Abstract
Hepatitis C virus (HCV) infects over 170 million people worldwide and is a leading cause of cirrhosis and hepatocellular carcinoma. Approximately 20% of those acutely infected clear the infection, whereas the remaining 80% progress to chronic infection. Hepatitis C thus provides a model in which successful and unsuccessful responses can be compared to better understand the human response to viral infection. Our laboratory studies the strategies by which HCV evades the adaptive immune response. This review describes the impact of viral mutation on T cell recognition, the role of cell surface inhibitory receptors in recognition of HCV, and the development of antibodies that neutralize HCV infection. Understanding what constitutes an effective immune response in the control of HCV may enable the development of prophylactic and therapeutic vaccines for HCV and other chronic viral infections.
Keywords: Hepatitis C virus, CD8 T cell, viral immunity, viral escape, epitope variant
Hepatitis C cellular Epidemiology
HCV infection is found in virtually every region of the world, and an estimated 170 million persons are infected with HCV worldwide (1). In the United States, there are approximately four million persons with persistent hepatitis C infection and more than 10 thousand HCV-related deaths each year, with mortality from HCV expected to double in this decade and possibly surpass that caused by HIV (2–4). Morbidity and mortality occur in the setting of chronic infection. Chronic HCV may cause cirrhosis and is the most common indication for liver transplantation in the United States. In addition, HCV is the major cause of hepatocellular carcinoma in the United States, Europe, Japan, Australia, and many other parts of the world (4–8). Although HCV induces both antibody (Ab) and T cell responses, the virus evades them effectively in most cases, with 75% of those exposed becoming chronically infected.
Hepatitis C cellular immunology
Most viral infections induce successful immune responses and do not persist. HCV has developed mechanisms to evade immune elimination, thereby allowing it to persist in the liver in the majority of infected individuals. The immune correlates that determine whether a patient resolves infection or proceeds to chronic infection are not well defined. Previous studies have shown that spontaneous clearance of HCV infection occurs in association with a broadly specific and vigorous cellular immune response (9–12). In contrast, chronic infection is characterized by low frequencies of specific CD8 T cells in peripheral blood (13–18). Experiments performed with in vitro model systems have demonstrated mechanisms by which HCV subverts the innate antiviral responses needed to stimulate lymphocyte effector functions (19–21). This may partially explain impaired generation of an effective immune response to HCV, but it is unclear how subversion of the innate immune response differs between hosts or how those differences would affect downstream adaptive immune responses.
CD4 T cell responses in humans are more frequently detected and more durable in those who control HCV infection than in those with chronic HCV infection and CD4 T cell responses seen in those who 3 progress to chronic infection have been associated with transient control of HCV RNA (11; 12; 22; 23). Chimpanzee data support the importance of CD4 T cell responses in control of infection (24). CD8 T cells are also critical to control of HCV and the appearance of HCV-specific CD8 T cells in liver and blood is kinetically associated with control of viremia (25; 26). While virus specific CD4 and CD8 T cell responses play a role, generation of a cellular immune response does not ensure control of infection. A detectable cellular immune response is usually present in early infection regardless of outcome and that response may even persist into chronic infection (27). It is unclear why those immune responses fail to control infection, but we and others have demonstrated that the responses generated in acute infection decline in subjects who remained persistently infected (11; 26–28). Most subjects with detectable cellular immune responses during the acute phase of infection had gradual loss of responses, in both breadth and magnitude, during the chronic phase of infection. Despite ongoing viremia and ample evidence that HCV sequence varies during acute and chronic infection, those persistently infected did not develop new epitope specificities after the first six months of infection. Taken together, these results suggest that development of HCV-specific T cells is arrested during the first year of chronic infection.
Hepatitis C escape from the immune response
The decline in T cell responses to HCV is poorly understood, but escape is a likely contributing factor. Because immune responses develop over weeks and pathogen replicate on the order of hours or days, it is well-recognized that immune escape mutations may blunt the effectiveness of the immune response (29). Mathematical models of viral kinetics suggest that up to 1012 virions are produced each day in a chronically HCV infected human (30). The high level of virion turnover, coupled with the absence of proofreading by the HCV RNA polymerase, results in frequent mutations within the viral genome. Mutation of class I or II major histocompatibility complex (MHC) restricted T cell epitopes may alter the outcome of infection by preventing or delaying clearance of infected hepatocytes (31). In the face of a vigorous multispecific cytotoxic lymphocyte (CTL) response, mutation of several epitopes, perhaps simultaneously, would be required for survival of the virus. In the chimpanzee model, antibody-mediated CD4 T cell depletion prior to HCV infection does not prevent initial CD8 T cell responses in a previously-exposed animal, but does impair viral control in association with epitope escape mutations in the viral sequence (24). Longitudinal analysis during chronic infection demonstrated a very low rate of amino acid substitution in CTL epitopes, suggesting that CTL escape that occurs may be limited to early infection (32).
HCV sequence variability
A major challenge in the study of HCV immunology in humans is the high variability of the antigen, which varies not only from person to person, but also at any instant and over time within an infected individual. HCV exists in each infected host as a swarm of genetically-related but distinct variants, collectively called a quasispecies (33–37). This characteristically diverse set of viruses in an individual is not completely random, but rather appears to be driven by the host immune system and balanced by functional constraints (38–41). As a result, each collection of HCV genomes in a quasispecies has a distinctive set of shared characteristics that make it distinct, allowing it to be distinguished from others (42). The random generation of sequences results in mutations that may be deleterious, neutral, or offer a selective advantage to the virus because they increase replication efficiency or permit evasion of the immune response. Our laboratory has worked with that of Dr. Stuart Ray to discover the immunological mechanisms underlying this evasion.
Selection of mutations within T cell epitopes with HCV sequence variation and T cell evasion
To test the hypothesis that immune escape occurs during progression to chronic infection, we compared sites of T cell epitopes with viral amino acid replacements (41). For subjects with persistent viremia and T cell responses, amino acid substitutions were a median of 16.7 fold more likely to occur within T cell epitopes than outside epitopes (range 11.4–24.9). In general, substitutions in recognized epitopes tested resulted in decreased recognition by T lymphocytes compared to recognition of the sequence present at initial viremia, indicating escape. Therefore, virus-specific T cells select for mutants that are not recognized as well by those T cells.
Mechanisms through which mutations permit T cell evasion
Reduced recognition may result from changes in the epitope sequence itself or in flanking residues that are involved in antigen processing (43). Evasion of the immune response via substitution within T cell epitopes occurs during HCV, HBV, SIV, and HIV infections via two known mechanisms: decreased MHC binding and impaired antigen processing or presentation (Figure 1, 41; 43–51). Substitutions within T cell epitopes that do not decrease MHC binding or impair antigen processing or presentation have been observed in multiple chronic viral infections, and substitutions affecting T cell receptor (TCR) engagement have been proposed to explain selection of these mutations. While impaired TCR engagement may allow immune escape, a new T cell repertoire should be available to recognize such neoepitiopes. We recently identified a novel mechanism for impaired T cell recognition of a mutation involving a TCR contact residue in HCV (52).
Figure 1.
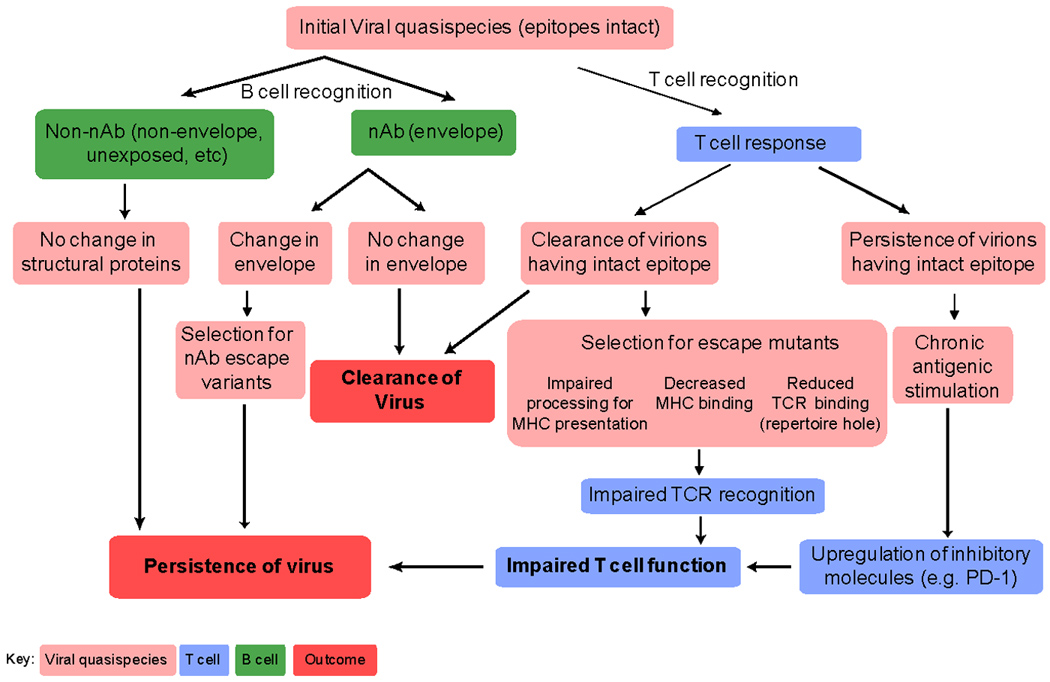
Viral evasion from the adaptive immune response. The constraints placed on the virus due to immune pressure lead to clearance or select for mutations that successfully evade the immune response. nAb = neutralizing antibody. TCR = T cell receptor.
A novel mechanism for impaired T cell recognition of a mutation involving a TCR contact residue
Substitutions in TCR contact residues would be expected to lead to only transient immune escape in immunocompetent hosts since the new epitope should be recognized by a distinct repertoire of T cells specific for the substituted epitope. In addition to identifying multiple mutations which decrease or abrogate HCV peptide binding to the human leukocyte antigen (HLA), we identified an amino acid substitution that reduces T cell recognition due to impaired engagement of the TCR with the variant peptide/A*0201 complex. The mutated peptide was not an antagonist, nor did it affect HLA binding affinity nor prevent antigen processing and presentation. However, it failed to expand T cells primed against the original sequence, poorly activated T cells specific for the original antigen to secrete cytokines, and weakly engaged the TCR when complexed to MHC (52). We determined that the failure to mount a better response to the peptide observed later in infection represented poor intrinsic immunogenicity of this variant because of a ‘hole in the repertoire’. This is reflected by an absence or paucity within the primary repertoire of T cells containing high affinity TCR with specificity for that variant. There was a dramatic difference between the ability of the original and variant peptides to prime responses from the primary naïve T cell repertoires in HLA A*0201+ individuals never exposed to HCV. The variant virus is highly inefficient at priming naïve T cells specific for that region relative to the virus at initial infection. Thus, in the setting of viral infection, the variant virus can exploit the relative hole in the T cell repertoire as an escape mechanism. This is the first example of a viral escape substitution resulting in evasion of the T cell response via exploitation of a hole in the T cell repertoire.
While substitution within T cell epitopes and the resultant loss of antigen may partially explain the decline of T cell responses generated to HCV in the acute phase of infection with progression to chronic infection, T cell responses to HCV epitopes that do not undergo substitution also decline. Additional evasion mechanisms must be employed to account for the decline observed when escape does not occur and the antigen is preserved.
Mechanisms for evasion of T cell responses other than sequence variation
Detectable levels of CD8 T cell recognition do not always result in escape substitutions and some CD8 T cell epitopes remain intact while others mutate within the same host (41; 53). Reversion of immune-escape mutations after transmission to a new host suggests that immune escape can be associated with a significant cost to the virus in terms of fitness (43). It is therefore possible that some substitutions result in virus with such significantly reduced fitness that substitution at that position is not observed. With preservation of viral sequence, alternative mechanisms to evade T cell responses must prevail.
T cell Inhibitory Receptors
The growing family of inhibitory (or regulatory) receptors may be one of the most important categories of cell membrane receptors participating in the hyporesponsiveness of HCV specific T cells. Although many of these were initially identified on CD4 cells, their expression on CD8 cells has been documented and their engagement appears to inhibit CD8 effector function. One example is programmed death-1 (PD-1). PD-1 is an ITIM-containing inhibitory receptor expressed on activated T cells that binds two known ligands: PDL-1 (also called B7-H1) and PDL-2 (also called B7-DC).(54–57). Anti-B7-H1/PDL-1 and anti-PD-1 antibodies partially restore activity in exhausted LCMV-specific T cells. PD-1 has recently been shown to be upregulated on HCV specific T cells and blockade of signaling through it to enhance their function(58–60). We found that surface PD-1 levels are significantly higher on HCV specific T cells in individuals who fail to control infection versus T cells from those who control infection in both early (<180 days) and later (>180days) infection. Although multivariant statistical analysis indicated PD-1 levels on HCV specific T cells are directly correlated with HCV RNA levels, high PD-1 levels are also associated with progression to chronic HCV infection independently of HCV RNA levels (61). Our comparative analyses of outcome of infection (clearance versus persistence) and PD-1 levels during acute infection demonstrate that PD-1 expression in HCV-specific T cells varies tremendously during acute infection and suggests that it is one of the independent determinants of outcome, a conclusion that bears relevance to intervention with blocking antibodies.
Factors Associated with PD-1 Regulation
In our statistical analysis, we determined that HCV RNA levels and outcome of infection are correlated with PD-1 levels, but not subject gender or age (61). Levels of PD-1 expression can also be dramatically affected by the presence versus absence of pro-inflammatory signals at the time of initial TCR engagement (62; 63). In addition to inflammatory stimuli, the presence of antigen is necessary to maintain PD-1 expression. A recent study examining PD-1 expression on CD8 T cells specific from SIV infected macaques demonstrated that PD-1 expression gradually declined on CD8 T cells specific for an SIV-derived epitope that had undergone mutational escape (64). We observed highly variable levels of PD-1 expression on tetramer+ HCV specific T cells during the chronic phase (>180 days infected) in patients who did not clear their infection.
Despite the association between high HCV RNA levels and high PD-1 levels, we observed T cells with low PD-1 levels in the setting of high circulating HCV RNA levels, even at later time points once chronicity has been established. We performed serial viral sequence analysis in subjects and correlated with PD-1 levels on tetramer+ HCV specific CD8 T cells. Viral sequence was assessed at initial infection and at multiple subsequent time points at which T cells specific for known antigens were detectable. Where PD-1 levels decreased despite persistently high HCV RNA levels, we detected substitutions representing T epitope escape mutations in the circulating virus. In contrast, PD-1 levels remained high on virtually all of the T cells specific for HCV epitopes that did not undergo sequence substitution over the period of evaluation. Furthermore, restoration of intact antigen after escape was associated with an increase in PD-1 levels (61). These results provide evidence that immune evasion mechanisms that allow HCV to persist either involve epitope escape or alternatively, signals that maintain high expression of inhibitory “checkpoint” receptors on virus-specific T cells (Figure 1). Validation of this hypothesis requires defining the functional roles of these inhibitory receptors, which can in part be determined by assessing the effects of in vivo antibody blockade of the PD-1 and other inhibitory pathways.
Durability and Protective Nature of Hepatitis C Immunity
Although the vast majority of those infected will fail to clear hepatitis C virus, about 20–30% of individuals will clear the virus spontaneously. However, spontaneous control of HCV does not always result in sterilizing immunity. Reinfection with a new strain of HCV is possible and sometimes results in persistent infection. Re-infection provides another opportunity to query parameters of protective immunity. Sequential inoculation of convalescent chimpanzees over a period of 3 years with different HCV strains of proven infectivity resulted in viremic infection with the challenge virus (65). Control of a second HCV infection in chimpanzees that had previously controlled their initial HCV infection was kinetically linked to rapid acquisition of virus-specific cytolytic activity by liver resident CD8 T cells and expansion of memory CD4 and CD8 T cells in blood (25). The importance of memory CD8 T cells in control of HCV infection was confirmed by antibody-mediated depletion of this lymphocyte subset before a third infection. Virus replication was prolonged despite the presence of memory CD4 T cells primed by the two prior infections and was not terminated until HCV-specific CD8 T cells recovered in the liver. The same group also found that memory CD4 T cells are critical to long-term immunity by showing that antibody-mediated depletion of CD4 T cells before re-infection of two immune chimpanzees resulted in persistent, low-level viremia despite functional intra-hepatic memory CD8 T cell responses (24). These experiments demonstrate an essential role for T cells in long-term protection from chronic hepatitis C.
Demonstration of protective immunity following reinfection has been limited within humans due to the low incidence of re-infection and the stringent requirements for longitudinal follow-up. However, a recent study suggests that reinfection with subsequent persistent viremia may not be uncommon (66). In two epidemiological studies that controlled for infection risk factors, individuals with previous clearance of HCV infection were less likely to develop infection than those infected for the first time and may have a lower risk of acquiring HCV despite ongoing exposure (67; 68). Another study found long periods of undetectable viremia in subjects who cleared infection even in the setting of ongoing injection drug use (6). To examine the hypothesis that protective immunity could be achieved in humans, we identified 164 people in the ALIVE cohort (69) who had no evidence of previous HCV infection and 98 individuals who had been previously, but were not currently, infected with HCV(68). We compared the incidence and persistence of HCV viremia in these two groups over four consecutive 6-month periods. Of participants without previous infection, the incidence of HCV infection was 21%. By contrast, people previously infected were half as likely to develop new viremia (12%), even after accounting for risk behavior (hazard ratio, 0.45; 95% CI 0.23–0.88). Furthermore, in HIV-1-negative people who developed detectable viremia, those previously infected were 12 times less likely than people infected for the first time to develop persistent infection (odds ratio 0.05, 95% CI 0.01–0.30), and the median peak HCV RNA concentration was two logs lower. These data suggest that immunity against viral persistence can be acquired, and that vaccines should be tested to reduce the burden of HCV-related liver disease.
We have begun to investigate the cellular and humoral adaptive responses associated with protective immunity through examination of reinfected subjects. A major challenge in the study of HCV immunology in humans is the typically asymptomatic nature of acute HCV infection. We have in part overcome this barrier by longitudinally following injection drug users (IDU’s) at risk for infection and with ongoing HCV exposure following control of the initial infection. Sequencing of the virus allows us to identify infection with heterologous virus. These two factors permit identification of members of the cohort who have been re-infected and the opportunity to study the role of the immune system in long-term protection from chronic HCV. Between 1997 and 2007, we identified 113 people who seroconverted with sufficient follow-up to evaluate outcome of infection. Reinfection occurred in 50% of those who controlled their first infection with variable virologic outcomes of subsequent infections. Although viral clearance occurs in approximately 25% of patients with primary infections, spontaneous viral clearance was observed in 83% of reinfected patients. The incident reinfection rate in our group of cleared seroconverters was very similar to the incident primary infection rate previously reported in the same cohort (70); strongly suggesting that prior clearance of HCV infection does not provide sterilizing immunity against reinfection. However, the clearance rate of reinfection was almost reversed from the clearance rate of primary infection in our cohort, suggesting protection from chronic infection.
Further evidence for the existence of protective immunity against HCV infection comes from analysis of infection kinetics during primary and reinfection. Frequent monitoring of HCV infection status allowed us to assess the kinetics of viremia during initial and subsequent infections within the same subjects. The duration of viremia during primary infection was nearly four-fold longer than in subsequent infections in reinfected individuals. Similarly, the maximum concentration of HCV RNA detected in blood during reinfection was approximately three logs lower compared to initial infection even when persistent reinfections were included in the analysis. The lower duration and magnitude of viremia in the subsequent infections suggest that prior exposure to HCV provides protective immunity against persistent reinfection since fixed genetic factors associated with control of infection would not be expected to improve with each infection.
Cellular immune responses following exposure to a new virus
Because reinfection is associated with altered infection kinetics, we investigated whether reinfection altered cellular immune responses to HCV, and specifically generation of new T cell responses not detected during the initial infection. In reinfected subjects, exposure to a new virus elicited new T cell responses in all subjects. In contrast, evolution of the same viral sequence in subjects with chronic infection resulted in new cellular responses in only one of 12 subjects tested, and new cellular responses were detected in only three out of nine subjects in whom multiple dominant viral sequences were detected during initial infection, significantly fewer than in reinfected subjects. These results suggest that exposure to a new virus is not sufficient to elicit new cellular immune responses, and that previous complete control of viremia is associated with enhanced generation of new cellular immune responses in response to a new HCV infection. However, one reinfected subject with new T cell responses developed a persistent reinfection, suggesting that development of new T cell responses does not provide absolute protection against persistence.
Humoral immune responses during reinfection
The development of new T cell responses was not associated with absolute protection from persistence and other factors must be important. Because the appearance of neutralizing antibodies (nAb) corresponds with clearance of initial infections in some individuals (71), we investigated the role of nAb in control of reinfection. Given the absence of a culture system for circulating HCV strains, the capacity of serum antibodies to broadly neutralize HCV envelope protein binding and cell entry has been assessed using HCV pseudoparticles as previously described (71). During acute infection, nAbs against heterologous viral pseudoparticles were detected in 60% of reinfected subjects. Those nAb drive sequence evolution in the envelope regions of the virus, allowing escape of viral variants that negate neutralization (Figure 1, 72). In contrast, cross-reactive nAbs are rarely detected in early infection in patients who progress to chronic infection (71). Compared with the initial infection, HCV reinfection is associated with a reduction in the magnitude and duration of viremia, broadened cellular immune responses, and the generation of cross-reactive humoral responses. These findings are consistent with the development of adaptive immunity that is not sterilizing but protects against chronic disease.
Future Directions
Fully human anti-human monoclonal antibodies against PD-1 have been tested in patients with chronic HCV infection in a Phase I clinical trial that was completed in fall of 2009. As part of the immunologic monitoring of this trial, analysis of the effects on T- and B-cells of blockade of PD-1 in vivo is planned and should provide insight into the effects of PD-1 blockade in humans. Ongoing analysis of the immunologic parameters of HCV control in acute infection continues in our laboratory, including in depth analysis of the neutralizing Ab and T cells responses produced in response to repeated infection in those who control an initial infection, but continue to expose themselves to HCV via high risk behavior. Individuals who repeatedly control HCV infection may provide valuable insight into what constitutes a protective immune response to infection and a goal for parameters in successful vaccine development.
Acknowledgments
This work was supported by NIH grants U19AI040035, R01DA024565, R01AI077757 and 5T32GM007309, as well as grants from The Damon Runyon Cancer Research Foundation and The Dana Foundation.
References
- 1.World Health Organization. Hepatitis C: global prevalence. Weekly Epidemiological Record. 1997;(No. 72):341–348.
- 2.Wong JB, McQuillan GM, McHutchison JG, Poynard T. Estimating future hepatitis C morbidity, mortality, and costs in the United States. American Journal of Public Health. 2000;Vol. 90(No. 10):1562–1569. doi: 10.2105/ajph.90.10.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, Kaslow RA, Margolis HS. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. New England Journal of Medicine. 1999;Vol. 341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR. 1998;Vol. 47(No. RR-19):1–39. [PubMed] [Google Scholar]
- 5.Alter MJ. Epidemiology of hepatitis C in the West. Seminars in Liver Disease. 1995;Vol. 15:5–14. doi: 10.1055/s-2007-1007259. [DOI] [PubMed] [Google Scholar]
- 6.Villano SA, Vlahov D, Nelson KE, Cohn S, Thomas DL. Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection. Hepatology. 1999;Vol. 29(No. 3):908–914. doi: 10.1002/hep.510290311. [DOI] [PubMed] [Google Scholar]
- 7.Cao J, Sullivan N, Desjardin E, Parolin C, Robinson J, Wyatt R, Sodroski J. Replication and neutralization of human immunodeficiency virus type 1 lacking the V1 and V2 variable loops of the gp120 envelope glycoprotein. Journal of Virology. 1997;Vol. 71(No. 12):9808–9812. doi: 10.1128/jvi.71.12.9808-9812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tong MJ, El-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. New England Journal of Medicine. 1995;Vol. 332:1463–1466. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- 9.Cooper S, Erickson AL, Adams EJ, Kansopon J, Weiner AJ, Chien DY, Houghton M, Parham P, Walker CM. Analysis of a successful immune response against hepatitis C virus. Immunity. 1999;Vol. 10(No. 4):439–449. doi: 10.1016/s1074-7613(00)80044-8. [DOI] [PubMed] [Google Scholar]
- 10.Gruner NH, Gerlach TJ, Jung MC, Diepolder HM, Schirren CA, Schraut WW, Hoffmann R, Zachoval R, Santantonio T, Cucchiarini M, Cerny A, Pape GR. Association of hepatitis C virus-specific CD8+ T cells with viral clearance in acute hepatitis C. Journal of Infectious Diseases. 2000;Vol. 181(No. 5):1528–1536. doi: 10.1086/315450. [DOI] [PubMed] [Google Scholar]
- 11.Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. Analysis of successful immune responses in persons infected with hepatitis C virus. J.Exp.Med. 2000;Vol. 191(No. 9):1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takaki A, Wiese M, Maertens G, Depla E, Seifert U, Liebetrau A, Miller JL, Manns MP, Rehermann B. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat.Med. 2000;Vol. 6(No. 5):578–582. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- 13.Hiroishi K, Kita H, Kojima M, Okamoto H, Moriyama T, Kaneko T, Ishikawa T, Ohnishi S, Aikawa T, Tanaka N, Yazaki Y, Mitamura K, Imawari M. Cytotoxic T lymphocyte response and viral load in hepatitis C virus infection. Hepatology. 1997;Vol. 25(No. 3):705–712. doi: 10.1002/hep.510250336. [DOI] [PubMed] [Google Scholar]
- 14.Rehermann B, Chang KM, McHutchison JG, Kokka R, Houghton M, Chisari FV. Quantitative analysis of the peripheral blood cytotoxic T lymphocyte response in patients with chronic hepatitis C virus infection. Journal of Clinical Investigation. 1996;Vol. 98(No. 6):1432–1440. doi: 10.1172/JCI118931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerny A, McHutchison JG, Pasquinelli C, Brown ME, Brothers MA, Grabscheid B, Fowler P, Houghton M, Chisari FV. Cytotoxic T lymphocyte response to hepatitis C virus-derived peptides containing the HLA A2.1 binding motif. J.Clin.Invest. 1995;Vol. 95(No. 2):521–530. doi: 10.1172/JCI117694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He XS, Rehermann B, Lopez-Labrador FX, Boisvert J, Cheung R, Mumm J, Wedemeyer H, Berenguer M, Wright TL, Davis MM, Greenberg HB. Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proceedings of the National Academy of Sciences of the United States of America. 1999;Vol. 96(No. 10):5692–5697. doi: 10.1073/pnas.96.10.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shirai M, Okada H, Nishioka M, Akatsuka T, Wychowski C, Houghten R, Pendleton CD, Feinstone SM, Berzofsky JA. An epitope in hepatitis C virus core region recognized by cytotoxic T cells in mice and humans. Journal of Virology. 1994;Vol. 68(No. 5):3334–3342. doi: 10.1128/jvi.68.5.3334-3342.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Battegay M, Fikes J, Di Bisceglie AM, Wentworth PA, Sette A, Celis E, Ching W-M, Grakoui A, Rice CM, Kurokohchi K, Berzofsky JA, Hoofnagle JH, Feinstone SM, Akatsuka T. Patients with chronic hepatitis C have circulating cytotoxic T cells which recognize hepatitis C virus-encoded peptides binding to HLA-A2.1 molecules. Journal of Virology. 1995;Vol. 69:2462–2470. doi: 10.1128/jvi.69.4.2462-2470.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foy E, Li K, Wang C, Sumpter R, Jr, Ikeda M, Lemon SM, Gale M., Jr Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science. 2003;Vol. 300(No. 5622):1145–1148. doi: 10.1126/science.1082604. [DOI] [PubMed] [Google Scholar]
- 20.Li K, Foy E, Ferreon JC, Nakamura M, Ferreon ACM, Ikeda M, Ray SC, Gale M, Lemon SM. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proceedings of the National Academy of Sciences of the United States of America. 2005;Vol. 102(No. 8):2992–2997. doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foy E, Li K, Sumpter R, Jr, Loo YM, Johnson CL, Wang C, Fish PM, Yoneyama M, Fujita T, Lemon SM, Gale M., Jr Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc.Natl.Acad.Sci.U.S.A. 2005;Vol. 102(No. 8):2986–2991. doi: 10.1073/pnas.0408707102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerlach JT, Diepolder HM, Jung MC, Gruener NH, Schraut WW, Zachoval R, Hoffmann R, Schirren CA, Santantonio T, Pape GR. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology. 1999;Vol. 117(No. 4):933–941. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]
- 23.Rosen HR, Miner C, Sasaki AW, Lewinsohn DM, Conrad AJ, Bakke A, Bouwer HG, Hinrichs DJ. Frequencies of HCV-specific effector CD4+ T cells by flow cytometry: correlation with clinical disease stages. Hepatology. 2002;Vol. 35(No. 1):190–198. doi: 10.1053/jhep.2002.30293. [DOI] [PubMed] [Google Scholar]
- 24.Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, Murthy KK, Rice CM, Walker CM. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;Vol. 302(No. 5645):659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 25.Shoukry NH, Grakoui A, Houghton M, Chien DY, Ghrayeb J, Reimann KA, Walker CM. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. Journal of Experimental Medicine. 2003;Vol. 197(No. 12):1645–1655. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. Journal of Experimental Medicine. 2001;Vol. 194(No. 10):1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox AL, Mosbruger T, Lauer GM, Pardoll D, Thomas DL, Ray SC. Comprehensive analyses of CD8+ T cell responses during longitudinal study of acute human hepatitis C. Hepatology. 2005;Vol. 42(No. 1):104–112. doi: 10.1002/hep.20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gruener NH, Lechner F, Jung MC, Diepolder H, Gerlach T, Lauer G, Walker B, Sullivan J, Phillips R, Pape GR, Klenerman P. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. Journal of Virology. 2001;Vol. 75(No. 12):5550–5558. doi: 10.1128/JVI.75.12.5550-5558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiner A, Erickson AL, Kansopon J, Crawford K, Muchmore E, Hughes AL, Houghton M, Walker CM. Persistent hepatitis C virus infection in a chimpanzee is associated with emergence of a cytotoxic T lymphocyte escape variant. Proceedings of the National Academy of Sciences of the United States of America. 1995;Vol. 92:2755–2759. doi: 10.1073/pnas.92.7.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neumann AU, Lam NP, Dahari H, Gretch DR, Wiley TE, Layden TJ, Perelson AS. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;Vol. 282(No. 5386):103–107. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- 31.Eckels DD, Wang H, Bian TH, Tabatabai N, Gill JC. Immunobiology of hepatitis C virus (HCV) infection: the role of CD4 T cells in HCV infection. Immunol.Rev. 2000;Vol. 174:90–97. doi: 10.1034/j.1600-0528.2002.017403.x. [DOI] [PubMed] [Google Scholar]
- 32.Chang KM, Rehermann B, McHutchison JG, Pasquinelli C, Southwood S, Sette A, Chisari FV. Immunological significance of cytotoxic T lymphocyte epitope variants in patients chronically infected by the hepatitis C virus. Journal of Clinical Investigation. 1997;Vol. 100(No. 9):2376–2385. doi: 10.1172/JCI119778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Domingo E, Martinez-Salas E, Sobrino F, de la Torre JC, Portela A, Ortin J, Lopez-Galindez C, Perez-Brena P, Villanueva N, Najera R. The quasispecies (extremely heterogeneous) nature of viral RNA genome populations: biological relevance--a review. Gene. 1985;Vol. 40(No. 1):1–8. doi: 10.1016/0378-1119(85)90017-4. [DOI] [PubMed] [Google Scholar]
- 34.Domingo E, Sabo D, Taniguchi T, Weissmann C. Nucleotide sequence heterogeneity of an RNA phage population. Cell. 1978;Vol. 13(No. 4):735–744. doi: 10.1016/0092-8674(78)90223-4. [DOI] [PubMed] [Google Scholar]
- 35.Eigen M. Self organization of matter and the evolution of biological macromolecules. Naturwissenschaften. 1971;Vol. 58(No. 10):465–523. doi: 10.1007/BF00623322. [DOI] [PubMed] [Google Scholar]
- 36.Kato N, Ootsuyama Y, Tanaka T, Nakagawa M, Nakazawa T, Muraiso K, Ohkoshi S, Hijikata M, Shimotohno K. Marked sequence diversity in the putative envelope proteins of hepatitis C viruses. Virus Res. 1992;Vol. 22(No. 2):107–123. doi: 10.1016/0168-1702(92)90038-b. [DOI] [PubMed] [Google Scholar]
- 37.Martell M, Esteban JI, Quer J, Genesca J, Weiner A, Esteban R, Guardia, Gomez J. Hepatitis C virus (HCV) circulates as a population of different but closely related genomes: quasispecies nature of HCV genome distribution. Journal of Virology. 1992;Vol. 66(No. 5):3225–3229. doi: 10.1128/jvi.66.5.3225-3229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray SC, Mao Q, Lanford RE, Bassett S, Laeyendecker O, Wang YM, Thomas DL. Hypervariable region 1 sequence stability during hepatitis C virus replication in chimpanzees. J Virol. 2000;Vol. 74(No. 7):3058–3066. doi: 10.1128/jvi.74.7.3058-3066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ray SC, Wang YM, Laeyendecker O, Ticehurst J, Villano SA, Thomas DL. Acute hepatitis C virus structural gene sequences as predictors of persistent viremia: hypervariable region 1 as decoy. Journal of Virology. 1998;Vol. 73:2938–2946. doi: 10.1128/jvi.73.4.2938-2946.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mao Q, Ray SC, Laeyendecker O, Ticehurst JR, Strathdee SA, Vlahov D, Thomas DL. Human immunodeficiency virus seroconversion and evolution of the hepatitis C virus quasispecies. Journal of Virology. 2001;Vol. 75(No. 7):3259–3267. doi: 10.1128/JVI.75.7.3259-3267.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cox AL, Mosbruger T, Mao Q, Liu Z, Wang XH, Yang HC, Sidney J, Sette A, Pardoll D, Thomas DL, Ray SC. Cellular immune selection with hepatitis C virus persistence in humans. J Exp.Med. 2005;Vol. 201(No. 11):1741–1752. doi: 10.1084/jem.20050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonzalez-Candelas F, Bracho MA, Moya A. Molecular epidemiology and forensic genetics: application to a hepatitis C virus transmission event at a hemodialysis unit. J Infect.Dis. 2003;Vol. 187(No. 3):352–358. doi: 10.1086/367965. [DOI] [PubMed] [Google Scholar]
- 43.Allen TM, Altfeld M, Yu XG, O'Sullivan KM, Lichterfeld M, Le Gall S, John M, Mothe BR, Lee PK, Kalife ET, Cohen DE, Freedberg KA, Strick DA, Johnston MN, Sette A, Rosenberg ES, Mallal SA, Goulder PJ, Brander C, Walker BD. Selection, transmission, and reversion of an antigen-processing cytotoxic T-lymphocyte escape mutation in human immunodeficiency virus type 1 infection. Journal of Virology. 2004;Vol. 78(No. 13):7069–7078. doi: 10.1128/JVI.78.13.7069-7078.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Timm J, Lauer GM, Kavanagh DG, Sheridan I, Kim AY, Lucas M, Pillay T, Ouchi K, Reyor LL, Zur Wiesch JS, Gandhi RT, Chung RT, Bhardwaj N, Klenerman P, Walker BD, Allen TM. CD8 epitope escape and reversion in acute HCV infection. Journal of Experimental Medicine. 2004;Vol. 200(No. 12):1593–1604. doi: 10.1084/jem.20041006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kimura Y, Gushima T, Rawale S, Kaumaya P, Walker CM. Escape mutations alter proteasome processing of major histocompatibility complex class I-restricted epitopes in persistent hepatitis C virus infection. J Virol. 2005;Vol. 79(No. 8):4870–4876. doi: 10.1128/JVI.79.8.4870-4876.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seifert U, Liermann H, Racanelli V, Halenius A, Wiese M, Wedemeyer H, Ruppert T, Rispeter K, Henklein P, Sijts A, Hengel H, Kloetzel PM, Rehermann B. Hepatitis C virus mutation affects proteasomal epitope processing. J.Clin.Invest. 2004;Vol. 114(No. 2):250–259. doi: 10.1172/JCI20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allen TM, O'Connor DH, Jing P, Dzuris JL, Mothe BR, Vogel TU, Dunphy E, Liebl ME, Emerson C, Wilson N, Kunstman KJ, Wang X, Allison DB, Hughes AL, Desrosiers RC, Altman JD, Wolinsky SM, Sette A, Watkins DI. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature. 2000;Vol. 407(No. 6802):386–390. doi: 10.1038/35030124. [DOI] [PubMed] [Google Scholar]
- 48.Jones NA, Wei X, Flower DR, Wong M, Michor F, Saag MS, Hahn BH, Nowak MA, Shaw GM, Borrow P. Determinants of human immunodeficiency virus type 1 escape from the primary CD8+ cytotoxic T lymphocyte response. Journal of Experimental Medicine. 2004;Vol. 200(No. 10):1243–1256. doi: 10.1084/jem.20040511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Draenert R, Le Gall S, Pfafferott KJ, Leslie AJ, Chetty P, Brander C, Holmes EC, Chang SC, Feeney ME, Addo MM, Ruiz L, Ramduth D, Jeena P, Altfeld M, Thomas S, Tang Y, Verrill CL, Dixon C, Prado JG, Kiepiela P, Martinez-Picado J, Walker BD, Goulder PJ. Immune selection for altered antigen processing leads to cytotoxic T lymphocyte escape in chronic HIV-1 infection. J Exp.Med. 2004;Vol. 199(No. 7):905–915. doi: 10.1084/jem.20031982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yokomaku Y, Miura H, Tomiyama H, Kawana-Tachikawa A, Takiguchi M, Kojima A, Nagai Y, Iwamoto A, Matsuda Z, Ariyoshi K. Impaired processing and presentation of cytotoxic-T-lymphocyte (CTL) epitopes are major escape mechanisms from CTL immune pressure in human immunodeficiency virus type 1 infection. J Virol. 2004;Vol. 78(No. 3):1324–1332. doi: 10.1128/JVI.78.3.1324-1332.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu.Rev.Immunol. 1995;Vol. 13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 52.Wolfl M, Rutebemberwa A, Mosbruger T, Mao Q, Li HM, Netski D, Ray SC, Pardoll D, Sidney J, Sette A, Allen T, Kuntzen T, Kavanagh DG, Kuball J, Greenberg PD, Cox AL. Hepatitis C virus immune escape via exploitation of a hole in the T cell repertoire. Journal of Immunology. 2008;Vol. 181(No. 9):6435–6446. doi: 10.4049/jimmunol.181.9.6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koibuchi T, Allen TM, Lichterfeld M, Mui SK, O'Sullivan KM, Trocha A, Kalams SA, Johnson RP, Walker BD. Limited sequence evolution within persistently targeted CD8 epitopes in chronic human immunodeficiency virus type 1 infection. Journal of Virology. 2005;Vol. 79(No. 13):8171–8181. doi: 10.1128/JVI.79.13.8171-8181.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat.Med. 1999;Vol. 5(No. 12):1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 55.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp.Med. 2000;Vol. 192(No. 7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp.Med. 2001;Vol. 193(No. 7):839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat.Immunol. 2001;Vol. 2(No. 3):261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 58.Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ, Altman JD, Rouse BT, Freeman GJ, Ahmed R, Grakoui A. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. Journal of Virology. 2007;Vol. 81(No. 6):2545–2553. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Penna A, Pilli M, Zerbini A, Orlandini A, Mezzadri S, Sacchelli L, Missale G, Ferrari C. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;Vol. 45(No. 3):588–601. doi: 10.1002/hep.21541. [DOI] [PubMed] [Google Scholar]
- 60.Urbani S, Amadei B, Tola D, Cavallo MC, Mezzadri S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus infection. Hepatology. 2006;Vol. 44(No. 4):292A–293A. [Google Scholar]
- 61.Rutebemberwa A, Ray SC, Astemborski J, Levine J, Liu L, Dowd KA, Clute S, Wang C, Korman A, Sette A, Sidney J, Pardoll DM, Cox AL. High-programmed death-1 levels on hepatitis C virus-specific T cells during acute infection are associated with viral persistence and require preservation of cognate antigen during chronic infection. Journal of Immunology. 2008;Vol. 181(No. 12):8215–8225. doi: 10.4049/jimmunol.181.12.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;Vol. 439(No. 7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 63.Goldberg MV, Maris CH, Hipkiss EL, Flies AS, Zhen L, Tuder RM, Grosso JF, Getnet D, Whartenby KA, Brockstedt DG, Dubensky TW, Jr, Chen L, Pardoll DM, Drake CG. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2007 doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petrovas C, Price DA, Mattapallil J, Ambrozak DR, Geldmacher C, Cecchinato V, Vaccari M, Tryniszewska E, Gostick E, Roederer M, Douek DC, Morgan SH, Davis SJ, Franchini G, Koup RA. SIV-specific CD8+T-cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood. 2007 doi: 10.1182/blood-2007-01-069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Farci P, Alter HJ, Govindarajan S, Wong DC, Engle R, Lesniewski RR, Mushahwar IK, Desai SM, Miller RH, Ogata N. Lack of protective immunity against reinfection with hepatitis C virus. Science. 1992;Vol. 258:135–140. doi: 10.1126/science.1279801. [DOI] [PubMed] [Google Scholar]
- 66.Schulze zur WJ, Lauer GM, Timm J, Kuntzen T, Neukamm M, Berical A, Jones AM, Nolan BE, Longworth SA, Kasprowicz V, McMahon C, Wurcel A, Lohse AW, Lewis-Ximenez LL, Chung RT, Kim AY, Allen TM, Walker BD. Immunologic evidence for lack of heterologous protection following resolution of HCV in subjects with nongenotype 1 infection. Blood. 2007 doi: 10.1182/blood-2007-01-069583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grebely J, Conway B, Raffa JD, Lai C, Krajden M, Tyndall MW. Hepatitis C virus reinfection in injection drug users. Hepatology. 2006;Vol. 44(No. 5):1139–1145. doi: 10.1002/hep.21376. [DOI] [PubMed] [Google Scholar]
- 68.Mehta SH, Cox A, Hoover DR, Wang XH, Mao Q, Ray S, Strathdee SA, Vlahov D, Thomas DL. Protection against persistence of hepatitis C. Lancet. 2002;Vol. 359(No. 9316):1478–1483. doi: 10.1016/S0140-6736(02)08435-0. [DOI] [PubMed] [Google Scholar]
- 69.Vlahov D, Anthony JC, Munoz A, Margolick J, Nelson KE, Celentano DD, Solomon L, Polk BF. The ALIVE study, a longitudinal study of HIV-1 infection in intravenous drug users: description of methods and characteristics of participants. NIDA.Res.Monogr. 1991;Vol. 109:75–100. 75–100. [PubMed] [Google Scholar]
- 70.Cox AL, Netski DM, Mosbruger T, Sherman SG, Strathdee S, Ompad D, Vlahov D, Chien D, Shyamala V, Ray SC, Thomas DL. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clinical Infectious Diseases. 2005;Vol. 40(No. 7):951–958. doi: 10.1086/428578. [DOI] [PubMed] [Google Scholar]
- 71.Dowd KA, Netski DM, Wang XH, Cox AL, Ray SC. Selection Pressure from Neutralizing Antibodies Drives Sequence Evolution during Acute Infection with Hepatitis C Virus. Gastroenterology. 2009;Vol. 136(No. 7):2377–2386. doi: 10.1053/j.gastro.2009.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Osburn WO, Fisher BE, Dowd KA, Urban G, Liu L, Ray SC, Thomas DL, Cox AL. Spontaneous Control of Primary Hepatitis C Virus Infection and Immunity against Persistent Reinfection. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.09.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


