Abstract
Study Objectives:
Although episodes of neck myoclonus (head jerks) in REM sleep have a characteristic appearance, they have so far not been described systematically in video-polysomnography. This study assesses the occurrence, frequency, and characteristics of neck myoclonus in REM sleep in a prospective sleep disorder cohort, and investigates clinical correlates and associations with medication.
Setting:
University hospital sleep disorders center.
Participants:
Two-hundred twenty-eight mixed sleep disorder patients.
Interventions:
Not applicable.
Measurements and Results:
REM sleep was screened visually for short “stripe-shaped” movement-induced artifacts visible vertically over the EEG leads in polysomnographic registration. If such artifact was present, the synchronized video was inspected for the presence of neck myoclonus. Out of 205 patients, 54.6% (n = 112) had neck myoclonus during REM sleep. The mean neck myoclonus index was 1.0 ± 2.7 /h REM sleep. Younger patients had a higher neck myoclonus index than older patients (< 45 years versus 45-60 years versus > 60 years: 1.8 ± 4.2 versus 0.6 ± 1.1 versus 0.5 ± 1.1; P = 0.004). Ninety-five percent of subjects < 45 years had a neck myoclonus index between 0 and 9.4 /h; 95% of subjects > 45 years had a neck myoclonus index between 0 and 2.7 /h. Patients on benzodiazepine treatment had no neck myoclonus (0 /112 vs. 13 /93; P < 0.001). In 23 patients, additional surface neck EMG was performed. EMG activation associated with neck myoclonus had a mean duration of 0.6 ± 0.4 sec. Correlation between duration of neck EMG activation and movement-induced EEG artifact duration was very high (rho = 0.96; P < 0.001).
Conclusions:
Neck myoclonus is common during REM sleep and more frequent in younger individuals. This could indicate that neck myoclonus during REM sleep is a physiological phenomenon. If there is a cut-off distinguishing normal from excessive has to be investigated in further studies.
Citation:
Frauscher B; Brandauer E; Gschliesser V; Falkenstetter T; Furtner MT; Ulmer H; Poewe W; Högl B. A descriptive analysis of neck myoclonus during routine polysomnography. SLEEP 2010;33(8):1091-1096.
Keywords: Polysomnography, REM sleep behavior disorder (RBD), movement disorder, head jerk, myoclonus, EEG artifact, normal variant, violent
DURING REM SLEEP, MOVEMENTS ARE RARE DUE TO PHYSIOLOGIC MUSCLE ATONIA. REM SLEEP MUSCLE ATONIA OR RATHER HYPOTONIA, AS FOUND BY A recent work analyzing muscle activity across sleep stages,1 is caused by an active glycinergic post-synaptic inhibition of the spinal alpha-motoneurons resulting in hyperpolarization,2 although this concept has been challenged recently.3 In addition, the caudal ventral mesopontine junction has been demonstrated to inhibit phasic motor activity during REM sleep.4 Nevertheless, even in physiological REM sleep, random myoclonic twitching, particularly in distal muscles of the limbs, has been described.5 During the past decades, different approaches have been applied in order to study motor activity in sleep. They ranged from the static charge sensitive bed6 over the electroencephalographic artifact method7 to direct observation, as well as photography and videotaping.8 Recently, time-synchronized video-polysomnography became widely available. The main advantage of this technology is that movements over the whole night can be directly correlated to polysomnographic signals.9,10
In clinical analysis of sleep recordings, neck myoclonus can be observed during REM sleep, but has so far not been described systematically. It typically presents in polysomnographic registration of REM sleep as short “stripe-shaped” movement-induced artifact visible vertically over the EEG leads. This study was designed to investigate in detail the occurrence, frequency, and characteristics of neck myoclonus during REM sleep, associations with clinical correlates and comedication, and night-to-night variability. In addition, we assessed the occurrence of neck myoclonus during NREM sleep.
METHODS
Patient Cohort and Study Design
Two-hundred five consecutive patients undergoing routine polysomnography at the sleep disorders unit of the Department of Neurology, Innsbruck Medical University, between January and June 2004 were included in this study. Sleep diagnoses according to ICSD criteria,11 comorbidity, and concomitant medication at the time of polysomnography were obtained by history and chart review. All patients gave written informed consent. The Innsbruck sleep laboratory is a tertiary sleep disorders referral center serving a population of about 2 million, mostly from western Austria and South Tyrol (Northern Italy). It is the only academic facility for diagnosis and treatment of sleep disorders in the area. Patients represent a broad clinical spectrum of sleep disorders. Data were gathered prospectively from all polysomnographies performed. A single scorer (EB), who also proposed the present project, performed sleep stage scoring and assessment of neck myoclonus. To check reproducibility of the findings, the analysis of 15% of episodes of neck myoclonus (n = 87) was cross-checked by a second rater (BF). The mean interscorer agreement was 0.91 (P = 0.001).
Polysomnography and Sleep Scoring
Eight-hour polysomnography was performed with a digital Schwarzer polygraph (Brainlab 4.0, Schwarzer Inc., Munich, Germany). Electroencephalography included C3, C4, O1, O2, A1, and A2 electrodes. Electrooculography included vertical and horizontal eye movements. Electromyography included submental, mental, and both tibialis anterior muscles. Cardiorespiratory variables included one channel electrocardiography, nasal pressure cannula, nasal airflow (thermocouple), tracheal microphone, thoracic and abdominal respiratory effort, and transcutaneous oxygen saturation. Infrared video was recorded by an Elbex camera (Elbex Inc., EX series, Regensburg, Germany) and digitally stored as a whole, with a data rate of 1.500.000 bits/sec. The screen resolution for video analysis was 1280 × 960 pixels. Sleep scoring was visually performed in 30-sec epochs according to standard criteria,12 with allowance to score REM sleep despite persistence of tonic or phasic muscle activity in case of REM sleep behavior disorder or REM sleep without atonia.13
Scoring of Neck Myoclonus
In all polysomnographic recordings, REM sleep was carefully investigated in the following way after regular sleep stage scoring: Each 30-sec epoch was screened for characteristic “stripe-shaped” movement-induced artifacts (up to 2 sec duration) visible vertically over the EEG leads by one experienced sleep rater (EB). Artifact is an external signal alteration visible over the EEG leads produced by body movements not by alteration of bioelectric cortical activity. To exclude movements associated with sleep stage shifts and arousals, we investigated only movement-induced EEG artifact not preceded by arousals. Movement-induced EEG artifact lasting > 2 sec (e.g., gross body movement, positional change, epochs with movement time due to restless legs syndrome) were excluded from the screening process. An illustration of a typical movement-induced EEG artifact is shown in Figure 1. If a characteristic short EEG artifact caused by neck myoclonus was present, the synchronized video was inspected for the presence of neck myoclonus. In the video, neck myoclonus presents as sudden myoclonic dorsal or ventral flexion or version of the head to one side, with varying amplitude from mild to intense, which is non-sustained. In order to describe characteristics of neck myoclonus in more detail, episodes of neck myoclonus of the first 50 patients (n = 99) were systematically analyzed. Based on videography, we distinguished between dorsal flexion, ventral flexion, and version of the head to one side. Moreover, we checked for neck myoclonus with simultaneous movements of other body parts. Based on polysomnography, the proportion of episodes of neck myoclonus with simultaneous activation in the chin EMG, presence of rapid eye movements, and the proportion of arousals following episodes of neck myoclonus were analyzed. In addition, we examined the occurrence of episodes of neck myoclonus during NREM sleep.
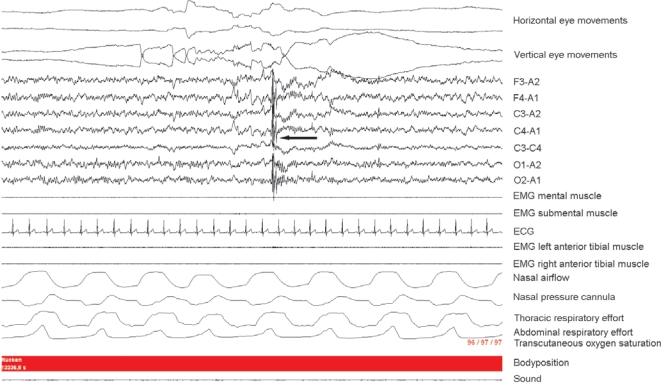
Figure 1.
Characteristic polysomnographic presentation of a neck myoclonus. Figure 1 represents a 30-sec REM sleep epoch containing a short-lasting “stripe-shaped” movement-induced artifact visible vertically over the EEG channels (black arrow). After visual inspection of the digitally synchronized video registration, the movement-induced EEG artifact could be identified as a neck myoclonus.
Polysomnographic Investigation Including Surface Neck Muscle EMG
For this part of the study, we performed standard polysomnography including surface neck muscle EMG in an additional sample of 23 patients. For the surface neck EMG, electrodes were placed exactly over the left and right splenius capitis muscles, with an inter-electrode distance of 3 cm. Surface neck EMG was high-pass filtered at 50 Hz and low-pass filtered at 300 Hz, amplified 10-fold, and sampled at a rate of 500 Hz. Sensitivity was set to 100 μV /cm and adjusted as needed for visual analysis. Impedance had to be lower than 10 kΩ. EMG activation was defined as any EMG elevation compared to background EMG activity. The EMG activation ends in case of > 0.5 sec with background EMG activity. This implies that a single EMG activation can consist of several phasic EMG events with an interactivity interval < 0.5 sec.
Statistics
SPSS for Windows, version 12.0 was used for data analysis. All values are presented as means ± standard deviation (range). The neck myoclonus index per individual patient was defined as the mean index calculated for every recording and given per hour of REM sleep. For assessing associations between neck myoclonus (presence, neck myoclonus index) and clinical correlates, as well as comedication, nonparametric statistics (χ2 tests and Mann-Whitney U tests) were performed. Bonferroni correction was used to account for multiple comparisons. In addition, we provided the range of neck myoclonus indices which were reached by 95% of investigated subjects. For the evaluation of night-to-night variability, registrations of patients with 2 consecutive nights of polysomnography during which REM sleep could be registered were analyzed. The neck myoclonus night-to-night variability index was calculated as the ratio of neck myoclonus indices of night 1 and night 2. For correlation analysis, Spearman correlation coefficient was calculated. P-values < 0.05 were considered to indicate statistical significance.
RESULTS
Characterization of the Study Population
The patient cohort consisted of 205 consecutive patients (28.3% women (n = 58), 71.7% men (n = 147). Mean age was 50.1 ± 14.4 (14 - 82) years. Patients' polysomnographic diagnoses are given in Table 1. Overall, 36.1% (n = 74) had arterial hypertension, 10.2% (n = 21) diabetes mellitus, 9.8% (n = 20) depression, 5.4% (n = 11) chronic obstructive pulmonary disease, and 3.9% (n = 8) coronary heart disease. Co-medication included antihypertensives (38 %, n = 78), lipid lowering drugs (17.6%, n = 36), antidepressants (15.1%, n = 31), thyroid medication (8.8%, n = 18), antiepileptic drugs (6.3%, n = 13), and benzodiazepines (6.3%, n = 13).
Table 1.
Polysomnographic diagnoses of the patient sample (n = 205)
| Frequency |
||
|---|---|---|
| Polysomnographic diagnoses | N | %* |
| Sleep related breathing disorders | 147 | 71.7 |
| Insomnia | 28 | 13.7 |
| Restless legs syndrome | 26 | 12.7 |
| Periodic leg movements in sleep > 5/h | 140 | 68.3 |
| Periodic leg movements in sleep > 15/h | 93 | 45.4 |
| REM sleep behavior disorder / REM sleep without atonia | 20 | 9.8 |
| Narcolepsy | 5 | 2.4 |
some patients had more than one diagnosis
In our sleep laboratory, every patient routinely undergoes 2 consecutive nights of polysomnography (adaption night, diagnostic night). In case of a relevant sleep related breathing disorder, nCPAP treatment is usually initiated in either night 2 or 3 during the same stay. Per patient, a mean of 2.3 ± 0.6 (1-4) polysomnograms were registered. All patients (n = 205) spent at least 1 night, 95.1% (n = 195) 2 nights, 31.7% (n = 65) 3 nights, and 3.4% (n = 7) 4 nights at the sleep laboratory. REM sleep was not detected in 7 polysomnograms. Mean REM sleep duration was 72.9 ± 31.5 (0 - 244) minutes.
Presence and Frequency of Neck Myoclonus during REM Sleep
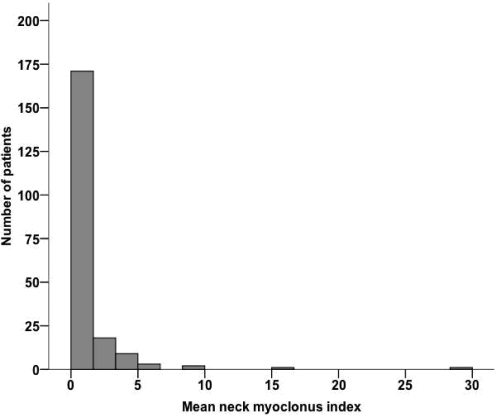
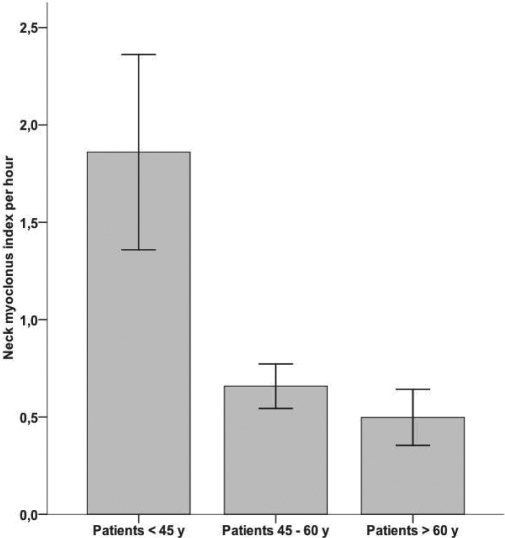
Neck myoclonus was present in 54.6% of 205 patients (n = 112); 45.4% (n = 93) had no neck myoclonus. Patients with episodes of neck myoclonus were younger than patients without neck myoclonus (mean age: 47.1 ± 13.9 vs. 53.5 ± 14.2; P = 0.001). In order to account for the neck myoclonus frequency, we calculated a neck myoclonus index defined as total number of episodes of neck myoclonus per hour of REM sleep. The mean neck myoclonus index was 1.0 ± 2.7 per hour of REM sleep. The distribution of neck myoclonus indices is illustrated in Figure 2. There was a significant association between the mean neck myoclonus index and the patients' age (< 45a vs. 45-60a vs. > 60a: 1.8 ± 4.2 vs. 0.6 ± 1.1 vs. 0.5 ± 1.1; P = 0.004). The mean neck myoclonus indices per hour of REM sleep for different age groups are given in Figure 3. Ninety-five percent of subjects < 45 years had a neck myoclonus index between 0 and 9.4 /h; 95% of subjects > 45 years had a neck myoclonus index between 0 and 2.7 /h.
Figure 2.
Distribution of episodes of neck myoclonus per hour over the whole patient sample.
Figure 3.
Neck myoclonus indices over different age groups.
Clinical correlates and Associations with Medication
Neck myoclonus was not observed in patients on benzodiazepines: 0 / 112 vs. 13 / 93 (0% vs. 14.1%; P < 0.001). All other sleep diagnoses, comorbidities, and co-medication did not reach statistical significance after correction for Bonferroni. For further details see Table 2.
Table 2.
Differences between patients with and without neck myoclonus
| Demographics | Patients with NM (n = 112) | Patients without NM (n = 93) | P-value |
|---|---|---|---|
| Age | 47.1 ± 13.9 | 53.5 ± 14.2 | 0.001* |
| Sex, n women (%) | 27 (24.1 %) | 31 (33.3 %) | 0.163 |
| PSG Diagnoses | |||
| Sleep related breathing disorders | 76 (67.9 %) | 71 (76.3 %) | 0.213 |
| Insomnia | 13 (11.6%) | 15 (16.1%) | 0.415 |
| Restless legs syndrome | 9 (8.0%) | 17 (18.3%) | 0.035 |
| PLMS > 5/h | 74 (66.1%) | 66 (71.0%) | 0.547 |
| PLMS > 15/h | 46 (41.1%) | 47 (50.5%) | 0.205 |
| REM sleep behavior disorder | 4 (3.6%) | 0 (0%) | 0.128 |
| REM sleep without atonia | 10 (8.9%) | 6 (6.5%) | 0.606 |
| Narcolepsy | 3 (2.7%) | 2 (2.2%) | 0.205 |
| Comorbidities | |||
| Hyperlipidemia | 38 (33.9%) | 40 (43.0%) | 0.196 |
| Hypertension | 38 (33.9%) | 36 (38.7%) | 0.559 |
| Diabetes mellitus | 9 (8.0%) | 12 (12.9%) | 0.355 |
| Depression | 10 (8.9%) | 10 (10.8%) | 0.814 |
| COPD | 5 (4.5%) | 6 (6.5%) | 0.551 |
| Coronary heart disease | 5 (4.5%) | 3 (3.2%) | 0.731 |
| Concomitant Medication | |||
| Antihypertensive medication | 39 (34.8%) | 39 (41.9%) | 0.315 |
| Lipid lowering drugs | 14 (12.5%) | 22 (24.4%) | 0.041 |
| Thyroid medication | 10 (8.9%) | 8 (8.7%) | 1.000 |
| Antidepressants | 16 (14.3%) | 15 (16.3%) | 0.700 |
| Antiepileptic drugs | 3 (2.7%) | 10 (10.9%) | 0.021 |
| Benzodiazepines | 0 | 13 (14.1%) | < 0.001* |
NM, neck myoclonus; PLMS, periodic leg movements in sleep; COPD, chronic obstructive pulmonary disease.
Significant P-values after correction for Bonferroni (P < 0.008 for PSG diagnoses, comorbidities, as well as concomitant medication; P < 0.05 for age)
There was a significant association between the mean neck myoclonus index and the use of benzodiazepines (benzodiazepines vs. no benzodiazepines: 0 vs. 1.1 ± 2.8; P < 0.001). Furthermore, it was remarkable that the 4 patients with REM sleep behavior disorder (none of whom were on benzodiazepine medication at time of polysomnographic investigation) had a higher mean neck myoclonus index than patients without REM sleep behavior disorder (2.8 ± 1.6 vs. 1.0 ± 2.7; P = 0.005), although this comparison is clearly of limited value due to the small sample size. All other sleep diagnoses, comorbidities, and co-medication did not reach significance after correction for Bonferroni.
Night-to-Night Variability of Neck Myoclonus Indices
For this analysis, registrations of 190 patients with 2 consecutive nights of polysomnography containing REM sleep were investigated. On night 1, neck myoclonus was present in 63 out of 190 patients (33.2%), and on night 2 in 77 out of 190 patients (40.5%). On both nights, the presence of neck myoclonus was associated with a longer REM sleep duration (REM sleep duration night 1, 76.6 ± 25.3 vs. 67.2 ± 29.5 min, P = 0.045; REM sleep duration night 2, 82.9 ± 25.3 vs. 75.1 ± 34.8 min, P = 0.024). The mean neck myoclonus index was 1.0 ± 2.8 on night 1, and 1.1 ± 2.9 on night 2. The neck myoclonus night-to-night variability index was 0.9 ± 0.6. The association between the neck myoclonus indices of both nights was significant (P < 0.001). The correlation, however, was moderate (Spearman rho = 0.53, P < 0.001).
Videopolysomnographic Properties of Neck Myoclonus
For this analysis, registrations (n = 115) of the first 50 consecutive patients were re-analyzed in more detail. Overall, 113 characteristic episodes of movement-induced artifact visible vertically over the EEG leads with a mean duration of 0.4 ± 0.4 seconds were detected. Ninety-nine of the 113 episodes (87.6%) corresponded to visible neck myoclonus.
In the video, 68 of 99 episodes of neck myoclonus (68.7%) contained brief versions of the head; 39 of 99 (39.4%) brief ventral flexions; and 36 of 99 (36.4%) contained brief dorsal flexions of the head. Forty-four of 99 episodes of neck myoclonus (44.4%) consisted of combinations of the above-mentioned head excursions. Amplitude was below 45 degrees in 91 out of 99 episodes of neck myoclonus (92.0%). Approximately two-thirds of episodes of neck myoclonus (n = 65, 65.7%) were associated with rapid eye movements; 73 (73.7%) were accompanied by muscle activation in the chin EMG; and a minority of episodes of neck myoclonus (n = 8, 8.1%) were accompanied by simultaneous movements of other body parts. Twenty of 99 (20.2%) episodes of neck myoclonus were followed by arousals.
Analysis of Neck Myoclonus during NREM Sleep
NREM sleep of the first 50 consecutive patients (n = 115) was analyzed. Overall, neck myoclonus was very rare during NREM sleep (mean ± SD, 0.04 ± 0.1) compared to REM sleep (mean ± SD, 0.7 ± 1.2; P < 0.001). Episodes of neck myoclonus occurred out of sleep stage 1 (n = 6 of 17), sleep stage 2 (n = 10 of 17) and sleep stage 3 (n = 1 of 17). Videographically, they were identical to those during REM sleep. We noticed that episodes of neck myoclonus during NREM sleep tended to occur often in association with rapid eye movements.
Detection of Neck Myoclonus in Surface Neck EMG
Twenty-three patients (15 men, 8 women) with a mean age of 52.7 ± 15.1 years were included in this additional part of the study. Age, sex, polysomnographic diagnoses, comorbidities, and co-medications were not different from the previously examined patient sample (P > 0.05). Neck myoclonus index per hour of REM sleep was not significantly different (P > 0.05). Overall, 65 episodes of neck myoclonus were registered during REM sleep. Neck EMG activation was present in 61 (93.8%) episodes of neck myoclonus. The mean neck myoclonus duration defined by a duration of neck EMG activation was 0.6 ± 0.4 sec. Thirty-five of 65 episodes of neck myoclonus had duration < 0.5 sec (54%); 30 had duration > 0.5 sec (46%). Mean movement-induced EEG artifact duration was 0.6 ± 0.4 sec. Correlation between neck EMG and movement-induced EEG artifact duration was very high (rho = 0.976, P < 0.001)
DISCUSSION
This study systematically describes and quantitatively assesses neck myoclonus during REM sleep. We found that occasional episodes of neck myoclonus during REM sleep are a common feature present in more than 50% of patients. The frequency of this phenomenon is low with a mean of 1.0 ± 2.8 episodes of neck myoclonus per hour of REM sleep. Neck myoclonus shows an inverse relation with age. Moreover, 20% are followed by arousals.
Visually, the described motor phenomenon has a characteristic appearance of myoclonus. According to the literature, some forms of subcortical myoclonus can last about 500 msec in EMG.14 This would imply that the brief head movements described here are truly myoclonic or may even represent a specific form of myoclonus. However, the literature on this is controversial, and many authors use the term myoclonus only for EMG events up to 100 msec.15 Neck myoclonus must be separated from fragmentary myoclonus described by Broughton et al.,16 which often goes along without a movement effect.
We do not know if episodes of neck myoclonus are a physiological phenomenon. The fact that they are common and occur in a wide variety of sleep disorders such as sleep related breathing disorders, restless legs syndrome, and insomnia may speak for a physiological rather than a pathological event. The inverse relation with age also points towards a physiological motor pattern. Interestingly, studies of body movements during the night in infancy revealed that twitch movements decreased with maturation to low base levels.17 In contrast, periodic leg movements in sleep (PLMS) increase with age, especially after the age of forty.18 Moreover, even in physiological REM sleep, random myoclonic twitching particularly in distal muscles of the limbs has been described.5 Neck EMG is not often performed in humans, while it is commonly used to monitor atonia in cats. In the seminal works of Jouvet et al., twitches of the whiskers and less frequent jerks of the jaw and tail, but not head or neck jerks, were reported in healthy cats during REM atonia.19 Hendricks et al. demonstrated that during recovery from paradoxical sleep without atonia, some cats exhibited minimal proximal and neck movements.20 It is unknown if this corresponds to residual pathological motor activity after REM sleep without atonia or to normal phasic motor activity, a fact that further supports the hypothesis of neck myoclonus at least as a non-relevant motor pattern. This hypothesis, however, has to be corroborated by further studies in healthy normals.
Defining pathological cut-offs is another crucial issue. Since neck myoclonus was common in our patient population, we provided the frequency range of neck myoclonus reached by 95% of all investigated subjects. Ninety-five percent of subjects younger than 45 years had a neck myoclonus index between 0 and 9.4 / h of REM sleep. Ninety-five percent of subjects older than 45 years had a neck myoclonus index between 0 and 2.8 /h of REM sleep. It has to be kept in mind that our study data were obtained from a mixed sleep disorder population and not from healthy controls, which represents real-life circumstances in the setting of routine polysomnographic testing. It has to be determined if neck myoclonus indices beyond the 95th percentile, which were present only in few patients, indicate a pathological condition. While there were too few RBD patients in this study to draw any valid statistical conclusions, our data could suggest that the frequency of neck myoclonus is higher in patients with REM sleep behavior disorder (RBD) than in patients without RBD. It is unknown if single episodes of neck myoclonus can evolve to full-blown RBD over time, but the inverse age-relation speaks against this. RBD behaviors typically involve the limbs,21 although some patients are reported who had predominantly head jerks as expression of RBD behavior during the video-polysomnographic recording.22
None of the 13 patients on benzodiazepine medication had neck myoclonus. Indeed, it is well known that clonazepam—a long acting benzodiazepine—is highly effective in reducing elaborate motor behaviors associated with RBD, but its effect on the phasic EMG activity during REM sleep has been shown to be quite modest.13 Therefore, the above-mentioned finding has to be interpreted with caution. Moreover, before investigating this issue, one would have to substantiate that neck myoclonus causes clinical complaints or has any adverse effect on health.
Although univariate analysis revealed additional associations between neck myoclonus and presence of restless legs syndrome, use of antiepileptic medication, antidepressants, and lipid-lowering agents, these associations did not withstand Bonferroni correction.
We found a moderate correlation, but no significant difference in night-to-night variability of the neck myoclonus index, suggesting relatively robust intraindividual findings.
Episodes of neck myoclonus occur typically during REM sleep; during NREM sleep they are detected very rarely. Interestingly, we noticed that episodes of neck myoclonus during NREM sleep tended to occur often in association with rapid eye movements. Although we did not formally evaluate this observation, one may speculate that these episodes of neck myoclonus represent REM features intruding into NREM sleep, similar to the intrusion of ponto-geniculo-occipital waves into NREM sleep.23
Neck EMG is activated in almost all cases of neck myoclonus. Interestingly, length of neck EMG activation highly correlated to the movement-induced artifact duration visible over the EEG leads, which therefore not only provides a good marker for the presence, but also for the duration of neck myoclonus.
In conclusion, this study demonstrates that neck myoclonus is common during REM sleep, especially in younger subjects. It occurs only sporadically (approximately 1 per hour of REM sleep). Arousal rates associated with episodes of neck myoclonus are low. Our results may indicate that neck myoclonus is a physiological rather than a pathological phenomenon. To finally solve this issue, studies in healthy controls are highly warranted.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Frauscher has consulted for Pfizer. Dr. Högl has participated in speaking engagements for Cephalon, Boehringer Ingelheim, Pfizer, and UCB and is on the advisory board of Merck, UCB, Jazz and Boehringer Ingelheim. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors wish to thank Heinz Hackner for his always excellent technical performance of polysomnographies.
This study was supported by the National Bank of Austria (Anniversary fund, 12594).
This work was performed at the Department of Neurology, Innsbruck Medical University, Innsbruck, Austria.
REFERENCES
- 1.Okura K, Kato T, Montplaisir JY, Sessle BJ, Lavigne GJ. Quantitative analysis of surface EMG activity of cranial and leg muscles across sleep stages in human. Clin Neurophysiol. 2006;117:269–78. doi: 10.1016/j.clinph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Chase MH, Morales FR. Control of motoneurons during sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia: Elsevier Saunders Company; 2005. pp. 154–68. [Google Scholar]
- 3.Brooks PL, Peever JH. Glycinergic and GABA(A)-mediated inhibition of somatic motoneurons does not mediate rapid eye movement sleep motor atonia. J Neurosci. 2008;28:3535–45. doi: 10.1523/JNEUROSCI.5023-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai YY, Hsieh KC, Nguyen D, Peever J, Siegel JM. Neurotoxic lesions at the ventral mesopontine junction change sleep time and muscle activity during sleep: an animal model of motor disorders in sleep. Neurosci. 2008;154:431–43. doi: 10.1016/j.neuroscience.2008.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldridge BJ, Whitman RM, Kramer M. The concurrence of fine muscle activity and rapid eye movements during sleep. Psychosom Med. 1965;27:19–26. doi: 10.1097/00006842-196501000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Laihinen A, Alihanka J, Raitasuo S, Rinne UK. Sleep movements and associated autonomic nervous activities in patients with Parkinson's disease. Acta Neurol Scand. 1987;76:64–8. doi: 10.1111/j.1600-0404.1987.tb03546.x. [DOI] [PubMed] [Google Scholar]
- 7.Wilde-Frenz J, Schulz H. Rate and distribution of body movements during sleep in humans. Percept Mot Skills. 1983;56:275–83. doi: 10.2466/pms.1983.56.1.275. [DOI] [PubMed] [Google Scholar]
- 8.Gardner R, Jr, Grossman WI. Normal motor patterns in sleep in man. Adv Sleep Res. 1975;2:67–107. [Google Scholar]
- 9.Frauscher B, Gschliesser V, Brandauer E, et al. Video analysis of motor events in REM sleep behavior disorder. Mov Disord. 2007;22:1464–70. doi: 10.1002/mds.21561. [DOI] [PubMed] [Google Scholar]
- 10.Frauscher B, Gschliesser V, Brandauer E, Ulmer H, Poewe W, Hogl B. The relation between abnormal behaviors and REM sleep microstructure in patients with REM sleep behavior disorder. Sleep Med. 2009;10:174–81. doi: 10.1016/j.sleep.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 11.American Sleep Disorders Association. Diagnostic and coding manual. Rochester, MN: American Sleep Disorders Association; 1990. The international classification of sleep disorders. [Google Scholar]
- 12.Rechtschaffen A, Kales A. Los Angeles: Brain Information Service/Brain Research Institute; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 13.Mahowald MW, Schenck CH. Principles and practice of sleep medicine. 4th ed. Philadelphia: Elsevier Saunders Company; 2005. REM sleep parasomnias. In: Kryger MH, Roth T, Dement WC, eds; pp. 897–916. [Google Scholar]
- 14.Marsden CD, Hallett M, Fahn S. The nosology and pathophysiology of myoclonus. In: Marsden CD, Fahn S, editors. Movement disorders. 1982. pp. 196–248. Butterworth International Medical Reviews. London. [Google Scholar]
- 15.Berardelli A, Thompson PD, Priori A. Myoclonus. In: Tolosa E, Koller WC, Gershanik OS, editors. Differential diagnosis and treatment of movement disorders. Boston: Butterworth-Heinemann; 1997. pp. 89–97. [Google Scholar]
- 16.Broughton R, Tolentino MA. Fragmentary pathological myoclonus in NREM sleep. Electroencephalogr Clin Neurophysiol. 1984;57:303–9. doi: 10.1016/0013-4694(84)90152-4. [DOI] [PubMed] [Google Scholar]
- 17.Fukumoto M, Mochizuki N, Takeishi M, Nomura Y, Segawa M. Studies of body movements during night sleep in infancy. Brain Dev. 1981;3:37–43. doi: 10.1016/s0387-7604(81)80004-6. [DOI] [PubMed] [Google Scholar]
- 18.Pennestri MH, Whittom S, Adam B, Petit D, Carrier J, Montplaisir J. PLMS and PLMW in healthy subjects as a function of age: prevalence and interval distribution. Sleep. 2006;29:1183–7. doi: 10.1093/sleep/29.9.1183. [DOI] [PubMed] [Google Scholar]
- 19.Jouvet M, Michel F, Courjon J. Sur un stade d'activité électrique cérébrale rapide au cours du somneil physiologique. C R Seances Soc Biol Fil. 1959;153:1024–1028. [Google Scholar]
- 20.Hendricks JC, Morrison AR, Mann GL. Different behaviors during paradoxical sleep without atonia depend on pontine lesion site. Brain Res. 1982;239:81–105. doi: 10.1016/0006-8993(82)90835-6. [DOI] [PubMed] [Google Scholar]
- 21.Schenck CH, Mahowald MW. REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep. 2002;25:120–38. doi: 10.1093/sleep/25.2.120. [DOI] [PubMed] [Google Scholar]
- 22.Kumru H, Santamaria J, Tolosa E, et al. Rapid eye movement sleep behavior disorder in parkinsonism with parkin mutations. Ann Neurol. 2004;56:599–603. doi: 10.1002/ana.20272. [DOI] [PubMed] [Google Scholar]
- 23.Fernández-Mendoza J, Lozano B, Seijo F, et al. Evidence of subthalamic PGO-like waves during REM sleep in humans: a deep brain polysomnographic study. Sleep. 2009;32:1117–26. doi: 10.1093/sleep/32.9.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]