Abstract
Angiogenesis is the formation of new capillaries from pre-existing vessels. A number of soluble and cell-bound factors may stimulate neovascularization. The perpetuation of angiogenesis involving numerous soluble and cell surface-bound mediators has been associated with rheumatoid arthritis (RA). These angiogenic mediators, among others, include growth factors, primarily vascular endothelial growth factor (VEGF) and hypoxia-inducible factors (HIFs), as well as pro-inflammatory cytokines, various chemokines, cell adhesion molecules, proteases and others. Among the several potential angiogenesis inhibitors, targeting of VEGF, HIF-1, angiopoietin and the αVβ3 integrin, as well as some endogenous or synthetic compounds including angiostatin, endostatin, paclitaxel, fumagillin analogues, 2-methoxyestradiol and thalidomide seems to be promising for the management of synovial inflammation and angiogenesis. A complete review of antiangiogenic drugs used in animal models of arthritis or human RA is available in a table.
Keywords: Angiogenesis, Rheumatoid arthritis, Vascular endothelial growth factor, Angiostasis
1. Introduction
Angiogenesis is the formation of new capillaries from pre-existing blood vessels. Angiogenesis has been associated with inflammation and inflammatory diseases. A perpetuation of angiogenesis leading to enhanced endothelial surface and perpetuated leukocyte ingress into inflamed tissues has been described in rheumatoid arthritis (RA), as well as other types of arthritis and connective tissue diseases [1–6]. Thus, increased angiogenesis and defective vasculogenesis have important clinical relevance for rheumatic diseases including RA. The control of inflammatory neovascularization attenuates synovitis in RA [1–6].
2. Angiogenesis: mechanisms and clinical relevances
2.1. Basic mechanisms
In RA, as well as in other types of arthritis, leukocytes migrate from the bloodstream through endothelia of vessels resembling high-endothelial venules generally found in lymphoid organs into the synovial tissue. Leukocyte-endothelial interactions are mediated by numerous cell adhesion receptors, primarily by integrins, selectins and their respective ligands [1,7]. As angiogenesis increases the number of blood vessels within a given compartment of the synovium, as well as the total endothelial surface, mechanistically, this process may result in the perpetuation of inflammatory leukocyte migration [7,8]. Synovial tissue macrophages and angiogenic mediators produced by these and other cells are primarily involved in RA-associated neovascularization [8].
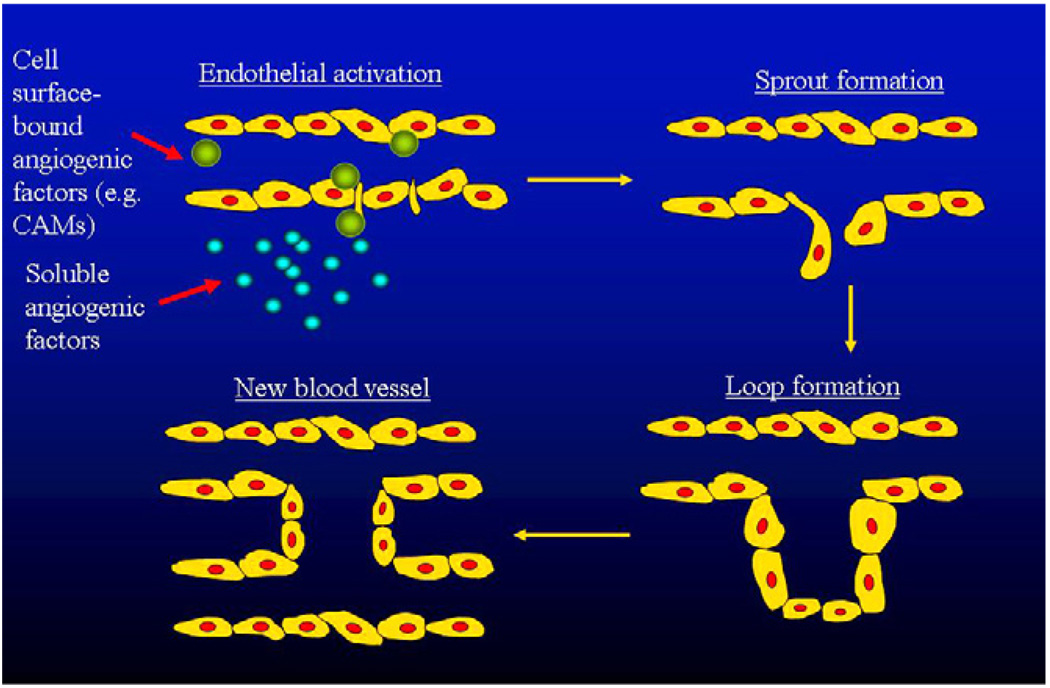
Angiogenesis is a programmed sequence of events. Angiogenic mediators produced by various cell types in the synovium activate endothelial cells, which, in turn, release proteolytic enzymes. These enzymes degrade the endothelial basement membrane and the perivascular extracellular matrix. ECs then proliferate and emigrate into the interstitial tissue and thus “primary sprouts” are formed. The lumen formation within these sprouts leads to the formation of “capillary loops” followed by synthesis of new basement membrane and finally, capillary formation [2,4] (Fig. 1).
Fig 1.
The process of angiogenesis. Both soluble and cell surface-bound angiogenic factors trigger endothelial activation, sprouting, lumen formation and then the formation of new vessels.
In vitro models used to investigate angiogenesis include EC cultures grown on extracellular matrix, such as Matrigel, as well as tissue culture systems or EC chemotaxis assays [2,4]. In vivo models include the animal corneal micropocket, chick embryo chorioallantoic membrane assays, aortic ring and sponge models, as well as other systems [2,4]. These models are used to investigate the role of angiogenesis in the pathogenesis of inflammatory diseases, as well as to design strategies for anti-angiogenic therapies.
2.2. Mediators of angiogenesis
Both soluble and cell surface-bound mediators may trigger neovascularization (Fig. 1). Angiogenic factors include growth factors, pro-inflammatory cytokines, chemokines, extracellular matrix molecules, matrix-degrading proteolytic enzymes, cellular adhesion molecules and others [1–5]. These mediators are primarily released by macrophages and ECs into the RA synovium [1–5,8]. Some of the most relevant molecules will be discussed in more detail (Table 1).
Table 1.
Some important mediators and inhibitors of angiogenesis in rheumatoid arthritisa.
| Mediators | Inhibitors | |
|---|---|---|
| Growth factors | VEGF, aFGF, bFGF, HGF, HIF-1a, HIF-2, PDGF, EGF, KGF, IGF-I, TGF-β | - |
| Cytokines | TNF-α, IL-1, IL-6a, IL-8, IL-15, IL-17, IL-18, G-CSF, GM-CSF, oncostatin M, MIF |
IFN-α, IFN-γ,IL-4, IL-12, LIF |
| Chemokines/receptors | IL-8/CXCL8, ENA-78/CXCL5, groα/CXCL1, CTAP-III/CXCL6, SDF-1/CXCL12a, MCP-1/CCL2, fractalkine/CX3CL1 CXCR2, CXCR4a, CCR2 |
PF4/CXCL4, Míg/CXCL9, IP-10/CXCL10, SLC/CCL21, CXCR3 |
| Matrix molecules | Type I collagen, fibronectin, laminin, vitronectin, tenascin, proteoglycan |
Thrombospondin-1 |
| Cell adhesion molecules | β1 and β3 integrinsa, E-selectina VCAM-1, ICAM-2, CD34, Lewisy/H, MUC18, PECAM-1, endoglin, JAM-A, JAM-C |
- |
| Proteolytic enzymes | MMPs, plasminogen activators | TIMPs, PAIs |
| Antirheumatic drugs | - | Dexamethasone, classical DMARDs, anti-TNF biologics |
| Antibiotic derivatives | - | Minocycline, fumagillin, deoxyspergualin, clarithromycin |
| Environmental factors | Hypoxia | - |
| Others | Angiopoietin 1 /Tie-2, angiotropin, pleiotrophin, angiogenin, survivin, COX/prostaglandin E2, PAF, Nitric oxide (NO), endothelin-1, Serum amyloid A, histamine, substance P, adenosine, erythropoietin, prolactin, thrombin, etc. |
Angiopoietin 2, angiostatin, endostatin, taxol, osteonectin, opioids, troponin I, chondromodulin, etc. |
VEGF: vascular endothelial growth factor; FGF: fibroblast growth factor; HGF: hepatocyte growth factor; HIF: hypoxia-inducible factors; PDGF: platelet-derived growth factor; EGF: epidermal growth factor; KGF: keratinocyte growth factor; IGF: insulin-like growth factor; TGF-β: transforming growth factor-β; TNF: tumor necrosis factor; G-CSF: granulocyte colony-stimulating factors; GM-CSF: granulocyte-macrophage colony-stimulating factors; MIF: migration inhibitory factor; JAM: junctional cell adhesion molecules; PECAM-1: platelet-endothelial cell adhesion molecule-1; MMP: matrix metalloproteinases; TIMP: tissue inhibitors of metalloproteinases; PAI: plasminogen activator inhibitors.
Also key regulators of vasculogenesis.
Growth factors are abundantly produced in synovial inflammation. These mediators are bound to heparin and heparan sulfate proteoglycans within the synovial extracellular matrix. Proteolytic enzymes, such as heparanase and plasmin release these growth factors from their binding during the process of angiogenesis [1,2]. With regards to angiogenesis and vasculogenesis, vascular endothelial growth factor (VEGF) is of outstanding importance. VEGF is probably the key regulator of neovascularization in inflammation. VEGF is produced within the synovium in response to pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1) released by perivascular cells and synovial fibroblasts [1,2,9,10]. VEGF induces EC proliferation and migration, and it also stimulates angiogenesis, at least in part via cyclooxygenase-2 (COX-2) induction [1,10].
Hypoxia is a major feature of the inflamed joint. Intraarticular hypoxia induces branching of acts in part by the stimulation of hypoxia-inducible factors (HIF-1 and HIF-2) and HIFs then act predominantly via upregulation of VEGF [11,12]. Hypoxia has been detected within the RA joint [11]. Recently, a novel, HIF-independent regulatory pathway involving peroxisome-proliferator-activated receptor-γ (PPARγ) and PPARγ coactivator 1α (PGC-1α) has been described. PGC-1α induces VEGF production and blood vessel reconstitution after an ischemic insult in a HIF-independent manner [13]. Apart from hypoxia and HIFs, other angiogenic mediators including HGF, prostaglandins and nitric oxide (NO) also act via VEGF during neovascularization [1]. Thus, the hypoxia-HIF-VEGF pathway is crucial in the regulation of inflammation-associated angiogenesis.
Interaction between VEGF and angiopoietin-1 (Ang1)/Tie-2 is critical for the stabilization of newly formed blood vessels. In contrast, Ang2 antagonizes Ang1 and thus inhibits vessel maturation [1,2]. One more player of this network is survivin, an apoptosis inhibitor, which is also involved in VEGF-induced angiogenesis [2]. VEGF, HIF-1, HIF-2, Ang1, Tie2 and survivin are all expressed in the RA synovium [2,12]. Moreover, overexpression of VEGF and Ang1 in the arthritic joints is associated with distinct vascular morphology [2].
Other growth factors involved in angiogenesis include the heparin-bound basic (bFGF), acidic fibroblast growth factors (aFGF) and hepatocyte growth factor (HGF), as well as the non-heparing bound platelet-derived growth factor (PDGF), epidermal growth factor (EGF), insulin-like growth factor-I (IGF-I), keratinocyte growth factor (KGF) and transforming growth factor-β (TGF-β) [1,2,14].
Some chemokines and chemokine receptors have also been implicated in synovial inflammation and angiogenesis [1,2,5]. Most CXC chemokines containing the glutamyl-leucyl-arginyl (ELR) amino acid sequence stimulate, while chemokines lacking this motif rather suppress neovascularization [15]. ELR+ angiogenic CXC chemokines include IL-8/CXCL8, epithelial neutrophil activating protein-78 (ENA-78)/CXCL5, growth-related oncogene α (groα)/CXCL1 and connective tissue activating protein-III (CTAP-III)/CXCL6 [5,15]. Stromal cell-derived factor-1 (SDF-1)/CXCL12, a key regulator of lymphoid neogenesis within the RA synovium, is an ELR−, but still angiogenic CXC chemokine [1,16]. Hypoxia stimulates the release of SDF-1/CXCL12 by RA synovial fibroblasts [16]. Among CC chemokines, MCP-1/CCL2 is involved in angiogenesis as this chemokine supports the angiogenic effects of growth factors [5,17]. Fractalkine/CX3CL1, a CX3C chemokine also promotes vessel formation [1]. All these chemokines are also involved in leukocyte recruitment into the inflamed synovium [5]. As CXCR2 is the most important endothelial receptor for ELR+ angiogenic CXC chemokines, this chemokine receptor plays a major role in synovial angiogenesis [1,2,5]. CXCR4, the receptor for SDF-1/CXCL12 and CCR2, the receptor for MCP-1/CCL2 are also involved in chemokine-induced angiogenesis [5,18].
Synovial inflammation is associated with chemokine-driven recruitment of inflammatory leukocytes into the synovium. This process involves cellular adhesion molecules (CAMs), synovial matrix macromolecules, as well as matrix-degrading proteolytic enzymes. Among extracellular matrix constituents, type I collagen, fibronectin, laminin, vitronectin, tenascin and proteoglycans are also involved in EC migration during angiogenesis [1,7,19]. Regarding CAMs, numerous β1 and β3 integrins, E-selectin, the L-selectin ligand CD34, selectin-related glycoconjugates including Lewisy/H and MUC18, vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-2 (ICAM-2), platelet-endothelial cell adhesion molecule-1 (PECAM-1; CD31), endoglin (CD105) and junctional cell adhesion molecules A (JAM-A) and C (JAM-C) are expressed by ECs and promote angiogenesis [5,12,20–23].
Among all these CAMs, the αVβ3 integrin seems to be of outstanding importance. This integrin mediates osteoclast-mediated bone resorption and thus the development of erosions, as well as synovial angiogenesis in RA [20,22]. Furthermore, the αV subunit of this integrin is encoded by the ITGAV gene. A significant association has been found between the ITGAV rs3738919-C allele and RA in the European Caucasian population [24]. Focal adhesion kinases (FAK) are predominant mediators of αVβ3 integrin signaling. FAKs have recently been detected in the RA synovium suggesting their role in synovial inflammation and angiogenesis [25].Mast cells are also involved in integrin-dependent angiogenesis and joint destruction as mast cell silencing with salbutamol or cromolyn prevented αVβ3 integrin activation and angiogenesis in mice [26]. Angiogenic proteases include some matrix metalloproteinases (MMPs), as well as plasminogen activators [2,5].
Pro-inflammatory cytokines may either directly induce neovascularization or may, as described above, act via VEGF-dependent pathways. Among these cytokines, TNF-α, IL-1, IL-6, IL-8, IL-15, IL-17, IL-18, oncostatin M, macrophage migration inhibitory factor (MIF), granulocyte (G-CSF) and granulocyte-macrophage colony-stimulating factors (GM-CSF) are involved in angiogenesis, as well as rheumatoid synovitis [1,2,14,27]. Among these cytokines, TNF-α may also regulate capillary formation via the Ang1-Tie2-VEGF system described above [28]. IL-6 may also act via the induction of VEGF production as blockade of the IL-6 receptor resulted in diminished serum VEGF levels in RA patients [29]. IL-17 synergizes with TNF- α in stimulating VEGF, EGF, HGF and KGF production by synovial fibroblasts [14]. IL-18 induces SDF-1/CXCL12, MCP-1/CCL2 and VEGF production by synovial fibroblasts [30]. Oncostatin M enhances ICAM-1 expression on RA synovial fibroblasts and endothelium and it also stimulates EC migration and tubule formation [31]. MIF has recently drawn a lot of attention as it is primarily produced by synovial macrophages and is involved in macrophage-dependent synovial angiogenesis [8,32,33]. MIF acts via the induction of VEGF and IL-8/CXCL8 chemokine release by RA synovial fibroblasts [8,33].
There are numerous other angiogenic mediators not classified above. The most relevant ones include endothelin 1 (ET-1), serum amyloid A (SAA), the cyclooxygenase-2 (COX-2)-prostaglandin E2 system, angiogenin, angiotropin, pleiotrophin, platelet-activating factor (PAF), substance P, histamine, erythropoietin, adenosine, prolactin and thrombin [1,2]. For example, ET-1, also released by ECs, induces VEGF production, EC proliferation and angiogenesis [34]. ET-1 is abundantly produced in RA [34]. SAA is an important acute-phase reactant, which has been implicated in the pathogenesis of arthritis. SAA acts through the formyl peptide receptor-like 1 (FPRL1). The binding of SAA to FPRL1 stimulates EC proliferation, migration, enhanced EC sprouting activity and neovascularization, as well as synovitis [35].
2.3. Angiogenesis inhibitors
There is a great number of naturally produced or synthetic angiostatic molecules. Some of them have already been tried to target tumor- or inflammation-associated neovascularization. Some examples for current or future anti-angiogenesis therapy will be presented later [1–3,6] (Table 1). Angiostatic cytokines include interferon-α (IFN-α), IFN-γ, IL-4, IL-12 and leukemia inhibitory factor (LIF). These cytokines inhibit the release of angiogenic mediators and thus they indirectly block neovascularization [2,3,6]. For example, IL-4 suppresses VEGF production by synovial fibroblasts [36].
As described above, CXC chemokines lacking the ELR sequence, such as platelet factor-4 (PF4)/CXCL4, monokine induced by interferon-γ (MIG)/CXCL9 and interferon-γ-inducible protein 10 (IP-10)/CXCL10 are angiogenesis inhibitors [2,5]. VEGF and these angiostatic chemokines may form a regulatory loop: VEGF stimulates the release of IP-10/CXCL10, on the other hand, IP-10/CXCL10 suppresses VEGF-induced angiogenesis [10,37]. Among CC chemokines, secondary lymphoid tissue chemokine (SLC)/CCL21 is angiostatic and it inhibits tumor progression [38]. Regarding chemokine receptors, as CXCR3 binds most angiostatic ELR− CXC chemokines, this receptor is primarily involved in angiogenesis inhibition [2,5].
Protease inhibitors including tissue inhibitors of metalloproteinases (TIMPs) and plasminogen activator inhibitors (PAIs) antagonize the effects of proteolytic enzymes during angiogenesis [2,3]. Thrombospondin-1 and the PF4/CXCL4 chemokine inhibit growth factor binding to heparin and thus synovial capillary formation [3].
Among currently used antirheumatic drugs, dexamethasone, chloroquine, sulfasalazine, methotrexate (MTX), azathioprine, cyclophosphamide, leflunomide, thalidomide, minocycline and some anti-TNF agents, apart from several other anti-inflammatory effects, also inhibit EC migration and synovial angiogenesis [3,6]. There are some conflicting results regarding MTX, as it suppressed cancer-related angiogenesis but failed to do so in psoriatic arthritis [6].
Some antibiotic derivatives including minocyclin, fumagillin analogues, deoxyspergualin and clarithromycin also inhibit the release of VEGF and other angiogenic mediators and thus neovascularization [2,3,6].
Further compounds not mentioned above include angiostatin (a fragment of plasminogen), endostatin (a fragment of type XIII collagen), paclitaxel, osteonectin, opioids, troponin I, and chondromodulin-1 [2,3,6]. Most angiostatic molecules block the action of angiogenic mediators, such as VEGF, HIFs or the αVβ3 integrin [2,3,6]. Some of these agents, particularly angiostatin and endostatin, gave promising results in cancer therapy trials, as well as preclinical arthritis studies [2,3,6,39].
2.4. Regulation of synovial angiogenesis
The central role of macrophages in inflammatory angiogenesis is indisputable [8]. These cells express CXCR2 and CXCR4, which recognize many angiogenic CXC chemokines described above [2,5,8,18]. Macrophages also release important soluble angiogenic mediators, such as IL-8/CXCL8, ENA-78/CXCL5, groα/CXCL1, CTAP-III/CXCL7, MCP-1/CCL2, TNF-α, IL-15, IL-18, VEGF, bFGF, aFGF, HGF, PDGF and MMPs [2,5,8,18]. On the other hand, macrophages are also involved in the down-regulation of neovascularization as they produce angiostatic IP-10/CXCL10, MIG/CXCL9, IFN-γ and TIMPs [5,8].
Several regulatory interactions exist in sites of angiogenesis and inflammation. For example, there is an imbalance between specific antagonistic pairs, such as ELR+ versus ELR− CXC chemokines, MMPs and TIMPs, pro-inflammatory, angiogenic versus anti-inflammatory, angiostatic cytokines [2,3]. Different angiogenic mediators may have additive effects. For example, the pro-inflammatory and angiogenic TNF-α stimulates the production of several angiogenic chemokines and growth factors, as well as the expression of angiogenic CAMs [2,3,7]. Furthermore, as described above, numerous angiogenic mediators indirectly promote neovascularization via VEGF- and Ang1/Tie-2-dependent mechanisms [2,10]. Regarding negative feedback, the autocrine loop between VEGF and IP-10/CXCL10 was described above [10,37]. Apart from intrinsic regulatory networks described here, external administration of angiostatic compounds may also interfere with neovascularization. As discussed later, these agents may be used clinically as anti-angiogenic therapy [3,6].
2.5. Therapeutic targeting of angiogenesis in arthritis
Inflammation and angiogenesis may be controlled simultaneously. Neovascularization may be targeted by the use of endogenous inhibitors or by specific targeting of angiogenic mediators [1–3,6]. Endogenous angiogenesis inhibitors including cytokines, chemokine, protease inhibitors and others produced within the synovial tissue were described above. These inhibitors try to counterbalance the effects of angiogenesis mediators, however, there is an excess of angiogenic factors within the inflamed synovium. Therefore, these endogenous angiostatic molecules need to be administered in excess in order to attenuate neovascularization [2,3,6] (Tables 2 and 3).
Table 2.
Inhibitors of angiogenesis.
| Endogenous inhibitors |
Angiostatin, endostatin and related agents |
| Thrombospondin 1 and 2 | |
| IL-4, IL-13 | |
| PF4/CXCL4 | |
| 2-methoxyestradiol | |
| Exogenous inhibitors | VEGF inhibitors |
| Ang1/Tie2 inhibitors | |
| Classical DMARDs | |
| Anti-TNF agents | |
| Thalidomide | |
| Fumagillin analogues | |
| Chemokine and chemokine receptor blockade (e.g. bicyclam) | |
| αVβ3 integrin inhibitors (e.g. vitaxin) | |
| Protease inhibitors | |
| Endothelin-1 antagonists | |
| Microtubule destabilizers (e.g. paclitaxel) | |
| Others (soluble FasL, PPARγ agonists, statins) |
Table 3.
Angiogenesis targeting strategies in animal models of arthritis and human RA.
| Compound | Animal (A)/Human (H) studya | Reference(s) |
|---|---|---|
| Endogenous inhibitors | ||
| Angiostatin | A | 5,54 |
| Endostatin | A | 5,53,55 |
| Protease-activated kringles 1–5 (K1–5) (angiostatin analogue) | A | 56 |
| Kallistatin | A | 57 |
| Thrombospondin-1-derived peptide | A | 59 |
| Thrombospondin-2 | A | 60 |
| Interleukin-4 gene | A | 61 |
| Interleukin-13 gene | A | 62 |
| PF4/CXCL4 chemokine | A | 4 |
| 2-methoxyestradiol (2-ME) | A | 64,65 |
| Exogenous inhibitors | ||
| Soluble VEGF receptor 1 | H (culture) | 67 |
| Vatalanib (VEGF-R tyrosine kinase inhibitor) | A | 68 |
| Soluble Fas ligand | H (culture) | 70 |
| Paclitaxel | H | 5 |
| Soluble Tie2 vector | A | 72 |
| Vitaxin (anti-αVβ3 integrin) | A, H (RA) | 1,5 |
| Traditional DMARDs (methotrexate, sulfasalazine, etc.) | H (RA) | 1,2,5,8 |
| Rofecoxib | H (culture) | 77 |
| Infliximab | H (RA) | 5,39 |
| Tocilizumab | H (RA) | 40 |
| Thalidomide | A, H (RA) | 5,80,81 |
| CC1069 (thalidomide analogue) | A | 5 |
| TNP-470 (fumagillin analogue) | A | 5,83 |
| PPI2458 (fumagillin analogue) | A | 84 |
| Statins | H (culture) | 85 |
| Pioglitazone | H (arthritis) | 86 |
VEGF: vascular endothelial growth factor.
Human studies include both in vitro studies with isolated cell cultures, as well as in vivo RA studies.
Among these compounds, angiostatin and endostatin block αVβ3 integrin-dependent angiogenesis [2,3]. Endostatin interferes with VEGF receptor 2 signaling [3]. The administration of either of these compounds abrogated arthritis in various rodent models [2,3,5,39]. For example, angiostatin gene transfer attenuated murine collagen-induced arthritis (CIA) [3], while endostatin also suppressed pannus formation and joint destruction in rodent arthritis models [3,39]. The angiostatin-like protease-activated kringles 1–5 (K1–5) suppressed CIA more effectively than angiostatin itself [40]. Intraarticular injection of kallistatin, an endogenous angiogenesis inhibitor also expressed within the RA joint, attenuated rat ankle arthritis using gene therapy [41]. Type IV collagen derivatives including arresten, canstatin and tumstatin also inhibit neovascularization [42]. Regarding human studies, both angiostatin and endostatin have been introduced to clinical trials in cancer [3,6].
Other endogenous angiogenesis inhibitors include thrombospondin-1 (TSP1) and TSP2 that are angiostatic extracellular matrix components naturally produced by RA synovial macrophages and fibroblasts [2,3]. A peptide derived from TSP1 suppressed synovial inflammation and angiogenesis in peptidoglycan-polysaccharide-induced rat arthritis, while TSP2 inhibited synovial neovascularization in the severe combined immunodeficiency (SCID) mouse model of arthritis [2,3,43]. IL-4 and IL-13 gene transfer inhibits synovial inflammation and angiogenesis in rats [2,44,45]. The PF4/CXCL4 angiostatic chemokine already described above has also been tried in rodent models of arthritis [2].
2-methoxyestradiol (2-ME) is a natural metabolite of estrogen with low affinity for estrogen receptors. 2-ME inhibits angiogenesis by disrupting microtubules and by suppressing HIF-1α activity [46]. In recent preclinical therapeutic studies, 2-ME suppressed VEGF and bFGF gene expression, as well as arthritis in the rat CIA model [2,3]. On the other hand, while 2-ME also suppressed rat adjuvant-induced arthritis, it did not affect synovial vascularity in this model [47]. Thus, 2-ME may also have angiogenesis-independent effects on synovitis.
Exogenously administered agents are not naturally found within the synovium. These synthetic compounds include antirheumatic drugs, biological monoclonal antibodies and receptor antagonists, as well as small molecule inhibitors that block the activities of VEGF, chemokines, integrins and other angiogenic mediators [2,3,6] (Table 3).
Certainly today targeting of VEGF and VEGF-induced angiogenesis, as well as the hypoxia-HIF-VEGF-Ang1-Tie2 system is in the focus of cancer and inflammation research. There have been several attempts to target VEGF by using synthetic VEGF and VEGFR inhibitors, anti-VEGF antibodies and inhibitors of VEGF and VEGFR signaling, primarily in colorectal, lung, renal and liver cancers [3,6,10]. The VEGF-Trap construct is a composite decoy receptor based on the fusion of VEGFR1 and VEGFR2 with IgG1-Fc [3,48]. Bevacizumab, a human monoclonal antibody to VEGF, has been approved for the treatment of various types of cancer [3]. A soluble VEGFR1 chimeric protein dose-dependently inhibited the proliferation of synovial ECs [48]. Vatalanib, sunitinib malate, sorafenib, vandetanib (ZD6474), cediranib (AZD2171), axatinib (AG013736), KRN-951 and CEP-7055 are small molecule inhibitors of VEGFR tyrosine kinases [3,10,48]. In cancer studies, these small molecules have been administered orally and exerted favorable safety profiles [3,48]. Among these VEGFR protein kinase inhibitors, vatalanib also inhibited knee arthritis in rabbits [49]. Semaphorin-3A blocks the function of the 165 amino-acid form of VEGF (VEGF165). This compound also suppressed EC survival and neovascularization [50]. Soluble Fas ligand (CD178) also inhibited VEGF165 production by RA synovial fibroblasts, as well as neovascularization [3].
Hypoxia-HIF-mediated neovascularization may also be targeted. YC-1, a superoxide-sensitive stimulator of soluble guanylyl cyclase originally developed to treat hypertension and thrombosis, is also a HIF-1 inhibitor [3,51]. Microtubule destabilizers, such as 2-ME mentioned above, as well as paclitaxel, an anti-cancer agent, also diminish HIF-1α expression and activity [3,46]. In a phase I human trial, paclitaxel was effective and safe in RA patients [3].
Regarding the Ang-Tie system, a soluble Tie2 receptor transcript was delivered via an adenoviral vector to mice with CIA. Tie2 inhibition attenuated the incidence and severity of arthritis [52].
The suppression of chemokine- and chemokine receptor-mediated neovascularization may also be feasible. For example, blockade of CXCR2 inhibited tumor-induced angiogenesis [53]. Mig/CXCL9 gene therapy and cytotoxic agents had additive effects in cancer trials [3]. Bicyclam (AMD3100), a highly selective antagonist of SDF-1/CXCL4, inhibits endothelial cell proliferation and migration [3,54].
As discussed above, the αVβ3 integrin may be a key adhesion molecule that stimulates angiogenesis. Vitaxin, a humanized antibody to this CAM, inhibited synovial angiogenesis in animal models of arthritis [1,3], however, it showed very little efficacy in a phase II human RA trial [3]. Numerous MMP inhibitors have been tried in angiogenesis models [55]. ET-1 antagonists currently used in the treatment of primary and scleroderma-associated pulmonary hypertension may also exert anti-angiogenic effects [34].
The angiostatic effects of currently used antirheumatic agents including classical disease-modifying drugs (DMARDs) and biologics are described above. In addition, the COX-2 inhibitor rofecoxib inhibited EC tube formation induced by RA synovial fibroblast culture supernatants [3]. Infliximab treatment in combination with MTX reduced synovial VEGF expression and vascularity [3,6,56]. Anti-TNF therapy in arthritic patients reduced Ang1-Tie2 and surviving but stimulated Ang2 expression [3,28]. Recently, the anti-IL-6 receptor antibody tocilizumab decreased serum levels of VEGF [29].
Thalidomide, currently in use to treat multiple myeloma but also tried in lupus and RA, is a potent TNF-α antagonist and angiogenesis inhibitor [3,57]. The effects of thalidomide on neovascularization may be controversial, as in one study it suppressed, while in a rat CIA study it did not affect VEGF production [3,57]. Nevertheless, thalidomide suppressed both synovitis and angiogenesis [3] suggesting that its anti-angiogenic effects may be, in part, VEGF-independent. CC1069, a thalidomide analogue, even more potently inhibited rat AIA than thalidomide itself [3]. Regarding human arthritis, thalidomide has been tried in RA showing little efficacy, while in systemic lupus erythematosus (SLE), it had some effect on disease activity [3].
Fumagillin is a naturally occurring product of Aspergillus fumigatus. TNP-470 and PPI2458 are synthetic derivatives of fumagillin that inhibit methionine aminopeptidase-2, an enzyme involved in angiogenesis [58]. These fumagillin analogues also act by inhibiting VEGF production and VEGF-induced capillary formation [3,58]. In rodent models, TNP-470 prevented arthritis when administered before the onset of the disease [3]. PPI2458 also suppressed the development of arthritis and joint erosions [3].
Statins and glitazones are currently used for the treatment of metabolic diseases, such as hyperlipidemia and diabetes, respectively. Statins modify EC function, as well as endothelial CAM expression [59]. The PPARγ agonist pioglitazone improved joint and skin symptoms in psoriatic arthritis [60].
Theoretically, most angiostatic agents may have therapeutic relevance for arthritis-associated angiogenesis and many of these compounds have already been introduced to either preclinical or clinical therapeutic trials (Table 3). However, it is unlikely that specific targeting of one single angiogenic mediator could exert dramatic effects on angiogenesis and inflammation. Non-specific approaches using DMARDs or anti-TNF-α biologics that suppress angiogenesis, as well as numerous other inflammatory mechanisms may be more effective in controlling synovitis and joint destruction [3,6].
3. Conclusions
In this review, we first discussed the putative role of angiogenesis in the pathogenesis of inflammatory conditions, such as RA. Neovascularization may increase the total vascular endothelial surface and thus may promote the ingress of leukocytes into inflammatory sites leading to the progression of the disease. Several soluble and cell surface-bound mediators of angiogenesis, including growth factors, cytokines, chemokines, CAMs, proteases and others have been described in relation to synovitis. On the other hand, naturally occurring angiogenesis inhibitors, as well as exogenously administered angiostatic compounds may counterbalance excessive neovascularization in inflammation, as well as in malignancies. Inhibitors of VEGF and VEGF receptor, angiogenic chemokines and chemokine receptors, integrins, currently used DMARDs and biologics, thalidomide, taxol, 2-ME, fumagillin analogues, angiostatin and endostatin are in the forefront in cancer-and arthritis-associated angiogenesis research.
Acknowledgement
This work was supported by NIH grants AR-048267 (A.E.K.), the William D. Robinson, M.D. and Frederick G.L. Huetwell Endowed Professorship (A.E.K.), funds from the Veterans’ Administration (A.E.K.); and grant No. T048541 from the National Scientific Research Fund (OTKA) (Z.S.).
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Szekanecz Z, Koch AE. Vascular involvement in rheumatic diseases: ‘vascular rheumatology’. Arthritis Res Ther. 2008;10:224. doi: 10.1186/ar2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szekanecz Z, Koch AE. Mechanism of disease: angiogenesis in inflammatory diseases. Nat Clin Pract Rheumatol. 2007;3:635–643. doi: 10.1038/ncprheum0647. [DOI] [PubMed] [Google Scholar]
- 3.Lainer-Carr D, Brahn E. Angiogenesis inhibition as a therapeutic approach for inflammatory synovitis. Nat Clin Pract Rheumatol. 2007;3:434–442. doi: 10.1038/ncprheum0559. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 5.Szekanecz Z, Koch AE. Chemokines and angiogenesis. Curr Opin Rheumatol. 2001;13:202–208. doi: 10.1097/00002281-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Veale DJ, Fearon U. Inhibition of angiogenic pathways in rheumatoid arthritis: potential for therapeutic targeting. Best Pract Res Clin Rheumatol. 2006;20:941–947. doi: 10.1016/j.berh.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal SK, Brenner MB. Role of adhesion molecules in synovial inflammation. Curr Opin Rheumatol. 2006;18:268–276. doi: 10.1097/01.bor.0000218948.42730.39. [DOI] [PubMed] [Google Scholar]
- 8.Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:289–295. doi: 10.1097/BOR.0b013e32805e87ae. [DOI] [PubMed] [Google Scholar]
- 9.Koch AE, Harlow LA, Haines GK, et al. Vascular endothelial growth factor. A cytokine modulating endothelial function in rheumatoid arthritis. J Immunol. 1994;152:4149–4156. [PubMed] [Google Scholar]
- 10.Kiselyov A, Balakin KV, Tkachenko SE. VEGF/VEGFR signaling as a target for inhibiting angiogenesis. Expert Opin Investig Drugs. 2007;16:83–107. doi: 10.1517/13543784.16.1.83. [DOI] [PubMed] [Google Scholar]
- 11.Taylor PC, Sivakumar B. Hypoxia and angiogenesis in rheumatoid arthritis. Curr Opin Rheumatol. 2005;17:293–298. doi: 10.1097/01.bor.0000155361.83990.5b. [DOI] [PubMed] [Google Scholar]
- 12.Giatromanolaki A, Sivridis E, Maltezos E, et al. Upregulated hypoxia inducible factor-1α and -2α pathway in rheumatoid arthritis and osteoarthritis. Arthritis Res Ther. 2003;5:R193–R198. doi: 10.1186/ar756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arany Z, Foo SY, Ma Y, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 14.Honorati MC, Neri S, Cattini L, et al. Interleukin-17, a regulator of angiogenic factor release by synovial fibroblasts. Osteoarthritis Cartilage. 2006;14:345–352. doi: 10.1016/j.joca.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Strieter RM, Polverini PJ, Kunkel SL, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 16.Pablos JL, Santiago B, Galindo M, et al. Synoviocyte-derived CXCL12 is displayed on endothelium and induces angiogenesis in rheumatoid arthritis. J Immunol. 2003;170:2147–2152. doi: 10.4049/jimmunol.170.4.2147. [DOI] [PubMed] [Google Scholar]
- 17.Salcedo R, Ponce ML, Young HA, et al. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- 18.Nanki T, Hayashida K, El-Gabalawy HS, et al. Stromal cell-derived factor-1-CXC chemokine receptor 4 interactions play a central role in CD4+ T-cell accumulation in rheumatoid arthritis synovium. J Immunol. 2000;165:6590–6598. doi: 10.4049/jimmunol.165.11.6590. [DOI] [PubMed] [Google Scholar]
- 19.Madri JA, Williams KS. Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol. 1983;97:153–165. doi: 10.1083/jcb.97.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol. 2004;4:432–444. doi: 10.1038/nri1375. [DOI] [PubMed] [Google Scholar]
- 21.Koch AE, Halloran MM, Haskell CJ, et al. Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule-1. Nature. 1995;376:517–519. doi: 10.1038/376517a0. [DOI] [PubMed] [Google Scholar]
- 22.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 23.Naik TU, Naik MU, Naik UP. Junctional adhesion molecules in angiogenesis. Front Biosci. 2008;13:258–262. doi: 10.2741/2676. [DOI] [PubMed] [Google Scholar]
- 24.Jacq L, Garnier S, Dieudé P, et al. The ITGAV rs3738919-C allele is associated with rheumatoid arthritis in the European Caucasian population: a family-based study. Arthritis Res Ther. 2007;9:R63. doi: 10.1186/ar2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shahrara S, Castro-Rueda HP, Haines GK, et al. Differential expression of the FAK family kinases in rheumatoid arthritis and osteoarthritis synovial tissues. Arthritis Res Ther. 2007;9:R112. doi: 10.1186/ar2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kneilling M, Hültner L, Pichler BJ, et al. Targeted mast cell silencing protects against joint destruction and angiogenesis in experimental arthritis in mice. Arthritis Rheum. 2007;56:1806–1816. doi: 10.1002/art.22602. [DOI] [PubMed] [Google Scholar]
- 27.Brennan F, Beech J. Update on cytokines in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:296–301. doi: 10.1097/BOR.0b013e32805e87f1. [DOI] [PubMed] [Google Scholar]
- 28.Markham T, Mullan R, Golden-Mason L, et al. Resolution of endothelial activation and down-regulation of Tie2 receptor in psoriatic skin after infliximab therapy. J Am Acad Dermatol. 2006;54:1003–1012. doi: 10.1016/j.jaad.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 29.Nakahara H, Song J, Sugimoto M, et al. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheum. 2003;48:1521–1529. doi: 10.1002/art.11143. [DOI] [PubMed] [Google Scholar]
- 30.Amin MA, Mansfield PJ, Pakozdi A, et al. Interleukin-18 induces angiogenic factors in rheumatoid arthritis synovial tissue fibroblasts via distinct signaling pathways. Arthritis Rheum. 2007;56:1787–1797. doi: 10.1002/art.22705. [DOI] [PubMed] [Google Scholar]
- 31.Fearon U, Mullan R, Markham T, et al. Oncostatin M induces angiogenesis and cartilage degradation in rheumatoid arthritis synovial tissue and human cartilage cocultures. Arthritis Rheum. 2006;54:3152–3162. doi: 10.1002/art.22161. [DOI] [PubMed] [Google Scholar]
- 32.Amin MA, Volpert OV, Woods JM, et al. Migration inhibitory factor mediates angiogenesis via mitogen-activated protein kinase and phosphatidylinositol kinase. Circ Res. 2003;93:321–329. doi: 10.1161/01.RES.0000087641.56024.DA. [DOI] [PubMed] [Google Scholar]
- 33.Morand EF, Leech M, Bernhagen J. MIF: a new cytokine link between rheumatoid arthritis and atherosclerosis. Nat Rev Drug Discov. 2006;5:399–410. doi: 10.1038/nrd2029. [DOI] [PubMed] [Google Scholar]
- 34.Koch AE, Distler O. Vasculopathy and disordered angiogenesis in selected rheumatic diseases: rheumatoid arthritis and systemic sclerosis. Arthritis Res Ther. 2007;9 Suppl. 2:S3. doi: 10.1186/ar2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mullan RH, Bresnihan B, Golden-Mason L, et al. Acute-phase serum amyloid A stimulation of angiogenesis, leukocyte recruitment and matrix degradation in rheumatoid arthritis through an NF-κB-dependent signal transduction pathway. Arthritis Rheum. 2006;54:105–114. doi: 10.1002/art.21518. [DOI] [PubMed] [Google Scholar]
- 36.Hong KH, Cho ML, Min SY, et al. Effect of interleukin-4 on vascular endothelial growth factor production in rheumatoid synovial fibroblasts. Clin Exp Immunol. 2007;147:573–579. doi: 10.1111/j.1365-2249.2006.03295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boulday G, Haskova Z, Reinders ME, et al. Vascular endothelial growth factor-induced signaling pathways in endothelial cells that mediate overexpression of the chemokine IFN-gamma-inducible protein of 10 kDa in vitro and in vivo. J Immunol. 2006;176:3098–3107. doi: 10.4049/jimmunol.176.5.3098. [DOI] [PubMed] [Google Scholar]
- 38.Vicari AP, Ait-Yahia S, Chemin K, et al. Antitumor effects of the mouse chemokine 6Ckine/SLC through angiostatic and immunological mechanisms. J Immunol. 2000;165:1992–2000. doi: 10.4049/jimmunol.165.4.1992. [DOI] [PubMed] [Google Scholar]
- 39.Yin G, Liu W, An P, et al. Endostatin gene transfer inhibits joint angiogenesis and pannus formation in inflammatory arthritis. Mol Ther. 2002;5:547–554. doi: 10.1006/mthe.2002.0590. [DOI] [PubMed] [Google Scholar]
- 40.Sumariwalla PF, Cao Y, Wu HL, et al. The angiogenesis inhibitor protease-activated kringles 1–5 reduces the severity of murine collagen-induced arthritis. Arthritis Res Ther. 2003;5:R32–R39. doi: 10.1186/ar608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang CR, Chen SY, Shiau AL, et al. Upregulation of kallistatin expression in rheumatoid joints. J Rheumatol. 2007;34:2171–2176. [PubMed] [Google Scholar]
- 42.Mundel TM, Kalluri R. Type IV collagen-derived angiogenesis inhibitors. Microvasc Res. 2007;74:85–89. doi: 10.1016/j.mvr.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park YW, Kang YM, Butterfield J, et al. Thrombospondin 2 functions as an endogenous regulator of angiogenesis and inflammation in rheumatoid arthritis. Am J Pathol. 2004;165:2087–2098. doi: 10.1016/S0002-9440(10)63259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haas CS, Amin MA, Allen BB, et al. Inhibition of angiogenesis by interleukin-4 gene therapy in rat adjuvant-induced arthritis. Arthritis Rheum. 2006;54:2402–2414. doi: 10.1002/art.22034. [DOI] [PubMed] [Google Scholar]
- 45.Haas CS, Amin MA, Ruth JH, et al. In vivo inhibition of angiogenesis by interleukin-13 gene therapy in a rat model of rheumatoid arthritis. Arthritis Rheum. 2007;56:2535–2548. doi: 10.1002/art.22823. [DOI] [PubMed] [Google Scholar]
- 46.Mabjeesh NJ, Escuin D, LaVallee TM, et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–375. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 47.Issekutz AC, Sapru K. Modulation of adjuvant arthritis in the rat by 2-methoxyestradiol: an effect independent of an anti-angiogenic action. Int Immunopharmacol. 2008;8:708–716. doi: 10.1016/j.intimp.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 48.Manley PW, Martiny-Baron G, Schlaeppi JM, et al. Therapies directed at vascular endothelial growth factor. Expert Opin Investig Drugs. 2002;11:1715–1736. doi: 10.1517/13543784.11.12.1715. [DOI] [PubMed] [Google Scholar]
- 49.Grosios K, Wood J, Esser R, et al. Angiogenesis inhibition by the novel VEGF tyrosine kinase inhibitor. PTK787/ZK222584, causes significant anti-arthritic effects in models of rheumatoid arthritis. Inflamm Res. 2004;53:133–142. doi: 10.1007/s00011-003-1230-4. [DOI] [PubMed] [Google Scholar]
- 50.Guttmann-Raviv N, Shraga-Heled N, Varshavsky A, et al. Semaphorin-3A and semaphorin-3F work together to repel endothelial cells and to inhibit their survival by induction of apoptosis. J Biol Chem. 2007;282:26294–26305. doi: 10.1074/jbc.M609711200. [DOI] [PubMed] [Google Scholar]
- 51.Yeo EJ, Chun YS, Cho YS, et al. YC-1: a potential anticancer drug targeting hypoxia-inducible factor 1. J Natl Cancer Inst. 2003;95:516–525. doi: 10.1093/jnci/95.7.516. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y, Donnelly E, Kobayashi H, et al. Gene therapy targeting the Tie2 function ameliorates collagen-induced arthritis and protects against bone destruction. Arthritis Rheum. 2005;52:1585–1594. doi: 10.1002/art.21016. [DOI] [PubMed] [Google Scholar]
- 53.Wente MN, Keane MP, Burdick MD, et al. Blockade of the chemokine receptor CXCR2 inhibits pancreatic cancer cell-induced angiogenesis. Cancer Lett. 2006;241:221–227. doi: 10.1016/j.canlet.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 54.Yin Y, Huang L, Zhao X, et al. AMD3100 mobilizes endothelial progenitor cells in mice, but inhibits its biological functions by blocking an autocrine/paracrine regulatory loop of stromal cell derived factor-1 in vitro. J Cardiovasc Pharmacol. 2007;50:61–67. doi: 10.1097/FJC.0b013e3180587e4d. [DOI] [PubMed] [Google Scholar]
- 55.Skotnicki JS, Zask A, Nelson FC, et al. Design and synthetic considerations of matrix metalloproteinase inhibitors. Ann NY Acad Sci. 1999;878:61–72. doi: 10.1111/j.1749-6632.1999.tb07674.x. [DOI] [PubMed] [Google Scholar]
- 56.Goedkoop AY, Kraan MC, Picavet DI, et al. Deactivation of endothelium and reduction in angiogenesis in psoriatic skin and synovium by low dose infliximab therapy in combination with stable methotrexate therapy. Arthritis Res Ther. 2004;6:R326–R334. doi: 10.1186/ar1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.D’Amato RJ, Loughnan MS, Flynn E, et al. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ingber D, Fujita T, Kishimoto S, et al. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature. 1990;348:555–557. doi: 10.1038/348555a0. [DOI] [PubMed] [Google Scholar]
- 59.McCarey DW, Sattar N, McInnes IB. Do the pleiotropic effects of statins in the vasculature predict a role in inflammatory diseases? Arthritis Res Ther. 2005;7:55–61. doi: 10.1186/ar1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bongartz T, Coras B, Vogt T, et al. Treatment of active psoriatic arthritis with the PPARγ ligand pioglitazone: an open-label pilot study. Rheumatology. 2005;44:126–129. doi: 10.1093/rheumatology/keh423. [DOI] [PubMed] [Google Scholar]