Abstract
Study Objectives:
Modafinil may promote wakefulness by increasing cerebral dopaminergic neurotransmission, which importantly depends on activity of catechol-O-methyltransferase (COMT) in prefrontal cortex. The effects of modafinil on sleep homeostasis in humans are unknown. Employing a novel sleep-pharmacogenetic approach, we investigated the interaction of modafinil with sleep deprivation to study dopaminergic mechanisms of sleep homeostasis.
Design:
Placebo-controlled, double-blind, randomized crossover study.
Setting:
Sleep laboratory in temporal isolation unit.
Participants:
22 healthy young men (23.4 ± 0.5 years) prospectively enrolled based on genotype of the functional Val158Met polymorphism of COMT (10 Val/Val and 12 Met/Met homozygotes).
Interventions:
2 × 100 mg modafinil and placebo administered at 11 and 23 hours during 40 hours prolonged wakefulness.
Measurements and Results:
Subjective sleepiness and EEG markers of sleep homeostasis in wakefulness and sleep were equally affected by sleep deprivation in Val/Val and Met/Met allele carriers (placebo condition). Modafinil attenuated the evolution of sleepiness and EEG 5-8 Hz activity during sleep deprivation in both genotypes. In contrast to caffeine, modafinil did not reduce EEG slow wave activity (0.75-4.5 Hz) in recovery sleep, yet specifically increased 3.0-6.75 Hz and > 16.75 Hz activity in NREM sleep in the Val/Val genotype of COMT.
Conclusions:
The Val158Met polymorphism of COMT modulates the effects of modafinil on the NREM sleep EEG in recovery sleep after prolonged wakefulness. The sleep EEG changes induced by modafinil markedly differ from those of caffeine, showing that pharmacological interference with dopaminergic and adenosinergic neurotransmission during sleep deprivation differently affects sleep homeostasis.
Citation:
Bodenmann S; Landolt HP. Effects of modafinil on the sleep EEG depend on Val158Met genotype of COMT. SLEEP 2010;33(8):1027-1035.
Keywords: EEG spectral analysis, sleep deprivation, pharmacogenetics, dopamine, adenosine
WAKEFULNESS AND SLEEP ARE REGULATED BY A CIRCADIAN PROCESS THAT SETS THE TIMING OF SLEEP, AND A HOMEOSTATIC PROCESS THAT TRACKS a sleep debt.1 Sleep deprivation provides the most powerful challenge to the mechanisms underlying sleep homeostasis and predictably affects waking performance, subjective state, and electrical brain activity in wakefulness and sleep. Prolonged wakefulness impairs neurobehavioral and cognitive performance, increases subjective and objective measures of sleepiness, and elevates low-frequency electroencephalogram (EEG) activity in wakefulness, NREM sleep, and REM sleep.2–4 High EEG slow wave activity or delta activity (spectral power within ∼ 0.5-4.5 Hz) is characteristic of deep NREM sleep and an established marker of sleep homeostasis.4,5 The neurophysiological mechanisms and the relationships among sleep deprivation-induced changes in neurocognitive measures, sleepiness, and brain activity are poorly understood. Recent studies suggest that distinct markers of accumulated sleep debt are closely related.6–9 By contrast, other studies indicate that they represent different entities and underlying mechanisms.10–14
Sleep pharmacogenetics provides a powerful novel approach in humans to identify molecular mechanisms of sleep homeostasis. For example, the competitive adenosine A1 and A2A receptor antagonist, caffeine, improves performance, reduces subjective sleepiness, and attenuates EEG markers of sleep homeostasis after sleep deprivation.15,16 In view of the EEG, the stimulant lowers theta activity (5-8 Hz) in waking and slow wave activity in NREM and REM sleep.7,17–21 Together with enhanced spindle frequency activity (∼ 11-15 Hz) and beta oscillations (> 16 Hz) in NREM recovery sleep, these changes indicate that caffeine attenuates the waking-induced build-up of sleep need. Interestingly, not only the increase in EEG beta activity but also self-rated sensitivity to the sleep-disrupting effects of caffeine depend on the genotype of the 1976T > C single nucleotide polymorphism (SNP; NCBI SNP-ID: rs5751876) of the adenosine A2A receptor gene (ADORA2A).21 The convergent pharmacological and genetic data strongly suggest that the adenosine neuro-modulator/receptor system plays an important role in behavioral, subjective, and electrophysiological effects of sleep loss. This hypothesis is further supported by the finding that a functional 22G > A polymorphism of the gene encoding adenosine deaminase (Online Mendelian Inheritance in Man database accession number: 608958) modifies the duration and intensity of deep slow wave sleep.22
To examine whether the effects of caffeine are specific or rather reflect nonspecific psychostimulant actions, the changes induced by other stimulants on markers of sleep homeostasis have to be studied. Modafinil promotes wakefulness and improves cognitive performance in healthy volunteers, and has demonstrated efficacy in treating excessive daytime sleepiness in narcolepsy and other diseases presenting with enhanced sleepiness and fatigue.15,23–25 Increasing evidence suggests that interference with dopamine re-uptake and D1/D2 receptors contributes to the stimulant actions of this drug.26–28 Consistent with this notion, we recently demonstrated that the functional Val158Met polymorphism of the gene encoding the monoamine metabolizing enzyme, catechol-O-methyltransferase (COMT), predicts the efficacy of modafinil in alleviating impaired subjective well-being, sustained vigilant attention, and executive functioning in healthy subjects following sleep loss.14 By contrast, neither COMT genotype nor modafinil affected the rebound of slow wave sleep and EEG < 2 Hz activity in recovery sleep.14
To investigate in more detail a role for dopamine in sleep homeostasis in humans, we performed the first quantification of modafinil-induced changes in waking and sleep EEG during and after sleep deprivation in Val/Val and Met/Met allele carriers of the Val158Met polymorphism of COMT. We show that in contrast to caffeine, modafinil does not attenuate slow wave activity in recovery sleep, but increases 3.0-6.75 Hz activity and beta oscillations in COMT genotype-specific manner. Together with our previous publication,14 we conclude that distinct mechanisms underlie sleep loss-induced changes in waking performance, subjective sleepiness, and electrical brain activity in wakefulness and sleep. Moreover, stimulants acting on dopaminergic and adenosinergic neurotransmission interact differently with sleep homeostasis.
MATERIALS AND METHODS
Study Participants, Genotyping, and Pre-Experimental Procedures
The study protocol and all experimental procedures were reviewed and approved by the local ethics committees for research on human subjects, and carried out in accordance with the Declaration of Helsinki. The study participants are the same as those in 2 previous reports,14,29 in which recruitment, genetic analyses, and all pre-experimental procedures have been described in detail. In summary, 88 respondents to public advertisements were genotyped for the Val158Met SNP of COMT (NCBI SNP-ID: rs4680). Twenty-two men were prospectively selected for participation in this study based on their Val158Met genotype. Ten were homozygous Val/Val allele carriers, and 12 were homozygous Met/Met allele carriers. All were nonsmokers, moderate consumers of alcohol and caffeine, good sleepers with no sleep disorders, free of neurological and psychiatric disorders, and denied taking medications or illicit drugs during ≥ 2 months before the study. The 2 genotypic groups were carefully matched for age, body mass index, trait anxiety, daytime sleepiness, and diurnal preference.14
During 2 weeks prior to the study, each participant wore a wrist activity monitor on the non-dominant arm, kept a sleep-wake diary, and was asked to abstain from all sources of caffeine. Three days before and during the experiment, participants were also requested to abstain from alcohol and to strictly maintain regular 8-h sleep/16-h wake cycles. Sleep and wake times were scheduled at 24:00 and 08:00, respectively. Participants were not allowed to deviate from these times by more than 1 hour. Compliance with these instructions was verified by inspection of rest-activity plots and sleep-wake diaries. Upon arrival in the sleep laboratory, saliva samples for caffeine determination were taken, and breath ethanol concentration was measured.
Study Protocol
All subjects completed 2 experimental blocks, consisting of 4 nights and 2 days in the sleep laboratory, separated by one week. After 2 consecutive 8-h nocturnal sleep recordings (adaptation and baseline nights, 24:00-08:00), subjects were kept awake for 40 h under constant supervision by members of the research team. Cognitive performance, subjective state, and waking EEG were intermittently recorded in 14 sessions, starting 15 min after lights-on following the baseline nights.14,29 After 11 h (at 19:00) and 23 h (at 07:00) of prolonged waking, 2 doses of 100 mg modafinil or placebo were administered to each subject in randomized, double-blind, crossover fashion. A 10-h recovery night (24:00-10:00) concluded each experimental block.
Psychomotor Vigilance Task
The psychomotor vigilance task (PVT) is a simple visual reaction time task with no learning curve and virtually independent of aptitude.2 The 10-min PVT used in the present study is described in detail in our previous publication.14
Subjective Sleepiness
A German translation of the Stanford Sleepiness Scale30 was administered at 3-h intervals before and after completion of the PVT.14 The 2 values were averaged for analyses. Due to technical problems, the sleepiness scores in the placebo condition at 08:00 and 11:00 on day 2 of prolonged waking are missing in one Met/Met allele carrier.
Waking EEG
The waking EEG was recorded under standardized conditions and the data were processed as previously described.29 In brief, subjects relaxed comfortably in a chair and placed their chin on an individually adjusted head-rest. Each recording consisted of a 3-min period with eyes closed and a 5-min period with eyes open while fixating a black dot attached to the wall. When signs of drowsiness were detected (e.g., reduced EEG alpha activity or rolling eyes), subjects were alerted by addressing them over the intercom. One hour before each waking EEG, subjects stayed in the laboratory (constant temperature, light intensity < 150 lux), and 15 min before each recording they were by themselves in their bedrooms. The bioelectric signals (including EEG [C3A2 derivation reported here], bipolar electrooculogram [EOG], mental electromyogram [EMG], and electrocardiogram [ECG]) were recorded with the polygraphic amplifier Artisan (Micromed, Mogliano Veneto, Italy) and Rembrandt Datalab (Version 8; Embla Systems, Broomfield, CO, USA). Analog signals were conditioned by a high-pass filter (EEG: −3 dB at 0.15 Hz; EMG: 10 Hz; ECG: 1 Hz) and an anti-aliasing low-pass filter (−3 dB at 67.2 Hz), digitized and transmitted via fiber-optic cables to a computer. Data were sampled with a frequency of 256 Hz.
The EEG spectra (Fast Fourier Transform, Hanning window) of artifact-free, 50% overlapping 2-s epochs were computed with MATLAB (The MathWorks Inc, Natick, MA, USA). Mean power spectra of the 5-min periods with eyes open are reported. To quantify the time course of relative EEG activity in the 5-8 Hz band, individual power values were expressed as a percentage of the mean value in the recordings at 11:00, 14:00, and 17:00 of day 1 (i.e., before the first modafinil/placebo administration). To compare the waking EEG in baseline and after sleep deprivation, mean absolute values recorded at 11:00, 14:00, and 17:00 on days 1 and 2, respectively, of prolonged waking were analyzed. The waking EEG data of 1 Val/Val allele carrier recorded in the placebo condition at 05:00 could not be used because of artifacts.
Sleep EEG
Continuous polysomnography of EEG, EOG, EMG, and ECG was performed during all experimental nights as previously reported.14,29 Standard sleep stage scoring31 of 20-s epochs (C3A2 derivation) was performed with Rembrandt Analysis Manager (Version 8; Embla Systems, Broomfield, CO, USA). Four-s EEG spectra (FFT routine, Hanning window, 0.25-Hz resolution) were calculated with MATLAB (The MathWorks Inc, Natick, MA, USA), averaged over 5 consecutive epochs and matched with the sleep scores. Twenty-s epochs with movement- and arousal-related artifact were visually identified and eliminated. For sleep and sleep EEG analyses, only the first 8 h of the recovery night were considered. To compute all-night power spectra in NREM sleep (stages 2-4) and REM sleep, all artifact-free 20-s values were averaged. In the recovery nights, the evolution of power in specific low-frequency EEG bands (0.75-4.5 Hz and 3.0-6.75 Hz) was calculated across consecutive NREM sleep episodes.
Data Analyses and Statistics
The effect of sleep deprivation and modafinil on subjective sleepiness, waking EEG, sleep variables, and the EEG in REM sleep and NREM sleep were analyzed in homozygous carriers of Val and Met alleles of the Val158Met polymorphism of COMT. To approximate a normal distribution, absolute power densities were log-transformed before statistical tests. The SAS 9.1 statistical software (SAS Institute, Cary, NC) was used. Three-way mixed-model analyses of variance (ANOVA) with the between-subjects factor “genotype” (Val/Val, Met/Met) and the within-subjects factors “treatment” (placebo, modafinil), “session” (14 time points across prolonged waking), and “condition” (baseline, sleep deprivation) served to estimate the effects of Val158Met genotype, sleep loss and modafinil. The significance level was set at α < 0.05. If not stated otherwise, only significant effects of factors and interactions are reported. Paired and unpaired, 2-tailed t-tests to localize differences within and between subjects were only performed if respective main effects or interactions of the ANOVA were significant. EEG power was computed for consecutive 0.5-Hz (in waking) and 0.25-Hz bins (in sleep) and for specific frequency bands. The frequency bins and bands are indicated by the encompassing frequency ranges (e.g., the 5-8 Hz band denotes 4.75-8.25 Hz in waking, and the 3.0-6.75 Hz band encompasses 2.875-6.875 Hz in sleep).
RESULTS
Effects of Sleep Deprivation and Modafinil on Vigilant Attention and Subjective Sleepiness
The time courses of sustained vigilant attention quantified as median reaction time on the PVT, subjective sleepiness, and EEG theta activity during prolonged wakefulness in placebo and modafinil conditions are illustrated in Figure 1. When receiving placebo, these variables evolved similarly in homozygous Val/Val and Met/Met allele carriers. Figure 1A recapitulates the striking genotype-dependent efficacy of modafinil to improve reduced vigilant attention following sleep deprivation.14 While the stimulant maintained baseline performance on the PVT throughout prolonged wakefulness in Val/Val genotype, the drug was hardly effective in Met/Met allele carriers (genotype × treatment × session: F26,203 = 1.71, P < 0.03).
Figure 1.
In contrast to sustained vigilant attention, modafinil attenuates the evolution of subjective sleepiness and theta activity in the waking EEG (C3A2 derivation, power within 5-8 Hz) during sleep deprivation independently of Val158Met polymorphism of COMT. (A) Median reaction times (RT), expressed as speed (1/RT) on the psychomotor vigilance task (PVT); (B) mean scores on the Stanford Sleepiness Scale (SSS); and (C) EEG theta activity are plotted for consecutive 3-h intervals. EEG power in placebo and modafinil conditions was expressed as a percentage of mean theta activity in the waking EEG recordings at 11:00, 14:00, and 17:00 on day 1 of prolonged wakefulness. Error bars represent SEM. Tick marks on the x-axes are rounded up to the nearest hour. Modafinil (100 mg) was administered at 11 and 23 h waking (vertical dashed lines). Black symbols: Val/Val genotype (open circles: placebo; closed circles: modafinil). Gray symbols: Met/Met genotype (open circles: placebo; closed circles: modafinil). Asterisks indicate the time intervals with significant differences between modafinil and placebo (P < 0.05, paired 2-tailed t-tests).
In contrast to vigilant attention, the Val158Met polymorphism of COMT did not modulate the effects of modafinil on subjective sleepiness. In both Val/Val and Met/Met genotypes, Stanford sleepiness scores increased during 40 h continuous wakefulness and were modulated by circadian influences (Figure 1B; session: F13,195 = 22.83, P < 0.001). Modafinil counteracted the sleep deprivation-induced changes independently of genotype (treatment: F1,56.3 = 17.92, P < 0.001; genotype × treatment × session: F26,206 = 1.25, P > 0.1).
Effects of Sleep Deprivation and Modafinil on the Waking EEG
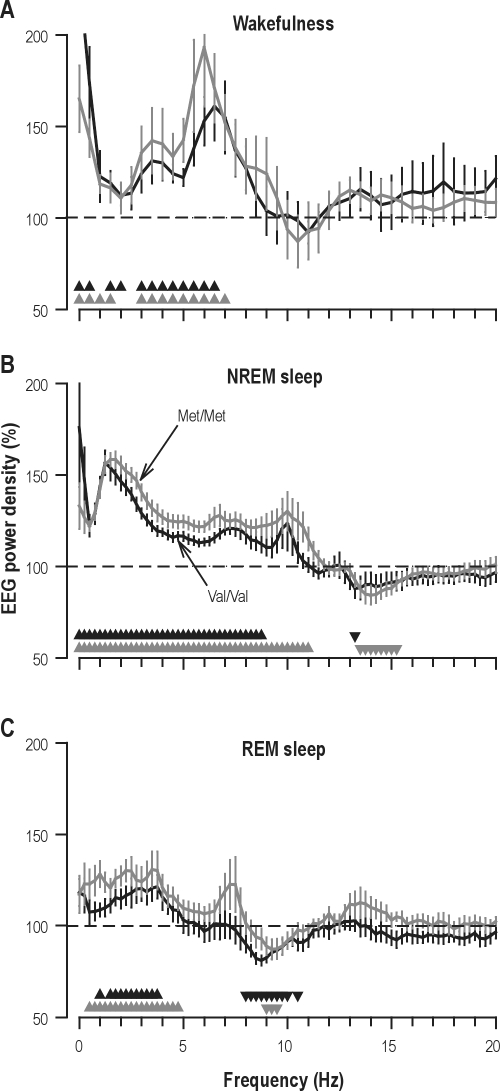
To quantify the effects of sleep loss on the waking EEG, spectral power averaged over 3 test sessions (11:00, 14:00, and 17:00) after sleep deprivation was compared to the corresponding values in baseline. Under placebo, prolonged waking affected the EEG in all bins below 8.0 Hz, as well as in the 10.5 and 12.0-13.5 Hz ranges (condition: F1,60 ≥ 4.86, P ≤ 0.04). Figure 2A illustrates the significant increase in most bins below 7.0 Hz in both Val/Val and Met/Met allele carriers. Except for the 10.5-11.0 Hz range (genotype × condition: F1,60 = 4.22, P < 0.05), the effect of sleep loss on the waking EEG was the same in both genotypes.
Figure 2.
Sleep deprivation affects EEG similarly in Val/Val (n = 10, black lines) and Met/Met (n = 12, gray lines) homozygotes of the Val158Met polymorphism of COMT. EEG power density (C3A2 derivation) in (A) wakefulness, (B) NREM sleep (stages 2-4), and (C) REM sleep in the placebo condition. Absolute power values in each frequency bin after sleep deprivation were expressed as percentage of the corresponding value in baseline (horizontal dashed line at 100%). To quantify the effects on the waking EEG, power values in the recording sessions at 11:00, 14:00, and 17:00 on day 2 of sleep deprivation were expressed as a percentage of the corresponding values of day 1. Means ± SEM are plotted for each 0.5 Hz bin in waking and for each 0.25 Hz bin in NREM and REM sleep. Black and gray triangles denote significant differences from baseline in Val/Val and Met/Met genotypes, respectively (P < 0.05, paired 2-tailed t-tests).
Because EEG theta activity in waking has been suggested to provide an objective measure of homeostatic sleep pressure and is attenuated after caffeine intake,6,7,32 the effects of modafinil on the time course of 5-8 Hz activity during prolonged waking were analyzed in Val/Val and Met/Met homozygotes. In both genotypes, theta power increased with increasing duration of wakefulness and also circadian modulation was present (Figure 1C; session: F13,178 = 11.00, P < 0.001). Similar to subjective sleepiness, modafinil reduced the sleep loss-induced increase in theta activity independently of Val158Met genotype (treatment: F1,62.8 = 6.9, P < 0.02; session × treatment × genotype: F26,196 = 0.67, P > 0.8).
Effects of Sleep Deprivation and Modafinil on Sleep and the Sleep EEG
Visually scored sleep variables
Sleep architecture in baseline was very similar in homozygous carriers of Val and Met alleles of COMT (Table 1). Compared to baseline, sleep episode duration, total sleep time, sleep efficiency, and stage 4 sleep increased in the recovery night after prolonged wakefulness. By contrast, sleep latency and stage 1 sleep were reduced. These typical sleep deprivation-induced changes in sleep structure were similar in Val/Val and Met/Met genotypes, and independent of placebo and modafinil intake during prolonged wakefulness (P > 0.1 for genotype and treatment main effects, and all interactions involving genotype).
Table 1.
Visually scored sleep variables in homozygous Val/Val and Met/Met genotypes of Val158Met polymorphism of COMT
| Val/Val genotype |
Met/Met genotype |
|||||||
|---|---|---|---|---|---|---|---|---|
| Placebo condition |
Modafinil condition |
Placebo condition |
Modafinil condition |
|||||
| Variable | Baseline | Recovery | Baseline | Recovery | Baseline | Recovery | Baseline | Recovery |
| Episode | 463.6 ± 3.9 | 477.7 ± 0.7** | 471.0 ± 1.4 | 476.4 ± 0.7** | 465.5 ± 3.7 | 477.1 ± 0.7** | 464.8 ± 4.0 | 474.9 ± 1.6** |
| TST | 445.2 ± 4.2 | 466.9 ± 2.2** | 450.8 ± 3.9 | 463.4 ± 2.5** | 449.4 ± 3.9 | 465.2 ± 1.5** | 450.1 ± 5.3 | 461.8 ± 2.2** |
| Efficiency | 92.8 ± 0.9 | 97.3 ± 0.5** | 93.9 ± 0.8 | 96.5 ± 0.5** | 93.7 ± 0.8 | 96.9 ± 0.3** | 93.8 ± 1.1 | 96.2 ± 0.5* |
| SL | 16.2 ± 4.0 | 2.3 ± 0.7** | 8.8 ± 1.5 | 3.6 ± 0.7** | 14.3 ± 3.7 | 2.9 ± 0.7** | 14.9 ± 4.0 | 5.1 ± 1.6** |
| RL | 65.9 ± 4.8 | 85.1 ± 15.7 | 61.2 ± 2.5 | 100.0 ± 19.8 | 69.1 ± 5.2 | 73.2 ± 8.4 | 68.0 ± 5.7 | 88.4 ± 11.2 |
| WASO | 6.6 ± 3.2 | 0.8 ± 0.3 | 8.7 ± 3.4 | 1.2 ± 0.6 | 4.9 ± 1.0 | 1.4 ± 1.1* | 4.1 ± 1.5 | 1.3 ± 0.5 |
| Stage 1 | 39.5 ± 2.8 | 22.7 ± 3.4*** | 37.1 ± 4.6 | 24.8 ± 4.3** | 37.9 ± 4.5 | 21.2 ± 5.3*** | 37.1 ± 3.9 | 26.0 ± 3.8*** |
| Stage 2 | 215.3 ± 13.1 | 207.8 ± 13.8 | 224.3 ± 12.3 | 201.8 ± 13.9** | 222.9 ± 7.9 | 202.3 ± 11.0* | 222.4 ± 10.7 | 214.0 ± 8.6 |
| Stage 3 | 38.1 ± 4.0 | 44.1 ± 4.5 | 38.4 ± 3.9 | 42.6 ± 4.0 | 34.7 ± 3.7 | 37.1 ± 3.5 | 31.9 ± 3.8 | 37.4 ± 2.5 |
| Stage 4 | 49.2 ± 11.5 | 98.2 ± 14.8*** | 47.2 ± 10.2 | 94.5 ± 15.0*** | 54.5 ± 6.0 | 104.1 ± 7.8*** | 56.7 ± 6.5 | 91.8 ± 7.7*** |
| REM sleep | 103.1 ± 6.0 | 94.3 ± 10.6 | 103.8 ± 7.6 | 99.7 ± 8.2 | 99.5 ± 4.2 | 100.5 ± 6.7 | 102.1 ± 6.7 | 92.6 ± 5.0 |
| MT | 11.8 ± 2.0 | 10.0 ± 1.8 | 11.5 ± 2.1 | 11.8 ± 2.1 | 11.3 ± 1.2 | 10.4 ± 1.2 | 10.6 ± 1.9 | 11.8 ± 1.1 |
Mean values ± SEM are in minutes (except sleep efficiency in %) for the first 480 minutes from lights-off. Ten Val/Val and 12 Met/Met allele carriers. Placebo condition, placebo intake during sleep deprivation; Modafinil condition, 2 x 100 mg modafinil intake during sleep deprivation; Baseline, baseline night; Recovery, recovery night after 40 hours extended waking; Episode, sleep episode (time after sleep onset until final awakening); TST, total sleep time; Efficiency, sleep efficiency (percentage of TST per 480 min); SL, sleep latency (time from lights-off to first occurrence of stage 2); RL, REM sleep latency (time from sleep onset to first occurrence of REM sleep); WASO, waking after sleep onset; Stages 1-4, NREM sleep stages; MT, movement time.
P < 0.001
P < 0.01
P < 0.05 compared to baseline (paired, two-tailed t-tests).
NREM sleep EEG
To characterize sleep loss and modafinil-induced changes during sleep in more detail, the spectral composition of the EEG in NREM sleep was quantified. After intake of placebo, prolonged waking enhanced EEG power in delta, theta, and alpha frequencies (0-10.25 Hz) and reduced activity in the frequency range of sleep spindles (13.25-15.75 Hz) irrespective of genotype (condition: F1,60 ≥ 5.05, P ≤ 0.03). The COMT genotype modulated the effect of sleep loss on the 10.5-11.25 Hz band (condition: F1,60 ≥ 8.86, P ≤ 0.005, genotype × condition: F1,60 ≥ 4.58, P ≤ 0.04). In the alpha and sigma range, respectively, EEG activity was increased up to 8.75 Hz and reduced in the 13.0-13.25 Hz range in Val/Val homozygotes, whereas the changes extended to 11.0 Hz and encompassed the 13.5-15.5 Hz range in Met/Met homozygotes (Figure 2B).
EEG slow wave activity (SWA, 0.75-4.5 Hz) in NREM sleep is a reliable marker of sleep homeostasis and is consistently reduced after caffeine.4,16 SWA did not differ between Val/Val and Met/Met genotypes in either baseline or recovery nights. In all recordings, SWA was highest in the first NREM sleep episode and declined across consecutive NREM sleep episodes (Figure 3). Moreover, the rebound of SWA after prolonged waking and its time course in recovery sleep were not altered by modafinil when compared to placebo. These data indicate that in both genotypes of COMT, the stimulant did not interfere with the homeostatic build-up and dissipation of sleep pressure.
Figure 3.
Intake of 2 x 100 mg modafinil does not affect the rebound of EEG slow wave activity (C3A2 derivation, power within 0.75-4.5 Hz) in NREM sleep (stages 2-4) after sleep deprivation in either Val/Val or Met/Met genotype of COMT. Mean slow wave activity in NREM sleep episodes 1-4 in baseline (mean of 2 nights) and recovery nights was expressed as a percentage of the mean all-night values in baseline (dashed vertical lines). Val/Val genotype: black symbols (dots: mean baseline; open triangles: placebo recovery; filled triangles: modafinil recovery). Met/Met genotype: gray symbols (dots: mean baseline; open triangles: placebo recovery; filled triangles: modafinil recovery). Means ± SEM are plotted.
*P < 0.01 (placebo-recovery vs. mean baseline; paired, 2-tailed t-tests)
+P < 0.03 (modafinil-recovery vs. mean baseline; paired, 2-tailed t-tests)
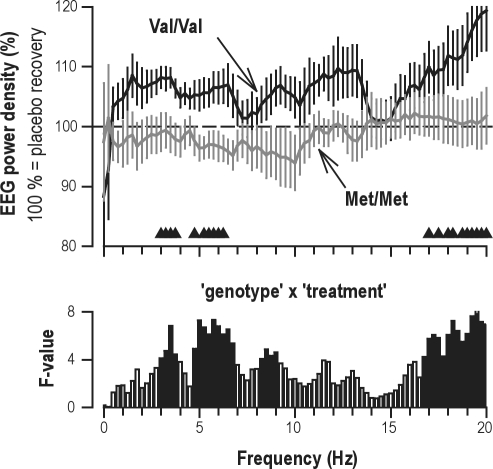
Despite the fact that sleep architecture and SWA were unaffected, modafinil induced subtle genotype- and state-specific changes in the NREM sleep EEG of the recovery night (Figure 4). Compared to placebo, EEG power was enhanced in 3.0-6.75 and 16.75-20.0 Hz ranges exclusively in Val/Val allele carriers (genotype × treatment: F1,60 ≥ 4.06, P ≤ 0.05). The stimulant induced no changes in the EEG in Met/Met allele carriers. Time course analyses revealed that the modafinil-induced increase in 3.0-6.75 Hz activity in the Val/Val homozygotes was restricted to the 3rd NREM sleep episode.
Figure 4.
Modafinil increases EEG 3.0-6.75 and 16.75-20.0 Hz activity in NREM sleep (stages 2-4) in Val/Val genotype but has no effect in Met/Met genotype. Top panel: In each frequency bin between 0.0-20.0 Hz, EEG power (C3A2 derivation) after modafinil was expressed as a percentage of the corresponding values after placebo (horizontal dashed line at 100%). Val/Val genotype: black line (n = 10); Met/Met genotype: gray line (n = 12). Means ± SEM are plotted for each 0.25 Hz bin. Black triangles indicate frequency bins which differed in the Val/Val genotype between modafinil and placebo (P < 0.05, paired 2-tailed t-tests). No significant differences were observed in the Met/Met genotype. Bottom panel: Significant (P < 0.05, black) and non-significant (P > 0.05, white) F-values of genotype × treatment interaction in 3-way mixed-model ANOVA with between-subjects factor genotype (Val/Val, Met/Met) and within-subjects factors condition (baseline, sleep deprivation) and treatment (placebo, modafinil).
REM sleep EEG
Sleep deprivation not only affected the EEG in wakefulness and NREM sleep, but also in REM sleep. Following intake of placebo, EEG activity in the recovery night was enhanced in REM sleep in delta frequencies (0-5.25 Hz) and reduced in alpha (8.0-10.75) and sigma (12.75-14.0 and 14.5 Hz) bands (condition: F1,60 ≥ 4.36, P ≤ 0.05). The changes were similar in both genotypes, and encompassed virtually all bins in 1.0-3.75 and 8.0-10.5 Hz ranges in Val/Val and in 0.5-4.75 and 9.0-9.5 Hz ranges in Met/Met homozygotes (Figure 2C). Modafinil had no effect on EEG activity in REM sleep in either genotype.
DISCUSSION
The Val158Met polymorphism of COMT reduces COMT enzymatic activity in prefrontal cortex,33 enhances dopamine D1 receptor availability in cortico-limbic structures,33–35 and modifies grey matter volume in hippocampus and dorsolateral prefrontal cortex.36 The main aim of the present study was to quantify the impact of this common genetic variation on the effects of sleep deprivation and modafinil on sleep and EEG markers of sleep homeostasis in wakefulness, NREM sleep, and REM sleep. We found in Val/Val and Met/Met genotypes that sleep loss induced similar alterations in subjective sleepiness, sleep structure, and EEG in wakefulness, NREM sleep, and REM sleep. Modafinil was equally effective in both genotypes in attenuating sleepiness and EEG theta activity in wakefulness. By contrast, the stimulant induced no changes in slow wave sleep and EEG slow wave activity in NREM sleep after sleep deprivation. This is in contrast to the adenosine receptor antagonist, caffeine, and demonstrates that modafinil leaves NREM sleep rebound after prolonged wakefulness unaffected. Nevertheless, in NREM sleep, the drug increased EEG activity in 3.0-6.75 and > 16.75 Hz frequencies exclusively in Val/Val allele carriers. Taken together, the data show that the promotion of wakefulness by pharmacological interference with dopaminergic and adenosinergic mechanisms differently affects sleep EEG markers of sleep homeostasis.
Prolonged wakefulness increases sleepiness, impairs performance, reduces vigilance, and alters the waking and sleep EEG.2–4 It is widely accepted that these changes reflect a wakefulness-induced increase in sleep pressure, which is homeostatically regulated. The mechanisms underlying sleep homeostasis are not well understood. Recent imaging studies in humans suggested that the brain dopamine system is involved in enhanced sleepiness and reduced cognitive performance after sleep deprivation.37,38 We investigated whether functional genetic variation in dopamine metabolism affects waking-induced changes in distinct markers of sleep homeostasis. Except for the 10.5-11 Hz range in the waking and NREM sleep EEG, the effects of sleep deprivation were the same in homozygous Val and Met allele carriers. While it is possible that genetic variation of COMT and sleep loss influence different aspects of dopamine neurotransmission/receptors (e.g., D1vs. D2/D3 receptors) and different brain areas (e.g., prefrontal cortex vs.striatum), further studies are needed to establish a potential role for dopamine in sleep homeostasis in humans.
Many stimulants and wake-promoting medications increase dopaminergic neurotransmission.39 Although the precise mode of action of modafinil remains unclear, data in animals and humans demonstrate that increased dopaminergic neurotransmission also contributes to the effects of modafinil.26–28 In support of this view, we recently reported that the Val158Met polymorphism of COMT strongly modulates the efficacy of modafinil to restore measures of well-being, executive functioning, and sustained attention after sleep loss.14 For example, modafinil maintained baseline performance on the psychomotor vigilance task measuring sustained vigilant attention throughout 40 hours without sleep in Val/Val homozygotes, whereas the same dose was virtually ineffective in Met/Met genotype (Figure 1A). By contrast, here we show that the stimulant reduced a subjective (Stanford Sleepiness Scale) and an objective (EEG 5-8 Hz activity) measure of sleepiness to a similar extent in both genotypes. This observation indicates that different mechanisms control neurobehavioral, subjective, and EEG markers of sleep homeostasis in wakefulness. This notion is consistent with recent findings in individuals who were either vulnerable or resistant to the neurobehavioral consequences of sleep deprivation.11 Our genetic and pharmacological data suggest that dopaminergic mechanisms contribute to the dissociation between performance and waking EEG measures of sleep homeostasis.
Similar to modafinil, caffeine improves neurobehavioral performance and subjective sleepiness and attenuates EEG theta activity in wakefulness.7,8 On the contrary, the effects of modafinil on the sleep EEG clearly differ from those of caffeine. Caffeine shortens slow wave sleep, reduces EEG low-delta activity, and increases spindle frequency activity and beta oscillations in NREM sleep.7,17,18,21 In support of the hypothesis that blockade of adenosine receptors during wakefulness attenuates the build-up of sleep pressure, caffeine consistently reduces EEG markers of sleep homeostasis in both wakefulness and sleep. Modafinil leaves delta and spindle frequency activity in NREM sleep unaffected when compared to placebo. The state-specific differences between modafinil and caffeine demonstrate that the changes in NREM sleep EEG reflect specific actions of different stimulant drugs. In addition, the data show that decreased theta power in wakefulness is not necessarily followed by reduced delta activity in NREM sleep. Different mechanisms thus also underlie stimulant-induced changes in waking and sleep EEG markers of sleep homeostasis.
Some studies in cats, rats, mice, and humans reported reduced or absent rebound sleep after modafinil-induced wakefulness.40–43 Because an ideal stimulant may promote wakefulness without subsequent sleep rebound,44 these reports raised widespread interest in modafinil. However, it may also be argued that a stimulant that lacks interference with sleep-wake regulation is advantageous. Indeed, other studies showed that modafinil-induced wakefulness enhances slow wave sleep duration and NREM sleep intensity as measured by EEG delta activity to a similar extent as the same duration of non-pharmacological sleep deprivation.14,44–48 These reports are consistent with the present data and demonstrate that modafinil does not attenuate slow wave sleep and slow wave activity in recovery sleep after sleep deprivation.
To investigate whether modafinil affects other markers of sleep homeostasis, we extended our analyses to the entire 0-20 Hz EEG power spectrum in wakefulness and sleep. In agreement with the preliminary results of another group,49 we found that during prolonged wakefulness, modafinil reduced theta activity (Figure 1C) and increased high alpha (11-13 Hz) oscillations29 when compared to placebo. These changes in the waking EEG were independent of COMT genotype. By contrast, the evolution across sleep deprivation of power in the 8-12 Hz band was not affected by modafinil (data not shown). This observation is opposite to a previous study, which suggested that modafinil inhibits the decrease of 8.5-11.5 Hz power present under placebo.50 Differences in drug doses, time of administration, and data analyses may underlie the discrepant findings.
Compared to placebo, modafinil increased 3.0-6.75 and > 16.75 Hz activity in NREM sleep in Val/Val allele carriers, whereas no such effects were observed in Met/Met homozygotes. The differences between the genotypes were not apparent from visual scoring because the scoring rules do not rely on EEG activity in these frequencies.31 It is well established that extension of prior wakefulness not only enhances delta activity but also theta oscillations in NREM sleep, whereas EEG power in the frequency range of sleep spindles is reduced4,51,52 (see also Figure 2B). To examine whether the genotype-specific modulation of 3.0-6.75 Hz activity reflects an interaction of modafinil with sleep homeostasis, a time course analysis was performed. Because the increase in the Val/Val genotype occurred in the third NREM sleep episode, when sleep pressure has largely dissipated, we suggest that it reflects a drug effect rather than an interaction with sleep regulation. This conclusion is further supported by the lack of effects of modafinil on low-delta and spindle frequencies in NREM sleep, and the absence of any EEG changes in REM sleep. Interestingly, also the dopamine D1/D2 receptor antagonist pergolide was reported to affect the sleep EEG in sleep state-specific manner.53 Comparison with this study, however, is difficult because it was conducted in patients with restless legs syndrome and did not include sleep deprivation. The functional significance of the genotype-dependent increase in 3.0-6.75 Hz activity in NREM sleep after modafinil remains to be elucidated in future studies. Notwithstanding, our data highlight the importance of genetic factors when investigating pharmacological changes in the sleep EEG.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank S. Xu, C. Stoll, T. Rusterholz, Dr. R. Dürr, V. Bachmann, Dr. E. Geissler, Dr. K. Jaggi, Dr. S. Regel, Dr. R. Khatami, Dr. U. Luhmann, Dr. N. Schäfer, Prof. W. Berger, and Prof. H. Jung, for their help with data collection and analysis, blood drawing, isolation of DNA, and genotyping. We are also indebted to Dr. C. Kopp and Prof. A. A. Borbély for helpful discussions and comments on the manuscript. This research was supported by Swiss National Science Foundation grants # 3100A0-107874 and 310000-120377, and EU Marie-Curie grant MCRTN-CT-2004-512362.
REFERENCES
- 1.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 2.Lim JL, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–22. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 3.Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29:320–39. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borbéely AA, Achermann P. Sleep homeostasis and models of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia, PA: Elsevier Saunders; 2005. pp. 405–17. [Google Scholar]
- 5.Borbély AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51:483–93. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- 6.Finelli LA, Baumann H, Borbély AA, Achermann P. Dual electroencephalogram markers of human sleep homeostasis: correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience. 2000;101:523–9. doi: 10.1016/s0306-4522(00)00409-7. [DOI] [PubMed] [Google Scholar]
- 7.Landolt HP, Rétey JV, Tönz K, et al. Caffeine attenuates waking and sleep electroencephalographic markers of sleep homeostasis in humans. Neuropsychopharmacology. 2004;29:1933–9. doi: 10.1038/sj.npp.1300526. [DOI] [PubMed] [Google Scholar]
- 8.Rétey JV, Adam M, Gottselig JM, et al. Adenosinergic mechanisms contribute to individual differences in sleep-deprivation induced changes in neurobehavioral function and brain rhythmic activity. J Neurosci. 2006;26:10472–9. doi: 10.1523/JNEUROSCI.1538-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt C, Collette F, Leclercq Y, et al. Homeostatic sleep pressure and responses to sustained attention in the suprachiasmatic area. Science. 2009;324:516–9. doi: 10.1126/science.1167337. [DOI] [PubMed] [Google Scholar]
- 10.Van Dongen HPA, Maislin G, Dinges DF. Dealing with inter-individual differences in the temporal dynamics of fatigue and performance: Importance and techniques. Aviat Space Environ Med. 2004;75:A147–a54. [PubMed] [Google Scholar]
- 11.Galliaud E, Taillard J, Sagaspei P, Valtati C, Bioulac B, Philip P. Sharp and sleepy: evidence for dissociation between sleep pressure and nocturnal performance. J Sleep Res. 2008;17:11–5. doi: 10.1111/j.1365-2869.2008.00629.x. [DOI] [PubMed] [Google Scholar]
- 12.Franzen PL, Siegle GJ, Buysse DJ. Relationships between affect, vigilance, and sleepiness following sleep deprivation. J Sleep Res. 2008;17:34–41. doi: 10.1111/j.1365-2869.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Axelsson J, Kecklund G, Akerstedt T, Donofrio P, Lekander M, Ingre M. Sleepiness and performance in response to repeated sleep restriction and subsequent recovery during semi-laboratory conditions. Chronobiol Int. 2008;25:297–308. doi: 10.1080/07420520802107031. [DOI] [PubMed] [Google Scholar]
- 14.Bodenmann S, Xu S, Luhmann UFO, et al. Pharmacogenetics of modafinil after sleep loss: catechol-o-methyltransferase genotype modulates waking functions but not recovery sleep. Clin Pharmacol Ther. 2009;85:296–304. doi: 10.1038/clpt.2008.222. [DOI] [PubMed] [Google Scholar]
- 15.Bonnet MH, Balkin TJ, Dinges DF, et al. The use of stimulants to modify performance during sleep loss: a review by the sleep deprivation and Stimulant Task Force of the American Academy of Sleep Medicine. Sleep. 2005;28:1163–87. doi: 10.1093/sleep/28.9.1163. [DOI] [PubMed] [Google Scholar]
- 16.Landolt HP. Sleep homeostasis: A role for adenosine in humans? Biochem Pharmacol. 2008;75:2070–9. doi: 10.1016/j.bcp.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 17.Landolt HP, Dijk DJ, Gaus SE, Borbély AA. Caffeine reduces low-frequency delta activity in the human sleep EEG. Neuropsychopharmacology. 1995;12:229–38. doi: 10.1016/0893-133X(94)00079-F. [DOI] [PubMed] [Google Scholar]
- 18.Landolt HP, Werth E, Borbély AA, Dijk DJ. Caffeine intake (200 mg) in the morning affects human sleep and EEG power spectra at night. Brain Research. 1995;675:67–74. doi: 10.1016/0006-8993(95)00040-w. [DOI] [PubMed] [Google Scholar]
- 19.Schwierin B, Borbély AA, Tobler I. Effects of N6-cyclopentyladenosine and caffeine on sleep regulation in the rat. Eur J Pharmacol. 1996;300:163–71. doi: 10.1016/0014-2999(96)00021-0. [DOI] [PubMed] [Google Scholar]
- 20.Drapeau C, Hamel-Hébert I, Robillard R, Selmaoui B, Filipini D, Carrier J. Challenging sleep in aging: the effects of 200 mg of caffeine during the evening in young and middle-aged moderate caffeine consumers. J Sleep Res. 2006;15:133–41. doi: 10.1111/j.1365-2869.2006.00518.x. [DOI] [PubMed] [Google Scholar]
- 21.Rétey JV, Adam M, Khatami R, et al. A genetic variation in the adenosine A2A receptor gene (ADORA2A) contributes to individual sensitivity to caffeine effects on sleep. Clin Pharmacol Ther. 2007;81:692–8. doi: 10.1038/sj.clpt.6100102. [DOI] [PubMed] [Google Scholar]
- 22.Rétey JV, Adam M, Honegger E, et al. A functional genetic variation of adenosine deaminase affects the duration and intensity of deep sleep in humans. Proc Natl Acad Sci U S A. 2005;102:15676–81. doi: 10.1073/pnas.0505414102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wesensten NJ. Effects of modafinil on cognitive performance and alertness during sleep deprivation. Curr Pharm Des. 2006;12:2457–71. doi: 10.2174/138161206777698819. [DOI] [PubMed] [Google Scholar]
- 24.Dauvilliers Y, Amulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007;369:499–511. doi: 10.1016/S0140-6736(07)60237-2. [DOI] [PubMed] [Google Scholar]
- 25.Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33:1477–502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- 26.Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21:1787–94. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qu WM, Huang ZL, Xu XH, Matsumoto N, Urade Y. Dopaminergic D-1 and D-2 receptors are essential for the arousal effect of modafinil. J Neurosci. 2008;28:8462–9. doi: 10.1523/JNEUROSCI.1819-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volkow ND, Fowler JS, Logan J, et al. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA. 2009;301:1148–54. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bodenmann S, Rusterholz T, Durr R, et al. The functional Val158Met polymorphism of COMT predicts interindividual differences in brain alpha oscillations in young men. J Neurosci. 2009;29:10855–62. doi: 10.1523/JNEUROSCI.1427-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sturm A, Clarenbach P. Schlafstörungen. Stuttgart, New York: Thieme Verlag; 1997. [Google Scholar]
- 31.Rechtschaffen A, Kales A. Bethesda, Maryland: National Institutes of Health; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 32.Cajochen C, Brunner DP, Kräuchi K, Graw P, Wirz-Justice A. Power density in theta/alpha frequencies of the waking EEG progressively increases during sustained wakefulness. Sleep. 1995;18:890–4. doi: 10.1093/sleep/18.10.890. [DOI] [PubMed] [Google Scholar]
- 33.Chen JS, Lipska BK, Halim N, et al. Functional analysis of genetic variation in catechol-o-methyltransferase (COMT): Effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–21. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–50. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Slifstein M, Kolachana B, Simpson EH, et al. COMT genotype predicts cortical-limbic D1 receptor availability measured with [C-11]NNC112 and PET. Mol Psychiatry. 2008;13:821–7. doi: 10.1038/mp.2008.19. [DOI] [PubMed] [Google Scholar]
- 36.Honea R, Verchinski BA, Pezawas L, et al. Impact of interacting functional variants in COMT on regional gray matter volume in human brain. Neuroimage. 2009;45:44–51. doi: 10.1016/j.neuroimage.2008.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volkow ND, Wang GJ, Telang F, et al. Sleep deprivation decreases binding of [C-11]raclopride to dopamine D-2/D-3 receptors in the human brain. J Neurosci. 2008;28:8454–61. doi: 10.1523/JNEUROSCI.1443-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volkow ND, Tomasi D, Wang GJ, et al. Hyperstimulation of striatal D2 receptors with sleep deprivation: Implications for cognitive impairment. Neuroimage. 2009;45:1232–40. doi: 10.1016/j.neuroimage.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boutrel B, Koob GF. What keeps us awake: The neuropharmacology of stimulants and wakefulness promoting medications. Sleep. 2004;27:1181–94. doi: 10.1093/sleep/27.6.1181. [DOI] [PubMed] [Google Scholar]
- 40.Lin JS, Gervasoni D, Hou Y, et al. Effects of amphetamine and modafinil on the sleep/wake cycle during experimental hypersomnia induced by sleep deprivation in the cat. J Sleep Res. 2000;9:89–96. doi: 10.1046/j.1365-2869.2000.00181.x. [DOI] [PubMed] [Google Scholar]
- 41.Touret M, Sallanon-Moulin M, Jouvet M. Awakening properties of modafinil without paradoxical sleep rebound: Comparative study with amphetamine in the rat. Neurosci Lett. 1995;189:43–6. doi: 10.1016/0304-3940(95)11448-6. [DOI] [PubMed] [Google Scholar]
- 42.Parmentier R, Anaclet C, Guhennec C, et al. The brain H3-receptor as a novel therapeutic target for vigilance and sleep-wake disorders. Biochem Pharmacol. 2007;73:1157–71. doi: 10.1016/j.bcp.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Buguet A, Montmayeur A, Pigeau R, Naitoh P. Modafinil, d-amphetamine and placebo during 64 hours of sustained mental work. II. Effects on two nights of recovery sleep. J Sleep Res. 1995;4:229–41. doi: 10.1111/j.1365-2869.1995.tb00173.x. [DOI] [PubMed] [Google Scholar]
- 44.Hasan S, Pradervand S, Ahnaou A, Drinkenburg W, Tafti M, Franken P. How to keep the brain awake? the complex molecular pharmacogenetics of wake promotion. Neuropsychopharmacology. 2009;34:1625–40. doi: 10.1038/npp.2009.3. [DOI] [PubMed] [Google Scholar]
- 45.Edgar DM, Seidel WF. Modafinil induces wakefulness without intensifying motor activity or subsequent rebound hypersomnolence in the rat. J Pharmacol Exp Ther. 1997;283:757–69. [PubMed] [Google Scholar]
- 46.Kopp C, Petit JM, Magistretti P, Borbely AA, Tobler I. Comparison of the effects of modafinil and sleep deprivation on sleep and cortical EEG spectra in mice. Neuropharmacology. 2002;43:110–8. doi: 10.1016/s0028-3908(02)00070-9. [DOI] [PubMed] [Google Scholar]
- 47.Saletu B, Frey R, Krupka M, Anderer P, Grunberger J, Barbanoj MJ. Differential-effects of a new central adrenergic agonist modafinil and d-amphetamine on sleep and early morning behavior in young healthy-volunteers. Int J Clin Pharmacol Res. 1989;9:183–95. [PubMed] [Google Scholar]
- 48.Wesensten NJ, Killgore WDS, Balkin TJ. Performance and alertness effects of caffeine, dextro amphetamine, and modafinil during sleep deprivation. J Sleep Res. 2005;14:255–66. doi: 10.1111/j.1365-2869.2005.00468.x. [DOI] [PubMed] [Google Scholar]
- 49.James LM, Dijk D, Boyle J, et al. Effects of a single dose of modafinil on EEG during the MWT during acute sleep deprivation. J Sleep Res. 2008;17:206. [Google Scholar]
- 50.Chapotot F, Pigeau R, Canini F, Bourdon L, Buguet A. Distinctive effects of modafinil and d-amphetamine on the homeostatic and circadian modulation of the human waking EEG. Psychopharmacology. 2003;166:127–38. doi: 10.1007/s00213-002-1315-8. [DOI] [PubMed] [Google Scholar]
- 51.Dijk DJ, Hayes B, Czeisler CA. Dynamics of electroencephalographic sleep spindles and slow wave activity in men: effect of sleep deprivation. Brain Res. 1993;626:190–9. doi: 10.1016/0006-8993(93)90579-c. [DOI] [PubMed] [Google Scholar]
- 52.Aeschbach D, Cajochen C, Landolt HP, Borbély AA. Homeostatic sleep regulation in habitual short sleepers and long sleepers. Am J Physiol. 1996;270:R41–R53. doi: 10.1152/ajpregu.1996.270.1.R41. [DOI] [PubMed] [Google Scholar]
- 53.Tagaya H, Wetter TC, Winkelmann J, et al. Pergolide restores sleep maintenance but impairs slee EEG synchronization in patients with restless legs syndrome. Sleep Med. 2002;3:49–54. doi: 10.1016/s1389-9457(01)00116-2. [DOI] [PubMed] [Google Scholar]