Abstract
Classic cadherins are synaptic adhesion proteins that have been implicated in synapse formation and targeting. Brief inactivation of classic cadherin function in young neurons appears to abrogate synapse formation when examined acutely. It remains unknown whether such abrogation is unique to young neurons, whether it occurs by stalling neuronal maturation or by directly interfering with the process of synapse assembly, and whether synapse targeting is altered. Here we asked whether sustained pan-cadherin blockade would prevent or alter the progression of axonal and dendritic outgrowth, synaptogenesis and the stereotypic distribution of excitatory and inhibitory synapses on cultured hippocampal neurons. While pre- and postsynaptic cadherins are required for synapse assembly in young neurons, we find that in neurons older than 10 days, classic cadherins are entirely dispensable for joining and aligning presynaptic vesicle clusters with molecular markers of the postsynaptic density. Further, we find the proportion and relative distributions of excitatory and inhibitory terminals on single neurons is not altered. However, synapse formation on neurons in which cadherin function is blocked are smaller; such synapses exhibit decreased synaptic vesicle recycling and a decreased frequency of spontaneous EPSCs. Moreover, such synapses fail to acquire resistance to F-actin depolymerization, a hallmark of mature, stable contacts. These data provide new evidence that cadherins are required to promote synapse stabilization and structural and functional maturation, but dispensable for the correct subcellular distribution of excitatory and inhibitory synapses.
Introduction
Mature synapses are thought to arise from several temporally and molecularly distinct stages of assembly that culminate in stable junctions. An important component of the process is the segregation of excitatory and inhibitory synapses to different regions of the somatodendritic domain (Blackstad and Flood, 1963, Andersen et al., 1966). Classic cadherins are synaptic adhesion proteins that have been implicated in initial stages of synapse formation based on brief periods of overexpression of dominant-negative mutants in young neurons (Togashi et al., 2002). Differential localization of cadherins implicates them in synapse targeting as well (Arndt and Redies, 1996, Fannon and Colman, 1996, Suzuki et al., 1997, Bekirov et al., 2002, Gil et al., 2002). It is an open question whether the actions of classic cadherins are required initially for synapse assembly in neurons of any age, whether their actions on assembly are exerted presynaptically, postsynaptically, or trans-synaptically and whether cadherins coordinate the subcellular localization of excitatory and inhibitory synapses.
Classic cadherins comprise a subfamily of the cadherin family of adhesion proteins whose members are suspected to be functionally similar (Tepass et al., 2000). They all engage in strong, calcium-dependent, homophilic binding interactions, and in epithelial cells they are the principal means for actin recruitment to adherens junctions by virtue of intracellular domain interactions with β- and α-catenin(Takeichi, 1991). Evidence consistent with the idea that cadherins are important for establishing synaptic junctions include the observations that cadherins and catenins are among the first proteins found concentrated at developing synapses (Benson and Tanaka, 1998, Huntley and Benson, 1999), and that presynaptic terminals fail to form on young neurons that express briefly a dominant negative N-cadherin mutant lacking the extracellular domain (NcadΔE) (Togashi et al., 2002). Furthermore, in young neurons, F-actin linked adhesion is critical for stabilizing young synapses (Zhang and Benson, 2001). These data have been broadly interpreted in form of the idea that classic cadherin adhesion across the synaptic cleft is required generally for synapse assembly (Fannon and Colman, 1996, Uchida et al., 1996). Nevertheless, a different view is emerging based on results of experiments carried out on more mature neurons. Transient introduction of NcadΔE into neurons after synapses have formed does not lead to synapse disassembly, but rather produces spikey-shaped dendritic spines (Togashi et al., 2002), similar to the spine morphology observed in mature neurons following actin depolymerization (Allison et al., 1998, Zhang and Benson, 2001), and in neurons cultured from mouse mutants lacking α-N-catenin (Abe et al., 2004). Together, these results suggest that the canonical classic cadherin interaction between the cadherin intracellular domain, β-catenin and α-catenin, and F-actin is critical for the generation of normal spine morphology, but given that synapses can form in the α-N-catenin mutant, is dispensable for initial stages of synapse assembly (Park et al., 2002, Togashi et al., 2002). Selective deletion of β-catenin following synaptogenesis decreases presynaptic vesicle reserve pool size in vivo and alters the shape of presynaptic vesicle clusters in culture (Bamji et al., 2003). Collectively, results from the manipulation of α- and β-catenins has been used to indirectly support the idea that cadherins are not required for synapse development and that cadherin action at synapses is principally modulatory (Bamji et al., 2003; Abe et al., 2004). However, it is important to note that in both α-N-catenin and β-catenin mouse mutants, N-cadherin remains appropriately localized to synapses (Togashi et al., 2002; Bamji et al, 2003), most likely by virtue of its interaction with a number of additional intracellular binding partners including δ-catenin, p120-catenin, presenilin and PTPμ each of which has been localized to synapses and some of which have links to the F-actin cytoskeleton (Yap et al., 1997, Brady-Kalnay et al., 1998, Georgakopoulos et al., 1999, Hirano et al., 2003, Martinez et al., 2003). Thus, it remains an open question whether cadherins are essential components for synapse assembly, being required in all neurons no matter what their age, or whether cadherin contributions to synapses are temporally regulated, being used for discrete purposes at different stages of either neural or synapse differentiation.
Recognition between pre- and postsynaptic elements is suspected to be a regulated stage of synapse assembly (Vaughn, 1989, Benson et al., 2001). While individual neurons express several cadherins simultaneously, particular cadherins tend to predominate within interconnected groups of neurons (Arndt and Redies, 1996, Suzuki et al., 1997, Bekirov et al., 2002, Gil et al., 2002) leading to the widely touted notion that adhesion between like-cadherins may be important for synapse recognition (Fannon and Colman, 1996, Serafini, 1999, Shapiro and Colman, 1999). In support of this idea, N-cadherin is important for generating layer specific connections (Inoue and Sanes, 1997, Poskanzer et al., 2003), N- and E-cadherin parse to different dendritic domains in hippocampus (Fannon and Colman, 1996), and N-cadherin becomes concentrated solely at excitatory synapses in several brain areas (Benson and Tanaka, 1998). Such specificity can be considered at many levels, one being that of subcellular position. For example, in adult hippocampus nearly all synapses on cell bodies are inhibitory while spine synapses are all excitatory (Blackstad and Flood, 1963, Andersen et al., 1966), a distribution of inputs that profoundly affects the firing behavior of individual neurons (Harris et al., 2002, Freund, 2003). This is a property that is at least partly intrinsic to hippocampal neurons in that when they are dissociated and allowed to form synapses in culture, inhibitory synapses predominate on cell somata while excitatory synapses predominate more distally (Benson and Cohen, 1996).
Here we have examined directly the temporal requirements for classic cadherins in synapse assembly and whether cadherins generate the ordered distribution of excitatory and inhibitory synapses upon individual neurons. Our data show there are maturationally distinct demands for cadherins at synapses. Cadherin function is required for the earliest stages of synapse assembly in young neurons, but in older neurons compensatory or alternative mechanisms suffice. Cadherins are also dispensable for the precise targeting of excitatory and inhibitory synaptic terminals to different regions of the somatodendritic domain. Beyond initial assembly, classic cadherin functions are required to generate synaptic complexes having normal levels of synaptic vesicle recycling and spontaneous neurotransmitter release, as well as for synapses to progress to a stage of maturation in which they become resistant to disassembly by actin depolymerizing reagents.
Materials and Methods
Hippocampal culture
Hippocampal neurons were cultured from embryonic day 18 Sprague-Dawley rats, using the methods described by Goslin and Banker, (Goslin and Banker, 1991), and plated at a density of 7,200 cells/cm2, on poly-L-lysine and laminin-coated coverslips unless indicated otherwise in the text. Neurons were inverted over a confluent layer of astrocytes and maintained in Neurobasal media (Invitrogen, Carlsbad, CA) supplemented with 10% B-27 (Invitrogen).
Transfections
Hippocampal neurons were transfected at times indicated in text using Effectene reagent (Qiagen, Valencia, CA) or Lipofectamine 2000 (Invitrogen) and 0.5 µg DNA for 2–5 hours at 37° C in 1 ml Neurobasal/B27, or by use of the Nucleoporator device (Amaxa, Gaithersberg, MD) at the time of plating. L cells were transfected using calcium phosphate-mediated gene transfer (Chen and Okayama, 1987).
Constructs
A mouse NcadΔE was derived from full length N-cadherin by deleting all of EC1–EC4 and most of EC5 and adding 6 myc tags at the C-terminus of the cytoplasmic domain and inserting into pCXN2, a derivative of pCAGGS which uses as an actin promoter. A similar Xenopus-based construct was obtained from Dr. C. Holt (Riehl et al., 1996) and then subcloned into pCXN2. Both mouse and Xenopus constructs inhibited adhesion in N-cadherin expressing mouse L cells, but the Xenopus construct gave greater signal to noise and was thus used more extensively in the study. Control vectors expressed the mouse N-cadherin pro-sequence directly ligated to 6 myc tags in frame (Shan et al., 2000), N-cadherin-GFP or pEGFP-N1 (Clontech, Palo Alto, CA). For electrophysiology and recycling studies, myc-tagged constructs were cotransfected with pEGFP-N1. Cotransfection efficiency in neurons for these plasmids was determined to be ~90%.
Antibodies
Polyclonal GFP antibody was obtained from Chemicon (Temecula, CA) and a monoclonal from Abcam (Cambridge, UK). Monoclonal mouse anti-myc antibody was a gift from Dr. Steve Salton, Mount Sinai Medical Center, New York, NY. A chicken anti-c-myc polyclonal antibody was purchased from Molecular Probes (Eugene, OR). Synaptic vesicle clusters were labeled using either a synaptophysin polyclonal antibody from Zymed Laboratories Inc. (San Francisco, CA), or monoclonals recognizing SV2 or GAD65 obtained from Developmental Studies Hybridoma Bank (DSHB), the University of Iowa (Buckley and Kelly, 1985). Postsynaptic sites were labeled with a mouse monoclonal anti-PSD-95 (Upstate Biotechnology (Lake Placid, NY), rabbit anti-proSAP which was kindly provided by Dr. C. C. Garner (Boeckers et al., 1999) or a mouse monoclonal anti-gephyrin(Alexis; Carlsbad, CA). Mouse monoclonals anti-L1 (ASCS4) was obtained from DSHB, monoclonal MAP2 antibody (AP14), and rabbit polyclonal β-catenin were gifts from Dr. E. Torre and Dr. B. Gumbiner, respectively (Binder et al., 1986, McCrea et al., 1993).
Labeling was visualized with either fluorophore conjugated secondary antibodies or biotinylated secondaries followed by avidin-tagged fluorophores. Anti-mouse, anti-rabbit Texas Red antibodies were obtained from Molecular Probes (Eugene, OR), fluorescein anti-mouse, from Vector Laboratories (Burlingame, CA) and fluorescein anti-rabbit, from Cappel (Aurora, Ohio). Biotinylated antibodies were obtained from Vector Laboratories and anti-chicken, from Jackson ImmunoResearch Laboratories, Inc (West Grove, PA). Oregon Green 488 streptavidin was obtained from Molecular Probes, and AMCA avidin D or streptavidin from Vector Labs and Cy5 avidin from Jackson Labs.
Immunocytochemistry and vesicle labeling
Cells were labeled as described previously (Zhang and Benson, 2001) following fixation in 4% paraformaldehyde/4% sucrose solution in phosphate-buffered saline (PBS), pH 7.3, for 20 minutes at 37°C. Subsequently, cells were washed in PBS and permeabilized with .25% Triton X in PBS for 5 minutes, washed, and then blocked in 10% BSA /PBS for 1 hour at 37°C. Cells were incubated in primary antibody diluted in a 1% BSA solution, and incubated overnight at 4°C. The following day, the cells were washed and incubated with appropriate secondary antibodies, then coverslipped in a 20% solution of Elvanol (Du Pont; Wilmington, De) or Mowiol 4–88 (Calbiochem; La Jolla, CA) with 1% Dabco in .2M Tris/HCL, pH 8.5.
FM4-64 uptake and release was carried out as described in Zhang and Benson (2001). Briefly neurons were placed in a chamber (Warner Instruments) perfused with extracellular recording medium (in mM) 124 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 30 Dextrose, 25 HEPES, pH7.3 adjusted to 300 mOsm and exposed to 15µM FM4-64 dye (Molecular Probes) for 45 seconds in high {K+} (same buffer with 60mMKCl, 39mM NaCl), washed for 10 minutes, then imaged using a Nikon Diaphot (see below). Neurons were then exposed to 4 alternating cycles of high {K+} and perfusion media to release the dye, then imaged again.
Microscopy
Analysis of fixed and labeled transfected cultures was performed using either a Zeiss Axiophot Photomicroscope (Carl Zeiss; Thornwood, NY), attached to a SPOT cooled CCD (Diagnostic Instruments), or a Nikon Diaphot (Morell Instrument Co, Inc; Melville, NY) attached to an ORCA ER cooled CCD (Hamamatsu). All images within a single experimental paradigm were captured using the same microscope/camera setup. Images were collected using the 25× and 40× Plan-Neofluar oil objectives on the Zeiss for analysis of axon and dendrite outgrowth and analyzed using Neurolucida (Microbrightfield) and on either the Zeiss or the Nikon at 60–63× or 100× Plan-Apochromat all with N.A. = 1.4 for analyzing synaptic puncta. To determine the number of synaptophysin labeled clusters per unit length of contacting axon, contact regions were defined as lengths of axons showing pixel overlap with dendrites or cell bodies. For this it is important to note that Neurolucida allows tracings to be made through z-axis progressions. Puncta were analyzed post hoc using Metamorph (Universal Imaging) and data were compared using Statview.
Immunoblot
Vectors were transfected into Cos7 cells with Fugene6 (Roche Diagnostics), cultured for 2 days, and harvested with SDS-sample buffer. Samples were run on an 8 % gel, transferred to nitrocellulose, and incubated with anti-myc Ab (OP10; Chemicon) at a dilution of 1:1000. The membrane was visualized with chemiluminescence (Roche).
Electrophysiology
Cultured neurons were transfected as described above with NcadΔE + GFP, myc + GFP or GFP only and allowed to express for several days (see Results), then transferred to a recording chamber, perfused with extracellular recording medium (see above) + 1 µM TTX and 25 µM bicuculline and bubbled with 95% O2/5% CO2, at room temperature. Cells expressing GFP were examined for mEPSCs with the whole-cell recording technique. Recording electrodes (4–5 MΩ) were filled with a solution consisting of cesium gluconate, 100; MgCl2, 5; EGTA, 2; HEPES, 40; Na2ATP, 2; NaGTP, 0.3 (in mM), adjusted to pH 7.35 and sterile-filtered. Data were acquired using an Axopatch 200B amplifier and PClamp software (Axon Instruments). Recordings were filtered at 2 kHz and sampled at 200 µsec. Capacitance and series resistance compensation was performed using the built-in amplifier circuitry.
Results
NcadΔE prohibits classic cadherin-mediated cell-cell interactions, synaptic accumulation of β-catenin and N-cadherin mediated outgrowth
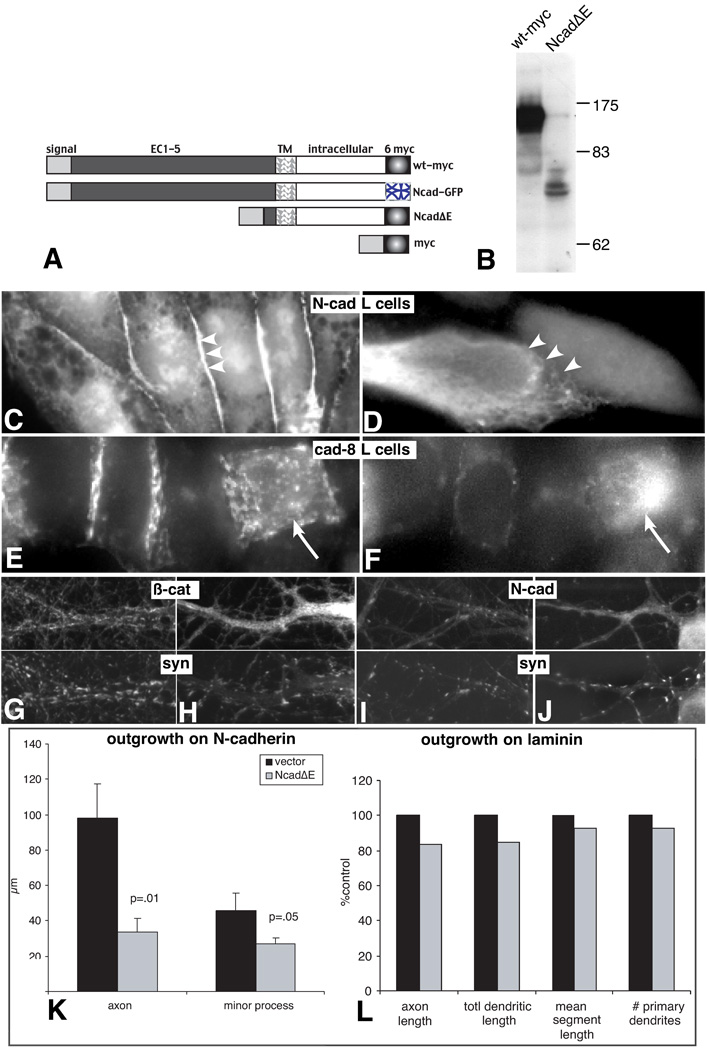
It is well established that overexpression of a membrane targeted, mutant N-cadherin lacking most or all of the extracellular domain effectively blocks N-cadherin mediated cell-cell adhesion by both competing for β-catenin binding at the membrane, which would disconnect cadherins from the actin cytoskeleton, as well as by downregulating cadherin production (Kintner, 1992, Fujimori and Takeichi, 1993, Nieman et al., 1999). We generated a similar construct from mouse N-cadherin, tagged at its C-terminus with myc (NcadΔE)(Fig. 1A, B) and tested its ability to block adhesion by stably transfecting it into mouse L-cells that were stably expressing N-cadherin (1C, D). Consistent with previous work, NcadΔE-expressing N-cadherin-L cells do not cluster β-catenin at sites of intercellular contact (compare 1D to 1C). The high degree of similarity between classic cadherins has led to the tacit view that NcadΔE is likely to block the action of all classic cadherins. To test this explicitly, we expressed NcadΔE in an L-cell line stably expressing cadherin 8, a type II classic cadherin that is also normally expressed in hippocampal neurons (Korematsu and Redies, 1997, Bekirov et al., 2002). In cells expressing NcadΔE, β-catenin accumulates in clusters throughout the cell and no longer congregates at sites of cell-cell contact (1E, F, arrow). These data indicate that NcadΔE acts as a dominant negative for all classic cadherins.
Figure 1. NcadΔE prevents cadherin dependent junction assembly, the synaptic accumulation of β-catenin and N-cadherin, and reduces N-cadherin dependent outgrowth.
Schematic (A) shows DNA constructs used in the study. Western blot (B) shows myc tagged wild type (wt-myc) and NcadΔE constructs expressed in cos 7 cells. A single band is evident for the wt-myc and the doublet at ~77 kD is the appropriate size for the NcadΔE and its partial proteolytic product. Similar results were observed in L-cells. Fluorescence photomicrographs show L-cells stably expressing N-cadherin-GFP (C,D) or cadherin-8 (E,F). In L-cells expressing N-cadherin, β-catenin immunolabeling concentrates at cell-cell junctions, often forming lines (arrowheads). When NcadΔE is stably expressed along with N-cadherin (E), β-catenin no longer clusters at sites of contact (arrowheads) and becomes diffusely localized throughout the cell. L-cells stably expressing cadherin-8 also show concentrations of β-catenin labeling at points of cell-cell contact. In contrast, cells transiently transfected with NcadΔE (arrow and shown by myc-labeling in F) show dispersed clusters of β-catenin and the junctional localization is lost (E). (G–J) 16 day old control (G, I) and transfected with NcadΔE on day 0 (H, J) and immunolabeled for β-catenin and synaptophysin or N-cadherin and synaptophysin as indicated. Expression of NcadΔE completely abrogates the synaptic clustering of β-catenin (H vs G, arrowheads) and greatly diminishes that of N-cadherin (I vs J, arrowheads). (K) Neurons were transfected with NcadΔE or vector control, plated onto a confluent layer of N-cadherin expressing L cells, fixed after 24 hours and immunolabeled for the axonal marker L1 and vector. Both axon and minor process length were significantly reduced (n= 10 neurons for each category). In contrast, neurons grown on laminin and expressing NcadΔE (L) show no significant differences (p > 0.33) in outgrowth relative to myc-expressing control neurons. Axons were labeled with L1 and measured in 4 day old neurons that had been expressing the constructs for 24 hours (n= 15 neurons for each category). Dendrites were labeled with MAP2 and measured in 5 day old neurons that had been expressing the constructs for 48 hours (n= 20 neurons for each category). In all cases, groups were compared using a two-tailed Student’s t-test. Bar = 6.2 µm
To determine the effect of NcadΔE expression on the synaptic accumulation of β-catenin and N-cadherin, hippocampal neurons were transfected on day 0 and at 16 div they were fixed and immunolabeled. In neurons expressing NcadΔE, β-catenin is diffusely distributed throughout the cytoplasm and no longer accumulates at synaptic sites identified by synaptophysin labeling (Fig. 1G vs. 1H). Nearly all synaptic clusters of N-cadherin are also lost (Fig. 1I vs. 1J). These data are distinct from results obtained in neurons lacking α-N-catenin or β-catenin (Togashi et al., 2002; Bamji et al., 2003) and support that NcadΔE expression disrupts cadherin function at the synapse and that this effect is sustained for weeks.
N-cadherin is also an excellent substrate for hippocampal neuron axonal (Blaschuk et al., 1990, Doherty et al., 1992) and dendritic outgrowth (Esch et al., 2000), and is required for thalamocortical axon ingrowth into neocortex (Poskanzer et al., 2003). Consistent with this role, when cultured neurons are transfected at the time of plating with myc-tagged NcadΔE and plated on N-cadherin expressing L-cells, axonal outgrowth is dramatically reduced within 24 hours relative to neurons expressing the myc tag only (Fig. 1K). Minor process length is only slightly reduced, but at this stage of development their growth and differentiation is minimal.
Since any effect on process outgrowth, positive or negative, will ultimately affect the number of axo-dendritic contacts and synapse formation, we tested the effect of NcadΔE on neurons cultured on laminin, an excellent substrate for outgrowth that does not engage cadherins. On laminin, NcadΔE expressing neurons elaborate axons and dendrites (Fig. 1L) and polarize axonal and dendritic markers (L1 and MAP2, respectively; not shown) in a manner indistinguishable from vector expressing control neurons. Control neurons expressing the myc tag and cadherin pro domain were quantitatively indistinguishable from those expressing GFP alone.
Short-term cadherin inactivation in either axons or dendrites disrupts synapse formation in young neurons
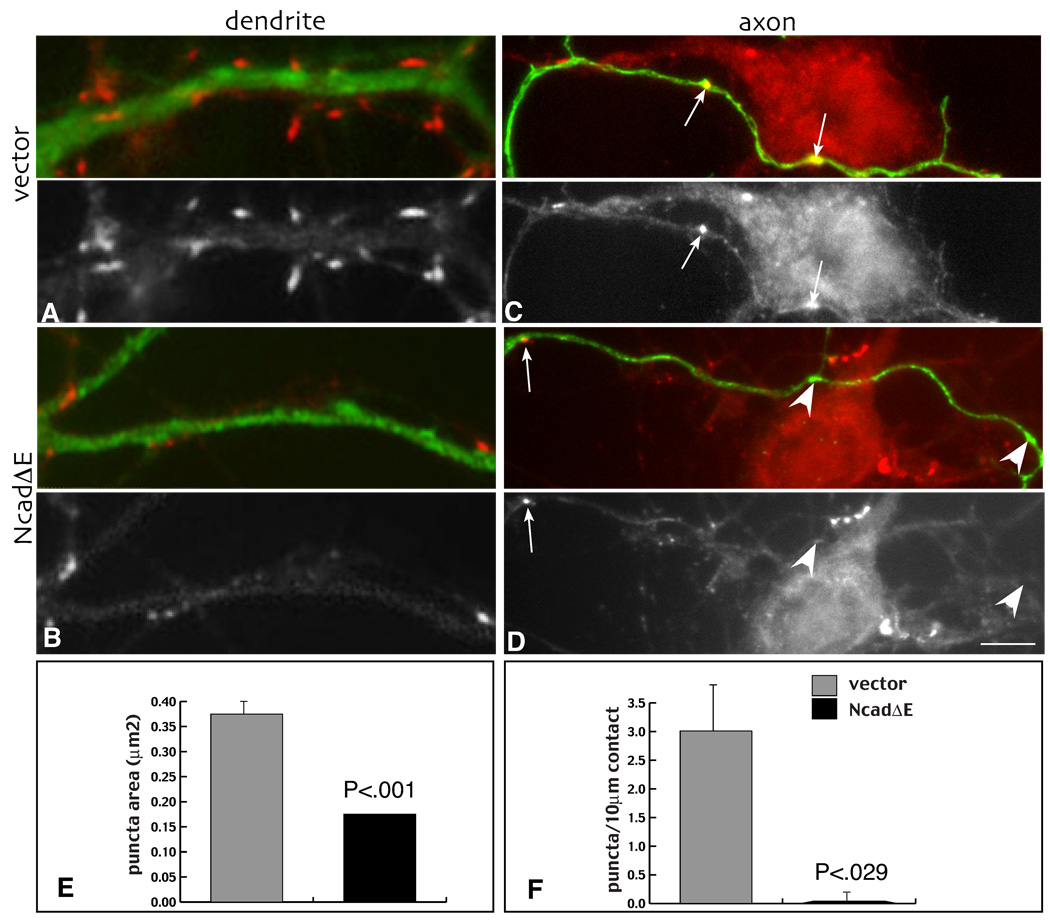
Previous work has demonstrated that the cell bodies and dendrites of young hippocampal neurons (7 days old) expressing NcadΔE receive few to no presynaptic boutons (Togashi et al., 2002). Since cadherins bind predominantly homophilically, the data suggest that formation of presynaptic terminals requires a trans-synaptic interaction between somatodendritic and axonal cadherins. To test this, we asked whether axons expressing NcadΔE were capable of forming boutons on wild type neurons. Four day old neurons were transfected with NcadΔE, myc, GFP or a myc-tagged wild type N-cadherin (Fig. 1A), and after two days of expression, fixed and immunolabeled for synaptophysin and the vector tag (myc or GFP). Axons expressing NcadΔE show an almost complete failure to form bouton-like swellings, and the swellings observed seldom correlate with accumulation of synaptophysin labeled vesicles relative to control neurons (Fig. 2C, D). Consistent with previous work, cell bodies and dendrites expressing NcadΔE (Fig. 2A) receive far fewer synaptophysin-labeled presynaptic terminals (Fig. 2B) (Togashi et al., 2002), and the boutons that form are also significantly smaller (Fig. 2E). Neurons expressing wild type N-cadherin, myc alone or GFP alone, showed no significant differences in the numbers of presynaptic terminals observed.
Figure 2. Transient cadherin blockade exerted either pre- or postsynaptically greatly inhibits presynaptic terminal formation.
Four day old neurons were transfected with vector control (A, C) or NcadΔE (B, D), fixed 48 hours later and immunolabeled for myc (green, upper image in each pair) and synaptophysin (red, upper image in each pair and grayscale, lower image). Relative to vector expressing controls (A), NcadΔE expressing dendrites (B) are contacted by fewer and smaller (E) clusters of synaptophysin. Similarly, NcadΔE expressing axons (D) form few synaptophysin clusters (arrows) relative to vector controls (C), even in relation to axon swellings (arrowheads in D), or when they contact potentially synaptogenic sites (F). Note that neuron contacted by NcadΔE expressing axon is associated with normal looking boutons arising from untransfected axons. Bar = 3.6 µm.
It is possible that NcadΔE expressing axons fail to form boutons because they contact fewer potential targets. To address this, numbers of boutons were counted per unit length of axon contacting cell bodies or dendrites. By this measure there were virtually no presynaptic boutons that formed in NcadΔE expressing axons relative to GFP or myc expressing axons (Fig. 2F). These data indicate that presynaptic vesicle clusters fail to develop over the normal time course in young neurons when cadherins are disrupted either pre- or postsynaptically and support the idea that trans-synaptic cadherin adhesion bi-directionally influences synapse assembly in young neurons.
With maturation, cadherins become dispensable for synapse assembly and alignment, but required for synapse growth
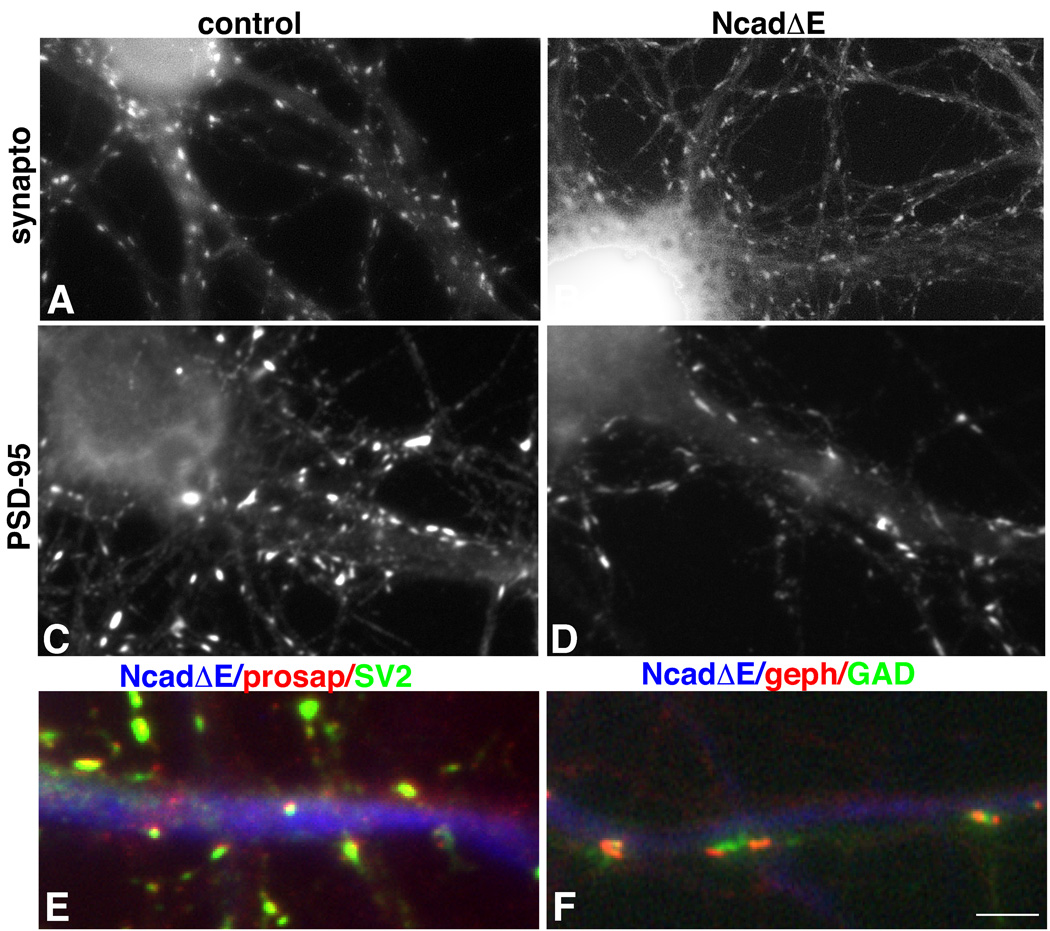
In contrast to results obtained from one week old neurons in culture, two days of NcadΔE expression in older neurons does not lead to an overt loss of presynaptic terminals (Togashi et al., 2002; data not shown). These data indicate that synapses, once formed, no longer require cadherins. It remains an open question whether synapses can eventually form as neurons in the continuous absence of cadherin function. To address this, hippocampal neurons were transfected with NcadΔE or vector controls between 0 and 5 days in culture (prior to the formation of most synapses) and analyzed after at least one week of expression, at stages when synaptogenesis is well under way or largely completed (10–20 div). Strikingly, such long-term NcadΔE-expressing neurons receive numerous synaptophysin labeled presynaptic boutons along their cell bodies and dendrites at densities similar to those observed on neurons expressing vector alone (Fig. 3 A, B) (0.95 ± .1 vs. 0.90 ± .1 puncta/10µm at 10 div; p = 0.7639; n = 40 neurons each category). The extent of inhibitory innervation appears unaffected as the number of GAD labeled clusters is not significantly different (0.59 ±. .06 vs 0.59 ± .08 puncta/10µm at 13 div). Postsynaptic clusters of PSD-95 form at densities comparable to vector expressing control neurons (Fig. 3 C, D) (1.5 ± .2 vs. 1.7 ± .2 puncta/10µm at 12 div, p = 0.548; n = 12 or more neurons each category). The presynaptic vesicle clusters that form on NcadΔE expressing neurons are likely to represent genuine excitatory and inhibitory synapses: SV2 labeled boutons lie in register with clusters of postsynaptic proSAP/Shank (Fig. 3E), a protein concentrated in excitatory postsynaptic densities (Boeckers et al., 1999, Naisbitt et al., 1999), and GAD65-labeled boutons associate with multiple, smaller clusters of postsynaptic gephyrin (Fig. 3F) similar to what has been described in untransfected neurons (Brunig et al., 2002). Thus, despite the near complete abrogation of synaptogenesis we observe in young neurons briefly expressing NcadΔE, the effect is clearly not maintained as neurons mature. These data indicate that classic cadherins are normally major participants in synapse assembly, but that its long term suppression reveals alternative mechanisms that appear to become fully operative following a delay (after 7 days). Interestingly, it takes little time to make up for this delay as normal presynaptic densities are detected within days.
Figure 3. Synapses form in the presence of NcadΔE, but they are smaller.
Neurons were transfected at 4 days in culture and fixed 8 days later. The relative density of both synaptophysin labeled (A and B) and PSD-95 labeled (C, D) clusters is similar between control and NcadΔE expressing neurons. However, synaptophysin puncta are smaller (A vs. B) and PSD95 puncta are lighter in intensity (C vs. D) (see text for values). In both cases, distributions were unimodal. Colocalization of SV2 (green) and proSAP (red) (E) and of GAD65 (green) and gephyrin (red) (F) show that excitatory and inhibitory pre- and postsynaptic clusters that form in NcadΔE expressing neurons are apposed and aligned in a manner similar to that observed in untransfected neurons. Bar = 4.7 µm (A–D); 2.5 µm (E–F).
Sustained cadherin inactivation greatly diminishes synapse size and function
We next asked whether the synapses forming on cadherin blocked neurons were normal. Presynaptic boutons forming on neurons expressing NcadΔE are on average, 25% smaller (0.13 ± .009 µm2 vs. 0.174 µm2± .006, p<.0001; n = 939 boutons or more each category; no significant difference in intensity) and the average intensity of PSD-95 labeled postsynaptic clusters is reduced 5.5 fold compared to vector transfected control neurons (Fig. 3A-D)(189.3 ± 1.5 vs. 34.4 ± 2, p<0.001; n = 324 clusters or more each category; no significant difference in area).
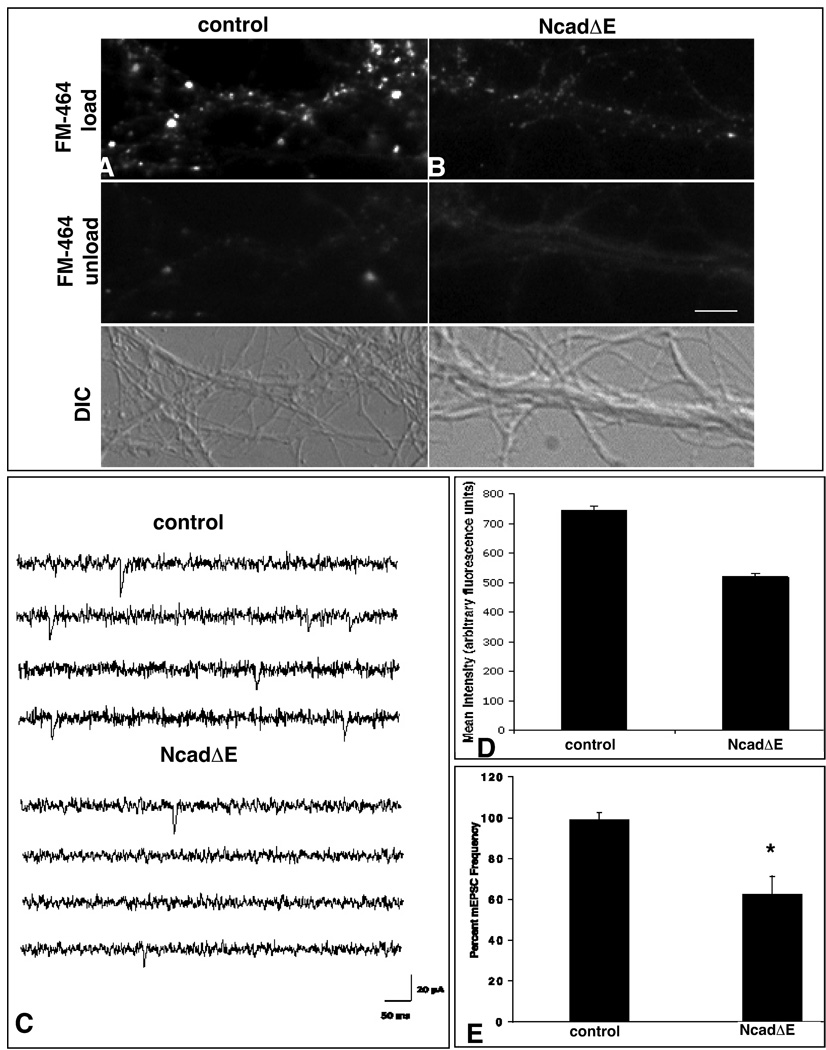
As a first measure of synapse function following sustained expression of NcadΔE, we examined synaptic vesicle recycling using the styryl dye FM4-64. The fluorescence intensity observed following activity-dependent FM4-64-uptake is diminished significantly relative to terminals impinging on control neurons (518.7 ± 14 versus 745.1 ± 11.7; mean arbitrary fluorescence units per terminal, p < 0.0001) (Fig. 4A, B, D). To further characterize the apparent defect in synapse function, we performed whole cell recordings to measure mEPSCs in neurons transfected with NcadΔE and GFP, GFP alone, or untransfected neurons (n = 4–7 neurons per condition). Consistent with a defect in presynaptic vesicle recycling, mEPSC frequencies recorded from NcadΔE expressing cells are significantly lower than the control neurons (1.4±0.3 Hz for control and 0.86±0.2 for NcadΔE respectively, p<0.05) (Fig. 4C, E). The amplitude of the mEPSCs does not differ: At −80 mV, the mean amplitudes are 26±7 pA and 25±10 pA for control and NcadΔE, respectively. mEPSC frequencies depend primarily on the number of synapses and their release probability, so these results indicate that sustained cadherin blockade negatively influences synaptic transmission. These data are consistent with the decrease in size of synaptophysin labeled puncta and the decrease in FM4-64 fluorescence, and together suggest that there is a smaller vesicle pool size that is less efficiently released (Blue and Parnavelas, 1983, Mohrmann et al., 2003).
Figure 4. Synapses that form in the presence of NcadΔE show greatly diminished presynaptic function.
Neurons expressing GFP alone show robust uptake of FM4-64 dye (A) compared to neurons expressing NcadΔE and GFP which show greatly attenuated recycling (B). Quantitative analysis shows this difference is significant (D), n≥95 boutons; 8 neurons each category, *p < 0.0001, t- test. C) Representative electrophysiological recordings show diminished mEPSC frequency from NcadΔE expressing neurons relative to control. E) Quantitative analysis shows mEPSC frequency is decreased by 40% in NcadΔE transfected neurons compared to control neurons (n ≥ 6; *p<0.05; t-test). Frequencies are normalized to the frequency during the control period. Bars = 4.7 µm (A, B) and 20 pA, 50 ms (C).
Cadherin blockade precludes acquisition of F-actin independence at most synapses
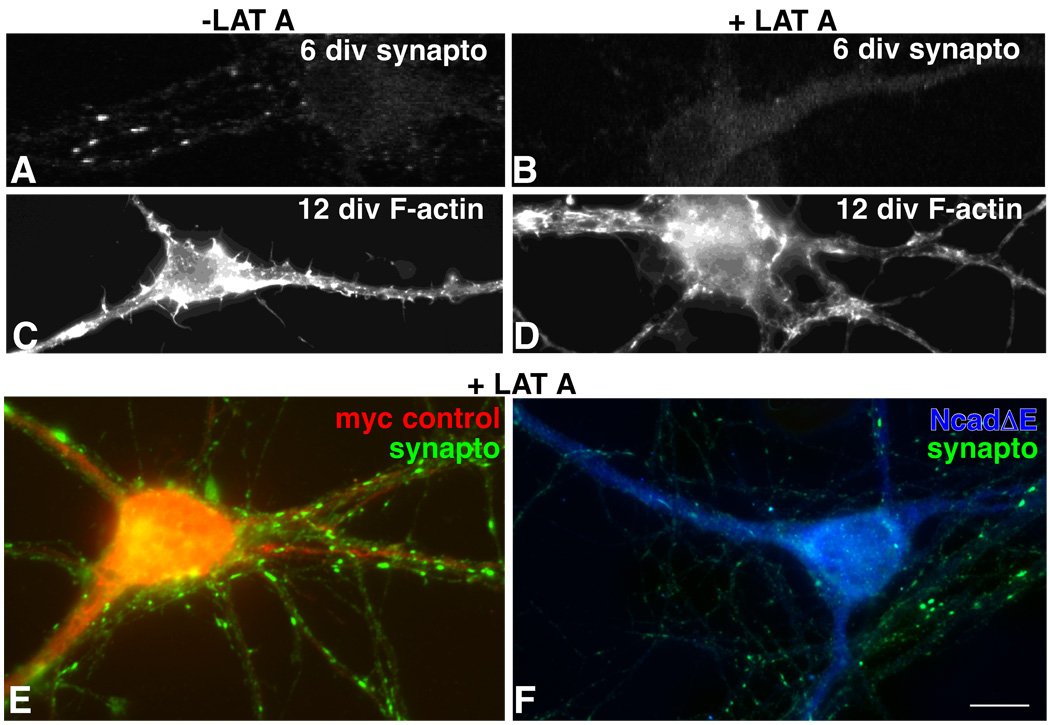
Previous work indicates that immature synapses completely disassemble following actin depolymerization, while mature synapses no longer require an intact actin cytoskeleton to maintain apposition (Zhang and Benson, 2001) (Fig. 5A, B). We asked whether the smaller synaptic terminals that form in neurons having sustained cadherin inactivation would remain susceptible to actin depolymerization, even though chronologically, such synapses would normally have acquired resistance to it. Neurons were transfected with NcadΔE or myc alone on day 4, and on day 12 they were exposed to latrunculin A for 4–6 hours, a time that is adequate for depolymerizing actin in 5 day old neurons, but insufficient (by 20 hours) to completely depolymerize actin in neurons older than 9 days (Allison et al., 1998, Zhang and Benson, 2001) (Fig. 5C, D). Following latrunculin A treatment NcadΔE-expressing neurons showed a 64% reduction in the number of presynaptic terminal clusters relative to control, latrunculin treated neurons (mean = 0.9 puncta/10 µm ± 0.3 vs. 2.6 puncta/10 µm ± 0.5, Students’ t-test, p= .01; n = 23 neurons each category) (Fig. 5). These data indicate that synapses do not become stabilized in neurons lacking cadherin function.
Figure 5. Classic cadherin adhesion is required for synapses to gain F-actin independence.
Six day old neurons following a 5 hour exposure to latrunculin A (LAT A) lose all synaptophysin labeled synaptic vesicle clusters (A vs. B). At 12 days, a similar exposure to latrunculin A leads to partial F-actin depolymerization as indicated by phalloidin staining (C vs. D), but a retention of synaptophysin clusters (E). Neurons in E and F were transfected with myc alone (E, red) or NcadΔE (F, blue) soon after plating and after 10 days were treated with latrunculin A for 5 hours, then fixed and immunolabeled as indicated. While control neurons receive and retain numerous synaptophysin clusters, the soma and dendrites of neurons expressing NcadΔE are nearly bare. Normal labeling of untransfected neighboring dendrites appears in F. Bar = 7 µm (A–D); 4.7 µm (E,F).
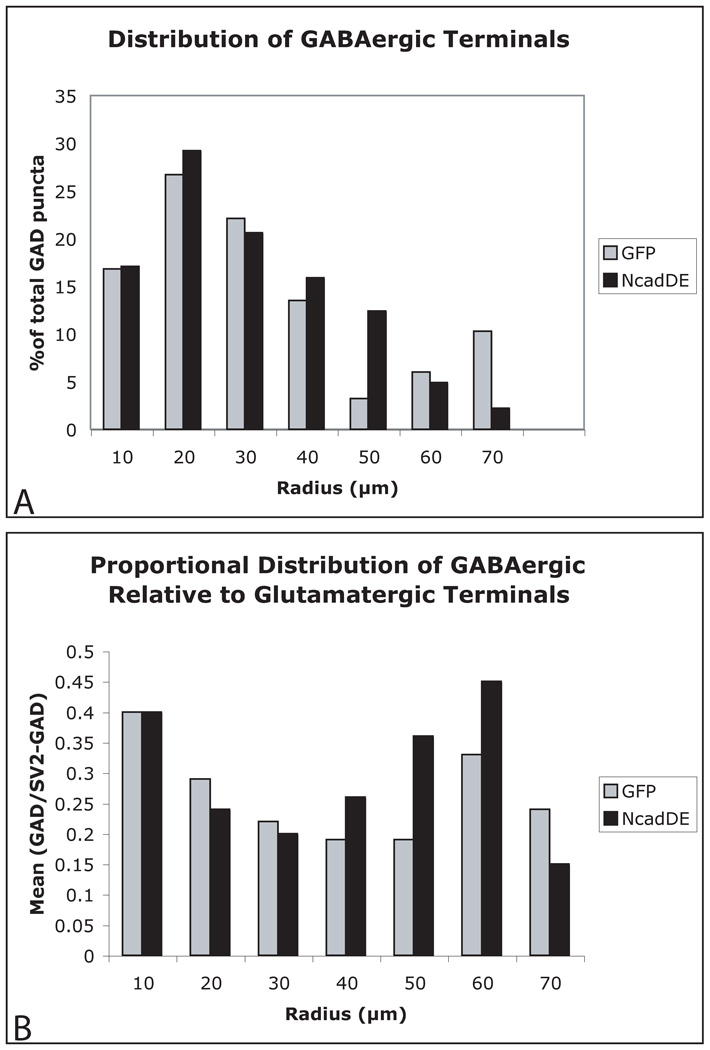
For what purpose might cadherin adhesion be used to trigger synapse differentiation and the acquisition of actin independence? A popular hypothesis is that cadherin adhesion may be required to generate synapse specificity at the level of individual neurons (Fannon and Colman, 1996, Benson and Tanaka, 1998). In hippocampal neurons grown in culture, N-cadherin becomes clustered exclusively at excitatory synapses (which predominate on spines). β-catenin remains clustered at inhibitory synapses (which predominate on cell bodies) suggesting the presence of an alternate cadherin (Benson and Cohen, 1996). We asked whether cadherin blockade alters the proportion and relative distributions of GABAergic and glutamatergic synaptic terminals on single neurons. Terminal locations were plotted on NcadΔE and GFP expressing neurons and data were grouped into areas defined by concentric circles of increasing radii, 10 µm apart beginning at the soma. To our surprise, there were no detectable differences between groups in either the distribution of GABAergic boutons or in the proportion of GABA/nonGABAergic terminals (Fig. 6). These data indicate that classic cadherins do not orchestrate intrinsically driven differences in the spatial distribution or proportion of excitatory and inhibitory synapses.
Figure 6. Cadherins are dispensable for synapse targeting on individual neurons.
In both GFP and NcadΔE transfected neurons, the distribution of GABAergic terminals is skewed proximally (A). The relative proportion of GABAergic to glutamatergic terminals (SV2 minus GAD) is also unchanged (B). A trend suggests there may be a greater proportion of GABAergic innervation distally, but this is not significant (p > .05).
Discussion
Here we report that functional interactions between pre- and postsynaptic classic cadherins are essential for normal synapse growth, for the acquisition of mature presynaptic function and for resistance to disassembly by actin depolymerizing reagents, all hallmarks of mature and stable synapses. While classic cadherins are important contributors to the initiation of synapse assembly, cadherin blockade in young neurons delays rather than prevents synapse formation. Over time, cadherin-based function becomes dispensable for the joining and alignment of presynaptic vesicle clusters with postsynaptic density markers as well as for orchestrating the distribution and proportion of excitatory and inhibitory synapses on individual neurons.
Cadherin function at the synapse
Our analysis shows that actions mediated by the intracellular domain of classic cadherins are required for synapse formation, maturation and stabilization. The requirement for synapse formation is likely to be mediated by trans-synaptic adhesion between cadherins as presynaptic terminals do not form whether NcadΔE is expressed pre- or postsynaptically (Togashi et al, 2002; current results). Much milder presynaptic terminal defects are observed in neurons in which β-catenin has been deleted after synaptogenesis is well under way (Bamji et al., 2003), supporting the idea that the complete dependence of presynaptic bouton formation on cadherins is a transient property unique to neurons younger than a week. Neurons that continue to develop in the presence of NcadΔE form normal numbers of synapses by 10 div. This time period correlates well with the transient dependence of young synaptic junctions on an intact actin cytoskeleton (Zhang and Benson, 2001) as well as with several lines of evidence which indicate that young synapses, while functional, have appreciably different synaptic vesicle release (Brenowitz and Trussell, 2001, Mozhayeva et al., 2002) and postsynaptic response properties (Wu et al., 1996, Zhang and Poo, 2001, Yasuda et al., 2003) compared to mature synapses.
The more sustained requirement observed for cadherins during synapse maturation and stabilization is likely to be conserved evolutionarily in that a targeted loss of Drosophila N-cadherin (the major neuronal cadherin in Drosophila) results in aberrant synapse differentiation in retina (Iwai et al., 2002). The decreased synapse stability we observe in more mature neurons, evidenced by the continued reliance on actin-based adhesion mechanisms, is likely to be due in part to a block in α-N-catenin recruitment. Mouse mutants lacking α-N-catenin develop immature looking dendritic spines that turnover with a 20% greater frequency than wild type controls (Abe et al., 2004). However, our results indicate that more than 60% of the synapses in neurons expressing NcadΔE remain dependent on F-actin based adhesion. The difference in magnitude may be attributed to different approaches, but is more likely due to the fact that in addition to α- and β-catenin, cadherins interact with a broad array of intracellular binding partners. In support of this, cadherin clustering is maintained in synapses in neurons lacking either α-N- or β-catenin (Togashi et al., 2002, Bamji et al., 2003), perhaps via the juxtamembrane binding protein, δ-catenin, which can interact with the actin cytoskeleton independent of α- and β-catenin (Martinez et al., 2003).
Coordinating adhesion and differentiation
That synaptic maturation requires cadherin function suggests that cadherin binding triggers a signaling pathway important for synapse differentiation. β-catenin is a likely focus for cadherin signaling. Its binding to cadherins can be rapidly and negatively regulated by phosphorylation which is associated with decreased adhesion as well as decreased levels in dendritic spines (Murase et al., 2002). β-catenin also binds directly to a variety of synaptic proteins. For example, members of the LAR family of receptor protein tyrosine phosphatases (RPTPs) can bind and potentially dephosphorylate β-catenin (Kypta et al., 1996). This interaction is intriguing in that coordinate actions of LAR-RPTP and liprin α, a binding partner, are important for presynaptic morphogenesis (Zhen and Jin, 1999, Kaufmann et al., 2002) and potentially for postsynaptic differentiation as well (Wyszynski et al., 2002). β-catenin also binds to the scaffolding protein S-SCAM (MAGI-2) (Nishimura et al., 2002) which binds a variety of postsynaptic molecules including neuroligin, GKAP/SAPAP, and NMDA receptors as well as presynaptically localized m-Lin 7 (MALS, Velis) (Perego et al., 2000). Both LAR and S-SCAM suggest means by which strengthened cadherin adhesion and synapse differentiation could be coordinately signaled. Cadherins may also directly effect transcriptional activity in that, like Notch, they can be cleaved by γ-secretase and the resultant intracellular fragment can sequester cytoplasmic β-catenin (Marambaud et al., 2002), an action predicted to negatively regulate β-catenin-mediated transcriptional activation (Sadot et al., 1998, Gottardi and Gumbiner, 2001). Although the downstream targets have yet to be identified, this action outlines a potential mechanism for the self-regulation of cadherin adhesion.
Differences between first forming and maturing synapses
Recent work (Togashi et al., 2002) together with data presented in this study demonstrates that classic cadherin adhesion is required for synapse assembly in young neurons. However, we find that as neurons mature, cadherin adhesion becomes dispensable for the initial stages of synapse assembly. These data indicate that cadherin blockade delays rather than prevents initial synapse formation, and they also underscore the idea that there is more than one way to assemble a CNS synapse. Given the essential nature of synapse formation for the integrity and survival of the organism, this is not surprising. A delay in synaptogenesis is also observed in neurons cultured from mice lacking synapsin I (Chin et al., 1995), but in this case, the synapses that eventually form appear to be normal while those forming during the sustained expression of NadΔE are not. Binding between neurexins and neuroligins or between synCAM and itself or its partners play key roles in synapse assembly (Scheiffele et al., 2000, Biederer et al., 2002, Dean et al., 2003), and it is likely that as neuronal maturation progresses additional cleft proteins become synthesized that can contribute to adhesion and assembly (Ethell and Yamaguchi, 1999, O'Brien et al., 1999). In light of this it will be important to determine whether cadherin adhesion directly triggers the addition of cleft proteins and whether synapses lacking matched cadherins might eventually regress. It will also be important to examine the coordinated or complementary effects of cadherins with activity dependent synapse competition and CaMKII activity which have been implicated in synapse stabilization over a similar time course (Burrone et al., 2002, Fink et al., 2003).
The timing and changes observed in the contribution of cadherins to CNS synapses closely mirrors data obtained for F-actin (Zhang and Benson, 2001; Togashi et al., 2002; present data) and strongly suggests that classic cadherins are major effectors of actin action at synapses. As cadherin adhesion and F-actin become dispensable for maintaining synapse structure (Zhang and Benson, 2001), they both become important for maintaining dendritic spine structure (Allison et al., 1998, Halpain et al., 1998, Togashi et al., 2002, Abe et al., 2004). At the same time cadherin actions through both actin and β-catenin remain important for regulating presynaptic vesicles (Bamji et al, 2003; Morales et al, 2000; present results). While precise links have yet to be made, a role for cadherins and actin in maintaining spine shape and presynaptic integrity correlates well with data showing that cadherin adhesion and F-actin are important for generating and maintaining long-term potentiation (Tang et al., 1998, Kim and Lisman, 1999, Bözdagi et al., 2000, Krucker et al., 2000, Colicos et al., 2001, Huntley et al., 2002).
Acknowledgments
This research was supported by NIH USPHS grants NS37731 and AA12971, and an Irma T. Hirschl Career Scientist Award. We thank Drs. G. W. Huntley, G. Phillips and T. Anderson for their comments on the manuscript and Insoo Kim and Pamela Shah for technical assistance. We thank Dr. S. T. Suzuki at the Institute for Developmental Research in Aichi, Japan for generously providing us with cadherin 8 expressing L-cells and Drs. Yoshinaga Saeki, E. A. Chiocca at Massachusetts General Hospital in Boston, MA for making viral vectors.
References
- Abe K, Chisaka O, Van Roy F, Takeichi M. Stability of dendritic spines and synaptic contacts is controlled by alphaN-catenin. Nat Neurosci. 2004;7:357–363. doi: 10.1038/nn1212. [DOI] [PubMed] [Google Scholar]
- Allison DW, Gelfand VI, Spector I, Craig AM. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J Neurosci. 1998;18:2423–2436. doi: 10.1523/JNEUROSCI.18-07-02423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Blackstad TW, Lömo T. Location and identification of excitatory synapses on hippocampal pyramidal cells. Experimental Brain Research. 1966;1:236–248. doi: 10.1007/BF00234344. [DOI] [PubMed] [Google Scholar]
- Arndt K, Redies C. Restricted expression of R-cadherin by brain nuclei and neural circuits of the developing chicken brain. Journal of Comparative Neurology. 1996;373:373–399. doi: 10.1002/(SICI)1096-9861(19960923)373:3<373::AID-CNE5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Bamji SX, Shimazu K, Kimes N, Huelsken J, Birchmeier W, Lu B, Rieichardt LF. Role of β-catenin in synaptic vesicle localization and presynaptic assembly. Neuron. 2003;40:719–731. doi: 10.1016/s0896-6273(03)00718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekirov IH, Needleman LA, Zhang W, Benson DL. Identification and localization of multiple classic cadherins in developing rat limbic system. Neuroscience. 2002;115:213–227. doi: 10.1016/s0306-4522(02)00375-5. [DOI] [PubMed] [Google Scholar]
- Benson DL, Cohen PA. Activity-independent segregation of excitatory and inhibitory synaptic terminals in cultured hippocampal neurons. Journal of Neuroscience. 1996;16:6424–6432. doi: 10.1523/JNEUROSCI.16-20-06424.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DL, Colman DR, Huntley GW. Molecules, maps and synapse specificity. Nat Rev Neurosci. 2001;2:899–909. doi: 10.1038/35104078. [DOI] [PubMed] [Google Scholar]
- Benson DL, Tanaka H. N-cadherin redistribution during synaptogenesis in hippocampal neurons. J Neurosci. 1998;18:6892–6904. doi: 10.1523/JNEUROSCI.18-17-06892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- Binder LI, Frankfurter A, Rebhun LI. Differential localization of MAP2 and tau in mammalian neurons in situ. Annals of the New York Academy of Sciences. 1986;466:145–167. doi: 10.1111/j.1749-6632.1986.tb38392.x. [DOI] [PubMed] [Google Scholar]
- Blackstad TW, Flood PR. Ultrastructure of hippocampal axo-somatic synapses. Nature. 1963;198:542–543. doi: 10.1038/198542a0. [DOI] [PubMed] [Google Scholar]
- Blaschuk OW, Sullivan R, David S, Pouliot Y. Identification of a cadherin cell adhesion recognition sequence. Developmental Biology. 1990;139:227–229. doi: 10.1016/0012-1606(90)90290-y. [DOI] [PubMed] [Google Scholar]
- Blue ME, Parnavelas JG. The formation and maturation of synapses in the visual cortex of the rat. I. qualitative analysis. Journal of Neurocytology. 1983;12:599–616. doi: 10.1007/BF01181526. [DOI] [PubMed] [Google Scholar]
- Boeckers TM, Kreutz MR, Winter C, Zuschratter W, Smalla KH, Sanmarti-Vila L, Wex H, Langnaese K, Bockmann J, Garner CC, Gundelfinger ED. Proline-rich synapse-associated protein-1/cortactin binding protein 1 (ProSAP1/CortBP1) is a PDZ-domain protein highly enriched in the postsynaptic density. J Neurosci. 1999;19:6506–6518. doi: 10.1523/JNEUROSCI.19-15-06506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bözdagi O, Shan W, Tanaka H, Benson DL, Huntley GW. Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites and required for potentiation. Neuron. 2000;28:245–259. doi: 10.1016/s0896-6273(00)00100-8. [DOI] [PubMed] [Google Scholar]
- Brady-Kalnay SM, Mourton T, Nixon JP, Pietz GE, Kinch M, Chen H, Brackenbury R, Rimm DL, Del Vecchio RL, Tonks NK. Dynamic interaction of PTPmu with multiple cadherins in vivo. J Cell Biol. 1998;141:287–296. doi: 10.1083/jcb.141.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz S, Trussell LO. Maturation of synaptic transmission at end-bulb synapses of the cochlear nucleus. J Neurosci. 2001;21:9487–9498. doi: 10.1523/JNEUROSCI.21-23-09487.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunig I, Suter A, Knuesel I, Luscher B, Fritschy JM. GABAergic terminals are required for postsynaptic clustering of dystrophin but not of GABA(A) receptors and gephyrin. J Neurosci. 2002;22:4805–4813. doi: 10.1523/JNEUROSCI.22-12-04805.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley K, Kelly RB. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J Cell Biol. 1985;100:1284–1294. doi: 10.1083/jcb.100.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrone J, O'Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin LS, Li L, Ferreira A, Kosik KS, Greengard P. Impairment of axonal development and of synaptogenesis in hippocampal neurons of synapsin I-deficient mice. Proc Natl Acad Sci U S A. 1995;92:9230–9234. doi: 10.1073/pnas.92.20.9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colicos MA, Collins BE, Sailor MJ, Goda Y. Remodeling of synaptic actin induced by photoconductive stimulation. Cell. 2001;107:605–616. doi: 10.1016/s0092-8674(01)00579-7. [DOI] [PubMed] [Google Scholar]
- Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P. Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci. 2003;6:708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P, Skaper SD, Moore SE, Leon A, Walsh FS. A developmentally regulated switch in neuronal responsiveness to NCAM and N-cadherin in the rat hippocampus. Development. 1992;115:885–892. doi: 10.1242/dev.115.3.885. [DOI] [PubMed] [Google Scholar]
- Esch T, Lemmon V, Banker G. Differential effects of NgCAM and N-cadherin on the development of axons and dendrites by cultured hippocampal neurons. J Neurocytol. 2000;29:215–223. doi: 10.1023/a:1026515426303. [DOI] [PubMed] [Google Scholar]
- Ethell IM, Yamaguchi Y. Cell surface heparan sulfate proteoglycan syndecan-2 induces the maturation of dendritic spines in rat hippocampal neurons. J Cell Biol. 1999;144:575–586. doi: 10.1083/jcb.144.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fannon AM, Colman DR. A model for central synaptic junctional complex formation based on the differential adhesive specificities of the cadherins. Neuron. 1996;17:423–434. doi: 10.1016/s0896-6273(00)80175-0. [DOI] [PubMed] [Google Scholar]
- Fink CC, Bayer KU, Myers JW, Ferrell JE, Jr, Schulman H, Meyer T. Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron. 2003;39:283–297. doi: 10.1016/s0896-6273(03)00428-8. [DOI] [PubMed] [Google Scholar]
- Freund TF. Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Fujimori T, Takeichi M. Disruption of epithelial cell-cell adhesion by exogenous expression of a mutated nonfunctional N-cadherin. Mol Biol Cell. 1993;4:37–47. doi: 10.1091/mbc.4.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgakopoulos A, Marambaud P, Efthimiopoulos S, Shioi J, Cui W, Li HC, Schutte M, Gordon R, Holstein GR, Martinelli G, Mehta P, Friedrich VL, Jr, Robakis NK. Presenilin-1 forms complexes with the cadherin/catenin cell-cell adhesion system and is recruited to intercellular and synaptic contacts. Mol Cell. 1999;4:893–902. doi: 10.1016/s1097-2765(00)80219-1. [DOI] [PubMed] [Google Scholar]
- Gil OD, Needleman L, Huntley GW. Developmental patterns of cadherin expression and localization in relation to compartmentalized thalamocortical terminations in rat barrel cortex. J Comp Neurol. 2002;453:372–388. doi: 10.1002/cne.10424. [DOI] [PubMed] [Google Scholar]
- Goslin K, Banker G. Rat hippocampal neurons in low density culture. In: Banker G, Goslin K, editors. Culturing Nerve Cells. vol. 1. Cambridge: MIT Press; 1991. pp. 251–282. [Google Scholar]
- Gottardi CJ, Gumbiner BM. Adhesion signaling: how beta-catenin interacts with its partners. Curr Biol. 2001;11:R792–R794. doi: 10.1016/s0960-9822(01)00473-0. [DOI] [PubMed] [Google Scholar]
- Halpain S, Hipolito A, Saffer L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J Neurosci. 1998;18:9835–9844. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Henze DA, Hirase H, Leinekugel X, Dragoi G, Czurko A, Buzsaki G. Spike train dynamics predicts theta-related phase precession in hippocampal pyramidal cells. Nature. 2002;417:738–741. doi: 10.1038/nature00808. [DOI] [PubMed] [Google Scholar]
- Hirano S, Suzuki ST, Redies CM. The cadherin superfamily in neural development: diversity, function and interaction with other molecules. Front Biosci. 2003;8:D306–D355. doi: 10.2741/972. [DOI] [PubMed] [Google Scholar]
- Huntley GW, Benson DL. N-Cadherin at developing thalamocortical synapses provides an adhesion mechanism for the formation of somatotopically organized connections. Journal of Comparative Neurology. 1999;407:453–471. [PubMed] [Google Scholar]
- Huntley GW, Benson DL, Colman DR. Structural remodeling of the synapse in response to physiological activity. Cell. 2002;108:1–4. doi: 10.1016/s0092-8674(01)00631-6. [DOI] [PubMed] [Google Scholar]
- Inoue A, Sanes JR. Lamina-specific connectivity in the brain: Regulation by N-cadherin, neurotrophins, and glycoconjugates. Science. 1997;276:1428–1431. doi: 10.1126/science.276.5317.1428. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Hirota Y, Ozaki K, Okano H, Takeichi M, Uemura T. DN-cadherin is required for spatial arrangement of nerve terminals and ultrastructural organization of synapses. Mol Cell Neurosci. 2002;19:375–388. doi: 10.1006/mcne.2001.1081. [DOI] [PubMed] [Google Scholar]
- Kaufmann N, DeProto J, Ranjan R, Wan H, Van Vactor D. Drosophila liprin-alpha and the receptor phosphatase Dlar control synapse morphogenesis. Neuron. 2002;34:27–38. doi: 10.1016/s0896-6273(02)00643-8. [DOI] [PubMed] [Google Scholar]
- Kim CH, Lisman JE. A role of actin filament in synaptic transmission and long-term potentiation. J Neurosci. 1999;19:4314–4324. doi: 10.1523/JNEUROSCI.19-11-04314.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintner C. Regulation of embryonic cell adhesion by the cadherin cytoplasmic domain. Cell. 1992;69:225–236. doi: 10.1016/0092-8674(92)90404-z. [DOI] [PubMed] [Google Scholar]
- Korematsu K, Redies C. Expression of cadherin-8 mRNA in the developing mouse central nervous system. J Comp Neurol. 1997;387:291–306. [PubMed] [Google Scholar]
- Krucker T, Siggins GR, Halpain S. Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus. Proc Natl Acad Sci U S A. 2000;97:6856–6861. doi: 10.1073/pnas.100139797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kypta RM, Su H, Reichardt LF. Association between a transmembrane protein tyrosine phosphatase and the cadherin-catenin complex. J Cell Biol. 1996;134:1519–1529. doi: 10.1083/jcb.134.6.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, Wisniewski T, Robakis NK. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. Embo J. 2002;21:1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez MC, Ochiishi T, Majewski M, Kosik KS. Dual regulation of neuronal morphogenesis by a delta-catenin-cortactin complex and Rho. J Cell Biol. 2003;162:99–111. doi: 10.1083/jcb.200211025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea PD, Brieher WM, Gumbiner BM. Induction of a secondary body axis in Xenopus by antibodies to beta-catenin. J Cell Biol. 1993;123:477–484. doi: 10.1083/jcb.123.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrmann R, Lessmann V, Gottmann K. Developmental maturation of synaptic vesicle cycling as a distinctive feature of central glutamatergic synapses. Neuroscience. 2003;117:7–18. doi: 10.1016/s0306-4522(02)00835-7. [DOI] [PubMed] [Google Scholar]
- Mozhayeva MG, Sara Y, Liu X, Kavalali ET. Development of vesicle pools during maturation of hippocampal synapses. J Neurosci. 2002;22:654–665. doi: 10.1523/JNEUROSCI.22-03-00654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase S, Mosser E, Schuman EM. Depolarization drives beta-Catenin into neuronal spines promoting changes in synaptic structure and function. Neuron. 2002;35:91–105. doi: 10.1016/s0896-6273(02)00764-x. [DOI] [PubMed] [Google Scholar]
- Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, Weinberg RJ, Worley PF, Sheng M. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–582. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- Nieman MT, Kim JB, Johnson KR, Wheelock MJ. Mechanism of extracellular domain-deleted dominant negative cadherins. J Cell Sci. 1999;112:1621–1632. doi: 10.1242/jcs.112.10.1621. [DOI] [PubMed] [Google Scholar]
- Nishimura W, Yao I, Iida J, Tanaka N, Hata Y. Interaction of synaptic scaffolding molecule and Beta -catenin. J Neurosci. 2002;22:757–765. doi: 10.1523/JNEUROSCI.22-03-00757.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P. Synaptic clustering of AMPA receptors by the extracellular immediate- early gene product Narp. Neuron. 1999;23:309–323. doi: 10.1016/s0896-6273(00)80782-5. [see comments] [DOI] [PubMed] [Google Scholar]
- Park C, Falls W, Finger JH, Longo-Guess CM, Ackerman SL. Deletion in Catna2, encoding alpha N-catenin, causes cerebellar and hippocampal lamination defects and impaired startle modulation. Nat Genet. 2002;31:279–284. doi: 10.1038/ng908. [DOI] [PubMed] [Google Scholar]
- Perego C, Vanoni C, Massari S, Longhi R, Pietrini G. Mammalian LIN-7 PDZ proteins associate with beta-catenin at the cell- cell junctions of epithelia and neurons. Embo J. 2000;19:3978–3989. doi: 10.1093/emboj/19.15.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskanzer K, Needleman LA, Bozdagi O, Huntley GW. N-cadherin regulates ingrowth and laminar targeting of thalamocortical axons. J Neurosci. 2003;23:2294–2305. doi: 10.1523/JNEUROSCI.23-06-02294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehl R, Johnson K, Bradley R, Grunwald GB, Cornel E, Lilienbaum A, Holt CE. Cadherin function is required for axon outgrowth in retinal ganglion cells in vivo. Neuron. 1996;17:837–848. doi: 10.1016/s0896-6273(00)80216-0. [DOI] [PubMed] [Google Scholar]
- Sadot E, Simcha I, Shtutman M, Ben-Ze'ev A, Geiger B. Inhibition of beta-catenin-mediated transactivation by cadherin derivatives. Proc Natl Acad Sci U S A. 1998;95:15339–15344. doi: 10.1073/pnas.95.26.15339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Serafini T. Finding a partner in a crowd: neuronal diversity and synaptogenesis. Cell. 1999;98:133–136. doi: 10.1016/s0092-8674(00)81008-9. [DOI] [PubMed] [Google Scholar]
- Shan WS, Tanaka H, Phillips GR, Arndt K, Yoshida M, Colman DR, Shapiro L. Functional cis-heterodimers of N- and R-cadherins. J Cell Biol. 2000;148:579–590. doi: 10.1083/jcb.148.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L, Colman DR. The diversity of cadherins and implications for a synaptic adhesive code in the CNS. Neuron. 1999;23:427–430. doi: 10.1016/s0896-6273(00)80796-5. [DOI] [PubMed] [Google Scholar]
- Suzuki SC, Inoue T, Kimura Y, Tanaka T, Takeichi M. Neuronal circuits are subdivided by differential expression of type-II classic cadherins in postnatal mouse brains. Mol Cell Neurosci. 1997;9:433–447. doi: 10.1006/mcne.1997.0626. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;25:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Tang L, Hung CP, Schuman EM. A role for the cadherin family of cell adhesion molecules in hippocampal long-term potentiation. Neuron. 1998;20:1165–1175. doi: 10.1016/s0896-6273(00)80497-3. [DOI] [PubMed] [Google Scholar]
- Tepass U, Truong K, Godt D, Ikura M, Peifer M. Cadherins in embryonic and neural morphogenesis. Nat Rev Mol Cell Biol. 2000;1:91–100. doi: 10.1038/35040042. [DOI] [PubMed] [Google Scholar]
- Togashi H, Abe K, Mizoguchi A, Takaoka K, Chisaka O, Takeichi M. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- Uchida N, Honjo Y, Johnson KR, Wheelock MJ, Takeichi M. The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. Journal of Cell Biology. 1996;135:767–779. doi: 10.1083/jcb.135.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn JE. Review: Fine structure of synaptogenesis in the vertebrate central nervous system. Synapse. 1989;3:255–285. doi: 10.1002/syn.890030312. [DOI] [PubMed] [Google Scholar]
- Wu G-Y, Malinow R, Cline HT. Maturation of a central glutamatergic synapse. Science. 1996;274 doi: 10.1126/science.274.5289.972. [DOI] [PubMed] [Google Scholar]
- Wyszynski M, Kim E, Dunah AW, Passafaro M, Valtschanoff JG, Serra-Pages C, Streuli M, Weinberg RJ, Sheng M. Interaction between GRIP and liprin-alpha/SYD2 is required for AMPA receptor targeting. Neuron. 2002;34:39–52. doi: 10.1016/s0896-6273(02)00640-2. [DOI] [PubMed] [Google Scholar]
- Yap AS, Brieher WM, Pruschy M, Gumbiner BM. Lateral clustering of the adhesive ectodomain: a fundamental determinant of cadherin function. Curr Biol. 1997;7:308–315. doi: 10.1016/s0960-9822(06)00154-0. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Barth AL, Stellwagen D, Malenka RC. A developmental switch in the signaling cascades for LTP induction. Nat Neurosci. 2003;6:15–16. doi: 10.1038/nn985. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Poo MM. Electrical activity and development of neural circuits. Nat Neurosci. 2001;(4 Suppl):1207–1214. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]
- Zhang W, Benson DL. Stages of synapse development defined by dependence on F-actin. J Neurosci. 2001;21:5169–5181. doi: 10.1523/JNEUROSCI.21-14-05169.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen M, Jin Y. The liprin protein SYD-2 regulates the differentiation of presynaptic termini in C. elegans. Nature. 1999;401:371–375. doi: 10.1038/43886. [DOI] [PubMed] [Google Scholar]