induces mesenchymal and transformed properties in epithelial cells. Journal of Biological Chemistry. 2003;278(46):45801–45810. doi: 10.1074/jbc.M308168200. [DOI] [PubMed] [Google Scholar]
- 40.Xu Y, Yu Q. E-cadherin negatively regulates CD44-hyaluronan interaction and CD44-mediated tumor invasion and branching morphogenesis. Journal of Biological Chemistry. 2003;278(10):8661–8668. doi: 10.1074/jbc.M208181200. [DOI] [PubMed] [Google Scholar]
- 41.Knudson CB. Hyaluronan receptor-directed assembly of chondrocyte pericellular matrix. Journal of Cell Biology. 1993;120(3):825–834. doi: 10.1083/jcb.120.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peebles KA, Lee JM, Mao JT, et al. Inflammation and lung carcinogenesis: applying findings in prevention and treatment. Expert Review of Anticancer Therapy. 2007;7(10):1405–1421. doi: 10.1586/14737140.7.10.1405. [DOI] [PubMed] [Google Scholar]
- 43.Fukuyama T, Ichiki Y, Yamada S, et al. Cytokine production of lung cancer cell lines: correlation between their production and the inflammatory/immunological responses both in vivo and in vitro. Cancer Science. 2007;98(7):1048–1054. doi: 10.1111/j.1349-7006.2007.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang M, Wang T, Liu S, Yoshida D, Teramoto A. The expression of matrix metalloproteinase-2 and -9 in human gliomas of different pathological grades. Brain Tumor Pathology. 2003;20(2):65–72. doi: 10.1007/BF02483449. [DOI] [PubMed] [Google Scholar]
- 45.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. Journal of the National Cancer Institute. 1997;89(17):1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 46.Grandis JR, Sok JC. Signaling through the epidermal growth factor receptor during the development of malignancy. Pharmacology and Therapeutics. 2004;102(1):37–46. doi: 10.1016/j.pharmthera.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Akita RW, Sliwkowski MX. Preclinical studies with erlotinib (Tarceva) Seminars in Oncology. 2003;30(3):15–24. [PubMed] [Google Scholar]
- 48.Normanno N, Bianco C, De Luca A, Salomon DS. The role of EGF-related peptides in tumor growth. Frontiers in Bioscience. 2001;6:685–707. doi: 10.2741/normano. [DOI] [PubMed] [Google Scholar]
- 49.Matanić D, Beg-Zec Z, Stojanović D, et al. Cytokines in patients with lung cancer. Scandinavian Journal of Immunology. 2003;57(2):173–178. doi: 10.1046/j.1365-3083.2003.01205.x. [DOI] [PubMed] [Google Scholar]
- 50.Hasegawa Y, Takanashi S, Kanehira Y, Tsushima T, Imai T, Okumura K. Transforming growth factor-beta1 level correlates with angiogenesis, tumor progression, and prognosis in patients with nonsmall cell lung carcinom. Cancer. 2001;91(5):964–971. [PubMed] [Google Scholar]
- 51.Jacobson A, Brinck J, Briskin MJ, Spicer AP, Heldin P. Expression of human hyaluronan syntheses in response to external stimuli. Biochemical Journal. 2000;348(1):29–35. [PMC free article] [PubMed] [Google Scholar]
- 52.Toole BP. Hyaluronan in morphogenesis. Seminars in Cell and Developmental Biology. 2001;12(2):79–87. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- 53.Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. Journal of the American Medical Association. 2003;290(16):2149–2158. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 54.Shepherd FA, Pereira JR, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. The New England Journal of Medicine. 2005;353(2):123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 55.Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) Journal of Clinical Oncology. 2003;21(12):2237–2246. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 56.Tsao M-S, Sakurada A, Cutz J-C, et al. Erlotinib in lung cancer—molecular and clinical predictors of outcome. The New England Journal of Medicine. 2005;353(2):133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 57.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. The New England Journal of Medicine. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 58.Thomson S, Petti F, Sujka-Kwok I, Epstein D, Haley JD. Kinase switching in mesenchymal-like non-small cell lung cancer lines contributes to EGFR inhibitor resistance through pathway redundancy. Clinical and Experimental Metastasis. 2008;25(8):843–854. doi: 10.1007/s10585-008-9200-4. [DOI] [PubMed] [Google Scholar]