Abstract
Purpose
A clinical trial was designed to test the hypothesis that a psychological intervention could reduce the risk for cancer recurrence. Newly diagnosed regional breast cancer patients (n=227) were randomized to Intervention with assessment or Assessment only arms. The intervention had positive psychological, social, immune, and health benefits, and after a median of 11 years the Intervention arm was found to have reduced risk of recurrence [hazard ratio (HR), 0.55; P=0.034]. In follow-up, we hypothesized that the Intervention arm might also show longer survival after recurrence. If observed, we then would examine potential biobehavioral mechanisms.
Experimental Design
All patients were followed; 62 recurred. Survival analyses included all 62. Upon recurrence diagnosis, those available for further biobehavioral study were accrued (n=41, 23 Intervention and 18 Assessment). For those 41, psychological, social, adherence, health, and immune (natural killer cell cytotoxicity; T-cell proliferation) data were collected at recurrence diagnosis and 4, 8, and 12 months later.
Results
Intent-to-treat analysis revealed reduced risk of death following recurrence for the Intervention arm (HR, 0.41; P=0.014). Mixed-effects follow-up analyses with biobehavioral data showed that all patients responded with significant psychological distress at recurrence diagnosis, but thereafter only the Intervention arm improved (P values<0.023). Immune indices were significantly higher for the Intervention arm at 12 months (P values<0.017).
Conclusions
Hazards analyses augment previous findings in showing improved survival for the Intervention arm after recurrence. Follow-up analyses showing biobehavioral advantages for the Intervention arm contribute to our understanding of how improved survival was achieved.
Keywords: psychological, intervention, survival, cancer, biobehavioral
Introduction
Meta analyses suggest that stress-related psychosocial factors1 and lower health-related quality of life2 are associated with poorer cancer survival, with a 13% increase in the hazard ratio in studies of breast cancer patients1. In 1994 a RCT (Stress and Immunity Breast Cancer Project; SIBCP) was designed to test the hypothesis that newly diagnosed breast cancer patients receiving a psychological intervention would have a reduced risk of recurrence and breast cancer death compared to patients who were only assessed. A conceptual model guided the development of the clinical trial. The Biobehavioral Model of Cancer Stress and Disease Course3 proposes that psychological stress leads to disruptions in quality of life, health behaviors, and immunity, which, in turn contribute to poorer disease outcomes. It was hypothesized that an intervention designed to reduce emotional distress and improve social adjustment, health behaviors, and adherence might also improve immunity and disease course. Analyses showed that positive intervention effects were achieved across the psychological and immune outcomes at four months4 with similar effects and improved health at 12 months5. Moreover, recent endpoint analyses show that after a median follow-up of 11 years, the Intervention arm had a reduced risk of breast cancer recurrence [hazard ratio (HR), 0.55; P=0.034] and breast cancer death (HR, 0.44; P=0.016) compared to the Assessment only arm6.
As cancer progresses, the ability of psychological intervention to affect disease course may change. The intervention was hypothesized to reduce the risk of recurrence, and it may have been solely through this reduction in recurrence risk that mortality was affected. However, if the intervention continued to affect psychological and immune functioning after the recurrence, it is plausible that survival benefits would have also persisted. Therefore, we test whether Intervention patients also showed a reduced risk of breast cancer death after recurrence. Of course, recurrence is devastating, with our previous studies showing patients’ stress to be equivalent to that reported at the time of the initial diagnosis7, 8. Patients who do not have the benefit of a psychological intervention at recurrence may have depressive symptoms and anxieties9, 10, and we anticipated that the Assessment only patients would respond similarly. In fact, we anticipated that all patients would be significantly distressed when learning of their recurrence. If, however, there were residual benefits from having earlier learned strategies for reducing stress and enhancing coping, the Intervention arm patients might evidence improved adaptation.
In summary, we first test for study arm differences in survival following recurrence. Secondly, we test for study arm differences in biobehavioral variables to determine if the Intervention arm evidenced more favorable trajectories in the 12 months following recurrence than the Assessment only arm.
Patients and Methods
Description of the SIBCP trial
Complete descriptions of accrual, sample characteristics, stratification and randomization, power estimates, assessment, intervention, and follow-up procedures have been published4. Briefly, patients (n=227) newly diagnosed with regional breast cancer and awaiting the start of adjuvant therapy after surgery were accrued. The majority had Stage II (90%) rather than Stage III (10%) disease, were estrogen/progesterone receptor (ER/PR) positive (68%), and premenopausal (54%). The majority (83%) received their treatment at the Ohio State University NCI-designated Comprehensive Cancer Center (OSUCCC). As previously reported4, Intervention with assessment (n=114) and Assessment only (n=113) arms did not differ with regard to sociodemographic characteristics, extent of disease, prognostic factors, or cancer treatments received (P values>0.23).
The intervention was conducted in groups of 8 to 12 patients with two clinical psychologist leaders. It included relaxation training, positive ways to cope with stress and cancer-related difficulties (e.g., fatigue), methods to maximize social support, and strategies for improving health behaviors (diet, exercise) and adherence to cancer treatments. A total of 26 sessions (39 therapy hours) were delivered over 12 months4. A detailed description is available11. Patients were reassessed every four months during year one, every 6 months during years 2–5, and annually thereafter.
Patients and procedures for recurrence substudy
As patients recurred, they were approached for accrual; see Figure 1. Recurrence was defined as the development of breast cancer in the treated breast or chest wall, or at a distant site. Second primary tumors (e.g., contralateral breast, endometrial) did not meet this criterion. Informed consent was obtained, with study approval from the local institutional review boards and USHHS assurances.
Figure 1.
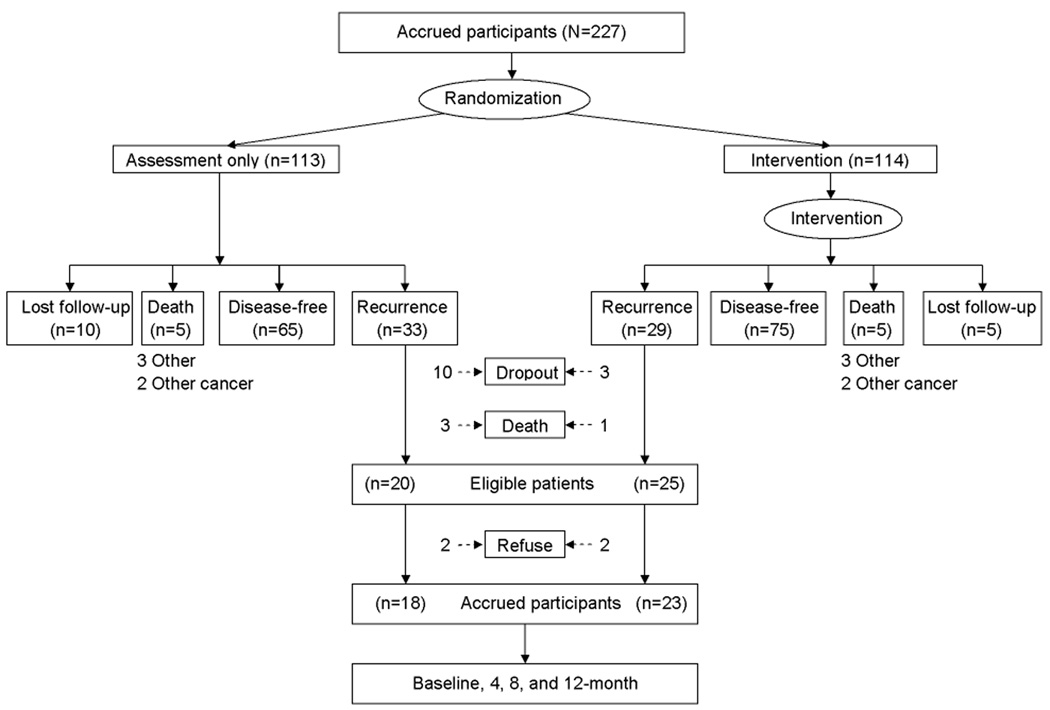
Study flow from the time of RCT accrual through a median of 11 years of follow-up. Note: Patients designated as “lost follow-up” are known to be alive; cancer status is not known, however.
By October of 2007, 62 patients had recurred. Of them, 17 (27%) were not available for substudy accrual; 4 patients (6%) progressed rapidly and died and 13 (21%) had previously discontinued SIBCP assessments. Of the remaining 45, 4 (6%) patients declined participation. For the 41 accrued, biobehavioral assessments were conducted a median of 11 weeks after diagnosis (baseline) and 4, 8, and 12 months later. Although biobehavioral data are available only for 41, sociodemographic, recurrence disease and treatments, and survival data are available for all 62 who recurred.
Measures
Psychological distress
The Profile of Mood States–Short Form (POMS)12, 13 is a 37-item inventory assessing negative mood. A Total Mood Disturbance score which ranges from −24 to 124 was used. Internal consistency was .92.
Social support
The Interpersonal Support Evaluation List (ISEL)14 assessed patients’ general perception of support. Scores range from 6 to 24. Internal consistency was .90. The Perceived Social Support Scales (PSS)15 assessed support from friends (PSS-Fr) and family (PSS-Fa) and was administered at baseline only. Scores range from 0 to 20 for each. Internal consistency was .85 and .92, respectively.
Adherence: Chemotherapy dose intensity
Dose intensity (DI) for chemotherapy regimens was calculated, as recommended16. Six Assessment and 13 Intervention patients received chemotherapy in the 12 months following recurrence diagnosis, with common agents being capecitabine (n=5), cisplatin/carboplatin (n=5), and the taxanes (n=13).
Immune assays
Procedures for blood separation, quantification of T lymphocytes and NK and T-cell subsets, and immune assay procedures have been detailed 4, 5. Natural killer cell cytotoxicity (NKCC) was expressed as the mean of standardized scores (Z-scores) from six E:T ratios, as previously described17. Blastogenesis responses were expressed as the mean of standardized scores from each of the three dilutions of concanavalin A (Con A) and phytohemagglutinin (PHA) as previously described17.
Health
A research nurse assessed patients. Items for evaluating symptoms, signs, illnesses, lab values, etc. (Symptoms/Signs) came from the Southwest Oncology Collaborative Group (1994 version) toxicity measure, as described5. Karnofsky Performance Status (KPS)18 was rated for use as a control variable in the analyses.
Other
Patients in both arms were queried about receipt of any psychotherapy or counseling as previously reported6. The same questions were used with the addition of 15 others assessing use of complimentary/alternative therapies (e.g., yoga, energy healing).
Statistical Analysis
Survival analysis
Descriptive data are provided (n=62). The prior SIBCP hazards analyses (n=227) showed advantages for the Intervention arm in risk of recurrence, defined as time from randomization to biopsy/study confirming first recurrence6. The current analyses test the period from biopsy/study confirming first recurrence to breast cancer death. Cox proportional hazards analysis19 was used to test for study arm differences. Known prognostic factors20 were examined as covariates. From the initial cancer diagnosis, we used cancer stage, ER/PR status, tumor grade, adjuvant therapy received, and measures with a significant group difference at study baseline (KPS, POMS4); and from the recurrence diagnosis, we used disease-free interval (time from randomization to biopsy/study confirming first recurrence), site of recurrence (loco-regional vs. distant), and type of cancer treatment received after recurrence. Of these possible covariates, if there were variables highly correlated with each other (r>0.6), only one was considered as a covariate to avoid multicollinearity. For model development, a backward elimination procedure was used as it has been recommended as one of the best methods for identifying important covariates when there are a large number of covariates to be considered with a relatively small number of events21, 22. The covariates that predicted the endpoint with P<0.1 were retained in the final model. The log linearity assumption for continuous covariates was tested using the procedures recommended by Hosmer, Lemeshow, and May23. The proportional hazards assumption was tested using the log minus log test.
Biobehavioral analysis
Preliminary analyses were conducted for the biobehavioral analyses. First, analyses contrasted the biobehavioral study participants (n=41) and non-participants (n=21). Second, analysis of variance or chi-square test, as appropriate, compared participants in the Assessment only (n=18) and Intervention (n=23) arms on sociodemographic, prognostic, and treatment variables. For the biobehavioral variables, mixed-effects modeling24 tested for study arm differences in baseline level and the trajectory of change following recurrence. Control variables were considered. To reduce variability due to cancer treatments, surgery, chemotherapy, and radiation and the treatment by time interactions were controlled in all models when relevant for specific assessments (e.g., surgery at baseline, chemotherapy at 4 months). In addition, baseline NK cell counts for the NKCC model and baseline total T-cell counts for the Con A and PHA models were included to control for absolute cell numbers in the analysis of functional responses. Non-significant control terms (P>0.1) were removed from each model until a final solution was reached, as recommended25.
For these analyses, the Study arm and Study arm × Time effects were of primary interest. The Study arm effect indicates a group difference in intercept, testing whether study arms differed at baseline (recurrence diagnosis). The Study arm × Time effect tests whether the rate of change differed between arms, with the prediction being more positive adaptation and outcomes for the Intervention arm. Bonferroni’s adjustment for multiple comparisons was used for the immune outcomes (i.e., reduction in α level to 0.017). Effect sizes, partial correlation coefficients (pr) computed using t values and degrees of freedom26, are provided. All statistical tests were two-sided.
Results
Survival Analyses
As of October 2007, 62 recurrence cases (29 Intervention arm and 33 Assessment arm) were confirmed through patient contact or cause of death determination from death certificates. Descriptive information is provided in Table 1. Patients (n=62) were primarily middle aged (M, 54 years; SD, 11), with some college (69%), and married/partnered (66%). 92% were Caucasian and 8% were African-American. At initial diagnosis, the majority (89%) had Stage II disease. At recurrence diagnosis, the median disease-free interval was 30 months (range, 2–144) and the majority (73%) had distant rather than loco-regional metastases. The majority (90%) were treated at OSU for their recurrence, receiving surgery (29%), chemotherapy (52%), radiation therapy (29%), hormonal therapy (48%), and/or bone marrow transplantation (7%).
Table 1.
Equivalence of the Assessment only and Intervention arms on sociodemographic, prognostic, and cancer treatments received variables (n=62).
| Assessment only (n=33) |
Intervention (n=29) |
|
|---|---|---|
| Variables | Mean (SD) / % | Mean (SD) / % |
|
Sociodemographic variables at recurrence |
||
| Age (years) | 52.2 (10.8) | 55.5 (11.9) |
| Race (% Caucasian) | 94 | 90 |
| Education (years) | 14.0 (2.3) | 14.9 (2.7) |
| Marital status (% married) | 67 | 66 |
| Family income (thousand $/year) | 51.6 (39.7) | 54.2 (40.6) |
| Initial diagnosis | ||
| Stage (II vs. III, % II) | 91 | 86 |
| Nodes (number positive) | 4.8 (7.3) | 4.9 (9.1) |
| ER/PR (% positive) | 55 | 55 |
| Treatments for initial diagnosis | ||
| Surgery (% modified radical mastectomy) | 61 | 62 |
| Chemotherapy (% yes) | 85 | 83 |
| Radiation therapy (% yes) | 49 | 45 |
| Hormonal therapy (% yes) | 73 | 62 |
| Recurrence diagnosis | ||
| Disease-free interval (months) | Median 26.0 | Median 33.0 |
| Site of recurrent disease (%) | ||
| Local | 29 | 18 |
| Regional | 0 | 7 |
| Distant | 71 | 75 |
| Bone only | 45 | 33 |
| Distant, other | 55 | 67 |
| Treatments for recurrence diagnosis | ||
| Surgery (% yes)* | 43 | 14 |
| Chemotherapy (% yes) | 47 | 57 |
| Radiation therapy (% yes) | 40 | 18 |
| Hormonal therapy (% yes) | 47 | 50 |
| Bone marrow transplantation (% yes) | 3 | 11 |
Significant group difference, P<0.05.
Of the 62 who recurred, 44 (71%; 19 Intervention arm and 25 Assessment arm) patients died, all from breast cancer. Median time to death was 2.8 years (range, 0.9–11.8) for the Intervention arm and 2.2 years (range, 0.2–12.0) for the Assessment arm. Multivariate comparison with the Cox proportional hazards analysis showed a significant effect of study arm adjusting for initial functional status, psychological distress, and recurrence site: patients in the Intervention arm had a lower risk of death after recurrence [HR, 0.41; 95% confidence interval (95% CI), 0.20–0.83; P=0.014; see Table 2 and Figure 2].
Table 2.
Final multivariate Cox proportional hazards model contrasting study arms in survival after recurrence (n=62).
| Variable | HR (95% CI) | P |
|---|---|---|
| Study arm (Intervention vs. Assessment only ) | 0.410 (0.202–0.832) | 0.014 |
| Initial functional status (KPS) | 0.346 (0.170–0.704) | 0.003 |
| Initial psychological distress (POMS) | 0.982 (0.967–0.997) | 0.022 |
| Recurrence site (loco-regional vs. distant) | 0.191 (0.080–0.458) | <0.001 |
Figure 2.

Predicted cumulative survival after recurrence of 62 breast cancer patients according to study arm, Intervention versus Assessment only.
Biobehavioral Analyses
Preliminary analyses
Two comparisons were made. First, analyses contrasted patients who recurred and participated in the follow-up (n=41) with patients who recurred but did not participate (n=21). The groups were not significantly different on sociodemographic (education, marital status, income, employment status), prognostic (stage, number of nodes positive, ER/PR status, menopausal status), previous cancer treatments received (surgery type, chemotherapy type and dose, radiation therapy, hormonal therapy), or disease-free interval (P values>0.128). The only difference was age (P=0.017), with participants being older when diagnosed initially (Mean age, 53) than the non-participants (Mean age, 46). The current sample might underestimate patients’ psychological distress as previous research27 indicated a faster quality of life recovery after recurrence among older patients (aged ≥54 years) compared to younger patients.
For the participants (n=41), analyses contrasted the Assessment (n=18) and Intervention (n=23) arms. There were no significant differences on sociodemographic, prognostic, and previous/current cancer treatments received variables (P values>0.059). The only difference between arms was receipt of surgery at recurrence (P=0.004). The higher rate of surgery in the Assessment arm reflects the higher rate of local recurrence, although differences between groups in site of recurrence was not statistically significant. For patients receiving surgery, surgical sites were as follows: Intervention (all 3 received breast surgery), Assessment (7 breast, 2 brain, and 1 lung). Therefore, receipt of surgery was controlled in the analyses regardless of significance. Lastly, study arms did not differ in their involvement in any type of counseling at any time (P values>0.457). Of the 15 complimentary therapies surveyed, study arms did not differ in use of the most commonly used (i.e., spiritual healing, megavitamin therapy, dietary changes) or any others (P values>0.147).
Main analyses
Descriptive statistics for all secondary measures at baseline and 12 months are available as supplementary information. Table 3 presents all mixed-effects model outcomes comparing study arms in biobehavioral trajectories during the 12 months following recurrence diagnosis.
Table 3.
Mixed-effects models comparing the Assessment only (n=18) versus Intervention arms (n=23) biobehavioral trajectories in the 12 months following recurrence diagnosis.
| Variable | Effect | Estimate | 95% CI | t | pr |
|---|---|---|---|---|---|
| Psychological distress | |||||
| POMS | Study arm | 5.811 | (−8.896, 20.518) | 0.798 | .12 |
| Time | 0.253 | (−0.553, 1.058) | 0.649 | .05 | |
| Study arm × Time | −1.282 | (−2.195, −0.370) | −2.908* | −.26 | |
| Social support | |||||
| ISEL | Study arm | 2.800 | (1.336, 4.264) | 3.925* | .60 |
| Time | −0.131 | (−0.206, −0.056) | −3.710* | −.68 | |
| Study arm × Time | 0.027 | (−0.049, 0.102) | 0.769 | .22 | |
| Health | |||||
| Symptoms /Signs |
Study arm | 0.019 | (−0.056, 0.094) | 0.508 | .08 |
| Time | −0.004 | (−0.008, −.0004) | −2.208* | −.35 | |
| Study arm × Time | −0.001 | (−0.005, 0.002) | −0.694 | −.14 | |
| Immunity | |||||
| NKCC | Study arm | 0.071 | (−0.427, 0.568) | 0.294 | .06 |
| Time | −0.169 | (−0.249, −0.089) | −4.292** | −.60 | |
| Study arm × Time | 0.101 | (0.024, 0.178) | 2.701** | .47 | |
| Con A | Study arm | −0.030 | (−0.631, 0.570) | −0.104 | −.02 |
| Time | −0.064 | (−0.146, 0.018) | −1.587 | −.27 | |
| Study arm × Time | 0.068 | (−0.030, 0.165) | 1.411 | .24 | |
| PHA | Study arm | 0.476 | (−0.081, 1.033) | 1.766 | .35 |
| Time | −0.016 | (−0.092, 0.059) | −0.456 | −.11 | |
| Study arm × Time | 0.049 | (−0.038, 0.136) | 1.192 | .28 | |
P<0.05.
P<0.017 with Bonferroni’s adjustment.
For psychological distress, there was no significant Study arm effect (P=0.430). As predicted, both arms responded to the diagnosis of recurrence with high levels of negative mood (POMS). The Time effect was not significant (P=0.523), indicating no reduction in negative mood from baseline to 12 months for the Assessment arm. However, the Intervention arm showed a decline in negative mood (i.e., emotional improvement), and the Study arm × Time effect indicated that the difference between the groups was significant (P=0.008; see Figure 3A).
Figure 3.
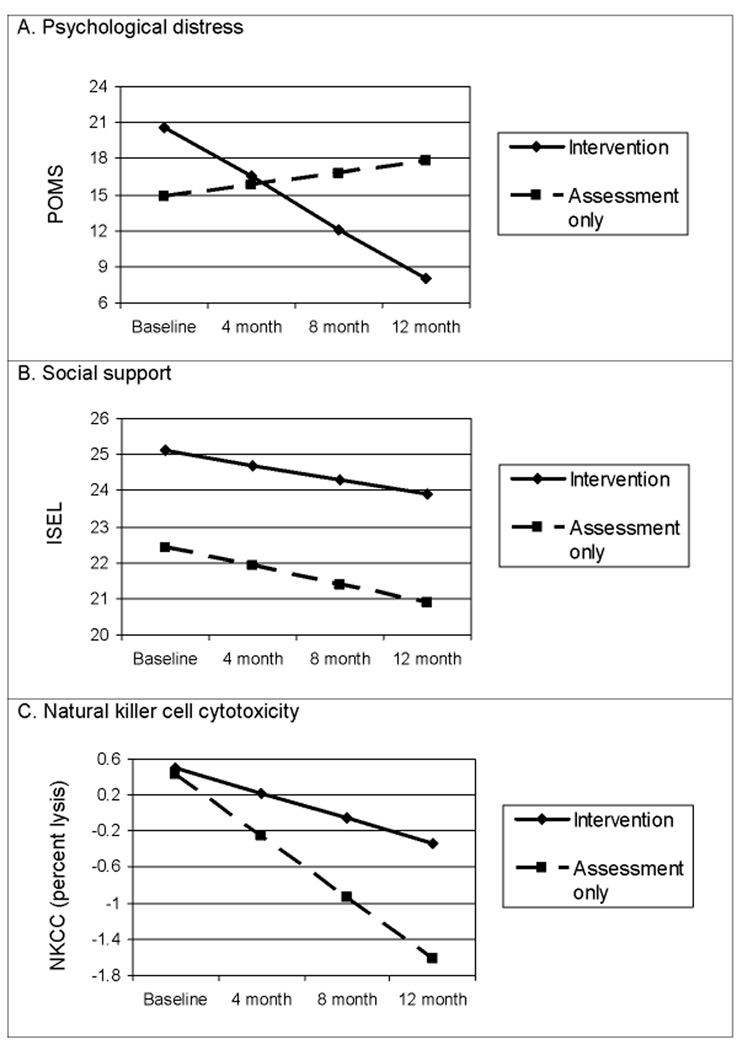
A) Significant study arm by time interaction showing a significantly greater improvement (reduction) in negative mood for the Intervention patients following the baseline distress of recurrence diagnosis.; B) Significant study arm effect with the Intervention arm reporting higher social support at baseline than the Assessment arm (P=0.001). The time effect indicates a significant decline in social support for the Assessment arm (P=0.002).; C) Significant study arm by time interaction showing greater NKCC for the Intervention arm 12 months after recurrence diagnosis. NKCC was expressed as the mean of standardized scores from six E:T ratios.
For social support, there was a significant Study arm effect, with patients in the Intervention arm reporting higher social support (ISEL) at baseline than those in the Assessment arm (P=0.001). The Time effect was significant (P=0.002), with the Assessment arm showing a decline (i.e., less social support). The non-significant Study arm × Time effect (P=0.456) indicates that the baseline group difference--significantly higher levels of social support for the Intervention arm--was maintained (see Figure 3B). Results for the Perceived Social Support scales (PSS) using analysis of covariance were consistent. Intervention arm patients perceived significantly more support at recurrence diagnosis from their family (PSS-Fa; P=0.007). The effect for PSS-Friends was similar, though P=0.086.
Chemotherapy dose intensity (DI) was compared using analysis of variance. The groups differed significantly (P=0.024), with a mean regimen DI of 94% (SD, 9; range, 74–100) for the Intervention arm and 81% (SD, 11; range, 63–92) for the Assessment arm. The significant group difference and the large effect size (Cohen’s d, 1.2) are notable, but they require replication to determine their reliability. Also, the relevance of DI to survival following breast cancer recurrence is unclear28, 29.
For health, there was no significant Study arm or Study arm × Time effect (P values>0.614). Both arms showed a significant reduction in Symptoms/Signs with time (P values<0.034).
With regard to immunity, there was no significant Study arm effect (P=0.771) for NKCC. The Time effect indicated a significant decline in NKCC for the Assessment arm (P<0.001). The Study arm × Time effect was significant (P=0.012), with the Intervention arm showing a slower rate of change than the Assessment arm (see Figure 3C). For Con A and PHA blastogenesis, none of the effects was significant (P values>0.090). As immunity was included in the trial as potential mechanism for improved disease course3, a follow-up analysis determined if the immune trajectories led to differential levels at 12 months. The same mixed-effects model contrasted the study arms with the intercept coded to be the 12-month assessment. Results showed that the Intervention arm had significantly higher NKCC (P=0.001), Con A blastogenesis (P=0.021), and PHA blastogenesis (P=0.007) at 12 months.
Discussion
Hypotheses were tested to illuminate survival effects from the SIBCP trial. The prior Cox proportional hazards analysis had shown that the Intervention arm had a 45% reduced risk for breast cancer recurrence6. The current analysis shows that following recurrence, the Intervention arm had a 59% reduction in the risk of dying of breast cancer. In combination, the data suggest that factors or mechanisms impacting disease progression occurred during both intervals: mechanisms influential from initial diagnosis to recurrence and mechanisms influential from recurrence to breast cancer death.
Collection of biobehavioral data following recurrence was planned shortly after the trial began. A majority of the recurrence patients (66%) were studied, and the availability of repeated assessments increased power and reliability for the analyses. The mixed-effects model analysis uses all available data, which enhances generalizability. Even considering these factors, we cannot be certain, of course, that identical biobehavioral data patterns would be observed with a sample size of 62 when compared with the 41. However, all findings were in the predicted direction, replicating, and in some cases extending, the effects observed at the time of intervention delivery4, 5 and with larger effect sizes.
For breast cancer patients, recurrence may be particularly stressful because currently available treatments are not curative30, posing a significant therapeutic challenge for the oncologist31. As in previous studies7, 27, all patients reported negative moods immediately following diagnosis. Thereafter, study arms diverged as they had when the intervention was delivered4, 5, with emotional distress significantly declining for the Intervention arm, yet remaining high for the Assessment arm. A plausible hypothesis for this pattern is that after the shock of diagnosis, the Intervention arm patients may have drawn upon strategies they had learned for reducing stress and coping positively. For them, a median of 18 months had passed from the conclusion of intervention to recurrence diagnosis. Importantly, ancillary data show that the decline in distress for the Intervention arm was not due to differential receipt of other counseling or complimentary therapies.
Intervention arm patients also appear to have been more successful at securing and maintaining social support. Significant gains in perceived social support had been found for the Intervention arm during intervention delivery4. This may have led to stable changes in their most important relationships, or at a minimum, changes helpful during times of stress. Upon recurrence, the social support advantage for the Intervention patients never diminished, and the lower support level reported by the Assessment patients never improved (and actually declined).
Regarding the immunity findings, our previous studies had shown that as patients entered the trial, stress covaried with lower levels of NKCC and T-cell blastogenesis32. During the intervention, T-cell blastogenesis was significantly higher for the Intervention arm4, 5. It remains to be seen whether immunity as measured here is critical to survival of breast cancer patients. High levels of NKCC covary with a better prognosis and overall survival in many different cancers33–35. Impaired NKCC has been found to correlate with stage of disease36, and NK cell infiltration into primary tumors is associated with less lymphatic invasion, fewer metastases, and better survival37.
The relationship between the psychological intervention effects and breast cancer survival will require further study. In the general case, however, clinical studies suggest that tumor cells remain dormant for varied periods of time following surgery and adjuvant chemotherapy, some of which are long38. The prevailing theory suggests that tumor cells may be quiescent and/or at equilibrium with the patient’s immune system. In support of this concept are studies showing that the maintenance of immune surveillance is important for long-term suppression of cancer cell growth. In these models, the immune system is able to control tumor growth, though it is unable to eliminate all of the tumor cells39–40. Theoretically, a cancer recurrence might occur and become clinically detectable when tumor cells overcome the multiple immune mechanisms (CD8+ T cells, NK cell surveillance, antibodies) that can inhibit tumor growth. Once tumor cells escape immune surveillance, immune effector cell function may be further impaired due to tumor-derived factors. For example, NK cell maturation in the bone marrow and NK cell recruitment to the tumor site is inhibited as tumor burden increases41, 42. The latter is consistent with Pierson and Miller’s43 comparison of cancer patient and healthy donor samples, which suggested both NK cell number and NKCC are distinctly and negatively affected by the malignant environment.
In summary, intent-to-treat analyses show improved survival for SIBCP Intervention arm patients following recurrence. Other recent trials have reported null survival effects when providing an intervention to patients recently diagnosed with metastatic breast cancer44–46. These trials and a similar one47 all used a therapy (supportive expressive48) that differs from that of the SIBCP11. Reviews of the literature49, 50 show that psychological interventions are typically delivered at the time of initial diagnosis, and it has not been considered that patients might derive benefits from them much later or at the time of recurrence. The findings suggest that if psychological interventions are offered early, they may provide enduring, late benefits and possibly longer survival.
Translational Relevance
This manuscript describes late translational research examining long-term benefits of a psychological intervention for newly-diagnosed breast cancer patients. Based on a theoretical model, the Biobehavioral Model of Cancer Stress and Disease Course, the intervention sought to reduce stress and distress, improve social support, improve health behaviors, including medical adherence, and ultimately improve disease course. The intervention was delivered using a session-by-session manual with the intent that the intervention could be easily disseminated. Thus, this psychological intervention could be incorporated into a comprehensive approach to cancer care.
Supplementary Material
Acknowledgments
Grant support: American Cancer Society (PBR-89; PF-07-169-01-CPPB); Longaberger Company-American Cancer Society Grant for Breast Cancer Research (PBR-89A, RSGPB-03-248-01-PBP); U.S. Army Medical Research Acquisition Activity (DAMD17-94-J-4165, DAMD17-96-1-6294, DAMD17-97-1-7062); National Institute of Mental Health (R01MH51487); and National Cancer Institute (R01CA92704, K05CA098133, P30CA16058).
Footnotes
Disclosure of potential conflicts of interest: None of the authors has conflicts of interest.
Trial registration: clinicaltrials.gov Identifier: SIBCP0350
REFERENCES
- 1.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychological factors contribute to cancer incidence and survival: A systematic review and meta-analysis. Nat Clin Pract Oncol. 2008;5(8):466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 2.Quinten C, Coens C, Mauer M, et al. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009;10(9):865–871. doi: 10.1016/S1470-2045(09)70200-1. [DOI] [PubMed] [Google Scholar]
- 3.Andersen BL, Kiecolt-Glaser JK, Glaser R. A biobehavioral model of cancer stress and disease course. Am Psychol. 1994;49(5):389–404. doi: 10.1037//0003-066x.49.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen BL, Farrar WB, Golden-Kreutz DM, et al. Psychological, behavioral, and immune changes after a psychological intervention: A clinical trial. J Clin Oncol. 2004;22(17):3570–3580. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen BL, Farrar WB, Golden-Kreutz DM, et al. Distress reduction from a psychological intervention contributes to improved health for cancer patients. Brain Behav Immun. 2007 October;21(7):953–961. doi: 10.1016/j.bbi.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen BL, Yang H-C, Farrar WB, et al. Psychologic intervention improves survival for breast cancer patients: A randomized clinical trial. Cancer. 2008;113:3450–3458. doi: 10.1002/cncr.23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen BL, Shapiro CL, Farrar WB, Crespin TR, Wells-Di Gregorio SM. Psychological responses to cancer recurrence: A controlled prospective study. Cancer. 2005;104:1540–1547. doi: 10.1002/cncr.21309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang H-C, Brothers BM, Andersen BL. Stress and quality of life in breast cancer recurrence: Moderation or mediation of coping? Ann Behav Med. 2008 Apr;35(2):188–197. doi: 10.1007/s12160-008-9016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahon SM, Cella DF, Donovan MI. Psychosocial adjustment to recurrent cancer. Oncol Nurs Forum. 1990;17(3):47–54. [PubMed] [Google Scholar]
- 10.Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: Characteristics and impact in patients with cancer pain. Pain. 1999;81(1–2):129–134. doi: 10.1016/s0304-3959(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 11.Andersen B, Golden-Kreutz DM, Emery CF, Thiel DL. Biobehavioral intervention for cancer stress: Conceptualization, components, and intervention strategies. Cognit Behav Pract. 2009;16:253–265. [Google Scholar]
- 12.Curran SL, Andrykowski KJ, Studts JL. Short form of the Profile of Mood States (POMS-SF): Psychometric information. Psychol Assess. 1995;7(1):80–83. [Google Scholar]
- 13.McNair DM, Lorr M, Droppleman LF. Profile of Mood States. San Diego, CA: Educational and Industrial Testing Sevices; 1971. [Google Scholar]
- 14.Cohen S, Mermelstein R, Kamarck T, Hoberman H. Measuring the functional components of social support. In: Sarason IG, Sarason BR, editors. Social support: Theory, research and application. The Hague, Holland: Martinus Nijhoff; 1985. pp. 73–94. [Google Scholar]
- 15.Procidano ME, Heller K. Measures of Perceived Social Support from friends and from family: Three validation studies. Am J Community Psychol. 1983;11(1):1–24. doi: 10.1007/BF00898416. [DOI] [PubMed] [Google Scholar]
- 16.Hryniuk WM. The importance of dose intensity in the outcome of chemotherapy. In: Winters R, Ewan H, editors. Important Advances in Oncology. Philadelphia, PA: J.B. Lippincott Company; 1988. pp. 121–141. [PubMed] [Google Scholar]
- 17.Carson WE, III, Shapiro CL, Crespin TR, Thornton LM, Andersen BL. Cellular immunity in breast cancer patients completing taxane treatment. Clin Cancer Res. 2004;10:3401–3409. doi: 10.1158/1078-0432.CCR-1016-03. [DOI] [PubMed] [Google Scholar]
- 18.Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: Macleod CM, editor. Evaluation of Chemotherapeutic Agents. New York, NY: Columbia University Press; 1949. pp. 199–205. [Google Scholar]
- 19.Cox DR. Regression models and life-tables. J R Stat Soc. 1972;34(2):187–220. [Google Scholar]
- 20.DeVita VT, Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg's Cancer: Principles & Practice of Oncology. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 21.Clark TG, Bradburn MJ, Love SB, et al. Survival analysis part IV: further concepts and methods in survival analysis. Br J Cancer. 2003;89(5):781–786. doi: 10.1038/sj.bjc.6601117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: NY: Springer-Verlag; 2001. [Google Scholar]
- 23.Hosmer DW, Lemeshow S, May S. Applied Survival Analysis: Regression modeling of time to event data. 2nd ed. New York, NY: Wiley; 2008. [Google Scholar]
- 24.Raudenbush SW, Bryk AS. Hierarchical Linear Models. Newbury Park, CA: Sage; 2002. [Google Scholar]
- 25.Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyze unbalanced repeated measures and longitudinal data. Stat Med. 1997;16(20):2349–2380. doi: 10.1002/(sici)1097-0258(19971030)16:20<2349::aid-sim667>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 26.Rosenthal R. Parametric measures of effect size. In: Cooper H, Hedges LV, editors. The Handbook of Research Synthesis. New York, NY: Russell Sage; 1994. pp. 231–244. [Google Scholar]
- 27.Yang H-C, Thornton LM, Shapiro CL, Andersen BL. Surviving recurrence: Psychological and quality of life recovery. Cancer. 2008;112(5):1178–1187. doi: 10.1002/cncr.23272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armstrong DK, Davidson NE. Dose intensity for breast cancer. Oncology. 2001;15(6):701–718. [PubMed] [Google Scholar]
- 29.Jones D, Ghersi D, Wilcken N. Addition of drug/s to a chemotherapy regimen for metastatic breast cancer. Cochrane Database Syst Rev. 2006;3:CD003368. doi: 10.1002/14651858.CD003368.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Carson WE, Liang MI. Current immunotherapeutic strategies in breast cancer. Surg Oncol Clin N Am. 2007;16(4):841–860. doi: 10.1016/j.soc.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Bernard-Marty C, Cardoso F, Piccart MJ. Facts and controversies in systemic treatment of metastatic breast cancer. Oncologist. 2004;9:617–632. doi: 10.1634/theoncologist.9-6-617. [DOI] [PubMed] [Google Scholar]
- 32.Andersen BL, Farrar WB, Golden-Kreutz D, et al. Stress and immune responses after surgical treatment for regional breast cancer. J Natl Cancer Inst. 1998;90(1):30–36. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liljefors M, Nilsson B, Hjelm Skog AL, Ragnhammar P, Mellstedt H, Frodin JE. Natural killer (NK) cell function is a strong prognostic factor in colorectal carcinoma patients treated with the monoclonal antibody 17-1A. Int J Cancer. 2003;105(5):717–723. doi: 10.1002/ijc.11139. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura H, Kawasaki N, Hagiwara M, Saito M, Konaka C, Kato H. Cellular immunologic parameters related to age, gender, and stage in lung cancer patients. Lung Cancer. 2000 May;28(2):139–145. doi: 10.1016/s0169-5002(99)00133-6. [DOI] [PubMed] [Google Scholar]
- 35.Taketomi A, Shimada M, Shirabe K, Kajiyama K, Gion T, Sugimachi K. Natural killer cell activity in patients with hepatocellular carcinoma: A new prognostic indicator after hepatectomy. Cancer. 1998;83(1):58–63. doi: 10.1002/(sici)1097-0142(19980701)83:1<58::aid-cncr8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 36.Levy S, Tempe JL, Caussade P, et al. Stage-related decrease in natural killer cell activity in untreated patients with mycosis fungoides. Cancer Immunology Immunotherapy. 1984;18(2):138–140. doi: 10.1007/BF00205749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takeuchi H, Maehara Y, Tokunaga E, Koga T, Kakeji Y, Sugimachi K. Prognostic significance of natural killer cell activity in patients with gastric carcinoma: A multivariate analysis. Am J Gastroenterol. 2001;96(2):574–578. doi: 10.1111/j.1572-0241.2001.03535.x. [DOI] [PubMed] [Google Scholar]
- 38.Meng S, Tripathy D, Frenkel EP, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004;10(24):8152–8162. doi: 10.1158/1078-0432.CCR-04-1110. [DOI] [PubMed] [Google Scholar]
- 39.DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2008;27(1):11–18. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- 40.Teng MW, Swann JB, Koebel CM, Schreiber RD, Smyth MJ. Immune-mediated dormancy: An equilibrium with cancer. J Leukoc Biol. 2008;84(4):988–993. doi: 10.1189/jlb.1107774. [DOI] [PubMed] [Google Scholar]
- 41.Richards JO, Chang X, Blaser BW, Caligiuri MA, Zheng P, Liu Y. Tumor growth impedes natural-killer-cell maturation in the bone marrow. Blood. 2006;108(1):246–252. doi: 10.1182/blood-2005-11-4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27(45):5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pierson BA, Miller JS. CD56+bright and CD56+dim natural killer cells in patients with chronic myelogenous leukemia progressively decrease in number, respond less to stimuli that recruit clonogenic natural killer cells, and exhibit decreased proliferation on a per cell basis. Blood. 1996;88(6):2279–2287. [PubMed] [Google Scholar]
- 44.Goodwin PJ, Leszcz M, Ennis M. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med. 2001;345(24):1719–1726. doi: 10.1056/NEJMoa011871. [DOI] [PubMed] [Google Scholar]
- 45.Kissane DW, Grabsch B, Clarke DM, et al. Supportive-expressive group therapy for women with metastatic breast cancer: Survival and psychosocial outcome from a randomized controlled trial. Psychooncology. 2007;16(4):277. doi: 10.1002/pon.1185. [DOI] [PubMed] [Google Scholar]
- 46.Spiegel D, Butler LD, Giese-Davis J, et al. Effects of supportive-expressive group therapy on survival of patients with metastatic breast cancer: A randomized prospective trial. Cancer. 2007;110(5):1130–1138. doi: 10.1002/cncr.22890. [DOI] [PubMed] [Google Scholar]
- 47.Kissane DW, Bloch S, Smith GC, et al. Cognitive-existential group psychotherapy for women with primary breast cancer: A randomized controlled trial. Psychooncology. 2003;12(6):532–546. doi: 10.1002/pon.683. [DOI] [PubMed] [Google Scholar]
- 48.Spiegel D, Classen C. Group therapy for cancer patients: A research-based handbook of psychosocial care. New York: Basic/Perseus Books; 2000. [Google Scholar]
- 49.Andersen BL. Biobehavioral outcomes following psychological interventions for cancer patients. J Consult Clin Psychol. 2002;70(3):590–610. doi: 10.1037//0022-006X.70.3.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meyer T, Mark M. Effects of psychosocial interventions with adult cancer patients: A meta-analysis of randomized experiments. Health Psychol. 1995;14:101–108. doi: 10.1037//0278-6133.14.2.101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


