Abstract
Objective:
Establish the dose-response relationship between increasing sleep durations in a single night and recovery of neurobehavioral functions following chronic sleep restriction.
Design:
Intent-to-treat design in which subjects were randomized to 1 of 6 recovery sleep doses (0, 2, 4, 6, 8, or 10 h TIB) for 1 night following 5 nights of sleep restriction to 4 h TIB.
Setting:
Twelve consecutive days in a controlled laboratory environment.
Participants:
N = 159 healthy adults (aged 22-45 y), median = 29 y).
Interventions:
Following a week of home monitoring with actigraphy and 2 baseline nights of 10 h TIB, subjects were randomized to either sleep restriction to 4 h TIB per night for 5 nights followed by randomization to 1 of 6 nocturnal acute recovery sleep conditions (N = 142), or to a control condition involving 10 h TIB on all nights (N = 17).
Measurements and Results:
Primary neurobehavioral outcomes included lapses on the Psychomotor Vigilance Test (PVT), subjective sleepiness from the Karolinska Sleepiness Scale (KSS), and physiological sleepiness from a modified Maintenance of Wakefulness Test (MWT). Secondary outcomes included psychomotor and cognitive speed as measured by PVT fastest RTs and number correct on the Digit Symbol Substitution Task (DSST), respectively, and subjective fatigue from the Profile of Mood States (POMS). The dynamics of neurobehavioral outcomes following acute recovery sleep were statistically modeled across the 0 h-10 h recovery sleep doses. While TST, stage 2, REM sleep and NREM slow wave energy (SWE) increased linearly across recovery sleep doses, best-fitting neurobehavioral recovery functions were exponential across recovery sleep doses for PVT and KSS outcomes, and linear for the MWT. Analyses based on return to baseline and on estimated intersection with control condition means revealed recovery was incomplete at the 10 h TIB (8.96 h TST) for PVT performance, KSS sleepiness, and POMS fatigue. Both TST and SWE were elevated above baseline at the maximum recovery dose of 10 h TIB.
Conclusions:
Neurobehavioral deficits induced by 5 nights of sleep restricted to 4 h improved monotonically as acute recovery sleep dose increased, but some deficits remained after 10 h TIB for recovery. Complete recovery from such sleep restriction may require a longer sleep period during 1 night, and/or multiple nights of recovery sleep. It appears that acute recovery from chronic sleep restriction occurs as a result of elevated sleep pressure evident in both increased SWE and TST.
Citation:
Banks S; Van Dongen HPA; Maislin G; Dinges DF. Neurobehavioral dynamics following chronic sleep restriction: dose-response effects of one night for recovery. SLEEP 2010;33(8):1013–1026.
Keywords: Recovery sleep, sleep dose response, chronic sleep restriction, neurobehavioral, homeostatic sleep drive, sleep duration, sleep deprivation, sleep loss, sleep need, PVT, KSS, MWT
RECOVERY OF NEUROBEHAVIORAL FUNCTIONS FROM CHRONIC CURTAILMENT OF SLEEP DURATION AS A RESULT OF WORK, MEDICAL CONDITIONS, OR lifestyle1 is not well understood. It has been rarely studied, despite the fact that a common sleep pattern for millions of people involves sleep restriction for 5 weekdays/workdays, followed by sleep extension on at least one weekend night (or day off from work).2–4 Much of what is known about recovery from sleep loss has been based on total sleep deprivation experiments, where robust NREM EEG slow wave activity (SWA, 0.5-4.5Hz) responses are the norm.5–9 Experiments in chronically sleep-restricted rats revealed increased recovery sleep duration, NREM and REM sleep durations, and elevated SWA, while only a small portion of the chronically lost sleep was actually recovered.10,11
Experiments in healthy humans have confirmed that chronic reduction of sleep can result in waking neurobehavioral deficits that become progressively worse over days;12–15 that the rate of accumulation of waking deficits is a function of the magnitude of the sleep restriction;12,15,16 and that measures of sleepiness, performance lapsing, and cognitive slowing can accumulate to deficit levels found for total sleep deprivation.15 These findings indicate that waking brain impairment from chronic sleep loss is sleep dose-dependent, that it can be as severe as that resulting from total sleep deprivation, and that the “sleep debt” is a result of prior sleep-wake history extending back in time more than a day. Thus, chronic sleep restriction appears to induce slow changes (spanning days to weeks) in neural processes mediating alertness, attention and other aspects of cognitive functioning, including learning and memory.17 How these slow (cumulative) changes are reversed via the dynamics of recovery sleep is not known.
Kleitman suggested “sleep debts” are “liquidated” by extending recovery sleep duration (p. 317).18 However, the primary model of human sleep homeostasis, the two-process model,19,20 posits that the intensity and temporal dynamics of NREM EEG SWA, more so than sleep duration, reflect the recovery process. For example, the two-process model predicts only an initial modest (∼10%-20%) elevation in SWA over the first few days of sleep restricted to 4 h per night, which has been experimentally confirmed,15,21 although increases of 50% have been reported for a broader EEG frequency band (1.25-7.75 Hz).22 The relatively modest increment in SWA during and following sleep restriction is not congruent with the large cumulative neurobehavioral deficits that develop across days of sleep restriction.15 The apparent uncoupling during chronic sleep restriction of the putative marker of homeostatic sleep drive (SWA) and waking neurobehavioral functions suggests that sleep duration and/or other aspects of sleep (e.g., REM sleep) may also have a critical role in recovery of neurobehavioral capability following chronic sleep restriction. On the other hand, the high degree of colinearity among SWA, TST and the duration of sleep stages may prevent attributing recovery from chronic sleep restriction to a specific physiological feature of sleep.
The dynamics of recovery of human waking alertness and neurobehavioral functions following chronic sleep restriction have not been systematically investigated. Experiments in healthy adults scheduled to 7 nights of sleep restricted to 3 h-7 h TIB12 or 5 nights of sleep restricted to 4 h TIB22 yielded data suggesting that some neurobehavioral functions may not return to baseline following up to 3 recovery sleep periods limited to 8 h TIB.12,23 Studying the dynamics of recovery from cumulative sleep loss is critical to a range of behavioral guidelines (e.g., days off duty for recovery from work schedules),24 biological questions (e.g., mechanisms and rates of homeostatic sleep drive build-up and dissipation),25 and theoretical issues (e.g., processes to instantiate into mathematical models predicting sleep and alertness).26–28The present experiment was designed to provide the first systematic, randomized, sleep dose-response data on the dynamic recovery of neurobehavioral functions when a single recovery sleep opportunity follows 5 days of nocturnal sleep restriction to 4 h TIB. The study tested the hypothesis that following sleep restriction, recovery of primary measures of neurobehavioral alertness would increase monotonically in relation to the duration of time allowed for recovery sleep. We also sought to determine the features of sleep that parallel this recovery.
METHODS
A total of 159 healthy adults completed a 12-day laboratory protocol (i.e., a total of 1,908 experimental days in the laboratory). The study was approved by the Institutional Review Board of the University of Pennsylvania, and all subjects were compensated for their participation. There were no serious adverse events during the study.
Subjects
Healthy individuals, aged 22-45 y, were recruited to the study in response to study advertisements. They reported habitual nightly sleep durations between 6.5 h and 8.5 h, and habitual morning awakenings between 06:00 and 09:00, with no evidence of habitual napping and no sleep disturbances (i.e., no complaints of insomnia, daytime sleepiness, or other sleep-wake disturbances). They were free of acute and chronic medical and psychological conditions, as established by interviews, clinical history, questionnaires, physical exams, and blood and urine tests. Subjects were monitored at home with actigraphy, sleep-wake diaries, and time-stamped phone records for time to bed and time awake during the week immediately before the 12-day laboratory phase and for 1 week after the laboratory phase. A behavioral estimate of circadian phase position was obtained with a morningness/eveningness questionnaire.29 Subjects recruited to the study were nonsmokers and had a body mass index (BMI) between 19 and 30. They did not participate in shift work, transmeridian travel, or irregular sleep/wake routines in the 60 days prior to the study. Sleep health was established by a night of laboratory PSG and oximetry measurements. Subjects were not permitted to use caffeine, alcohol, tobacco, and medications (except oral contraceptives) in the week before the laboratory experiment, as verified by blood and urine screens. Out of a total of 171 eligible subjects empanelled into the 12-day laboratory protocol, n = 6 (3.5%) withdrew before the protocol was completed for personal reasons (primarily related to time commitment required), and n = 6 (3.5%) were withdrawn for non-serious health reasons (e.g., gastrointestinal problems, headache, etc.).
Laboratory Protocol
Subjects were studied in small groups for 12 consecutive days (continuously for 288 h) in the Sleep and Chronobiology Laboratory at the Hospital of the University of Pennsylvania, with daily clinical checks of vital signs and symptoms by nurses (with an independent physician on call). They were randomized as a group (n = 4 to 5 per group) to either the sleep restriction condition (N = 142) or control condition (N = 17). In the sleep restriction condition, subjects had 2 initial baseline nights (B1, B2) of 10 h TIB per night (22:00–08:00), followed by 5 nights (SR1-SR5) of sleep restricted to 4 h TIB per night (04:00–08:00). Sleep restriction to 4 h for 5 consecutive nights was selected because this degree of sleep restriction produces cumulative neurobehavioral deficits in most healthy adults15 and it is within the range of sleep restriction that can occur as a result of lifestyle factors.
To model recovery of neurobehavioral measures of alertness across a dynamic range of recovery sleep opportunities, subjects were then randomized to one of 6 doses of sleep on the recovery (REC) night: 0 h TIB (no sleep), 2 h TIB (06:00-08:00), 4 h TIB (04:00-08:00), 6 h TIB (02:00-08:00), 8 h TIB (00:00-08:00), or 10 h TIB (22:00-08:00). The remaining 4 nights of the study involved other conditions not reported here. The control condition involved all the same procedures as the sleep restriction condition, except that subjects were allowed 10 h TIB (22:00–08:00) every night of the protocol. Subjects were informed of their randomization to the sleep restriction or control condition on the second baseline night (B2), and if they were assigned to the sleep restriction condition they were informed of their recovery sleep dose during the afternoon of sleep restriction day 4 (SR4). Subjects were behaviorally monitored by trained staff continuously throughout the protocol to ensure adherence to the experimental protocol. They had daily contact with the Project Manager and/or the Principal Investigators. Demand characteristics were controlled by ensuring subjects remained blinded to their recovery condition, by continuous monitoring of subjects' performance for adherence, and by sustained encouragement of subjects to perform to the best of their ability at all times.
Subjects wore a wrist actigraph throughout the 12-day laboratory protocol (as well as 1 week before and after the laboratory protocol). On protocol days B1, B2, SR1, SR5, and REC, they wore ambulatory EEG and ECG recording equipment throughout the day and night. During the days without EEG, subjects were given shower opportunities between 14:30 and 16:00. Meals were provided at regular times throughout the protocol (08:30-10:00; 12:30-14:00; 18:30-20:00). The light levels in the laboratory were held constant at < 50 lux during scheduled wakefulness and < 1 lux during scheduled sleep periods. Ambient temperature was maintained between 22°-24°C.
Neurobehavioral Outcomes
Subjects completed 30-min bouts of computerized neurobehavioral assessments every 2 h during scheduled wakefulness, beginning at 08:00 each day. Those randomized to the sleep restriction condition had additional, abbreviated test bouts (20 min) after 20:00 during the sleep restriction phase of the study, and also on the day before recovery sleep if they were randomized to a sleep dose < 10 h TIB. The 30-min neurobehavioral assessment bouts included a 10-min Psychomotor Vigilance Test (PVT; ISI 2-10s),30–32 a 3-min Digit Symbol Substitution Task (DSST),33 the Karolinska Sleepiness Scale (KSS),34 and the Profile of Mood States (POMS).35 Daily averages of outcome variables (see below) were computed over the test bouts administered during a 12-h period from morning to early evening (08:00, 10:00, 12:00, 14:00, 16:00, 18:00, 20:00). Between neurobehavioral test bouts, subjects were permitted to read, watch movies and television, play card/board games, and interact with laboratory staff to help them stay awake, but no naps/sleep or vigorous activities (e.g., exercise) were allowed.
A modified MWT, used to measure the ability to stay awake in a soporific environment, was completed on baseline day 2 (B2), sleep restriction day 5 (SR5) and on the recovery day (REC). The MWT procedure was modified from that described in earlier studies,36–38 in that a single MWT trial limited to 30 min was conducted between 14:30 and 16:00. All other aspects of the test including EEG montage (C3/A2, O2/A1), environment, and sleep onset definition were as previously described.36 Before each trial, subjects were instructed to “keep eyes open and try not to fall asleep,” and the lights were dimmed to 10 lux. Each trial was terminated at the first occurrence of a microsleep (10 s of EEG theta activity) or at 30 min if sleep onset was not achieved.36,37 The latency to the onset of the microsleep was measured, or if sleep onset was not reached, a value of 30 min was recorded.
Neurobehavioral outcomes for this study were selected for their representativeness of different waking functional domains of alertness and for their known sensitivity to sleep-wake dynamics, including chronic partial sleep deprivation.1 Primary outcomes for hypothesis testing included well-validated behavioral, subjective, and physiological measures of alertness. The behavioral measure was the number of lapses of attention (RT ≥ 500ms) on the PVT; the subjective measure was sleepiness on the KSS; and the physiological measure was latency to the first microsleep on the modified MWT. Secondary neurobehavioral outcomes addressed additional functional domains that were less well validated for sensitivity to chronic sleep restriction. These included psychomotor speed as measured by the mean of the PVT fastest 10% RTs (PVTf10), cognitive speed (number correct) measured by the DSST, and subjective fatigue from the POMS (POMSf).
Sleep Physiology
PSG recordings (EEG derivations C3-A2, Fz-A1, O2-A1; EOG LOC/ROC; submental EMG) were conducted with the Sandman Suzanne portable digital recording system (128-Hz sampling) on B1, B2, SR1, SR5, and REC. The EEG from the C3-A2 derivation was scored according to the criteria of Rechtschaffen and Kales39 by trained technicians. Sleep onset was conservatively defined by the occurrence of ≥ 3 consecutive 30-s epochs of stage 2-4 or REM sleep. Total sleep time, sleep efficiency (TST percentage of TIB), REM sleep, stage 2 sleep, and slow wave sleep (SWS; stages 3+4) were determined for each recorded night. Subjects with missing or artifact-ridden PSG data on the final baseline night or the recovery night were not included in the PSG analyses, which yielded a total of N = 118 sleep-restricted subjects and N = 17 control subjects for PSG analyses.
Slow wave energy (SWE) was calculated as integrated power in the delta band (0.5-4.5 Hz) totaled over all 30-s epochs of NREM sleep (stages 2-4). The NREM sleep EEG was analyzed in 5-s bins using fast Fourier transform, after visually determined EEG artifacts were removed. Only data from the C3-A2 derivation are presented here. Total SWE for each night was expressed as percentage of the B2 night for each subject. In n = 16 subjects the EEG quality prevented reliable SWE analysis, and in n = 23 subjects B2 data were missing, leaving a total of N = 104 sleep-restricted subjects and N = 16 control condition subjects for SWE analyses.
Statistical Methods
Data analyses were performed using the intent-to-treat framework40 in order to retain the bias-reducing benefits of randomization and to maximize relevance of the experiment to the practical question of recovery sleep scheduling. Before proceeding with linear and non-linear dose-response analyses of recovery sleep duration, the effects of the 5-day sleep restriction protocol were characterized by estimation of relevant parameters including standardized effect sizes. Mixed model repeated measures (MMR) analyses were used to demonstrate the effects of restricting sleep-induced changes on neurobehavioral outcomes as compared to the control condition. To avoid making any assumptions concerning the shape of changes across days, time was considered a categorical variable.
Analyses of recovery involved formal testing of the existence of a dose response relationship (for the 0 h to 10 h TIB recovery sleep doses) for 3 primary neurobehavioral outcomes (PVT lapses, KSS score, and modified MWT sleep latency) followed by: (a) assessment of non-linearity of dose responsiveness; (b) prediction of minimum recovery sleep duration necessary to reach pre-sleep restriction baseline; and (c) prediction of minimum recovery sleep duration necessary to reach performance levels exhibited by sleep-satiated controls treated similarly in the laboratory but with no sleep restriction. The study design was optimized to test the primary research hypothesis that following sleep restriction to 4 h TIB for 5 nights, recovery of neurobehavioral function would increase monotonically in relation to the duration of time allowed for recovery sleep. A linear dose-response was assumed for the purpose of testing the overall experiment-wise primary hypothesis. This approach was selected for its generally good statistical power under a variety of underlying monotonic shapes. For the purpose of type I error control, the primary null and alternative hypotheses were formulated in terms of absence versus presence of a significant linear recovery-sleep dose-response relationship. Subsequent analyses assessed theory-driven hypotheses relative to whether the shape of the predicted recovery dose-response relationships could be better characterized through the use of exponential and sigmoidal models.
The primary hypothesis implied that a significant percentage of variance in each of the primary neurobehavioral outcomes (PVT lapses, KSS score, and modified MWT sleep latency) would be explainable by a linear association with duration of recovery sleep. For each of these outcomes, least squares estimation was used to determine parameters of an analysis of covariance (ANCOVA) regression model that included a fixed set of a priori chosen covariates. Appropriate model modifications were made for the MWT data, which were acquired only at B2 and SR5. Covariates included the pre-sleep restriction baseline value of the outcome variable, a factor reflecting the cumulative effects of the sleep restriction protocol (determined as the area under the curve of changes from baseline during the sleep restriction days—i.e., subjects' individual rates of accumulating sleepiness during 5 days of sleep restriction), age and sex. One set of a priori covariates was chosen for all analyses in order to maximize generalizability. Continuous covariates were centered on their grand means, and sex was centered on male so as to make model intercepts interpretable as the predicted value for a representative enrolled male. Since three primary outcomes were tested, overall experiment-wise significance required P < 0.05/3 = 0.0167 for at least one of the 3 primary endpoints. The ANCOVA models used to test the primary hypotheses incorporated recovery sleep dose (0 h, 2 h, 4 h, 6 h, 8 h, 10 h) as a single degree-of-freedom continuous variable.
Shapes of the recovery sleep dose-response curves were determined in subsequent analyses. Four candidate models were compared for each parameter including linear (model 1), exponential (models 2 and 3), and sigmoidal (model 4) functional forms. Exponential forms were specified with (model 3) and without (model 2) restricting the asymptote to be equal to baseline (a difference of one parameter). Maximum likelihood estimation (MLE, SAS Proc NLMIXED) was used to evaluate parameters for each dose response functional form. Akaike's information criterion41 (AIC) was used to select the best-fitting functional form for each outcome variable. AIC penalizes the better fit of more complex models (i.e., those with more parameters) by a factor proportional to the number of additional parameters. AIC is scaled such that the model with the minimum AIC is identified as the one that most adequately fits the data. The functional forms of the models and summaries of the AIC model comparisons are provided in the Supplement Tables (supplementary material is available online only at www.journalsleep.org).
Analyses were performed to determine the duration of recovery sleep necessary for predicted performance to return to pre-deprivation levels (i.e., baseline) levels. Separate analyses that included controls were performed to determine the duration of recovery sleep necessary for those who underwent 4 h TIB for 5 days to reach a predicted performance level equal to that of control subjects (10 h TIB per night) in the same experimental context on the same day of the protocol. Models involving comparisons to controls did not include the covariate for the subject-specific cumulative effects of prior sleep loss since controls did not undergo experimental sleep restriction. The same analyses were performed on secondary neurobehavioral outcomes (DSST, PVTf10, POMSf). Analyses were also performed for all 6 neurobehavioral outcomes by replacing TIB with TST as the primary independent variable. There was a statistically significant correlation between TIB and TST on all nights of the study (all r > 0.95, all P < 0.001), and no new findings were observed using TST that were not found using TIB. Therefore, this report emphasizes primarily TIB-based analyses and results. TST results are reported for some analyses to present the amount of physiological sleep acquired during recovery TIB following sleep restriction.
Adjusted linear dose-response ANCOVA models, in which covariates included baseline sleep, age, and gender, were used to evaluate sleep parameters across recovery nights. Categorical sleep duration was used to avoid making linearity assumptions when testing these models. Shapes of the recovery sleep-dose response curves were determined in subsequent analyses, as described above, across the 2 h-10 h TIB recovery conditions.
RESULTS
Subjects were 30.4 y ± 7.0 y (mean ± SD) of age (median = 29 y). They had a BMI of 25.1 ± 3.5, and pre-laboratory nocturnal sleep durations of 7.9 h ± 0.6 h; 49% were female; and 63% were from racial/ethnic minorities. Table 1 shows the demographics of subjects randomized to the control and sleep restriction conditions, and among the latter, of subjects randomized to each of the recovery sleep doses. There were no statistically significant differences in age, BMI, pre-laboratory sleep duration, or Morningness-Eveningness scores among the randomized subgroups.
Table 1.
Demographic and subject characteristics by group and sleep dose.
| All Subjects n = 159 | Control 10h TIB n = 17 | Sleep Restricted n = 142 | Recovery Sleep Dose |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0h TIB n = 13 | 2h TIB n = 27 | 4h TIB n = 29 | 6h TIB n = 25 | 8h TIB n = 21 | 10h TIB n = 27 | ||||
| Age (y)1 | 30.4 ± 7.0 | 29.8 ± 6.8 | 30.6 ± 7.0 | 31.3 ± 7.1 | 32.1 ± 8.2 | 31.9 ± 6.9 | 30.0 ± 6.7 | 29.5 ± 5.9 | 28.7 ± 6.7 |
| Females (%) | 49.1 | 52.9 | 48.6 | 61.5 | 40.7 | 48.3 | 48.0 | 52.4 | 48.1 |
| BMI | 25.1 ± 3.5 | 25.5 ± 3.3 | 24.7 ± 3.6 | 25.0 ± 4.2 | 24.9 ± 3.8 | 24.1 ± 2.8 | 24.5 ± 3.6 | 24.4 ± 4.3 | 25.3 ± 3.5 |
| Caucasian (%) | 36.5 | 17.6 | 38.7 | 53.8 | 40.7 | 41.4 | 36.0 | 38.1 | 29.6 |
| AA2 (%) | 57.2 | 64.7 | 56.3 | 23.1 | 55.6 | 55.2 | 64.0 | 61.9 | 63.0 |
| Hispanic (%) | 3.8 | 5.9 | 3.5 | 15.4 | 3.7 | 3.4 | 0 | 0 | 3.7 |
| Asian (%) | 2.5 | 11.8 | 1.4 | 7.7 | 0 | 0 | 0 | 0 | 3.7 |
| Pre-lab TST (h)1 | 7.9 ± 0.6 | 7.8 ± 0.4 | 8.1 ± 0.7 | 8.3 ± 0.6 | 8.0 ± 0.4 | 8.0 ± 1.0 | 8.2 ± 0.5 | 8.1 ± 0.6 | 8.0 ± 0.7 |
| MEQc1 | 38.9 ± 5.6 | 38.2 ± 5.6 | 39.7 ± 5.6 | 39.2 ± 6.7 | 40.5 ± 5.7 | 40.4 ± 5.3 | 38.2 ± 6.5 | 38.7 ± 5.4 | 40.9 ± 4.9 |
Mean ± SD.
African American.
BMI, body mass index; Pre-lab TST, actigraph + sleep diary estimated mean nightly sleep duration during the week prior to the laboratory study. MEQc, Morningness-Eveningness Composite Score (all means are intermediate between evening and morning types).26
Effect of Sleep Restriction on Sleep Physiology
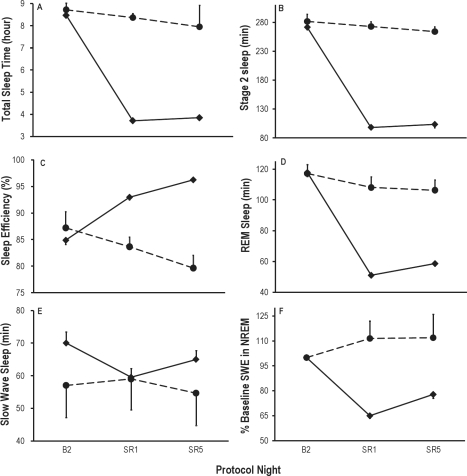
Figure 1 displays the sleep responses at baseline (B2; 10 h TIB) and on the first (SR1) and fifth (SR5) nights for N = 118 subjects in the sleep restriction condition (solid line), and N = 17 control subjects (10 h TIB on all nights) on equivalent protocol nights. The effect size analyses for the sleep parameters illustrated in Figure 1 are summarized in Table S1 of the Supplement. Figure 1A shows the expected reduction in TST from 8.47 h at baseline to 3.72 h on SR1 and 3.85 h on SR5. The mean changes from baseline to first and fifth restriction nights were 4.73 h and 4.64 h, respectively, corresponding to standardized effect sizes (ES) of −4.57 and −4.31, respectively (both P < 0.0001; see Supplement Table S1). The mean increase in TST from SR1 to SR5 was 7.51 minutes (ES = 0.46, P < 0.0001). The pattern for stage 2 sleep (Figure 1B) was similar to that of TST except that there was no significant change in stage 2 time from SR1 to SR5. Sleep efficiency (Figure 1C) had a mean increase from 84.6% at baseline to 93.0% on the first restriction night (ES = 0.88, P < 0.0001), and increased further from SR1 to SR5 by 3.8%, (ES = 0.52, P < 0.0001). REM sleep time (Figure 1D) significantly decreased from 117.3 min at baseline to 51.0 min on SR1, then increased from SR1 to SR5 by 7.3 min (ES = 0.41, P < 0.0001).
Figure 1.
Mean ± SEM for sleep variables at baseline (B2 = 10 h TIB) and on the first (SR1) and fifth (SR5) nights of sleep restriction to 4 h TIB (04:00-08:00) for N = 118 sleep-restricted subjects (solid line), and N = 17 control subjects (dashed line) who received 10 h TIB (22:00-08:00) on all protocol nights. The effect sizes for the sleep parameters illustrated in Figure 1 are summarized in Supplement Table S1. As expected, sleep restriction decreased TST (graph A, P < 0.0001), stage 2 sleep (B, P < 0.0001), REM sleep (D, P < 0.0001), SWS (E, P < 0.0001), SWE (F, P < 0.0006), and increased sleep efficiency (C, P < 0.0001). There were small but reliable increases from SR1 to SR5 in TST (A, P < 0.0001), sleep efficiency (C, P < 0.0001), REM sleep (D, P < 0.0001), SWE (F, P < 0.0006), and SWS (E, P < 0.0006) but not stage 2 sleep (B, P > 0.05). The control group did not differ from the sleep restriction group on any of the sleep variables at baseline (all P > 0.2). Subjects in the control condition had a reduction in mean TST from 8.74 h on B2 to 7.95 h (P = 0.02) on the seventh night of 10 h TIB (equivalent night to SR5), and thus an 8% decrease in sleep efficiency across these nights, P = 0.02. No one specific aspect of sleep physiology accounted for the decreased TST across protocol nights (stage 2 sleep, P = 0.26; REM sleep, P = 0.85; SWS, P = 0.38; and SWE, P = 0.96).
Table S1.
Standardized effect sizes (ES) for chronic sleep restriction changes in physiological sleep parameters
| BL to SR1 | BL to SR5 | SR1 to SR5 | |
|---|---|---|---|
| TST | −4.57** | −4.31** | +0.46** |
| Stage 2 | −3.83** | −3.44** | −0.10 |
| Sleep Efficiency | +0.88** | +1.18** | +0.52** |
| REM (minutes) | −2.38** | −2.01** | +0.41** |
| SWS (minutes) | −0.41** | −0.14 | +0.32* |
| SWE | −2.15* | −0.91* | +0.50* |
BL, baseline sleep (10 h time in bed [TIB]); SR1, sleep restriction (4 h TIB) night 1; SR5, sleep restriction (4 h TIB) night 5; TST, total sleep time; SWE, slow wave energy during NREM sleep.
paired t-test P < 0.0006;
paired t-test P < 0.0001.
SWS (Figure 1E) showed a pattern unlike other sleep variables. Although it decreased from a mean of 69.0 min at baseline to 59.5 min at SR1 (ES = −0.41, P < 0.0001), and increased to 64.9 min at SR5 (ES = 0.32, P < 0.0006), the mean change from baseline to SR5 was not significantly different from zero (ES = −0.14, P = 0.14), due to an increase in SWS from SR1 to SR5 (ES = 0.32, P < 0.0006).
SWE (Figure 1F) was by definition 100% at baseline. It decreased to a mean of 64.8% on SR1 (ES = −2.15, P < 0.0001), then increased to 77.8% on SR5 (ES = 0.50, P < 0.0001). Unlike SWS, SWE remained significantly below baseline levels at SR5 (ES = −0.91, P < 0.0001), although there was an increase in SWE from SR1 to SR5 (ES = 0.50, P < 0.0001)—see Supplement Table S1.
The control group did not differ from the sleep restriction group on any of the sleep variables at baseline (all P > 0.2). TST, stage 2 sleep, REM sleep, and SWE were significantly greater in the control group relative to the sleep restriction group on both nights SR1 and SR5 (all P < 0.001). Sleep efficiency was higher in the sleep restriction group than in the control group on both SR1 and SR5 nights (P < 0.001). SWS did not differ significantly between the 2 groups on any night (P > 0.05).
Subjects in the control condition slept a mean of 8.74 h of the 10 h TIB on baseline night B2, but by the eighth night of 10 h TIB (equivalent to SR5 in the sleep-restricted condition), they slept on average 7.95 h, a mean decrease of 46 min (P = 0.02). No other control group sleep variable exhibited a significant change from baseline to the eighth night in the control group (stage 2 sleep, P = 0.26; REM sleep, P = 0.85; SWS, P = 0.38; and SWE, P = 0.96).
Effect of Sleep Restriction on Neurobehavioral Functions
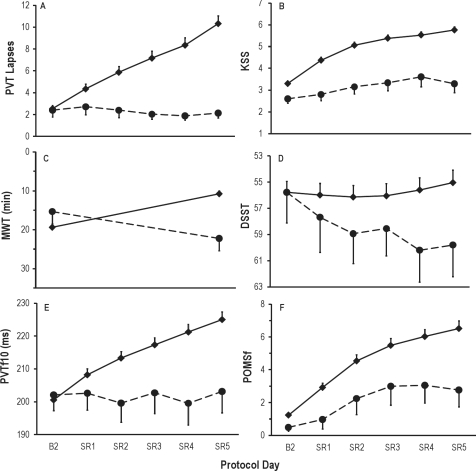
Figure 2 displays the cumulative effects on neurobehavioral outcomes of 5 nights of sleep restricted to 4 h TIB relative to the control condition (i.e., 10 h TIB each night). Sleep restriction effects were evident in the interaction between condition (sleep restriction vs. control) and time (across protocol days). These interactions were statistically significant for all primary and secondary neurobehavioral outcomes shown in Figure 2 (PVT number of lapses, F5,785 = 12.6, P < 0.0001; KSS sleepiness score, F5,785 = 10.3, P < 0.0001; MWT sleep latency, F1,295 = 4.8, P < 0.0001; DSST number correct, F5,785 = 4.8, P < 0.0001; PVT fastest 10%, F5,785 = 14.4, P < 0.0001; POMS fatigue score, F5,784 = 2.9, P = 0.01). The main effect of time was also significant (P < 0.0001) for all outcomes in Figure 2, except for DSST number correct (P = 0.052). This was the result of learning countering the effects of sleep restriction on DSST performance across days. The control group displayed this performance improvement due to learning over time (F5,80 = 5.5, P = 0.002; Figure 2D). Changes across days were not significant in control subjects for any other variables except the MWT, which displayed a significant increase in sleep latency from B2 to SR5 (P = 0.02) in response to the extended (10 h) TIB provided to controls each day (Figure 2C).
Figure 2.
Daily means (± SEM) of 6 neurobehavioral assessments in the sleep restriction group (N = 142, 4 h TIB for 5 nights [SR1-SR5], solid line), and the control group (N = 17, 10 h TIB on all nights, dashed line). All subjects had 10 h TIB (22:00-08:00) on baseline day 2 (B2). Sleep restriction on SR1 to SR5 was from 04:00 to 08:00. Data are plotted to show deficits in neurobehavioral functions increasing (upward) on the ordinate. Relative to the control condition, sleep restriction degraded all neurobehavioral functions over days (graph A, increased PVT lapses, P < 0.0001; B, increased KSS scores, P < 0.0001; C, decreased MWT sleep latency [assessed on B2 and SR5], P < 0.0001; D, decreased DSST number correct, P < 0.0001; E, increased PVT fastest RTs, P < 0.0001; F, increased POMS fatigue, P = 0.01). Control group performance on the DSST improved significantly (P = 0.002) across days due to learning (D), while MWT sleep latency increased significantly (P = 0.02) across days due to the extended (10h) TIB provided to control subjects each day (C).
Neurobehavioral Outcomes as a Function of Recovery Sleep Dose
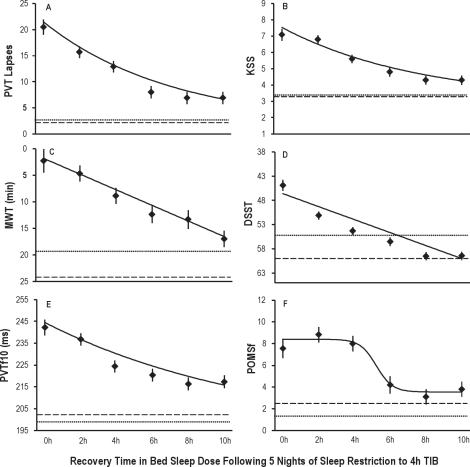
Figure 3 displays the 0 h-10 h recovery sleep dose effects on neurobehavioral responses. For comparison, the mean baseline values for the sleep restriction group and the mean values for the control group (on the day equivalent to REC) are shown as horizontal lines. Controlling for baseline performance, cumulative deficit during sleep restriction, age, and sex, the estimated linear slope (standard error over sleep doses) for PVT lapses was −1.38 (0.10), shown in Figure 3A (t115 = −8.89, P < 0.0001, R2 = 74.98%). KSS ratings had an adjusted slope of −0.32 (0.04), shown in Figure 3B (t115 = −8.41, P < 0.0001, R2 = 67.89%). MWT latencies had an adjusted slope of 1.47 (0.21), shown in Figure 3C (t115 = 6.99, P < 0.0001, R2 = 34.33%). Thus, for the primary outcome variables, a significant linear recovery sleep dose response was found beyond chance (i.e., a Bonferroni corrected type 2 threshold of P < 0.0167). The same results were obtained for the secondary neurobehavioral outcomes. DSST number correct had an adjusted slope (SE) of 1.34 (0.12), shown in Figure 3D (t115 = 11.20, P < 0.0001, R2 = 89.79%). PVT fastest 10% RTs had an adjusted slope of −2.67 (0.38), shown in Figure 3E (t115 = −7.04, P < 0.0001, R2 = 81.86%). POMS fatigue had an adjusted slope of −0.60 (0.10), shown in Figure 3F (t115 = −6.05, P < 0.0001, R2 = 64.59%).
Figure 3.
Neurobehavioral outcomes as a function of increasing TIB dose (0 h-10 h) following the acute recovery (REC) night. N = 142 sleep restricted subjects were randomized to either 0 h TIB (n = 13), 2 h TIB (n = 27), 4 h TIB (n = 29), 6 h TIB (n = 25), 8 h TIB (n = 21), or 10 h TIB (n = 27). Least squares means (± SEM) are shown as diamonds for each REC sleep dose subgroup, controlling for covariates (i.e., baseline, cumulative deficits during sleep restriction, age, and sex). For comparison, horizontal dotted lines show baseline night (B2, 10 h TIB) values, and horizontal dashed lines show control group (N = 17) means on day 8 (10 h TIB), which is the day equivalent to REC. All neurobehavioral outcomes showed improvement as recovery sleep doses increased (graph A, PVT lapses decreased, P < 0.0001; B, KSS sleepiness decreased, P < 0.0001; C, MWT latencies increased, P < 0.0001; D, DSST number correct increased, P < 0.0001; E, PVT fastest RTs shortened, P < 0.0001; F, POMS fatigue decreased, P < 0.0001). Best-fitting recovery sleep dose-response functions (from AIC) are shown as the solid lines in each graph (see Supplement Tables S2 to S8). These functions are exponential with asymptote set to baseline (graphs A, B, E), linear (graphs C, D) and sigmoidal (graph F). Least squares means (diamonds) represent the overall covariate-controlled group means; best-fitting functions are shown for males with the other covariates set to the sample means.
Analyses of Non-Linearity of Dose Response Curves
To determine the functional shape of how recovery sleep dose affected the response curves for each of the neurobehavioral outcomes, four models that included the recovery sleep dose condition were estimated—linear dose response (model 1), exponential dose response (model 2), exponential dose response restricting the asymptote to baseline (model 3) and sigmoidal dose response (model 4). The model that best approximated the reality of the functional recovery of each neurobehavioral outcome was identified as the one with the smallest value for Akaike's information criterion41 (AIC). The functional forms of the four models, and the null model (no recovery condition effect included), which was estimated for comparison purposes, are provided in Supplement Table S2, and AIC comparisons are provided for the primary (Tables S3 to S5) and secondary outcomes (Tables S6 to S7).
Table S2.
Summary of the four models considered to describe the recovery sleep dose-response curves, and the null model estimated for comparison purposes.
| Model | Model description* | df |
|---|---|---|
| 0 | Null model, constant (mean) plus 4 covariates | 1 + 4 = 5 |
| 1 | Linear dose response | 2 + 4 = 6 |
| 2 | Exponential dose response | 3 + 4 = 7 |
| 3 | Exponential dose response with asymptote set to baseline | 2 + 4 = 6 |
| 4 | Sigmoidal dose response | 4 + 4 = 8 |
The following 4 covariates were used: Baseline level, cumulative changes in the prior days of sleep restriction (neurobehavioral responses only, except MWT), age, and sex.
Table S3.
Akaike's information criterion (AIC) for the Models in Table S2 fit to Psychomotor Vigilance Test (PVT) lapses across recovery sleep doses (smallest AIC values are shown first as they indicate the most adequately fitting models).
| Model | AIC | Estimate | R | R2 |
|---|---|---|---|---|
| 3 | 750.4 | 25.743 | 0.8387 | 0.7034 |
| 2 | 752.1 | 25.687 | 0.8737 | 0.7635 |
| 4 | 753.1 | 25.458 | 0.8750 | 0.7656 |
| 1 | 756.9 | 27.167 | 0.8659 | 0.7498 |
| 0 | 818.1 | 45.804 | 0.7604 | 0.5783 |
Table S5.
Akaike's information criterion (AIC) for the Models in Table S2 fit to Maintenance of Wakefulness (MWT) sleep latency scores across recovery sleep doses (smallest AIC values are shown first as they indicate the most adequately fitting models).
| Model | AIC | Estimate | R | R2 |
|---|---|---|---|---|
| 1 | 998.2 | 59.937 | 0.5860 | 0.3433 |
| 3 | 999.2 | 60.343 | 0.5642 | 0.3184 |
| 2 | 1001.1 | 60.718 | 0.5823 | 0.3391 |
| 0 | 1039.8 | 81.479 | 0.3277 | 0.1074 |
Model 4 was not estimable.
Table S6.
Akaike's information criterion (AIC) for the Models in Table S2 fit to Digit Symbol Substitution Test (DSST) number correct across recovery sleep doses (smallest AIC values are shown first as they indicate the most adequately fitting models).
| Model | AIC | Estimate | R | R2 |
|---|---|---|---|---|
| 1 | 692.6 | 15.967 | 0.9476 | 0.8979 |
| 3 | 696.7 | 16.519 | 0.9171 | 0.8411 |
| 0 | 779.9 | 33.381 | 0.8869 | 0.7867 |
Models 2 and 4 were not estimable.
Table S7.
Akaike's information criterion (AIC) for the Models in Table S2 fit to Psychomotor Vigilance Test (PVT) fastest 10% RTs across recovery sleep doses (smallest AIC values are shown first as they indicate the most adequately fitting models).
| Model | AIC | Estimate | R | R2 |
|---|---|---|---|---|
| 3 | 972.5 | 161.30 | 0.8910 | 0.7939 |
| 1 | 975.9 | 166.02 | 0.9047 | 0.8186 |
| 2 | 977.0 | 163.48 | 0.9055 | 0.8199 |
| 0 | 1017.3 | 237.55 | 0.8604 | 0.7404 |
Model 4 was not estimable.
Models fit to PVT lapses revealed that the exponential dose response function with asymptote set to baseline (i.e., model 3) had the lowest (most adequate) AIC = 750.4 (R2 = 70.3%; Supplement Table S3). The R2 value for the null model that included only covariates for the mean recovery and change from baseline was 57.8% (model 0; Supplement Table S3). The difference between the R2 values of models 0 and 3 reflects the incremental predictive capacity of the exponential dose response function with asymptote set to baseline (i.e., model 3) when accounting for the recovery dose response. The corresponding squared partial correlation for model 3 was computed as (0.703-0.587)/(1-0.587) = 0.281, which indicates that exponential model 3 accounted for 28.1% of the unexplained variance in PVT lapses relative to the null hypothesis model. Figure 3A presents the predicted values from model 3 as a smooth line plotted over the least squares estimates (means ± SEM) from an unrestricted categorical dose response relationship. Although the best fitting model was the exponential dose response with asymptote set to pre-sleep-restriction baseline, the squared partial correlation for the linear model 1 accounted for 41.1% of the unexplained variance in the recovery dose-response function. Thus, while the pattern of unrestricted least square means as well as the AIC analyses suggest an exponential recovery dose response, on an individual basis, the assumption of linear dose response appears to perform at least as well for PVT lapses.
The model with the lowest AIC value for KSS scores was also the exponential function with asymptote set to baseline (model 3, Supplement Table S4). The R2 for exponential model 3 was 67.6%, and the R2 for linear model 1 was 67.9%. The R2 for null hypothesis model 0 was 48.2%. Thus, after accounting for baseline KSS, age, gender, and the subjects' individual rates of accumulating sleepiness during 5 days of sleep restriction (4h/day), the duration of recovery sleep modeled exponentially with asymptote to baseline, explaining 37.6% (squared partial correlation) of the unexplained variance in KSS scores. As for PVT lapses, the squared partial correlation for the KSS linear model 1 also explained a comparable portion of the residual variance (i.e., 37.9%) in the recovery sleep dose-response function. Figure 3B displays the predicted values from model 3 as a smooth line plotted over the least squares estimates (means ± SEM) from an unrestricted categorical sleep dose-response relationship.
Table S4.
Akaike's information criterion (AIC) for the Models in Table S2 fit to Karolinska Sleepiness Scale (KSS) scores across recovery sleep doses (smallest AIC values are shown first as they indicate the most adequately fitting models).
| Model | AIC | Estimate | R | R2 |
|---|---|---|---|---|
| 3 | 406.4 | 1.502 | 0.8224 | 0.6764 |
| 2 | 408.5 | 1.501 | 0.8283 | 0.6862 |
| 1 | 409.3 | 1.536 | 0.8239 | 0.6789 |
| 4 | 410.8 | 1.504 | 0.8279 | 0.6855 |
| 0 | 465.3 | 2.480 | 0.6939 | 0.4815 |
The linear model had the lowest AIC for MWT sleep latency (model 1; Supplement Table S5). The R2 for this model was 34.3%. The R2 for null hypothesis model 0 was 10.7%. The squared partial correlation revealed the additional variance explained by the linear model over the null model was 26.4%. Figure 3C displays the predicted values from model 1 relative to the least squares estimates (means ± SEM) from an unrestricted categorical sleep dose-response relationship.
The models with the lowest AIC values for the secondary outcomes of DSST number correct (Figure 3D), PVT fastest 10% (Figure 3E), and POMS fatigue (Figure 3F) were linear (model 1), exponential with asymptote set to baseline (model 3), and sigmoidal (model 4), respectively (see Supplement Tables S6, S7, S8). The additional variance explained in DSST performance by linear model 1 over null model 0 was 52.1%. The additional variance explained in PVT fastest 10% RTs by exponential model 3 over null model 0 was 20.6%. The additional variance explained in POMS fatigue scores by sigmoidal model 4 over null model 0 was 31.9%. As with all 3 primary outcomes, for DSST and PVT fastest 10% the proportion of total variance explained by linear model 1 was larger than or only slightly smaller than the optimal model selected by AIC (see Supplement Tables S6, S7, S8).
Table S8.
Akaike's information criterion (AIC) for the Models in Table S2 fit to Profile of Mood States (POMS) fatigue subscale scores across recovery sleep doses (smallest AIC values are shown first as they indicate the most adequately fitting models).
| Model | AIC | Estimate | R | R2 |
|---|---|---|---|---|
| 4 | 642.6 | 10.214 | 0.8257 | 0.6819 |
| 1 | 651.5 | 11.369 | 0.8037 | 0.6459 |
| 3 | 654.0 | 11.602 | 0.7900 | 0.6242 |
| 0 | 683.0 | 14.993 | 0.7301 | 0.5330 |
Model 2 was not estimable.
Analyses of Neurobehavioral Recovery to Baseline and Control Levels
The extent of recovery from the chronic sleep restriction conditions was assessed in two ways: (1) by comparing the neurobehavioral outcomes after each recovery sleep dose to the pre-sleep-restriction baseline values (within-subjects comparisons), and (2) by projecting the linear sleep-dose recovery model to determine an intersection of each neurobehavioral outcome with the control group's data after the same number of days in the laboratory. Linear models (rather than exponential models) were used to ensure intersection values could be extrapolated for neurobehavioral dose-response functions (except POMS fatigue), because intersection with control group values was not guaranteed with exponential models.
Post Recovery Neurobehavioral Outcomes Relative to Baseline
Table 2 displays the results of pairwise comparisons between each recovery (REC) sleep dose subgroup (condition) and the subjects' respective baseline values (B2). These comparisons revealed that among the 10 h TIB recovery group, PVT lapses and KSS ratings remained elevated above baseline (P = 0.008 and P = 0.007, respectively). This was also the case for the secondary outcomes of PVT fastest 10% RTs (P = 0.005) and POMS fatigue scores (P = 0.021). MWT sleep latency was the third primary outcome, but as revealed in Table 2, unlike PVT lapses and KSS scores, it was not significantly different from baseline at the 8 h and 10 h TIB recovery conditions. The final (secondary) outcome—DSST number correct—showed apparent recovery to baseline at 6 h TIB, but the recovery function on this outcome was affected by substantial learning over days, making the comparisons to baseline uninterpretable.
Table 2.
Pairwise comparison of each recovery sleep dose (REC) subgroup condition to their baseline (B2) values on each neurobehavioral outcome.
| REC TIB = | 0h |
2h |
4h |
6h |
8h |
10h |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | SD2 | P3 | M | SD | P | M | SD | P | M | SD | P | M | SD | P | M | SD | P | ||
| PVT lapses4 | B2 | 2.2 | 2.7 | < 0.001 | 1.8 | 1.9 | < 0.001 | 2.8 | 3.0 | < 0.001 | 1.7 | 1.6 | < 0.001 | 3.4 | 6.2 | 0.004 | 3.1 | 3.5 | 0.008 |
| REC | 21.3 | 11.0 | 15.2 | 13.2 | 13.0 | 9.6 | 6.9 | 6.3 | 7.6 | 8.1 | 5.6 | 7.2 | |||||||
| KSS4 | B2 | 4.42 | 1.61 | < 0.001 | 3.5 | 1.1 | < 0.001 | 3.1 | 1.5 | < 0.001 | 3.0 | 1.1 | < 0.001 | 3.3 | 1.3 | < 0.001 | 3.0 | 1.5 | 0.007 |
| REC | 7.68 | 1.33 | 7.1 | 1.8 | 5.5 | 2.1 | 4.5 | 1.9 | 4.3 | 1.7 | 3.8 | 1.6 | |||||||
| MWT4 | B2 | 20.4 | 10.1 | < 0.001 | 22.1 | 10.7 | < 0.001 | 19.3 | 10.3 | < 0.001 | 18.5 | 9.6 | 0.009 | 16.9 | 10.4 | 0.072 | 18.8 | 9.9 | 0.558 |
| REC | 1.7 | 1.1 | 5.8 | 4.6 | 8.8 | 8.9 | 11.5 | 9.8 | 12.8 | 9.2 | 17.3 | 10.9 | |||||||
| DSST5 | B2 | 60.3 | 13.8 | < 0.001 | 55.6 | 8.8 | 0.013 | 54.8 | 8.4 | < 0.037 | 55.3 | 11.0 | 0.225 | 56.7 | 14.2 | 0.0266 | 56.0 | 8.5 | < 0.0016 |
| REC | 42.3 | 10.07 | 51.5 | 10.4 | 52.0 | 11.8 | 57.0 | 11.6 | 60.8 | 13.2 | 60.0 | 9.3 | |||||||
| PVTf105 | B2 | 198 | 15.9 | < 0.001 | 199 | 19.3 | < 0.001 | 207 | 24.9 | < 0.001 | 200 | 17.4 | < 0.001 | 196 | 16.4 | < 0.001 | 197 | 20.9 | 0.005 |
| REC | 245 | 24.0 | 234 | 34.1 | 230 | 33.3 | 220 | 25.8 | 215 | 23.5 | 207 | 27.9 | |||||||
| POMSf5 | B2 | 2.1 | 1.6 | < 0.003 | 1.1 | 1.4 | < 0.001 | 1.6 | 2.4 | < 0.001 | 0.7 | 1.5 | 0.002 | 1.4 | 1.6 | 0.006 | 0.9 | 1.2 | 0.021 |
| REC | 7.5 | 5.98 | 9.2 | 6.0 | 7.2 | 6.5 | 3.7 | 3.8 | 3.5 | 3.5 | 2.2 | 2.9 | |||||||
Mean,
Standard Deviation,
P value from pairwise t-test between B2 and REC.
Primary outcome.
Secondary outcome.
REC > B2.
PVT lapses, Psychomotor Vigilance Test number of lapses; KSS, Karolinska Sleepiness Scale scores; MWT, Maintenance of wakefulness test sleep latency; DSST, Digit Symbol Substitution Task number correct, PVTf10, Psychomotor Vigilance Test fastest 10% of reaction times; POMSf, Profile of Mood States fatigue subscale. Pairwise comparisons were made between baseline and each recovery dose condition (0 h n = 13, 2 h n = 27, 4 h n = 29, 6 h n = 25, 8 h n = 21, 10 h n = 27).
Post recovery neurobehavioral outcomes relative to the control condition.
Linear functions fit to the recovery sleep dose-adjusted means from the ANCOVA analyses (which controlled for baseline performance, cumulative deficit during sleep restriction, age, and sex) yielded estimated slopes that were extrapolated to find the point of intersection with the levels of neurobehavioral functioning observed in the non-sleep-restricted control group (10 h TIB/night) on the equivalent day in the laboratory.
As summarized in Table 3, for the 3 primary outcomes (PVT lapses, KSS sleepiness, and MWT sleep latency) the estimated dose of sleep (both TIB and TST) necessary to intersect the functional levels of the control group exceeded the maximum recovery dose of sleep provided in the experiment (i.e., > 10 h TIB). For all 3 primary outcomes, the intersection point estimated using TIB was close to that found using TST. While PVT lapses and KSS scores resulted in similar intersection points for TIB (10.66 h and 10.62 h, respectively) and for TST (10.00 h and 10.29 h, respectively), MWT sleep latency was projected to require much more sleep time for recovery to intersect with the control levels. It is noteworthy, however, that the control condition (10 h TIB) showed increasing MWT latencies from B2 to SR5 (i.e., control subjects were more alert near the end of the experiment than at baseline; Figure 2C). Among the 3 secondary outcomes, DSST number correct and POMS fatigue had intersection points of less than 10 h TIB, indicating that recovery to control group levels occurred at a sleep dose within the range of conditions examined in this study. PVT fastest reaction times were projected to require a sleep dose of more than 13 h TIB (11 h TST) to intersect the control condition. However, the confidence intervals for intersection with control group levels were large for all outcomes presented in Table 3.
Table 3.
Predicted recovery sleep dose at which sleep-restricted subjects would intersect the levels of neurobehavioral function of control subjects on each outcome.
| Time in Bed intersection with control group |
Total Sleep Time intersection with control group |
|||
|---|---|---|---|---|
| Intersection (h) | CI (h) | Intersection (h) | CI (h) | |
| PVT lapses | 10.66 | 7.97-13.34 | 10.00 | 7.32-12.68 |
| KSS score | 10.62 | 7.80-13.44 | 10.29 | 7.10-13.51 |
| MWT sleep latency | 15.16 | 10.41-19.90 | 15.76 | 10.10-21.44 |
| DSST # correct | 8.07 | 5.99-10.15 | 7.69 | 5.45-9.93 |
| PVT fastest 10% | 13.09 | 8.64-17.54 | 11.75 | 7.75-15.75 |
| POMS fatigue | 8.95 | 4.95-12.96 | 8.32 | 4.47-14.18 |
Intersection (h) refers to the projected recovery sleep time at which the linear model (fit to the sleep dose adjusted means from the ANCOVA analyses) intersects with the non-sleep-restricted control group (10 h TIB/night) for each outcome. CI (h), 95% confidence intervals on the intersection points. PVT lapses, Psychomotor Vigilance Test number of lapses; KSS, Karolinska Sleepiness Scale scores; MWT, Maintenance of Wakefulness Test sleep latency; DSST, Digit Symbol Substitution Task number correct, PVTf10, Psychomotor Vigilance Test fastest 10% of reaction times; POMSf, Profile of Mood States fatigue subscale.
Sleep Architecture as a Function of Recovery Sleep Dose
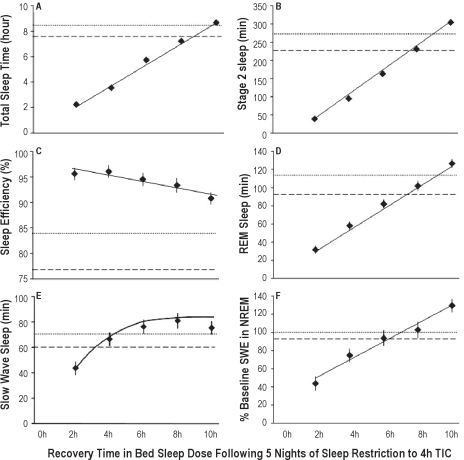
Figure 4 displays the adjusted mean (± SEM) physiological sleep characteristics (from ANCOVA analyses controlling for baseline performance, cumulative effect of the sleep restriction, age, and sex) as a function of sleep dose (TIB) on the recovery night (REC) in the sleep restriction condition, with horizontal lines showing the baseline and control condition (on the equivalent day of the protocol to REC). Total sleep time was 1.88 h (0.04 h) in the 2-h TIB group, 3.84 h (0.03 h) in the 4-h TIB group, 5.74 h (0.04 h) in the 6-h TIB group, 7.51 h (0.10 h) in the 8-h TIB group, and 8.96 h (0.18 h) in 10-h TIB group. Raw mean sleep efficiencies were 96.4% (2 h group), 95.9% (4 h group), 95.7% (6 h group), 93.9% (8 h group), and 89.6% (10 h group), which were above the mean sleep efficiencies of sleep-restricted subjects at baseline (B2 = 84.6%), and above the control group's mean sleep efficiency on the day equivalent to REC (protocol day 8 = 77.2%) (Figure 4C). (Note that the values listed above are the raw means, while those shown in Figure 4 are the adjusted means from the ANCOVA.)
Figure 4.
Recovery night (REC) sleep variables as a function of increasing TIB dose from 2 h to 10 h. Least squares means (± SEM; data from N = 118 subjects with complete PSG data) are shown as diamonds for each REC sleep dose subgroup, controlling for covariates (i.e., baseline, age and sex). For comparison, horizontal dotted lines show baseline night (B2, 10 h TIB) data, and horizontal dashed lines show the control group (N = 17) means on day 8 (10 h TIB), which is the day equivalent to REC. Increasing REC TIB dose increased TST (graph A, P < 0.0001), stage 2 sleep (B, P < 0.0001), REM sleep (D, P < 0.0001), SWS (E, P < 0.0001), and SWE (F, P < 0.0001). Sleep efficiency decreased with increasing TIB (C, P = 0.039). Best-fitting recovery sleep dose-response functions (from AIC) are shown as the solid lines in each graph (see Supplement Table S10). These were linear for TST, stage 2, sleep efficiency, REM sleep, and SWE (graphs A, B, C, D, F, respectively) and exponential for SWS (graph E). Least squares means (diamonds) represent the overall covariate-controlled group means; best-fitting functions are shown for males with the other covariates set to the sample means.
Sleep-restricted subjects randomized to 10 h TIB on REC had a mean (± SEM) of 51.5 min (12.3 min) more TST than on their 10 h baseline night B2 (t25 = 4.17, P < 0.001) and 7.2% (1.86%) higher sleep efficiency than at B2 (t25 = 3.87, P = 0.001). They also had an average of 31.2 min (10.2 min) more stage 2 sleep (t25 = 3.05, P = 0.005) than at baseline, but not more REM sleep (mean difference = 12.5 min [7.1 min], t25 = 1.76, P = 0.091). They did not average significantly more SWS relative to baseline (7.4 min [5.5 min], t25 = 1.46, P = 0.16), but they averaged 29.6% (11.3%) more SWE than at B2 (t23 = 2.60, P = 0.016).
As expected, increasing recovery sleep dose had an effect on all sleep parameters (see Supplement Table S9). The TIB condition effect in the ANCOVA models was significant for TST, stage 2 sleep, SWS, REM sleep, and SWE (all P < 0.0001). Decreasing sleep efficiency with increasing sleep dose was significant at P = 0.0039. Although the effects of age and gender were generally small and not significant (except for sleep efficiency, which decreased with age), baseline sleep contributed significantly to recovery sleep architecture for all sleep variables (Supplement Table S9)—the exception was SWE for which the covariate was irrelevant since SWE was already normalized to baseline (i.e., set to 100% on B2).
Table S9.
ANCOVA results for time in bed (TIB) dose response effect on sleep parameters across the recovery night (REC).
| Effect of TIB | Covariates |
||||||
|---|---|---|---|---|---|---|---|
| Baseline | Age | Sex | |||||
| Variable | F ratio (df)1 | P | R2 | P | P | P | P |
| TST | 98.36 (7, 106) | < 0.0001 | 0.867 | < 0.0001 | 0.018 | 0.066 | 0.246 |
| SE | 8.44(7, 106) | < 0.0001 | 0.358 | 0.0039 | < 0.001 | 0.022 | 0.079 |
| S2 | 124.68(7, 106) | < 0.0001 | 0.892 | < 0.0001 | < 0.001 | 0.130 | 0.054 |
| SWS | 27.93(7, 106) | < 0.0001 | 0.648 | < 0.0001 | < 0.001 | 0.055 | 0.060 |
| REM | 46.38(7, 106) | < 0.0001 | 0.754 | < 0.0001 | 0.006 | 0.435 | 0.117 |
| SWE | 12.17(7, 106) | < 0.0001 | 0.429 | < 0.0001 | —* | 0.094 | 0.822 |
SWE was normalized to baseline (i.e., set to 100% on B2).
The best-fitting functions to the recovery dose response curves (from 2 h to 10 h TIB) for each of the physiological sleep variables shown in Figure 4 were established for 3 models used to evaluate the shape of the neurobehavioral recovery sleep dose functions (linear; exponential; and exponential restricting the asymptote to baseline; see Supplement Table S2). The smallest AIC was the linear model 1 for TST (Figure 4A), stage 2 sleep (Figure 4B), sleep efficiency (Figure 4C), REM sleep (Figure 4D), and SWE (Figure 4F). Exponential model 2 best fit SWS (Figure 4E)—AIC values are shown in Supplement Table S10.
Table S10.
Akaike's information criterion (AIC) for Models 1-3 in Table S2 fit to sleep physiology outcomes across REC recovery sleep doses (lowest AIC = best-fitting model).*
| TST |
SE |
S2 |
SWS |
REM |
SWE |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AIC | model | AIC | model | AIC | model | AIC | model | AIC | model | AIC | model |
| 1267.5 | 17 | 730.3 | 1 | 1136.4 | 1 | 1079.9 | 28 | 1029.1 | 1 | 1044.5 | 1 |
| 1270.5 | 2 | 730.9 | 39 | 1136.8 | 2 | 1084.0 | 1 | 1034.4 | 2 | 1046.8 | 2 |
| 1583.5 | 3 | 734.8 | 2 | 1149.3 | 3 | 1086.9 | 3 | 1145.7 | 3 | 1069.6 | 3 |
See Table S2 for model definitions. Results based on only those subjects with complete polysomnographic data on protocol nights B2, SR1, SR5, and REC: REC = 2 h (n = 25), REC = 4 h (n = 23), REC = 6 h (n = 21), REC = 8 h (n = 16), REC = 10 h (n = 23).
Across recovery sleep-dose conditions, TST, stage 2, REM sleep, and SWE were intercorrelated (r <183> 0.59, P < 0.001), but these parameters had lower correlations with SWS (r = 0.02 to r = 0.36, P < 0.001)—see Supplement Table S11. Partial correlations among the sleep variables were calculated, controlling for recovery (REC) night categorical sleep dose (i.e., 2 h-10 h). Colinearity among sleep parameters decreased but remained statistically significant for TST and REM sleep (r = 0.36, P < 0.001), TST and SWS (r = 0.32, P = 0.002), SWS and stage 2 sleep (r = <23>0.68, P < 0.001), and SWS and SWE (r = 0.22, P = 0.027)—see Supplement Table S12.
Table 11.
Pearson correlations among physiological sleep variables on the recovery night (REC) for N=100 to 120* subjects randomized to between 2 h and 10 h TIB following chronic sleep restriction.
| Stage 2 |
REM |
SWS |
SWE |
|||||
|---|---|---|---|---|---|---|---|---|
| r | (P) | r | (P) | r | (P) | r | (P) | |
| TST | 0.921 | (< 0.0001) | 0.866 | (< 0.0001) | 0.346 | (< 0.0001) | 0.651 | (< 0.0001) |
| Stage 2 | 0.765 | (< 0.0001) | 0.021 | (0.819) | 0.608 | (< 0.0001) | ||
| REM sleep | 0.278 | (< 0.002) | 0.593 | (< 0.0001) | ||||
| SWS | 0.362 | (< 0.0001) | ||||||
Correlations between SWE and all other variables based on N = 100; correlations among TST, stage 2, REM sleep, and SWS based on N = 120. The sample size difference was due an inability to extract reliable SWE data from N = 20 records, for technical reasons.
Table S12.
Partial correlations among physiological sleep variables, controlling for recovery night (REC) categorical sleep dose (TIB), for N = 93 subjects randomized to between 2 h and 10 h TIB following chronic sleep restriction.
| Stage 2 |
REM |
SWS |
SWE |
|||||
|---|---|---|---|---|---|---|---|---|
| r | (P) | r | (P) | r | (P) | r | (P) | |
| TST | 0.089 | (0.39) | 0.369 | (0.0003) | 0.320 | (0.002) | 0.144 | (0.16) |
| Stage 2 | −0.087 | (0.40) | −0.688 | (< 0.0001) | 0.039 | (0.70) | ||
| REM sleep | 0.027 | (0.79) | 0.113 | (0.27) | ||||
| SWS | 0.226 | (0.027) | ||||||
DISCUSSION
This was the first experiment to systematically examine the relationship of the duration of sleep dose to the recovery of neurobehavioral deficits from sustained sleep restriction. It offers original data, in a large cohort of healthy adult sleepers, aged 22-45 y, on the dynamics of functional recovery relative to sleep duration. Our hypothesis that the degree of recovery of neurobehavioral functions would increase monotonically in relation to the duration of time allowed for recovery sleep, as tested on primary outcome measures of alertness and sleepiness (i.e., PVT lapses, KSS, MWT), was supported by the results of linear and exponential recovery functions for 5 of 6 neurobehavioral outcomes (all but POMS fatigue). However, for the level of sleep restriction used in this experiment (5 days at 4 h TIB), recovery to either subjects' own baseline values, or to values recorded for the sleep-satiated control group, was not achieved at the maximum recovery sleep dose examined (i.e., 10 h TIB, ∼9 h TST) for measures of behavioral alertness (PVT lapses and fastest 10% RTs) and for subjective measures of sleepiness (KSS). We deliberately used both within-subject (comparison to baseline) and between-subject (comparison to control condition) to evaluate at what sleep dose recovery was achieved, in order to determine if there was agreement between these two approaches on the sleep dose needed to achieve recovery. We reduced the sleep debt subjects may have had coming into the laboratory by providing 10 h TIB on the 2 baseline nights, and evaluated the effects of the laboratory procedures without sleep debt by sleep-satiating the control group via 10 h TIB per night and comparing them on the same day in the laboratory. Despite these differences, the two comparison standards resulted in remarkably similar results.
Our findings that the recovery functions for PVT and KSS outcomes were statistically projected to intersect with baseline and control group values when TST was 10 h or longer would suggest that the sleep period would have to be > 10 h TIB to achieve recovery, although it is uncertain whether circadian constraints on sleep duration would permit enough sleep to achieve recovery within 1 night. If this were not possible, we speculate that the residual neurobehavioral deficits still present after a single recovery sleep could potentiate the effects of a subsequent sleep restriction period. Some support for this speculation comes from a report on chronic sleep loss in a 42.85-h forced desynchrony protocol, which found that being awake during the circadian night exposed the cumulative detrimental effects of prior chronic sleep loss on PVT performance that were not apparent during the first several hours of wakefulness.42
Results for the third primary outcome—the modified MWT measure of physiological sleepiness—indicated recovery to baseline was nearly complete by 8 h TIB (∼7.5 h TST) and definitely complete by 10 h TIB. However, relative to the control group, recovery was projected to require a much longer sleep period (∼15.7 h TIB). This large discrepancy in MWT recovery dynamics relative to baseline versus controls, not seen for other outcomes, was due to the sensitivity of the MWT to repeated 10 h TIB sleep periods in the control condition, which produced increased latencies to the occurrence of the first microsleep. It is noteworthy that the MSLT—another measure of physiological sleepiness—also has been reported to be sensitive to extending TIB in normal subjects (from 6.5 h to 10 h per night).43 This suggests that physiological measures based on the latency to microsleep or sleep onset may have sensitivity to variations within what is widely regarded as the normal range of behavioral alertness.
Historically, it has not been clear what scientific or clinical significance should be given to sleep restriction within what is considered to be a normal range of behavioral alertness. However, a recent experiment found that the extent to which sleep restriction generated (and recovery sleep reversed) deficits in behavioral alertness (PVT lapses) and physiological sleepiness (modified MWT) was influenced by the amount of nightly sleep in the range from 7 h to 10 h TIB obtained prior to the sleep restriction period.44 Consequently, 10 h TIB periods not only resulted in more TST than 7 h-8 h TIB periods, but these longer sleep opportunities further reduced physiological sleepiness and improved behavioral alertness during subsequent sleep restriction (whether or not subjects experienced the benefits subjectively).44
In our experiment, cognitive throughput performance, as measured by DSST number correct, showed dynamic improvements in the control condition, but these were due to learning. Relative to control group performance, DSST performance in the sleep-restricted group was comparable by the 8 h TIB recovery condition (∼7.7 h TST). Thus cognitive throughput, which is primarily a measure of neurocognitive speed in healthy adults, recovered more quickly than did measures of behavioral alertness (PVT) and sleepiness (KSS, MWT). This finding also suggests that DSST learning was occurring during sleep restriction, and that the DSST deficit was one of slowed cognitive processing (more so than a learning deficit), which was improved by increasing sleep duration to 8 h TIB, and which normalized at a lower recovery sleep dose than other neurobehavioral outcomes.
A key goal of this experiment was to statistically model the most parsimonious shape of the relationship between recovery sleep dose and neurobehavioral functions. Akaike's information criterion indicated exponential models with asymptote set to baseline best characterized recovery sleep dose-response profiles for the primary outcomes of PVT lapses and KSS scores, and for the secondary outcome of PVT fastest 10% RTs (Supplement Tables S3, S4, S7), while a linear relationship best captured the recovery of MWT and DSST outcomes (Supplement Tables S5, S6). AIC parsimoniously models the functional form of the recovery data by penalizing the better fit of more complex models (i.e., those with more parameters) by a factor proportional to the number of additional parameters. Although for all primary and secondary neurobehavioral outcomes, the proportion of total variance explained by a linear model was modestly larger than the optimal model selected by AIC, the exponential models with asymptote set to baseline provided a more parsimonious fit to the results for PVT and KSS outcomes. It is noteworthy that the PVT and KSS outcomes had significant advantages over the other neurobehavioral measures. In addition to reflecting functional alertness and subjective sleepiness, both measures are uncontaminated by aptitude and learning, and the values of these measures were virtually identical between baseline in the sleep-restricted group and the control group, despite increased sleep time in the latter. Based on the PVT and KSS outcomes, we conclude that recovery was incomplete at 10 h TIB in this experiment.
In contrast to exponential models selected by AIC for three of the six neurobehavioral variables, linear models selected by AIC best described the following four recovery sleep variables as increasing relative to increasing recovery sleep duration from 2 h to 10 h TIB: TST, stage 2 sleep, REM sleep, and NREM SWE as a percentage of baseline. When considered relative to the neurobehavioral outcomes, this suggests that the more time one has available for sleep following sleep restriction, the greater the likelihood of recovery, and that additional time for sleep beyond habitual sleep duration has significant recovery benefits. The increases in TST, stage 2, REM sleep time, and SWE that we observed as recovery dose was increased from 2 h to to 10 h TIB are consistent with recovery sleep findings from experiments in chronically sleep-restricted rats.10,11 We observed a high degree of co-linearity among these sleep parameters across recovery sleep doses, although this was markedly reduced when we controlled for categorical sleep dose. Importantly, the 10 h TIB recovery condition had significantly more TST, stage 2 sleep, and percentage of SWE on the recovery night (post chronic sleep restriction) than at the 10 h TIB baseline night (pre chronic sleep restriction). These findings suggest caution in assuming that sleep intensity (measured by NREM EEG slow wave dynamics) and sleep duration are only “marginally related,” and in generalizing the observation that “sleep loss is primarily recovered by increasing sleep intensity and not necessarily by sleep duration.”45 Our data suggest that sleep intensity and sleep duration are intimately related in recovery sleep following chronic sleep restriction, and that sleep intensity from the prior sleep restriction may be reflected in both variables. This is similar to recovery sleep after total sleep deprivation, which results in increases in multiple sleep variables including TST, NREM and REM sleep time, sleep efficiency and SWE relative to baseline.46,47
Since we were interested in the cumulative integrated power in the delta band as a function of different recovery sleep durations, we focused our data analyses on SWE rather than SWA. Using SWA could give a skewed perspective on the recovery process when nights of different TIB/TST are compared (i.e., the longer the night, the less the marginal increase in delta power with increasing time of NREM sleep, and thus the smaller SWA). SWA could therefore lead one to conclude that the longer the TIB, the less sleep homeostatic recovery occurs as judged by delta power. In contrast, SWE reflects the actual delta power cumulatively across each sleep dose, revealing the continued presence of slow wave activity in the longer recovery sleeps. Similarly, the 0h condition was not included in the recovery sleep physiology data analyses (i.e., Figure 4) because no sleep was allowed in that condition. To include it would produce a nonvariant (artificial) anchor point on the dose response functions for sleep variables, which would distort model fit and potentially the theoretical interpretation of the modeled function.
The two-process model of sleep regulation posits that the time constant for the elevation of homeostatic pressure for sleep during deprivation is much longer than the decline in homeostatic pressure during recovery sleep, due to the rapid intensification and exponential decay rate of sleep homeostasis as expressed in slow wave activity.20,48 The model predicts only an initial modest (∼10%-20%) elevation in slow wave activity (SWA) over the first few days of sleep restricted to 4 h TIB per night, which we found in SWE in this study, and others have also observed.15,21 Following chronic sleep restriction to 4 h TIB, during which SWE was in deficit on SR5 (mean = −22.2% relative to baseline), delta power accumulated linearly as recovery sleep duration increased, ultimately reaching 29.6% above baseline when recovery sleep duration was extended to 10 h TIB. We speculate that this SWE increase may also serve to prolong sleep20 for 1 or more hours past habitual sleep duration, depending on the severity of the prior deprivation (e.g., recovery sleep can extend to 14 h TST under extreme conditions of deprivation).20,48 Therefore the prolongation of sleep duration by 1 h-2 h following chronic sleep restriction should be considered part of the sleep homeostatic response, which is further supported by the fact that a few hours of additional sleep contributed substantially to increasing normalization of waking neurobehavioral functions, at least for the diurnal portion of the day after the recovery sleep. We believe this conceptualization is consistent with the original two-process model of sleep regulation.20 Thus SWE may not only serve to protect the continuity and intensity of the first 4 h-6 h of sleep, but also to extend the continuity (duration and efficiency) of sleep beyond the habitual (basal) sleep duration following chronic partial sleep deprivation. This conclusion does not abrogate the possibility that REM sleep homeostasis—evident in linear increases in REM sleep time as a function of recovery sleep dose—may also have a role in recovery of neurobehavioral capability following chronic sleep restriction.
It is noteworthy that the maximum extended recovery sleep period we studied (10 h TIB) could not fully normalize behavioral alertness and subjective sleepiness. Either a longer recovery sleep period, or one or more additional night(s) of recovery sleep would be needed to fully recover neurobehavioral functioning. This suggests that any model of sleep recovery based on the intensity and temporal dynamics of NREM EEG slow waves19,20,50 needs to also include extended sleep duration (and possibly repeated sleep periods) for recovery from chronic sleep restriction. An exclusive focus on sleep intensity without regard for sleep duration would miss the critical role of sleep homeostatic processes in extending sleep duration. Furthermore, this perspective suggests that when modeling sleep regulation it should not be assumed that recovery of waking neurobehavioral functions is complete when the exponential function for the dissipation of homeostatic pressure approaches zero.
Our results indicate that a period of recovery sleep time greater than 10 h TIB would be needed for full recovery after 5 nights of sleep restriction to 4 h TIB per night. This finding is consistent with the conclusion of Belenky and colleagues,12 who provided 3 recovery nights of 8 h TIB to subjects who had their sleep chronically restricted for a week to between 3 h and 7 h TIB. Other recent experiments have also found that following 5-7 nights of sleep restriction to 3 h-6 h TIB, aspects of performance similar to the PVT were not normalized when recovery sleep TIB was less than 9 h TIB.8,23,44 This is in agreement with predictions from a new model of the homeostatic effects of sleep loss on neurobehavioral performance27 based on the findings of our earlier study of chronic sleep restriction.15
It is worth noting that our study involved restricting the sleep of the largest number of subjects (N = 142) in a single laboratory-based experiment conducted to date, and that the effects of 5 nights at 4 h TIB per night on neurobehavioral functions measured between 08:00 and 20:00 were consistent with what we reported in the past on smaller sample sizes.14,15 Thus, increases of PVT lapses and decreases on fastest RTs were cumulative and exhibited near-linear profiles across days, while increases in KSS sleepiness and POMS fatigue were cumulative but displayed nonlinear (saturating) profiles. The robustness of this inconsistency between objective and subjective outcomes during chronic sleep restriction suggests the need to find ways to help people identify their behavioral vulnerability to chronic sleep restriction,15,32 as their self-evaluation of sleepiness and fatigue during chronic sleep restriction may not reflect the continuing development of deficits in neurobehavioral functions.
At a theoretical level, our results provide some support for the idea that the effects of chronic sleep loss and the effects of recovery sleep on waking neurobehavioral functions should be interpreted in the context of shifts in physiologic balance that occur over much longer periods than a day or two.27 This perspective encompasses both the idea of sleep debt and the theory of homeostatic recovery responses to sleep loss, but emphasizes that equilibrium between these dynamics is what determines integrity of waking neurobehavioral functions.27
Finally, some limitations of this experiment need to be recognized. Our conclusions are based on one type of sleep restriction (i.e., 4 h TIB for 5 nights). There is ample evidence in healthy adults that more severe sleep restriction (i.e., less sleep time and/or more nights of restriction at 4 h TIB) would result in greater waking deficits.12,15 Under such circumstances, we speculate that the same dynamic range of recovery sleep doses would yield a monotonic relationship between TST and neurobehavioral improvements, but make acute recovery even less likely in a single night. Similarly, less severe sleep restriction would be expected to yield a monotonic relationship but make acute recovery more likely in a single night.
We averaged waking neurobehavioral measures of alertness between 08:00 and 20:00, but did not report data for specific times of day. Assessments made for individual hours of the day, as well as at other times of day, may yield additional insights into the recovery of neurobehavioral functions following sleep restriction.51 Moreover, our study design resulted in subjects randomized to the longer duration recovery sleep doses going to bed earlier during the recovery night than during the sleep restriction nights, so that all neurobehavioral assessments could be kept at the same times each day. Subjects may have been somewhat phase delayed by the chronic sleep restriction protocol since it was conducted in dim light.52 However, this did not noticeably affect sleep onset for those randomized to an extended recovery sleep dose.
Our data generalize to healthy adults aged 22 y-45 y who habitually sleep from 6.5 h to 8.5 h per night. It is not known whether our findings generalizable to those who need less than 6.5 h or more than 8.5 h habitual sleep, to younger (< 22 y) or older (> 45 y) subjects, or to those suffering from insomnia or other sleep disorders. Our recovery responses may have been influenced by the degree of sleep debt prior to restriction.44 While control group decreases in TST across days of 10 h TIB suggest that some sleep pressure was present even after 2 baseline nights of 10 h TIB, the degree of this debt was small and it was not evident in waking neurobehavioral outcomes during baseline. Lastly, this experiment did not reveal whether subjects could have slept longer than they did in the 10 h TIB recovery condition if we had permitted even more TIB, and whether any additional sleep would have led to further recovery—although the recovery dose-response functions we found predict this might be possible.
In conclusion, in healthy adults between 22 y and 45 y of age, objective and subjective neurobehavioral functions following 5 nights of sleep restricted to 4 h TIB (04:00-08:00) improved monotonically as sleep duration, sleep stages, and sleep intensity (SWE) increased across recovery sleep doses from 2 h to 10 h TIB. However, behavioral alertness and subjective sleepiness did not fully recover even with an extended night of sleep (10 h TIB resulting in ∼9 h TST). It appears, therefore, that a simulated workweek with 5 nights of chronic partial sleep loss can continue to adversely affect waking neurobehavioral functions even after a night of extended duration recovery sleep. This suggests the presence of residual sleep pressure that may potentiate neurobehavioral deficits if exposed to a subsequent period of sleep restriction. It highlights the importance of ensuring recovery sleep periods are sufficient in occupations and lifestyles where chronic partial sleep loss is frequent or cyclic.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Dinges has received research support and consulted for Cephalon and Merck & Co. and consulted for Actelion, Cephalon, Eli Lilly, Mars Inc., Merck & Co, Sanofi-Aventis, and Somaxon; he is compensated by the Associated Professional Sleep Societies for serving as Editor in Chief of SLEEP. Dr. Van Dongen has received support from The Boeing Company and Continental Airlines; he has participated in a speaking engagement for Clayton Sleep Institute, Saint Louis, MO; and he has consulted for FedEx Corporation. Dr. Banks has no financial conflicts of interest. Mr. Maislin directs a statistical consulting firm that contracts with industry and academia.
ACKNOWLEDGMENTS
We thank Michele Carlin, Adrian Ecker, Olivera Crenshaw, Claire Fox, Deepa Avinash, Chris Jones, Eric Hyder, Julieanne Muto, Adrian DiAntonio, Robert Hachadoorian, and Christos Ballas for their contributions to this study, and Mathias Basner and Namni Goel for review of the manuscript. Supported primarily by NIH grants NR04281 and CTRC UL1 RR0241340, and in part by the National Space Biomedical Research Institute through NASA NCC 9-58 (DFD). SB was supported in part by Women in Science Fellowship from University of South Australia. HVD was supported by AFOSR grants FA9550-05-1-0086, FA9550-06-1-0055 and FA9550-09-1-0136.
Work performed at Division of Sleep and Chronobiology, Unit for Experimental Psychiatry, Department of Psychiatry, University of Pennsylvania School of Medicine, Philadelphia, PA.
SUPPLEMENTAL MATERIAL
Effect of Sleep Restriction Protocol on Sleep Architecture
Figure 1 in the published paper displays the sleep responses at baseline and on the first and fifth sleep restriction nights for N = 118 sleep-restricted subjects (solid line) and N = 17 control subjects (dashed line). Tables S1 displays the effect sizes (ES) for the effect of 5 nights of sleep restriction to 4 h TIB, relative to the control group with 10 h TIB nightly, for the sleep parameters illustrated in Figure 1.
Neurobehavioral Outcomes as a Function of Recovery Sleep Dose
To determine the best-fitting function to the recovery sleep dose-response curves for each of the neurobehavioral outcomes shown in Figure 3 in the published paper, 4 models were evaluated. Table S2 provides details of functional forms of the 4 models (1-4) and of the null (0) model estimated for comparison purposes. Models evaluated included linear (model 1), exponential (models 2 and 3), and sigmoidal (model 4) functional forms. Exponential forms were specified with (model 2) and without (model 3) restricting the asymptote to be equal to baseline (thereby reducing the number of parameter estimates by 1 for model 2 relative to model 3).
Maximum likelihood estimation (MLE, SAS Proc NLMIXED) was used to estimate the parameters of each dose response functional form. Akaike's information criterion1 (AIC) was used to select the most adequately fitting functional form for each outcome variable. AIC is scaled such that the model with the minimum AIC is identified as “best-fitting.” Tables S3–S8 summarize the AIC model comparisons for each neurobehavioral outcome examined in the published paper.
Sleep Architecture as a Function of Recovery Sleep Dose
Figure 4 in the published paper displays the physiological sleep characteristics as a function of TIB sleep dose on the recovery night (REC) in the sleep restriction condition. Adjusted linear dose-response ANCOVA models, in which covariates included baseline sleep, age and gender, were used to evaluate each of the 6 sleep parameters considered. Significance levels are reported for the categorical sleep duration model to avoid the necessity of making assumptions about functional relationships when testing these models. Table S9 reveals that, as expected, increasing recovery sleep dose (TIB) significantly increased all 6 physiological sleep parameters.
Best-fitting functions to the recovery sleep dose-response curves for each of the physiological sleep variables shown in Figure 4 in the published paper were established for 4 models that included linear and the 2 exponential models—the sigmoidal model did not fit any recovery sleep parameter (see Table S2). The “best fitting” model was identified as the one with the smallest AIC. Tables S10 summarizes the results of these analyses, which revealed that a linear model best fit TST, sleep efficiency, stage 2 sleep, REM sleep, and SWE as percent of B2. An exponential model best fit SWS time.
Table S11 shows the results of Pearson correlations among physiological sleep variables on the recovery night (REC) for N = 100 to 120 subjects randomized to between 2 h and 10 h TIB following chronic sleep restriction. Table S12 displays the results of partial correlations among the sleep variables, controlling for recovery (REC) night categorical sleep dose (i.e., 2 h-10 h).
REFERENCES
- 1.Akaike H. A new look at the statistical model identification. In: Childers DG, editor. Modern spectrum analysis. New York: IEEE Press; 1978. pp. 234–41. [Google Scholar]
REFERENCES
- 1.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–28. [PMC free article] [PubMed] [Google Scholar]
- 2.Basner M, Fomberstein KM, Razavi FM, et al. American time use survey: sleep time and its relationship to waking activities. Sleep. 2007;30:1085–95. doi: 10.1093/sleep/30.9.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinges DF, Maislin G, Brewster RM, et al. Journal of the Transportation Research Board. Washington, DC: Transportation Research Board of the National Academies; 2005. Pilot test of fatigue management technologies; pp. 175–182. No. 1922. [Google Scholar]
- 4.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: Daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 5.Dijk D-J, Hayes B, Czeisler CA. Dynamics of electroencephalographic sleep spindles and slow wave activity in men: effect of sleep deprivation. Brain Res. 1993;626:190–9. doi: 10.1016/0006-8993(93)90579-c. [DOI] [PubMed] [Google Scholar]
- 6.Franken P, Dijk D-J, Tobler I, Borbely AA. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol. 1991;261:R198–R208. doi: 10.1152/ajpregu.1991.261.1.R198. [DOI] [PubMed] [Google Scholar]
- 7.Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28:1446–54. doi: 10.1093/sleep/28.11.1446. [DOI] [PubMed] [Google Scholar]
- 8.Lamond N, Jay SM, Dorrian J, et al. The dynamics of neurobehavioural recovery following sleep loss. J Sleep Res. 2007;16:33–41. doi: 10.1111/j.1365-2869.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 9.Mander BA, Reid KJ, Baron KG, et al. EEG measures index neural and cognitive recovery from sleep deprivation. J Neurosci. 2010;30:2686–93. doi: 10.1523/JNEUROSCI.4010-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.KimY, Laposky AD, Bergmann BM, Turek FW. Repeated sleep restriction in rats leads to homeostatic and allostatic responses during recovery sleep. Proc Natl Acad Sci U S A. 2007;104:10697–702. doi: 10.1073/pnas.0610351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rechtschaffen A, Bergmann BM, Gilliland MA, Bauer K. Effects of method, duration, and sleep stage on rebounds from sleep deprivation in the rat. Sleep. 1999;22:11–31. doi: 10.1093/sleep/22.1.11. [DOI] [PubMed] [Google Scholar]
- 12.Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 13.Carskadon MA, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107–13. doi: 10.1111/j.1469-8986.1981.tb02921.x. [DOI] [PubMed] [Google Scholar]
- 14.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 15.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 16.Cote KA, Milner CE, Smith BA, et al. CNS arousal and neurobehavioral performance in a short-term sleep restriction paradigm. J Sleep Res. 2009;18:291–303. doi: 10.1111/j.1365-2869.2008.00733.x. [DOI] [PubMed] [Google Scholar]
- 17.Hairston IS, Little MTM, Scanlon MD, et al. Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J Neurophyiol. 2005;94:4224–33. doi: 10.1152/jn.00218.2005. [DOI] [PubMed] [Google Scholar]
- 18.Kleitman N. Sleep and wakefulness. Chicago: University of Chicago Press; 1963. [Google Scholar]
- 19.Achermann P, Borbely AA. Simulation of daytime vigilance by the additive interaction of a homeostatic and a circadian process. Biol Cybern. 1994;71:115–21. doi: 10.1007/BF00197314. [DOI] [PubMed] [Google Scholar]
- 20.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 21.Brunner DP, Dijk DJ, Borbely AA. Repeated partial sleep deprivation progressively changes in EEG during sleep and wakefulness. Sleep. 1993;16:100–13. doi: 10.1093/sleep/16.2.100. [DOI] [PubMed] [Google Scholar]
- 22.Åkerstedt T, Kecklund G, Ingre M, et al. Sleep homeostasis during repeated sleep restriction and recovery: support from EEG dynamics. Sleep. 2009;32:217–22. doi: 10.1093/sleep/32.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Axelsson J, Kecklund G, Åkerstedt T, et al. Sleepiness and performance in response to repeated sleep restriction and subsequent recovery during semi-laboratory conditions. Chronobiol Int. 2008;25:297–308. doi: 10.1080/07420520802107031. [DOI] [PubMed] [Google Scholar]
- 24.Åkerstedt T, Kecklund G, Gillberg M, et al. Sleepiness and days of recovery. Transp Res Part F Traffic Psychol Behav. 2000:251–61. [Google Scholar]
- 25.Modirrousta M, Mainville L, Jones BE. Dynamic changes in GABAA receptors on basal forebrain cholinergic neurons following sleep deprivation and recovery. BMC Neurosci. 2007;8:15. doi: 10.1186/1471-2202-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinges DF. Sleep debt and scientific evidence. Sleep. 2004;27:1–3. [PubMed] [Google Scholar]
- 27.McCauley P, Kalachev LV, Smith AD, et al. A new mathematical model for the homeostatic effects of sleep loss on neurobehavioral performance. J Theor Biol. 2009;256:227–39. doi: 10.1016/j.jtbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Åkerstedt T, Ingre M, Kecklund G, et al. Accounting for partial sleep deprivation and cumulative sleepiness in the Three-Process Model of alertness regulation. Chronobiol Int. 2008;25:309–19. doi: 10.1080/07420520802110613. [DOI] [PubMed] [Google Scholar]
- 29.Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 1989;74:728–38. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- 30.Doran SM, Van Dongen HPA, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001;139:253–67. [PubMed] [Google Scholar]
- 31.Dorrian J, Rogers NL, Dinges DF. Psychomotor vigilance performance: A neurocognitive assay sensitive to sleep loss. In: Kushida C, editor. Sleep deprivation: Clinical issues, pharmacology and sleep loss effects. New York: Marcel Dekker; 2005. pp. 39–70. [Google Scholar]
- 32.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–22. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 33.Wechsler Adult Intelligence Scale 3 - Technical Manual. San Antonio: Hardcourt Brace and Company; 1997. [Google Scholar]
- 34.Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 35.McNair DM, Lorr M, Drupplemann LF. EITS manual for the profile of mood states. San Diego: Educational and Industrial Test Services; 1971. [Google Scholar]
- 36.Banks S, Barnes M, Tarquinio N, et al. The maintenance of wakefulness test in normal healthy subjects. Sleep. 2004;27:799–802. [PubMed] [Google Scholar]
- 37.Doghramji K, Mitler MM, Sangal RB, et al. A normative study of the maintenance of wakefulness test (MWT) Electroencephalogr Clin Neurophysiol. 1997;103:554–62. doi: 10.1016/s0013-4694(97)00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitler MM, Gujavarty KS, Browman CP. Maintenance of wakefulness test: a polysomnographic technique for evaluation treatment efficacy in patients with excessive somnolence. Electroencephalogr Clin Neurophysiol. 1982;53:658–61. doi: 10.1016/0013-4694(82)90142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rechtschaffen A, Kales A. Public Health Service. Washington, DC: U.S. Government Printing Office; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 40.Knickerbocker RK. Intention-to-treat analyses. In: Chow SC, editor. Encyclopedia of biopharmaceutical statistics. New York: Marcel Dekker; 2000. pp. 271–275. [Google Scholar]
- 41.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974:716–23. [Google Scholar]
- 42.Cohen D, Wang W, Wyatt JK, et al. Uncovering residual effects of chronic sleep loss on human performance. Sci Transl Med. 2010;2:14ra3. doi: 10.1126/scitranslmed.3000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roehrs T, Shore E, Papineau K, et al. A two-week sleep extension in sleepy normals. Sleep. 1996;19:576–82. [PubMed] [Google Scholar]
- 44.Rupp TL, Wesensten NJ, Bliese PD, Balkin TJ. Banking sleep: realization of benefits during subsequent sleep restriction and recovery. Sleep. 2009;32:311–21. doi: 10.1093/sleep/32.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hor H, Tafti M. How much sleep do we need? Science. 2009;325:825–6. doi: 10.1126/science.1178713. [DOI] [PubMed] [Google Scholar]
- 46.Borbely AA, Baumann F, Brandeis D, et al. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51:483–95. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- 47.Tucker AM, Dinges DF, Van Dongen HPA. Trait interindividual differences in the sleep physiology of healthy young adults. J Sleep Res. 2007;16:170–80. doi: 10.1111/j.1365-2869.2007.00594.x. [DOI] [PubMed] [Google Scholar]
- 48.Borbely AA, Achermann P. Concepts and models of sleep regulation: an overview. J Sleep Res. 1992;1:63–79. doi: 10.1111/j.1365-2869.1992.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 49.Gulevich G, Dement W, Johnson L. Psychiatric and EEG observations on a case of prolonged (264 hours) wakefulness. Arch Gen Psychiatry. 1966;15:29–35. doi: 10.1001/archpsyc.1966.01730130031005. [DOI] [PubMed] [Google Scholar]
- 50.Daan S, Beersma DGM, Borbély AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246:R161–R178. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- 51.Mollicone DJ, Van Dongen H., Rogers NL, et al. Time of day modulates the effects of chronic sleep restriction on neurobehavioral performance. Aviat, Space & Environ Med. doi: 10.3357/asem.2756.2010. in press. [DOI] [PubMed] [Google Scholar]
- 52.Goel N. Late-night presentation of an auditory stimulus phase delays human circadian rhythms. Am J Physiol Regul Integr Comp Physiol. 2005;289:R209–16. doi: 10.1152/ajpregu.00754.2004. [DOI] [PubMed] [Google Scholar]