Abstract
Objective
To provide an overview of the NIMH Multisite HIV/STD Prevention Trial for African American Couples conducted in four urban areas: Atlanta, Los Angeles, New York, and Philadelphia. The rationale, study design methods, proposed data analyses, and study management are described.
Design
This is a two arm randomized Trial, implementing a modified randomized block design, to evaluate the efficacy of a couples based intervention designed for HIV serodiscordant African American couples.
Methods
The study phases consisted of formative work, pilot studies, and a randomized clinical trial. The sample is 535 HIV serodiscordant heterosexual African American couples. There are two theoretically derived behavioral interventions with eight group and individual sessions: the Eban HIV/STD Risk Reduction Intervention (treatment) versus the Eban Health Promotion Intervention (control). The treatment intervention was couples based and focused on HIV/STD risk reduction while the control was individual based and focused on health promotion. The two study conditions were structurally similar in length and types of activities. At baseline, participants completed an Audio Computer-assisted Self Interview (ACASI) interview as well as interviewer-administered questionnaire, and provided biological specimens to assess for STDs. Similar follow-up assessments were conducted immediately after the intervention, at 6 months, and at 12 months.
Results
The Trial results will be analyzed across the four sites by randomization assignment. Generalized estimating equations (GEE) and mixed effects modeling (MEM) are planned to test: (1) the effects of the intervention on STD incidence and condom use as well as on mediator variables of these outcomes, and (2) whether the effects of the intervention differ depending on key moderator variables (e.g., gender of the HIV-seropositive partners, length of relationship, psychological distress, sexual abuse history, and substance abuse history).
Conclusions
The lessons learned from the design and conduct of this clinical trial provide guidelines for future couples based clinical trials in HIV/STD risk reduction and can be generalized to other couples based behavioral interventions.
Keywords: HIV, AIDS, STDs, behavioral intervention, HIV serodiscordant couples, clinical trial
Introduction
The National Institute of Mental Health (NIMH) Multisite HIV/STD Prevention Trial for African American Couples is a randomized trial of a couples based HIV prevention intervention conducted in four US urban areas: Atlanta, Los Angeles, New York, and Philadelphia. If successful, the Trial will provide evidence for an intervention urgently needed to reduce risk behaviors in HIV serodiscordant African American couples. There are few evidence-based interventions services addressed to their specific HIV/STD risk reduction needs.
Background and Significance
The impact of HIV/AIDS in the African American community continues to be a health crisis. Although African Americans constitute approximately 12% of the US population, they represent 42.4% of people living with AIDS in the US.1 During 2000-2005, African Americans represented more than half (51%) of the 184,991 reported new cases of HIV in the 33 states with confidential name-based reporting.2 Rates of HIV/AIDS among African American men were seven times higher than those among non-Hispanic white men and three times higher than those among Hispanic men in the years 2000-2003.1 During the same period, the HIV/AIDS rate for African American women was 19 times the rate for non-Hispanic white women and five times the rate for Hispanic women.2
Of special concern is the fact that African Americans experienced a shorter interval between testing HIV positive and being diagnosed with AIDS due to a delay in seeking treatment associated with poverty and stigma.3 According to the Centers for Disease Control and Prevention, in 2001, HIV/AIDS was among the three leading causes of death for African American men aged 25-54 years, among the four leading causes of death for African American women aged 20-54 years, and the leading cause of death among African American women 25-34 years of age.4
In the four cities selected for this Trial, the HIV/AIDS prevalence per 100,000 is disproportionately higher for African Americans (27.1 in Atlanta; 25.9 in Los Angeles; 59.2 in New York City; and 24.9 in Philadelphia).5 The cumulative number of HIV/AIDS cases in the four cities represents 25% of US cases.5 The prevalence of Chlamydia, the most common of reported STDs per 100,000, was varied (726.8 in Atlanta; 407.1 in Los Angeles; 437.5 in New York City; and 1,219 in Philadelphia.5 The large proportion of HIV/STDs cases and geographic diversity represented by the four cities enhances the generalizability of the proposed study data.5
This Trial is designed for couples because heterosexual contact is the leading route of HIV transmission among African American women and the second leading transmission mode among African American men.2 Wyatt and her colleagues reported that almost three of four HIV positive African American women were infected by their husbands or steady partners.6 Studies have noted low rates of condom use among African Americans,7 African American women with steady male partners,8,9,10,11 HIV positive African American women,6 and the HIV negative partners of HIV positive African American women.11 Evidence suggests that STDs facilitate the spread of HIV.12 A person with an STD is 2-5 times more likely to be HIV positive than a person without an STD.13
A recent review of condom efficacy studies led by the National Institutes of Health found that consistent condom use reduces the probability of HIV transmission per sex act by as much as 95%. Considerable evidence suggests that behavioral interventions can reduce HIV sexual risk behavior in various populations.14 A meta-analysis of 14 behavioral HIV/STD prevention interventions among heterosexual adults found statistically significant increases in condom use and reductions in incidence of biologically confirmed STDs.15 Interventions with small groups showed more favorable effects than those with individuals.
While no couples based interventions were included in the meta-analysis, studies with couples have provided compelling descriptive evidence of the utility of interventions for dyads. In a study examining couples, Allen and colleagues offered a confidential HIV testing and condom program for 1,458 childbearing women in Rwanda.16 While not originally designed as a couples study, 26 per cent of the male partners volunteered to view the educational videotape and receive an HIV test. Couples in whom both partners were tested were twice as likely to use condoms. The man’s participation was also associated with significant reductions in HIV and gonorrhea rates among the women. Of concern was the finding that seropositive women with untested partners comprised the group least likely to use condoms, which accounts for the rate of HIV seroconversion in this group being more than twice that for women whose partners were tested and received counseling. The strongest predictors of condom use were a seropositive test result in the woman and HIV testing and counseling of the male partner. At the 2-year follow-up, HIV negative women whose partners had participated were 50% less likely to become seropositive than were those whose partners had not participated. Allen continued to corroborate these results and this program is now being supported by the Global Health Program at the Centers for Disease Control and Prevention.17
In one of the first studies to evaluate couples based voluntary counseling and testing (VCT) in the US, Padian corroborated these results in a longitudinal intervention with mixed serostatus couples.18 The intervention focused on building skills for correctly using condoms and providing social support, and included role plays for problem solving how to implement safer sex behavior strategies. Although the study lacked a control group, the data suggest that the intervention had a positive impact. The proportion of couples reporting consistent condom use increased from 49 per cent at baseline to 88 per cent at follow-up. Among those couples at the 16-month follow-up no seronegative partner had become positive.
The European Study Group on Heterosexual Transmission of HIV documented similar patterns, suggesting that condom use was associated with fewer seroconversions in 304 serodiscordant couples.19 The couples were counseled and tested for HIV every six months over 20 months. Nearly half of the couples (48.4%) used condoms consistently, and experienced no seroconversions. Among those who did not use condoms consistently, 9.9 per cent seroconverted (4.8/100 person years).
Further evidence of the utility of couples interventions comes from a study in Haiti with 476 patients with HIV and their non-infected regular sex partners, who were evaluated at 3 and 6 months for HIV infection, sexually transmitted diseases, and sexual practices.20 Counseling and free condoms were provided. Only one seroconversion occurred among the 42 sexually active couples (23.7% of the 177 sexually active couples) who reported always using condoms. In contrast, the incidence in sexually active couples who infrequently used or did not use condoms was 6.8 per 100 person years (CI, 6.49 to 7.14 per 100 person years).21 Transmission of HIV was associated with genital ulcer diseases, syphilis, and vaginal or penile discharge in the HIV negative partner and with syphilis in the HIV infected partner.
The first multi-country randomized controlled trial (RCT) to test the efficacy of VCT with couples was conducted in Nairobi, Kenya, Dar es Salaam, Tanzania, and Port of Spain, Trinidad.22 Although it was not specifically designed for couples, the trial enrolled 586 couples, and randomly assigned them to receive either a couples based VCT or a basic health information intervention. The couples assigned to VCT reduced unprotected intercourse with their enrollment partners significantly more than couples assigned to the health information group. The proportion of individuals reporting unprotected intercourse with non-primary partners declined significantly more for those receiving VCT compared to those receiving health information (men, 35% reduction with VCT vs.13% reduction with health information; women, 39% reduction with VCT vs. 17% reduction with health information) and these results were maintained at the second follow-up. Individual HIV infected men were more likely than uninfected men to reduce unprotected intercourse with primary and non-primary partners, whereas HIV infected women were more likely than infected women to reduce unprotected intercourse with primary partners.
El Bassel and colleagues conducted one of the first RCTs to test the efficacy of a relationship based HIV/STD prevention intervention with low-income urban couples in the US.23,24,25 For this study, 217 low-income urban couples at elevated risk of HIV/STDs were randomized to one of three conditions: (1) a 6-session relationship based HIV/STD prevention intervention provided to couples; (2) the same 6 sessions provided to the women alone; or (3) a 1-session HIV/STD information session provided to the women alone, which served as the control condition. The 6-session interventions for couples or women alone were efficacious in reducing unprotected sex at both the 3-and 12-month follow-up assessments. This study provides additional evidence of the sustained efficacy of a relationship based intervention for increasing condom use among low-income urban couples.
While conducted in different settings with African and West Indian samples whose history of health care access utilization differs from African American couples in the US, the findings highlight the important of involving couples in HIV prevention efforts. In addition, all of these studies have one or more of the following methodological drawbacks: not being designed specifically for couples; relatively small samples sizes; and lacking a randomized control design, biological confirmed outcomes, or an attention control group. However, accumulating research has suggested that couples based HIV prevention interventions may be more efficacious in promoting condom use among HIV serodiscordant couples than traditional HIV prevention interventions aimed at individuals or groups.16,26,27,28,29,30,31,32 Couples based approaches have been found to be associated with increasing commitment in a relationship to protecting each other from HIV/STDs, reducing gender power imbalances that impact condom use, and increasing sexual communication and negotiation skills.23,33,34,35,36,37,38,39,40,41 The collective results from these studies support the development of a couples based intervention for African American HIV serodiscordant heterosexual couples.
The primary objective of this Trial was to develop a culturally congruent, couples based intervention and test its efficacy in African American serodiscordant couples in four US cities. The couples were randomized to one of two interventions: an eight session couple focused Eban HIV/STD Risk Reduction Intervention (treatment) or an eight session individual focused Eban Health Promotion Intervention (control), addressing health issues unrelated to sexual behavior. Both interventions involved couple and group sessions led by trained male and female co facilitators. The treatment intervention focused on couple goals on how to reduce HIV related risk behaviors and teach condom use and communication skills. In contrast, the control intervention focused on individual goals related to health promotion and providing factual information about health screening and teaching skills in exercise, diet management, and medication adherence.
There are four urban performance sites: Atlanta (Emory University), Los Angeles (University of California, Los Angeles), New York (Columbia University), and Philadelphia (University of Pennsylvania), and one data coordinating center (DCC) (University of Pennsylvania). Because this Trial is funded through a Cooperative Agreement, NIMH has a major role in the conduct of the study. Representatives from the sites, DCC, and NIMH formed a Scientific Steering Committee that developed a common protocol and procedures for the conduct of the Trial including the interventions; assessment questionnaire; biological specimen collection, storage, and analysis; study procedures and materials. The Steering Committee was also responsible for the conduct of all aspects of the Trial. A Data Safety and Monitoring Board (DSMB), appointed by NIMH, reviewed and approved the Protocol. The Trial organization appears in Figure 1.
Figure 1.
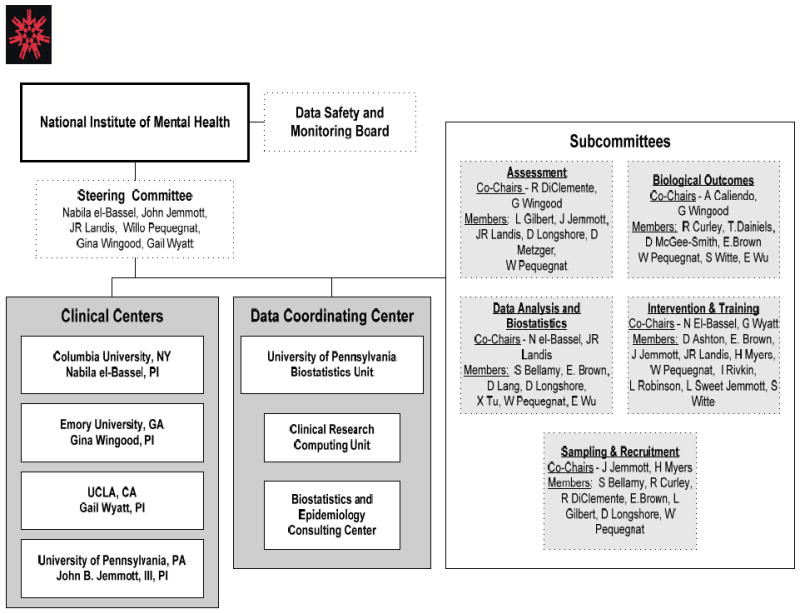
Trial Organization
Formative Research
To inform the design of the interventions and study procedures, one four session focus group was held in Los Angeles, two focus groups were conducted in New York, and two additional groups were held in Philadelphia. The focus groups were used to inform and refine the content of the intervention sessions, and barriers to participation in the intervention, such as transportation and child care. The themes addressed in the focus groups were: (1) stigma and distress associated with HIV; (2) barriers to condom use; (3) insufficient support from family and HIV community services; and (4) the lack of skill based interventions that emphasize valuing self and relationship protection. (See “Formative Study to Develop the NIMH Multisite HIV/STD Prevention Trial for African American Couples” in this issue.)
The paper and pencil version of the baseline assessment instrument was pilot tested in Atlanta and Philadelphia. Based on these data, an Audio Computer Assisted Self Interview (ACASI) version of the questionnaire was developed. All four sites pilot tested the ACASI version with a new sample of participants and revisions were made. All four sites then conducted a full pilot study with African American HIV serodiscordant couples using all the materials developed for the Trial. Six-month follow-up assessments were conducted in Atlanta and Philadelphia and twelve-month follow-up was conducted in Philadelphia. The participants were debriefed, and their comments regarding how the study might be improved were solicited and incorporated into the final questionnaire.
Centralized training programs were conducted for all four performance sites, including: (1) three-day training for recruiters; (2) three-day training for data collectors; and (3) two-day training for clinical research coordinators. The facilitators for the two interventions each received two-part training. The first was onsite where the facilitators watched videos, read and discussed background materials, and practiced role-playing and implementation of each session. Then, four days of centralized training was conducted at NIMH for each intervention separately. All staff was certified after the training by senior staff who participated in all training.
Randomized Controlled Trial
Research Questions
The primary aims of the Trial were to test the hypothesis that across all four study sites couples who received the Eban HIV/STD Risk Reduction Intervention (n = 260) relative to the control couples (n= 275) would have (1) a greater increase in the self reported rate of condom protected sexual intercourse at immediate post intervention and at 6-and 12-month follow-up, and (2) a lower incidence of biologically confirmed STDs (Chlamydia, gonorrhea, and trichomoniasis) at immediate post-intervention and at 6-and 12-month follow-up.
The secondary aims of the Trial were (1) to test whether key variables moderate the effects of the treatment intervention, and (2) to test whether the theoretical mediator variables changed.
The Trial also tested the hypothesis that across all four urban sites couples who receive the Eban Health Promotion Intervention relative to the couples in the treatment condition would increase their health promotion and screening activities.
Research Participants
Participants were 535 English-speaking, African American, HIV serodiscordant, heterosexual couples recruited in each of the four urban sites (Atlanta = 117 couples, Los Angeles = 100 couples, New York = 221 couples, and Philadelphia = 97 couples). Three hundred twenty-three couples had HIV positive female partners and 212 couples had HIV positive male partners. There were specific inclusion and exclusion criteria associated with the intervention’s target population.
Eligibility
Couples were eligible to participate if: (1) each partner was at least 18 years of age; (2) each partner agreed that the relationship had lasted at least 6 months; (3) each partner intended to stay in the relationship for at least 12 months (i.e., stated independently that he or she was confident or very confident that they would remain together); (4) at least one partner reported having unprotected intercourse at least once in the previous 90 days; (5) each partner had no plans to relocate beyond a reasonable distance from the study site; (6) at least one partner self identified as African American or black; (7) at least one partner agreed that they were not planning pregnancy within the next 18 months; (8) each partner was aware of his/her partner’s HIV status; (9) only one partner was HIV positive and had known their status for at least three months; and (10) each partner was committed to attending all of the sessions.
Couples were ineligible if: (1) one or both partners did not have an address where they could receive mail, which would have made tracking and follow-up difficult; (2) one or both manifested significant psychiatric, physical or neurological impairment that would limit their effective participation as confirmed on an MMSE and/or Quick Test; (3) there was a history of severe physical or sexual abuse in the past year in the current relationship (i.e., significant enough to require medical, psychological and/or legal intervention); (4) one or both partners were unwilling or unable to commit to participating in the study through to completion; (5) both partners had not previously participated in an HIV sexual risk reduction intervention for couples in the past 12 months; (6) one or both were not fluent in English as determined by the Informed Consent process; or (7) both partners were planning to get pregnant in the next 18 months.
Recruitment and Retention
Because these couples were not receiving services in the traditional recruitment sites and are a hidden population, it was impossible to determine in advance the best sites or recruitment methods. To achieve the recruitment targets and to ensure that the results would be generalizable to multiple settings, research subjects were recruited from HIV care clinics; HIV testing and counseling centers; primary care clinics; AIDS service organizations; substance abuse treatment programs; churches with HIV/AIDS ministries; HIV/AIDS provider networks, coalitions, and advocacy organizations; HIV/AIDS hotline services; and community based organizations.42,43 The members of the Community Advisory Boards (CABs) identified specific sites and provided credibility for the recruitment efforts. (See “The Role of Community Advisory Boards (CABs) in Project Eban” in this issue.)
Traditional strategies included: presentations and face-to-face recruitment in HIV clinics, health centers, and CBOs; snowball sampling by eliciting referrals from former participants who had completed the 12-month follow-up; targeted street outreach; a Project Website; and brochures and handouts distributed at potential sites with toll free numbers.2
Sources of potential participants were tracked across the sites and appear in Table 1.
Table 1.
Recruitment Sources
| Clinical Center |
Depart. Of Health |
VA | Hospital Based Clinic |
Non Hospital Based Clinic |
Community Based Org. |
AIDS Service Org. |
Physician Referral |
Church Services Org. |
Media | Word of Mouth |
Housing Facility |
Other Research Studies |
CFAR | Conferences | Community Outreach |
Partner | Other | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Columbia | 3 | 7 | 206 | 17 | 287 | 306 | 7 | 17 | 487 | 661 | 37 | 29 | 0 | 0 | 4 | 24 | 103 | 2195 |
| Emory | 93 | 98 | 360 | 54 | 102 | 2 | 1 | 4 | 890 | 159 | 15 | 2 | 0 | 82 | 42 | 0 | 174 | 2078 |
| UCLA | 5 | 14 | 71 | 34 | 97 | 273 | 16 | 17 | 86 | 29 | 30 | 12 | 0 | 0 | 15 | 0 | 69 | 768 |
| UPENN | 37 | 22 | 70 | 53 | 36 | 147 | 9 | 6 | 298 | 78 | 0 | 25 | 74 | 2 | 0 | 94 | 0 | 951 |
| Total | 138 | 139 | 669 | 156 | 454 | 645 | 32 | 42 | 1668 | 883 | 75 | 66 | 74 | 84 | 59 | 118 | 690 | 5992 |
To increase the number of eligible couples, some sites expanded the recruitment strategies to nontraditional recruitment approaches such as media. At Emory University and UCLA, the investigators implemented a media recruitment campaign. Table 2 illustrates the increase in the percentage of couples under 30 recruited using these methods. Specifically, the two sites that implemented media campaigns, observed a 160% increase (Atlanta/Emory) and 260% increase (UCLA/Los Angeles) in the percentage of new study participants recruited who were < 30 years of age. It was important to recruit younger couples in order to increase the baseline prevalence of STDs in the sample. The sources of participants who were randomized appears in Table 3.
Table 2.
Increase in Couples Enrolled who are Less than 30 Years of Age Before and After Implementing a Media Campaign
| Site | Percent < 30 Years of Age Prior to Mass Media Recruitment Efforts | Percent < 30 Years of Age After Mass Media Recruitment Efforts | Percentage Increase |
|---|---|---|---|
| Atlanta | 5.9% | 9.8% | 160% |
| Los Angeles | 7.1% | 18.2% | 260% |
Table 3.
Randomized Participant’s Recruitment Sources
| Dept. Of Health |
VA | Hospital Based Clinic |
Non Hospital Based Clinic |
Community Based Org. |
AIDS Service Org. |
Church Services Org. |
Media | Word Of Mouth |
Housing Facility |
Other Research Studies |
Community Outreach |
Other | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site 10-Columbia | 0 | 2 | 44 | 0 | 22 | 108 | 4 | 132 | 94 | 24 | 8 | 4 | 0 | 442 |
| Site 20-Emory | 24 | 2 | 99 | 0 | 15 | 0 | 0 | 85 | 7 | 0 | 0 | 0 | 2 | 234 |
| Site 30-UCLA | 0 | 0 | 4 | 4 | 16 | 140 | 0 | 20 | 6 | 8 | 2 | 0 | 200 | |
| Site 40-UPENN | 2 | 0 | 38 | 0 | 20 | 46 | 0 | 64 | 20 | 0 | 2 | 0 | 2 | 194 |
| Total | 26 | 4 | 185 | 4 | 73 | 294 | 4 | 301 | 127 | 24 | 18 | 6 | 4 | 1070 |
Categorical Definitions:
Randomized participants’ includes all individuals that were eligible and randomized into the study at visit 3.
The Word of Mouth category also includes partner and participant referrals.
Design and Methods
There was a detailed Project Eban Participant Visit Schedule that was followed across the sites, which appears in Figure 2. There were three phases of the Trial: (1) the pre-intervention phase; (2) intervention phase; and (3) follow-up phase.
Figure 2.
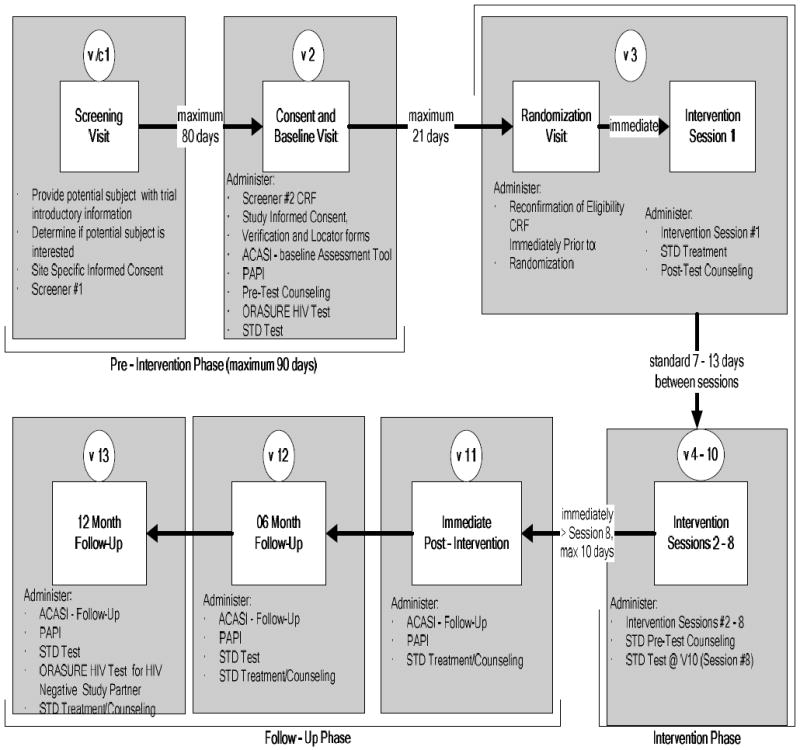
Schedule of Study Sites
Schedule of Study Visits
Pre-Intervention-Phase
This phase was a maximum of 90 days. At Visit 1, couples were initially screened, their interest in participating was assessed, and they received Informed Consent. At Visit 2, each participant was screened to determine couple eligibility. No additional data were collected for ineligible couples. Refusal data were collected for eligible couples that declined to participate. Eligible couples that wished to continue completed a Short Locator Form (for each individual), which was used to maintain contact with each participant throughout the trial. Participants were also asked to provide picture identification. The Data Collector gave each participant the ACASI instructions and practice questions. After completing the ACASI, participants received face-to-face interviews in private rooms. Pretest HIV/STD counseling preceded collection of specimens, which were shipped to a centralized testing facility at Emory University. Specimens for Neisseria gonorrhoeae (GC) and Chlamydia trachomatis (CT) were assessed using the Becton Dickinson ProbeTec ET Amplified DNA Assay, (Becton, Dickinson and Co., Sparks, MD) and Trichomonas vaginalis (TV) was assessed using Taq-Man PCR. At the end participants were then scheduled for randomization and the initial intervention session within 10-14 days, and reimbursed.
After completing baseline measures at Visit 2, participants were asked to provide an oral specimen, using the OraSure test procedures, as defined by the Epitope system, to confirm their HIV status. Testing with OraSure requires no blood or needles. The accuracy of the OraSure HIV-1 oral specimen test ranged from 97.7% in high-risk populations to 99.6% in low risk populations. If an OraSure specimen tests repeatedly reactive with the Oral Fluid Vironostika HIV Microelisa System, that specimen undergoes further testing for HIV-1 antibodies with the more specific OraSure HIV-1 Western Blot Kit. All participants received standard pre- and posttest counseling from the site CRC, who was trained and certified to conduct such counseling. Participants who were identified as HIV positive were informed in private and referred to clinical sites for further diagnostic evaluation and care.
Detected STDs were treated according to CDC guidelines. After notification, participants were treated immediately or scheduled to return to a designated clinical site for treatment by a project clinician. For both ethical and methodological reasons, it was imperative that treatment was completed promptly and that confirmation of treatment was obtained. Participants were treated with directly observable, state-of-the-art, single dose, oral therapy to minimize potential non-adherence to multi-dose medication regimens.
Intervention Phase
This phase occurred within 21 days of Visit 2. At Visit 3 couples were reconfirmed for eligibility, randomized to either the treatment or control intervention, and participated in the first intervention session. Also, posttest counseling and test results were provided and, if required, the couples were treated for any positive results. Treatment procedures followed local or CDC guidelines as appropriate. Participants randomized to both conditions received HIV/STD education when the biospecimens were collected and treatment was rendered.
During this period Intervention sessions 2 to 8 were delivered, allowing only 7 to 13 days between sessions.
Follow-up Phase
This phase began within 10 days after the intervention ended. At Visits 11-13 the data collection activities in Visit 2 were repeated at immediate post-intervention, 6 months, and 12 months, respectively.
Randomization
In contrast to traditional randomized trials, which randomize individuals to intervention conditions, this couples based, randomized trial assigned intact couples to either the treatment or control condition in groups of three to five couples. The gender of the HIV positive partner was used as a blocking factor to ensure that the distribution of HIV positive males and females was equal across interventions. (See paper on the randomization procedures.44)
Interventions
Previous studies have established that eight sessions ensure that problems emerge and the skills required for behavior change can be acquired. Male and female co-facilitator pairs delivered a standardized intervention based on the criteria in the manual that was reinforced by training and weekly supervision. supervision. (See “Supervision Model Used to Ensure Fidelity of Intervention Implementation” in this issue.)
Eban HIV/STD Prevention Intervention (Treatment)
The Eban HIV/STD Risk Reduction Intervention was based on three integrated theoretical foundations (i.e., social cognitive theory, an ecological framework, and an Afro centric paradigm), which were incorporated into the structure, format and content of the curriculum. (See Figure 3.) The couples focused intervention, which consisted of eight weekly two-hour sessions, was structured and addressed on personal, interpersonal, community, and societal levels (ecological model). The facilitators modeled self-protection, working together, and communication, and supported all aspects of healthy interactions. The intervention relied on dyadic and group processes and allowed facilitators to use strategies that addressed the broad array of interpersonal, social, and cultural factors influencing risk behavior among HIV affected couples.
Figure 3.
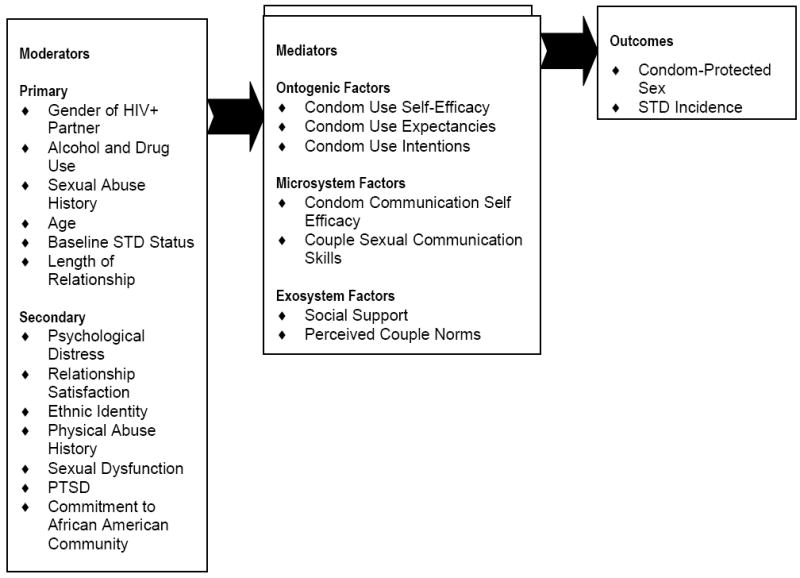
Conceptual Framework for Eban HIV/STD Risk Reduction Intervention
The intervention was tailored to the realities of urban African American couples and focused on the enhancement of positive evaluations of self-worth, self-esteem, ethnic pride, and risk avoidance. The content of this culturally congruent curriculum combined skills building exercises guided by Nguza Saba principles that promote valuing health and working together to preserve families and communities. The intervention incorporated Eban, a traditional African concept meaning “fence,” a symbol of safety, security, and love within one’s family and relationship space. Group sessions promoted a “village concept” of collective support to a community surrounded and protected by Eban. Group cohesion was encouraged through certain group activities, including opening and closing rituals, the “talking circle,” and the setting of group rules for safety and respect. Elements of the intervention that foster application of the Nguza Saba for practicing safer sex were followed by exercises that promote sexual risk reduction skills. (See “Eban HIV/STD Prevention Intervention: Conceptual Basis and Procedures” in this issue.)
Eban Health Promotion Intervention (Control)
The Eban Health Promotion Intervention was the control condition that was also an active intervention with health related endpoints. In contrast to the treatment condition, this intervention was guided by a social cognitive approach to developing health promotion skills without providing cultural context or gender tailoring. (See Figure 4.) It focused on setting goals for individuals and did not encourage partners to work together in choosing healthier behaviors.
Figure 4.
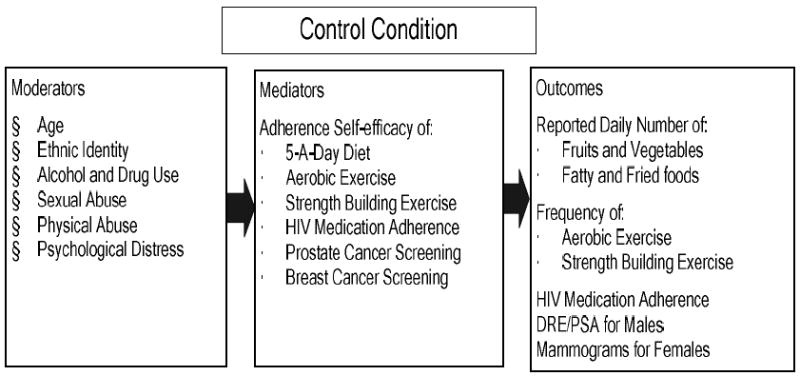
Conceptual Framework for Eban Health Promotion Intervention
Although the intervention did not focus on HIV/STD risk reduction, because of the ethical obligations to serodiscordant couples, HIV/STD testing and counseling were provided during all assessment visits. Also, in the intervention sessions, participants were presented information and skills to increase their adherence to medication regimens, including those for HIV. The presentation focused on behaviors related to risk for certain cancers, hypertension, and heart disease, which are the leading causes of morbidity and mortality among African Americans. Participants were taught that changing personal behaviors can prevent these diseases. The behaviors included increasing physical activity and healthy dietary practices, cessation of cigarette smoking and abusing alcohol and drugs, and practicing early detection and screening behaviors (e.g., breast and testicular self-examinations and Pap smears). Because all couples received an active intervention and participated in VCT, this intervention addressed the ethical issues and minimized differential attrition between conditions. (See “Eban Health Promotion Intervention: Conceptual Basis and Procedures” in this issue.)
Assessment of Outcomes
The assessment activities were designed to measure the constructs central to the theoretical frameworks on which the interventions were designed. (See Figures 3 and 4 for these constructs.) The behavioral questionnaire in ACASI included 296 questions for baseline, 217 for IPT, and 299 for the 6 month and 12 month and 50 questions in the face-to-face interview. There were six questions focused on the behavior outcome.45 (See “Developing an Audio Computer-Assisted-Self-Interview (ACASI) for a Multisite Trial” in this issue.)
Data on childhood sexual abuse, physical abuse, relationship satisfaction, and social desirability were collected using a face-to-face survey. If a participant displayed significant distress, the Data Collector halted the assessment and the participant was provided brief counseling and referred for mental health services.
There were intervention specific primary endpoints of interests including two primary endpoints (behavioral and biological) for the treatment intervention. The primary behavioral endpoint for the treatment intervention was reduction in HIV/STD risk behavior that was assessed independently for each couple member using six self report questions about sexual practices with study partners) The primary biological endpoint was reduction in incident STD cases (Chlamydia, gonorrhea, and trichomoniasis) during any of the follow up phases (IPT, 6 and 12 months). The primary endpoint for the control intervention was an increase in health promoting behaviors (screening for cancer, diet, exercise).
The assessment sessions involved approximately two hours each at baseline, 6-and 12-month follow-up. To maintain the integrity of the intervention, the Data Collector was blinded to treatment condition and interacted with couples only on assessment issues. A Data Collector was available to assist with ACASI.
(See “Challenges of Selecting Outcome Measures for the NIMH Multisite HIV/STD Prevention Trial for African American Couples” in this issue.)
Data Analysis Plans
The Trial will not complete final data collection until August 2008 and therefore the plans for analysis are presented below.
The primary objective of this study was to test the efficacy of a couples based HIV sexual risk reduction intervention versus a health promotion control group in increasing condom use and reducing STD incidence in a sample of African American HIV serodiscordant couples. More than 500 couples were randomized to each of the two interventions. Data were collected at baseline, IPT, 6-and 12-months post-intervention. The aims were concerned with within couple specific variation and between treatment differences. Parallel intent-to-treat and completer analyses will be performed for all study aims. Attrition rates will be examined for comparability between the two treatment conditions. If the attrition rates differ, variables related to attrition might constitute confounding factors that limit internal validity; accordingly, such variables will be used as covariates. General statistical design issues for the proposed study, including topics that cut across aims, are highlighted.
Analysis of Baseline Data
Baseline data for eligible enrolled participants will be compared to data for participants not enrolled, in order to temper the generalizability of the trial results. Additionally, baseline data were compared across intervention conditions, to ensure that the couples randomized to each intervention were comparable. These summaries across the Trial and by sites were prepared by the DCC for review by the Steering Committee on a quarterly basis and at least every six months by the DSMB, which permitted monitoring the progress and data quality of the Trial.
Analysis of Primary Endpoints at Follow-up
The primary aims focus on testing for significant differences in sexual risk behaviors and STD incidence between the two conditions. Outcome measures for primary analysis are couple specific incident rates of condom protected sexual contact and STD incidence over the 12-month follow-up period. In addition, the secondary aims are to: (1) identify variables that moderate the intervention induced changes in the primary outcome variables and (2) determine variables that mediate the effects of the intervention. Additionally, the effects of moderators and mediators in examining other secondary behavioral outcomes (e.g., number of condom protected oral sex acts, etc.) will be examined.46 Major statistical challenges in testing these hypotheses arise in the proper handling of repeated clustered outcomes. Each variable of interest was completed by each partner of each couple, thus creating correlated outcomes. Each couple was nested within a cohort of 3 to 5 couples, which was further nested within one of the four study sites. Multiple assessments of each variable over the study period produce repeated outcomes for each couple. Thus, the analytic strategy accommodated the unique multilevel nested structures of the data.
The primary challenge in the analysis of such data will be to make appropriate adjustments for the differential treatment means between clusters, and the correlations among the observations within a cluster (cluster effects). Each nested level of the study design will form a cluster. Most statistical models assume stochastic independence among observations, and thus are inappropriate for clustered data. The two most common approaches for handling clustered data are mixed effects modeling (MEM) and generalized estimating equations (GEE) modeling. The MEM approach employs normal variates (random effects) to account for the within cluster correlations, and uses standard likelihood methods for statistical inferences, whereas GEE modeling avoids explicit modeling of the within cluster correlations by basing statistical inferences of model parameters on marginalized likelihood or generalized estimating equations. Although MEM is inappropriate for non-normal data because it uses normal variates for both cluster effects and model errors, it is appropriate if the data are approximately normal. The normality assumption was examined in this study using various descriptive statistics such as histogram and quartile plots based on the standardized residuals. GEE requires a relatively large sample size (e.g., large numbers of small clusters), which this study satisfied. The implementation of both approaches for this study is relatively straightforward. For both approaches, between cluster variations such as treatment differences are modeled by fixed effects. Modeling of the within cluster correlations is not required for GEE; for MEM, such correlations are modeled by random effects. Although site differences may be treated as either fixed or random effects, such differences will be modeled using fixed effects, because such a strategy will enable examination of possible site-by-treatment interactions. Ultimately, the choice of MEM and/or GEE will be based on each approaches strengths and limitations, based on the underlying assumptions of each modeling approach.
An intent-to-treat analysis, for which all available data on all randomized participants are included, will be used for the primary control of the two intervention conditions. Every attempt will be made to keep missing data to a minimum, and participants who fail to attend intervention sessions were strongly encouraged to complete follow-up assessments. Analyses to examine whether screened eligible non-participants and participants differ on screened variables will be performed. Attrition analyses will be performed on whether participants and dropouts differ on key variables (informative censoring), and whether variables on which they differ interact with the intervention to affect outcome measures. A few randomly missing observations have only a slight impact upon study power and do not introduce biases. Larger numbers of nonrandom missing data (e.g., if distressed participants are not compliant) can bias study findings. Various methods were used to determine the severity of missing data on the results of the analyses. Sensitivity analyses for all hypotheses under various missing data correction methods were examined. If a finding was not dependent on the missing data mechanism, then significant findings should be consistent across all imputation methods. The number and proportion of missing data points estimated by these methods will be reported. Analyses were conducted with and without interpolated data and any differences in results were reported.
There are two major outcomes for the primary hypotheses, one behavioral and the other biological. Since these two outcomes may be correlated, it is difficult to control for Type I error arising from using them in testing the primary hypotheses. The common Bonferroni procedure for adjusting Type I error within such a multiple control setting is likely to be unnecessarily conservative. Thus, the general principle is to use multiple control procedures, mostly for the secondary analyses, since they are based on weak a priori hypotheses. By using multiple comparison procedures selectively, the study will guard against Type I errors and avoid being too conservative, thereby protecting against missing important statistical findings.
Human Subjects
Because this Trial recruited HIV serodiscordant African American couples, there was a strong imperative to design a trial adhering to the highest ethical standards for research subjects. (See “Ethical Issues in Designing and Conducting a Trial for Serodiscordant Couples” in this issue.)
Informed Consent
At Visit 1 the study was preliminarily discussed and informed consent was provided. At baseline (Visit 2), the interviewer explained the aims, requirements, risks and benefits of the study. The interviewer assisted participants in understanding the informed consent form, which had been reviewed by both the Institution’s IRB and the NIMH appointed Data Safety Monitoring Board (DSMB). Participants were asked to sign and date the form and were given a copy. Signed informed consent forms were kept in locked files in a locked office with restricted access.
After consent, the interviewer reconfirmed eligibility and completed a Short Locator Form. Each subject was then escorted to a gender specific testing room and given instructions on how to complete the ACASI. When it was completed, each person was escorted to a private room for a face-to-face interview. Next, each subject received protesting counseling and provided urine specimens. Women also provided a self-administered vaginal swab. Participants were then reimbursed and scheduled within a 21-day window for randomization.
Reimbursement
Each subject received a total of $395 for participating in all eight intervention sessions and four data collection sessions over 14 months. Participants received $5 for initial screening and $5 for confirmatory screening. Also, participants received $25 for post-intervention assessment, $50 for 6-and 12-month follow-up assessments, and $10 for each urine or vaginal specimen provided at any of the assessment points.
Data Safety and Monitoring Plan
Ongoing monitoring ensured that there were no undue risks to participants, that participants in one arm were not experiencing more adverse events than the other, and that the data were being collected in a valid and reliable way. All staff and investigators had been trained to identify and report potential adverse events and to implement corrective actions. Three entities provided ongoing monitoring of the trial: (1) Steering Committee and the DCC; (2) the local IRB and in country IRB; and (3) the NIMH and the DSMB. Because there was limited experience with adverse events when working with African American serodiscordant couples, the Steering Committee decided to cast a broad net and collect information on all unusual events and classify them later. All events were reviewed by each site’s respective PI within 24 hours of the event, reported to the DCC and then reviewed by the SC on its next conference call. The Steering Committee determined whether the event was related to the study in any way, whether it met criteria for an adverse event and what corrective action should be taken. Adverse events must be reported to the IRB. The IRB reviewed and approved all local informed consent forms and procedures. Each time a change was made in the protocol, the IRB was asked to approve it. To date, the Steering Committee has not identified any adverse events.
Quality Control/Quality Assurance Procedures
In this Trial, quality control (QC) procedures are the methods used to ensure that data were collected in a standardized way and interventions were implemented in a similar fashion across sites with fidelity to the Protocol. This included development of a detailed Protocol, using standard criteria for hiring staff, consistent staff training and supervision, and development of manualized interventions.47 Quality assurance (QA) procedures addressed adherence to the protocol and study procedures, assessment, and interventions, as well as whether the quality control procedures were effective. They included onsite quality assurance led by PIs or designees and central monitoring (coordinated by the DCC and designated sites). There are three major components of a QC/QA model adopted in this Trial: (1) personnel, (2) manuals for the multisite study, and (3) ongoing monitoring of adherence to study procedures. (See Table 3 for the model.)
Study Personnel
For each role in the Trial there was a detailed job description, educational requirements, and practical experience. All study personnel were trained both at the site and centrally. Ongoing supervision by a well trained research staff at each site and ongoing feedback across sites on conference calls were found to be effective ways to maintain a high level of adherence to the Protocol.
Manuals for the Multisite Study
A complete Protocol was developed that detailed how all aspects of the Trial should be implemented. There were also complete training manuals for implementing the Assessment, Biospecimen Collection, and the Interventions.
Ongoing Monitoring of Adherence to Study Procedures
Site visits were conducted for all aspects of the Trial. The Quality Assurance Monitor for the interventions, observed sessions, interviewed facilitators, and reviewed files. Ten percent of the sessions in each cohort were reviewed via audio recordings. The Session Adherence Scale, developed for this study and using the same quantitative items as the Facilitator Session Implementation Form, was tailored to the content of each session for each arm. It was used to assess how adequately the key elements of the session were addressed and the time spent delivering each element. The Facilitation Skills Scale, also developed for this study, was a quantitative measure with a different version for each arm. It assessed facilitators’ adherence to the intended delivery style. To ensure sufficient reliability of these two measures, two raters reviewed 50% of the selected audio recordings for the first cohort and interpreter reliability was checked.
The data collectors were trained by the DCC that provided feedback to them after review of the ACASI and face-to-face interview data.
A team at Emory University provided feedback and generates detailed reports on adherence to biospecimen procedures. Site visits are conducted annually to observe CRCs conducting biospecimen procedures. A standard form outlining feedback was provided to the PI, DCC, and the SC for review. Significant deviation from the biospecimen protocol was an indication that the site needed retraining.
Summary
This Trial is an innovative example of how social, behavioral, and biological scientists collaborate to tackle the unrelenting HIV epidemic among African American HIV serodiscordant couples. The successful completion of this Trial will advance the knowledge base on couples based HIV prevention intervention research with the following innovative achievements, as the Trial: (1) addressed previous methodological limitations by using a rigorous RCT design to test the efficacy of the intervention in increasing self reported condom use and decreasing the incidence of biologically confirmed STDs over a 12-month follow-up period among more than 500 African American HIV serodiscordant couples; (2) adapted an Afro centric, culturally congruent approach to build self efficacy to protect oneself, one’s partner, and the African American community from HIV/AIDS and to reduce stigma and social isolation experienced by African American HIV serodiscordant couples, a population which remains underserved and at very high risk of HIV/AIDS transmission; (3) employed a multisite design replicating a common protocol in four geographically diverse cities, which represent 25% of all HIV/AIDS cases nationally, increasing the generalizability of the findings; (4) used a novel mixed modality approach in which couples benefit both from sessions as individual couples and from sessions with groups of couples; (5) adopted an innovative strategy to ensure that STDs acquired over the follow-up period are incident infections (many HIV prevention trials use STDs to determine intervention efficacy); and (6) allowed for progress reports to be made available to CABs and the collaborating agencies on a regular basis and a summary of the major findings of the study were made available to the four groups at its completion.
In individual level interventions. the participant may become reinfected if they have unsafe sex with a partner who is not treated. The Eban protocol, by testing and treating both partners of a couple, ensured that the HIV and STD cases detected in the couple were incident cases.
This study has the potential to significantly contribute to the prevention of HIV/STDs in the African American community and with serodiscordant couples. African American couples affected by HIV have been ignored by the research community, especially in the context of designing culturally tailored and theoretically sound risk reduction interventions. Whether the couples were randomized to the treatment or control condition, they benefited by learning how to reduce their health risks and to enhance their overall health. If both interventions demonstrate efficacy, the investigators will collaborate with the CABs and the agencies from which the Trial recruited to facilitate adoption of these evidence based prevention programs for these underserved populations in order to stop the dual epidemics of HIV/STDs and chronic illnesses.
Table 4.
Components of Model of Quality Control/Quality Assurance Plan
| Study Personnel |
|
| Manuals for the multisite study |
|
| Ongoing monitoring of adherence to study procedures |
|
References
- 1.Centers for Disease Control and Prevention (CDC) HIV/AIDS Surveillance Report. 2003, Divisions of HIV/AIDS Prevention. 2004 [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) HIV/STD Surveillance Report, 2003. Vol. 15. Atlanta: US Department of Health and Human Services, CDC; 2004. pp. 1–46. [Google Scholar]
- 3.Silverberg MJ, Wegner SA, Milazzo MJ, McKaig RG, Williams CF, Agan BK, Armstrong AW, Gange SJ, Hawkes C, O’Connell RJ, Ahuja SK, Dolan MJ, for the Tri-Service AIDS Cooinical Consortium Natural History Study Group Effectiveness of highly active antiretroviral therapy by race/ethnicity. AIDS. 2006;20:1531–1538. doi: 10.1097/01.aids.0000237369.41617.0f. [DOI] [PubMed] [Google Scholar]
- 4.Anderson RN, Smith BL. Deaths: Leading causes for 2001. National Vital Statistics Reports. 2003;52(9):27–33. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) HIV/AIDS Surveillance Report (Tech Rep No 11) Atlanta, GA: U.S. Department for Health and Human Services; 1999. [Google Scholar]
- 6.Wyatt G, Moe A, Guthrie D. The gynecological, reproductive, and sexual health of HIV positive women. Cultural Diversity and Ethnic Minority Psychology. 1999;5(3):183–196. [Google Scholar]
- 7.Catania J, Coates T, Kegeles S, et al. Condom use in multiethnic neighborhoods of San Francisco: The population based AMEN (AIDS in multiethnic neighborhoods) study. Am J Public Health. 1992;82(2):284–287. doi: 10.2105/ajph.82.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Overby KJ, Kegeles SM. The impact of AIDS on an urban population of high risk female minority adolescents: Implications for intervention. Journal of Adolescent Health. 1994;15(3):216–217. doi: 10.1016/1054-139x(94)90507-x. [DOI] [PubMed] [Google Scholar]
- 9.Wingood G, DiClemente R. Partner influences and gender related factors associated with non-condom use among young adult African American women. American Journal of Community Psychology. 1998;26(1):29–51. doi: 10.1023/a:1021830023545. [DOI] [PubMed] [Google Scholar]
- 10.Wingood GM, DiClemente RJ. The influence of psychosocial factors, alcohol, drug use on African American women’s high risk sexual behavior. American Journal of Preventive Medicine. 1998;15(1):54–59. doi: 10.1016/s0749-3797(98)00027-0. [DOI] [PubMed] [Google Scholar]
- 11.Hunt WK, Myers HF, Dyche M. Living with risk: Male partners of HIV positive women. Cultural Diversity and Ethnic Minority Psychology. 1999;5:276–289. [Google Scholar]
- 12.Grosskurth H, Gray R, Hayes R, Mabey D, Wawer M. Control of sexually transmitted diseases for HIV-1 prevention: Understanding the implications of the Mwanza and Rakai trials. Lancet. 2000;355:1981–1987. doi: 10.1016/S0140-6736(00)02336-9. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC) HIV/AIDS Surveillance Report. Atlanta, GA: U.S. Department for Health and Human Services; 2000. [Google Scholar]
- 14.NIH/NHLBI. Archives of Internal Medicine. 21. Vol. 157. NIH/NHLBI; 1997. The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure; pp. 2413–2446. [DOI] [PubMed] [Google Scholar]
- 15.Neumann MS, Johnson WD, Semaan S, et al. Review and meta-analysis of HIV prevention intervention research for heterosexual adult populations in the United States. Journal of Acquired Immune Deficiency Syndrome. 2002;30:S106–S117. [PubMed] [Google Scholar]
- 16.Allen S, Serufilira A, Bogaerts J, et al. Confidential HIV testing and condom promotion in Africa. Impact on HIV and gonorrhea rates. JAMA. 1992;268(23):3338–3343. [PubMed] [Google Scholar]
- 17.Allen S, Meinzen-Derr J, Kautzman M, Zulu I, Treask S, Fideli U, Musonda R, Kasolo F, Gao F, Haworth A. Sexual behavior of discordant couples after HIV counseling and testing. AIDS. 2003;17:733–740. doi: 10.1097/00002030-200303280-00012. [DOI] [PubMed] [Google Scholar]
- 18.Padian NS, O’Brien TR, Chang YC, Glass S, Francis D. Prevention of heterosexual transmission of human immunodeficiency virus through couple counseling. Journal of Acquired Immune Deficiency Syndromes. 1993;6:1043–1048. [PubMed] [Google Scholar]
- 19.De Vincenzi I, for the European Study Group on Heterosexual Transmission of HIV A longitudinal study of human immunodeficiency virus transmission by heterosexual partners. N Engl J Med. 1994;331:341–66. doi: 10.1056/NEJM199408113310601. [DOI] [PubMed] [Google Scholar]
- 20.Deschamps M, Pape J, Haffner A, Hyppolite R, Johnson W. Heterosexual activity in at risk couples for HIV infection. Abstract WC3089 presented at the Seventh International AIDS Conference; Florence, Italy. 1991. p. 318. [Google Scholar]
- 21.Deschamps MM, Paper JW, Hafner A, Johnson WD. Heterosexual Transmission of HIV in Haiti. 1996 August 15;125(4):324–330. doi: 10.7326/0003-4819-125-4-199608150-00011. [DOI] [PubMed] [Google Scholar]
- 22.Voluntary HIV-1 Counseling and Testing Efficacy Study Group. Efficacy of voluntary HIV-1 counseling and testing in individuals and couples in Kenya, Tanzania and Trinidad: A randomized trial. Lancet. 2000;356:103–112. [PubMed] [Google Scholar]
- 23.El-Bassel N, Witte S, Gilbert L, et al. HIV prevention for intimate couples: A relationship based model. Families, Systems, and Health. 2001;19(4):379–395. [Google Scholar]
- 24.El-Bassel N, Witte SS, Gilbert L, et al. The efficacy of a relationship based HIV/STD prevention program for heterosexual couples. Am J Public Health. 2003;93(6):963–969. doi: 10.2105/ajph.93.6.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Bassel N, Witte SS, Gilbert L, et al. Long-term effects of an HIV/STI sexual risk reduction intervention for heterosexual couples. AIDS & Behavior. 2005;9(1):1–13. doi: 10.1007/s10461-005-1677-0. [DOI] [PubMed] [Google Scholar]
- 26.Glick P. Scaling up HIV voluntary counseling and testing in Africa. Evaluation Review. 2005;29(4):331–357. doi: 10.1177/0193841X05276437. [DOI] [PubMed] [Google Scholar]
- 27.van der Straten A, Vernon K, Knight K, Gomez C, Padian N. Managing HIV among serodiscordant heterosexual couples: Serostatus, stigma and sex. AIDS Care. 1998;10(5):533. doi: 10.1080/09540129848406. [DOI] [PubMed] [Google Scholar]
- 28.van der Straten A, King R, Grinstead O, Serufilira A, Allen S. Couple communication, sexual coercion and HIV risk reduction in Kigali, Rwanda. AIDS. 1995;9:935–944. doi: 10.1097/00002030-199508000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Kamenga M, Ryder RW, Jingu M, et al. Evidence of marked sexual behavior change associated with low HIV-1 seroconversion in 149 married couples with discordant HIV-1 serostatus: experience at an HIV counseling center in Zaire. AIDS. 1991;5:61–67. doi: 10.1097/00002030-199101000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Weinhardt LS, Carey M, Johnson B, Bickham N. Effects of HIV counseling and testing on sexual risk behavior: A meta-analytic review of published research, 1985-1997. Am J Public Health. 1999;89(9):1397–1405. doi: 10.2105/ajph.89.9.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wyatt GE, Myers HF, Loeb TB. Women, Trauma, and HIV: An Overview. AIDS and Behavior. 2004;8(4):401–403. doi: 10.1007/s10461-004-7324-3. [DOI] [PubMed] [Google Scholar]
- 32.Wyatt GE, Longshore D, Chin D, et al. The Efficacy of an Integrated Risk Reduction Intervention for HIV Positive Women with Child Sexual Abuse Histories. AIDS and Behavior. 2004;8(4):453–462. doi: 10.1007/s10461-004-7329-y. [DOI] [PubMed] [Google Scholar]
- 33.O’Leary A, Wingood GM. Interventions for sexually active heterosexual women. In: Peterson JL, DiClemente RJ, editors. Handbook of HIV Prevention. Plenum Publishing Corp; New York: 2000. pp. 179–197. [Google Scholar]
- 34.Kelly JA. Advances in HIV/AIDS education and prevention. Family Relations. 1995;44:345–352. [Google Scholar]
- 35.Tanner WM, Pollack RH. The effect of condom use and erotic instructions on attitudes toward condoms. Journal of Sex Research. 1988;25:537–541. [Google Scholar]
- 36.Ehrhardt AA. Narrow vs. broad targeting of HIV/AIDS education: Response. Am Public Health. 1994;84(3):498–499. doi: 10.2105/ajph.84.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basen-Engquist K. Psychosocial predictors of “safer sex” behaviors in young adults. AIDS Education and Prevention. 1992;4(2):120–134. [PubMed] [Google Scholar]
- 38.Fisher JD, Fisher WA. Changing AIDS risk behavior. Psychological Bulletin. 1992;111(3):455–474. doi: 10.1037/0033-2909.111.3.455. [DOI] [PubMed] [Google Scholar]
- 39.Nadler A, Fisher JD. Volitional personal change in an interpersonal perspective. In: Klar Y, Fisher JD, Chinsky J, Nadler A, editors. Initiating Self Change: Social Psychological and Clinical Perspectives. SpringerVerlag; New York: 1992. pp. 213–230. [Google Scholar]
- 40.Kalichman SC, Kelly JA, Hunter TL, Murphy DA, Tyler R. Culturally tailored HIV/AIDS risk reduction messages targeted to African American urban women: Impact on risk sensitization and risk reduction. Journal of Consulting and Clinical Psychology. 1993;61:291–295. doi: 10.1037//0022-006x.61.2.291. [DOI] [PubMed] [Google Scholar]
- 41.Sormanti M, Pereira L, El Bassel N, Witte S, Gilbert L. The role of community consultants in designing an HIV prevention intervention. AIDS Education and Prevention. 2001;13(4):311–328. doi: 10.1521/aeap.13.4.311.21431. [DOI] [PubMed] [Google Scholar]
- 42.Witte S, el-Bassel N, Gilbert L, Wu E, Chang M, Steinglass P. Recruitment of minority women and their main sexual partners in an HIV/STI prevention trial. Journal of Women’s Health. 2004;13(10):1137–1147. doi: 10.1089/jwh.2004.13.1137. [DOI] [PubMed] [Google Scholar]
- 43.Wu E, el Bassel N, Witte S, Gilbert L, Chang M, Morse P. Enrollment of minority women and their main sexual partners in an HIV/STI prevention trial. AIDS Education and Prevention. 2005;17(1):41–52. doi: 10.1521/aeap.17.1.41.58685. [DOI] [PubMed] [Google Scholar]
- 44.Bellamy SL, for the NIMH Multisite HIV/STD Prevention Trial for African American Couples Study Group A dynamic block randomization algorithm for group randomized clinical trials when the composition of blocking factors is not known in advance. Contemporary Clinical Trials. 2005;26(4):469–479. doi: 10.1016/j.cct.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Metzger DS, Koblin B, Turner C, Navaline HM, Sheon A, Miller H, Cooley P, Seage G, and the HIVNET Vaccine Preparedness Study Protocol Team Randomized controlled trial of audio computer-assisted self-interviewing: Utility and acceptability in longitudainal studies. Am J of Epidemiology. 2000;152:2. doi: 10.1093/aje/152.2.99. [DOI] [PubMed] [Google Scholar]
- 46.Baron RM, Kenny DA. The moderator and mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of Personality & Social Psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 47.NIMH Multisite HIV/STD Prevention Trial. Quality control/quality assurance. In: Coutinho R, Fishbein M, editors. AIDS. 1997. [Google Scholar]
- 48.NIMH Collaborative HIV/STD Prevention Trial. Role of the data safety and monitoring board in an international trial. In: van Griensven F, Kalichman SC, editors. AIDS. Vol. 2007. 2007. pp. 99–102. [Google Scholar]


