Abstract
Aim
To examine relationships between subgingival biofilm composition and levels of gingival crevicular fluid (GCF) cytokines in periodontal health and generalized aggressive periodontitis (GAP).
Materials and methods
Periodontal parameters were measured in 25 periodontally healthy and 31 GAP subjects. Subgingival plaque and GCF samples were obtained from 14 sites from each subject. 40 subgingival taxa were quantified using checkerboard DNA-DNA hybridization and the concentrations of 8 GCF cytokines measured using Luminex. Cluster analysis was used to define sites with similar subgingival microbiotas in each clinical group. Significance of differences in clinical, microbiological and immunological parameters among clusters was determined using the Kruskal-Wallis test.
Results
GAP subjects had statistically significantly higher GCF levels of interleukin-1β (IL-1β) (p<0.001), granulocyte-macrophage colony-stimulating factor (GM-CSF) (p<0.01), and IL-1β/IL-10 ratio (p<0.001) and higher proportions of Red and Orange complex species than periodontally healthy subjects. There were no statistically significant differences in the mean proportion of cytokines among clusters in the periodontally healthy subjects, while the ratio IL-1β/IL-10 (p<0.05) differed significantly among clusters in the aggressive periodontitis group.
Conclusions
Different subgingival biofilm profiles are associated with distinct patterns of GCF cytokine expression. Aggressive periodontitis subjects were characterized by a higher IL-1β/IL-10 ratio than periodontally healthy subjects, suggesting an imbalance between pro- and anti-inflammatory cytokines in aggressive periodontitis.
Keywords: Generalized aggressive periodontitis, microbiota, gingival crevicular fluid, biomarkers
Clinical Relevance.
Scientific rationale for study: Periodontal diseases result from interactions between specific subgingival microbial species and the susceptible host. However, very little is known regarding the impact of the subgingival biofilm composition on the secretion of biomarkers by the adjacent periodontal tissues.
Principal findings: Generalized aggressive periodontitis (GAP) subjects were characterized by a higher IL-1β/IL-10 ratio than periodontally healthy subjects, suggesting an imbalance between pro-and anti-inflammatory cytokines in GAP. A high IL-1β/IL-10 ratio in GAP was accompanied by higher proportions of Red and Orange complex species.
Practical implications: Clarification of mechanisms involved in the inflammatory-immune response to polymicrobial biofilms could lead to the development of novel therapies for periodontal infections.
Introduction
Periodontal diseases result from interactions between specific subgingival microbial species and the susceptible host, leading to the release of inflammatory mediators that mediate tissue destruction (Socransky & Haffajee 2005, Kornman et al. 1997). The host immune responses to subgingival biofilms are orchestrated by cytokines. These molecular messengers are determinants of efficient innate and adaptive responses and allow fine tuning of the interplay among the various components of the immune system (Dinarello 2007). Shifts in cytokine levels appear to be guided by the microbiota present at each stage of periodontitis; however, there are very limited data regarding the changes in cytokine levels in relation to the total mass and composition of subgingival biofilms (Albandar et al. 1998, Apatzidou et al. 2005, Jin et al. 2000). It is also recognized that subgingival biofilms present great variability in their microbial composition (Kumar et al. 2005, Kumar et al. 2006). Neighboring sites on different teeth or even on the same tooth may differ considerably in levels and proportions of colonizing bacterial species (Socransky & Haffajee 2005). Far less appreciated has been the effect of this microbial diversity on the cytokine milieu released by the adjacent periodontal tissues. Clarification of the key immunological mechanisms involved in the response to polymicrobial biofilms associated with periodontal diseases holds immense promise to unlock novel diagnostic and therapeutic approaches for these infections.
Previous attempts to investigate the interplay between subgingival species and the host have focused on examining the effects of single bacterial species or in vitro models of simple mixed biofilms composed of a few species on different host cell types (Hasegawa et al. 2007, Darveau et al. 1998, Dongari-Bagtzoglou & Ebersole 1996, Vernal et al. 2008). This approach has been instrumental in determining several possible mechanisms of interference of subgingival species on the secretion and function of inflammatory mediators, which may be potentially involved in periodontal diseases. However, these in vitro models cannot mimic the microbial diversity found in vivo in periodontal health and disease. In addition, they only explore the effects that the bacteria have on single cell types or on simple mixtures of cell types studied in co-culture. Since the expression of cytokines are ultimately determined by an interplay between the microbial challenge and interactions among all cells present at the lesion site, these in vitro models underestimate potential cell interactions that might be crucial to determine the levels and composition of the inflammatory mediators that are released.
Our hypothesis was that in periodontal disease we would find a greater diversity in the composition of the subgingival microbiota and that this diversity would be accompanied by a greater variety in the profiles of cytokine expression, when compared to periodontal health. The goal of this study was to examine in vivo associations among the composition of subgingival biofilms and the levels of cytokines released by the adjacent tissues and cells, measured in the gingival crevicular fluid (GCF). The data generated also allowed us to make inferences on the potential involvement of the biomarkers examined in the pathogenesis of periodontal diseases.
Materials and methods
Study approach
Associations among the subgingival microbiota and the GCF biomarkers: GM-CSF, interleukin-2 (IL-2), interferon-γ (IFN-γ), IL-10, IL-13, IL-6, IL-1β and tumor necrosis factor-α (TNF-α) were examined in periodontally healthy and generalized aggressive periodontitis subjects. Samples of subgingival biofilm and GCF were collected from the same sites allowing for comparisons on a site by site basis. Since a large number of sites were samples, they could be grouped according to distinct microbial profiles, identidyed using cluster analysis. This approach allowed us to examine the interplay between the complex subgingival microbiota and the expression of multiple local host biomarkers.
Subject population and clinical monitoring
Twenty five periodontally healthy and 31 generalized agressive periodontitis subjects were recruited at the Guarulhos University periodontal clinic (Guarulhos, SP, Brazil). The Guarulhos University’s Ethics Committee in Clinical Research approved the study protocols including the periodontal examination and the taking of GCF and subgingival plaque samples. Each subject read and signed an informed consent before entering the study. To be included in the study, the periodontally healthy subjects had to be ≥ 18 and ≤ 30 years of age, have at least 24 natural teeth and no probing pocket depth (PD) and clinical attachment level (CAL) > 3 mm and have less than 20% of the sites with bleeding on probing (BOP). The generalized aggressive periodontitis subjects had to be ≥ 18 and ≤ 30 years of age, have at least 20 natural teeth and a minimum of 6 teeth with an interproximal site with PD ≥ 5 mm and CAL ≥ 5 mm and ≤ 10 mm.
The diagnosis of generalized aggressive periodontitis also required familial aggregation, i.e. at least one other family member presenting or with a history of periodontal disease. Subjects were excluded if they had any systemic condition which would influence the course of periodontal disease or treatment, and medical conditions which would require antibiotic prophylaxis for routine dental procedures. Individuals who smoked, had taken antibiotics in the previous 3 months or were either pregnant or nursing were also excluded. The baseline clinical parameters of the study subjects are presented in Table 1.
Table 1.
Mean (± SD) demographic and clinical parameters for periodontally healthy and generalized aggressive periodontitis (GAP) subjects
| Health (n = 25) | GAP (n = 31) | |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| Age (years) | 26 ± 3 | 25 ± 4 |
| % malesa | 45 | 44 |
| % sites with: | ||
| visible plaque*** | 40 ± 14 | 59 ± 31 |
| gingival redness* | 6 ± 6 | 26 ± 35 |
| bleeding on probing*** | 8 ± 9 | 72 ± 28 |
| Pocket depth (mm)*** | 2.3 ± 0.5 | 4.5 ± 1.2 |
| Clinical attachment level (mm)*** | 1.7 ± 0.4 | 4.7 ± 1.1 |
| Number of missing teeth*** | 0.5 ± 1.2 | 3.6 ± 3.4 |
p<0.05,
p<0.001, Mann-Whitney test
non-significant, Fisher’s exact test
Plaque accumulation (0 or 1), gingival redness (0 or 1), suppuration (0 or 1), BOP (0 or 1), PD (mm) and CAL (mm) were measured at 6 sites per tooth (mesiobuccal, buccal, distobuccal, mesiolingual, lingual and distolingual) for all teeth present (excluding third molars), for a maximum of 168 sites per subject, by a calibrated examiner. PD and CAL were recorded to the nearest millimeter using a North Carolina periodontal probe.
Gingival crevicular fluid sampling
Gingival crevicular fluid samples were obtained from the mesiobuccal site of every tooth present (excluding third molars) from two randomly selected contralateral quadrants (maximum of 14 teeth per subject), for a total of 551 samples. Following isolation of the site with cotton rolls to prevent contamination with saliva, supragingival plaque was removed, the tooth air dried and a 30-second GCF sample was collected on filter strips (Periopaper®, Interstate Drug Exchange, Amityville, NY). Periopaper strips were gently inserted into the orifice of the periodontal pocket, 1–2 mm subgingivally. GCF volume was determined using a Periotron 8000® (Oraflow Inc., Plainview, NY), calibrated following the protocol described by Chapple et al. (1999). Samples were immediately placed in Eppendorf tubes on ice, transported to the laboratory and stored at −80°C. GCF samples were collected prior to clinical measurements and samples visibly contaminated with blood were discarded. All GCF samples were shipped to the Forsyth Institute for analysis.
Quantification of cytokines using multiplexed bead immunoassay (Luminex)
Cytokine levels were determined using the high sensitivity human cytokine 8-plex by Millipore (Millipore Corporation, Billerica, MA). Prior to assay, GCF samples were eluted using 60 μl of the assay buffer provided in the Millipore kit by vortexing for 30 minutes and then centrifuging for 10 minutes at 10,000 rpm. Eight cytokines: GM-CSF, IL-2, IFN-γ, IL-10, IL-13, IL-6, IL-1β and TNF-α were measured. The assays were performed in 96-well filter plates following the manufacturer’s instructions. Briefly, the filter plate was pre-wetted with washing buffer and the solution was aspirated from the wells using a vacuum manifold (Millipore Corporation, Billerica, MA). Microsphere beads coated with monoclonal antibodies against the 8 different target analytes were added to the wells. Samples and standards (ranging from 0.13 to 2,000 pg/ml for each analyte) were pipetted into the wells and incubated overnight at 4°C. The wells were washed using a vacuum manifold (Millipore Corporation, Billerica, MA) and a mixture of biotinylated secondary antibodies was added. After incubation for 1 h, streptavidin conjugated to the fluorescent protein, R-phycoerythrin (streptavidin-RPE) was added to the beads and incubated for 30 min. After washing to remove the unbound reagents, sheath fluid (Luminex®, MiraiBio, Alameda, CA) was added to the wells and the beads (minimum of 100 per analyte) were analyzed in the Luminex 100™ instrument (MiraiBio, Alameda, CA). The Luminex 100™ monitors the spectral properties of the beads to distinguish the different analytes, while simultaneously measuring the amount of fluorescence associated with R-phycoerythrin, reported as median fluorescence intensity. The concentrations of the unknown samples (antigens in GCF samples) were estimated from the standard curve using a third order polynomial equation and the GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA) and expressed as pg/ml. Samples below the detection limit of the assay were recorded as zero, while samples above the upper limit of quantification of the standard curves were assigned the highest value of the curve.
Checkerboard DNA-DNA hybridization
Subgingival plaque samples were taken from the same sites sampled for GCF immediately after GCF collection. The subgingival plaque samples were collected using sterile Gracey curettes (Hu-Friedy®, Chicago, IL, USA) after removal of supragingival plaque. The samples were placed in separate microcentrifuge tubes containing 0.15 ml TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.6), and 0.1 ml of 0.5 M NaOH was added. All samples were processed at The Forsyth Institute. Samples were individually analyzed for their content of 40 bacterial species using the checkerboard DNA-DNA hybridization technique (Socransky et al. 2004). In brief, the samples were lysed and the DNA placed in lanes on a nylon membrane using a Minislot device (Immunetics, Cambridge, MA, USA). After fixation of the DNA to the membrane, the membrane was placed in a Miniblotter 45 (Immunetics), with the lanes of DNA at 90° to the lanes of the device. Digoxigenin-labeled whole genomic DNA probes to 40 subgingival species were hybridized in individual lanes of the Miniblotter. After hybridization, the membranes were washed at high stringency and the DNA probes detected using antibody to digoxigenin, conjugated with alkaline phosphatase and chemifluorescence detection. Signals were detected using AttoPhos substrate (Amersham Life Sciences, Arlington Heights, Illinois) and were read using a Storm Fluorimager (Molecular Dynamics, Sunnyvale, CA), a computer-linked instrument that reads the intensity of the fluorescence signals resulting from the probe-target hybridization. Two lanes in each run contained standards at the concentration of 105 and 106 cells of each species. The sensitivity of the assay was adjusted to permit the detection of 104 cells of a given species by adjusting the concentration of each DNA probe. Signals were converted to absolute counts by comparison with standards on the same membrane. Failure to detect a signal was recorded as zero. A total of 551 subgingival samples were evaluated.
Data analysis
Clinical data were available from 6 sites per tooth for visible plaque, gingival redness, BOP, suppuration, PD and CAL. The levels of each inflammatory mediator were measured for up to 14 GCF samples per subject and expressed as pg/ml. The total level of cytokines was calculated by adding the levels of the 8 biomarkers assessed. The proportion of each cytokine was computed by dividing the level of each cytokine by the total level of cytokines for each site. Microbiological data available were the counts of each of the 40 test species for up to 14 subgingival plaque samples per subject. The data for each species were expressed as counts x 105 and as percentage of total DNA probe count at each site. For overall analyses, the data for each clinical, microbiological and immunological parameter were averaged within a subject and then averaged across subjects in each clinical group separately. Significance of differences between healthy and generalized aggressive periodontitis subjects for each clinical, microbiological and immunological parameter was determined using the Mann-Whitney test. For microbiological and immunological data, significance of differences between groups was tested adjusting for multiple comparisons (Socransky et al. 1991). Significance of difference between groups for percentage of males was tested using Fisher’s exact test.
Correlations among mean proportions of subgingival species and mean proportions of GCF cytokines were examined using the Spearman rank correlation coefficient. Hierarchical cluster analysis of site-specific levels of 40 subgingival species employing the chord coefficient and an averaged unweighted linkage sort (Sneath & Sokal, 1973) was used to differentiate sites with similar subgingival microbiotas in each clinical group separately. Clinical, microbiological and immunological parameters were averaged across sites in each cluster in each subject and then across subjects for each cluster in the two clinical groups separately. Significance of differences in clinical, microbiological and immunological parameters among clusters in each clinical group was determined using the Kruskal-Wallis test. For microbiological data, significance of differences among groups was tested adjusting for multiple comparisons (Socransky et al. 1991).
Results
Table 1 presents the mean demographic and clinical parameters for the two groups. The generalized aggressive periodontitis subjects exhibited significantly higher mean PD, CAL, number of missing teeth, and percentage of sites with visible plaque, gingival redness and bleeding on probing. Mean age and gender distribution did not differ between clinical groups.
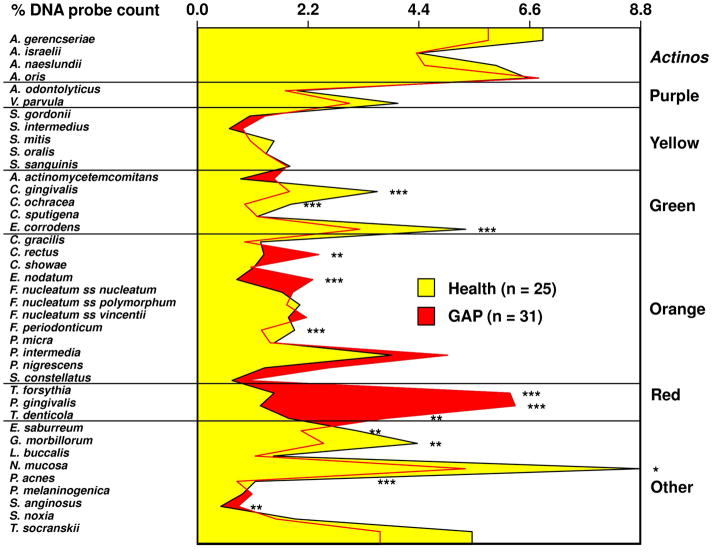
Figure 1 shows the mean proportions of each species for the two clinical groups. Generalized aggressive subjects presented statistically significantly higher proportions of 6/40 species, including Campylobacter rectus, Streptococcus anginosus, and the periodontal pathogens Eubacterium nodatum, Tannerella forsythia, Porphyromonas gingivalis and Treponema denticola. The periodontally healthy subjects presented statistically significantly higher proportions of 7/40 taxa, including Capnocytophaga gingivalis, Capnocytophaga ochracea, Eikenella corrodens, Fusobacterium periodonticum, Eubacterium saburreum, Gemella morbillorum, Neisseria mucosa, and Propionibacterium acnes.
Fig. 1.
Mean percentage of the total DNA probe count of the 40 test species in subgingival biofilm samples from up to 14 periodontal sites from each periodontally healthy and generalized aggressive periodontitis subject. Mean % DNA probe count of each species was computed at each site, averaged for each subject, and then averaged across subjects in each group separately. Significance of differences for each species between clinical groups was sought using the Mann Whitney test; * p < 0.05, ** p < 0.01, *** p < 0.001 and adjusted for 40 comparisons (Socransky et al. 1991). The species were ordered and grouped according to the complexes described by Socransky et al. (1998).
Table 2 presents the mean levels of each cytokine, mean total cytokine level, mean proportion of each cytokine, and the IL-1β/IL-10 ratios for the two clinical groups. Generalized aggressive periodontitis subjects presented statistically significantly higher mean levels (pg/ml) of IL-1β (p<0.001) and GM-CSF (p<0.01) compared to periodontally healthy subjects. Although not statistically significant, there was a trend for IL-2 and IL-13 to be elevated in aggressive periodontitis and IL-10 to be elevated in periodontally healthy subjects. The mean ratio IL-1β/IL-10 was compared between groups, the generalized aggressive periodontitis group had a statistically significantly higher IL-1β/IL-10 ratio than the periodontally healthy subjects (p<0.001). When the data for the cytokines were expressed as percentage of the total level of cytokines, aggressive periodontitis subjects presented a statistically significantly higher percentage of IL-1β (p<0.001) and a statistically significantly lower percentage of IL-10 (p<0.001) and TNF-α (p<0.05), compared to periodontally healthy subjects.
Table 2.
Mean (± SD) immunological parameters determined in GCF samples from the two clinical groups
| Health (n = 25) | GAP (n = 31) | |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| GM-CSF (pg/ml)** | 2.2 ± 1.2 | 4.4 ± 2.6 |
| IL-2 (pg/ml) | 0.7 ± 1.2 | 1.8 ± 2.6 |
| IFN-γ (pg/ml) | 0.6 ± 1.2 | 0.7 ± 1.2 |
| IL-10 (pg/ml) | 23.7 ± 19.9 | 19.9 ± 13.3 |
| IL-13 (pg/ml) | 1.7 ± 2.1 | 12.0 ± 23.1 |
| IL-6 (pg/ml) | 1.9 ± 3.9 | 2.9 ± 5.1 |
| IL-1β (pg/ml)*** | 7.0 ± 3.9 | 20.9 ± 11.0 |
| TNF-α (pg/ml) | 1.9 ± 1.4 | 2.0 ± 1.9 |
| Total cytokines** | 39.7 ± 24.6 | 64.5 ± 34.5 |
| IL-1/IL-10*** | 0.5 ± 0.4 | 2.5 ± 2.3 |
| GM-CSF (%) | 7.0 ± 3.2 | 7.5 ± 4.0 |
| IL-2 (%) | 1.9 ± 2.8 | 3.0 ± 4.0 |
| IFN-γ (%) | 1.4 ± 1.7 | 1.0 ± 1.2 |
| IL-10 (%)*** | 56.6 ± 12.1 | 34.6 ± 17.2 |
| IL-13 (%) | 3.8 ± 2.7 | 9.1 ± 13.5 |
| IL-6 (%) | 4.4 ± 2.9 | 4.9 ± 6.9 |
| IL-1β (%)*** | 18.9 ± 8.4 | 36.3 ± 17.8 |
| TNF-α (%)* | 5.9 ± 2.6 | 3.6 ± 2.3 |
p<0.001;
p<0.01;
p<0.05 using Mann-Whitney and adjusted for multiple comparisons
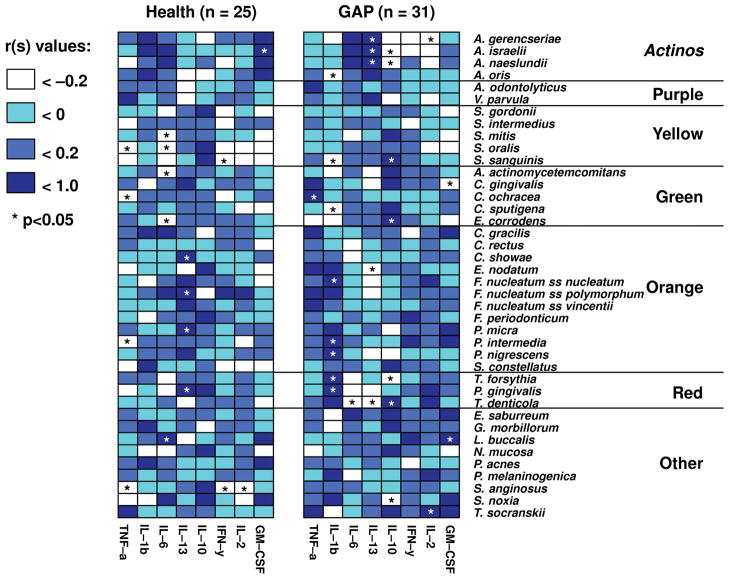
Associations among the mean proportions of each of the 40 subgingival species and the percentage of each GCF cytokine were examined in each group separately. Figure 2 demonstrates that these associations differed between the two clinical groups. In generalized aggressive periodontitis subjects, IL-1β presented statistically significantly positive correlations with periodontal pathogens such as T. forsythia (rs = 0.64; p<0.001) and P. gingivalis (rs = 0.54; p<0.01) and with several members of the Orange complex, while there was a statistically significant negative association with Actinomyces oris (formerly Actinomyces naeslundii 2) (rs = −0.49; p<0.01), a health associated species. In the periodontally healthy group there were non-statistically significant positive associations with A. oris, and either negative or weak positive correlations with Red and Orange complex species.
Fig. 2.
Grid-plots of the Spearman rank correlation coefficients among the mean proportions of 8 cytokines and the mean proportions of 40 subgingival microbial species for periodontally healthy (left panel) and generalized aggressive periodontitis subjects (right panel). Mean % DNA probe count of each species and the proportion of each cytokine were computed by averaging the data within each subject, and then averaging across subjects in each group separately. The color code indicates the level of the Spearman rank coefficient. Asterisks indicate significance at p < 0.05.
IL-13 was statistically significantly positively associated with Campylobacter showae (rs = 0.67; p<0.01), Fusobacterium nucleatum ss. polymorphum (rs = 0.45; p<0.05), Parvimonas micra (rs = 0.43; p<0.05), and P. gingivalis (rs = 0.46; p<0.05) in the periodontally healthy subjects. In contrast, in aggressive periodontitis subjects, there were statistically significant positive correlations between IL-13 and health associated Actinomyces species and statistically significant negative correlations with the periodontal pathogens E. nodatum (rs = −0.39; p<0.05) and T. denticola (rs = −0.51; p<0.001).
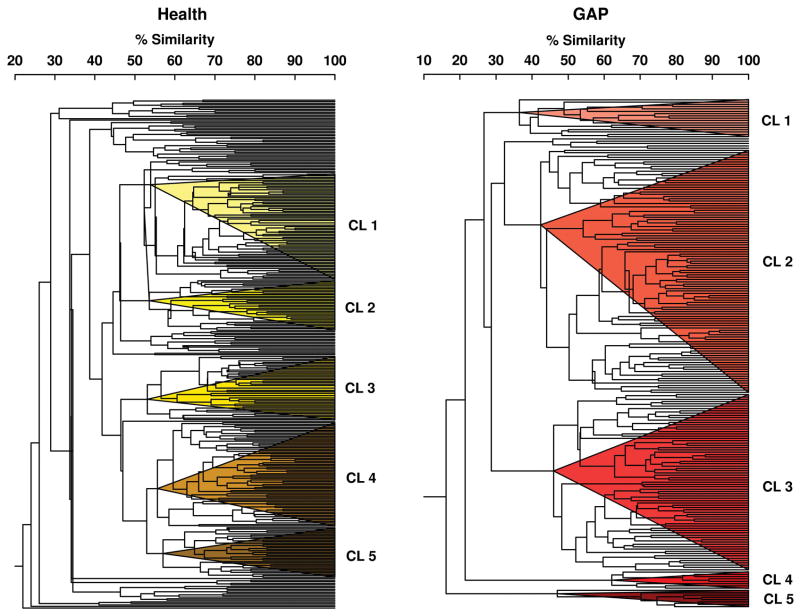
Since the two clinical groups differed in their associations between GCF biomarkers and subgingival bacterial species, sites were grouped in each clinical group separately according to their subgingival microbial composition using cluster analysis. Fig. 3 illustrates the results of the cluster analyses of mean microbial counts of 40 subgingival species in subgingival plaque samples from 330 sites from 25 periodontally healthy subjects (left panel) and 221 sites from 31 aggressive periodontitis subjects (right panel). The colored triangles within the dendrograms indicate the 5 clusters of sites that were formed at > 53% similarity for the periodontally healthy subjects and > 33% similarity for the aggressive periodontitis group.
Fig. 3.
Dendrograms of cluster analyses of mean microbial counts of 40 subgingival species in subgingival plaque samples from 330 sites from 21 periodontally healthy subjects (left panel) and 221 sites from 31 generalized aggressive periodontitis subjects (right panel). The counts of 40 bacterial species were determined at each of up o 14 sites in each subject. The counts of the 40 taxa were employed in cluster analyses using the chord coefficient and an average unweighted linkage sort. The colored triangles within each dendrogram indicate the 5 clusters of sites that were formed at > 53% similarity for the periodontally healthy group and at > 33% similarity for the aggressive periodontitis group.
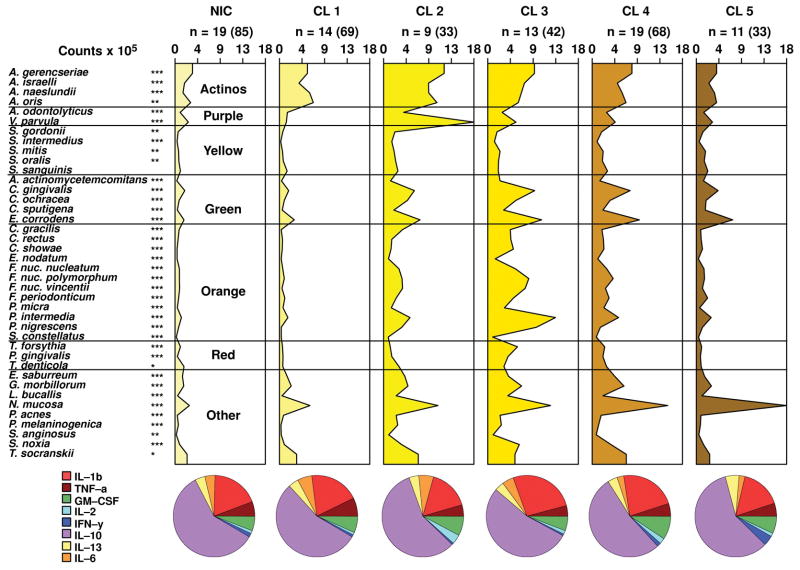
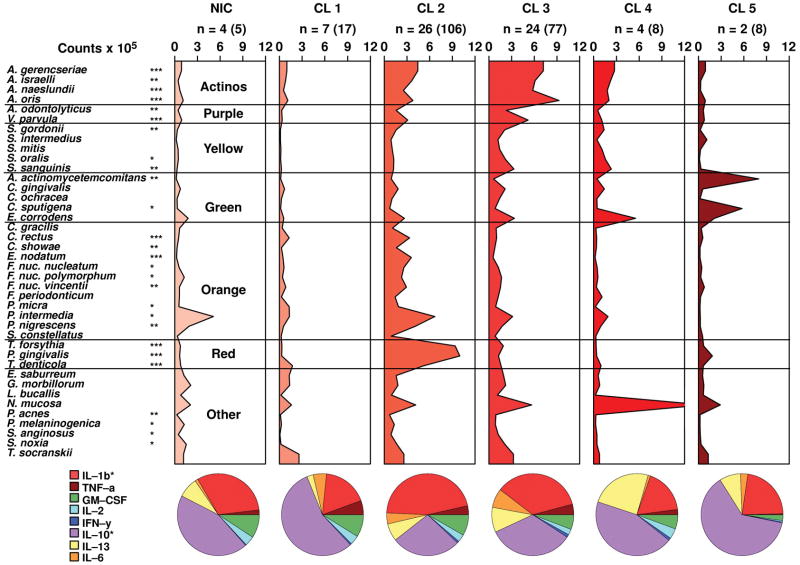
Figs. 4 and 5 present the microbial profiles and cytokine expression profiles for the clusters and not-in-cluster groups defined on the basis of the 40 subgingival bacterial species in the periodontally healthy and generalized aggressive periodontitis groups, respectively. There were statistically significant differences among clusters for the mean levels of 39/40 species in the periodontally healthy group and for the mean levels of 27/40 species in the aggressive periodontitis group. When clinical data were compared among cluster groups the only statistically significant difference found was for the percentage of sites with BOP among clusters in the aggressive periodontitis group (Tables 3 and 4). There were no statistically significant differences in the mean proportion of the cytokines among clusters in the periodontally healthy subjects (Table 3), while the percentage of IL-10 and IL-1β (p<0.05) differed significantly among clusters in the aggressive periodontitis group (Table 4). The levels of GM-CSF were statistically significantly different among clusters in the periodontally healthy group (p<0.01) (Table 3). These differences in levels and proportions of cytokines among clusters should be interpreted with caution because p-values were not adjusted for multiple comparisons in this analysis. The ratio IL-1β/IL-10 was also statistically significantly different among clusters in the aggressive periodontitis group (Table 4).
Fig. 4.
Mean counts x 105 of subgingival taxa in the clusters and not-in-cluster group (NIC) detected in 25 periodontally healthy subjects. The counts of 40 subgingival species were measured at each of up to 14 sites in each subject and employed in a cluster analysis using the chord coefficient and an average unweighted linkage sort. Five clusters were formed at > 53% similarity. Nineteen subjects presented at least one site with a microbial profile that did not fit any cluster. After the clusters were identified, data were averaged within a subject and then averaged across subjects in each cluster group separately. The numbers above each panel represent the number of subjects with at least one site with the microbial profile that defined that cluster. The numbers in parentheses represent the total number of sites in each cluster. The pie diagrams indicate the mean proportion of each cytokine in the 5 cluster groups and in the NIC subjects. Significance of differences for each species among cluster groups was sought using the Kruskal-Wallis test; * p < 0.05, ** p < 0.01, *** p < 0.001 and adjusted for 40 comparisons.(Socransky et al. 1991) The species were ordered and grouped according to the complexes described by Socransky et al. (1998).(Socransky et al. 1998) Significance of differences for the proportion of each cytokine among clusters was also examined using the Kruskal-Wallis test.
Fig. 5.
Mean counts x 105 of subgingival taxa in the clusters and not-in-cluster group (NIC) detected in 31 generalized aggressive periodontitis subjects. The counts of 40 subgingival species were measured at each of up to 14 sites in each subject and employed in a cluster analysis using the chord coefficient and an average unweighted linkage sort. Five clusters were formed at > 33% similarity. Four subjects presented at least one site with a microbial profile that did not fit any cluster. The numbers above each panel represent the number of subjects with at least one site with the microbial profile that defined that cluster. The numbers in parentheses represent the total number of sites in each cluster. The pie charts and the statistical analyses were as described for Fig. 4.
Table 3.
Mean (± SD) clinical and immunological parameters for the not in cluster group (NIC) and 5 microbiological clusters defined in the periodontally healthy group.
| NIC | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | |
|---|---|---|---|---|---|---|
| n subjects (sites): | 19 (85) | 14 (69) | 9 (33) | 13 (42) | 19 (68) | 11 (33) |
| Variable: | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean SD | Mean SD |
| PD (mm) | 2.2 ± 0.3 | 2.6 ± 0.2 | 2.5 ± 0.1 | 2.3 ± 0.9 | 2.4 ± 0.6 | 2.3 ± 0.6 |
| CAL (mm) | 1.6 ± 0.4 | 1.8 ± 0.4 | 1.5 ± 0.7 | 1.7 ± 0.7 | 1.9 ± 0.6 | 1.9 ± 0.4 |
| BOP (%) | 10 ± 18 | 10 ± 20 | 4 ± 10 | 0 ± 0 | 10 ± 10 | 3 ± 6 |
| GM-CSF (pg/ml)** | 1.9 ± 1.3 | 1.6 ± 1.1 | 1.5 ± 2.0 | 2.2 ± 1.9 | 3.0 ± 1.4 | 3.4 ± 1.9 |
| IL-2 (pg/ml) | 0.3 ± 0.1 | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.4 ± 0.2 | 0.8 ± 1.1 | 0.9 ± 1.9 |
| IFN-γ (pg/ml) | 0.9 ± 3.1 | 0.3 ± 0.4 | 0.1 ± 0.1 | 0.2 ± 0.2 | 0.4 ± 0.4 | 1.6 ± 4.2 |
| IL-10 (pg/ml) | 29.0 ± 32.3 | 23.0 ± 21.0 | 18.3 ± 24.7 | 20.3 ± 16.3 | 25.5 ± 23.8 | 30.0 ± 18.5 |
| IL-13 (pg/ml) | 1.4 ± 1.2 | 1.0 ± 0.8 | 0.6 ± 0.6 | 2.1 ± 2.8 | 1.5 ± 0.9 | 4.2 ± 8.9 |
| IL-6 (pg/ml) | 1.2 ± 1.3 | 2.5 ± 6.3 | 1.0 ± 1.3 | 1.4 ± 1.9 | 0.9 ± 0.7 | 1.0 ± 1.3 |
| IL-1β (pg/ml) | 6.5 ± 4.7 | 5.8 ± 3.3 | 4.5 ± 4.5 | 9.4 ± 6.2 | 8.3 ± 5.2 | 8.1 ± 5.5 |
| TNF-α (pg/ml) | 2.0 ± 1.7 | 2.0 ± 1.8 | 0.8 ± 0.7 | 1.6 ± 1.3 | 1.8 ± 0.1 | 2.4 ± 3.1 |
| Total cytokines | 43.1 ± 37.3 | 36.4 ± 27.7 | 27.1 ± 29.3 | 37.5 ± 24.0 | 42.3 ± 24.8 | 51.6 ± 30.6 |
| IL-1/IL-10 | 0.6 ± 0.7 | 0.5 ± 0.5 | 0.4 ± 0.4 | 0.7 ± 0.6 | 0.7 ± 0.6 | 0.4 ± 0.3 |
p<0.001 using the Kruskal-Wallis test.
Table 4.
Mean (± SD) clinical and immunological parameters for the not in cluster group (NIC) and 5 microbiological clusters defined in the aggressive periodontitis group.
| NIC | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | |
|---|---|---|---|---|---|---|
| n subjects (sites): | 4 (5) | 7 (17) | 26 (106) | 24 (77) | 4 (8) | 2 (8) |
| Variable: | Mean ± SD | Mean ± SD | Mean SD | Mean SD | Mean SD | Mean SD |
| PD (mm) | 4.6 ± 1.1 | 3.6 ± 0.1 | 4.6 ± 1.5 | 4.2 ± 1.1 | 4.4 ± 1.8 | 8.9 ± 1.8 |
| CAL (mm) | 4.6 ± 1.1 | 3.5 ± 1.1 | 4.9 ± 1.6 | 4.4 ± 1.2 | 4.4 ± 1.8 | 9.2 ± 2.6 |
| BOP (%)** | 10 ± 0 | 30 ± 40 | 80 ± 30 | 60 ± 40 | 40 ± 50 | 80 ± 20 |
| GM-CSF (pg/ml) | 3.3 ± 2.7 | 3.6 ± 2.8 | 5.1 ± 3.6 | 3.7 ± 3.0 | 7.2 ± 2.3 | 2.2 ± 2.3 |
| IL-2 (pg/ml) | 2.1 ± 3.3 | 1.6 ± 2.6 | 2.2 ± 4.2 | 1.2 ± 2.0 | 3.1 ± 5.3 | 0.4 ± 0.2 |
| IFN-γ (pg/ml) | 0.2 ± 0.3 | 0.3 ± 0.4 | 0.7 ± 1.2 | 0.9 ± 2.8 | 0.9 ± 1.3 | 0.3 ± 0.3 |
| IL-10 (pg/ml) | 20.3 ± 15.2 | 22.6 ± 19 | 16.4 ± 15.2 | 18.8 ± 16.1 | 42.8 ± 34.0 | 53.2 ± 4.2 |
| IL-13 (pg/ml) | 4.6 ± 7.9 | 0.9 ± 0.7 | 9.0 ± 20.4 | 10.4 ± 19.5 | 72.0 ± 127.2 | 11.3 ± 15.8 |
| IL-6 (pg/ml) | 0.5 ± 0.5 | 4.3 ± 9.2 | 2.7 ± 5.3 | 4.6 ± 8.8 | 0.8 ± 0.4 | 2.2 ± 1.1 |
| IL-1β (pg/ml) | 14.4 ± 13.3 | 9.6 ± 10.7 | 27.2 ± 17.3 | 17.7 ± 11.6 | 26.2 ± 17.2 | 18.4 ± 3.2 |
| TNF-α (pg/ml) | 0.8 ± 0.3 | 3.9 ± 5.4 | 2.0 ± 2.0 | 2.3 ± 3.0 | 1.9 ± 1.0 | 0.4 ± 0.0 |
| Total cytokines | 46.1 ± 14.0 | 46.8 ± 40.1 | 65.3 ± 35.8 | 59.4 ± 36.5 | 154.8 ± 127.0 | 88.4 ± 17.4 |
| IL-1/IL-10* | 1.6 ± 1.8 | 0.6 ± 0.6 | 4.1 ± 4.7 | 2.1 ± 2.5 | 1.7 ± 1.62 | 0.5 ± 0.1 |
p<0.05,
p<0.001 using the Kruskal-Wallis test.
The cytokine profiles of the clusters in the periodontally healthy subjects were more homogeneous and presented a similar pattern with IL-10 composing over 50% of the total GCF cytokines, while the expression of IL-1β ranged from 16.2% to 26.3% (Figs. 4 and 5). In contrast, the generalized aggressive periodontitis group presented a more diverse pattern of cytokine expression among the microbial clusters. For instance, the proportions of IL-10 and IL-1β in the aggressive periodontitis group ranged from 27.3% to 62.7% and 17.5% to 45.9%, respectively. In order to confirm that healthy subjects presented less diversity in their cytokine expression, we used the F-test to compare the standard deviations of the percentage of each cytokine between the two clinical groups. The results demonstrated that the generalized aggressive periodontitis subjects presented statistically significantly greater standard deviations for the proportions of IL-13, IL-6 and IL-1β (p<0.001) (data not shown).
Discussion
The main goal of the present study was to examine the interplay between subgingival biofilms and the host immune-inflammatory response on a site-by-site basis in periodontally healthy and generalized aggressive periodontitis subjects. In order to achieve this goal we employed two high-throughput techniques to characterize the bacterial composition of subgingival plaque samples and to determine the GCF cytokine expression profile in a large number of subgingival sites. In addition, we used hierarchical cluster analysis to define groups of sites based on the composition of their subgingival biofilm. Site categories were not based on their clinical parameters since this would limit our ability to identify sites that differed microbiologically. It has been well established that subgingival microbial biofilm samples exhibit a great deal of diversity and may present different compositions even when located on adjacent sites within the same oral cavity (Socransky & Haffajee 2005). One of our goals was to determine if this microbiological diversity would be associated with different kinds of cytokine profiles.
Our data indicated that there was a clear association between the subgingival microbial composition and the local cytokine milieu. It also became apparent that there is less diversity in the profile of cytokine expression in periodontal health, compared to aggressive periodontitis. Mean cytokine expression profiles were quite similar for 6 completely distinct microbial profiles identified in periodontally healthy subjects. In fact, there were no statistically significant differences among the proportions of any of the 8 cytokines measured for the microbial clusters in the healthy group. Conversely, the cytokine profiles associated with microbial clusters defined in the generalized aggressive periodontitis subjects differed considerably, with differences being statistically significant for the proportions of IL-1β and IL-10. The statistically significantly higher standard deviations for the proportions of IL-13, IL-6 and IL-1β in aggressive periodontitis compared to periodontally healthy subjects provided added evidence of the greater diversity of patterns of cytokine expression in the generalized aggressive periodontitis group. This observation implies that although there is a great deal of variety in the subgingival microbial composition in health, the host seems to respond to each type of microbial challenge in a similar fashion. Based on these observations, we hypothesize that these microbiotas were all commensal. In this context, the host does not need to release specific cytokine profiles tailored to the response to the specific challenge. In support of this interpretation, it has been suggested that the immune system may recognize certain bacterial types as non-threatening and choose not to activate a clearance response, permitting the colonization of host-compatible species (Dixon et al. 2004).
When examining the different cytokine expression profiles associated with microbial clusters in the aggressive periodontitis group, cluster 1 presented a GCF cytokine milieu quite similar to the cytokine profiles associated with periodontally healthy subjects, with IL-10 accounting for over 50% of its cytokines. This cluster presented low levels of most pathogens in a microbial profile more typical of healthy sites. Clinically, these sites were also “healthier” than the sites in the other clusters in the aggressive periodontitis group, with a mean PD of 3.6 mm and a mean CAL of 3.5 mm.
Clusters 2, 3 and 4 illustrate the advantage of defining site categories based on their microbial profile for the investigation of host-microbial interactions. These 3 clusters presented clearly distinct microbiotas but rather similar clinical characteristics with mean PD of 4.6, 4.2 and 4.4 mm for clusters 2, 3 and 4, respectively. However, the clusters differed considerably in their cytokine profiles. Cluster 2 presented a microbial profile typically associated with chronic periodontitis, with high proportions of Red and Orange complex species. This microbiota was accompanied by a pro-inflammatory cytokine profile with the highest IL-1β/IL-10 ratio (4.1) of all clusters. Cluster 3 presented a somewhat less pathogenic microbiota, with higher levels and proportions of health-associated species of Actinomyces and members of the Purple, Yellow and Green complexes. This microbial profile was associated with a lower proportion of IL-1β and a higher proportion of IL-10, resulting in an IL-1β/IL-10 ratio of 2.1. In addition, IL-13 was also somewhat elevated in cluster 3 compared to cluster 2 (9.9% vs. 6.9%). In contrast, cluster 4 had the least pathogenic microbial profile, with low levels of microorganisms, particularly members of the Red complex, and high levels of N. mucosa, a species more associated with health. This cluster presented the lowest IL-1β/IL-10 ratio among the 3 clusters (1.7) and the highest proportion of IL-13 (24.3%) of all clusters. Our findings suggest that IL-10 is associated with periodontal health, with its levels and proportions elevated in periodontally healthy subjects and statistically significantly lower IL-1β/IL-10 ratios in this group, compared to aggressive periodontitis subjects. In fact, logistic regression analysis of microbiological and immunological parameters associated with the diagnosis of generalized aggressive periodontitis, revealed that the ratio IL-1β/IL-10 presented an odds ratio of 7.2 with a 95% confidence interval of 2.1 – 24.8 (p<0.001) (data not shown). Taken together, these data reinforce the previously reported role of IL-10 as a regulator of inflammation and alveolar bone loss in periodontal diseases (Garlet et al. 2004, Sasaki et al. 2004, Bozkurt et al. 2006).
Cluster 5 of the aggressive periodontitis group presented a very high proportion of IL-10 (62.7) and a very low IL-1β/IL-10 ratio (0.5). Interestingly, the most diseased sites were in this cluster, with mean PD and CAL of 8.9 and 9.2 mm, respectively. These sites also presented a very distinct microbial profile with overall low levels of microorganisms and very high levels of Aggregatibacter actinomycetemcomitans and Capnocytophaga sputigena. This microbiota would fit the classic description of the biofilm associated with localized forms of aggressive periodontitis (Yang et al. 2004, Fine et al. 2007, Haubek et al. 2002). The high levels and proportions of IL-10 associated with this microbial cluster, present in very deep pockets, is somewhat puzzling. There are several possible explanations for this finding. First, this cluster was associated with very high levels of total cytokines. Therefore, despite a low IL-1β/IL-10 ratio, these sites still expressed relatively high mean concentrations of the pro-inflammatory IL-1β (18.4 pg/ml), compared to the mean for healthy subjects (7.0 pg/ml). It is also possible that IL-10 was inactivated in these sites, through microbial mechanisms. It has been demonstrated, for instance, that an antigen of A. actinomycetemcomitans may bind the IL-10 receptor, potentially functioning as an antagonist (Kato & Okuda 2001). In this situation, despite its high concentration, IL-10 is ineffective in counteracting the pro-inflammatory effects of IL-1β. Alternatively, since it is not possible to determine the level of activity of periodontal disease of the sites sampled, it is conceivable that these sites were sampled at a moment when the inflammatory reaction was under control and the sites were in a state of relative quiescence.
In summary, our data support the notion that subgingival biofilms of different microbial compositions are associated with distinct patterns of GCF cytokine expression. Aggressive periodontitis subjects were characterized by a higher IL-1β/IL-10 ratio, compared to periodontally healthy subjects, suggesting that an imbalance between pro- and anti-inflammatory cytokines is associated with the onset and progression of this disease. The expression of a high IL-1β/IL-10 ratio in GCF was accompanied by high levels and proportions of periodontal pathogens of the Red and Orange complexes. Interleukin-10 might be involved in controlling the inflammatory process at periodontally healthy sites.
The present manuscript was an initial attempt to examine the complex interplay between the subgingival microbiota and the host response. It was revealing in indicating that the interactions appeared to be different in subjects that were periodontally healthy when compared with subjects with aggressive periodontitis.
Acknowledgments
The authors would like to express their appreciation to Dr. Alpdogan Kantarci at the Boston University Goldman School of Dental Medicine for his invaluable support with the Luminex technique. This work was supported in part by research grants DE-016700 and T32 DE-07327 from the National Institute of Dental and Craniofacial Research and grant # 4137-07-8 from the Coordenação de Aperfeicoamento de Pessoal de Nível Superior (CAPES), Brazil.
References
- Albandar JM, Kingman A, Lamster IB. Crevicular fluid level of beta-glucuronidase in relation to clinical periodontal parameters and putative periodontal pathogens in early-onset periodontitis. J Clin Periodontol. 1998;25:630–639. doi: 10.1111/j.1600-051x.1998.tb02499.x. [DOI] [PubMed] [Google Scholar]
- Apatzidou DA, Riggio MP, Kinane DF. Impact of smoking on the clinical, microbiological and immunological parameters of adult patients with periodontitis. J Clin Periodontol. 2005;32:973–983. doi: 10.1111/j.1600-051X.2005.00788.x. [DOI] [PubMed] [Google Scholar]
- Bozkurt FY, Yetkin AZ, Berker E, Tepe E, Akkus S. Anti-inflammatory cytokines in gingival crevicular fluid in patients with periodontitis and rheumatoid arthritis: a preliminary report. Cytokine. 2006;35:180–185. doi: 10.1016/j.cyto.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Chapple IL, Landini G, Griffiths GS, Patel NC, Ward RS. Calibration of the Periotron 8000 and 6000 by polynomial regression. J Periodontal Res. 1999;34:79–86. doi: 10.1111/j.1600-0765.1999.tb02226.x. [DOI] [PubMed] [Google Scholar]
- Darveau RP, Belton CM, Reife RA, Lamont RJ. Local chemokine paralysis, a novel pathogenic mechanism for Porphyromonas gingivalis. Infect Immun. 1998;66:1660–1665. doi: 10.1128/iai.66.4.1660-1665.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Historical insights into cytokines. Eur J Immunol. 2007;37(Suppl 1):S34–S45. doi: 10.1002/eji.200737772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DR, Bainbridge BW, Darveau RP. Modulation of the innate immune response within the periodontium. Periodontol 2000. 2004;35:53–74. doi: 10.1111/j.0906-6713.2004.003556.x. [DOI] [PubMed] [Google Scholar]
- Dongari-Bagtzoglou AI, Ebersole JL. Production of inflammatory mediators and cytokines by human gingival fibroblasts following bacterial challenge. J Periodontal Res. 1996;31:90–98. doi: 10.1111/j.1600-0765.1996.tb00469.x. [DOI] [PubMed] [Google Scholar]
- Fine DH, Markowitz K, Furgang D, Fairlie K, Ferrandiz J, Nasri C, McKiernan M, Gunsolley J. Aggregatibacter actinomycetemcomitans and its relationship to initiation of localized aggressive periodontitis: longitudinal cohort study of initially healthy adolescents. J Clin Microbiol. 2007;45:3859–3869. doi: 10.1128/JCM.00653-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlet GP, Martins W, Jr, Fonseca BA, Ferreira BR, Silva JS. Matrix metalloproteinases, their physiological inhibitors and osteoclast factors are differentially regulated by the cytokine profile in human periodontal disease. J Clin Periodontol. 2004;31:671–679. doi: 10.1111/j.1600-051X.2004.00545.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Mans JJ, Mao S, Lopez MC, Baker HV, Handfield M, Lamont RJ. Gingival epithelial cell transcriptional responses to commensal and opportunistic oral microbial species. Infect Immun. 2007;75:2540–2547. doi: 10.1128/IAI.01957-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubek D, Ennibi OK, Abdellaoui L, Benzarti N, Poulsen S. Attachment loss in Moroccan early onset periodontitis patients and infection with the JP2-type of Actinobacillus actinomycetemcomitans. J Clin Periodontol. 2002;29:657–660. doi: 10.1034/j.1600-051x.2002.290711.x. [DOI] [PubMed] [Google Scholar]
- Jin L, Soder B, Corbet EF. Interleukin-8 and granulocyte elastase in gingival crevicular fluid in relation to periodontopathogens in untreated adult periodontitis. J Periodontol. 2000;71:929–939. doi: 10.1902/jop.2000.71.6.929. [DOI] [PubMed] [Google Scholar]
- Kato T, Okuda K. Actinobacillus actinomycetemcomitans possesses an antigen binding to anti-human IL-10 antibody. FEMS Microbiol Lett. 2001;204:293–297. doi: 10.1111/j.1574-6968.2001.tb10900.x. [DOI] [PubMed] [Google Scholar]
- Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 1997;14:33–53. doi: 10.1111/j.1600-0757.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Kumar PS, Griffen AL, Moeschberger ML, Leys EJ. Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J Clin Microbiol. 2005;43:3944–3955. doi: 10.1128/JCM.43.8.3944-3955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar PS, Leys EJ, Bryk JM, Martinez FJ, Moeschberger ML, Griffen AL. Changes in periodontal health status are associated with bacterial community shifts as assessed by quantitative 16S cloning and sequencing. J Clin Microbiol. 2006;44:3665–3673. doi: 10.1128/JCM.00317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Okamatsu Y, Kawai T, Kent R, Taubman M, Stashenko P. The interleukin-10 knockout mouse is highly susceptible to Porphyromonas gingivalis-induced alveolar bone loss. J Periodontal Res. 2004;39:432–441. doi: 10.1111/j.1600-0765.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- Sneath PHA, Sokal RR. Numerical taxonomy. The principles and practice of numerical classification. San Francisco: WH Freeman & Co; 1973. [Google Scholar]
- Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005;38:135–187. doi: 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Smith C, Dibart S. Relation of counts of microbial species to clinical status at the sampled site. J Clin Periodontol. 1991;18:766–775. doi: 10.1111/j.1600-051x.1991.tb00070.x. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Smith C, Martin L, Haffajee JA, Uzel NG, Goodson JM. Use of checkerboard DNA-DNA hybridization to study complex microbial ecosystems. Oral Microbiol Immunol. 2004;19:352–362. doi: 10.1111/j.1399-302x.2004.00168.x. [DOI] [PubMed] [Google Scholar]
- Vernal R, Leon R, Herrera D, Garcia-Sanz JA, Silva &, Sanz M. Variability in the response of human dendritic cells stimulated with Porphyromonas gingivalis or Aggregatibacter actinomycetemcomitans. J Periodontal Res. 2008;43:689–697. doi: 10.1111/j.1600-0765.2007.01073.x. [DOI] [PubMed] [Google Scholar]
- Yang HW, Asikainen S, Dogan B, Suda R, Lai CH. Relationship of Actinobacillus actinomycetemcomitans serotype b to aggressive periodontitis: frequency in pure cultured isolates. J Periodontol. 2004;75:592–599. doi: 10.1902/jop.2004.75.4.592. [DOI] [PubMed] [Google Scholar]