Abstract
Purpose
To investigate intracortical inhibition and facilitation in response to unilateral dominant, nondominant and bilateral biceps activation and short-term upper extremity training in right and left-handed adults.
Methods
Paired-pulse transcranial magnetic stimulation was used to measure intracortical excitability in motor dominant and nondominant cortices of 26 nondisabled adults. Neural facilitation and inhibition were measured in each hemisphere during unilateral dominant, nondominant and bilateral arm activation and after training in each condition.
Results
No differences were seen between right and left- handed subjects. Intracortical facilitation and decreased inhibition were seen in each hemisphere with unilateral activation/training of contralateral muscles and bilateral muscle activation/training. Persistent intracortical inhibition was seen in each hemisphere with ipsilateral muscle activation/training. Inhibition was greater in the nondominant hemisphere during dominant hemisphere activation (dominant arm contraction).
Conclusion
Strongly dominant individuals show no difference in intracortical responses given handedness. Intracortical activity with unilateral and bilateral arm activation and short-term training differs based on hemispheric dominance, with the motor dominant hemisphere exerting a larger inhibitory influence over the nondominant hemisphere. Bilateral activation and training has a disinhibitory effect in both dominant and nondominant hemispheres.
Keywords: motor cortex, neuronal excitability, motor control
Introduction
Upper extremity hemiparesis presents a major challenge to rehabilitation after stroke. Differences in neural control mechanisms for unilateral and bilateral upper extremity tasks are of interest because of the use of novel active bilateral and unilateral training paradigms in stroke rehabilitation. These training approaches have demonstrated a positive impact on return of paretic limb function (1–5) but little is known about the specific neural mechanisms that underlie those functional changes. In this study we investigate a particular aspect of function that is uniquely developed in the upper extremity, namely handedness. After unilateral lesion several factors related to handedness may impact functional response to upper extremity (UE) rehabilitation training. Among these are (a) differences between the dominant and nondominant hemispheres, (b) influences of interhemispheric connections during unilateral vs. bilateral tasks, and (c) possible differences in neural control mechanisms in left- vs. right-handed individuals.
The ability of the brain to adapt neural firing in relation to various unilateral muscle activations and/or training has been documented using single and paired pulsed transcranial magnetic stimulation (TMS). Increased excitability in homologous upper extremity muscles has been demonstrated in response to 50% maximal voluntary contraction (6) and with complex motor sequence tasks (7). Inhibitory modulation has also been seen in the ipsilateral cortex during tasks of increasing difficulty, indicating inhibition of muscles in the nonmoving hand. Liepert et al. reported that intracortical inhibitory and facilitory modulation is related to the selective requirements of a motor task (2). For example, muscles targeted for activation were facilitated whereas intentional relaxation increased inhibition. Taken together these studies show motor effort of one arm can impact the excitability of contralateral and ipsilateral cortex affecting homologous arm muscles as well other ipsilateral muscles. Questions remain, however, as to whether these responses differ with dominant versus nondominant hemisphere activity and furthermore if handedness impacts the degree of the response.
Tinazzi and Zanette reported the presence of hemispheric asymmetries of the ipsilateral cortex during unilateral tasks in which homologous muscle excitability was significantly less when the dominant hand was the task hand (7). This indicates greater motor influence from one hemisphere to another when the nondominant hand was moving; however, this study was limited to right hand dominant subjects. Asymmetries in neural excitability have been shown to differ based on hand dominance by Yahagi and Kasai who found that in right-handed subjects, motor evoked potential (MEP) amplitudes of the dominant hand were significantly greater than those in the nondominant hand while in left-handed subjects, there was no significant difference between the dominant and nondominant hands (8).
In summary, these studies show the presence of interhemispheric neural firing (inhibitory and facilitory) with unilateral activation, modulation of neural firing based on task and task complexity, and asymmetry of neural firing depending on hand dominance. One aspect that has not been investigated is the response of intracortical inhibition and excitation to bilateral activation and following short-term bilateral training. This is of interest for post-stroke rehabilitation where approaches to UE rehabilitation typically target the movement and use of the paretic limb. In addition, compensatory training of the nonparetic limb is often used in acute care during periods of flaccidity or when UE paresis is severe and may contribute to learned nonuse. Bilateral training incorporating the use of both limbs in therapy has also shown functional gains in patients with mild and moderate stroke severity (9–17). In this training paradigm the emphasis is on exercising the proximal muscles and targeting patients with moderate levels of impairment. In bilateral training approaches in subjects with more severe paresis that involved whole arm training (18) or training involving copying tasks with a digitized pen (19), the bilateral training has not shown a benefit over unilateral training.
In fact, there is some controversy regarding the benefit of bilateral arm training because, for example, there is evidence of brief performance improvements in the contralateral hand if the other hand has reduced somatosensory input from cutaneous anesthesia. This occurs in healthy adults (20) and in stroke patients when the intact hand is anesthetized (21) and argues against activating the non-paretic arm and hand while training the paretic arm and hand. Furthermore, there is evidence that some patients with chronic stroke and larger impairments have an abnormally high interhemispheric inhibitory drive from M1 in the contralesional hemisphere to M1 in the ipsilesional hemisphere (22). Therefore, down-regulation of activity in the ipsilateral intact motor cortex may reduce abnormal inhibition from the contralesional to the ipsilesional hemisphere suggesting that increased activity of the non-paretic arm is potentially detrimental to paretic arm and hand (23, 24) However, these studies have only involved unilateral activation. Cortical facilitation and inhibition in response to bilateral arm activation and training have not been investigated.
Examining the differences between left and right-handed subjects in terms of cortical processes, and comparing unilateral and bilateral motor task conditions, may provide a foundation for determining the underlying cortical control mechanisms and the influence of hand dominance on these mechanisms. In this initial study, we examine intracortical inhibition and excitation in healthy nondisabled adults to provide a comparison with future studies on individuals with stroke. Based on previous literature, we hypothesized that there would be a neural firing asymmetry between the dominant and nondominant hemispheres in right-handed subjects for biceps muscles, seen to a lesser extent in the left-handed. We anticipated that bilateral training would have a disinhibitory effect on intracortical inhibition when compared to unilateral training of either arm individually in both right and left-handed. We selected the biceps as our target muscle for training to extend our previous work using BATRAC (bilateral arm training with rhythmic auditory cueing) which targets more proximal muscles used in reach and return tasks.
Methods
Subjects included 26 adults with no neurological impairment or impaired arm function. Of these, 10 had strong left-hand dominance (36± 12 yrs; 4 female) and 16 had strong right-hand dominance (42±15 yrs; 6 female). Strength of hand preference was determined by scores on the Edinburgh Inventory(25), with criteria for strong preference set between +85 to +100 for right-handed and −85 to −100 for left-handed. Exclusion criteria included: 1) ambidexterity or forced change of hand dominance that precludes unilateral motor dominance, 2) metal implants in the brain or skull, 3) history of seizures, 4) pregnancy, 5) medical history of neuromuscular disease including neuropathy /myopathy affecting the arms and/or report of numbness or tingling in the arms, 6) drug use that may influence excitability threshold (antispastics, anxiolytics, hypnotics, antiepileptics), and 7) active cardiac disease by patient report. All subjects signed informed consent approved by the University of Maryland School of Medicine and Baltimore VAMC joint Internal Review Board.
Preliminary work to establish testing and training parameters
Because the main investigation targeted proximal muscles used in a novel arm training protocol, we conducted a pilot study with 3 subjects to define appropriate testing and training parameters. Specifically we identified 1) optimal interstimulus (ISI) settings for inhibition and facilitation of proximal arm muscles used in the training, 2) the necessary and sufficient training durations to elicit intracortical effects, and 3) the duration of the post-training period required for a return of MEP amplitudes to baseline values.
1. Determination of ISIs
It has been reported by some that the ideal ISI for inhibition is 2.5– 5 (26) while other studies demonstrate that ISIs of 1 ms elicit maximal inhibition (27, 28). We conducted a battery of tests with ISIs between 1 – 5 ms. The magnitudes of the conditioning and test stimuli were held constant throughout this testing. A 1ms ISI consistently elicited the largest inhibitory responses, as described in other studies, and thus was selected as the inhibitory ISI parameter. There is some debate that an ISI of 1 ms may not elicit pure neuronal inhibition because the targeted neurons are still in a refractory state. However, Roshan, Paradiso and Chen have shown that intracortical inhibition (ICI) at 1 ms cannot be fully explained by axonal refractoriness and conclude that synaptic inhibition is likely involved. ISIs of 10 – 15 ms were also compared to obtain ideal facilitory responses(29). Intracortical facilitation (ICF) from ISIs between 12 – 15 ms did not differ, but were greater than ISIs of 10 and 11 ms. Thus an ISI 15 ms was selected as the facilitory ISI parameter for the main investigation.
2. Necessary and sufficient training time
Each pilot subject participated in sessions of unilateral dominant arm training of 5, 10, and 15 minute durations to determine the necessary and sufficient training time to obtain a training effect. Inconsistent responses in MEP inhibition and facilitation were seen after 5 and 15 minute sessions. It is possible that 5 minutes was not a sufficient amount of time to achieve a training effect and that fatigue was a factor at the 15 minute training time. Because all subjects produced appropriate responses after 10 minutes of training, that interval was selected for all subsequent training conditions.
3. Post-training washout period
On a separate day each subject participated in 10 minutes of training in both unilateral dominant and bilateral training with TMS post-testing at 5, 10, 15 and 30 minutes post training. All subjects returned to baseline values for facilitory and inhibitory responses by the 10 minute testing point with no further changes at the 15 and 30 minute time points. The need to minimize testing burden precluded use of these repeated tests to establish baseline with the larger subject sample. However, since all pilot subjects achieved complete washout after 10 minutes, this recovery interval was used between training modes in the subsequent protocols.
TMS Testing: Unilateral dominant, unilateral nondominant and bilateral activations
Subjects were seated in a cushioned semi-reclining chair with elbows in 90° flexion and bipolar surface EMG electrodes spaced 1 cm apart on the belly of the biceps brachii. The ground electrode was placed on the right medial malleolous. Force transducers (Transducer Techniques, CA) were mounted on the armrests, and the subject’s arms were stabilized in cuffs attached to the armrests. The head was stabilized in a support to standardize head orientation and minimize extraneous movement.
Baseline CNS corticospinal excitation was determined using single, suprathreshold magnetic pulses delivered transcranially via a system with two magnetic stimulators connected through a Bistim device (Magstim Company, Dyfed, UK) with a figure - 8 coil. For each hemisphere the best stimulation site to elicit MEPs in the contralateral biceps was determined by stimulating cortex in 1 cm coordinates relative to Cz and marked with a felt tip pen on the subject’s head. Precise location and threshold values for eliciting MEPs were determined for each individual by convention with threshold defined as lowest intensity producing MEP amplitudes exceeding 50µV in > 5 of 10 consecutive stimulations (30). Both active and passive threshold measurements were obtained. To standardize the muscle contraction for active threshold testing, subjects used real time visual feedback to generate the force required to offset the resting weight of the forearm on the armrest. This was easily accomplished and provided a reproducible standardized low-force isometric contraction for facilitated TMS.
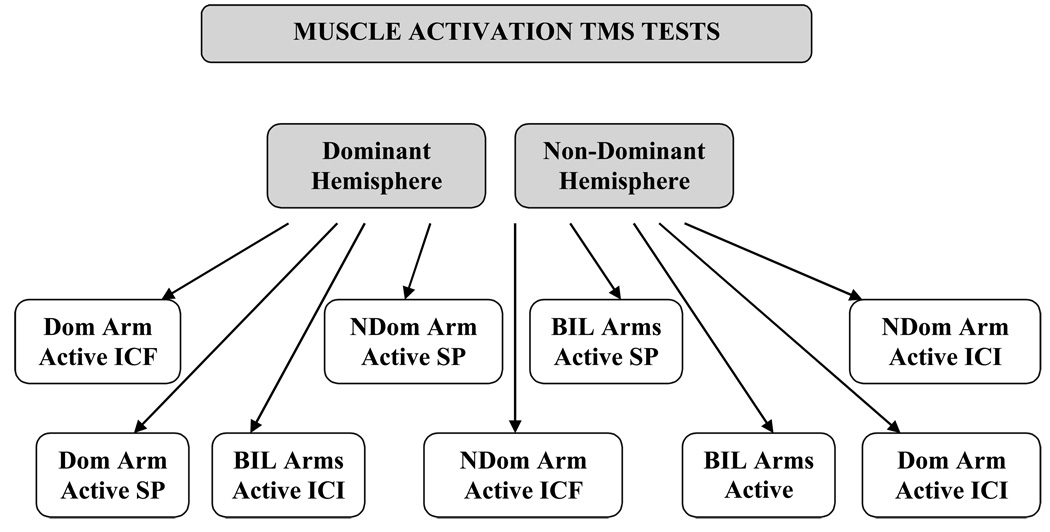
Intracortical excitability was measured using a paired-pulsed paradigm as described by Kujirai et al.(31) and Muelbacher et al.(6) In this TMS paradigm a subthreshold conditioning stimulus (CS-80% active motor threshold) was followed by a suprathreshold test–stimulus (TS) at different ISIs. The intensity of the TS was 120 % of the threshold. Active threshold was used for all tests in which the contralateral biceps was activated (e.g. dominant hemisphere testing with dominant arm or bilateral arm activation). Although, ICI and ICF testing is usually done without muscle activation, Kujari et al. report that ICI and ICF can still be seen with muscle activation though it may be suppressed (31) We chose to use an active threshold because we anticipated taking this paradigm to a stroke population where an active threshold would likely be necessary for eliciting MEPS particularly for proximal biceps muscle. The TS was 120% of passive threshold for all tests in which the contralateral biceps were inactive (e.g. dominant hemisphere testing with nondominant arm active). As previously described, ISIs of 1 (ICI) and 15 (ICF) ms were utilized to elicit inhibitory and facilitory responses respectively (31). A trial consisted of 5 stimulations for each ISI setting and the single pulse suprathreshold stimulus. The inter-trial interval was set at 5 seconds. Single pulse recording ( to obtain control MEP) and paired-pulsed recording (at ISI 1 and 15) were completed for each hemisphere during low force activation of the target muscle in each of the following conditions; 1) contralateral biceps contraction, 2) ipsilateral biceps contraction and, 3) bilateral biceps contraction. The order of the ISI (1 ms or 15 ms) and the single pulse (control MEP stimulus) was randomized to control for order effect. The order of hemispheres tested was counter-balanced across subjects. See Figure 1 for clarification of the arm activation conditions schedule.
Fig. 1.
Schematic of TMS testing under unilateral dominant, unilateral non-dominant and bilateral muscle activation conditions. Testing of each hemisphere was counterbalanced but the order of SP, ICI and ICF stimulations were completely crossed and randomized with the three biceps activation conditions.
Short-term Training
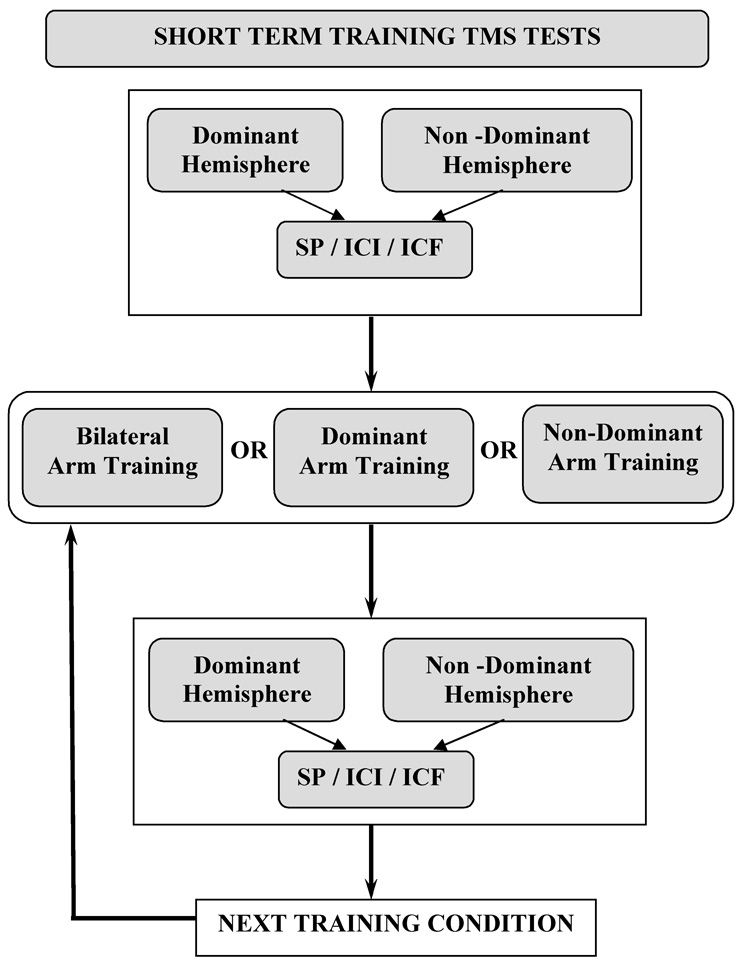
Short-term training consisted of 3 separate 10-minute bouts of arm exercise using a device employed for bilateral arm training with auditory cueing (BATRAC see Whitall et al., (4) for details of the device) immediately followed by repeat paired-pulse TMS testing. All subjects completed the training timing their movements to a metronome set at the subject’s preferred rate of the first training condition. This same rate of movement was maintained for all 3 training sessions to ensure dose matching across training. A rest period was given between testing and the subsequent training sessions. This resulted in a 10- minute break between the end of one training session and the beginning of the next to avoid after affects. During training, the subject remained positioned in the testing chair and was brought to an upright position and asked to complete the 10-minute training exercise. Subjects were asked to move at a preferred speed, paced with an auditory cue, for all training sessions. All EMG electrodes remained attached and were monitored during training to ensure biceps activity during the training period. The training conditions were as follows: 1) repetitive reach and return movement of the dominant arm, 2) repetitive reach and return movement of the nondominant arm, 3) repetitive reach and return movement of both arms simultaneously (inphase movement). The order of the training conditions was randomized across subjects to avoid an order effect.
Post training testing
Post training tests occurred immediately following each of the training bouts. The subject was returned to the semi-reclined position. The location of stimulation was the same location scouted and determined to be the hot spot during baseline testing. Post testing consisted of the stimulation at 120 % active threshold with the single pulse TMS and the intracortical excitability testing (at ISIs 1 and 15 ms). Subjects were again instructed to contract sufficiently to overcome the weight of the arm as done prior to training. Each hemisphere received 5 stimulations for each single-pulse stimulation at 120% and paired –pulse stimulation at ISI 1 ms and 15 ms in the same order randomly assigned at the start of training. The exact order of all tests remained constant for pre and post training tests for a given subject. The hemisphere tested first, alternated from one subject to the next. See Figure 2 for clarification of the protocol for the training conditions.
Fig. 2.
Schematic of TMS testing before and after short-term unilateral dominant, unilateral non-dominant and bilateral training. Testing of each hemisphere was counterbalanced and the order of training conditions randomized. The order of SP, ICI and ICF was randomized across subjects for the pre-test and then maintained in the same order for a given subject across the remaining testing conditions.
Data reduction and analysis
EMG data were collected, amplified at a gain of 1000 and filtered using a band pass filter set between 30 and 1000 HZ (Bioamp, James Long Company, Caroga Lake, NY). Using data from the unconditioned MEP, the stimulus intensity, thresholds, locations required to elicit the MEP, and MEP amplitudes (peak-to-peak in millivolts) were quantified. The mean MEP amplitude after single unconditioned stimulations during each activation condition was defined as 100% for that condition. MEP amplitudes after paired pulse stimulation were expressed as a percentage of the unconditioned MEP amplitude. The paired-pulse MEP percentages were then compared for each interstimulus interval under each testing condition (unilateral dominant, unilateral nondominant, bilateral biceps activation) and before and after each training condition (unilateral dominant, unilateral nondominant and bilateral training) to generate profiles of ICI and ICF responses.
Baseline data were initially analyzed using separate 3- way ANOVA 2 (dominance) × 2 (hemisphere) × 3 (muscle activation) with repeated measures on the last two factors for inhibitory and facilitory responses. A separate 3-way ANOVA 2 (dominance) × 2 (hemisphere) × 4 (pre and 3 post training conditions) with repeated measures on the last two factors was used for inhibitory and facilitory responses on pre- post-training data. No significant effects or interactions were found as a result of hand dominance (p> .51). Data were then pooled for comparisons across handedness for both baseline muscle activation conditions and post training conditions and were subsequently analyzed using separate 2- way repeated measures ANOVA 2 (hemisphere) × 3 (muscle activation) and 2 (hemisphere) × 4 (training condition) for inhibition and facilitation. The adjusted Tukey test was used for post hoc comparisons. Two- way analyses of covariance (ANCOVA), second effect repeated (hemisphere), were used to determine if potential variance in intergroup baseline measures influenced the post training results.
Results
Comparison of Arm Activation Conditions
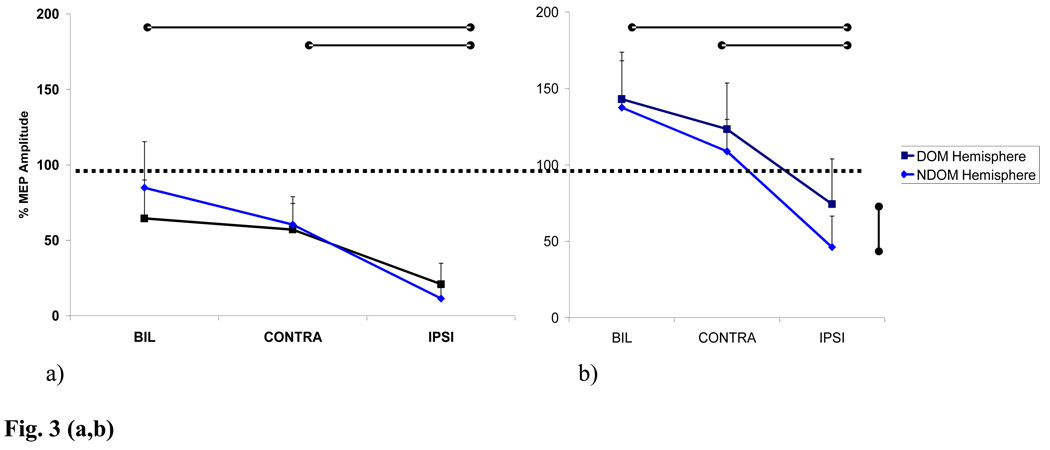
For inhibitory responses there were main effects for muscle activation (F = 51.7, p <.0001), hemisphere (F = 6.4, p < .01) and an interaction effect (F = 72.6, p <.0001). For facilitory responses there were main effects for muscle activation (F = 56.5, p < .0001), hemisphere (F = 6.2, p < .04) and an interaction effect (F = 83.4, p < .0001). Figure 3 illustrates the mean percentages of control MEP amplitude (MEP ratio) and the standard deviations for both inhibitory (a) and facilitatory (b) responses during each activation condition for both dominant (DH) and nondominant hemisphere (NDH) testing. There were no significant differences in the ICI or ICF in both NDH and DH with contralateral or bilateral biceps activation. During ipsilateral biceps activation the MEP ratios for both NDH and DH were significantly diminished for both ICI ( p<.0001) and ICF (p<.0001). Furthermore the suppression of the MEP ratio was significantly greater in the ICF responses in the NDH during dominant biceps contraction compared to those in the DH during nondominant biceps contraction (p<. 004). This same trend was seen in the ICI responses but did not reach significance (p< .07).
Fig. 3.
Inhibitory (a) and Facilitory(b) responses for dominant and nondominant hemispheres during each muscle activation condition ( mean % of single pulse MEP, and SD). Control/ single pulse MEP indicated by dashed line. CONTRA for dominant hemisphere = dominant biceps activation, for nondominant hemisphere = nondominant biceps activation, IPSI for dominant hemisphere = non-dominant biceps activation; for non-dominant hemisphere = dominant biceps activation
Post Training Comparisons
Table 1 shows the mean single pulse (or control MEP) average amplitude for each hemisphere at baseline and across each training conditions. These MEP averages were used to derive the inhibitory and facilitory ratios in the study. There were no significant differences among the mean single pulse MEP amplitudes at baseline or across training bouts Indicating that corticospinal excitability was stable across conditions.
TABLE 1.
Mean Amplitudes for Single Pulse MEPs across Training Conditions for Each Hemisphere
| Nondominant Hemisphere ( nondominant biceps tested) | |||
| Baseline | Post NDOM biceps Training | Post DOM biceps training | Post BIL biceps training |
| 1.645±.845 | 1.747±.830 | 1.656±.594 | 1.740±.789 |
| Dominate Hemisphere ( dominant biceps tested) | |||
| Baseline | Post DOM biceps Training | Post NDOM biceps training | Post BIL biceps training |
| 1.723±.743 | 1.783±.695 | 1.604±.592 | 1.758±.703 |
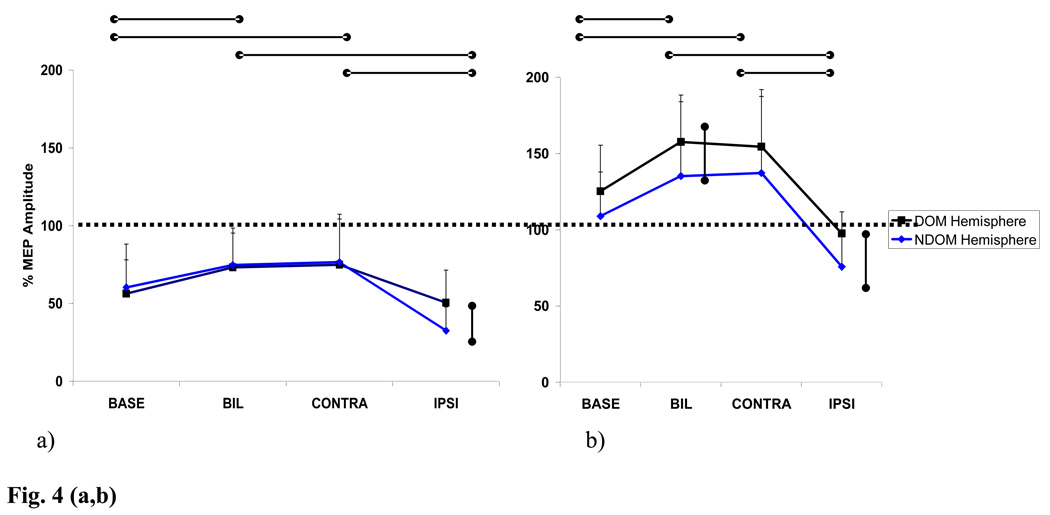
For inhibitory responses there was a main effect for training group (F = 27.1, p < .0001), but not hemisphere (F = 18.6, p < .08) and an interaction effect (F = 33.0, p <.0001). For facilitory responses there were main effects for training group (F = 16.4, p < .0001), hemisphere (F = 30.1, p < .0001) and an interaction effect (F = 30.0, p < .0001). Figure 4 illustrates the mean percentages of control MEP amplitude (MEP ratio) and the standard deviations for both inhibitory (a) and facilitatory (b) responses after each training session for both dominant (DH) and nondominant hemisphere (NDH) testing. A suppression or disinhibition in ICI responses (a) from baseline as well as a significant increase of the ICF responses ( b) after bilateral and contralateral arm training were seen in both NDH and DH. Following ipsilateral biceps training, there was an increase in ICI (a) and a decrease in ICF (b) in both NDH and DH which was not significantly different from baseline responses but significantly different from responses seen after contralateral and bilateral training. Additionally, there was a significant difference between the effects seen on ICI and ICF after ipsilateral training between the NDH and DH. Greater inhibition and suppressed facilitation was seen in the NDH (ICI: p<. 05; ICF: p< .01) suggesting a differential inhibition from the DH on the NDH. This differential effect was also seen after bilateral training for ICF only (p<.01), however both hemispheres still demonstrated a significant facilitory training effect from baseline.
Fig. 4.
Post Training Inhibitory (a) and Facilitory (b) responses for the dominant and nondominant hemisphere for each training condition. Control / single pulse MEP indicated by the dashed line. CONTRA training for dominant hemisphere = dominant arm training, IPSI = nondominant arm training. CONTRA training for nondominant hemisphere = non dominant arm training, IPSI =dominant arm training.
Discussion
In this study we examined the neural ICI and ICF responses to unilateral dominant, unilateral nondominant and bilateral biceps muscle activation and training in the motor dominant and nondominant hemispheres of healthy left and right hand dominant subjects using paired-pulsed TMS. No differences were seen in the responses based on hand dominance at baseline or post training. After pooling data from all subjects, comparison at baseline of unilateral dominant, unilateral nondominant and bilateral biceps activation showed persistent inhibition and reduced facilitation in both the DH and NDH during ipsilateral arm activation (contralateral hemispheric activation). Furthermore, the DH activation had a greater neural suppression effect on the NDH than vice versa. Neural response with bilateral arm activation had a “disinhibitory” affect in comparison to ipsilateral arm activation in both DH and NDH. Post training results showed a similar pattern of adaptive changes to those seen during the muscle activation conditions..
In terms of the original hypotheses, the lack of handedness effect was surprising given previous studies. Yahagi and Kasai reported differences in MEP amplitudes induced with motor imagery in the distal muscles between right and left handed adults (8). Right- handed adults demonstrated greater neural asymmetry between dominant and nondominant hemispheres compared to left handed adults. Similarly Netz et al. (32) found that in right handed adults the inhibition after stimulation of the dominant hemisphere was greater than that seen in the nondominant hemisphere; however in left handed subjects this marked asymmetry in inhibition was not seen. Civardi et al.(33)demonstrated asymmetry between dominant and nondominant hemispheres in right, but not in left-handed subjects. Their left- handed group however, contained only 6 subjects and all were not strongly left-handed which may explain the lack of similar asymmetry in this group. Recently, Ilic et al. reported an asymmetry in motor threshold and short latency intracortical inhibition between dominant and nondominant hemispheres in right but not left-handers (34). However, the laterality index of the left-handers was much lower (68.7±3.9) compared to the right-handers (92.5±2.4). In our study we selected only subjects who were “strongly” left or right handed with little to no ability to use their nondominant hand functionally in traditionally dominant hand functions. We speculate that the strong laterality of our left handed could explain the lack of differential response seen in our group of subjects. Another possibility is that our study compared proximal muscles while previous studies showing differential effects between left and right handed subjects investigated distal muscles. Distal muscles may be more likely to show a difference in dominant versus nondominant function and in turn neural activation because there is a predominant contralateral neural innervation found in distal musculature compared to bilateral pathways that project to more proximal musculature (35).
Our results for the baseline muscle activation are consistent with previous studies showing facilitation and disinhibition in the active cortex (2, 36). An extension to these earlier studies is the inclusion of a bilateral activation of the biceps indicating a disinhibitory effect of bilateral activation compared to ipsilateral activation in which persistent inhibition was exhibited. To our knowledge, this is the first demonstration of the neural intracortical inhibitory and facilitory effects during bilateral task activation although Marchand-Pauvert et al., report asymmetrical MEP suppression on the dominant side with peripheral stimulation not found during a bilateral contraction (37). The persistent inhibition and suppression of facilitation in each hemisphere during ipsilateral biceps activation is consistent with findings of Liepert et al., in which non-active muscles in the same hand underwent inhibition. In our results non-active homologous muscles in the opposite limb showed MEP inhibition as well as suppression of facilitation (2). These findings contradict those of Muellbacher et al., who found that contraction of a distal hand muscle facilitated the MEP response (decreased cortical inhibition) in the contralateral homologous muscle(6) In their study, however, the contraction intensity was > 50 % of maximal voluntary contraction and in contrast to Liepert et al , they demonstrated changes in F-wave responses indicating changes in spinal level activity(2). In fact, Muellbacher et al. report that at that intensity of contraction, the homologous muscle did not remain at rest, which could also have influenced the facilitation response(6). In our study, as in Liepert et al., the force of contraction is minimal and may not invoke such spinal excitatory paths(2).
Similarly, Lewis and Perrault (38) report increased excitability to passive paretic biceps with activation of the ipsilateral nonparetic biceps when compared to rest stimulation intensities of 100 and 120% of resting threshold. In this single pulse TMS study, motor evoked potentials were measured for the resting biceps at 80% , 100% and 120% of the resting threshold. Given that they were able to elicit MEPs at 80% of resting threshold, the stimulus intensity used in their study may have been much larger than ours. It is possible that at a certain force level of contraction or stimulus intensity, excitatory pathways at the level of the spinal cord are recruited resulting in facilitation.
Our results are more consistent with activation of intrinsic inhibitory pathways related to interhemispheric GABAergic connections previously reported in a number of animal and human studies(39–42) Based on the work of Kujirai et al.(31), Rothwell et al.(43), and Liepert et al.(2) who have reported ICF and ICI changes to similar TMS protocols with no change in F waves, we suggest that our results are likely representing intracortical neural activity but we cannot absolutely rule out influence of spinal level changes.
Post training results after contralateral and bilateral arm training showed practice dependent plasticity as previously demonstrated by Liepert et al.(3), in a short term contralateral training paradigm using the distal hand muscles. In this study, muscles that were used repetitively also demonstrated decreased inhibition and increased facilitation. In addition to our use of proximal muscles, the novel finding is that bilateral training had a disinhibitory effect on the ipsilateral cortex particular in comparison to unilateral arm training with the ipsilateral arm. The inhibitory effects seen in both DH and NDH with training of the ipsilateral arm are not present when both arms are moving indicating differing mechanisms of neural control between unilateral and bilateral movements.
Our results of a differential inhibition seen with DH activation influencing the NDH in both our baseline and post training results are consistent with previous findings of Ziemann and Hallet (44). Using single pulse TMS they found that the left motor cortex exerts more effective inhibitory control over the right motor cortex than vice versa in healthy right-handed subjects. They suggested that this hemispheric asymmetry is one property of motor dominance of the left motor cortex. Similarly, Ilic et al., found that the motor dominant cortex was controlled by less inhibitory tone than was the motor nondominant cortex in right-handed subjects only (34). We found the differential inhibition to be present for both left and right-handed adults suggesting that in strongly dominant subjects similar mechanisms of neural control are present. In fact the strength of dominance may actually be a contributing factor for the significant differences in the level of interhemispheric inhibitory influence. Two studies support this assertion. Using function magnetic resonance imaging, Dassonville et al., demonstrated a linear relationship between the degree of handedness and the amount of contralateral hemispheric activation in both left and right-handed healthy subjects (45). Triggs et al., found a lower MEP threshold for one hand in both right and left- handed subjects to be strongly associated with greater ability with that hand (46). These studies suggest that asymmetries between dominant and nondominant hemispheres may be a function of the degree of hand dominance, which would support our rationale for lack of dominance effect in our study. In addition, our study showed that both ICI and ICF processes appear to be affected by this motor dominance asymmetry. Since ICI and ICF processes have been shown to function independently (2) this result is not predictable a priori.
Our results have potential implications for rehabilitation particularly in patients with unilateral brain lesions if similar neural mechanisms of motor control are retained after lesion. For example, training of the paretic limb alone, as proposed in the Constraint Induced Training methods (1, 47–50, 51) , would decrease the ICI and increase the ICF in the lesioned hemisphere both of which might be beneficial for use dependent plasticity. Compensatory training of the nonparetic arm may be detrimental to lesioned hemisphere plasticity since training of the limb ipsilateral to the lesion would increase ICI and decrease ICF to this hemisphere. This could potentially explain the phenomenon of learned nonuse from a neural perspective and is consistent with the findings of Murase(21) and Floel (22) On the other hand, bilateral training has the advantage of involving both limbs in the therapeutic approach with a disinhibition of ICI and an increase in ICF in both hemispheres. Theoretically, neural plasticity might be elicited in both hemispheres. In addition, the differential inhibitory effect of DH activation on the NDH may play a role in responsiveness to a particular treatment approach. In our previous work we found a response advantage to bilateral training in those patients with lesions of the motor dominant hemisphere {McCombe Waller, 2005 #164) (10). This group may have responded to bilateral training to a greater extent due to the loss of inhibition from the dominant hemisphere. These applications to treatment are at this point hypothetical, as one cannot assume the same responses would be present in a subject with a brain lesion. Specific investigation of ICI and ICF with unilateral and bilateral training in this population is warranted.
Conclusions
In individuals with strong hand dominance our data suggest that there are no differences in the neural mechanisms between those who are right and left handed. Bilateral activation and training has a disinhibitory and facilitory affect in both dominant and nondominant hemispheres to the same extent as contralateral limb activation and after short- term training. Ipsilateral arm activation and training however, result in persistent intracortical inhibition likely through interhemispheric inhibition with the dominant cortex having a greater inhibitory affect on the nondominant cortex than vice versa. Our findings may have future relevance in the development of rehabilitation strategies for patients with upper extremity paresis. Further research is needed to compare the benefit of unilateral versus bilateral arm training in individuals with unilateral hemispheric lesion to determine if subjects with particular lesions (motor dominant versus motor nondominant) may benefit from one approach versus the other.
Acknowledgements
This study was funded by a Claude D. Pepper Center Pilot Grant awarded to the first author (National Institute on Aging, Claude D. Pepper Older Americans Independence Center; #P60AG 12583). Support was also provided by a Veterans Affairs Rehabilitation Research & Development Career Development Award (#B3390K) to the second author. We would like to thank our participants for their time and wish to recognize Doug Savin, Jennifer Sulin-Stair, and Wei Lui for their assistance in data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sandy McCombe Waller, University of Maryland, School of Medicine, Department of Physical Therapy and Rehabilitation Science, 100 Penn Street, Baltimore, MD 21201.
Larry Forrester, University of Maryland, School of Medicine, Department of Physical Therapy and Rehabilitation Science and VA Maryland Healthcare System, 100 Penn Street, Baltimore, MD 21201.
Federico Villagra, University of Maryland, School of Medicine, Department of Physical Therapy and Rehabilitation Science, 100 Penn Street, Baltimore, MD 21201.
Jill Whitall, University of Maryland, School of Medicine, Department of Physical Therapy and Rehabilitation Science, 100 Penn Street, Baltimore, MD 21201.
References
- 1.Wolf S, Lecraw D, Barton L, Jann B. Forced Use of Hemiplegic Extremities to Reverse the Effect of Learned Nonuse among Chronic Stroke and Head Injured Patients. Experimental Neurology. 1989;104:125–132. doi: 10.1016/s0014-4886(89)80005-6. [DOI] [PubMed] [Google Scholar]
- 2.Liepert J, Classen J, Cohen L, Hallett M. Task-dependent changes of intracortical inhibition. Experimental Brain Research. 1998;118:421–426. doi: 10.1007/s002210050296. [DOI] [PubMed] [Google Scholar]
- 3.Liepert J, Miltner WHR, Bauder H, Sommer M, Dettmers C, Taub E, et al. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neuroscience Letters. 1998;250:5–8. doi: 10.1016/s0304-3940(98)00386-3. [DOI] [PubMed] [Google Scholar]
- 4.Whitall J, McCombe Waller S, Silver KH, Macko RF. Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke. 2000;31(10):2390–2395. doi: 10.1161/01.str.31.10.2390. [DOI] [PubMed] [Google Scholar]
- 5.Luft AR, McCombe-Waller S, Whitall J, Forrester LW, Macko R, Sorkin JD, et al. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. Jama. 2004;292(15):1853–1861. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muellbacher W, Facchini S, Boroojerdi B, Hallett M. Changes in motor cortex excitability during ipsilateral hand muscle activation in humans. Clinical Neurophysiology. 2000;111:344–349. doi: 10.1016/s1388-2457(99)00243-6. [DOI] [PubMed] [Google Scholar]
- 7.Tinazzi M, Zanette G. Modulation of ipsilateral motor cortex in man during unimanual finger movements of differenct complexities. Neuroscience Letters. 1998;244:121–124. doi: 10.1016/s0304-3940(98)00150-5. [DOI] [PubMed] [Google Scholar]
- 8.Yahagi S, Tatsuya K. Motor evoked potentials induced by motor imagery reveal a functional asymmetry of cortical motor control in left-and right-handed human subjects. Neuroscience Letters. 1999;276:185–188. doi: 10.1016/s0304-3940(99)00823-x. [DOI] [PubMed] [Google Scholar]
- 9.McCombe Waller S, Whitall J. Fine motor control in adults with and without chronic hemiparesis: baseline comparison to nondisabled adults and effects of bilateral arm training. Arch Phys Med Rehabil. 2004;85(7):1076–1083. doi: 10.1016/j.apmr.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 10.McCombe Waller S, Whitall J. Hand dominance and side of stroke affect rehabilitation in chronic stroke. Clin Rehabil. 2005;19(5):544–551. doi: 10.1191/0269215505cr829oa. [DOI] [PubMed] [Google Scholar]
- 11.Cauraugh JH, Kim S. Two coupled motor recovery protocols are better than one: electromyogram-triggered neuromuscular stimulation and bilateral movements. Stroke. 2002;33(6):1589–1594. doi: 10.1161/01.str.0000016926.77114.a6. [DOI] [PubMed] [Google Scholar]
- 12.Cauraugh JH, Kim SB. Stroke motor recovery: active neuromuscular stimulation and repetitive practice schedules. J Neurol Neurosurg Psychiatry. 2003;74(11):1562–1566. doi: 10.1136/jnnp.74.11.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cauraugh JH. Coupled rehabilitation protocols and neural plasticity: upper extremity improvements in chronic hemiparesis. Restor Neurol Neurosci. 2004;22(3–5):337–347. [PubMed] [Google Scholar]
- 14.Cauraugh JH, Kim SB, Duley A. Coupled bilateral movements and active neuromuscular stimulation: intralimb transfer evidence during bimanual aiming. Neurosci Lett. 2005;382(1–2):39–44. doi: 10.1016/j.neulet.2005.02.060. [DOI] [PubMed] [Google Scholar]
- 15.Hesse S, Werner C, Pohl M, Rueckriem S, Mehrholz J, Lingnau ML. Computerized arm training improves the motor control of the severely affected arm after stroke: a single-blinded randomized trial in two centers. Stroke. 2005;36(9):1960–1966. doi: 10.1161/01.STR.0000177865.37334.ce. [DOI] [PubMed] [Google Scholar]
- 16.Mudie MH, Matyas TA. Can simultaneous bilateral movement involve the undamaged hemisphere in reconstruction of neural networks damaged by stroke? Disabil Rehabil. 2000;22(1–2):23–37. doi: 10.1080/096382800297097. [DOI] [PubMed] [Google Scholar]
- 17.Summers JJ, Kagerer FA, Garry MI, Hiraga CY, Loftus A, Cauraugh JH. Bilateral and unilateral movement training on upper limb function in chronic stroke patients: A TMS study. J Neurol Sci. 2007;252(1):76–82. doi: 10.1016/j.jns.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Lewis GN, Byblow WD. Neurophysiological and behavioural adaptations to a bilateral training intervention in individuals following stroke. Clin Rehabil. 2004;18(1):48–59. doi: 10.1191/0269215504cr701oa. [DOI] [PubMed] [Google Scholar]
- 19.Tijs E, Matyas TA. Bilateral training does not facilitate performance of copying tasks in poststroke hemiplegia. Neurorehabil Neural Repair. 2006;20(4):473–483. doi: 10.1177/1545968306287900. [DOI] [PubMed] [Google Scholar]
- 20.Werhahn KJ, Mortensen J, Kaelin-Lang A, Boroojerdi B, Cohen LG. Cortical excitability changes induced by deafferentation of the contralateral hemisphere. Brain. 2002;125(Pt 6):1402–1413. doi: 10.1093/brain/awf140. [DOI] [PubMed] [Google Scholar]
- 21.Floel A, Nagorsen U, Werhahn KJ, Ravindran S, Birbaumer N, Knecht S, et al. Influence of somatosensory input on motor function in patients with chronic stroke. Ann Neurol. 2004;56(2):206–212. doi: 10.1002/ana.20170. [DOI] [PubMed] [Google Scholar]
- 22.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55(3):400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- 23.Floel A, Cohen LG. Strategies in motor stroke rehabilitation. Stroke. 2005;36(3):530. doi: 10.1161/01.str.0000154867.01422.76. author reply 530. [DOI] [PubMed] [Google Scholar]
- 24.Ward N, Brown M, Thompson AJ, Frackowiak R. The influence of time after stroke on brain activations during a motor task. Ann. Neurol. 2004;55:829–834. doi: 10.1002/ana.20099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 26.Chen R, Tam A, Butefisch C, Corwell B, Ziemann U, Rothwell JC, et al. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol. 1998;80(6):2870–2881. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- 27.Maeda F, Gangitano M, Thall M, Pascual-Leone A. Inter-and intra-individual variability of paired-pulse curves with transcranial magnetic stimulation (TMS) Clin Neurophysiol. 2002;113(3):376–382. doi: 10.1016/s1388-2457(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 28.Nordstrom MA, Butler SL. Reduced intracortical inhibition and facilitation of corticospinal neurons in musicians. Exp Brain Res. 2002;144(3):336–342. doi: 10.1007/s00221-002-1051-7. [DOI] [PubMed] [Google Scholar]
- 29.Roshan L, Paradiso GO, Chen R. Two phases of short-interval intracortical inhibition. Exp Brain Res. 2003;151(3):330–337. doi: 10.1007/s00221-003-1502-9. [DOI] [PubMed] [Google Scholar]
- 30.Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91(2):79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 31.Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Netz J, Lammers T, Homberg V. Reorganization and motor output in the non-affected hemisphere after stroke. Brain. 1997;120:1579–1586. doi: 10.1093/brain/120.9.1579. [DOI] [PubMed] [Google Scholar]
- 33.Civardi C, Cavalli A, Naldi P, Varrasi C, Cantello R. Hemispheric asymmetries of cortico-cortical connections in human hand motor areas. Clinical Neurophysiology. 2000;111:624–629. doi: 10.1016/s1388-2457(99)00301-6. [DOI] [PubMed] [Google Scholar]
- 34.Ilic TV, Jung P, Ziemann U. Subtle hemispheric asymmetry of motor cortical inhibitory tone. Clin Neurophysiol. 2004;115(2):330–340. doi: 10.1016/j.clinph.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 35.Kuypers H. Handbook of Physiology, Section I: Nervous System. In: Brooks V, editor. Handbook of Physiology. Bethesda: American Physiological Society; 1981. pp. 597–666. [Google Scholar]
- 36.Matsunaga K, Uozumi T, Tsuji S, Murai Y. Age-dependent changes in physiological threshold asymmetries for the motor evoked potential and silent period following transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1998;109(6):502–507. doi: 10.1016/s1388-2457(98)00020-0. [DOI] [PubMed] [Google Scholar]
- 37.Marchand-Pauvert V, Mazevet D, Pierrot-Deseilligny E, Pol S, Pradat-Diehl P. Handedness-related asymmetry in transmission in a system of human cervical premotoneurones. Exp Brain Res. 1999;125(3):323–334. doi: 10.1007/s002210050688. [DOI] [PubMed] [Google Scholar]
- 38.Lewis GN, Perreault EJ. Side of lesion influences bilateral activation in chronic, post-stroke hemiparesis. Clin Neurophysiol. 2007;118(9):2050–2062. doi: 10.1016/j.clinph.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 39.Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991;251(4996):944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- 40.Keller A. Intrinsic synaptic organization of the motor cortex. Cereb Cortex. 1993;3(5):430–441. doi: 10.1093/cercor/3.5.430. [DOI] [PubMed] [Google Scholar]
- 41.Matsumura M, Sawaguchi T, Kubota K. GABAergic inhibition of neuronal activity in the primate motor and premotor cortex during voluntary movement. J Neurophysiol. 1992;68(3):692–702. doi: 10.1152/jn.1992.68.3.692. [DOI] [PubMed] [Google Scholar]
- 42.Ziemann U, Lonnecker S, Paulus W. Inhibition of human motor cortex by ethanol. A transcranial magnetic stimulation study. Brain. 1995;118(Pt 6):1437–1446. doi: 10.1093/brain/118.6.1437. [DOI] [PubMed] [Google Scholar]
- 43.Rothwell JC, Thompson PD, Day BL, Boyd S, Marsden CD. Stimulation of the human motor cortex through the scalp. Exp Physiol. 1991;76(2):159–200. doi: 10.1113/expphysiol.1991.sp003485. [DOI] [PubMed] [Google Scholar]
- 44.Ziemann U, Hallett M. Hemispheric asymmetry of ipsilateral motor cortex activation during unimanual motor tasks: further evidence for motor dominance. Clinical Neurophysiology. 2001;112:107–113. doi: 10.1016/s1388-2457(00)00502-2. [DOI] [PubMed] [Google Scholar]
- 45.Dassonville P, Lewis SM, Zhu XH, Ugurbil K, Kim SG, Ashe J. Effects of movement predictability on cortical motor activation. Neurosci Res. 1998;32(1):65–74. doi: 10.1016/s0168-0102(98)00064-9. [DOI] [PubMed] [Google Scholar]
- 46.Triggs WJ, Calvanio R, Levine M. Transcranial magnetic stimulation reveals a hemispheric asymmetry correlate of intermanual differences in motor performance. Neuropsychologia. 1997;35(10):1355–1363. doi: 10.1016/s0028-3932(97)00077-8. [DOI] [PubMed] [Google Scholar]
- 47.Taub E, Wolf SW. Constraint Induced Movement Techniques to Facilitate Upper Extremity Use in Stroke Patients. Top Stroke Rehabilitation. 1997;3:38–61. doi: 10.1080/10749357.1997.11754128. [DOI] [PubMed] [Google Scholar]
- 48.Taub E, Crago JE, Uswatte G. Constraint-induced movement therapy: a new approach to treatment in physical rehabilitation. Rehabil Psychol. 1998;43:152–170. [Google Scholar]
- 49.Taub E, Uswatte G, Pidikiti R. Constraint-Induced Movement Therapy: a new family of techniques with broad application to physical rehabilitation--a clinical review. J Rehabil Res Dev. 1999;36(3):237–251. [PubMed] [Google Scholar]
- 50.Taub E, Uswatte G, King DK, Morris D, Crago JE, Chatterjee A. A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke. 2006;37(4):1045–1049. doi: 10.1161/01.STR.0000206463.66461.97. [DOI] [PubMed] [Google Scholar]
- 51.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. Jama. 2006;296(17):2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]