Abstract
Rationale:
The most common single channel devices used for obstructive sleep apnea (OSA) screening are nasal airflow and oximetry. No studies have directly compared their role in diagnosing OSA at home.
Study Objectives:
To prospectively compare the diagnostic utility of home-based nasal airflow and oximetry to attended polysomnography (PSG) and to assess the diagnostic value of adding oximetry to nasal airflow for OSA.
Design:
Cross-sectional study
Setting:
Laboratory and home
Participants:
Sleep clinic patients with suspected OSA.
Interventions:
All patients had laboratory PSG and 2 sets of 3 consecutive nights on each device; nasal airflow (Flow Wizard, DiagnoseIT, Australia) and oximetry (Radical Set, Masimo, USA) at home in random order.
Results:
Ninety-eight of the 105 patients enrolled completed home monitoring. The accuracy of nasal airflow respiratory disturbance index (NF RDI) was not different from oximetry (ODI 3%) for diagnosing OSA (area under the ROC curve (AUC) difference, 0.04; 95% CI of difference −0.05 to 0.12; P = 0.43) over 3 nights of at-home recording. The accuracy of NF RDI was higher after 3 nights compared to one night (AUC difference, 0.05; 95% CI of difference, 0.01 to 0.08; P = 0.04). Addition of oximetry to nasal airflow did not increase the accuracy for predicting OSA compared to nasal airflow alone (P > 0.1).
Conclusions:
Nasal flow and oximetry have equivalent accuracy for diagnosing OSA in the home setting. Choice of device for home screening of sleep apnea may depend on logistical and service delivery issues.
Citation:
Makarie Rofail L; Wong KKH; Unger G; Marks GB; Grunstein RR. Comparison between a single-channel nasal airflow device and oximetry for the diagnosis of obstructive sleep apnea. SLEEP 2010;33(8):1106-1114.
Keywords: Oximetry, nasal airflow pressure transducer, home diagnosis, oxygen desaturation index, obstructive sleep apnea
RECOGNITION OF THE PREVALENCE OF OBSTRUCTIVE SLEEP APNEA (OSA) AND THE NEED TO RESTRAIN INCREASING HEALTH CARE EXPENDITURE has resulted in development of simplified diagnostic approaches in investigating patients with suspected sleep disordered breathing.1,2 One approach has been the development of a range of devices that monitor one or more of the non-EEG based parameters used in polysomnography (PSG). Previous studies examining the utility of these types of multichannel portable monitors for the diagnosis of OSA have typically been performed in sleep laboratories at highly specialized centers. While this approach limits the impact of factors such as differences in sleep quality and night-to-night variability, it may not yield results that are representative of how these devices will perform in the home setting.
In contrast to multi-channel devices, single-channel devices, usually measuring either oximetry or nasal flow, have the advantage of increased accessibility, ease of operation and ease of deployment in the home setting. These factors are particularly advantageous for patients living in regional and remote areas. These devices tend to employ automated analysis thus decreasing technician time and cost of operation.
Device utility studies in the home setting have investigated either multi-parameter monitors that measure 2 or more bioparameters3,4 or single channel monitors (predominantly involving oximeters).5–10 Although nocturnal oximetry has been extensively studied at home, the results have shown a wide range of specificity (41% to 100%) and sensitivity (31% to 98%) when compared to conventional PSG.7,11 This is largely due to differences in study populations, study design and the reference standard used to diagnose OSA as well as the use of older generations of pulse oximeters with different operating characteristics such as averaging time, sampling rate and artifact detection capability. With the advent of new generation pulse oximeters with faster averaging time and sampling rate and better artifact detection technology (patient motion, low perfusion conditions, and hypotension),12 further studies into the utility of pulse oximetry for diagnosing OSA at home are needed.
There is limited data available for single channel measurement of nasal flow, either by thermistors and thermal sensors13–15 or by nasal pressure transducers16,17 at home. In the studies examining nasal pressure transducers, nasal flow has been shown to have a high accuracy in diagnosing OSA when compared to conventional in-laboratory PSG, with high sensitivity (82% to 91%) and specificity (75% to 96%).16–18
Although a small recent study has compared agreement between nasal flow (using a thermal sensor) and oximetry in the laboratory concurrent with PSG; between-device accuracy comparisons were not performed.15 Studies comparing the diagnostic utility of single-channel oximetry and single-channel nasal flow in the same patient group at home are lacking, and no studies have compared diagnostic accuracy of single-night to multiple-night recording of either device.
The primary aim of the present study was to assess and compare the validity (with respect to in-laboratory PSG) of nasal flow and oximetry recordings (using separate single-channel monitors) at home for 3 nights each. Our secondary aims were to compare the diagnostic utility of one and 3 nights of monitoring for each device and to assess the additional value of combining the 2 signals in diagnosing OSA.
METHODS
We conducted a cross-sectional study to evaluate the test characteristics of 2 single-channel devices, nasal flow monitors and oximetry, for the diagnosis of OSA in the home setting. In-laboratory polysomnography was the reference for OSA diagnosis. The study was approved by the Human Research Ethics Committee of the Sydney South West Area Health Service (study number X05-0105) and was registered with the Australian New Zealand Clinical Trials Registry (ACTRN012605000120673).
Study Population
We recruited consecutive patients who were referred to the Sleep Disorders Clinic for evaluation of possible OSA between July 2006 and October 2007. Patients with complex unstable medical conditions, such as severe congestive heart failure, severe chronic obstructive pulmonary disease, interstitial lung disease, dependency on home oxygen, severe obesity (BMI > 45 kg/m2), neuromuscular disorder, inability to apply the diagnostic device (e.g., severe osteoarthritis), unstable psychiatric illness and/or history of current or previous drug and alcohol dependence including those in drug and alcohol rehabilitation, shift workers, known history of other sleep disorders, patients unable to understand the patient information sheets and those enrolled in other clinical research studies were excluded. In addition patients who lived in remote areas (> 40 km away from study site), and those who presented when all of the available nasal flow monitors and oximeters were in use could not be recruited for the home study.
Study Design
Detailed history examination and demographic data were collected at baseline. Height (to the nearest 0.5 cm), weight (to the nearest 0.5 kg) were measured and body mass index (BMI) was calculated. The Epworth Sleepiness Scale (ESS)19 was also administered at baseline. Patients were given written instruction about the application and operation of both devices. Research staff also gave a detailed standard demonstration of the application and the operation of each device. Patients performed home recordings for 2 consecutive 3-night sequences. The 2 sequences, which were preformed in random order, were 3 nights on the nasal flow monitor (Flow Wizard, DiagnoseIT, Sydney, Australia) and 3 nights on the oximeter (Radical Set, Masimo, CA, USA). They were instructed to use each device for a minimum of 6 h per night. The 2 sequences conducted at home and the in-laboratory PSG were performed in random order within an 8-week period. The patients, research staff, and their physician were blinded to all the results until the completion of all components of the study.
In-Laboratory PSG
Computerized attended full PSG recordings were performed (Alice 5, Respironics, Murrysville PA, USA) and included electroencephalography (EEG) (C2-A1, C3-A2, O1-A2, O2-A1); electro-oculography (EOG), and submental and tibialis anterior electromyography (EMG) for sleep staging according to Rechtschaffen and Kales criteria.20 Also, thoracic and abdominal piezoelectric respiratory movement sensors, oxygen saturation, nasal pressure via adult nasal cannulae (Pro-Tech, Washington, USA), body position, snoring, and electrocardiogram were monitored. A PSG apnea hypopnea index (AHI) ≥ 5 classified patients as having OSA and PSG AHI ≥ 30 as having severe OSA according to the American Academy of Sleep Medicine Task Force diagnostic criteria.21 Apneas were defined as complete cessation of airflow and hypopneas were defined as flow reduction > 50% associated with either a 3% desaturation or an arousal. The PSG recordings were scored independently by trained sleep technicians blinded to the portable monitor results.
Nasal Flow Monitor
The nasal flow monitor (Flow Wizard) recorded nasal airflow pressure via nasal cannulae.17 Automated nasal flow respiratory disturbance index (NF RDI) calculations were based on the artefact-free flow recording time (Figure 1). Respiratory disturbances included apneas, defined as a decrease in the amplitude of the airflow signal by ≥ 90% for ≥ 10 sec, and hypopneas, a reduction in the amplitude of the respiratory signal ≥ 50% for ≥ 10 sec. The recordings were automatically scored without manual editing. In the home, 2 types of nasal cannula were used: the Comfort Plus Soft Tip adult nasal cannula (Westmed, Arizona, USA) was used in the first 53 patients, and the Pro-Flow adult nasal cannula (Pro-Tech, Washington, USA) was used in the following 52 patients.
Figure 1.
Flow Wizard and analysis software
Oximetry
Finger pulse oximeter (Radical Set) was set to a short (2-sec) averaging time and a high sampling rate (80 Hz). The oxygen desaturation index (ODI 3%) was calculated as the number of desaturation events ≥ 3% divided by the total time in bed. Download 2001 v. 2.6.0 (Stowood Scientific Instruments, Oxford, UK) was used to analyze the tracing. The recordings were automatically scored without manual editing (Figure 2)
Figure 2.
The Radical Set Oximeter with a pulse probe attached and the oxygen tracing analysed by the Download 2001 program.
Data Quality
The reference standard (in-lab PSG) was included in the analysis and regarded as sufficient if ≥ 3 h of total sleep time was obtained. For nasal flow and oximetry the data were included in the analysis and regarded as sufficient if ≥ 3 h of good quality recording was obtained over one study night and ≥ 6 h over all 3 nights combined. For nasal flow, the duration of good quality recording was defined as the total recording duration minus poor quality signal time (defined by very low mean maximum pressure for 20 breaths and prolonged loss of flow signal > 2 min as per software algorithm). For oximetry good quality recording duration was the analysis duration minus artefact time as per the software algorithm. The data reported for all 3 nights was the total number of events divided by total good quality time over the 3 nights.
Statistical Methods
All analyses were carried out using SPSS, Version 14 software (Chicago, IL, USA). Values are expressed as mean (SD) and median (interquartile range, IQR) according to their distribution. Paired t-test and McNemar test were used for testing within-subject differences in continuous and categorical variables, respectively. The statistical level of significance was set as P < 0.05. The mean bias and limits of agreement between the PSG and nasal flow and oximetry were calculated.22 Reproducibility of NF RDI and ODI 3% was evaluated using a mixed effect intraclass correlation coefficient (ICC) among the data from each of the 3 nights at home. Areas under ROC curves (AUC) were compared for nasal flow and oximetry with respect to PSG AHI ≥ 5 and ≥ 30 events per hour.23 Sensitivity (Sn), specificity (Sp), positive (LR+) and negative (LR−) likelihood ratios, as well as the post-test probability of having OSA, were calculated at different thresholds.
A sample size calculation prior to the study initiation was performed based on the comparison of the sensitivities of the 2 monitors, and a formula derived from McNemar test was used (described by Li et al.).24 A pilot study of the Flow Wizard used in a sleep center population yielded a sensitivity of 96% and specificity of 71%. There were no data on the use of the Masimo oximeter to detect OSA, but a meta-analysis of apnea screening devices pooled values from 17 studies of various oximeters, and quotes a sensitivity of 87% and specificity of 65%.25 To demonstrate that the devices had a significantly different sensitivity in the detection of OSA with a 2-tailed significance level of 0.05 and a power of 0.8, 84 subjects with OSA were needed. Assuming a prevalence of OSA of 80% in our clinic population, a total of 105 subjects was required.
RESULTS
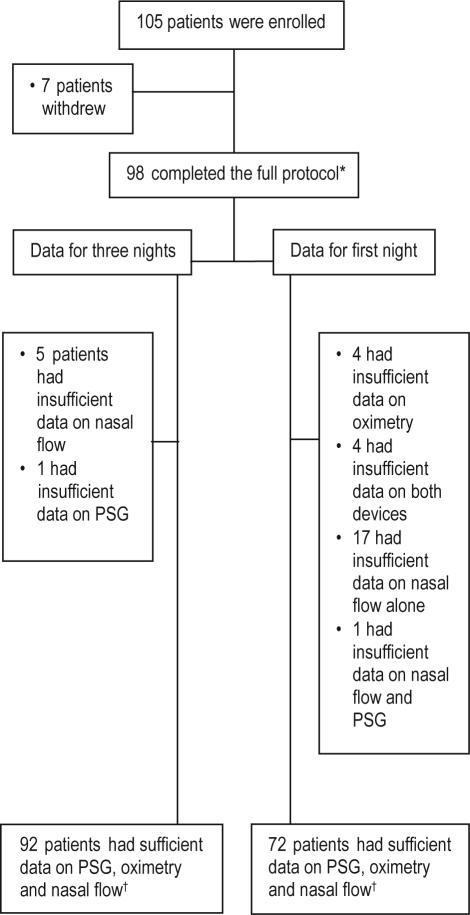
Between July 2006 and October 2007, 173 sleep disorders clinic patients were eligible; of this consecutive sample 128 were approached at random when devices were available. Of the 105 patients who consented, 98 (93.3%) completed the protocol, 92 (87.6%) had sufficient data on all 3 modalities at home after 3 nights, and 72 (68.6%) had sufficient data on the first night alone (Figure 3). Patients were predominantly Caucasian, middle-aged, obese, male, and had moderate OSA (Table 1).
Figure 3.
Subject participation and data sufficiency at various stages of the study. PSG, polysomnography. *denotes data included in the quality analysis, †denotes data included in the nasal flow and oximetry accuracy analysis.
Table 1.
Subject characteristics
| Mean (Median) | SD | |
|---|---|---|
| Age (y) | 46.0 (46.0) | 11.7 |
| BMI (kg/m2) | 29.7 (29.3) | 5.1 |
| ESS | 9.7 (10.0) | 5.0 |
| PSG AHI (Events/h) | 18.7 (15.2) | 21.2 |
| Proportion | ||
| Males | 77.1% | |
| Caucasian | 89.5% | |
| AHI ≥ 5 | 70.5% | |
| AHI ≥ 30 | 24.8% |
BMI, body mass index; ESS, Epworth Sleepiness Scale; PSG, poly-somnography; AHI, Apnea hypopnea index
Data Completeness and Reproducibility
Three nights of data compared to only the first night data resulted in better data sufficiency for nasal flow (failure = 5.1% (5/98) vs. 22.4% (22/98), P < 0.0001) and for oximetry (0% (0/97) vs. 8.1% (8/98), P = 0.04). There was no significant difference in the rate of insufficient data between nasal flow and oximetry when recorded across 3 nights (P = 0.6).
Both NF RDI and ODI 3% were highly reproducible between the 3 nights ICC = 0.86 (95% CI 0.79-0.91) and 0.90 (95% CI 0.86-0.93), respectively.
Agreement of Nasal Flow and Oximetry with PSG
Figure 4 shows the agreement between PSG AHI and both NF RDI and ODI 3%, when used at home for one night and for 3 nights. Overall nasal flow overestimated the PSG AHI while oximetry underestimated PSG AHI (Table 2). The mean bias of PSG AHI and each device for the first night was not different from that obtained over 3 nights (P = 0.58 for nasal flow and P = 0.29 for oximetry).
Figure 4.
Bland Altman plots showing the mean difference (thin lines) and the limits of agreement (2 SD; thick lines) for polysomnography apnea hypopnea index (PSG AHI), nasal flow respiratory disturbance index (NF RDI) and oxygen desaturation index ≥3% (ODI 3%) on the first night (A and B) and after 3 nights (C and D) at home. NF RDI overestimated AHI while ODI 3% underestimated AHI. The limits of agreement were comparable for both devices and were generally lower at low AHI.
Table 2.
Mean (SD) difference between PSG AHI and NF RDI and between PSG AHI and ODI 3% (events/h)
| PSG AHI minus NF RDI, Mean (SD) | PSG AHI minus ODI 3%, Mean (SD) | |
|---|---|---|
| N1 | −6.1 (14.3) | 6.8 (15.0) |
| N1-3 | −5.7 (13.6) | 6.5 (14.3) |
SD, standard deviation; PSG, polysomnography; AHI, apnea hypopnea index; NF RDI, nasal flow respiratory disturbance index; ODI 3%, oxygen desaturation index ≥ 3%; N1, first night; N1-3, 3 nights
Diagnostic Accuracy of Nasal Flow and Oximetry
ROC curves were used to examine the relation between the diagnostic tests (NF-RDI and ODI 3%) and the reference standard definition of OSA (PSG AHI > 5) and severe OSA (PSG AHI > 30). The overall diagnostic performance was quantified as the area under the ROC curve (AUC). Finally, the performance characteristics of each of these thresholds were assessed.
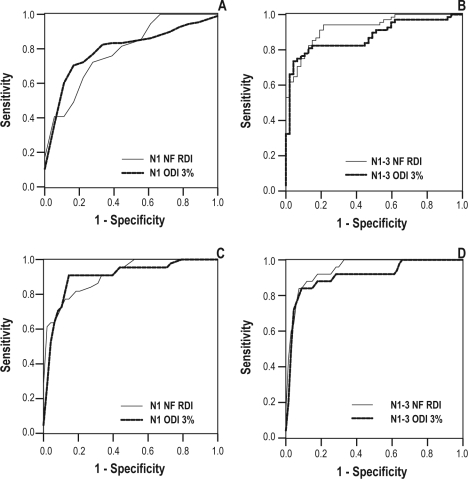
For diagnosing OSA, the AUC for NF RDI and for 3% ODI were not significantly different from each other when recorded over 3 nights or after the first night (Table 3 and Figure 5). Similarly for diagnosing severe OSA, there was no difference between the AUC for NF RDI and 3% ODI recorded over the first night and over 3 nights. Both devices had a high specificity, sensitivity, and positive likelihood ratio and a low negative likelihood ratio at the optimal thresholds shown in Table 4 and 5.
Table 3.
AUC for nasal flow and oximetry at home for diagnosing OSA (PSG AHI ≥ 5 events/h) and severe OSA (PSG AHI ≥ 30 events/h)
| PSG AHI ≥ 5 |
PSG AHI ≥ 30 |
|||
|---|---|---|---|---|
| N1 AUC (95% CI) | N1-3 AUC (95% CI) | N1 AUC (95% CI) | N1-3 AUC (95% CI) | |
| NF RDI | 0.80 (0.70-0.91) | 0.85 (0.76-0.91) | 0.94 (0.87-1.00) | 0.95 (0.90-0.98) |
| ODI 3% | 0.80 (0.69-0.91) | 0.81 (0.72-0.90) | 0.91 (0.82-0.99) | 0.91 (0.83-0.98) |
| Difference in AUC | < 0.01 (−0.1 to 0.01) | 0.04 (−0.05 to 0.12) | 0.03 (−0.08 to 0.08) | 0.04 (−0.04 to 0.10) |
| P value | 0.97 | 0.43 | 0.98 | 0.35 |
PSG, polysomnography; AHI, apnea hypopnea index; AUC, area under the receiver operator characteristics curve; NF RDI, nasal flow respiratory disturbance index; ODI 3%, oxygen desaturation index ≥ 3%; N1, first night; N1-3, 3 nights; CI, confidence interval
Figure 5.
Receiver operator characteristics (ROC) curves of NF RDI and ODI 3% at home for the first night (A) and for 3 nights (B) for diagnosing OSA as defined by polysomnography Apnea Hypopnea index (PSG AHI) ≥5 events per hour and for the first night (C) and 3 nights (D) for diagnosing severe OSA defined as PSG AHI ≥30 events per hour. The accuracy for diagnosing OSA and severe OSA was high for both devices. There is no difference in the AUC between devices on the first night or after 3 nights for diagnosing OSA or for severe OSA.
Table 4.
Operating characteristics of nasal flow (NF RDI) oximetry (ODI 3%) at home on the first night and after 3 nights for diagnosing OSA (PSG AHI ≥ 5)
| Post-test probability |
|||||||
|---|---|---|---|---|---|---|---|
| NF RDI | Cut Off | Sensitivity (%) | Specificity (%) | LR+ | LR− | OSA (Pre-test = 0.75) | no OSA (Pre-test = 0.25) |
| N1 | 20 | 0.75 (0.63-0.85) | 0.79 (0.61-0.97) | 3.6 | 0.30 | 0.91 | 0.51 |
| N1-3 | 20 | 0.80 (0.67-0.93) | 0.87 (0.77-0.97) | 6.3 | 0.23 | 0.95 | 0.59 |
| 3% ODI | |||||||
| N1 | 7 | 0.63 (0.66-0.86) | 0.83 (0.74-0.80) | 3.7 | 0.45 | 0.92 | 0.43 |
| N1-3 | 7 | 0.77 (0.63-0.91) | 0.89 (0.80-0.98) | 7.2 | 0.26 | 0.95 | 0.56 |
PSG AHI, polysomnography derived apnea hypopnea index; LR+, positive likelihood ratio; LR−, negative likelihood ratio; NF RDI, nasal flow respiratory disturbance index; ODI 3%, oxygen desaturation index ≥ 3%; N1, First night; N1-3, 3 nights
Table 5.
Operating characteristics of nasal flow (NF RDI) oximetry (ODI 3%) at home on the first night and after 3 nights for diagnosing severe OSA (PSG AHI ≥ 30)
| Post-test probability |
|||||||
|---|---|---|---|---|---|---|---|
| NF RDI | Cut Off | Sensitivity (%) | Specificity (%) | LR+ | LR− | OSA (Pre-test = 0.28) | no OSA (Pre-test = 0.72) |
| N1 | 30 | 0.90 (0.84-0.98) | 0.83 (0.76-0.87) | 5.3 | 0.12 | 0.67 | 0.96 |
| N1-3 | 30 | 0.90 (0.83-0.98) | 0.85 (0.78-0.89) | 6.0 | 0.12 | 0.70 | 0.96 |
| 3% ODI | |||||||
| N1 | 10 | 0.90 (0.86-0.96) | 0.88 (0.75-0.94) | 7.5 | 0.11 | 0.74 | 0.96 |
| N1-3 | 10 | 0.90 (0.87-0.97) | 0.85 (0.73-0.92) | 6.0 | 0.11 | 0.70 | 0.96 |
PSG AHI, polysomnography derived apnea hypopnea index; LR+, positive likelihood ratio; LR−, negative likelihood ratio; NF RDI, nasal flow respiratory disturbance index; ODI 3%, oxygen desaturation index ≥ 3%; N1, First night; N1-3, 3 nights
Diagnostic Accuracy: One Night Vs. Three Nights at Home
The patients who had sufficient data after the first night for NF RDI and ODI 3% were used to compare accuracy of the first night versus 3 nights on each device. For diagnosing OSA, nasal flow had a higher AUC after 3 nights than after the first night (difference in AUC = 0.05; 95% CI (0.01 to 0.08); P = 0.04). However, there was no significant difference in the AUC for 3% ODI recorded over 3 nights compared to after the first night (difference in AUC = 0.01; 95% CI (−0.04 to 0.05); P = 0.76). For predicting severe OSA, there was no difference in the accuracy of 3 nights, as opposed to the first night only for both NF-RDI or for ODI 3%. Overall for nasal flow the specificity increased with the number of nights recorded. The operating characteristics of oximetry did not change with the number of nights recorded.
The Addition of Oximetry to Nasal Flow
If either nasal flow and/or oximetry were regarded as sufficient 93/96 patients would have sufficient data after one night recording on each device and no patients would have insufficient data after 3 nights on each device.
Using logistic regression analysis with PSG AHI as a binary variable being predicted by nasal flow and oximetry as continuous variables, the addition of N1 (first night) ODI 3% to N1 NF RDI did not significantly contribute to the model. Similarly the addition of N1-3 (3 nights) ODI 3% to N1-3 NF RDI did not increase the probability of diagnosing OSA above that obtained by N1-3 NF RDI alone (Table 6).
Table 6.
Odds ratio for the combination of oximetry and nasal flow after one night and after 3 nights
| Model 1* | Odds Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| N1 NF RDI | 1.11 (1.03-1.18) | 0.002 |
| N1 ODI 3% | 1.15 (0.95-1.39) | 0.14 |
| Model 2** | ||
| N1-3 NF RDI | 1.12 (1.03-1.22) | 0.008 |
| N1-3 ODI 3% | 1.16 (0.971-1.4) | 0.10 |
NF RDI, nasal flow respiratory disturbance index; ODI 3%, oxygen desaturation index ≥ 3%; N1, First night; N1-3, 3 nights;
Combining one night of nasal flow and one night of oximetry;
Combining 3 nights of nasal flow and 3 nights of oximetry
DISCUSSION
Our data demonstrate that both automated analysis of single-channel nasal flow (Flow Wizard) or oximetry (Radical Set) have high accuracy when recorded at home in patients referred to a sleep disorders clinic with suspected OSA. Nasal flow had a higher accuracy when used over three nights than over a single night for diagnosing OSA (PSG AHI ≥ 5). Data sufficiency increased with the number of nights recorded on both devices. The addition of oximetry to nasal flow did not increase the accuracy of OSA diagnosis but increased the rate of data sufficiency if either device data was used.
One of the main issues surrounding ambulatory device testing is the rate of data sufficiency, which has both utility and cost implications. Our study suggests that with three nights of recording there is a much lower rate of data insufficiency on each device. For example, the rate of data loss or inadequacy was much lower after 3 nights than after the first night of home recording for nasal flow (5.1% vs. 22.4%) and for oximetry (0% vs. 8.1%). These results are consistent with previously reported on data inadequacy or loss rate of 18% to 20% with full polysomnography at home.26–28 While the rate of data loss with nasal flow recorded on the first night was greater than that of oximetry, the cost of additional nights of recording was minimal compared to additional nights of conventional PSG, as there are no additional consumables used and no extra technician time is required. However there remains the potential cost of acquiring a larger number of devices in the clinic to accommodate the demand if the device is to be used for up to 3 nights. Given that most of the patients with insufficient data on one device on the first night had sufficient data on the other device, another possibility is a system where there is sequential use of devices or employing 2-channel devices, but these may be more costly. It is also possible that rates of data sufficiency may be higher for a single channel nasal flow device such as the Flow Wizard if the nasal prongs are taped to the face but this method was not utilized in this study.
Nasal flow at home tended to overestimate the severity of OSA compared to in-laboratory PSG. This degree of overestimation did not change after 3 nights of recording. This difference in agreement may have occurred due to the automatic scoring algorithm classifying respiratory events at a lower threshold than the PSG event criteria. Other factors that may have contributed to the difference in agreement between PSG AHI and NF RDI include the presence of nasal obstruction, initiation of mouth or partial mouth breathing,29 and the inclusion of events that may have occurred when the patient was awake. On the other hand oximetry tended to underestimate the degree of OSA. This is not surprising, as the ODI 3% requires desaturation to identify events and some events can be scored on PSG without episodes of oxygen desaturation (as flow-based AHI was used in our reference standard) thus ultimately leading to a degree of underestimation of the severity of OSA. Of note, the differences between PSG AHI, NF RDI, and ODI 3% are not evenly distributed across the Bland Altman plot, with smaller difference at lower PSG AHI and larger differences at high PSG AHI. Thus it is important to look at the Bland Altman plot in conjunction with mean difference or degree of bias. These findings have been reported previously by other groups examining the agreement of diagnostic devices.10,16,17,30
The accuracy of diagnosing OSA (PSG AHI ≥ 5) by nasal flow recorded for three nights increased compared to that recorded for the first night. This may be due to the large number of patients with mild OSA in our study. Night-to-night variability and first night effect have more profound effects at milder degrees of sleep apnea or patients with predominant hypopnea. Thus our data suggests that if suspicion is high for mild OSA then one night of nasal flow recording may not be sufficient to rule in or rule out OSA. However, a significant number of patients with OSA can be missed even with the reference standard in-laboratory PSG when only one night is recorded.31 On the other hand, the accuracy of ODI 3% did not increase with the number of nights recorded; thus if suspicion for OSA is high, a negative oximetry study is not sufficient to rule out OSA, and there is no extra diagnostic gain by repeating the study. The patient should progress to other diagnostic modalities, such as nasal flow recording or multichannel monitoring at home or formal laboratory PSG.
Both nasal flow and oximetry have a high accuracy for diagnosing severe OSA which was not increased by additional nights of recording; thus repeating the study will not yield any additional diagnostic information unless insufficient data was obtained on the first night. Tables 7 and 8 summarize the published studies on single channel oximetry and nasal flow performed at home. Our data is in keeping with that published on nasal airflow, with high sensitivity, specificity and overall accuracy. The data on oximetry is more difficult to compare because of differences in the parameters measured by oximetry and differences in the reference standard. Suffice to say that, as noted by our study, most of the studies reported high specificity with the parameters measured.
Table 7.
Studies of single-channel nocturnal oximetry performed at home
| Author, Year, Country | Number of subjects | Name of Device | Reference Diagnostic Criteria | Oximeter scoring criteria | Sn (%) | Sp (%) | LR+ | LR− |
|---|---|---|---|---|---|---|---|---|
| Williams et al,5 1991, USA | 40 referred to sleep clinic | Biox 3700 IV*, Nelllcor N100† | AI > 10 | ODI 4% | 58 | 92 | 10.6 | 0.16 |
| Oximetry plus clinical score | 85 | 92 | 7.2 | 0.46 | ||||
| Series et al,6 1993, Canada | 240 sleep clinic patients with possible OSA | Biox IVa‡ | AHI ≥ 10 | Visual scoring | 98 | 48 | 1.9 | 0.04 |
| Gyulay et al,7 1993, Australia | 98 with possible OSA | Biox 3700§ | AHI ≥ 15 | Clinical assessment | 79 | 50 | 1.6 | 0.42 |
| ODI 4% | 40 | 98 | 20.0 | 0.61 | ||||
| ODI 3% | 51 | 90 | 5.1 | 0.54 | ||||
| ODI 2% | 65 | 74 | 2.5 | 0.47 | ||||
| CT < 90 | 93 | 51 | 1.9 | 0.14 | ||||
| Visual inspection | 72 | 88 | 6.0 | 0.32 | ||||
| Ryan et al,8 1995, UK | 69 with possible OSA | Minolta Pulsox-7** | AHI ≥ 15 | ODI 4% > 15 | 32 | 100 | 0.68 | |
| Olson et al,9 1999, Australia | 793 with possible OSA | Biox 4700†† | AHI ≥ 15 | Delta Index (< 0.6) | 88 | 40 | 1.5 | 0.29 |
| CT 90 ≥ 1% | 75 | 46 | 1.4 | 0.54 | ||||
| AHI ≥ 30 | Delta Index (< 0.6) | 93 | 34 | 1.4 | 0.21 | |||
| CT 90 ≥ 1% | 84 | 44 | 1.5 | 0.36 | ||||
| Wiltshire et al,10 2001, England | 100 with possible OSA | Biox 3740‡‡ | ODI ≥ 10 | ODI ≥ 10 | 41 | 100 | – | 0.59 |
| ODI ≥ 15 | ODI ≥ 15 | 35 | 100 | – | 0.65 | |||
AHI, Apnea hypopnea Index; AI, Apnea Index; ODI 4%, 3%, 2%, oxygen desaturation index per hour to 4%, 3%, 2%; CT < 90, cumulative time below 90% saturation; Delta Index, the average of the absolute differences of oxygen saturation between successive intervals; Sn, sensitivity; Sp, specificity; LR+, positive likelihood ratio; LR−, negative likelihood ratio;
Ohmeda, Boulder, CO, USA;
Nellcor Inc, Hayward, CA, USA;
Ohmeda, Boulder, CO, USA;
Ohmeda, Boulder, CO, USA;
Minolta, Diessenhofen, Switzerland;
Ohmeda, Boulder, CO, USA;
Ohmeda, Boulder, CO, USA
Table 8.
Studies of nasal flow using thermal sensors and nasal pressure transducers performed at home
| Author, Year, Country | Subjects | Device | Diagnostic Criteria | Sn (%) | Sp (%) | LR+ | LR− | AUC |
|---|---|---|---|---|---|---|---|---|
| Hollingworth et al,13 2003, UK | 48 with suspected OSA | Thermal sensor* | PSG vs. home nasal airflow | |||||
| AHI ≤ 10 | NR | 56 | ||||||
| AHI ≥ 20 | 14 | NR | ||||||
| Pang et al,14 2006, USA | 39 with suspected OSA | Thermal sensor† | PSG vs. home nasal airflow | |||||
| AHI > 15 | 55 | 70 | 1.8 | 0.64 | ||||
| AHI > 25 | 44 | 81 | 2.3 | 0.69 | ||||
| AHI > 40 | 33 | 95 | 6.6 | 0.71 | ||||
| Nakano et al,15 2008, Japan | 100 with suspected OSA | Thermal sensor‡ | PSG vs. in lab nasal airflow | |||||
| AHI ≥ 5 | 96 | 82 | 5.3 | 0.05 | 0.95 | |||
| AHI ≥ 15 | 91 | 82 | 5.1 | 0.11 | 0.96 | |||
| AHI ≥ 30 | 89 | 96 | 22.3 | 0.11 | 0.98 | |||
| PSG vs. home nasal airflow | NR | NR | NR | NR | NR | |||
| Erman et al,16 2007, USA | 59 from Diabetic clinic | Nasal pressure transducer§ | PSG vs. in lab nasal airflow | |||||
| AHI > 5 | 85 | 50 | 1.7 | 0.3 | 0.86 | |||
| AHI > 10 | 82 | 84 | 5.1 | 0.2 | 0.86 | |||
| AHI > 15 | 91 | 95 | 18.2 | 0.09 | 0.98 | |||
| AHI ≥ 20 | 83 | 93 | 11.9 | 0.18 | 0.98 | |||
| PSG vs. home nasal airflow | NR | NR | NR | NR | NR | |||
| Wong et al,17 2008, Australia | 34 with suspected OSA | Nasal pressure transducer** | PSG vs. home nasal airflow | |||||
| AHI ≥ 10 | 92 | 86 | 6.2 | 0.10 | 0.96 | |||
| AHI ≥ 30 | 91 | 75 | 3.6 | 0.12 | 0.85 | |||
PSG, polysomnography; AHI, apnea hypopnea index; NR, not reported; Sn, sensitivity; Sp, specificity; LR+, positive likelihood ratio; LR−, negative likelihood ratio; AUC, Area under the receiver operator characteristics curve;
SleepStrip S.L.P. Ltd., Israel;
SleepStrip S.L.P. Ltd., Israel;
SOMNIE, NGK sparkPlug Co. Ltd, Nagoya, Japan;
ApneaLink™, ResMed Corporation, Poway, CA, USA;
Flow Wizard, DiagnoseIT, Sydney, New South Wales, Australia
The strengths of our study includes the prospective patient recruitment, the random order of laboratory PSG and home testing as well as the random order of portable device usage at home. Further, a high percentage of patients completed the protocol at home, and there was an overall low rate of data loss after 3 nights at home. All patients had the reference attended in-laboratory PSG as well as nasal flow and oximetry to avoid verification bias and to provide an appropriate standard for comparison and we performed the unattended home studies over a few nights to avoid possible confounders, such as the first-night effect and night-to-night variability, and also to assess the utility of one versus three nights of recording.
Also, the laboratory and home data were independently and blindly scored and the patients were not selected but consecutively recruited to minimize selection bias. We have calculated the operating characteristics of both single-channel devices at the optimal threshold for ease of comparison. However, in practice disease prevalence as well as the cost of indeterminate studies, unnecessary investigation (false positive) and undiagnosed disease (false negative) need to be considered when determining the relevant thresholds.
Limitations
One limitation of our study was the use of automated scoring for the portable devices. There have been reports of the superiority of manual over automated scoring. Recent studies have shown that automatic analysis of nasal flow at home compares favorably with in laboratory manual PSG scoring.32 The use of the manufacturer-provided automatic scoring software helped delineate the performance of these devices in the manner in which they could be commonly used in practice. Although there was no difference in the accuracy for OSA diagnosis using oximetry or nasal airflow monitors compared to PSG, differences in patient outcomes may indeed depend on parameters that may not have been reported in this study such as severity of oxygen desaturation. The relative importance of respiratory event frequency, desaturation frequency, and severity of desaturation, and how these relate to outcomes is controversial and the subject of ongoing study. Mortality in sleep apnea certainly relates to frequency of disordered breathing events but the scoring of these may depend on a combination of information from airflow and oximetry. For these reasons, the devices used in this study need to be interpreted in a clinical context and more detailed methods of diagnosis used in management algorithms. Future research is needed to study prospectively different diagnostic criteria of OSA severity using portable monitors compared to patient related outcomes, such as morbidity including cardiovascular and metabolic consequences.
Our study also used a single night in-center PSG as the reference standard rather than multiple nights of in-center or home PSG. This was chosen for pragmatic reasons, as it has been used as the reference standard in most sleep apnea test evaluations and is the recommended reference standard.33,34 Also, our study was conducted in highly selected patient groups (patients from sleep laboratories and sleep clinics) specifically referred for the possibility of OSA. While our data may support applications in other populations with high pre-test probability of OSA, it must be noted that we excluded subjects with existing sleep disorders or severe cardiovascular and respiratory conditions. The study did not address the utility of these devices for population screening of OSA and thus cannot be extrapolated to non-clinic populations. However, they provide potential for developing newer pathways utilizing single-channel monitors in other populations, such as primary care, where there is greater demand for clinical screening for OSA.
CONCLUSION
Due to the high prevalence of OSA in the community and the increasing demand for diagnostic services our findings may contribute to better resource allocation within the sleep laboratory and clinic populations through establishing rapid and accurate diagnostic pathways. We have compared the accuracy of two commonly used single-channel devices, illustrated the problems that can occur in obtaining data in the home setting and outlined their equivalence in diagnosing OSA. The accuracy for diagnosing OSA was greater when recordings were made over three nights instead of a single night for nasal flow. The addition of oximetry to nasal flow did not increase the accuracy of OSA diagnosis but improved the rate of data sufficiency. Ultimately the choice of which modality to use lies in cost of diagnosis and misdiagnosis, ease of deployment, local experience and availability and as monitoring devices have a diversity of characteristics and limitations, the operating characteristics of each device needs to be understood before they can be advocated for general clinical application.35–37
DISCLOSURE STATEMENT
This was not an industry supported study. Mr. Unger has an unpaid directorship and financial interest in DiagnoseIt which provided the nasal airflow pressure transducer devices (Flow Wizard) and analysis software. Dr. Grunstein's department has received research support from Respironics, Resmed, Covidien, Fisher-Paykel, Sanofi-Aventis, Actelion, Impax, DiagnoseIT, and Arena and has consulted for and has financial interests in DiagnoseIT. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are indebted to all the patients, the technical and nursing staff for participating and assisting in this study. The work for this study was perfomed at NHMRC CCRE in Respiratory and Sleep Medicine, Woolcock Institute of Medical Research, Royal Prince Alfred Hospital and the University of Sydney, Australia.
Also the National Health Medical Research Council Centre of Clinical Research Excellence (CCRE) in Respiratory and Sleep Medicine has granted Dr. Makarie Rofail a research fellow scholarship to support this work and provided the Radical Set Masimo oximeters and Download 2001 Software and consumables. Dr. Wong received a clinical fellowship from the CCRE and Professors Marks and Grunstein are both National Health Medical Research Council (NHMRC) Practitioner Fellows. DiagnoseIT (Sydney, Australia) has donated the use of the Flow Wizard pressure transducer devices and propriety analysis software.
REFERENCES
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea - A population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Bearpark H, Elliott L, Grunstein R, et al. Snoring and sleep-apnea - a population study in Australian men. Am J Respir Crit Care Med. 1995;151:1459–65. doi: 10.1164/ajrccm.151.5.7735600. [DOI] [PubMed] [Google Scholar]
- 3.Flemons WW, Littner MR, Rowley JA, et al. Home diagnosis of sleep apnea: a systematic review of the literature. An evidence review cosponsored by the American Academy of Sleep Medicine, the American College of Chest Physicians, and the American Thoracic Society. Chest. 2003;124:1543–79. doi: 10.1378/chest.124.4.1543. [DOI] [PubMed] [Google Scholar]
- 4.Ghegan MD, Angelos PC, Stonebraker AC, et al. Laboratory versus portable sleep studies: A meta-analysis. Laryngoscope. 2006;116:859–64. doi: 10.1097/01.mlg.0000214866.32050.2e. [DOI] [PubMed] [Google Scholar]
- 5.Williams AJ, Yu G, Santiago S, et al. Screening for sleep-apnea using pulse oximetry and a clinical score. Chest. 1991;100:631–5. doi: 10.1378/chest.100.3.631. [DOI] [PubMed] [Google Scholar]
- 6.Series F, Marc I, Cormier Y, et al. Utility of nocturnal home oximetry for case-finding in patients with suspected sleep-apnea hypopnea syndrome. Ann Intern Med. 1993;119:449–53. doi: 10.7326/0003-4819-119-6-199309150-00001. [DOI] [PubMed] [Google Scholar]
- 7.Gyulay S, Olson LG, Hensley MJ, et al. A comparison of clinical-assessment and home oximetry in the diagnosis of obstructive sleep-apnea. Am Rev Respir Dis. 1993;147:50–3. doi: 10.1164/ajrccm/147.1.50. [DOI] [PubMed] [Google Scholar]
- 8.Ryan PJ, Hilton MF, Boldy DAR, et al. Validation of British-Thoracic-Society guidelines for the diagnosis of the sleep-apnea hypopnea syndrome - can polysomnography be avoided. Thorax. 1995;50:972–5. doi: 10.1136/thx.50.9.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olson LG, Ambrogetti A, Gyulay SG. Prediction of sleep-disordered breathing by unattended overnight oximetry. J Sleep Res. 1999;8:51–5. doi: 10.1046/j.1365-2869.1999.00134.x. [DOI] [PubMed] [Google Scholar]
- 10.Wiltshire N, Kendrick AH, Catterall JR. Home oximetry studies for diagnosis of sleep apnea/hypopnea syndrome - Limitation of memory storage capabilities. Chest. 2001;120:384–9. doi: 10.1378/chest.120.2.384. [DOI] [PubMed] [Google Scholar]
- 11.Chiner E, Signes-Costa J, Arriero JM, et al. Nocturnal oximetry for the diagnosis of the sleep apnoea hypopnoea syndrome: a method to reduce the number of polysomnographies? Thorax. 1999;54:968–71. doi: 10.1136/thx.54.11.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barker SJ, Shah NK. The effects of motion on the performance of pulse oximeters in volunteers. Anesthesiology. 1997;86:101–8. doi: 10.1097/00000542-199701000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Hollingworth L, Tooby M, Roberts D, et al. Practicality of the Sleepstrip (TM) in postal screening for obstructive sleep apnoea. J Sleep Res. 2003;12:157–9. doi: 10.1046/j.1365-2869.2003.00349.x. [DOI] [PubMed] [Google Scholar]
- 14.Pang KP, Gourin CG, Podolsky R, et al. A comparison of polysomnography and the SleepStrip in the diagnosis of OSA. Otolaryngology-Head and Neck Surgery. 2006;135:265–8. doi: 10.1016/j.otohns.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 15.Nakano H, Tanigawa T, Ohnishi Y, et al. Validation of a single-channel airflow monitor for screening of sleep-disordered breathing. Eur Respir J. 2008;32:1060–7. doi: 10.1183/09031936.00130907. [DOI] [PubMed] [Google Scholar]
- 16.Erman MK, Stewart D, Einhorn D, et al. Validation of the ApneaLink for the screening of sleep apnea: a novel and simple single-channel recording device. J Clin Sleep Med. 2007;3:387–92. [PMC free article] [PubMed] [Google Scholar]
- 17.Wong KKH, Jankelson D, Reid A, et al. Diagnostic test evaluation of a nasal flow monitor for obstructive sleep apnea detection in sleep apnea research. Behav Res Methods. 2008;40:360–6. doi: 10.3758/brm.40.1.360. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Teschler T, Weinreich G, et al. Validation of microMESAM as screening device for sleep disordered breathing. Pneumologie. 2003;57:734–40. doi: 10.1055/s-2003-812423. [DOI] [PubMed] [Google Scholar]
- 19.Johns MW. A new method for measuring daytime sleepiness - the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 20.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Service; 1977. [DOI] [PubMed] [Google Scholar]
- 21.American Academy of Sleep Medicine Taskforce. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 22.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 23.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–43. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 24.Li JL, Fine J. On sample size for sensitivity and specificity in prospective diagnostic accuracy studies. Stat Med. 2004;23:2537–50. doi: 10.1002/sim.1836. [DOI] [PubMed] [Google Scholar]
- 25.Ross SD, Sheinhait IA, Harrison KJ, et al. Systematic review and meta-analysis of the literature regarding the diagnosis of sleep apnea. Sleep. 2000;23:519–32. [PubMed] [Google Scholar]
- 26.Portier F, Portmann A, Czernichow P, et al. Evaluation of home versus laboratory polysomnography in the diagnosis of sleep apnea syndrome. Am J Respir Crit Care Med. 2000;162:814–8. doi: 10.1164/ajrccm.162.3.9908002. [DOI] [PubMed] [Google Scholar]
- 27.Whittle AT, Finch SP, Mortimore IL, et al. Use of home sleep studies for diagnosis of the sleep apnoea/hypopnoea syndrome. Thorax. 1997;52:1068–73. doi: 10.1136/thx.52.12.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dingli K, Coleman EL, Vennelle M, et al. Evaluation of a portable device for diagnosing the sleep apnoea/hypopnoea syndrome. Eur Respir J. 2003;21:253–9. doi: 10.1183/09031936.03.00298103. [DOI] [PubMed] [Google Scholar]
- 29.Oeverland B, Akre H, Skatvedt O. Oral breathing in patients with sleep-related breathing disorders. Acta Otolaryngol. 2002;122:651–4. doi: 10.1080/000164802320396349. [DOI] [PubMed] [Google Scholar]
- 30.De Almeida F, Ayas N, Otsuka R, et al. Sleep Breath. 2006. Nasal pressure recordings to detect obstructive sleep apnea; pp. 1–8. [DOI] [PubMed] [Google Scholar]
- 31.Meyer TJ, Eveloff SE, Kline LR, et al. One Negative Polysomnogram Does Not Exclude Obstructive Sleep-Apnea. Chest. 1993;103:756–60. doi: 10.1378/chest.103.3.756. [DOI] [PubMed] [Google Scholar]
- 32.Grover SS, Pittman SD. Automated detection of sleep disordered breathing using a nasal pressure monitoring device. Sleep Breath. 2008;12:339–45. doi: 10.1007/s11325-008-0181-y. [DOI] [PubMed] [Google Scholar]
- 33.Chesson AL, Berry RB, Pack A. Practice parameters for the use of portable monitoring devices in the investigation of suspected obstructive sleep apnea in adults. Sleep. 2003;26:907–13. doi: 10.1093/sleep/26.7.907. [DOI] [PubMed] [Google Scholar]
- 34.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]
- 35.Attin M, Cardin S, Dee V, et al. An educational project to improve knowledge related to pulse oximetry. Am J Crit Care. 2002;11:529–34. [PubMed] [Google Scholar]
- 36.Kruger PS, Longden PJ. A study of a hospital staff's knowledge of pulse oximetry. Anaesth Intens. 1997;25:38–41. doi: 10.1177/0310057X9702500107. [DOI] [PubMed] [Google Scholar]
- 37.Teoh L, Epstein A, Williamson B, et al. Medical staff's knowledge of pulse oximetry: A prospective survey conducted in a tertiary children's hospital. J Paediatr Child H. 2003;39:618–22. doi: 10.1046/j.1440-1754.2003.00238.x. [DOI] [PubMed] [Google Scholar]