Abstract
Thirteen to 20% of lung cancer patients continue to smoke after diagnosis. Guided by Self-Regulation theory, the purpose of this study was to examine illness perceptions over time in a sample of lung cancer patients.
This prospective one-group descriptive longitudinal design study included participants 18 years or older, with a lung cancer diagnosis within the past 60 days who self-reported smoking within the past 7 days. At baseline patients completed a sociodemographics and tobacco use history questionnaire. The Illness Perception Questionnaire-Revised (IPQ-R) was repeated at 3 time points (baseline, 2–4 weeks, 6 months).
Fifty-two participants provided data for the IPQ-R at baseline, 47 at 2–4 weeks, and 29 at 6 months. Differences between mean scores for each illness representation attribute of the IPQ-R at repeated time points were calculated by within-subject repeated measures analysis of variance and Wilcoxon Signed-Rank Tests. Identity (baseline vs. 2–4 weeks: p=0.026; baseline vs. 6 months: p=0.005) and acute/chronic timeline (p=0.018) mean scores significantly increased over time; personal and treatment control mean scores significantly decreased over time (p=0.007 and p=0.047, respectively). Understanding the context in which a patient perceives disease and smoking behavior may contribute to developing interventions that influence behavior change.
Keywords: Self-Regulation theory, common-sense model, illness cognitions, smoking behavior, lung cancer, illness representation
Introduction
Lung cancer is responsible for most cancer deaths in the United States for both men and women 1. For all stages of the disease, the 5 year survival rate of lung cancer is approximately 15% 2. It is well established that quitting smoking after a diagnosis of lung cancer improves survival, side effects of disease and treatment, and decreases the risk of developing a second smoking-related lung cancer 3, 4.
In patients who continue to smoke after diagnosis, tobacco may act as a carcinogenesis promoter in previously initiated cancer sites 5. Patients who survive lung cancer and continue to smoke risk further compromise of lung function that is diminished due to surgical resection, pulmonary toxicity from chemotherapy, and/or chest irradiation 5. Results from the Nurses Health Study (n=158,734) indicated that current smokers reported lower health-related quality of life than former and never smokers 6. Among non-small cell lung cancer patients of all stages (n=206), performance status (PS) (i.e., quality of life measure) was significantly related to patient smoking status. Patients who quit smoking maintained a better PS at 0 to 6 months (OR=7.09, 95% CI=1.99–25.3) and at 0 to 12 months (OR=6.99, 95% CI=1.76–27.7), than those who continued to smoke after diagnosis, when controlling for stage, demographics, treatment, and comorbidities 7.
Patients who quit smoking prior to and at the time of a lung cancer diagnosis (all stages) have a significantly better prognosis than those who continued to smoke during and subsequent to treatment 3, 4. Smoking cessation after initial treatment decreases the risk of developing a second, smoking-associated primary tumor 8. Among early stage lung cancer patients (n=569), smoking status (current versus former) was a significant predictor of the development of a second, smoking-associated primary lung cancer 9. Despite known benefits of quitting, 13% to 20% of lung cancer patients continue to smoke after diagnosis 5, 10–12.
Head and neck and lung cancer patients that continued smoking after diagnosis had higher nicotine dependence, reported more perceived ‘cons of quitting’, fatalism, and emotional distress; and lower self efficacy, perceptions of risk and perceived ‘pros of quitting’ 13. In female lung cancer patients, younger age, living with another smoker, and depressive symptoms are significantly associated with continued smoking after diagnosis 14. While these findings contribute interesting preliminary data for this population, conceptual understanding that exclusively examines characteristics of smoking behavior in lung cancer patients is lacking. Furthermore, an understanding of how a lung cancer diagnosis impacts patient perceptions and behavior such as smoking could be useful in designing future smoking cessation interventions.
Limited research has been conducted to examine the psychosocial and behavioral influences that contribute to continued smoking following a lung cancer diagnosis. Understanding a patient’s perception of illness may improve the day-to-day management of illness and disease 15 as patient beliefs about illness are known to influence health behavior outcomes 16. Illness perceptions over time among smoking lung cancer patients as guided by Self-Regulation theory is the focus of the next section.
Background
Overview of the Self-Regulation Model of Illness
The Self-Regulation Model of Illness (SRMI), initially described in 1980 as the “common sense model of illness representation” by Leventhal and colleagues, provides a framework for understanding how individual symptoms and emotions experienced during a health threat or diagnosis influence perception of illness and guide subsequent coping behavior 17, 18. The SRMI may be useful to further understanding of why individuals diagnosed with lung cancer continue to smoke. This model has been examined within multiple illnesses and health-related behaviors including coronary heart disease 19, human immunodeficiency syndrome (HIV) medication adherence 20, and diabetes self management 21. Components of the SRMI have been examined among oncology patients 22–25, however, there has been no examination of the SRMI among lung cancer patients who smoke at the time of diagnosis.
The SRMI theory suggests that individuals search to understand their illness or disease threat by developing an understanding of what the illness is, what it means, its causes, its consequences, how long it will last, and whether it can be cured or controlled. This understanding (or illness representation) is not necessarily scientifically or medically validated, but formulated from personal experience (physical symptoms and emotions), social influences, and/or interaction with healthcare providers. Individuals are thought to reduce their health risk or change their health behavior in ways consistent with their own illness representation. The model in Figure 1 suggests that a lung cancer patient’s decision to quit or continue to smoke following diagnosis will be influenced by whether it ‘makes sense’ given the patient’s own illness representation. The discussion in the following section will focus on how the theoretical components of the SRMI can be conceptually applied to continued smoking following a lung cancer diagnosis.
Figure 1.
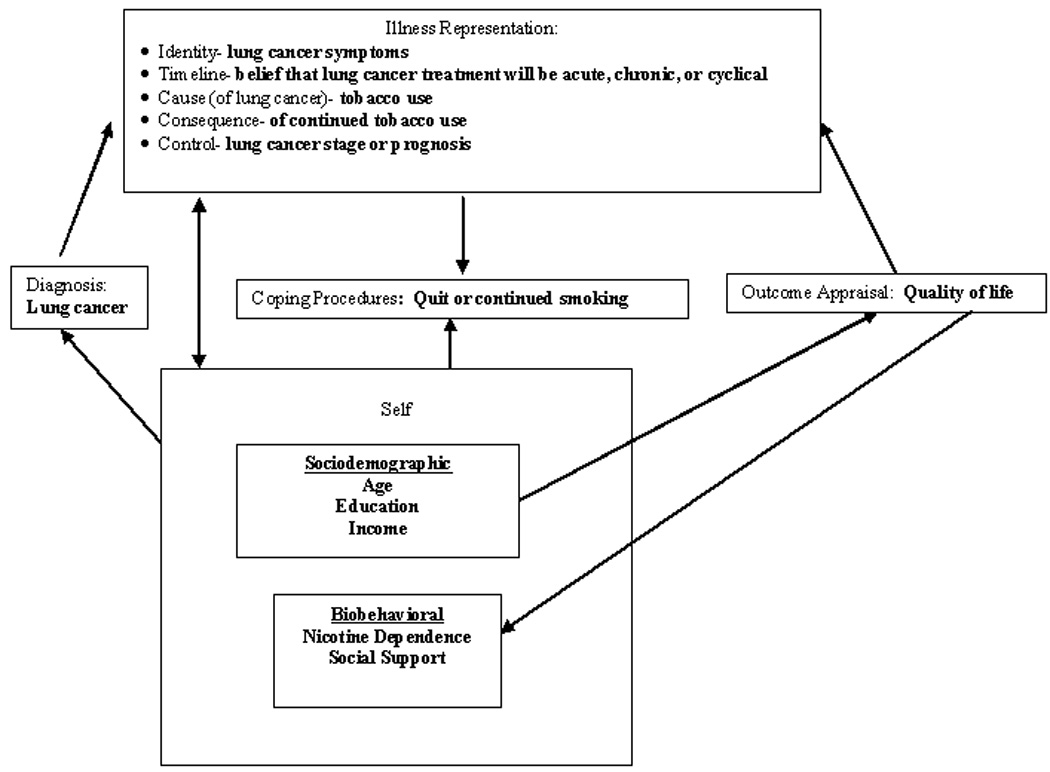
Self-regulation model applied to tobacco use in smokers recently diagnosed with lung cancer
SRMI as applied to lung cancer
Illness representation
Following a diagnosis such as lung cancer, the individual analyzes, internalizes, and interprets the meaning of the illness. The individual becomes an active problem solver and simultaneously deals with two phenomena: the perceived reality of the illness and their emotional reaction. Internal and external stimuli such as previous experience with the illness and social and societal influences operate to influence the development of the illness representation 17, 18. For example, a newly diagnosed lung cancer patient who is currently smoking may interpret their increased cough and worsening shortness of breath as symptoms of lung cancer. Depending on past experiences with these symptoms and influences from healthcare providers, the media, family and/or friends, an illness representation is formed by the patient with respect to his or her continued smoking after this new diagnosis (see Figure 1). Aside from recognizing the signs and symptoms (identity) of the disease, other components of this illness representation are cause, consequence, control, and timeline 17, 18. The patient’s perception of lung cancer will influence the interpretation of the cause of the disease, linking past or continued smoking with the cause of the disease. The consequences of continuing or stopping smoking will be internalized and made part of the smoker’s illness representation. Perceived control of lung cancer symptoms and disease and whether quitting or continuing smoking will have an effect upon the symptoms and the stage of the lung cancer, become part of the illness representation. In addition, the smoker’s ability to change the overall timeline or prognosis of the diagnosis, with respect to quitting or continuing smoking, will be an important component of the illness representation.
Illness representations are complex and dynamic. The internal and external stimuli of the individual changes the illness representation over time and further guides actions of the individual in response to the health threat 17, 18. Internal stimuli such as side effects of lung cancer treatment and external stimuli such as public opinion on causes of lung cancer and prognosis will shape the illness representation over the course of the disease. Emotions are also integral to illness representation and develop simultaneously with the cognitive component. Emotional experiences such as fear, anger, depression or anxiety can motivate the individual to develop an action plan (coping procedure), or can be so overwhelming, resulting in less or no action taken (with respect to the disease) 17. After a patient has had the opportunity to consider the diagnosis, prognosis, and proposed medical treatment, the patient’s illness representation may change. Receiving a cancer diagnosis can evoke a range of emotions, contributing to the formation of the illness representation that influences the coping procedures.
Coping procedure
The illness representation drives the individual’s coping strategies. A response to the illness representation is instituting a behavior, or coping procedure. The individual develops a response plan or procedure to cope with the illness representation, and the selection of a coping procedure is guided by the illness representation concept 18. A smoker may choose to cope with the new diagnosis of lung cancer by either quitting or continuing to smoke. The consequences of quitting smoking may involve physical and psychological factors (both positive and negative) such as decreased shortness of breath 26, increased nicotine withdrawal symptomatology 27, increased family support 13, and improved survival 3, 4. Although the consequences of continued smoking may result in worsening pulmonary symptoms and possible decreased long-term survival, the patient may continue to smoke to avoid the additional stress of quitting smoking during this already stressful time.
Outcome appraisal
The individual will engage in ongoing outcome appraisal, or the analysis of the consequence or efficacy of the coping procedure. The outcome appraisal is the repeated evaluation of the coping procedure (continued or quitting smoking) and may be influenced by such variables as quality of life, clinical response to lung cancer treatment and lung cancer symptomatology, in addition to the consequences of quitting or continuing to smoke. Each variable influences the patient’s evaluation of the coping procedure. Information gained during the coping procedure feeds back to the other constructs. If an individual perceives that a coping procedure is ineffective, an alternative coping procedure may be selected. Thus the model is fluid and dynamic, with continuous feedback between each component 18. A patient may use his or her own quality of life assessment as a proxy indicator for outcome appraisal when evaluating their smoking behavior (coping procedure).
Representation of self
The individual’s cognitive and emotional processes that form illness representations do not occur in isolation, they are influenced by the representation of self. A lung cancer patient’s representation of self is defined as their self-perception (“Who am I?” or “How do I define myself?”) and self-meaning or ‘importance of self’ (“What value do I place on myself?” or “Why do I matter?”). After a disease threat or illness (i.e., lung cancer), the representation of self is redefined within the context of the illness and is influenced by the individual’s social interactions (e.g., family, friends, society, and healthcare professionals). Thus, the individual ‘looks’ at or interprets the illness ‘through the eyes of the self’ 17, 18, 28. There are many sociodemographic and biobehavioral characteristics that are specific to an individual (self), and are known to be associated with continued smoking.
Sociodemographic characteristics and self
Age, education, and income are several known sociodemographic characteristics associated with continued smoking behavior 29. Smoking is often initiated in adolescence, and once dependent, continues throughout adulthood 30. Level of education is inversely correlated with smoking prevalence, as those with a higher education are least likely to smoke and are most successful in quitting 29, 31. There is an inverse relationship between lower socioeconomic status (income) and smoking, those living at or below the poverty line have a higher prevalence of smoking 29, 31. These sociodemographic characteristics (age, education, and income), that are specific to an individual, can further influence and guide the patient’s perception of illness and coping procedure (quitting or continuing to smoke).
Biobehavioral characteristics and self
Biobehavioral characteristics such as higher nicotine dependence and less social support are also associated with smokers who are unable to quit 13, 32. Nicotine is the psychoactive drug in tobacco that causes acute and chronic dependence 32, and nicotine dependence often requires repeated intervention to assist individuals to successfully quit smoking 33. Smokers who have increased dependence on nicotine often require many quit attempts and have higher relapse rates before achieving permanent abstinence 27. Most tobacco users express the desire to quit smoking and many make unsuccessful attempts to quit 27, 33. The level of nicotine dependence and social support can further influence the illness representation and coping procedure.
Living with other smokers and having family and/or caregiver support to quit smoking can greatly influence the outcome of a patient’s success at quitting smoking. Among those diagnosed with cancer (n=74), it has been observed that having a family member at home who smokes increased the likelihood that patients will continue to smoke 13.
This study was designed to examine the natural course of a smoking behavior once a diagnosis of lung cancer has been made. Its purpose was to describe changes in illness representations over time and answer the following questions: Do the components of illness representations change from baseline to 2–4 weeks and at 6 months among newly diagnosed lung cancer patients who smoke at the time of diagnosis? And, how do lung cancer patients’ reasons for smoking or quitting align with Self-Regulation theory constructs?
Methods
Design/sample
This was a prospective one-group descriptive longitudinal design. Eligibility criteria included those participants who were age 18 years or older, had a confirmed diagnosis of lung cancer (non-small cell or small cell) within the past 60 days, and self-reported current smoking within the past seven days. Participants had to be able to understand English and provide informed consent.
Procedure
Recruitment took place within the thoracic oncology outpatient clinics at an urban, academic comprehensive cancer center. At baseline, patients completed a sociodemographic, medical and tobacco use history questionnaire, and the Illness Perception Questionnaire (Revised) (IPQ-R) 34. At 2–4 weeks and 6 months following enrollment, the IPQ-R was re-administered. The 2–4 time point was selected to examine illness representation after allowing the patient time to consider the medical information regarding diagnosis, prognosis, and treatment, after the initial medical center visit. Patients also completed the medical and tobacco use history questionnaire again at 6 months post enrollment. Data collection at all time points took place either while waiting for a clinic appointment, through mail correspondence, or during a chemotherapy visit. All data was collected via self-administered, written questionnaires. At 6 months, patients who self-reported as a non-smoker provided a saliva cotinine sample for biochemical verification. Verbal and written instructions for the questionnaires were given to each patient. As part of usual care, all patients in these clinics were routinely asked their smoking status and advised to quit smoking at the time of each visit. Appropriate pharmacotherapy was recommended, according to the U.S. Public Health Service Guideline recommendations 33. This study was approved by and in compliance with the institution’s Human Subjects Cancer Review Board.
Study Measures
A sociodemographic (age, gender, insurance, education, race, marital status, and household income), tobacco use history (cigarettes per day (CPD), years smoked, previous quit attempts, and living with another smoker), and medical history (pathology, stage, previous cancer treatment at study entry, and date of diagnosis) questionnaires were administered. Tobacco use history questions were obtained from other standardized questionnaires 35, 36. The IPQ-R is a quantitative measure of illness representation, containing five scales that assess each component of illness representation. It is intended to be used in a variety of diseases, inserting the specific disease or health threat where appropriate 34. The identity scale includes 14 symptoms that the patient is asked to state if present. This provides a simple measure of the number of symptoms perceived by the patient to be associated with the illness. A higher score indicates a greater number of symptoms attributable to the disease 34. The remaining scales of the IPQ-R include acute/chronic timeline (6 items), cyclical timeline (4 items), consequences (6 items), personal control (6 items), treatment control (5 items), illness coherence (5 items), and emotional representation (6 items), and are rated by the patient on a five-point Likert-type scale ranging from “strongly disagree” to “strongly agree” 34.
Higher scores on the timeline scales, acute/chronic and cyclical, indicate a strong belief that the illness is chronic or cyclical in nature. A stronger belief that the illness has negative consequences is represented by a higher score on the consequence scale. Higher scores on the personal and treatment control scales suggest a strong belief in personal and treatment control of the disease. A greater personal understanding of the disease is represented by a higher score on the illness coherence scale and a higher score on the emotional representation scale suggests that the illness has a greater emotional meaning 34. Estimates of Cronbach’s alpha coefficients for the IPQ-R range from 0.79–0.89 34.
During baseline data collection, each patient was asked, “What is the primary reason you have not quit smoking?” At the study completion, each patient was asked: 1) “What is the primary reason you have not quit smoking?” Or, 2) “What was the primary reason you were successful in quitting smoking?” These open-ended questions were investigator designed.
Smoking Status
Smokers were defined as self-reported users of cigarettes in the past 7 days. Non-smokers (i.e., quitters) were defined as those who self-report no use of cigarettes in the past 7 days 37 AND a saliva cotinine concentration <14ng/mL 38. Cotinine is a reliable and valid measure of tobacco smoke exposure 39. Jarvis and others 38 reported a 96% sensitivity rate and a 99% specificity rate when using 14ng/mL as a cutoff level in discriminating tobacco users from non-users. Biochemical verification of self-reported smoking status is recommended as additional confirmation that self-reporting is accurate. The window for precise biochemical verification of smoking status by cotinine is within 7 days 40. Saliva samples were collected with Salivettes® (Sarstedt, Newton, NC). The samples were processed at the outpatient clinic, stored frozen at −80 degrees Celsius until analyses were conducted. Cotinine was extracted from the saliva using a technique described by Hariharan et al., (1991). Next, saliva cotinine levels were quantified by a high-performance reversed-phase ion exchange liquid chromatographic technique.
Statistical Analyses
Descriptive statistics (means, standard deviations, percents) were calculated on all sociodemographic, medical history, tobacco use, and illness representation data. Missing data on the IPQ-R were imputed with the mean score for the corresponding question. Only 0.6% (6 cases) of the data had to be imputed. Internal consistency was assessed by estimating Cronbach’s alpha coefficient on each IPQ-R attribute except identity 41. Differences between mean scores for each attribute of the IPQ-R at repeated time points were calculated by within-subject repeated measures analysis of variance (ANOVA). Tukey-Kramer post-hoc tests were conducted to indicate pairs of data that had significantly different means. Histograms and quantile-quantile (Q-Q) plots were constructed and examined to determine if the residuals met the assumption of normality, and compound symmetry was assessed with Mauchly’s Test of Sphericity. For data that violated the assumption of normality, Wilcoxon Signed-Rank Tests for non-parametric data were calculated 42. Data were analyzed using SPSS 14.0 (SPSS Inc, Chicago, IL) and SAS version 9.1 (SAS Institute Inc., Cary, NC). Open-ended responses were independently reviewed by 2 nurse experts and percent agreement of selected patient response categories was reported. Typology of smoking relapse 43 and the Horn-Waingrow smoking typology 44served as the framework for selection of the open-ended patient response categories.
Results
Fifty-two participants provided data for the IPQ-R at baseline, 47 at the 2–4 week time point, and 29 at 6 months. Five participants did not provide data at the 2–4 week time point because they were not able to be contacted. Of the participants who did not provide data at 6 months (n=23), 21.7% (5) were lost to follow up, 73.9% (17) were deceased, and 4.4% (1) withdrew. Fifty percent of the sample was female and most were married (61.5%) and were Non-Hispanic white (84.6%) (see Table 1). Twenty-seven percent of the sample had Medicaid and 5.8% had no insurance; 42.9% reported an annual household income of <$25,000. (see Table 1 for other sociodemographic characteristics.) There was no statistical difference in sociodemographic characteristics or medical history between patients who provided data at all time points and those who provided data only at baseline or baseline and the 2–4 week time point.
Table 1.
Sociodemographic characteristics for entire sample
| Variables | n | % | mean (SD) | range |
|---|---|---|---|---|
| Age | 52 | 56.6 (10.1) | 25–80 | |
| Gender (n=52) | ||||
| Male | 26 | 50.0 | ||
| Female | 26 | 50.0 | ||
| Insurance (n=52) | ||||
| Private/Medicare | 35 | 67.3 | ||
| Medicaid | 14 | 26.9 | ||
| No insurance | 3 | 5.8 | ||
| Education (n=51) | ||||
| Some HS | 11 | 21.6 | ||
| HS graduate | 13 | 25.4 | ||
| GED | 6 | 11.8 | ||
| Post HS education | 17 | 33.4 | ||
| College graduate | 4 | 7.8 | ||
| Race (n=52) | ||||
| Non Hispanic black | 7 | 13.5 | ||
| Non Hispanic white | 44 | 84.6 | ||
| Other | 1 | 1.9 | ||
| Marital Status (n=52) | ||||
| Married/Living with partner | 32 | 61.5 | ||
| Widowed | 4 | 7.7 | ||
| Divorced | 11 | 21.2 | ||
| Never married | 5 | 9.6 | ||
| Household Income (n=49) | ||||
| <$25K | 21 | 42.9 | ||
| $25K-$50K | 10 | 20.4 | ||
| >$50K | 9 | 18.4 | ||
| Refused | 5 | 10.2 | ||
| Don’t know | 4 | 8.2 |
The majority of the sample was diagnosed with non-small cell lung cancer (78.8%) and were in the late stages of disease (68.6%) (see Table 2). Forty percent (n=31) of the sample had already begun cancer treatment (chemotherapy, radiation therapy, and/or surgery) at baseline. At study entry, the mean time since diagnosis (date of pathology) was 26.1 days. The sample reported smoking 16 cigarettes per day on average and the average number of years smoked were 36.8. The average number of quit attempts was 5.1. A higher number of previous quit attempts were reported at 6 months than at baseline. (See Table 2 for other tobacco and medical history data.) At 6 months 27 out of 29 patients (93.1%) reported receiving some treatment for lung cancer (chemotherapy, radiation therapy, and/or surgery) and the remaining two patients had surgery or received radiation prior to study entry. Twenty-four patients (89.7%) received chemotherapy as a component of their lung cancer treatment. The majority of patients (51.7%, n=15) received chemotherapy plus radiation therapy for their prescribed lung cancer treatment (data not presented in Table 2).
Table 2.
Smoking history and lung cancer characteristics at baseline categorized by the entire sample and participants who provided data at 6 months
| Entire Sample | Participants Who Provided Data at 6 Months | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | n | % | mean (SD) | range | n | % | mean (SD) | range |
| CPD | 51 | 16.0 (11.01) | 0–40 | 29 | 14.2 (10.57) | 0–40 | ||
| Years smoked | 51 | 36.8 (11.24) | 8–65 | 29 | 35.2 (11.95) | 8–60 | ||
| Previous quit attempts | 50 | 5.1 (14.52) | 0–100 | 28 | 7.3 (19.52) | 0–100 | ||
| Living with a smoker (n=51, n=28) | 27 | 52.9 | 15 | 53.6 | ||||
| Pathology (n=52, n=29) | 7 | |||||||
| Small cell | 11 | 21.2 | 22 | 24.1 | ||||
| Non-small cell | 41 | 78.8 | 75.9 | |||||
| Stage (n=52, n=29) | ||||||||
| Early (I-IIIA, limited) | 16 | 31.3 | 12 | 41.4 | ||||
| Late (IIIB, IV, extensive) | 35 | 68.7 | 17 | 58.6 | ||||
| Cancer treatment at baseline (n=52) | ||||||||
| No treatment | 31 | 59.6 | ||||||
| Surgery | 6 | 11.5 | ||||||
| Radiation (XRT) | 6 | 11.5 | ||||||
| Chemotherapy | 4 | 7.7 | ||||||
| Chemotherapy + XRT | 5 | 9.7 | ||||||
| Time since diagnosis (days) | 52 | 26.1 (14.20) | 3–58 | 28 | 27.0 (14.13) | 4–58 | ||
Smoking Status
Twenty-nine patients completed data collection at 6 months. Twenty-two patients were self-reported smokers and seven patients self-reported quitting smoking. Of these, five (17.2%) were biochemically confirmed to be abstinent by saliva cotinine. The 2 misclassified patients reported no use of nicotine replacement therapy. Most patients (76.7%) made at least one attempt to quit smoking in the previous 6 months, and smokers reported a mean of 2.9 quit attempts in the past 6 months (data not present in table). The lung cancer staging of the 5 non-smokers included 4 early stage (one IB, one IIIA, two limited stage) and 1 late stage (IIIB).
Illness Perception at Baseline, 2–4 Weeks and 6 Months
The results of the IPQ-R at baseline and second time point are summarized in Table 3. The acute/chronic timeline attribute mean scores indicated that patients believed their disease was more chronic than acute. The cyclical timeline scores showed that patients had a stronger belief in the cyclical nature of their lung cancer. Patients held strong beliefs about the personal and treatment controllability of lung cancer. The reported mean emotional representation attribute was high at both time points. There were no significant differences detected between IPQ-R baseline group means of those who provided data at 6 months and those who did not provide data at 6 months.
Table 3.
Mean scores (SD) and range of the IPQ-R attributes at baseline and second time point for entire sample*
| IPQ-R Attributes | Possible Range |
Baseline | Second Time Point** | ||||
|---|---|---|---|---|---|---|---|
| n | mean (SD) | range | n | mean (SD) | range | ||
| Identity | (0–14) | 52 | 5.4 (3.83) | 0–12 | 47 | 6.6 (4.34) | 0–14 |
| Timeline (acute/chronic) | (0–30) | 52 | 17.8 (5.61) | 3–29 | 47 | 18.2 (5.02) | 10–30 |
| Timeline (cyclical) | (0–20) | 52 | 11.6 (2.28) | 6–18 | 47 | 12.0 (2.59) | 7–18 |
| Personal control | (0–30) | 52 | 22.4 (4.00) | 12–30 | 47 | 21.4 (4.16) | 6–30 |
| Treatment control | (0–25) | 52 | 18.8 (2.88) | 13–25 | 47 | 18.7 (2.36) | 13–24 |
| Consequence | (0–30) | 52 | 23.7 (3.60) | 14–30 | 47 | 23.6 (4.67) | 9–30 |
| Illness coherence | (0–25) | 52 | 16.3 (4.23) | 6–24 | 47 | 17.3 (4.39) | 6–25 |
| Emotional representation | (0–30) | 52 | 21.5 (4.53) | 10–30 | 47 | 20.5 (4.44) | 13–29 |
No significant differences between time points
2–4 weeks
IPQ-R descriptive results for patients who provided data for all of the time points (baseline, second time point, and 6 months) and results of the within-subjects repeated measures ANOVAs and Wilcoxon Signed-Rank Tests can be found in Table 4. All data had a normal distribution, except for the identity attribute. The identity data (at all 3 time points) had a mixed distribution. There appeared to be a binary response, where many patients reported having either zero or 14 symptoms, with a more normal distribution of data in between. The identity mean scores were significantly higher at the second time point (p=0.03) and 6 month time point (p=0.01) as compared to baseline mean scores. Significant differences between mean scores of the person control attribute were detected, with the 6 month mean score significantly lower than the sample’s baseline mean score. The acute/chronic timeline mean scores were significantly different; the 6 month mean score indicated a more chronic belief by patients as compared to their mean scores at baseline and the second time point. The sample’s 6 month treatment control attribute mean score was significantly lower than the mean score at baseline.
Table 4.
| IPQ-R Attributes | Possible Range |
Baseline | Second Time Point | 6 Months | |||
|---|---|---|---|---|---|---|---|
| mean (SD) | range | mean (SD) | range | mean (SD) | Range | ||
| Identity***, d | (0–14) | 4.7 (3.55) | 0–12 | 6.2 (4.29) | 0–14 | 6.6 (3.94) | 0–12 |
| Timeline (acute/chronic)a | (0–30) | 17.8 (4.59) | 10–29 | 17.2 (4.67) | 10–27 | 19.8 (5.50) | 8–30 |
| Timeline (cyclical) | (0–20) | 11.2 (2.35) | 6–16 | 11.8 (2.63) | 7–18 | 11.9 (2.64) | 7–16 |
| Personal controlb | (0–30) | 22.7 (3.51) | 12–28 | 21.8 (3.18) | 15–28 | 20.4 (3.89) | 12–26 |
| Treatment controlc | (0–25) | 19.2 (2.47) | 13–24 | 19.0 (1.88) | 15–24 | 17.8 (3.06) | 9–23 |
| Consequence | (0–30) | 23.7 (3.17) | 15–30 | 22.6 (5.03) | 9–30 | 23.1 (4.54) | 10–29 |
| Illness coherence | (0–25) | 15.9 (4.70) | 6–24 | 16.9 (4.46) | 6–23 | 16.9 (3.87) | 9–23 |
| Emotional representation | (0–30) | 21.0 (5.01) | 10–30 | 19.6 (3.92) | 14–27 | 19.6 (4.03) | 12–29 |
Differences between means calculated by within-subjects repeated measures ANOVA except for identity attribute
baseline, 2–4 weeks, 6 months
Differences between means calculated by Wilcoxon Signed-Rank Tests
F=4.310, df=(2,28), p=0.018
F=5.495, df=(2,28), p=0.007
F=3.239, df=(2,28), p=0.047
Baseline vs. second time point: s=59, p=0.026; baseline vs. 6 months: s=75.5, p=0.005
The internal consistency coefficients for the acute/chronic timeline, personal control, illness coherence and emotional representation attributes at all 3 time points ranged from 0.80–0.91. The internal consistency coefficients for the cyclical timeline ranged from 0.60–0.73 over the 3 time points. Treatment control ranged from 0.56–0.81 and consequence ranged from 0.61–0.82.
Illness Perception Questionnaire at 6 months
Descriptive results of the IPQ-R at 6 months by smoking status are summarized in Figure 2. Smokers reported higher mean identity, chronic and cyclical timeline, and emotional representation scores and quitters reported higher mean treatment control scores. The personal consequences and illness coherence mean scores were about the same for both groups. Four of the 5 non-smokers were female, 2 had surgery and chemotherapy, 2 had radiation therapy and chemotherapy, and 1 had surgery, radiation therapy, and chemotherapy.
Figure 2.
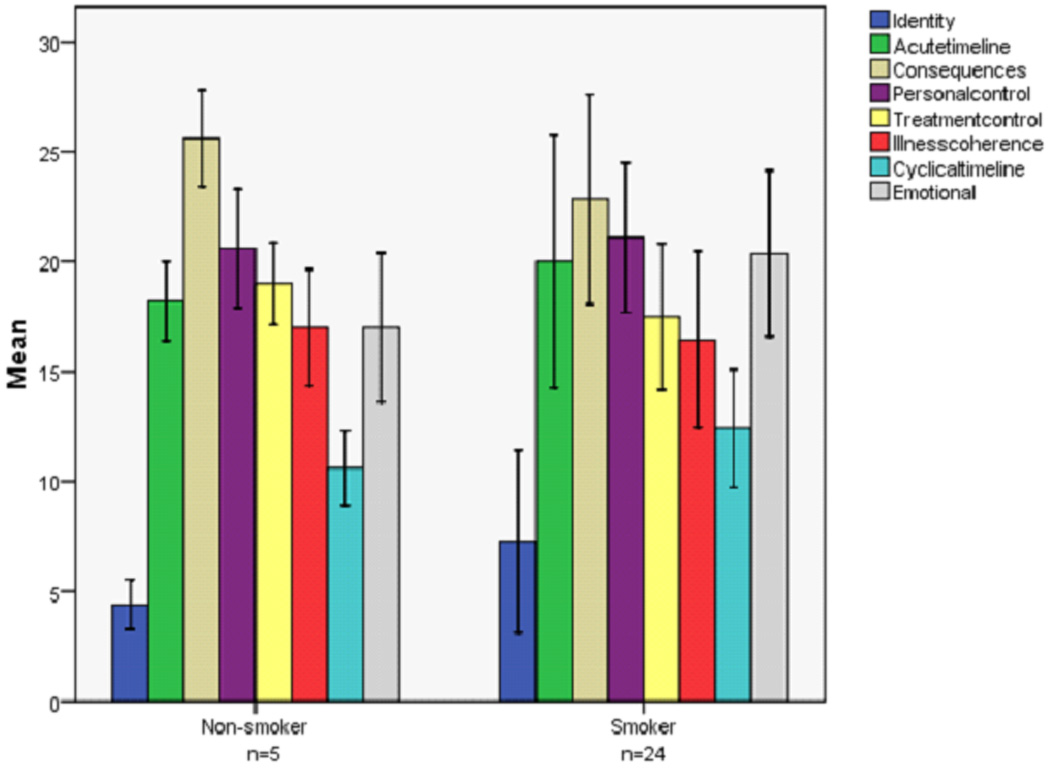
Six month mean scores (SD) of the IPQ-R attributes by smoking status as 6 months
Reason for Smoking or Quitting
At baseline, patients were asked, “What is the primary reason you have not quit smoking?”, at 6 months smokers were asked, “What is the primary reason you have not quit smoking?” and non-smokers were asked, “What is the primary reason you were successful in quitting smoking?” There was a 91.7% agreement by expert reviewers on selected patient response categories. Patient responses were organized according to corresponding SRMI constructs (see Table 5). For example, nicotine dependence and family support related responses were categorized under the SRMI construct ‘self’. ‘Quality of life’ type statements (e.g., enjoyment, fear, nervousness) were categorized under outcome appraisal. Statements that corresponded with ‘attempting to quit’ or ‘not wanting to quit’ were categorized as illness representation.
Table 5.
| SRMI Construct |
Patient Reasons for Not Quitting Smoking at Baseline |
Patient Reasons for Quitting and Continuing Smoking at 6 months |
|||||
|---|---|---|---|---|---|---|---|
| n | % | Reasons for Quitting | n | Reasons for Continuing Smoking | n | ||
| Illness representation |
don’t want to not ready interested but unsuccessful |
9 | 25.9 | lung cancer surgery, hospitalization for lung cancer treatment, started chemotherapy, cancer |
5 | don’t want to, not ready, cutting down or trying to quit, starting medication soon |
6 |
| 4 | |||||||
| 1 | |||||||
| Outcome Appraisal (quality of life) |
fear, scared, or overwhelmed enjoyment fear of weight gain calming or relaxing |
3 | 27.8 | makes cancer treatment harder, to feel better |
2 | nervous or anxiety, stress |
8 |
| 8 | |||||||
| 1 | |||||||
| 3 | |||||||
| Self | addiction or habit no will power or can’t quit depression stupid, weakness or hardheaded |
11 | 46.3 | family is supportive of quitting |
1 | addiction or habit, no will power or can’t quit, depression, family smokes |
10 |
| 10 | |||||||
| 1 | |||||||
| 3 | |||||||
| Total | 54 | ||||||
n=48
n=23
Categories were not mutually exclusive
Discussion
This is the first paper to integrate smoking behavior characteristics of lung cancer patients within the context of Self-Regulation theory, and the first to describe changes in illness representations over time, among lung cancer patients who smoke. The SRMI provided a framework to help guide our understanding of the complexity of illness representation formation as it applies to lung cancer patients who smoke. The components of illness representation, identity, acute/chronic timeline, and personal and treatment control, were found to significantly change over time. Nicotine dependence was the most frequently cited reason for smoking at study entry and at 6 months.
Changes in illness representations over time have been characterized in head and neck cancer and coronary artery bypass graft surgery patients. In these patients, illness representation findings have been useful in predicting patient quality of life, psychological distress, and return to work following a medical procedure 45–47. Changes in symptom representations as a result of psychoeducational interventions have been identified among ovarian cancer patients 25. Illness representation information from lung cancer patients that smoke could be useful in developing specific smoking cessation interventions. Understanding the context in which a patient perceives disease and smoking behavior can translate to developing specific smoking cessation interventions that contribute to successful quitting. For instance, smokers report worse quality of life than non-smokers 48, 49. If a lung cancer patient quits smoking at any point after the diagnosis, this may improve quality of life.
In this study, a significant increase in the identity attribute over time was consistent with a patient experiencing increased disease and treatment-related symptomatology. Lung cancer patients identified their symptoms to be related to their disease. The majority of patients in this study continued to smoke, which may also have contributed to increased symptoms. Interestingly, non-smokers at 6 months reported less symptoms of their lung cancer than smokers (although not able to be statistically compared).
A strong belief in the personal and treatment controllability of lung cancer was exhibited by patients at baseline. This is appropriate for patients who were actively undergoing lung cancer treatment or just had lung cancer surgery. Both attributes significantly decreased over time and could have been attributed to patient realization of the serious nature and poor prognosis of the disease. Unsuccessful attempts to quit smoking may also have decreased patient’s beliefs in the personal and treatment controllability of the disease. Furthermore, smokers reported lower personal and treatment controllability at 6 months than non-smokers (not able to statistically compare).
The significantly increased belief among patients over time that lung cancer was a chronic disease (timeline acute/chronic) suggested that at diagnosis, patients may not have understood the nature of living with a chronic disease, and over 6 months, patients had an increased understanding of the chronicity of their disease. Chronic disease refers to “living with a disease for lifetime” versus having a relative quick disease cure. Although not statistically compared, smokers reported a chronic disease belief that was stronger than non-smokers. Continued smoking behavior may have accentuated patients’ beliefs in the chronicity of their lung cancer. The increased trend (although not significant) of the cyclical nature of lung cancer was consistent with patients who had recently completed or were receiving chemotherapy. In general, chemotherapy treatment for lung cancer is given for 1–3 days during a 21 day cycle, and the symptoms experienced by the patient also follow the same cyclical pattern 50.
The patient’s consistent, strong belief that lung cancer produced negative consequences was expected, given the known toxicity of lung cancer treatment and overall poor prognosis of the disease. Illness coherence scores (understanding of illness) were relatively stable over time, perhaps suggesting that patients could benefit from further education. The decreased trend (although not significant) of the emotional representation attribute reflected a decreased emotional response to lung cancer among patients, perhaps suggesting that at 6 months, the ‘shock’ of the cancer diagnosis had ‘worn off’ and patients were more accepting of their diagnosis and/or treatment.
Reliability estimates for acute/chronic timeline, personal control, illness coherence, and emotional representation attributes all demonstrated good reliability and comparable to other reports of internal consistency scores 34. The cyclical timeline, treatment control and consequences internal consistency scores were lower than those previously reported 34.
Study limitations
A sizable portion of this sample was deceased at the 6 month time point which reduced the investigators’ ability to examine illness perception among study participants. The 6 month study endpoint was chosen because it is a reasonable amount of time to have completed initial lung cancer treatment and not too long to exclude patients with advanced stage. In addition, due to fewer participants completing the study, comparison of illness perception among early and late stage lung cancer patients was unable to be completed. Illness perception may have been influenced by type of cancer treatment the patient received (i.e., chemotherapy plus radiation may cause more symptoms than a single modality of treatment). A patient’s perception of symptoms may have been influenced by treatment or disease related symptoms rather than explicating the role of tobacco use behavior.
The study included current smokers and their natural course of behavior following a lung cancer diagnosis. Never and former smokers and recent quitters may have very different smoking behavior and illness representation characteristics.
Implications for future research
Interventions to aid the lung cancer patient in coping with lung cancer treatment-related symptoms in addition to the emotional distress that living with a lung cancer diagnosis causes, all while quitting smoking, is essential. Illness representation and ‘representation of self’ may be the most interesting attributes of the SRMI to examine in future research with smoking behavior among lung cancer patients. Understanding the context in which a patient perceives disease and smoking behavior may contribute to influencing behavior change. These characteristics deserve consideration as interventions are designed. The emotional stress and treatment-related side effects that accompany therapy must be addressed as lung cancer patients attempt to stop smoking.
Further research investigating the influence of illness representations on the lung cancer patient’s decision to quit or continue to smoke is needed. In addition, interventions that are tailored to a patient’s illness representation should be developed and tested. Early stage lung cancer patients who continue to smoke after a diagnosis represent a group who may benefit substantially from these types of studies. Well controlled multisite trials that increase patient accrual should be considered.
Conclusion
Smoking cessation after a diagnosis of lung cancer is an important health-related behavior change. Characteristics that contribute to continued smoking among lung cancer patients are not well understood. In order for oncology nurses and physicians to deliver the most effective smoking cessation interventions to lung cancer patients, more empirical research is warranted. The model proposed in this paper was constructed to illustrate the components that may underlay smoking behavior after a diagnosis of lung cancer. The model’s constructs deserve further consideration in the development of future smoking cessation interventions.
Acknowledgements
This study was funded by NIH/NINR F 31 NR008978 (Browning) and the Walther Cancer Institute, Indianapolis, IN predoctoral fellowship (Browning).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Reis LAG, Melbert D, Krapcho M, et al. [Accessed July 2, 2007];SEER cancer statistics review, 1974–2004. Available at: http://seer.cancer.gov.proxy.lib.ohio-state.edu/csr/1975_2004/
- 3.Zhou W, Heist RS, Liu G, et al. Smoking cessation before diagnosis and survival in early stage non-small cell lung cancer patients. Lung Cancer. 2006;53:375–380. doi: 10.1016/j.lungcan.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 4.Kawahara M, Ushijima S, Koama N, et al. Second primary tumours in more than 2-year disease-free survivors of small-cell lung cancer in Japan: The role of smoking cessation. Br J Cancer. 1998;78:409–412. doi: 10.1038/bjc.1998.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evangelista LS, Sarna L, Brecht ML, Padilla G, Chen J. Health perceptions and risk behaviors of lung cancer survivors. Heart Lung. 2003;32:131–139. doi: 10.1067/mhl.2003.12. [DOI] [PubMed] [Google Scholar]
- 6.Sarna L, Bialous SA, Cooley ME, Jun HJ, Feskanich D. Impact of smoking and smoking cessation on health-related quality of life in women in the nurses' health study. Qual Life Res. 2008 doi: 10.1007/s11136-008-9404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baser S, Shannon VR, Eapen GA, et al. Smoking cessation after diagnosis of lung cancer is associated with a beneficial effect on performance status. Chest. 2006;130:1784–1790. doi: 10.1378/chest.130.6.1784. [DOI] [PubMed] [Google Scholar]
- 8.Erickson M, Kondo A. Smoking cessation for cancer patients: Rationale and approaches. Health Education Research Theory & Practice. 1989;4:489–494. [Google Scholar]
- 9.Rice D, Kim H, Sabichi A, et al. The risk of second primary tumors after resection of stage I nonsmall cell lung cancer. Ann Thorac Surg. 2003;76:1001–1008. doi: 10.1016/s0003-4975(03)00821-x. [DOI] [PubMed] [Google Scholar]
- 10.Cox L, Sloan J, Patten C, et al. Smoking behavior of 226 patients with diagnosis of stage IIIA/IIIB non-small cell lung cancer. Psychooncology. 2002;11:472–478. doi: 10.1002/pon.612. [DOI] [PubMed] [Google Scholar]
- 11.Dresler CM, Bailey M, Roper CR, Patterson GA, Cooper JD. Smoking cessation and lung cancer resection. Chest. 1996;110:1199–1202. doi: 10.1378/chest.110.5.1199. [DOI] [PubMed] [Google Scholar]
- 12.Sridhar K, Raub W. Present and past smoking history and other predisposing factors in 100 lung cancer patients. Chest. 1992;101:19–25. doi: 10.1378/chest.101.1.19. [DOI] [PubMed] [Google Scholar]
- 13.Schnoll R, Malstom M, James C, et al. Correlates of tobacco use among smokers and recent quitters diagnosed with cancer. Patient Educ Couns. 2002;46:137–145. doi: 10.1016/s0738-3991(01)00157-4. [DOI] [PubMed] [Google Scholar]
- 14.Cooley ME, Sarna L, Brown JK, et al. Tobacco use in women with lung cancer. Annals of Behavioral Medicine. 2007;33:242–250. doi: 10.1007/BF02879906. [DOI] [PubMed] [Google Scholar]
- 15.Weinman JA, Petrie KJ. Introduction to the perceptions of health and illness. In: Petrie KJ, Weinman JA, editors. Perceptions of Health and Illness: Current Research and Applications. Australia: Harwood Academic Publishers; 1997. pp. 1–17. [Google Scholar]
- 16.Hagger MS, Orbell S. A meta-analytic review of common-sense model of illness representations. Psychology and Health. 2003;18:141–184. [Google Scholar]
- 17.Diefenebach MA, Leventhal H. The common-sense model of illness representation: Theoretical and practical considerations. Journal of Social Distress and the Homeless. 1996;5:11–38. [Google Scholar]
- 18.Leventhal H, Hudson S, Robitaille C. Health, coping, and well-being perspectives from social comparison theory. In: Buunk BP, Gibbons FX, editors. Social Comparison and Health: A Process Model. Mahweh, New Jersey: Lawrence Erlbaum Associates; 1997. pp. 411–432. [Google Scholar]
- 19.Petrie KJ, Weinman J, Sharpe N, Buckley J. Role of patients' view of their illness in predicting return to work and functioning after myocardial infarction: Longitudinal study. BMJ. 1996;312:1191–1194. doi: 10.1136/bmj.312.7040.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynolds NR. The problem of antiretroviral adherence: A self-regulatory model for intervention. AIDS Care. 2003;15:117–124. doi: 10.1080/0954012021000039815. [DOI] [PubMed] [Google Scholar]
- 21.Lange LJ, Piette JD. Personal models for diabetes in context and patients' health status. J Behav Med. 2006;29:239–253. doi: 10.1007/s10865-006-9049-4. [DOI] [PubMed] [Google Scholar]
- 22.Donovan HS, Ward S. Representations of fatigue in women receiving chemotherapy for gynecologic cancers. Oncol Nurs Forum. 2005;32:113–116. doi: 10.1188/05.ONF.113-116. [DOI] [PubMed] [Google Scholar]
- 23.Ward S, Donovan HS, Owen B, Grosen E, Serlin R. An individualized intervention to overcome patient-related barriers to pain management in women with gynecologic cancers. Research in Nursing and Health. 2000;23:393–405. doi: 10.1002/1098-240x(200010)23:5<393::aid-nur6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 24.Johnson JE, Lauver DR, Nail LM. Process of coping with radiation therapy. Journal of Consulting & Clinical Psychology. 1989;57:358–364. doi: 10.1037//0022-006x.57.3.358. [DOI] [PubMed] [Google Scholar]
- 25.Donovan HS, Ward S, Sherwood P, Serlin RC. Evaluation of the symptom representation questionnaire (SRQ) for assessing cancer-related symptoms. Journal of Pain and Symptom Management. 2008;35:242–257. doi: 10.1016/j.jpainsymman.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knudsen N, Schulman S, Fowler R, van den Hoek J. Why bother with stop-smoking education for lung cancer patients? Oncol Nurs Forum. 1984;11:30–33. [PubMed] [Google Scholar]
- 27.Benowitz NL. Nicotine addiction. Prim Care. 1999;26:611–631. doi: 10.1016/s0095-4543(05)70120-2. [DOI] [PubMed] [Google Scholar]
- 28.Buick DL. Illness representations and breast cancer: Coping with radiation and chemotherapy. In: Petrie KJ, Weinman JA, editors. Perceptions of Health and Illness. 1st ed. Australia: Harwood Academic Publishers; 1997. pp. 379–409. [Google Scholar]
- 29.CDC. Cigarette smoking among adults- United States. MMWR. 2007;56:1157–1161. [PubMed] [Google Scholar]
- 30.CDC. Youth tobacco surveillance- United States. MMWR. 2001;50:1–84. [PubMed] [Google Scholar]
- 31.Pierce JP, Fiore MC, Novotny TE, Hatziandreu EJ, Davis RM. Trends in cigarette smoking in the united states. educational differences are increasing. JAMA. 1989;261:56–60. [PubMed] [Google Scholar]
- 32.USDHHS. The health consequences of smoking, nicotine addiction: A report of the surgeon general. Rockville, Maryland: 1988. [Google Scholar]
- 33.Fiore MC, Jaén CRBT, et al. Treating Tobacco use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services Public Health Service; 2008. [Google Scholar]
- 34.Moss-Morris R, Weinman J, Petrie K, Horne R, Cameron L, Buick D. The revised illness perception questionnaire (IPQ-R) Psychology and Health. 2002;17:1–16. [Google Scholar]
- 35.Pomerleau CF, Carton SM, Lutzke ML, Flessland KA, Pomerleau OF. Reliability of the Fagerström tolerance questionnaire and the Fagerström test for nicotine dependence. Addict Behav. 1994;19:33–39. doi: 10.1016/0306-4603(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 36.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: A revision of the Fagerström tolerance questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 37.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond R, Swan GE. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine Tobacco Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 38.Jarvis M, Tunstall-Pedoe H, Feyerabend C, Vessy C, Saloohee Y. Comparison of tests used to distinguish smokers from nonsmokers. Am J Public Health. 1987;77:1235–1238. doi: 10.2105/ajph.77.11.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benowitz N. Pharmacological aspects of cigarette smoking and nicotine addiction. N Engl J Med. 1988;319:1318–1330. doi: 10.1056/NEJM198811173192005. [DOI] [PubMed] [Google Scholar]
- 40.Benowitz ND, Jacob P, Ahijevych K, et al. Biochemical verification of tobacco use and cessation. Nicotine Tobacco Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 41.Nunnally J, Bernstein I. Psychometric Theory. New York: McGraw-Hill, Inc; 1994. [Google Scholar]
- 42.Pagano M, Gauvreau K. Principles of Biostatistics. Australia: Duxbury Thomson Learning; 2000. [Google Scholar]
- 43.Shiffman S, Read L, Jarvik ME. Smoking relapse situations: A preliminary typology. Int J Addict. 1985;20:311–318. doi: 10.3109/10826088509044913. [DOI] [PubMed] [Google Scholar]
- 44.Gottlieb NH. The determination of smoking types: Evidence for a sociological-pharmacological continuum. Addict Behav. 1983;8:47–51. doi: 10.1016/0306-4603(83)90055-2. [DOI] [PubMed] [Google Scholar]
- 45.Hermele S, Olivo EL, Namerow P, Oz MC. Illness representations and psychological distress in patients undergoing coronary artery bypass graft surgery. Psychological Health Medicine. 2007;12:580–591. doi: 10.1080/13548500601162705. [DOI] [PubMed] [Google Scholar]
- 46.Llewellyn CD, McGurk M, Weinman J. Illness and treatment beliefs in head and neck cancer: Is Leventhal's common sense model a useful framework for determining changes in outcomes over time? Journal of Psychosomatic Research. 2007;63:17–26. doi: 10.1016/j.jpsychores.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 47.Scharloo M, Baatenburg de Jong RJ, Langeveld TPM, et al. Quality of life and illness perceptions in patients with recently diagnosed head and neck cancer. Head Neck. 2005;27:857–863. doi: 10.1002/hed.20251. [DOI] [PubMed] [Google Scholar]
- 48.Garces YI, Yang P, Parkinson J, et al. The relationship between cigarette smoking and quality of life after lung cancer diagnosis. Chest. 2004;126:1733–1741. doi: 10.1378/chest.126.6.1733. [DOI] [PubMed] [Google Scholar]
- 49.Wilson D, Parsons J, Wakefield M. The health-related quality-of-life of never smokers, ex-smokers, and light, moderate, and heavy smokers. Prev Med. 1999;29:139–144. doi: 10.1006/pmed.1999.0523. [DOI] [PubMed] [Google Scholar]
- 50.Chabner BA, Lynch TJ Jr, Longo DL, editors. Harrison's Manual of Oncology. New York: McGraw-Hill Medical Pub. Division; 2008. [Google Scholar]


