Summary
Objective
Neuroendocrine-immune models have been proposed to account for the frequent co-occurrence of pain, depression, and fatigue (PDF) among cancer patients.
Design
In a cross-sectional observational study of advanced cancer patients (N=104), we test the hypothesis that the PDF cluster covaries with proposed biological mediators: hormones of the sympathetic nervous system (SNS) and the hypothalamic-pituitary-adrenal (HPA) axis.
Main Outcome Measures
PDF symptoms were measured using the Brief Pain Inventory, Fatigue Severity Index, and the Center for Epidemiological Studies–Depression scales. HPA activation was indicated by plasma levels of cortisol and adrenocorticotropic hormone, and SNS activation was indicated by plasma epinephrine and norepinephrine.
Results
Preliminary analyses supported the use of covariance structure modeling to test whether shared variance among hormone levels predicted shared variance among PDF symptoms. Latent variable analysis indicated neuroendocrine levels predicted PDF (standardized β=.23, p=.039), while controlling for important disease and demographic variables.
Conclusion
Previous studies have linked individual symptoms to individual biomarkers. The observed significant paring of the four hormones to the PDF cluster provides the first evidence suggestive of stress hormones as a common mechanism for the co-occurrence of pain, depression, and fatigue symptoms.
Keywords: symptom clusters, depression, cancer, hypothalamic-pituitary-adrenal axis, sympathetic nervous system
Pain, depression and fatigue (PDF) are among the most common and distressing symptoms in advanced breast cancer, and they often occur concurrently (Cleeland, et al., 2003; Miller, Ancoli-Israel, Bower, Capuron, & Irwin, 2008). Their co-occurrence may indicate a common etiological mechanism, and neuroendocrine-immune models have been proposed (Cleeland, et al., 2003; Dantzer, O'Connor, Johnson, & Kelley, 2008; Miller, et al., 2008). The models differ slightly, but all propose that the clustering of symptoms results from an inflammatory reaction: Proinflammatory cytokines, such as interleukin (IL)-6, IL-1, and tumor necrosis factor (TNF)-α, trigger a complex neurochemical pathway in the brain, activating the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system (SNS), among other effects (e.g., altered neurotransmitter metabolism, disrupted circadian rhythms; see Cleeland, et al., 2003; Dantzer, et al., 2008; Elenkov, Wilder, Chrousos, & Vizi, 2000; Miller, et al., 2008). Elevated sympathetic activity, in turn, could promote further inflammation, for example by promoting secretion of IL-6 (Charmandari, Tsigos, & Chrousos, 2005). Thus, according to proposed models, patients experiencing the symptoms would also exhibit observable endocrine changes in the periphery: elevations in HPA and SNS hormones.
To date, no study has examined multiple symptoms comprising a cluster in relation to biological markers, and theorists argue that such data are needed (Cleeland, et al., 2003). Published data have shown correlations between some symptoms individually and some of the HPA/SNS hormones: Depression has been linked to overall elevations of plasma cortisol, epinephrine, and norepinephrine, as well as dysregulation of cortisol (nonsuppression of cortisol in response to dexamethasone, lack of diurnal variability) (Cohen, de Moor, Devine, Baum, & Amato, 2001; Lechin, van der Dijs, & Benaim, 1996; Miller, et al., 2008). Fatigue correlates with a loss of diurnal variation in cortisol (Miller, et al., 2008). Although not studied among those with cancer, pain correlates with elevated cortisol in humans and animals (Vierck, 2006). The present study extends these findings by examining the relationships between the three symptoms and hormones of the HPA and SNS. Whereas prior studies have correlated individual symptoms with individual hormones, the goal of the present study is to test whether the hormone systems predict the symptom cluster. We do so using structural equation modeling, which allows us to test their shared variance.
Methods
Participants and Procedures
Consecutive cases from a medical oncology clinic at a university-affiliated National Cancer Institute-designated Comprehensive Cancer Center were sought. Those newly diagnosed with advanced breast cancer (i.e., metastatic or recurrent) were eligible. Exclusion criteria included prior cancer diagnosis other than initial breast cancer; refusal of cancer treatment; age <21 or >85 years; residence >70 miles from the research site; diagnoses of mental retardation, severe or untreated psychopathology, neurological disorders, dementia, or any immunologic condition/disease. One hundred thirty two patients were eligible and 104 (79%) were enrolled. Reasons for declining participation were as follows: Not interested (n=12), no time/too much stress (n=11), transportation problems (n=4), and too ill (n=1). There were no significant differences between study participants (N =104) and non-participants (n = 33) with respect to patient characteristics (age, race, menopausal status), disease characteristics (cancer stage, hormone receptor status, nodal status at original diagnosis, presence of distant disease), or cancer treatments received (surgery, radiation, hormonal, and chemotherapy; ps > .10).
The sample was primarily Caucasian (89%; 11% African-American), middle aged (mean=53, SD=11 years), and post-menopausal (78%). Twenty four (23%) patients had received an initial diagnosis of stage IV breast cancer, whereas the remainder were cases of breast cancer recurrence (n=80; 77%). Of the latter, 39% (31 of 80) had loco-regional disease and 61% (n=49) had distant metastasis. The mean disease free interval was 53 months (SD = 52 months; median=34). Patients were assessed a median of 8 weeks following diagnosis, by which time the majority (85%) had begun treatment (surgery 24%, chemotherapy 61%, radiation 15%, and/or hormonal therapy 22%).
The protocol complied with American Psychological Association ethical standards and was approved by local Institutional Review Boards. Following informed consent, assessments were conducted between the hours of 7 AM and 10 AM during a regularly scheduled clinic visit. A female research assistant conducted an individual structured interview that included questionnaire completion, and a nurse completed a health assessment with a 60 mL blood draw, with patients resting first in a supine position for 15 minutes. Participants had been instructed to avoid caffeinated beverages for 12 hours prior to the draw. Patients were paid $40 per assessment.
Measures
Pain
The 7-item Pain Interference scale from the Brief Pain Inventory (BPI) (Cleeland & Ryan, 1994) was used. Patients rate the degree to which pain interferes with general activity, mood, walking, work, relationships, sleep, and enjoyment of life on 11-point scales ranging from 0 = does not interfere to 10 = completely interferes. Scores can range from 0 to 70. Coefficient alpha reliability was .96.
Depression
The Center for Epidemiological Studies Depression Scale (CES-D) (Radloff, 1977) contains 20 depressive symptom items rated on a 4-point scale (0 = Rarely or none of the time to 3 = Most or all of the time). Scores can range from 0 to 60. Coefficient alpha reliability was .93. CES-D data from a subset of the present sample (n=67) has been previously described (Brothers & Andersen, 2009).
Fatigue
The 7-item Disruption Index from the Fatigue Symptom Inventory (Hann, et al., 1998) was used. Patients indicate the degree to which fatigue interferes with daily activity on 11-point scales ranging from 0 = no interference to 10 = extreme interference. Scores can range from 0 to 70. Coefficient alpha reliability was .94.
Functional Status
The nurse-rated Karnofsky Performance Status Scale (Karnofsky & Burchenal, 1949) is an index of patient function. It ranges from 100 (Normal, no complaints, no evidence of disease) to 0 (Dead) in 10 point intervals.
Plasma Catecholamines (Norepinephrine and Epinephrine)
Determinations were made by HPLC with ElectroChemical Detection using Standards and Chemistry [Alumina extraction] purchased from ChromSystems, Munich, Germany (U.S .affiliate Thermo-Alko, Beverly, MA). C-18 Columns were purchased from the Waters Corporation (Waters Corporation, Milford, MA). Intra-assay variation, inter-assay variation, and sensitivity for norepinephrine were 3%, 6%, and 15 pg/ml, respectively. For Epinephrine, values were 6%, 13%, and 6 pg/ml, respectively.
Plasma Adrenocorticotropic Hormone (ACTH)
ACTH was measured using the Immulite 1000 with reagents manufactured specifically for this instrument (Diagnostic Products Corporation, Los Angeles, CA). Intra-assay coefficient of variation was 5.6% and Inter-assay coefficient of variation was 7.8%. Sensitivity was 9 pg/ml. This assay was read and calculated with the System Luminometer 400 (Nichols Institute, San Clemente, CA).
Plasma Cortisol
Determinations were made using the Cortisol Coat-A-Count RIA (Diagnostic Products Corporation, Los Angeles, CA). Intra-assay variation was 4.3% and inter-assay variation was 5.2%. Sensitivity was 0.2 μg/dl.
Analytic Strategy
Correlations among behavioral symptoms and endocrine levels were examined. We then used structural equation modeling to test whether hormones contributed to pain, depression, and fatigue symptoms after accounting for health and treatment-related variables. To that end, two latent variables were defined. “PDF” had three indicators: pain, depression, and fatigue. “HPA/SNS” had four indicators: cortisol, ACTH, epinephrine, and norepinephrine. Next, control variables (demographic [age, partner status], disease [local vs. distant, primary vs. recurrent disease], treatment [surgery, chemotherapy, radiation therapy and/or hormonal therapy in the past 4 months] and performance status [KPS]) were included as predictors of PDF. Control variables which approached significance (p<.20) were retained in the final model. The study hypothesis was tested with a directional path from the HPA/SNS variable to the PDF variable. In summary, we tested whether HPA/SNS activity significantly predicted the PDF cluster after controlling for demographic, disease, treatment, and health status variables. The covariance matrix was analyzed using full information maximum likelihood (FIML), which permits full use of available data, even in the presence of missing data. We used Analysis of Moment Structures (AMOS) 16.0 software Arbuckle (Arbuckle, 2007). Alpha was set at .05 (two sided).
Results
Table 1 displays descriptive data as well as correlations among endocrine and psychological variables. Correlations among psychological symptoms were positive and high, ranging from .62 to .70. Among endocrine variables, correlations ranged from .31 to .75. The consistently positive intercorrelations supported the planned use of latent variables for HPA/SNS and PDF.
Table 1. Correlations of Symptom Reports and Endocrine Measures among Breast Cancer Patients (N=104).
| Mean (SD) | 1. | 2. | 3. | 4. | 5. | 6. | |
|---|---|---|---|---|---|---|---|
| Behavioral | |||||||
| 1. Pain (BPI) | 14.25 (19.04) | ||||||
| 2. Depression (CES–D) | 13.57 (9.12) | .620 | |||||
| 3. Fatigue (FSI) | 3.06 (2.56) | .649 | .700 | ||||
| Endocrine | |||||||
| 4. Cortisol | 9.97 (5.73) | .339 | .353 | .288 | |||
| 5. ACTH | 20.01 (24.07) | .202 | .156 | .224 | .556 | ||
| 6. Epinephrine | 86.05 (129.4) | .298 | .281 | .373 | .446 | .746 | |
| 7. Norepinephrine | 555.4 (299.5) | -.005 | .057 | -.026 | .311 | .473 | .468 |
Note: Correlations greater than .230 are significant at the α=.05 level. BPI=Brief Pain Inventory, CES-D=Center for Epidemiological Studies – Depression Scale, FSI=Fatigue Symptom Inventory ACTH=adrenocorticotropin hormone.
Regarding the relationships between psychological and endocrine variables, a consistent pattern emerged. Cortisol and epinephrine showed consistent positive correlations of “moderate” strength with PDF (Cohen, 1988). Correlations between PDF and ACTH were positive but of low magnitude (.20-.22). Norepinephrine was unrelated to PDF.
Preliminary analyses tested the assumptions of maximum likelihood by examining skewness and kurtosis statistics. Non-normal distributions were observed for ACTH, norepinephrine, and epinephrine; however normalizing the data using log-transformation did not affect the study results. Therefore, we present analyses based on non-transformed data. Identifiability of the model was secured using the method of independent clusters (McDonald & Ho, 2002). Across variables, 11% of data points were missing, primarily due to insufficient blood samples. This is within recommended limits for FIML, and all participants had at least partial data. Therefore, all data were used, as planned.
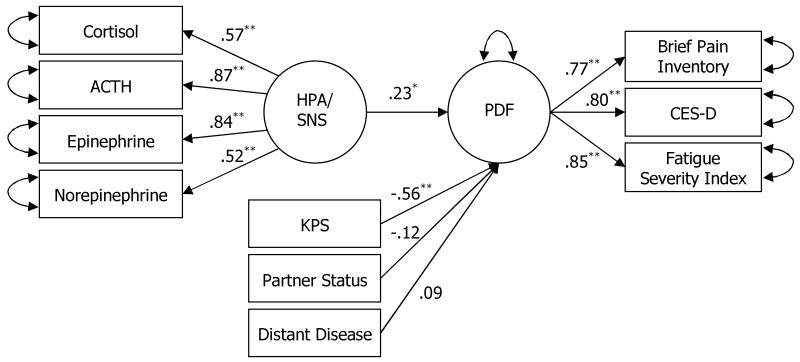
The final latent variable model is shown in Figure 1. Paths from PDF to all three psychological variables were positive and significant (standardized β=.77, .80, and .85 for pain, depression, and fatigue, respectively; ps<.001; see Figure 1). Thus, the latent variable PDF reflected the shared variance among the three symptoms. Paths from the HPA/SNS latent variable to all four endocrine measures were also significant (standardized β = .57, .87, .84, and .52 for cortisol, ACTH, epinephrine, and norepinephrine, respectively; ps < .001), suggesting that the HPA/SNS latent variable reflected the shared variance among endocrine measures. For the control variables, partner status, presence of distant metastases, and functional status were included in the final model. That is, the presence of a partner, an absence of distant disease, and better functional status were all associated with lowered symptomatology.
Figure 1.
Structural equation model testing the relationship between the SNS and HPA axes and the pain, depression, and fatigue (PDF) symptom cluster.
Note: ACTH=adrenocorticotropic hormone, HPA=hypothalamic-pituitary-adrenal axis, PDF=pain, depression, and fatigue, CES-D=Center for Epidemiological Studies – Depression Scale, KPS=Karnofsky Performance Status scale.
* p < .05 ** p < .001
The final model showed good fit to the data, as indicated by multiple criteria (Chi-square[df=31] = 39.486, p = .141; Root Mean Square Error of Approximation [RMSEA]= 0.052; Tucker-Lewis Index [TLI] = 0.944; Comparative Fit Index [CFI] = 0.969). The test of perfect fit was not rejected (p=.141), indicating that the model is a plausible representation of the data. The final analyzed covariance matrix, parameter estimates, and discrepancies are available from the publisher as supplemental information. As hypothesized, HPA/SNS significantly predicted PDF after controlling for relevant demographic and disease variables (standardized β = .23, p = .039).
Discussion
This is the first study to explore the relationship between a specific symptom cluster and proposed biological markers for it. As has been hypothesized, elevated SNS and HPA axis hormones explained significant shared variance between pain, depression, and fatigue, after inclusion of relevant controls. The strength of the relationship between the hormonal and behavioral variables (standardized beta =.23), was comparable to those between depression and inflammation markers [.15, .25, .35, and .25 for CRP, IL-6, IL-1, and IL-1ra, respectively, (Howren, Lamkin, & Suls, 2009)] and those between chronic stress and immune function [-.22, -.12, -.13, -.16, and -.21 for antibody response to influenza vaccine, natural killer cell cytotoxicity, lymphocyte proliferation to Con A, PHA, and cytokine-stimulated IL-2 production, respectively; (Segerstrom & Miller, 2004)].
These data confirm hypotheses derived from the neuroendocrine-immune models of cancer symptoms (Cleeland, et al., 2003; Dantzer, et al., 2008; Miller, et al., 2008). In such models, elevated SNS and HPA axis hormones are thought to occur in the context of a system of neuroendocrine-immunologic alterations. Specifically, the SNS and the HPA axis are activated concurrent with alterations in neurotransmitter metabolism (e.g., serotonin, dopamine, norepinephrine) which are thought to be primarily responsible for the psychological/behavioral symptoms (pain sensitivity, depression, fatigue). As these are cross-sectional data, directionality cannot be shown. Pain, depression, and fatigue could contribute to dysregulation of endocrine activity via behavioral pathways as well. Indeed, the theoretical model proposes a cyclical relationship among these variables. Another alternative involves direct effects from the individual hormones to the symptoms. For example, epinephrine can act directly on peripheral nerve fibers to decrease pain threshold and enhance pain sensation (Janssen, Arntz, & Bouts, 1998). Persistent elevations in cortisol, such as in Cushing's syndrome, can produce severe fatigue, muscle weakness, and depression (Nieman & Ilias, 2005). Regarding alternative statistical models, we considered the possibility of separating HPA and SNS hormones into two latent variables, but with only two measured variables for each latent variable, such a model could not be fit to data. Further, we felt the preliminary analyses showed no evidence of differential effects for the HPA versus SNS hormones, supporting the use of one latent variable for all four hormones.
Consistent with the definition of a symptom cluster, our study observed positive relationships among the pain, depression, and fatigue symptoms. The control variables eventually included in the analyses were the presence of distant metastases, nurse ratings of patients' functional status, and the absence of a partner, all with positive relationships with PDF symptoms. The latter correlation is consistent with other data from this laboratory showing that the absence of a partner confers added vulnerability for later depressive symptoms among patients who respond to the recurrence diagnosis with feelings of hopelessness (Brothers & Andersen, 2009).
It is notable that recent cancer treatments were not consistently related to the PDF latent variable. This may reflect differences in how the treatments affected each symptom for different patients. Specifically, treatment may increase fatigue or pain for some but be palliative for others. This is consistent with data reported in other samples (Ciaramella & Poli, 2001; Prue, Rankin, Allen, Gracey, & Cramp, 2006; van den Beuken-van Everdingen, et al., 2007).
Advantages of this test included accrual of a large, homogenous sample with significant symptom burden, the use of validated measures of psychological and endocrine status, and consideration of multiple disease, treatment, health status, and sociodemographic control variables. Data were collected as part of a larger observational study, and for this reason the measures for the present investigation were not ideal. Morning collection of plasma hormone levels served as a modest control of diurnal variation, but the length of the window in which sample collections were allowed may have introduced additional error into the data. In addition, a single measure of plasma hormones may not generalize to tonic levels. Care was taken to standardize the blood collection process through a consistent blood draw location, mandatory rest period, and limitations on caffeine prior to the appointment; however, the data may still not be as reliable as multiple measurements would have been. Thus, replication of these results is warranted. It should also be noted that, as this analysis was only performed in patients with recurrent or advanced stage breast cancer, additional studies will need to determine if the model holds for individuals with early-stage disease or other types of cancer. Further, these data may not generalize to men with cancer, as there are gender differences in HPA axis regulation (Kirschbaum, Kudielka, Gaab, Schommer, & Hellhammer, 1999). Another limitation was the sample size relative to the complexity of the model. The ratio of participants to free parameters was smaller than recommended. A simplified model which omitted the control variables resulted in an acceptable ratio (5:1; model not shown) and yielded identical results. Thus, we believe the results presented here are reliable. However, they will need to be tested in a study designed for this purpose as opposed to a convenience sample, as presented here.
Poorly controlled symptoms can be one of the greatest obstacles to breast cancer patients' quality of life (Yang, Thornton, Shapiro, & Andersen, 2008), and patients with recurrent or metastatic cancer face significant symptoms (Andersen, Shapiro, Farrar, Crespin, & Wells-Di Gregorio, 2005; Munkres, Oberst, & Hughes, 1992; Yang, et al., 2008). The identification of symptom clusters – three or more concurrent, subjective indicators of physiological or psychological disturbance that are consistently related to one another (Barsevick, Whitmer, Nail, Beck, & Dudley, 2006; Dodd, Miaskowski, & Lee, 2004) – could aid symptom management. Discovery of the psychological or biological processes that underlie a symptom cluster could inform the development of targeted interventions to treat the whole cluster.
The present study represents an early step in identifying the biological correlates of pain, depression, and fatigue. To the extent that the neuroendocrine-immune models are borne out by future research, targeted interventions may include normalization of neuroendocrine function using corticotropin-releasing hormone, anti-depressants, or melatonin-receptor agonists, for example (Cleeland, et al., 2003; Miller, et al., 2008). Data from our lab suggest that improvements in pain, depression, and fatigue following psychological intervention can precipitate reduction in inflammation (Thornton, Andersen, Schuler, & Carson, 2009). Thus, psychological or behavioral treatments should also be considered as a way to normalize the system. Future research may extend these findings in a repeated measures study or examine other hypotheses of the model, such as test whether symptoms correlate positively with proinflammatory cytokines, acute phase proteins, or other indicators of inflammation.
Supplementary Material
Supplemental Tables
Sample correlations, sample variances, and discrepancies (N=104)
Parameter estimates
Acknowledgments
We thank the patients and the professional and research staff of the Stress and Immunity Cancer Projects. Special thanks to Dr. Tim Crespin for his work on an earlier version of the manuscript.
Preparation of this manuscript was supported by the American Cancer Society (PF-07-169-01-CPPB), the Longaberger Company-American Cancer Society Grant for Breast Cancer Research (RSGPB-03-248-01-PBP), the Ohio State University General Clinical Research Center M01-RR0034), and the National Cancer Institute (K05 CA098133).
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/hea
References
- Andersen BL, Shapiro CL, Farrar WB, Crespin TR, Wells-Di Gregorio SM. Psychological responses to cancer recurrence: A controlled prospective study. Cancer. 2005;104:1540–1547. doi: 10.1002/cncr.21309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuckle JL. Amos 16.0 [computer software] (Version 16.0) Spring House, PA: SPSS Inc.; 2007. [Google Scholar]
- Barsevick AM, Whitmer K, Nail LM, Beck SL, Dudley WN. Symptom cluster research: conceptual, design, measurement, and analysis issues. Journal of Pain and Symptom Management. 2006;31:85–95. doi: 10.1016/j.jpainsymman.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Brothers BM, Andersen BL. Hopelessness as a predictor of depressive symptoms for cancer patients coping with recurrence. Psycho-Oncology. 2009;18:267–275. doi: 10.1002/pon.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annual Review of Physiology. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- Ciaramella A, Poli P. Assessment of depression among cancer patients: the role of pain, cancer type and treatment. Psycho-Oncology. 2001;10(2):156–165. doi: 10.1002/pon.505. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Bennett GJ, Dantzer R, Dougherty PM, Dunn AJ, Meyers CA, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? Cancer. 2003;97(11):2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Annals of the Academy of Medicine, Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc.; 1988. [Google Scholar]
- Cohen L, de Moor C, Devine D, Baum A, Amato RJ. Endocrine levels at the start of treatment are associated with subsequent psychological adjustment in cancer patients with metastatic disease. Psychosomatic Medicine. 2001;63:951–958. doi: 10.1097/00006842-200111000-00014. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JCF, G G, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd MJ, Miaskowski C, Lee KA. Occurrence of symptom clusters. Journal of the National Cancer Institute. Monographs. 2004;(32):76–78. doi: 10.1093/jncimonographs/lgh008. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve—An integrative interface between two supersystems: The brain and the immune system. Pharmacological Reviews. 2000;52(4):595–638. [PubMed] [Google Scholar]
- Hann DM, Jacobsen PB, Azzarello LM, Martin SC, Curran SL, Fields KK, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Smptom Inventory. Quality of Life Research. 1998;7:301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-Reactive Protein, IL-1, and IL-6: A meta-analysis. Psychosomatic Medicine. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Janssen SA, Arntz A, Bouts S. Anxiety and pain: epinephrine-induced hyperalgesia and attentional influences. Pain and Headache. 1998;76:309–316. doi: 10.1016/S0304-3959(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: Macleod CM, editor. Evaluation of Chemotherapeutic Agents. New York, NY: Columbia University Press; 1949. pp. 199–205. [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, mentrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adernal axis. Psychosomatic Medicine. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Lechin F, van der Dijs B, Benaim M. Stress versus depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1996;20:899–950. doi: 10.1016/0278-5846(96)00075-9. [DOI] [PubMed] [Google Scholar]
- McDonald RP, Ho MHR. Principles and practice in reporting structural equation analyses. Psychological Methods. 2002;7(1):64–82. doi: 10.1037/1082-989x.7.1.64. [DOI] [PubMed] [Google Scholar]
- Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. Journal of Clinical Oncology. 2008;26(6):971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munkres A, Oberst M, Hughes SH. Appraisal of illness, symptom distress, self-care burden, and mood states in patients receiving chemotherapy for initial and recurrent cancer. Oncology Nursing Forum. 1992;19(8):1201–1209. [PubMed] [Google Scholar]
- Nieman LK, Ilias I. Evaluation and treatment of Cushing's syndrome. American Journal of Medicine. 2005;118(12):1340–1346. doi: 10.1016/j.amjmed.2005.01.059. [DOI] [PubMed] [Google Scholar]
- Prue G, Rankin J, Allen J, Gracey J, Cramp F. Cancer-related fatigue: A critical appraisal. European Journal Of Cancer. 2006;42(7):846–863. doi: 10.1016/j.ejca.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin. 2004;130(4):601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton LM, Andersen BL, Schuler TA, Carson WE. A psychological intervention reduces inflammation by alleviating depressive symptoms: Secondary analysis of a randomized controlled trial. Psychosomatic Medicine. 2009 doi: 10.1097/PSY.0b013e3181b0545c. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Beuken-van Everdingen MHJ, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Annals of Oncology. 2007;18(9):1437–1449. doi: 10.1093/annonc/mdm056. [DOI] [PubMed] [Google Scholar]
- Vierck CJ., Jr Mechanisms underlying development of spatially distributed chronic pain (fibromyalgia) Pain. 2006;124:242–263. doi: 10.1016/j.pain.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Yang HC, Thornton LM, Shapiro CL, Andersen BL. Surviving recurrence: Psychological and quality of life recovery. Cancer. 2008;112(5):1178–1187. doi: 10.1002/cncr.23272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables
Sample correlations, sample variances, and discrepancies (N=104)
Parameter estimates