Diabetes mellitus and its associated complications are major health problems in the developed world. Diabetes mellitus is associated with an increased risk of cardiovascular disease (CVD) even in the presence of intensive glycemic control. Indeed, 75% of diabetic patients will die of CVD. Patients with type 1 and type 2 diabetes mellitus have an increased risk for the 3 major types of macrovascular disease (coronary heart disease, peripheral vascular disease, and stroke).1 A striking feature of diabetic cardiovascular complications is the appearance of accelerated atherosclerosis, which anatomically resembles atherosclerosis in nondiabetic individuals but is more extensive and occurs at an earlier age. Substantial clinical and experimental evidence suggests that endothelial dysfunction is a crucial early step in the development of atherosclerosis. Evidence also suggests that it participates in plaque progression and the clinical emergence of cardiovascular events. Endothelial dysfunction is characterized by impaired endothelium-dependent vasodilation and “endothelial activation,” which is associated with a proinflammatory, proliferative, and procoagulatory milieu that promotes initiation and complications of atherogenesis.2 Endothelial dysfunction associated with insulin resistance appears to precede the development of overt hyperglycemia in patients with type 2 diabetes mellitus.3 Therefore, endothelial dysfunction may be a critical early target for the prevention of atherosclerosis and CVD in patients with diabetes mellitus or insulin resistance.2 A synergistic cross talk exists among the conventional cardiovascular risk factors associated with diabetes mellitus, and such cross talk contributes to disruption of endothelial integrity and acceleration of atherosclerosis. However, the biochemical and cellular links between elevated blood glucose levels and endothelial dysfunction remain incompletely understood. This review will focus on the multifactorial nature of endothelial dysfunction in diabetes mellitus, the relationship between endothelial dysfunction and conventional cardiovascular risk factors, and the translational potential of molecular targets such as the AMP-activated protein kinase (AMPK) for treating endothelial dysfunction in diabetes mellitus.
Endothelial Function and Cardiovascular Health
Endothelial Function and Vascular Homeostasis
Originally considered to be simply a physical barrier between the blood and vascular wall, the endothelium is now recognized as the most important component of normal vascular homeostasis, because it serves to maintain the anticoagulant, antiplatelet, and fibrinolytic phenotypes of vascular cells. The maintenance of vascular homeostasis is accomplished through the release of numerous dilator and constrictor substances (the endothelium is regarded as a complex endocrine and paracrine organ4; Figure 1). The most important factor released by the endothelium is nitric oxide (NO), originally identified as endothelium-derived relaxing factor. Other endothelium-derived vasodilators include prostacyclin, bradykinin, and an uncharacterized endothelium-derived hyperpolarizing factor. Prostacyclin acts synergistically with NO to inhibit platelet aggregation. Bradykinin stimulates release of NO, prostacyclin, and endothelium-derived hyperpolarizing factor to inhibit platelet aggregation. Bradykinin also stimulates production of tissue plasminogen activator and thus may play an important role in fibrinolysis. The endothelium also produces vasoconstrictor substances such as endothelin (the most potent endogenous vasoconstrictor identified to date), reactive oxygen species (ROS), prostaglandin H2 (PGH2), thromboxane A2 (TXA2), and angiotensin II. Angiotensin II is not only a vasoconstrictor but also a pro-oxidant that stimulates production of endothelin. Endothelin and angiotensin II promote proliferation of smooth muscle cells and in this way contribute to plaque formation. Large amounts of endothelin are produced by activated macrophages and vascular smooth muscle cells, the cellular components of atherosclerotic plaques. Because the endothelium plays a key role in vascular homeostasis, damage to this cellular layer will upset the balance between vasoconstriction and vasodilation, initiating a number of events/processes that promote or exacerbate atherosclerosis. These include endothelial permeabilization, platelet aggregation, leukocyte adhesion, and cytokine production.
Figure 1.
Endothelium, as a complex endocrine and paracrine organ, plays a crucial role in the maintenance of vascular homeostasis. Far beyond being simply a physical barrier between the blood and vascular wall, the endothelium is now recognized as the most important component of normal vascular homeostasis, through which the anticoagulant, antiplatelet, and fibrinolytic phenotypes of vascular cells can be maintained. Essentially, the maintenance of vascular homeostasis by endothelium is accomplished through the release of vasoprotective factors (eg, NO/endothelium-derived relaxing factor, prostacyclin, bradykinin, and endothelium-derived hyperpolarizing factor [EDHF]) and harmful substances (eg, endothelin [ET], ROS, endothelium-derived COX-dependent vasoconstricting factor [EDCF], and angiotensin II [Ang II]). Damage to endothelium will disrupt the balance between vasoprotective factors and harmful substances, initiating a number of events/processes that promote or exacerbate atherosclerosis via increased endothelial permeabilization, platelet aggregation, leukocyte adhesion, and cytokine production.
Assays of Endothelial Function in the Clinic
An improved understanding of the vascular biology of the endothelium and appreciation of the central role of the endothelium in vascular disease has enabled the development of clinical tests to evaluate the functional properties of normal and activated endothelium.5 Ideally, such tests should be safe, noninvasive, reproducible, inexpensive, and standardized between laboratories. The results should also reflect the dynamic biology of the endothelium throughout the natural history of atherosclerotic disease, define subclinical disease processes, and provide prognostic information for risk stratification in the later clinical phase.6 No single test currently fulfills these requirements, which necessitates the use of several tests to characterize the multiple aspects of endothelial biology. Because endothelial function cannot be measured directly in humans, estimates may be obtained indirectly by measuring the endothelium-dependent vasodilation (vasoregulation), plasma levels of endothelium-derived regulatory proteins (NO, endothelium-derived hyperpolarizing factor, prostacyclin [PGI2], TXA2, and endothelin-1 [ET-1]), and possibly microalbuminuria. Additional aspects of endothelial function that can be measured are coagulation, fibrinolysis, inflammation, and angiogenesis.7 Other vascular properties such as arterial stiffness and intima/media thickness of the carotid artery are likely only endothelium dependent in part.8
Endothelial function was first measured in humans in 1986 by assessing responses of the epicardial arteries to infused acetylcholine, an invasive approach.9 The first noninvasive approach was described in 1992.10 In this test, the diameter of an artery was measured by noninvasive ultrasound before and after an increase in shear stress (provided by reactive hyperemia), with the degree of dilation reflecting (in large part) arterial endothelial NO release.11 Measurement of ultrasound-based flow-mediated dilation in the brachial artery has intrinsic appeal,12 because it approaches certain criteria for endothelial function assessment. Nevertheless, it is not yet ready for widespread use in risk stratification (see Deanfield et al6 and Schalkwijk et al13 for the advantages and disadvantages of the available methods). Other noninvasive vascular testing approaches have been proposed in recent years and include pulse-wave analysis, pulse-wave velocity measurement, and pulse amplitude tonometry,12 such as reactive hyperemia pulse amplitude tonometry measured in the fingertips.14 Overall, clinical assessments of endothelial function not only have provided novel insights into pathophysiology but also have enabled detection of early disease, quantification of risk, judgment of response to interventions designed to prevent progression of early disease, and reduction of later adverse events in patients.6
Endothelial Dysfunction and CVD Prognosis
The term “endothelial dysfunction” encompasses several pathological conditions, including altered anticoagulant and antiinflammatory properties of the endothelium, impaired modulation of vascular growth, and dysregulation of vascular remodeling.15 However, in much of the literature, this term often refers to reduced bioavailability of endothelium-derived relaxation factors (such as NO, prostacyclin, or endothelium-derived hyperpolarizing factor) and, consequently, impairment in the vasodilatory effects of these relaxation factors.1,16 The concept of endothelial dysfunction has attracted considerable attention, because the identification of a measurable parameter for endothelial dysfunction may link complex molecular phenomena to vascular pathologies such as atherosclerosis.2,17
Numerous experimental studies have demonstrated that maintenance of the functional integrity of the endothelium exerts potent antiatherosclerotic and antithrombotic effects.8 Endothelial injury and dysfunction are associated with a number of known vascular diseases.18 For example, traditional CVD risk factors such as advanced age, hypertension, smoking, and low HDL cholesterol are associated with endothelial dysfunction.18 Importantly, these risk factors typically produce endothelial dysfunction before they generate true atherosclerosis, which suggests that a unifying mechanism links endothelial dysfunction to atherosclerosis and vascular disease.18 Although endothelial dysfunction is clearly a feature of atherosclerosis, it has been difficult to prove that it is required for the development and clinical manifestation of vascular disease. Nevertheless, several studies have proven the prognostic value of endothelial function, which is most often clinically assessed as a vasodilator response to pharmacological or mechanical stimuli. For example, impaired vasodilation in either coronary8,19 or peripheral20 vessels identifies those individuals at increased risk for future cardiovascular events. In addition, impaired brachial endothelial function predicts both short-term21 and long-term22 outcome in patients undergoing vascular surgery. Thus, prospective studies clearly indicate that impaired endothelial function identifies patients at increased vascular risk, providing a “barometer” for vascular health.23
Endothelial Dysfunction in Diabetes Mellitus
Vascular injury is the principal complication of all forms of diabetes mellitus. One of the main events underlying vascular injury is the development of endothelial dysfunction, which predisposes diabetic patients to cardiovascular complications and microthrombus formation. Multiple studies in patients and in vitro have revealed that hyperglycemia alters endothe-lial metabolism and function in such a way that could lead to vascular disease. In diabetic patients and animals, endothelial dysfunction precedes clinically significant atherosclerotic vascular disease and may play a role in its pathogenesis. An overwhelming mass of data demonstrates that endothelial dysfunction occurs in animal models of diabetes and in human blood vessels from diabetic patients.24,25 Abnormalities in endothelium-dependent relaxation have been described in patients with diabetes,26 and impaired glucose tolerance has been described in young normoglycemic relatives of patients with type 2 diabetes mellitus.27 Similar abnormalities have been detected in individuals with acutely increased blood glucose and insulin levels resulting from glucose infusion.28
Clinical Relevance of Endothelial Dysfunction in Diabetic Patients
Accumulating evidence suggests that endothelial dysfunction is an early marker for atherosclerosis and can be detected before angiographic or ultrasound-assisted detection of structural changes to the vessel wall. Multiple risk factors have been found to predict endothelial dysfunction, and many of these risk factors are also associated with a predisposition to atherosclerosis.
Accelerated Atherosclerosis
The most common and devastating complications of diabetes mellitus are cardiovascular complications, with these events being the major cause of hospital admissions. A striking feature of diabetic cardiovascular complications is accelerated atherosclerosis, which is associated with metabolic syndrome, insulin resistance, and oxidative stress.29–37 The Diabetes Control and Complications Trial for type 1 diabetes mellitus and the United Kingdom Prospective Diabetes Study38 for type 2 diabetes mellitus have established the importance of intensive glycemic control in dramatically reducing the devastating complications that result from poorly controlled diabetes mellitus. Both trials demonstrated the efficacy of intensive glucose control in reducing the risk of microvascular complications such as retinopathy, neuropathy, and nephropathy.39,40 Recent findings from the long-term follow-up of participants in the Diabetes Control and Complications Trial reveal that study participants who intensively controlled blood glucose had a decreased incidence of atherosclerosis,25 which suggests that hyperglycemia is important in the development of macrovascular diseases in diabetes mellitus. Accordingly, endothelium-dependent relaxation is impaired in patients with type 2 diabetes mellitus and individuals with an acute elevation of blood glucose and insulin levels due to glucose infusion.24–26,28
Dysfunctional Endothelial Progenitor Cells
The presence of endothelial progenitor cells (EPCs) was first demonstrated by showing that CD34+ hematopoietic progenitor cells purified from adults can differentiate, acquire an endothelial phenotype ex vivo, and be incorporated into neovessels at sites of ischemia.41 Circulating bone marrow–derived endothelial progenitor cells were then identified in the adult. Most convincingly, bone marrow–transplanted genetically tagged cells were found to colonize implanted Dacron grafts.42 Regardless of the origin of circulating EPCs, this pool of circulating endothelial cells may function in an endogenous repair mechanism that serves to maintain the integrity of the endothelial monolayer by replacing denuded parts of the artery.43 Increasing evidence supports the notion that maintenance of the endothelial monolayer may prevent thrombotic complications and atherosclerotic lesion development. Transplantation of apolipoprotein E–deficient mice with wild-type bone marrow reduces atherosclerotic lesion formation. Various risk factors for coronary artery disease, such as diabetes mellitus, hypercholesterolemia, hypertension, and smoking, affect the number and functional activity of EPCs in healthy volunteers44 and in patients with coronary artery disease.45 Likewise, diabetic mice and patients exhibit reduced functional activity of EPCs.46–48 In addition, factors that reduce cardiovascular risk, such as statins49–52 or exercise,53 elevate the number of EPCs, which enhances endothelial repair. Thus, the balance of atheroprotective and proatherosclerotic factors may influence EPC numbers and subsequently reendothelialization capacity.43 A few studies have shown that injected EPCs home to sites of ischemia, incorporate into the newly formed capillaries, and augment neovascularization.54 A strong inverse correlation exists between EPC number and cardiovascular risk, as assessed by the combined Framingham risk factor score.44 Consistent with this finding, analysis of flow-mediated brachial artery reactivity has revealed a significant relation between endothelial function and EPC number. Together, these findings suggest that EPCs are critical to the maintenance of endothelial integrity, and EPC dysfunction contributes to the pathogenesis of vascular disease.47
Wound Healing
More than 100 known physiological factors contribute to wound-healing deficiencies in individuals with diabetes mellitus. These factors include growth factor production, angiogenic response, macrophage function, collagen accumulation, epidermal barrier function, quantity of granulation tissue, keratinocyte and fibroblast migration and proliferation, number of epidermal nerves, bone healing, and the balance between extracellular matrix production and remodeling by matrix metalloproteinases (see Brem and Tomic-Canic55 for a detailed discussion). Optimum healing of a cutaneous wound requires orchestration of complex biological and molecular events such as cell migration, cell proliferation, and extracellular matrix deposition and remodeling.56 Wound healing is a cellular response that must be appropriate and precise to the injury. This response involves subsequent activation of keratinocytes, fibroblasts, endothelial cells, macrophages, and platelets. However, this orderly progression of the healing process is impaired in chronic wounds, including those that result from diabetes mellitus. Several pathogenic abnormalities ranging from disease-specific intrinsic flaws (blood supply, angiogenesis, and matrix turnover) to extrinsic factors (infection and continued trauma) contribute to the failed healing. Impaired healing of diabetic ulcers is caused by several intrinsic factors (neuropathy, vascular problems, and other complicating diabetes-induced systemic effects) and extrinsic factors (wound infection, callus formation, and excessive pressure to the site). Recently, identification of key factors that participate in EPC-mediated diabetic wound healing has gained increasing attention. Gallagher and colleagues57 demonstrated that in diabetic mice, hyperoxia enhances mobilization of circulating EPCs from the bone marrow to the peripheral circulation. Local injection of the chemokine stromal cell-derived factor-1α then recruits these EPCs to the cutaneous wound site to accelerate wound healing.
Causes of Endothelial Dysfunction in Diabetes Mellitus
Hyperglycemia
Prolonged exposure to hyperglycemia is now recognized as a major factor in the pathogenesis of diabetic complications, including atherosclerosis. Hyperglycemia induces a large number of cellular alterations58 in vascular tissue that potentially accelerate the atherosclerotic process. Animal and human studies have identified at least 5 major mechanisms that account for most of the pathological alterations observed in the diabetic vasculature25,58: (1) Nonenzymatic glycosylation of proteins and lipids interferes with normal protein function by disrupting molecular conformation, altering enzymatic activity, reducing degradative capacity, and interfering with receptor recognition. In addition, glycosylated proteins interact with a specific receptor present on all cells relevant to the atherosclerotic process, including monocyte-derived macrophages, endothelial cells, and smooth muscle cells. The interaction of glycosylated proteins with their receptor induces oxidative stress and proinflammatory responses. (2) Protein kinase C activation alters growth factor expression. (3) Shunting of excess intracellular glucose into the hexosamine pathway leads to O-linked glycosylation of various enzymes and perturbs normal enzyme function. (4) Hyperglycemia increases oxidative stress through several pathways. A major mechanism appears to be superoxide (O2•−) overproduction by the mitochondrial electron transport chain. (5) Hyperglycemia promotes inflammation through induction of cytokine secretion by several cell types, including monocytes and adipocytes. Importantly, hyperglycemia-induced oxidative stress is tightly linked with other hyperglycemia-dependent mechanisms of vascular damage described above, namely, formation of advanced glycation end products, protein kinase C activation, and increased flux through the hexosamine pathway (Figure 2).
Figure 2.
Origin of endothelial dysfunction in diabetes mellitus. Endothelial dysfunction in diabetes can be induced solely by or by a combination of (1) hyperglycemia, (2) fatty acids, (3) inflammation, and (4) insulin resistance. Prolonged exposure to hyperglycemia is now recognized as a major factor in the pathogenesis of diabetic complications, including atherosclerosis, mechanistically involving enhanced enzymatic and nonenzymatic protein/lipid glycosylation, protein kinase C activation, inflammation, and ROS production. Other factors including dyslipidemia, elevated FFAs, inflammation, and insulin resistance, can cause endothelial dysfunction. RNS indicates reactive nitrogen species; EDCF, endothelium-derived COX-dependent vasoconstricting factor; AGE, advanced glycation end products; and PKC, protein kinase C.
Hyperglycemia might not be the sole factor responsible for endothelial dysfunction in diabetes mellitus. Other factors such as dyslipidemia, elevated free fatty acids (FFAs), inflammation, and insulin resistance can cause endothelial dysfunction. For example, endothelial dysfunction might precede the development of overt hyperglycemia in type 2 diabetes mellitus and prediabetic states (family history of type 2 diabetes mellitus and prior gestational diabetes, as well as in obese but nondiabetic individuals).59 Consistent with these observations, inflammation and endothelial activation are evident at birth in offspring of women with type 1 diabetes mellitus.60 In the nonobese diabetic (NOD) mouse, the preferred and accepted animal model of spontaneous autoimmune type 1 diabetes mellitus, there is endothelial dysfunction characterized by a paradoxical vasoconstriction that precedes the hyperglycemia, which suggests a hyperglycemia-independent effect on endothelial function in type 1 diabetes mellitus. This could explain the clinical observation that certain people are very prone to complications (despite good glycemic control), whereas others are not, regardless of glycemic status.
Fatty Acids
Both type 1 and type 2 diabetes mellitus are associated with an increase in circulating FFAs.61 Elevation of plasma FFA levels through infusion of a lipid emulsion and heparin has been shown to impair endothelium-mediated vasodilation (and NO production) in human volunteers undergoing a hyperinsulinemic-euglycemic clamp.62 Interestingly, such elevations in plasma FFAs during a clamp have also been shown to induce insulin resistance and large increases in intramyocellular triglyceride in skeletal muscle,63 which suggests that the accumulation of esterified lipid, insulin resistance, and vascular dysfunction are linked. In insulin-resistant states of obesity and type 2 diabetes mellitus, the action of FFAs on phosphatidylinositol 3-kinase (PI3K)–mediated mechanisms may diminish NO release.64 FFAs may also decrease NO bioavailability through 2 other important mechanisms.61 First, NO bioavailability may be decreased by oxidative stress, which is increased by obesity and type 2 diabetes mellitus. These conditions not only increase generation of ROS but also lead to oxidative damage to lipids, proteins, amino acids, and DNA.64 The major ROS generated by leukocytes is O2•−, which binds NO to form peroxynitrite (ONOO−). ONOO− is toxic and is not vasodilative. In accordance with the idea that oxidative stress decreases NO bioavailability, acute increases in FFAs have recently been shown to diminish postischemic flow-mediated vasodilation of the brachial artery.65 In an in vivo model, FFA infusion leads to insulin resistance and endothelial dysfunction.66 This probably happens owing to an increased ROS via enhanced FFA oxidation, which in turn suppresses NO bioavailability.67 Thus, FFAs could account for abnormalities in the vascular reactivity of both arterial and venous vascular beds observed in diabetes mellitus and obesity.68,69
Inflammation
The discovery that metabolic conditions such as obesity and type 2 diabetes mellitus (insulin resistance) are associated with inflammation was first described in a seminal publication by Hotamisligil et al in 1993.70 This group demonstrated that adipocytes constitutively express the proinflammatory cytokine tumor necrosis factor-α (TNF-α), and adipocyte TNF-α expression is markedly increased in obese animals. They also showed that neutralization of TNF-α with soluble TNF-α receptor decreases insulin resistance in these animals.70 These observations provided the first link between increased expression and plasma concentrations of a proinflammatory cytokine and insulin resistance.71 Further work in humans has confirmed that obesity, a major risk factor for type 2 diabetes mellitus, and diabetes itself are inflammatory conditions, as indicated by increased plasma concentrations of C-reactive protein, interleukin-6, and plasminogen activator inhibitor-1 (see Dandona et al71,72 for details). At least 2 mechanisms might promote inflammation. First, glucose and macronutrient intake increase both oxidative stress and inflammatory changes. Thus, chronic overnutrition (obesity) might induce a proinflammatory state with oxidative stress. Second, increases in TNF-α and interleukin-6 associated with obesity and type 2 diabetes mellitus might interfere with insulin action by suppressing insulin signal transduction and thus the antiinflammatory effect of insulin. This, in turn, may promote inflammation.72 Hyperglycemia exacerbates the inflammation associated with type 2 diabetes mellitus. Notably, atherosclerosis links inflammation, obesity, insulin resistance, and type 2 diabetes mellitus. Moreover, it is responsible for the major cause of death (acute myocardial infarction) in this patient population and is itself an inflammatory process.72 Therefore, inflammation is an effector of not only endothelial dysfunction3,73,74 but also insulin resistance and atherosclerosis.
Insulin Resistance
Endothelial cells express the cognate insulin receptor (IR), which belongs to a family of membrane-bound receptors with intrinsic tyrosine kinase activity, whose ligands include growth factors such as insulin-like growth factor-1, vascular endothelial growth factor, platelet-derived growth factor, and epidermal growth factor. In addition to crucial metabolic actions, insulin plays a critical role in the maintenance of physiological endothelial function through its ability to stimulate NO release via a cascade of signaling that involves activation of the PI3K-Akt axis and the downstream serine phosphorylation of endothelial NO synthase (eNOS). In addition to NO-dependent vasodilatory actions, insulin stimulates endothelial release of the vasoconstrictor ET-1, as suggested by increased insulin vasodilatory effects in humans under ET-1 receptor blockade. Thus, insulin has multiple opposing hemodynamic actions, the net effect of which on blood pressure is negligible in normal individuals.
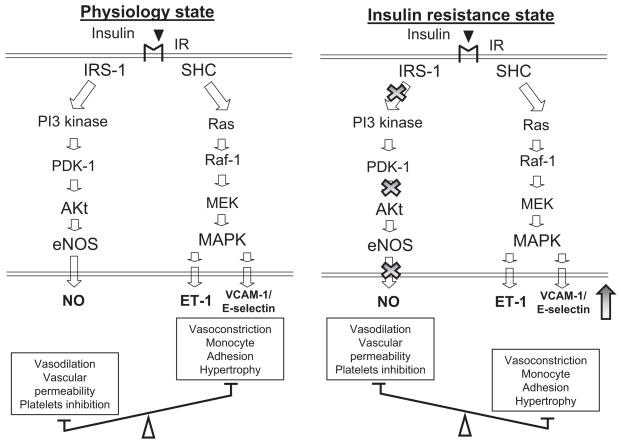
Insulin resistance is characterized by specific impairment in PI3K-dependent signaling pathways, whereas other insulin-signaling branches, including Ras/mitogen-activated protein kinase–dependent pathways, are unaffected (Figure 3). In addition, metabolic insulin resistance is usually paralleled by a compensatory hyperinsulinemia to maintain euglycemia. Thus, consequent hyperinsulinemia in insulin-resistant states will overdrive unaffected mitogen-activated protein kinase–dependent pathways. In endothelium, decreased PI3K signaling and increased mitogen-activated protein kinase signaling in response to insulin may lead to decreased production of NO and increased secretion of ET-1, a characteristic of endothelial dysfunction. Indeed, insulin-resistant patients have elevated plasma ET-1 levels, and hyperinsulinemia increases ET-1 secretion in humans. Pharmacological blockade of ET-1 receptors (ET-A isoform) improves endothelial function in obese and diabetic patients but not in lean, insulin-sensitive subjects.
Figure 3.
Insulin resistance induces endothelial dysfunction in diabetes. In addition to crucial metabolic actions, insulin plays a critical role in the maintenance of physiological endothelial function through its ability to stimulate NO release via a cascade of signaling, initiated by binding to its cognate receptor (IR) expressed on endothelial cells. The cascade involves activation of the PI3K-Akt axis and downstream serine 1177 phosphorylation of eNOS for NO-dependent vasodilator actions or stimulation of the endothelial release of ET-1 for its vasoconstrictor effect. In insulin-resistance vessels, pathway-specific impairments in PI3K-dependent signaling decrease the expression and activity of eNOS, whereas compensatory secretion of insulin augment its mitogen-activated protein kinase pathways, which results in both the overexpression of adhesion molecules (vascular cell adhesion molecule-1 and E-selectin) and increased secretion of ET-1. Insulin-resistant endothelium becomes highly inflammatory, with impaired blood supply, which in turn worsens insulin resistance. SHC indicates Src[sarcoma] Homology domain C-terminal; PDK-1, phosphoinositide-dependent kinase-1; MEK, MAPK (mitogen-activated protein kinase)/ERK (extracellular signal-regulated kinase) kinase; MAPK, mitogen-activated protein kinase; VCAM, vascular cell adhesion molecule;.
Endothelial dysfunction might also play a causal role in the development of insulin resistance. Insulin can relax resistance vessels and increase blood flow to skeletal muscle. Insulin acts on the vasculature in 3 discrete steps to enhance its own delivery to muscle/fat tissues: (1) Relaxation of resistance vessels to increase total blood flow; (2) relaxation of precapillary arterioles to increase the microvascular exchange surface perfused within skeletal muscle (microvascular recruitment); and (3) the transendothelial transport of insulin. Indeed, insulin resistance is associated with functional disturbances of the coronary circulation. Conversely, insulin infusion improves coronary flow, even in the setting of type 2 diabetes mellitus and coronary artery disease. Thus, such an imbalance between production of NO and secretion of ET-1 leads to decreased blood flow, which worsens insulin resistance. The reciprocal relationship of insulin resistance and endothelial dysfunction has been a subject of several excellent reviews.75
Mediators of Vascular Endothelial Dysfunction in Diabetes Mellitus
NO is the single most important factor for maintaining vascular endothelial function. NO is a gaseous free radical molecule and is synthesized by the action of the enzyme NO synthase (NOS). In endothelial cells, NO is quickly quenched and inactivated by O2•− to form ONOO−. Thus, NO bioactivity is determined by the rate of NO production by NOS and the rate of O2•− production.
Decreased NO Release
The influence of hyperglycemia and diabetes on the synthesis and release of NO by cells and tissues has been the subject of intense investigation. A number of studies suggest that decreased NO bioactivity associated with these conditions is due to either quenching of normally released NO or impairment of NOS activity.17,76 In the glomeruli of diabetic rats, TXA2 and protein kinase C mediate the impairment of NO-dependent cGMP generation and are thought to do so by decreasing NO production.77 In vitro studies of human umbilical vein endothelial cells show that elevated glucose inhibits NO production.78
Loss of endothelium-dependent relaxation has been described in various animal models of diabetes and in patients. Several mechanisms, including activation of the protein kinase pathway,79 the posttranslational modification of eNOS through the hexosamine pathway,67 downregulation of the expression of eNOS (as opposed to inhibition of its catalytic activity),80 and S-nitrosylation of eNOS,81 have also been ascribed to diabetic endothelial dysfunction, respectively.
On the other hand, a number of studies have demonstrated that NO release is increased under hyperglycemic and diabetic conditions. Similarly, NOS activity in heart endothelium is increased in diabetic rats.82 Increased NO production due to inducible NOS (NOS-II) upregulation has been reported in pancreatic islet cells,83,84 murine macrophages,85 glomerular mesangial cells,85,86 and placental tissues of diabetic individuals.87 This paradoxical phenomenon might be best explained by a phenomenon called “eNOS uncoupling.” Increasing evidence suggests that in diabetes mellitus, the function of eNOS (or NOS-III) is altered, which leads to production of O2•− by this enzyme rather than NO (eNOS uncoupling). This transformation of eNOS from a protective enzyme to a contributor of oxidative stress has been observed in several in vitro models, in animal models of CVDs, and in patients with cardiovascular risk factors (reviewed in Cai et al88 and Forstermann and Munzel89). Several studies from us90,91 and others92 suggest that 2 interrelated mechanisms, including oxidation of the zinc-thiolate center of eNOS and tetrahydrobiopterin (BH4), an essential cofactor for eNOS, might play a particularly important role in regulating NO and O2•− production by eNOS in diabetes mellitus (see below).
Reactive Oxygen Species
Recent studies indicate that the endothelial dysfunction associated with diabetes mellitus results from the formation of oxidants and free radicals within and near the vascular endothelium.93,94 A number of functional studies have demonstrated that diabetic blood vessels respond with an improved endothelium-dependent relaxant response when treated with various antioxidant agents, including superoxide dismutase (SOD).95–98 The role of O2•− in the development of diabetic endothelial dysfunction is supported by the fact that many biochemical pathways strictly associated with hyperglycemia (glucose auto-oxidation, the polyol pathway, prostanoid synthesis, and protein glycation) can increase the production of free radicals and oxidants. Both hyperglycemia and FFAs have also been shown to alter cellular oxidative stress.36,61,99 Exposure of endothelial cells to high glucose or to FFAs augments O2•− production. In vivo studies of diabetic BB rats show that endothelium-mediated relaxation is diminished in these animals, possibly owing to NO quenching by increased O2•− production.94 Intense investigation has identified several sources of O2•− in vascular endothelial cells. Studies performed by Inoguchi et al99 suggest that oxidative stress is secondary to activation of NAD(P)H oxidase and is protein kinase C-β–dependent, whereas work by Brownlee58 suggests that hyperglycemia-induced oxidative stress from mitochondria is the earliest event in the development of cardiovascular complications in diabetes mellitus.
Reactive Nitrogen Species
The half-life of NO and therefore its biological activity are decisively determined by oxygen-derived free radicals such as O2•−. O2•− rapidly reacts with NO to form the highly reactive intermediate ONOO−.100,101 The rapid bimolecular reaction between NO and O2•− that yields ONOO− (ONOO−, rate constant equal to 5 to 10 ×109 mol · L−1 ·· s−1) is 3 to 4 times faster than the dismutation of superoxide by SOD. Therefore, ONOO− formation represents a major potential pathway of NO reactivity that relies on the rates of tissue O2•− generation (reviewed in Zou et al1,102,103), and many of the biological effects attributed to NO are in fact mediated by ONOO− (Figure 4).
Figure 4.
Generation of ROS and reactive nitrogen species in endothelial cells. ONOO− is formed by the diffusion-limited radical-radical reaction between O2•− and NO. The diffusion-limited reaction means that every time NO bumps into O2•− (which is controlled by diffusion), the 2 produce ONOO−. Because NO is 1000 times smaller than CuZn-SOD, it diffuses faster and therefore reacts with superoxide at least 10 times faster than SOD can possibly scavenge O2•−. Thus, many of the biological effects attributed to NO are in fact mediated by ONOO−.
The production of O2•− has been long known to play a causal role in the development of endothelial dysfunction and cardiovascular complications in diabetes mellitus. From a chemical point of view, neither NO nor O2•− alone is considered a strong oxidant of most types of biological molecules.100,101 Recent evidence indicates that reaction of NO with O2•−, which was initially viewed as a route for NO inactivation, yields the potent oxidizing species ONOO−. ONOO− exhibits direct oxidative reactivity and becomes protonated to form peroxynitrous acid (ONOOH) at physiological pH (pKa=6.8). Direct bimolecular reaction of NO with O2•− produces ONOO− at near–diffusion-limited rates (5 to 10 × 109 mol ·· L−1s−1).100,101 This rate is at least 3 times faster than the rate of enzymatic dismutation of O2•− catalyzed by SOD at neutral pH (Ksod=2 × 109 mol · L−1s−1).100 Thus, ONOO− formation, which is potentially a major pathway governing NO reactivity, depends on local rates of NO and O2•− production. ONOO− is a potent oxidant capable of directly oxidizing sulfhydryl groups and thioethers. It also nitrates and hydroxylates aromatic groups, including those of tyrosine, tryptophan, and guanine. This process is mediated by both 1- and 2-electron transfer reactions. Finally, ONOO− is capable of reaction with metal centers to yield a species that has the reactivity of nitronium (NO2+), an oxidizing and nitrating intermediate. All of these reactions occur when ONOO− encounters biological molecules such as enzymes and lipids, and they result in altered functions of these molecules.100,101
A characteristic reaction of ONOO− is the nitration of protein-bound tyrosine residues. Antibodies against nitrated tyrosine can be used to detect the ONOO− “footprint.”104 ONOO− reaction products have been detected in many conditions, including diabetes mellitus. Moreover, decomposition of ONOO− or pharmacological inhibition of ONOO− formation has been shown to be of benefit in these conditions. Recent clinical data reveal that vascular tissues from diabetic patients and animals exhibit significantly more intense 3-nitrotyrosine staining than tissues from normal controls.1,101,103,105 There are multiple lines of evidence demonstrating the formation of ONOO− in the diabetic vasculature, both in experimental models and in humans (reviewed in Zou et al1,102,103). The tissues and species in which ONOO− has been identified in experimental animals (rodent and nonrodent species) and in humans include plasma, kidney, blood vessels (especially endothelium), retina, heart, and peripheral nerves and have been reviewed recently.105
Increased 3-nitrotyrosine levels have also been described in plasma from humans infused with glucose.106 Importantly, Frustaci et al107 analyzed ventricular myocardial biopsy samples from diabetic patients and found that apoptosis was increased 61-fold in endothelial cells and 85-fold in cardiomyocytes. In both cell types, apoptosis was strongly associated with positive 3-nitrotyrosine staining, which suggests that apoptosis is likely caused by ONOO− generation. Accumulating evidence suggests that ONOO− might play a central role in the initiation of vascular endothelial dysfunction by altering the balance of endothelial cell–derived dilators/constrictors.
Prostacyclin Deficiency
Prostanoids are produced when arachidonic acid is released from the plasma membrane by phospholipases and metabolized by cyclooxygenases (COXs) and specific isomerases. The profile of the prostanoids that are produced can vary dramatically during the course of a response. Prostanoid production depends on the activity of the 2 COX isoenzymes within cells. PGH2 is produced by both COX isoforms (ie, COX-1 and COX-2) and is the common substrate for a series of specific synthase enzymes that produce prostaglandin (PG) D2 (PGD2), PGE2, PGF2α, PGI2, and TXA2. Importantly, the differential expression of these enzymes within the cells situated at inflammation sites determines the profile of prostanoid production.
Like other prostaglandins and TXA2, PGI2 is produced by the 2 rate-limiting cyclooxygenases, COX-1 and COX-2. These COX enzymes form the prostaglandin endoperoxide, PGH2, which is then enzymatically transformed into PGI2 by prostacyclin synthase (PGIS) in blood vessels or into TXA2 by TXA2 synthase in platelets. In blood vessels, particularly larger arteries, the predominant prostaglandin is PGI2.108 The ability of blood vessels to generate PGI2 is essential to the integrity of the endothelium.108,109 Vascular PGI2 synthesis and release are reduced in diabetes mellitus.108–115 Blood vessels obtained from animals with streptozotocin- or alloxan-induced diabetes show a reduced production of PGI2, whereas platelets from these animals release more TXA2 than normal platelets. In diabetic patients, PGI2 production by blood vessels is depressed. In patients with proliferative retinopathy and pregnancy, urinary and circulating levels of 6-keto-PGF1α are reduced.116–119 Decreased PGI2 has been linked to platelet hyperaggregability, increased platelet adhesiveness, and increased release of PGH2/TXA2 in diabetes mellitus.108–118 Moreover, a reduction in PGI2 production has been proposed to accelerate atherosclerosis during the pathogenesis of macroangiopathy and microangiopathy in diabetic patients.109,111 In mice, PGI2 deficiency elicited by PGIS gene depletion results in the development of vascular disorders and thickening of vascular walls,119 which suggests that PGI2 is important in the homeostasis of blood vessels.
Endothelium-Derived COX-Dependent Vasoconstriction Factor
Increased release of the endothelium-derived COX-dependent vasoconstriction factor in high glucose–exposed aortas was first described by Tesfamariam and colleagues.120,121 Endothelium-derived COX-dependent vaso-constriction factor was subsequently identified as prostaglandin endoperoxide, PGG2 or PGH2 (reviewed in Zou et al1). Both TXA2 and PGH2 stimulate contraction of vascular smooth muscle via activation of the TXA2/PGH2 receptor (TPr).122–124 Activation of TPr in vascular cells also induces apoptosis,125,126 abnormal expression of adhesion molecules (intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and endothelial-leukocyte adhesion molecule-1),127,128 and mitogenic or hypertrophic activities.129–132 The actions of PGH2/TXA2 are opposed by prostacyclin. The major sources of TXA2 are platelets, polymorphonuclear leukocytes, and monocytes.
One of the hallmarks of diabetic endothelium is its propensity to produce and release vasoconstrictors, as manifested by the presence of endothelium-dependent contractions. These findings suggest that alterations in arachidonic acid metabolism via the COX pathway are important for the synergism between vascular disease and diabetes mellitus. Aortic release of PGH2/TXA2-like vasoconstrictors is increased in hyperglycemic animals with alloxan- or streptozotocin-induced diabetes.97,120,121,133–142 Furthermore, in the same models, the impaired relaxation is restored by COX inhibitors such as indomethacin or TPr blockers but not by TXA2 synthase inhibitors.97,120,121,133–142 This suggests that constrictor responses are mediated by PGH2 rather than by TXA2. In contrast, in blood vessels of normal animals, PGH2 is metabolized primarily by PGIS to yield PGI2, which minimizes PGH2-mediated responses in the aorta. Decreased activity of PGIS or increased COX activity likely causes PGH2-dependent vasoconstriction that contributes to impaired relaxation in diabetes mellitus. Generation of PGH2 appears to be related to hyperglycemia rather than elevated lipids. Both in vitro and in vivo, elevated glucose reduces the production of 6-keto-PGF1α and promotes generation of PGH2/TXA2, which results in PGI2/TXA2 imbalance.97,120,121,133–142 Interestingly, a number of free radical scavengers, such as SOD, prevent and restore impaired endothelium-dependent relaxation, which suggests that free radicals play a major role in the generation of the vasoconstrictor PGH2 in diabetes mellitus.97,120,121,133–142 Furthermore, endothelium-independent vasodilation in response to sodium nitroprusside does not differ between aortic rings exposed to control and elevated glucose levels, which suggests that hyperglycemia-associated endothelial dysfunction is related to endothelium-derived NO.
More recent data reveal that S18886, an orally active TXA2 receptor antagonist in clinical development for use in secondary prevention of thrombotic events in CVD, inhibits inflammation and the accelerated atherogenesis caused by diabetes mellitus, most likely by counteracting the effects on endothelial function and adhesion molecule expression of eicosanoids stimulated by the diabetic milieu.143 Although potential endogenous ligands for TPr remain to be identified, the study by Zuccollo et al143 provides important evidence for TPr-dependent atherogenesis in diabetic cardiovascular complications. How TPr enhanced atherogenesis remains unknown. Interestingly, we and others have observed a TPr-dependent ROS production in several cell types144 (M. Zhang, MD, PhD, M.H. Zou, MD, PhD, unpublished observations, 2009) and in vivo.145,146 Thus, it is highly likely that TPr and ROS might mutually promote their effects in vascular cells to worsen endothelial dysfunction and atherosclerosis. Thus, the way in which TPr triggers ROS warrants further investigation.
Increased Production of Endothelins
ET-1 is primarily synthesized by vascular endothelial cells and acts on the vascular smooth muscle. This factor participates in the development of vascular diseases through its vasoconstrictor and mitogenic effects.147 In addition, ET-1 is reported to exert its pathological effects by increasing the formation of ROS and reactive nitrogen species in vascular endothelial cells.148 Interestingly, ROS are reported to increase endothelin.149 Thus, ONOO− and endothelin might form a feed-forward loop to worsen endothelial dysfunction in diabetes mellitus.
Clinical evidence has demonstrated that alterations in plasma ET-1 concentrations are associated with various physiological abnormalities. In a cross-sectional study, ET-1 was associated with urinary albumin excretion, with plasma ET-1 levels progressively increasing in patients with normoalbuminuria, microalbuminuria, and macroalbuminuria.150 ET-1 levels are also higher in patients with type 2 diabetes mellitus than in healthy subjects, and this increase in ET-1 levels is accompanied by increased levels of oxidative stress markers, proinflammatory markers, and (downstream) adhesion molecules.151 ET-1 is a marker of endothelial dysfunction and is responsible in part for the endothelial dysfunction associated with diabetes mellitus. Therefore, it may also play an important role in the development of diabetic arterial disease.147 This vascular peptide is released from human adipose tissue and provides a link between fat accumulation and insulin resistance.152 Increasing evidence suggests that chronic activation of the ET-1 system can lead to heterologous desensitization of the glucose-regulatory and mitogenic actions of insulin, with subsequent development of glucose intolerance, hyperinsulinemia, impaired endothelial function, and exacerbation of CVD. However, prospective trials are needed to assess whether ET-1 antagonists are better than conventional therapies in preventing the development of insulin resistance and the progression of diabetes mellitus.153
Peroxynitrite as the Unifying Mechanism for Endothelial Dysfunction in Diabetes Mellitus
ONOO− Increases Tyrosine Nitration of Prostacyclin Synthase
Our recently published work86,154,155 has provided new insights into how hyperglycemia increases reactive nitrogen species and affects cell function. Exposure of cultured human aortic endothelial cells to clinically relevant concentrations of high glucose increases the production of both NO and O2•− and consequently decreases the bioactivity of NO, as seen by decreased levels of cGMP. This suggests that NO is inactivated through its reaction with O2•−. Simultaneously, high glucose increases PGIS nitration and decreases PGIS activity. We have also shown that activation of TPr in human aortic endothelial cells modulates both adhesion molecule expression and apoptosis, with each being significantly attenuated by a TPr antagonist or COX inhibition. Thus, TPr may be activated by PGH2 as a result of PGIS nitration and inactivation. Consistent with this observation, S18886, a potent TPr antagonist, markedly attenuated diabetes-enhanced aortic lesions in vivo.143 We also found that blocking TPr prevents the dramatic enhancement in atherogenesis caused by diabetes mellitus in the Apo-E–deficient mouse with a concomitant endothelium-dependent constriction sensitive to the TPr antagonist SQ29548 (Zou et al, unpublished observations). This observation may explain not only why diabetes mellitus decreases PGI2 levels but also why increases occur in its precursor, PGH2, which activates TPr.
Thus, diabetes mellitus likely acts via hyperglycemia/hyperlipidemia to increase O2•− and then ONOO−, resulting in PGIS nitration and consequent TPr stimulation. This, in turn, contributes to the initiation and progression of vascular complications in diabetes mellitus through downregulation of the protective actions of NO and PGI2 and accumulation of nonmetabolized PGH2, which tips the balance toward platelet aggregation, atheroma accumulation, and thrombus formation (Figure 5).
Figure 5.
ONOO−-mediated tyrosine nitration of prostacyclin synthase contributes to vascular complications in diabetes mellitus. In diabetes, hyperglycemia or hyperlipidemia increases O2•− and ONOO− generation, which results in PGIS nitration and subsequent TPr stimulation. The formation of ONOO− not only decreases the protective actions of both NO and PGI2 but also releases endothelium-derived COX-dependent vasoconstriction factor (EDCF) via the accumulation of nonmetabolized PGH2. Thus, ONOO−-mediated PGIS nitration tips the balance toward platelet aggregation, atheroma accumulation, and thrombus formation. AA indicates arachidonic acid; TP receptor, TXA2/PGH2 receptor.
ONOO− Causes eNOS Uncoupling
When we studied diabetes-enhanced PGIS nitration, we found that both diabetic eNOS−/− mice and mice overexpressing human SOD (hSOD+/+ mice) exhibited less PGIS nitration and released less O2•− than their diabetic littermates.156 Conversely, PGIS activity was significantly preserved in both eNOS−/− and hSOD+/+ mice.156 Thus, tyrosine nitration of PGIS is most likely mediated by ONOO− formed endogenously from NO and O2•− in diabetes mellitus, because both eNOS−/− or hSOD+/+ mice had significantly attenuated diabetes-enhanced PGIS nitration and inhibition. Although we cannot totally exclude the possibility of peroxidase-catalyzed PGIS nitration, our data strongly suggest that ONOO− derived from eNOS is likely to be responsible for the increased PGIS nitration caused by diabetes mellitus in vivo.
All 3 NOS isoforms are catalytically active only in dimeric form. In the NOS dimer, a tetra-coordinated zinc ion is held by 4 thiols (cysteine 94 and 99 in human eNOS), with 2 thiols being contributed by each 135-kDa monomer. Because it remains partially positively charged, the zinc-thiolate center is subject to attack by anionic oxidants such as ONOO−. ONOO− reacts with zinc-thiolate clusters (5.2 × 105 mol · L−1s−1)100,101 at least 1000 times faster than it reacts with cysteine thiols (6 × 102 mol ·· L−1s−1) and 100 times faster than it reacts with BH4 (6 × 103 mol · L−1s−1). Recently, we found that oxidation of the eNOS zinc-thiolate cluster by a small amount of ONOO− precipitates oxidative stress in cells exposed to elevated glucose.90 The catalytic activities of recombinant eNOS are exquisitely sensitive to ONOO−-induced uncoupling, which decreases NO synthesis and increases O2•− production by the enzyme. An analysis of recombinant eNOS in endothelial cells revealed that exposure of these cells to elevated glucose induces loss of zinc from eNOS, disruption of SDS-resistant dimers, and uncoupling of eNOS activity.90 The same observations have been made in diabetic mouse tissues, which underscores the significance of this process under in vivo conditions.90
Regulation of BH4 synthesis and bioavailability is a topic of great interest, because BH4 is essential in NOS function and vascular NO synthesis. BH4 is an essential cofactor for activity of all NOS enzymes. The exact role of BH4 in NOS catalysis is incompletely understood, but BH4 appears to facilitate electron transfer from the eNOS reductase domain and maintain the heme prosthetic group in its redox active form. BH4 also stabilizes the NOS dimer complex, increases dimerization of eNOS monomers, and lowers the Km for L-arginine binding. Recent studies suggest that in diabetes mellitus, reduced BH4 availability is an important contributor to reduced NO production and increased endothelial O2•− production. In vessel rings from diabetic or atherosclerotic animals and in mammary artery rings from diabetic patients, supplementation with high concentrations of BH4 improves some features of endothelial dysfunction. Accordingly, acute BH4 administration appears to augment NO-mediated effects on forearm blood flow in patients with diabetes mellitus or hypercholesterolemia. In addition, deficiency in BH4 has been reported in diabetes mellitus, and BH4 supplementation partially restores endothelial function.157,158
The BH4 pool in endothelial cells is variable, and a continuous supply of BH4 is needed to maintain basal BH4 levels.159 BH4 is synthesized from GTP de novo. The first and rate-limiting step in this biosynthetic pathway is catalyzed by guanosine 5′-triphosphate cyclohydrolase I (GTPCH).159,160 Thus, GTPCH levels and activity are crucial for maintenance of BH4 bioavailability and endothelial function under both physiological and pathological conditions.160 Indeed, inhibition of GTPCH rapidly decreases BH4. GTPCH is constitutively expressed in endothelial cells, and endothelial GTPCH expression is markedly reduced in type 1 and type 2 diabetes mellitus rat models.158 In addition, aortic GTPCH activity is significantly lower in the insulin-resistance rat model than in control rats.157,158 Consistent with these results, GTPCH gene transfer or endothelial cell–specific GTPCH overexpression in mice increases BH4 bioavailability and improves endothelial function.157,158,161,162 In contrast, acute GTPCH knockdown by small interfering RNA technology uncouples eNOS and elevates blood pressure in mice.163 We have found that either ONOO− or high glucose significantly lowers BH4 levels by increasing 26S proteasome–dependent degradation of GTPCH.164 Furthermore, ONOO− releases zinc from the zinc-thiolate cluster of eNOS, which presumably leads to the formation of disulfide bonds between monomers.90 As a result, disruption of the SDS-resistant eNOS dimers occurs under reducing conditions. These exciting observations suggest that hyperglycemia might initially generate “kindling radicals” (O2•−) and then a “bonfire” oxidant (ONOO−) that uncouples eNOS through 26S proteasome–dependent degradation of GTPCH and oxidizes the zinc-thiolate center of eNOS, which is required for NO production (Figure 6).
Figure 6.
ONOO− causes eNOS uncoupling through multiple pathways. Diabetes via hyperglycemia- or dyslipidemia-derived O2•− and then ONOO− enhances (1) BH4 oxidation into dihydrobiopterin (BH2), (2) oxidation of the zinc-thiolate cluster of eNOS, which results in zinc-depleted eNOS dimers, which have reduced affinity toward both BH4 and L-arginine, and (3) ubiquitination and proteasomal degradation of GTPCH1, a rate-limiting enzyme in de novo synthesis of BH4, an essential cofactor of eNOS.
ONOO− and Insulin Resistance
Insulin resistance is characterized by pathway-specific impairment in PI3K-dependent signaling in both metabolic and vascular insulin target tissues. Increasing evidence suggests that ROS and reactive nitrogen species play a causal role in the development of insulin resistance and consequent endothelial dysfunction. ONOO− inhibited Akt signaling in endothelial cells. Furthermore, ONOO− nitrates a critical tyrosine residue in the p85 regulatory subunit of PI3K, preventing its association with the catalytic p110 subunit and subsequent PI3K activation. ONOO− causes the nitration of tyrosine residues in rat insulin receptor substrate-1, including Tyr939, a critical site for the association of insulin receptor substrate-1 with the p85 subunit of PI3K. Thus, ONOO− reduces the protein levels of insulin receptor substrate-1 and associated PI3K activity, upstream of Akt/protein kinase B.165
Our recent work might have provided an alternative mechanism for ONOO−-mediated insulin resistance and endothelial dysfunction in diabetes mellitus.166 In that study, we found that endogenous ONOO− generated during hyperglycemia exposure inhibits insulin-enhanced protein kinase B/Akt signaling in endothelial cells by increasing the phosphorylation and activity of the phosphatase and tensin homologue deleted on chromosome 10 (PTEN), elicited by the tumor suppressor LKB1, eventually leading to an inhibition of PI3K and apoptotic cell death. In vivo, the aortas of diabetic mice exhibited increased levels of nitrotyrosine together with increased phosphorylation of PTEN and LKB1 but reduced protein kinase B/Akt phosphorylation. These findings, therefore, suggest that hyperglycemia may promote insulin resistance and endothelial cell apoptosis in endothelial cells through protein kinase B/Akt downregulation via an ONOO−-mediated, LKB1-dependent PTEN activation (Figure 7).
Figure 7.
Mechanism of ONOO−-mediated insulin resistance in diabetic vessels. ONOO− can cause tyrosine nitration of PI3K or insulin receptor substrate-1 (IRS-1) to prevent their activation, which results in a reduction of both IRS-1 and PI3K-Akt signaling. in addition, ONOO− generated during hyperglycemia exposure via the tumor suppressor LKB1 via Rho/Rho-associated kinase–dependent signaling pathways causes insulin resistance by increasing the phosphorylation and activity of PTEN. PDK indicates phosphoinositide-dependent kinase.
ONOO− and the Formation of Advanced Glycation End Products
Nε-(carboxymethyl)lysine (CML) is a major antigenic advanced glycation end-product structure. CML concentration, adjusted for age and duration of diabetes mellitus, is also increased in patients with diabetes mellitus. CML is also recognized by the receptor for advanced glycation end product (RAGE), and CML-RAGE interaction activates cell-signaling pathways such as nuclear factor-κB (NF-κB) and enhances the expression of vascular cell adhesion molecule-1 in human umbilical vein endothelial cells. CML formation was increased with increasing ONOO− concentrations when glycated human serum albumin (Amadori-modified protein) was incubated with ONOO−. CML was inhibited by aminoguanidine, a trapping reagent for α-oxoaldehydes.167 Thus, ONOO− can induce protein modification by oxidative cleavage of the Amadori product and by generation of reactive α-oxoaldehydes from glucose.
Pharmacological Therapies for Treating Endothelial Dysfunction in Diabetes Mellitus
If one accepts the premise that the endothelium is a “gateway” to vascular disease, then one would expect that therapies known to reduce cardiovascular risk would also improve endothelial function. This expectation has been fulfilled, except in rare instances. Cholesterol lowering unequivocally reduces cardiovascular events,168,169 and multiple studies indicate this effect is accompanied by improved endothelial function.170,171 Angiotensin-converting enzyme inhibitors also reduce cardiovascular risk172 and improve endothelial function.173 Finally, patients with type 2 diabetes mellitus have abnormal endothelial function that is improved by metformin,174 a drug that reduces the myocardial infarction risk in overweight patients.175 Several pharmacological agents are thought to achieve vascular protection through mechanisms that go beyond their primary therapeutic actions (eg, hypotensive or hypocholesterolemic actions).
Lipid-Lowering Drugs
Statin, a well-known cholesterol-lowering drug, has been shown to execute its vascular protective effect at least in part through AMPK activation.102,176–179 Berberine, an alkaloid isolated from a Chinese herb widely used as a drug to treat gastrointestinal infections, has recently been described as a new cholesterol-lowering drug.180 The cholesterol-lowering effect of berberine is distinct from that of the statin drugs in that it elevates expression of the LDL receptor through a posttranscriptional mechanism that stabilizes the mRNA.180 Surprisingly, the increasingly emerging evidence links many of its beneficial effects on CVDs, such as suppression of proinflammatory responses in macrophage,181 improvement of glucose metabolism through induction of glycolysis,182 and prevention of hyperglycemia-induced endothelial injury and enhancement of vasodilation,183 to AMPK.
Antioxidants
Given the consistent and promising findings from experimental, clinical, and epidemiological investigations, the overall results of large-scale clinical studies investigating antioxidant (principally vitamin E and C) effects on CVD have been disappointing.184,185 At least several explanations may account for this outcome. First, the type of antioxidants used may be ineffective and nonspecific. For example, ONOO3 is a highly reactive oxidant with proteins, lipids, and DNA that causes tissue injury. The reaction of ONOO− is at least 1000 times faster with the zinc-thiolate complex (5.2 × 105 mol · L−1s−1) than with cysteine thiols (6 × 102 mol · L−1s−1) and 100 times faster than with BH4 (6 × 103 mol · L−1s−1) or vitamin C or E. Thus, antioxidants such as vitamin E or C require extremely high concentrations to compete for ONOO− with endogenous targets. Therefore, negative results with antioxidant vitamins cannot be generalized to all antioxidants. Second, the dosing regimens and duration of therapy may have been insufficient.186,187 Third, some antioxidants, such as vitamin E and vitamin C, might possess a pro-oxidant property. Fourth, there is insufficient evidence to demonstrate that vitamin E reaches target cells. Fifth, the antioxidant potency of vitamins such as C and E is limited, because these antioxidants work as scavengers of existing excess reactive species in a stoichiometric manner, and this approach represents a symptomatic approach to oxidative stress-associated clinical problems. Sixth, the trial design did not allow for recruitment of subjects based on evidence of elevated ROS formation.188 Indeed, the failed clinical trials of vitamin E may have not addressed possible benefits to subgroups with increased oxidative stress. Seventh, as has been eloquently argued elsewhere, treating the antioxidant vitamins as a single class of compounds with expected similar effects inappropriately disregards their wide range of chemical properties and pharmacodynamics. Clinical trials to date have been conducted without any real understanding of the mechanisms of action or the concentrations of the various agents seen at different physiological sites. Eighth, in cohorts included in large trials, most subjects have significant CVD, in which damaging effects of oxidative stress may be irreversible. Finally, carefully designed clinical studies have revealed that vitamin E supplementation appears to reduce cardiovascular events in individuals with diabetes mellitus and the Hp 2-2 (haptoglobin, a major antioxidant protein) genotype.189
On the basis of the new developments in our understanding of the pathophysiology of oxidative stress, it is clear that strategies to block the formation of reactive oxygen radicals will provide conclusive evidence on whether antioxidants should be part of the cardiovascular treatment plan in diabetes mellitus. Over the last decade, several classes of ONOO− decomposition catalysts (eg, ebselen and the metalloporphyrin compounds FP-15, FeTPPS, and FeTMPS) have been tested in a variety of experimental models of diabetic complications in various animal models of diabetes (reviewed in Szabo190). The results from these studies have demonstrated that the neutralization of ONOO− significantly delays the development of endothelial dysfunction, cardiomyopathy, retinopathy, nephropathy, and neuropathy in diabetes mellitus (reviewed in Szabo190).
Thiazolidinediones
Treatment with thiazolidinediones has been shown to improve endothelium-dependent vasodilation in patients with diabetes mellitus and in individuals with metabolic syndrome but without diabetes after 8 weeks of rosiglitazone treatment. Moreover, in obese men with normal glucose tolerance, 12 weeks of pioglitazone treatment reduced markers of endothelial function, such as intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin. Finally, in nondiabetic patients with major cardiovascular risk factors, pioglitazone treatment improved both insulin sensitivity and endothelial vasodilator function. Consistently, thiazolidinediones have also been reported to reduce the levels of biomarkers (eg, C-reactive protein, TNF, and cytokines), intimamedia thickness, restenosis, and plaque stability in diabetic and nondiabetic patients. The PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events)191–196 also reported that pioglitazone treatment led to a nonsignificant 10% reduction of the combined primary end point (mortality, nonfatal myocardial infarction, acute coronary syndrome, stroke, percutaneous or surgical coronary revascularization, peripheral revascularization, or leg amputation), although thiazolidinedione treatment significantly reduced the predefined, principal secondary end point of mortality, nonfatal myocardial infarction, and stroke by 16%. These data suggest that thiazolidinediones may have beneficial vascular properties independent of their effect on insulin sensitivity and inflammation. PROactive also showed an increase in hospitalization due to cardiac decompensation, which most likely stems from fluid retention in the kidney. Thus, the difference of improved endothelial function and increased cardiac incidence in thiazolidinedione-treated patients might be explained by the off-target effects of thiazolidinediones in other organs such as hearts and kidneys.
Metformin
The biguanide metformin is widely used in type 2 diabetes mellitus. Metformin is known to have beneficial vascular effects in animal studies and most importantly in clinical studies. Data from the United Kingdom Prospective Diabetes Study provided the first substantive prospective evidence that early and intensive intervention with metformin could reduce the incidence of myocardial infarction and increase survival in overweight patients with type 2 diabetes mellitus. Moreover, this apparently vasoprotective effect of metformin could not be explained by its blood glucose–lowering efficacy, because intensive therapy with a sulfonylurea or insulin produced similar glycemic control but afforded less protection against myocardial infarction and other macrovascular events. A large, randomized, double-blind trial with tranilast to prevent restenosis after percutaneous coronary intervention (the Presto trial) provided prospective data from patients with type 2 diabetes mellitus who were receiving metformin (n=887) versus other antidiabetic therapies that excluded a thiazolidinedione (n=1110). After 9 months, metformin reduced the incidence of myocardial infarctions by 69% and of all-cause mortality by 61%.197
Metformin is known to improve sensitivity to the glycemic effects of insulin; however, metformin appears to have a direct protective effect on endothelial cells. Metformin is reported to reduce monocyte adhesion to human endothelial cells.198 Furthermore, several studies have shown that metformin therapy improves vascular reactivity. Metformin therapy for 3 months increased acetylcholine-induced endothelium-dependent vasodilation, whereas there was no significant increase in nitroprusside-induced endothelium-independent vasodilation in patients with type 2 diabetes mellitus.174 Other studies have further demonstrated that metformin-increased endothelium-dependent vasodilation appears to be independent of glycemia.199,200 Conversely, there are conflicting reports that metformin has no significant effects on endothelium-dependent vasodilation in patients with type 2 diabetes mellitus.201,202 The reasons for these discrepancies remain unknown, but they are likely related to the difference in the methods used for the assays of the endothelium-dependent relaxation and the patient populations studied.
AMPK as an Emerging Therapy for Promoting Vascular Health in Diabetes Mellitus
The pharmacological effects of metformin have recently been ascribed to its activation of AMPK. Certain beneficial effects of agents such as physical exercise, statins, thiazolidinediones, leptin, adiponectin, and rosiglitazone are mediated, at least in part, by activation of AMPK in endothelial cells.203 Although these effective therapies have distinct effects in improving endothelial function in diabetes mellitus, AMPK activation appears to be a shared molecular target in vascular tissues.
AMPK was initially identified as a sensor of cellular energy and is also likely a sensor of cellular redox status.102,204 AMPK is a heterotrimeric enzyme composed of a catalytic (α1 or α2) subunit and 2 regulatory (β1 or β2 and γ1, γ2, and γ3) subunits, all of which are encoded by separate genes, which makes it possible to form a total of 12 complexes.205 AMPKs are activated by 2 distinct signals: A Ca2+-dependent pathway mediated by calcium calmodulin-dependent kinase kinase-β (CaMKK-β) and an AMP-dependent pathway mediated by LKB1.102 These and other upstream kinases, including TGF-β–activated kinase-1 (Tak1), phosphorylate Thr172 on the α-subunit. Binding of AMP to the γ-subunit leads to allosteric activation of AMPK and protection of Thr172 from dephosphorylation, thereby maintaining the enzyme in the activated state. AMPK is a key regulator of carbohydrate and lipid metabolism, and activation of this protein kinase is expected to have multiple beneficial effects for diabetes mellitus, including suppression of lipogenesis and gluconeogenesis, as well as augmentation of fatty acid oxidation and glucose uptake.203 AMPK has also been shown to mediate the glucose-lowering efficacy of metformin in both experimental animals and humans.153,206,207 Thus, AMPK activation might indirectly improve vascular endothelial function via its improvement in metabolic profiles and insulin sensitivity. Because the effects of AMPK on other key metabolically relevant tissues such as liver, skeletal muscle, adipose, and hypothalamus have been a subject of several recent reviews, in the present report, we will focus the discussion on the beneficial effects of AMPK activation in endothelium and how AMPK activation could be used as a strategy to reverse endothelial dysfunction in diabetes mellitus and other CVDs (Figure 8). This hypothesis is strengthened by the findings from us208–211 and others212–215 that AMPK is essential for maintaining endothelial homeostasis by increasing NO bioactivity and mitochondrial biogenesis and by suppressing inflammation and the production of ROS/ONOO− in endothelial cells.
Figure 8.
AMPK as an emerging therapeutic target for promoting vascular health in diabetes mellitus.
AMPK-eNOS-NO Axis: AMPK Is Important for NO Bioactivity and Endothelial Function
The notion that AMPK contributes to eNOS bioactivity is supported by in vitro and in vivo work by our group and others. We were among the first to observe that blood vessels from α1-AMPK–null mice exhibit defective eNOS-mediated NO production in response to estradiol.212 We also showed that AMPK promotes eNOS association with heat shock protein 90 (HSP90) and in this way is required for estradiol-induced eNOS activation.212 Similarly, in vivo activation of AMPK with the antidiabetic drug metformin stimulates NO synthesis, which is promoted by association of HSP90 with eNOS.208 The pharmacological AMPK activator 5-aminoimidazole-4-carboxamide ribose (AICAR) stimulates eNOS activity.216 Adiponectin, which is reduced in the plasma of patients with type 2 diabetes mellitus, protects against myocardial contractile dysfunction and limits infarct size after ischemia and reperfusion. The mechanism underlying this protective effect involves activation of AMPK and production of NO.217 Interestingly, adiponectin-mediated eNOS activation depends on AMPK-mediated eNOS association with HSP90. Ghrelin is a newly discovered gastric peptide that improves endothelial function and inhibits proatherogenic changes. A recent study of cultured endothelial cells and mice aortas revealed that AMPK-dependent eNOS activation underlies the vascular actions of ghrelin.218 Cilostazol, a selective inhibitor of phosphodiesterase 3, increases intracellular cAMP and activates protein kinase A, which leads to inhibition of platelet aggregation and induction of peripheral vasodilation. Administration of cilostazol to diabetic rats significantly restores endothelium-dependent vasodilation, an effect that is attributed at least in part to AMPK-dependent amelioration of eNOS activation and biopterin metabolism in the aorta.213 Finally, AMPK is critical for eNOS activation by vascular endothelial growth factor,219 shear stress,220 and insulin.221 Thus, abundant data link AMPK to NO bioactivity.
AMPK Suppression of Inflammation
Vascular inflammation is a feature of endothelial dysfunction, and NF-κB activation is a major player in the development of vascular inflammation. AMPK activation via several mechanisms suppresses endothelial inflammation. Recent data suggest that AMPK is a master regulator of macrophage differentiation to an antiinflammatory phenotype.222 Such an antiinflammatory role has been suggested by data obtained with the AMPK activators in endothelial cells. Cacicedo and colleagues223 have reported that pharmacological or genetic activation of AMPK blunts palmitate- or TNF-induced NF-κB activation and consequent expression of adhesion molecules in endothelial cells. Furthermore, treatment of mice with AICAR reduces the severity of experimental autoimmune encephalomyelitis.224 AICAR has also been shown to reduce NOS-II synthesis by adipocytes, macrophages, myocytes, and glia.225,226 Similarly, metformin-induced AMPK activation inhibits cytokine-induced NF-κB activation in vascular endothelial cells and thus suppresses inflammation.227 Overall, AMPK appears to be a natural suppressor of NF-κB and vascular inflammation in endothelial cells.
AMPK Suppression of ROS/ONOO−
Although nontoxic levels of ROS/ONOO− modulate AMPK or AMPK-dependent pathways,179,210,228–231 activation of AMPK may suppress ROS/ONOO− generation. For example, rosiglitazone quenches oxidative stress elicited by high glucose by preventing NAD(P)H oxidase activation in an AMPK-dependent manner.232 Accumulating data also support the notion that AMPK activation exerts antiinflammatory responses, likely via suppression of the production of ROS such as ONOO−. Indeed, mice with deficient AMPKα2 exhibited increased ROS in parallel with impaired endothelium-dependent vasorelaxation in isolated aortas.211 Consistently, mice deficient in both the AMPKα2 and apolipoprotein E genes exhibit worse atherosclerosis that involves an alteration in NF-κB activation and NADP(H) oxidase expression (M. Zhang, MD, PhD, M.H. Zou, MD, PhD, unpublished observations, 2009), which further supports a key role of AMPK in improving atherosclerosis and maintaining endothelium homeostasis.
AMPK activation also suppresses endothelial dysfunction by increasing cellular endogenous antioxidant potentials. Pharmacological activation of AMPK with AICAR markedly increased mitochondrial UPC-2 (uncoupling protein-2) expression and reduced both O2− and PGIS nitration in diabetic wild-type mice but not in their AMPKα2-deficient counterparts in vivo.233 Similarly, Kukidome et al234 reported that AMPK activation by metformin normalizes hyperglycemia-induced mitochondria-derived ROS production by induction of Mn-SOD and promotion of mitochondrial biogenesis through activation of the AMPK-PGC-1α (peroxisome proliferator–activated receptor-γ coactivator-1α) pathway. This is important because mitochondria are a principal site for hyperglycemia-driven O2− in endothelial cells.
AMPK Increases Mitochondrial Biogenesis in Endothelial Cells
AMPK activation has been shown to induce mitochondrial biogenesis via PGC-1α induction in the endothelium,214 consistent with another report.235 In parallel, AMPK increases cellular NAD+ levels and enhances sirtuin 1 (SIRT1) activity, which results in the deacetylation and activation of PGC-1α.236 Indeed, chronic administration of AICAR in vivo prevented angiotensin II– or endotoxin-mediated JNK activation and endothelial injury, a process dependent on oxidative stress and vascular ROS production. This protective effect of AICAR was lost in mice lacking AMPK and PGC-1α.214 Thus, these data indicate that AMPK can direct adaptive changes in the mitochondria via PGC-1α, which enhances mitochondrial biogenesis and cellular resistance to stress.
Concluding Remarks
Macrovascular and microvascular disorders are the principal causes of morbidity and mortality in patients with diseases that involve the cardiovascular system (eg, atherosclerosis and diabetes mellitus). The endothelium has emerged as the key regulator of vascular homeostasis because it not only serves a barrier function but also acts as an active signal transducer that regulates vessel wall phenotype. Abnormal vasomotor responses and impaired endothelium-dependent vasodilation have been demonstrated in a number of vessels in a variety of animal models and in humans with CVDs. Endothelial dysfunction plays a key role in the development of these diseases, although the genesis of this endothelial dysfunction and its associated vasomotor abnormalities remains poorly understood. Hyperglycemia, fatty acids, inflammation, and insulin resistance are major inducers of endothelial dysfunction in diabetes mellitus and likely act through common mechanisms that involve reactive nitrogen species. Increasing evidence suggests that AMPK activation suppresses ROS but increases both NO release and mitochondrial biogenesis in endothelial cells. Thus, AMPK appears to be a therapeutic target for improving endothelial function in patients with diabetes mellitus and other forms of CVD.
Acknowledgments
We sincerely apologize to our colleagues whose original contributions were not cited owing to page limitations. We thank all current and former members of Dr Zou’s laboratory for the work described in this review.
Sources of Funding
Work described in this review was supported in part or as a whole by grants from the National Institutes of Health (HL079584, HL074399, HL080499, HL089920, and HL096032), as well as by research awards from the American Diabetes Association, Juvenile Diabetes Research Foundation, Oklahoma Center for Advancement of Science and Technology, and Travis Endowed Chair in Endocrinology, University of Oklahoma Health Sciences Center. Dr Zou is a recipient of the National Established Investigator Award of the American Heart Association.
Footnotes
Disclosures
None.
References
- 1.Zou MH, Cohen R, Ullrich V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium. 2004;11:89–97. doi: 10.1080/10623320490482619. [DOI] [PubMed] [Google Scholar]
- 2.Brunner H, Cockcroft JR, Deanfield J, Donald A, Ferrannini E, Halcox J, Kiowski W, Luscher TF, Mancia G, Natali A, Oliver JJ, Pessina AC, Rizzoni D, Rossi GP, Salvetti A, Spieker LE, Taddei S, Webb DJ. Endothelial function and dysfunction, part II: association with cardiovascular risk factors and diseases: a statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:233–246. doi: 10.1097/00004872-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Caballero AE. Endothelial dysfunction, inflammation, and insulin resistance: a focus on subjects at risk for type 2 diabetes. Curr Diab Rep. 2004;4:237–246. doi: 10.1007/s11892-004-0074-9. [DOI] [PubMed] [Google Scholar]
- 4.Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323:27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 5.Deanfield J, Donald A, Ferri C, Giannattasio C, Halcox J, Halligan S, Lerman A, Mancia G, Oliver JJ, Pessina AC, Rizzoni D, Rossi GP, Salvetti A, Schiffrin EL, Taddei S, Webb DJ. Endothelial function and dysfunction, part I: methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:7–17. doi: 10.1097/00004872-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 7.Abdu TA, Elhadd T, Pfeifer M, Clayton RN. Endothelial dysfunction in endocrine disease. Trends Endocrinol Metab. 2001;12:257–265. doi: 10.1016/s1043-2760(01)00425-8. [DOI] [PubMed] [Google Scholar]
- 8.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 9.Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 10.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 11.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 12.Celermajer DS. Reliable endothelial function testing: at our fingertips? Circulation. 2008;117:2428–2430. doi: 10.1161/CIRCULATIONAHA.108.775155. [DOI] [PubMed] [Google Scholar]
- 13.Schalkwijk CG, Stehouwer CD. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin Sci (Lond) 2005;109:143–159. doi: 10.1042/CS20050025. [DOI] [PubMed] [Google Scholar]
- 14.Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (2) N Engl J Med. 1992;326:310–318. doi: 10.1056/NEJM199201303260506. [DOI] [PubMed] [Google Scholar]
- 16.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 17.Rask-Madsen C, King GL. Mechanisms of disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3:46–56. doi: 10.1038/ncpendmet0366. [DOI] [PubMed] [Google Scholar]
- 18.De Meyer GR, Herman AG. Vascular endothelial dysfunction. Prog Cardiovasc Dis. 1997;39:325–342. doi: 10.1016/s0033-0620(97)80031-x. [DOI] [PubMed] [Google Scholar]
- 19.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 20.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 21.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 22.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 23.Vita JA, Keaney JF., Jr Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106:640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 24.McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Henry WR, Andrews JW, Hayes JR. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:771–776. doi: 10.1007/BF00429099. [DOI] [PubMed] [Google Scholar]
- 25.Nathan DM, Lachin J, Cleary P, Orchard T, Brillon DJ, Backlund JY, O’Leary DH, Genuth S. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003;348:2294–2303. doi: 10.1056/NEJMoa022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27:567–574. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- 27.Perseghin G, Ghosh S, Gerow K, Shulman GI. Metabolic defects in lean nondiabetic offspring of NIDDM parents: a cross-sectional study. Diabetes. 1997;46:1001–1009. doi: 10.2337/diab.46.6.1001. [DOI] [PubMed] [Google Scholar]
- 28.Williams SB, Goldfine AB, Timimi FK, Ting HH, Roddy MA, Simonson DC, Creager MA. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998;97:1695–1701. doi: 10.1161/01.cir.97.17.1695. [DOI] [PubMed] [Google Scholar]
- 29.Ruderman NB, Haudenschild C. Diabetes as an atherogenic factor. Prog Cardiovasc Dis. 1984;26:373–412. doi: 10.1016/0033-0620(84)90011-2. [DOI] [PubMed] [Google Scholar]
- 30.Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes. 2002;51:159–167. doi: 10.2337/diabetes.51.1.159. [DOI] [PubMed] [Google Scholar]
- 31.Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest. 1999;104:447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacob SRK, Haring HU. Oxidative stress and insulin action: a role for antioxidants? In: Packer L, Rösen P, Tritschler HJ, King GL, editors. Antioxidants in Diabetes Management. New York, NY: Marcel Decker; 2000. pp. 319–338. [Google Scholar]
- 33.Jacob S, Machann J, Rett K, Brechtel K, Volk A, Renn W, Maerker E, Matthaei S, Schick F, Claussen CD, Haring HU. Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes. 1999;48:1113–1119. doi: 10.2337/diabetes.48.5.1113. [DOI] [PubMed] [Google Scholar]
- 34.Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 35.Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities: the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334:374–381. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]
- 36.Reaven GM. Banting lecture 1988: role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 37.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Jr, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 38.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 39.Magill SDJ. Effects of hyperglycemia on vascular endothelium nitric oxide metabolism. In: Sowers JR, editor. Endocrinology of the Vasculature. Totowa, NJ: Humana Press; 1986. pp. 145–156. [Google Scholar]
- 40.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 41.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 42.Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- 43.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 44.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 45.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 46.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 47.Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 48.Tamarat R, Silvestre JS, Le Ricousse-Roussanne S, Barateau V, Lecomte-Raclet L, Clergue M, Duriez M, Tobelem G, Levy BI. Impairment in ischemia-induced neovascularization in diabetes: bone marrow mononuclear cell dysfunction and therapeutic potential of placenta growth factor treatment. Am J Pathol. 2004;164:457–466. doi: 10.1016/S0002-9440(10)63136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Llevadot J, Murasawa S, Kureishi Y, Uchida S, Masuda H, Kawamoto A, Walsh K, Isner JM, Asahara T. HMG-CoA reductase inhibitor mobilizes bone marrow–derived endothelial progenitor cells. J Clin Invest. 2001;108:399–405. doi: 10.1172/JCI13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–3024. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 51.Werner N, Junk S, Laufs U, Link A, Walenta K, Bohm M, Nickenig G. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res. 2003;93:e17–e24. doi: 10.1161/01.RES.0000083812.30141.74. [DOI] [PubMed] [Google Scholar]
- 52.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, Dimmeler S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–2890. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 53.Laufs U, Werner N, Link A, Endres M, Wassmann S, Jurgens K, Miche E, Bohm M, Nickenig G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109:220–226. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 54.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23:1185–1189. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- 55.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219–1222. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 57.Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG, Nedeau A, Thom SR, Velazquez OC. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 59.Caballero AE. Endothelial dysfunction in obesity and insulin resistance: a road to diabetes and heart disease. Obes Res. 2003;11:1278–1289. doi: 10.1038/oby.2003.174. [DOI] [PubMed] [Google Scholar]
- 60.Nelson SM, Sattar N, Freeman DJ, Walker JD, Lindsay RS. Inflammation and endothelial activation is evident at birth in offspring of mothers with type 1 diabetes. Diabetes. 2007;56:2697–2704. doi: 10.2337/db07-0662. [DOI] [PubMed] [Google Scholar]
- 61.Tripathy D, Aljada A, Dandona P. Free fatty acids (FFA) and endothelial dysfunction: role of increased oxidative stress and inflammation. Diabetologia. 2003;46:300–301. doi: 10.1007/s00125-002-1027-y. [DOI] [PubMed] [Google Scholar]
- 62.Capaldo B, Napoli R, Di Marino L, Picardi A, Riccardi G, Sacca L. Quantitation of forearm glucose and free fatty acid (FFA) disposal in normal subjects and type II diabetic patients: evidence against an essential role for FFA in the pathogenesis of insulin resistance. J Clin Endocrinol Metab. 1988;67:893–898. doi: 10.1210/jcem-67-5-893. [DOI] [PubMed] [Google Scholar]
- 63.Boden G, Chen X, Rosner J, Barton M. Effects of a 48-h fat infusion on insulin secretion and glucose utilization. Diabetes. 1995;44:1239–1242. doi: 10.2337/diab.44.10.1239. [DOI] [PubMed] [Google Scholar]
- 64.Smith U, Axelsen M, Carvalho E, Eliasson B, Jansson PA, Wesslau C. Insulin signaling and action in fat cells: associations with insulin resistance and type 2 diabetes. Ann N Y Acad Sci. 1999;892:119–126. doi: 10.1111/j.1749-6632.1999.tb07790.x. [DOI] [PubMed] [Google Scholar]
- 65.Saloranta C, Groop L. Interactions between glucose and FFA metabolism in man. Diabetes Metab Rev. 1996;12:15–36. doi: 10.1002/(SICI)1099-0895(199603)12:1<15::AID-DMR153>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 66.Boden G, She P, Mozzoli M, Cheung P, Gumireddy K, Reddy P, Xiang X, Luo Z, Ruderman N. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappaB pathway in rat liver. Diabetes. 2005;54:3458–3465. doi: 10.2337/diabetes.54.12.3458. [DOI] [PubMed] [Google Scholar]
- 67.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boden G, Chen X. Effects of fat on glucose uptake and utilization in patients with non-insulin-dependent diabetes. J Clin Invest. 1995;96:1261–1268. doi: 10.1172/JCI118160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leiter LA, Lewanczuk RZ. Of the renin-angiotensin system and reactive oxygen species type 2 diabetes and angiotensin II inhibition. Am J Hypertens. 2005;18:121–128. doi: 10.1016/j.amjhyper.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 70.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 71.Dandona P, Aljada A, Chaudhuri A, Mohanty P. Endothelial dysfunction, inflammation and diabetes. Rev Endocr Metab Disord. 2004;5:189–197. doi: 10.1023/B:REMD.0000032407.88070.0a. [DOI] [PubMed] [Google Scholar]
- 72.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 73.Stenvinkel P. Endothelial dysfunction and inflammation: is there a link? Nephrol Dial Transplant. 2001;16:1968–1971. doi: 10.1093/ndt/16.10.1968. [DOI] [PubMed] [Google Scholar]
- 74.Vallance P, Collier J, Bhagat K. Infection, inflammation, and infarction: does acute endothelial dysfunction provide a link? Lancet. 1997;349:1391–1392. doi: 10.1016/S0140-6736(96)09424-X. [DOI] [PubMed] [Google Scholar]
- 75.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 76.Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 77.Craven PA, Studer RK, DeRubertis FR. Impaired nitric oxide-dependent cyclic guanosine monophosphate generation in glomeruli from diabetic rats: evidence for protein kinase C-mediated suppression of the cholinergic response. J Clin Invest. 1994;93:311–320. doi: 10.1172/JCI116961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Graier WF, Simecek S, Kukovetz WR, Kostner GM. High D-glucose-induced changes in endothelial Ca2+/EDRF signaling are due to generation of superoxide anions. Diabetes. 1996;45:1386–1395. doi: 10.2337/diab.45.10.1386. [DOI] [PubMed] [Google Scholar]
- 79.Beckman JA, Goldfine AB, Gordon MB, Garrett LA, Creager MA. Inhibition of protein kinase Cβ prevents impaired endothelium-dependent vasodilation caused by hyperglycemia in humans. Circ Res. 2002;90:107–111. doi: 10.1161/hh0102.102359. [DOI] [PubMed] [Google Scholar]
- 80.Veves A, Akbari CM, Primavera J, Donaghue VM, Zacharoulis D, Chrzan JS, DeGirolami U, LoGerfo FW, Freeman R. Endothelial dysfunction and the expression of endothelial nitric oxide synthetase in diabetic neuropathy, vascular disease, and foot ulceration. Diabetes. 1998;47:457–463. doi: 10.2337/diabetes.47.3.457. [DOI] [PubMed] [Google Scholar]
- 81.Wadham C, Parker A, Wang L, Xia P. High glucose attenuates protein S-nitrosylation in endothelial cells: role of oxidative stress. Diabetes. 2007;56:2715–2721. doi: 10.2337/db06-1294. [DOI] [PubMed] [Google Scholar]
- 82.Raij L. Nitric oxide in the pathogenesis of cardiac disease. J Clin Hypertens (Greenwich) 2006;8:30–39. doi: 10.1111/j.1524-6175.2006.06025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rabinovitch A, Suarez-Pinzon WL, Sorensen O, Bleackley RC. Inducible nitric oxide synthase (iNOS) in pancreatic islets of nonobese diabetic mice: identification of iNOS- expressing cells and relationships to cytokines expressed in the islets. Endocrinology. 1996;137:2093–2099. doi: 10.1210/endo.137.5.8612552. [DOI] [PubMed] [Google Scholar]
- 84.Eizirik DL, Leijerstam F. The inducible form of nitric oxide synthase (iNOS) in insulin-producing cells. Diabetes Metab. 1994;20:116–122. [PubMed] [Google Scholar]
- 85.Sharma K, Danoff TM, DePiero A, Ziyadeh FN. Enhanced expression of inducible nitric oxide synthase in murine macrophages and glomerular mesangial cells by elevated glucose levels: possible mediation via protein kinase C. Biochem Biophys Res Commun. 1995;207:80–88. doi: 10.1006/bbrc.1995.1156. [DOI] [PubMed] [Google Scholar]
- 86.Zou MH, Klein T, Pasquet JP, Ullrich V. Interleukin 1beta decreases prostacyclin synthase activity in rat mesangial cells via endogenous peroxynitrite formation. Biochem J. 1998;336(part 2):507–512. doi: 10.1042/bj3360507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schonfelder G, John M, Hopp H, Fuhr N, van Der Giet M, Paul M. Expression of inducible nitric oxide synthase in placenta of women with gestational diabetes. FASEB J. 1996;10:777–784. doi: 10.1096/fasebj.10.7.8635695. [DOI] [PubMed] [Google Scholar]
- 88.Cai S, Khoo J, Mussa S, Alp NJ, Channon KM. Endothelial nitric oxide synthase dysfunction in diabetic mice: importance of tetrahydrobiopterin in eNOS dimerisation. Diabetologia. 2005;48:1933–1940. doi: 10.1007/s00125-005-1857-5. [DOI] [PubMed] [Google Scholar]
- 89.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 90.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu J, Xie Z, Reece R, Pimental D, Zou MH. Uncoupling of endothelial nitric oxidase synthase by hypochlorous acid: role of NAD(P)H oxidase-derived superoxide and peroxynitrite. Arterioscler Thromb Vasc Biol. 2006;26:2688–2695. doi: 10.1161/01.ATV.0000249394.94588.82. [DOI] [PubMed] [Google Scholar]
- 92.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pieper GM. Acute amelioration of diabetic endothelial dysfunction with a derivative of the nitric oxide synthase cofactor, tetrahydrobiopterin. J Cardiovasc Pharmacol. 1997;29:8–15. doi: 10.1097/00005344-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 94.Pieper GM, Moore-Hilton G, Roza AM. Evaluation of the mechanism of endothelial dysfunction in the genetically-diabetic BB rat. Life Sci. 1996;58:PL147–PL152. doi: 10.1016/0024-3205(95)02360-7. [DOI] [PubMed] [Google Scholar]
- 95.Diederich D, Skopec J, Diederich A, Dai FX. Endothelial dysfunction in mesenteric resistance arteries of diabetic rats: role of free radicals. Am J Physiol. 1994;266:H1153–H1161. doi: 10.1152/ajpheart.1994.266.3.H1153. [DOI] [PubMed] [Google Scholar]
- 96.Taylor PD, Poston L. The effect of hyperglycaemia on function of rat isolated mesenteric resistance artery. Br J Pharmacol. 1994;113:801–808. doi: 10.1111/j.1476-5381.1994.tb17064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tesfamariam B. Selective impairment of endothelium-dependent relaxations by prostaglandin endoperoxide. J Hypertens. 1994;12:41–47. [PubMed] [Google Scholar]
- 98.Voinea M, Georgescu A, Manea A, Dragomir E, Manduteanu I, Popov D, Simionescu M. Superoxide dismutase entrapped-liposomes restore the impaired endothelium-dependent relaxation of resistance arteries in experimental diabetes. Eur J Pharmacol. 2004;484:111–118. doi: 10.1016/j.ejphar.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 99.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C–dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 100.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 101.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zou MH, Wu Y. AMP-activated protein kinase activation as a strategy for protecting vascular endothelial function. Clin Exp Pharmacol Physiol. 2008;35:535–545. doi: 10.1111/j.1440-1681.2007.04851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zou MH. Peroxynitrite and protein tyrosine nitration of prostacyclin synthase. Prostaglandins Other Lipid Mediat. 2007;82:119–127. doi: 10.1016/j.prostaglandins.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 104.Schulz E, Anter E, Keaney JF., Jr Oxidative stress, antioxidants, and endothelial function. Curr Med Chem. 2004;11:1093–1104. doi: 10.2174/0929867043365369. [DOI] [PubMed] [Google Scholar]
- 105.Pacher P, Szabo C. Role of peroxynitrite in the pathogenesis of cardiovascular complications of diabetes. Curr Opin Pharmacol. 2006;6:136–141. doi: 10.1016/j.coph.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, Boemi M, Giugliano D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57:1349–1354. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 107.Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123–1132. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- 108.Harrison HE, Reece AH, Johnson M. Decreased vascular prostacyclin in experimental diabetes. Life Sci. 1978;23:351–355. doi: 10.1016/0024-3205(78)90020-6. [DOI] [PubMed] [Google Scholar]
- 109.Johnson M, Harrison HE, Raftery AT, Elder JB. Vascular prostacyclin may be reduced in diabetes in man. Lancet. 1979;1:325–326. doi: 10.1016/s0140-6736(79)90737-2. [DOI] [PubMed] [Google Scholar]
- 110.Dollery CT, Friedman LA, Hensby CN, Kohner E, Lewis PJ, Porta M, Webster J. Circulating prostacyclin may be reduced in diabetes. Lancet. 1979;2:1365. doi: 10.1016/s0140-6736(79)92844-7. [DOI] [PubMed] [Google Scholar]
- 111.Harrison HE, Reece AH, Johnson M. Effect of insulin treatment on prostacyclin in experimental diabetes. Diabetologia. 1980;18:65–68. doi: 10.1007/BF01228305. [DOI] [PubMed] [Google Scholar]
- 112.Silberbauer K, Clopath P, Sinzinger H, Schernthaner G. Effect of experimentally induced diabetes on swine vascular prostacyclin (PGI2) synthesis. Artery. 1980;8:30–36. [PubMed] [Google Scholar]
- 113.Silberbauer K, Schernthaner G, Sinzinger H, Piza-Katzer H, Winter M. Decreased vascular prostacyclin in juvenile-onset diabetes. N Engl J Med. 1979;300:366–367. [PubMed] [Google Scholar]
- 114.Davis TM, Bown E, Finch DR, Mitchell MD, Turner RC. In-vitro venous prostacyclin production, plasma 6-keto-prostaglandin F1 alpha concentrations, and diabetic retinopathy. BMJ (Clin Res Ed) 1981;282:1259–1262. doi: 10.1136/bmj.282.6272.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Karpen CW, Pritchard KA, Jr, Merola AJ, Panganamala RV. Alterations of the prostacyclin-thromboxane ratio in streptozotocin induced diabetic rats. Prostaglandins Leukot Med. 1982;8:93–103. doi: 10.1016/s0262-1746(82)80001-2. [DOI] [PubMed] [Google Scholar]
- 116.Naveh-Floman N, Moisseiev J. Prostanoids and thromboxane A2 involvement in diabetic retinopathy. Metab Pediatr Syst Ophthalmol. 1982;6:321–325. [PubMed] [Google Scholar]
- 117.Stuart MJ, Sunderji SG, Allen JB. Decreased prostacyclin production in the infant of the diabetic mother. J Lab Clin Med. 1981;98:412–416. [PubMed] [Google Scholar]
- 118.Yokoyama C, Yabuki T, Shimonishi M, Wada M, Hatae T, Ohkawara S, Takeda J, Kinoshita T, Okabe M, Tanabe T. Prostacyclin-deficient mice develop ischemic renal disorders, including nephrosclerosis and renal infarction. Circulation. 2002;106:2397–2403. doi: 10.1161/01.cir.0000034733.93020.bc. [DOI] [PubMed] [Google Scholar]
- 119.Somova L, Kamenov V, Doncheva M, Kirilov G, Vassileva M. Pathogenesis of cardiovascular disorders in streptozotocin-induced diabetes in rat, II: correlation between lipid peroxides, thromboxane A2/prostacyclin, and platelet aggregation in different stages of diabetes. Acta Physiol Pharmacol Bulg. 1988;14:57–62. [PubMed] [Google Scholar]
- 120.Tesfamariam B, Brown ML, Cohen RA. Elevated glucose impairs endothelium-dependent relaxation by activating protein kinase C. J Clin Invest. 1991;87:1643–1648. doi: 10.1172/JCI115179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tesfamariam B, Brown ML, Deykin D, Cohen RA. Elevated glucose promotes generation of endothelium-derived vasoconstrictor prostanoids in rabbit aorta. J Clin Invest. 1990;85:929–932. doi: 10.1172/JCI114521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mais DE, Saussy DL, Jr, Chaikhouni A, Kochel PJ, Knapp DR, Hamanaka N, Halushka PV. Pharmacologic characterization of human and canine thromboxane A2/prostaglandin H2 receptors in platelets and blood vessels: evidence for different receptors. J Pharmacol Exp Ther. 1985;233:418–424. [PubMed] [Google Scholar]
- 123.Ogletree ML, Harris DN, Greenberg R, Haslanger MF, Nakane M. Pharmacological actions of SQ 29,548, a novel selective thromboxane antagonist. J Pharmacol Exp Ther. 1985;234:435–441. [PubMed] [Google Scholar]
- 124.Fitzgerald DJ, Fragetta J, FitzGerald GA. Prostaglandin endoperoxides modulate the response to thromboxane synthase inhibition during coronary thrombosis. J Clin Invest. 1988;82:1708–1713. doi: 10.1172/JCI113784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jariyawat S, Takeda M, Kobayashi M, Endou H. Thromboxane A2 mediates cisplatin-induced apoptosis of renal tubule cells. Biochem Mol Biol Int. 1997;42:113–121. doi: 10.1080/15216549700202491. [DOI] [PubMed] [Google Scholar]
- 126.Ushikubi F, Aiba Y, Nakamura K, Namba T, Hirata M, Mazda O, Katsura Y, Narumiya S. Thromboxane A2 receptor is highly expressed in mouse immature thymocytes and mediates DNA fragmentation and apoptosis. J Exp Med. 1993;178:1825–1830. doi: 10.1084/jem.178.5.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ishizuka T, Suzuki K, Kawakami M, Hidaka T, Matsuki Y, Nakamura H. Thromboxane A2 receptor blockade suppresses intercellular adhesion molecule-1 expression by stimulated vascular endothelial cells. Eur J Pharmacol. 1996;312:367–377. doi: 10.1016/0014-2999(96)00478-5. [DOI] [PubMed] [Google Scholar]
- 128.Ishizuka T, Kawakami M, Hidaka T, Matsuki Y, Takamizawa M, Suzuki K, Kurita A, Nakamura H. Stimulation with thromboxane A2 (TXA2) receptor agonist enhances ICAM-1, VCAM-1 or ELAM-1 expression by human vascular endothelial cells. Clin Exp Immunol. 1998;112:464–470. doi: 10.1046/j.1365-2249.1998.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sachinidis A, Flesch M, Ko Y, Schror K, Bohm M, Dusing R, Vetter H. Thromboxane A2 and vascular smooth muscle cell proliferation. Hypertension. 1995;26:771–780. doi: 10.1161/01.hyp.26.5.771. [DOI] [PubMed] [Google Scholar]
- 130.Zucker TP, Bonisch D, Muck S, Weber AA, Bretschneider E, Glusa E, Schror K. Thromboxane A2 potentiates thrombin-induced proliferation of coronary artery smooth muscle cells. Adv Exp Med Biol. 1997;433:387–390. doi: 10.1007/978-1-4899-1810-9_84. [DOI] [PubMed] [Google Scholar]
- 131.Ko FN. Low-affinity thromboxane receptor mediates proliferation in cultured vascular smooth muscle cells of rats. Arterioscler Thromb Vasc Biol. 1997;17:1274–1282. doi: 10.1161/01.atv.17.7.1274. [DOI] [PubMed] [Google Scholar]
- 132.Jones DA, Benjamin CW, Linseman DA. Activation of thromboxane and prostacyclin receptors elicits opposing effects on vascular smooth muscle cell growth and mitogen-activated protein kinase signaling cascades. Mol Pharmacol. 1995;48:890–896. [PubMed] [Google Scholar]
- 133.Gonzalez E, Franchi AM, Jawerbaum A, Gimeno AL, Gimeno MA. Effects of U46619 and of inhibitors of the synthesis of TXA2 on insulin action on the metabolism of labelled glucose in uteri isolated from ovariectomized-diabetic rats. Prostaglandins. 1994;48:129–137. doi: 10.1016/0090-6980(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 134.Hodgson WC, King RG, Boura AL. Augmented potentiation of renal vasoconstrictor responses by thromboxane A2 receptor stimulation in the alloxan-diabetic rat. J Pharm Pharmacol. 1990;42:423–427. doi: 10.1111/j.2042-7158.1990.tb06583.x. [DOI] [PubMed] [Google Scholar]
- 135.Jawerbaum A, Franchi AM, Gonzalez ET, Novaro V, de Gimeno MA. Hyperglycemia promotes elevated generation of TXA2 in isolated rat uteri. Prostaglandins. 1995;50:47–56. doi: 10.1016/0090-6980(95)00086-p. [DOI] [PubMed] [Google Scholar]
- 136.Tajiri Y, Umeda F, Inoguchi T, Nawata H. Effects of thromboxane synthetase inhibitor (OKY-046) on urinary prostaglandin excretion and renal function in streptozotocin-induced diabetic rat. J Diabetes Complications. 1994;8:126–132. doi: 10.1016/1056-8727(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 137.Masumura H, Kunitada S, Irie K, Ashida S, Abe Y. A thromboxane A2 synthetase inhibitor retards hypertensive rat diabetic nephropathy. Eur J Pharmacol. 1992;210:163–172. doi: 10.1016/0014-2999(92)90667-s. [DOI] [PubMed] [Google Scholar]
- 138.Tesfamariam B, Jakubowski JA, Cohen RA. Contraction of diabetic rabbit aorta caused by endothelium-derived PGH2-TxA2. Am J Physiol. 1989;257:H1327–H1333. doi: 10.1152/ajpheart.1989.257.5.H1327. [DOI] [PubMed] [Google Scholar]
- 139.Shimizu K, Muramatsu M, Kakegawa Y, Asano H, Toki Y, Miyazaki Y, Okumura K, Hashimoto H, Ito T. Role of prostaglandin H2 as an endothelium-derived contracting factor in diabetic state. Diabetes. 1993;42:1246–1252. doi: 10.2337/diab.42.9.1246. [DOI] [PubMed] [Google Scholar]
- 140.Dai FX, Diederich A, Skopec J, Diederich D. Diabetes-induced endothelial dysfunction in streptozotocin-treated rats: role of prostaglandin endoperoxides and free radicals. J Am Soc Nephrol. 1993;4:1327–1336. doi: 10.1681/ASN.V461327. [DOI] [PubMed] [Google Scholar]
- 141.Jaschonek K, Faul C, Weisenberger H, Kronert K, Schroder H, Renn W. Platelet thromboxane A2/endoperoxide (TXA2/PGH2) receptors in type I diabetes mellitus. Thromb Haemost. 1989;61:535–536. [PubMed] [Google Scholar]
- 142.Morinelli TA, Tempel GE, Jaffa AA, Silva RH, Naka M, Folger W, Halushka PV. Thromboxane A2/prostaglandin H2 receptors in streptozotocin-induced diabetes: effects of insulin therapy in the rat. Prostaglandins. 1993;45:427–438. doi: 10.1016/0090-6980(93)90119-r. [DOI] [PubMed] [Google Scholar]
- 143.Zuccollo A, Shi C, Mastroianni R, Maitland-Toolan KA, Weisbrod RM, Zang M, Xu S, Jiang B, Oliver-Krasinski JM, Cayatte AJ, Corda S, Lavielle G, Verbeuren TJ, Cohen RA. The thromboxane A2 receptor antagonist S18886 prevents enhanced atherogenesis caused by diabetes mellitus. Circulation. 2005;112:3001–3008. doi: 10.1161/CIRCULATIONAHA.105.581892. [DOI] [PubMed] [Google Scholar]
- 144.Zhang M, Dong Y, Xu J, Xie Z, Wu Y, Song P, Guzman M, Wu J, Zou MH. Thromboxane receptor activates the AMP-activated protein kinase in vascular smooth muscle cells via hydrogen peroxide. Circ Res. 2008;102:328–337. doi: 10.1161/CIRCRESAHA.107.163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kawada N, Dennehy K, Solis G, Modlinger P, Hamel R, Kawada JT, Aslam S, Moriyama T, Imai E, Welch WJ, Wilcox CS. TP receptors regulate renal hemodynamics during angiotensin II slow pressor response. Am J Physiol Renal Physiol. 2004;287:F753–F759. doi: 10.1152/ajprenal.00423.2003. [DOI] [PubMed] [Google Scholar]
- 146.Xu S, Jiang B, Maitland KA, Bayat H, Gu J, Nadler JL, Corda S, Lavielle G, Verbeuren TJ, Zuccollo A, Cohen RA. The thromboxane receptor antagonist S18886 attenuates renal oxidant stress and proteinuria in diabetic apolipoprotein E-deficient mice. Diabetes. 2006;55:110–119. [PubMed] [Google Scholar]
- 147.Sarman B, Toth M, Somogyi A. Role of endothelin-1 in diabetes mellitus. Diabetes Metab Rev. 1998;14:171–175. doi: 10.1002/(sici)1099-0895(199806)14:2<171::aid-dmr207>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 148.Wedgwood S, McMullan DM, Bekker JM, Fineman JR, Black SM. Role for endothelin-1-induced superoxide and peroxynitrite production in rebound pulmonary hypertension associated with inhaled nitric oxide therapy. Circ Res. 2001;89:357–364. doi: 10.1161/hh1601.094983. [DOI] [PubMed] [Google Scholar]
- 149.Kaehler J, Sill B, Koester R, Mittmann C, Orzechowski HD, Muenzel T, Meinertz T. Endothelin-1 mRNA and protein in vascular wall cells is increased by reactive oxygen species. Clin Sci (Lond) 2002;103(suppl 48):176S–178S. doi: 10.1042/CS103S176S. [DOI] [PubMed] [Google Scholar]
- 150.Zanatta CM, Gerchman F, Burttet L, Nabinger G, Jacques-Silva MC, Canani LH, Gross JL. Endothelin-1 levels and albuminuria in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2008;80:299–304. doi: 10.1016/j.diabres.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 151.el-Mesallamy H, Suwailem S, Hamdy N. Evaluation of C-reactive protein, endothelin-1, adhesion molecule(s), and lipids as inflammatory markers in type 2 diabetes mellitus patients. Mediators Inflamm. 2007;2007:73635. doi: 10.1155/2007/73635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.van Harmelen V, Eriksson A, Astrom G, Wahlen K, Naslund E, Karpe F, Frayn K, Olsson T, Andersson J, Ryden M, Arner P. Vascular peptide endothelin-1 links fat accumulation with alterations of visceral adipocyte lipolysis. Diabetes. 2008;57:378–386. doi: 10.2337/db07-0893. [DOI] [PubMed] [Google Scholar]
- 153.Shaw SG, Boden PJ. Insulin resistance, obesity and the metabolic syndrome: is there a therapeutic role for endothelin-1 antagonists? Curr Vasc Pharmacol. 2005;3:359–363. doi: 10.2174/157016105774329471. [DOI] [PubMed] [Google Scholar]
- 154.Zou MH, Ullrich V. Peroxynitrite formed by simultaneous generation of nitric oxide and superoxide selectively inhibits bovine aortic prostacyclin synthase. FEBS Lett. 1996;382:101–104. doi: 10.1016/0014-5793(96)00160-3. [DOI] [PubMed] [Google Scholar]
- 155.Zou MH, Bachschmid M. Hypoxia-reoxygenation triggers coronary vasospasm in isolated bovine coronary arteries via tyrosine nitration of prostacyclin synthase. J Exp Med. 1999;190:135–139. doi: 10.1084/jem.190.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nie H, Wu JL, Zhang M, Xu J, Zou MH. Endothelial nitric oxide synthase-dependent tyrosine nitration of prostacyclin synthase in diabetes in vivo. Diabetes. 2006;55:3133–3141. doi: 10.2337/db06-0505. [DOI] [PubMed] [Google Scholar]
- 157.Alp NJ, Mussa S, Khoo J, Cai S, Guzik T, Jefferson A, Goh N, Rockett KA, Channon KM. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I overexpression. J Clin Invest. 2003;112:725–735. doi: 10.1172/JCI17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Meininger CJ, Cai S, Parker JL, Channon KM, Kelly KA, Becker EJ, Wood MK, Wade LA, Wu G. GTP cyclohydrolase I gene transfer reverses tetrahydrobiopterin deficiency and increases nitric oxide synthesis in endothelial cells and isolated vessels from diabetic rats. FASEB J. 2004;18:1900–1902. doi: 10.1096/fj.04-1702fje. [DOI] [PubMed] [Google Scholar]
- 159.Katusic ZS, d’Uscio LV. Tetrahydrobiopterin. mediator of endothelial protection. Arterioscler Thromb Vasc Biol. 2004;24:397–398. doi: 10.1161/01.ATV.0000121569.76931.0b. [DOI] [PubMed] [Google Scholar]
- 160.Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:413–420. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
- 161.Cai S, Khoo J, Channon KM. Augmented BH4 by gene transfer restores nitric oxide synthase function in hyperglycemic human endothelial cells. Cardiovasc Res. 2005;65:823–831. doi: 10.1016/j.cardiores.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 162.Du YH, Guan YY, Alp NJ, Channon KM, Chen AF. Endothelium-specific GTP cyclohydrolase I overexpression attenuates blood pressure progression in salt-sensitive low-renin hypertension. Circulation. 2008;117:1045–1054. doi: 10.1161/CIRCULATIONAHA.107.748236. [DOI] [PubMed] [Google Scholar]
- 163.Wang S, Xu J, Song P, Wu Y, Zhang J, Chul Choi H, Zou MH. Acute inhibition of guanosine triphosphate cyclohydrolase 1 uncouples endothelial nitric oxide synthase and elevates blood pressure. Hypertension. 2008;52:484–490. doi: 10.1161/HYPERTENSIONAHA.108.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xu J, Wu Y, Song P, Zhang M, Wang S, Zou MH. Proteasome-dependent degradation of guanosine 5′-triphosphate cyclohydrolase I causes tetrahydrobiopterin deficiency in diabetes mellitus. Circulation. 2007;116:944–953. doi: 10.1161/CIRCULATIONAHA.106.684795. [DOI] [PubMed] [Google Scholar]
- 165.Nomiyama T, Igarashi Y, Taka H, Mineki R, Uchida T, Ogihara T, Choi JB, Uchino H, Tanaka Y, Maegawa H, Kashiwagi A, Murayama K, Kawamori R, Watada H. Reduction of insulin-stimulated glucose uptake by peroxynitrite is concurrent with tyrosine nitration of insulin receptor substrate-1. Biochem Biophys Res Commun. 2004;320:639–647. doi: 10.1016/j.bbrc.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 166.Song P, Wu Y, Xu J, Xie Z, Dong Y, Zhang M, Zou MH. Reactive nitrogen species induced by hyperglycemia suppresses Akt signaling and triggers apoptosis by upregulating phosphatase PTEN (phosphatase and tensin homologue deleted on chromosome 10) in an LKB1-dependent manner. Circulation. 2007;116:1585–1595. doi: 10.1161/CIRCULATIONAHA.107.716498. [DOI] [PubMed] [Google Scholar]
- 167.Nagai R, Unno Y, Hayashi MC, Masuda S, Hayase F, Kinae N, Horiuchi S. Peroxynitrite induces formation of N(epsilon)-(carboxymethyl) lysine by the cleavage of Amadori product and generation of glucosone and glyoxal from glucose: novel pathways for protein modification by peroxynitrite. Diabetes. 2002;51:2833–2839. doi: 10.2337/diabetes.51.9.2833. [DOI] [PubMed] [Google Scholar]
- 168.Lipid Research Clinics Program. The Lipid Research Clinics Coronary Primary Prevention Trial results, I: reduction in incidence of coronary heart disease. JAMA. 1984;251:351–364. doi: 10.1001/jama.1984.03340270029025. [DOI] [PubMed] [Google Scholar]
- 169.Smith SC, Jr, Allen J, Blair SN, Bonow RO, Brass LM, Fonarow GC, Grundy SM, Hiratzka L, Jones D, Krumholz HM, Mosca L, Pasternak RC, Pearson T, Pfeffer MA, Taubert KA. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006;113:2363–2372. doi: 10.1161/CIRCULATIONAHA.106.174516. [DOI] [PubMed] [Google Scholar]
- 170.Anderson TJ, Meredith IT, Yeung AC, Frei B, Selwyn AP, Ganz P. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Engl J Med. 1995;332:488–493. doi: 10.1056/NEJM199502233320802. [DOI] [PubMed] [Google Scholar]
- 171.Tamai O, Matsuoka H, Itabe H, Wada Y, Kohno K, Imaizumi T. Single LDL apheresis improves endothelium-dependent vasodilatation in hypercholesterolemic humans. Circulation. 1997;95:76–82. doi: 10.1161/01.cir.95.1.76. [DOI] [PubMed] [Google Scholar]
- 172.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 173.Anderson TJ, Elstein E, Haber H, Charbonneau F. Comparative study of ACE-inhibition, angiotensin II antagonism, and calcium channel blockade on flow-mediated vasodilation in patients with coronary disease (BANFF study) J Am Coll Cardiol. 2000;35:60–66. doi: 10.1016/s0735-1097(99)00537-9. [DOI] [PubMed] [Google Scholar]
- 174.Mather KJ, Verma S, Anderson TJ. Improved endothelial function with metformin in type 2 diabetes mellitus. J Am Coll Cardiol. 2001;37:1344–1350. doi: 10.1016/s0735-1097(01)01129-9. [DOI] [PubMed] [Google Scholar]
- 175.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 176.Choi HC, Song P, Xie Z, Wu Y, Xu J, Zhang M, Dong Y, Wang S, Lau K, Zou MH. Reactive nitrogen species is required for the activation of the AMP-activated protein kinase by statin in vivo. J Biol Chem. 2008;283:20186–20197. doi: 10.1074/jbc.M803020200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 177.Sun W, Lee TS, Zhu M, Gu C, Wang Y, Zhu Y, Shyy JY. Statins activate AMP-activated protein kinase in vitro and in vivo. Circulation. 2006;114:2655–2662. doi: 10.1161/CIRCULATIONAHA.106.630194. [DOI] [PubMed] [Google Scholar]
- 178.Fisslthaler B, Fleming I, Keseru B, Walsh K, Busse R. Fluid shear stress and NO decrease the activity of the hydroxy-methylglutaryl coenzyme A reductase in endothelial cells via the AMP-activated protein kinase and FoxO1. Circ Res. 2007;100:e12–e21. doi: 10.1161/01.RES.0000257747.74358.1c. [DOI] [PubMed] [Google Scholar]
- 179.Choi HC, Song P, Xie Z, Wu Y, Xu J, Zhang M, Dong Y, Wang S, Lau K, Zou MH. Reactive nitrogen species is required for the activation of the AMP-activated protein kinase by statin in vivo. J Biol Chem. 2008;283:20186–20197. doi: 10.1074/jbc.M803020200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 180.Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, Wang Y, Wang Z, Si S, Pan H, Wang S, Wu J, Wang Y, Li Z, Liu J, Jiang JD. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–1351. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 181.Jeong HW, Hsu KC, Lee JW, Ham M, Huh JY, Shin HJ, Kim WS, Kim JB. Berberine suppresses pro-inflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009;296:E955–E964. doi: 10.1152/ajpendo.90599.2008. [DOI] [PubMed] [Google Scholar]
- 182.Yin J, Gao Z, Liu D, Liu Z, Ye J. Berberine improves glucose metabolism through induction of glycolysis. Am J Physiol Endocrinol Metab. 2008;294:E148–E156. doi: 10.1152/ajpendo.00211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang Y, Huang Y, Lam KS, Li Y, Wong WT, Ye H, Lau CW, Vanhoutte PM, Xu A. Berberine prevents hyperglycemia-induced endothelial injury and enhances vasodilatation via adenosine monophosphate-activated protein kinase and endothelial nitric oxide synthase. Cardiovasc Res. 2009;82:484–492. doi: 10.1093/cvr/cvp078. [DOI] [PubMed] [Google Scholar]
- 184.Heart Protection Study Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 185.Huang HY, Caballero B, Chang S, Alberg AJ, Semba RD, Schneyer CR, Wilson RF, Cheng TY, Vassy J, Prokopowicz G, Barnes GJ, II, Bass EB. The efficacy and safety of multivitamin and mineral supplement use to prevent cancer and chronic disease in adults: a systematic review for a National Institutes of Health state-of-the-science conference. Ann Intern Med. 2006;145:372–385. doi: 10.7326/0003-4819-145-5-200609050-00135. [DOI] [PubMed] [Google Scholar]
- 186.Tribble DL. AHA science advisory: antioxidant consumption and risk of coronary heart disease: emphasis on vitamin C, vitamin E, and beta-carotene: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;99:591–595. doi: 10.1161/01.cir.99.4.591. [DOI] [PubMed] [Google Scholar]
- 187.Siekmeier R, Steffen C, Marz W. Role of oxidants and antioxidants in atherosclerosis: results of in vitro and in vivo investigations. J Cardiovasc Pharmacol Ther. 2007;12:265–282. doi: 10.1177/1074248407299519. [DOI] [PubMed] [Google Scholar]
- 188.Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care. 2008;31(suppl 2):S170–S180. doi: 10.2337/dc08-s247. [DOI] [PubMed] [Google Scholar]
- 189.Milman U, Blum S, Shapira C, Aronson D, Miller-Lotan R, Anbinder Y, Alshiek J, Bennett L, Kostenko M, Landau M, Keidar S, Levy Y, Khemlin A, Radan A, Levy AP. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2–2 genotype: a prospective double-blinded clinical trial. Arterioscler Thromb Vasc Biol. 2008;28:341–347. doi: 10.1161/ATVBAHA.107.153965. [DOI] [PubMed] [Google Scholar]
- 190.Szabo C. Role of nitrosative stress in the pathogenesis of diabetic vascular dysfunction. Br J Pharmacol. 2009;156:713–727. doi: 10.1111/j.1476-5381.2008.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Erdmann E, Dormandy J, Wilcox R, Massi-Benedetti M, Charbonnel B. PROactive 07: pioglitazone in the treatment of type 2 diabetes: results of the PROactive study. Vasc Health Risk Manag. 2007;3:355–370. [PMC free article] [PubMed] [Google Scholar]
- 192.Riche DM, Dale KM. A perspective on coronary revascularization in the PROactive 05 study. J Am Coll Cardiol. 2007;50:1705–1706. doi: 10.1016/j.jacc.2007.06.050. [DOI] [PubMed] [Google Scholar]
- 193.Erdmann E, Charbonnel B, Wilcox RG, Skene AM, Massi-Benedetti M, Yates J, Tan M, Spanheimer R, Standl E, Dormandy JA. Pioglitazone use and heart failure in patients with type 2 diabetes and preexisting cardiovascular disease: data from the PROactive study (PROactive 08) Diabetes Care. 2007;30:2773–2778. doi: 10.2337/dc07-0717. [DOI] [PubMed] [Google Scholar]
- 194.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Koranyi L, Laakso M, Mokan M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 195.Yki-Jarvinen H. The PROactive study: some answers, many questions. Lancet. 2005;366:1241–1242. doi: 10.1016/S0140-6736(05)67504-6. [DOI] [PubMed] [Google Scholar]
- 196.Charbonnel B, Dormandy J, Erdmann E, Massi-Benedetti M, Skene A. The prospective pioglitazone clinical trial in macrovascular events (PROactive): can pioglitazone reduce cardiovascular events in diabetes? Study design and baseline characteristics of 5238 patients. Diabetes Care. 2004;27:1647–1653. doi: 10.2337/diacare.27.7.1647. [DOI] [PubMed] [Google Scholar]
- 197.Kao J, Tobis J, McClelland RL, Heaton MR, Davis BR, Holmes DR, Jr, Currier JW. Relation of metformin treatment to clinical events in diabetic patients undergoing percutaneous intervention. Am J Cardiol. 2004;93:1347–1350. A1345. doi: 10.1016/j.amjcard.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 198.Mamputu JC, Wiernsperger NF, Renier G. Antiatherogenic properties of metformin: the experimental evidence. Diabetes Metab. 2003;29(part 2):6S71–6S76. doi: 10.1016/s1262-3636(03)72790-6. [DOI] [PubMed] [Google Scholar]
- 199.de Aguiar LG, Bahia LR, Villela N, Laflor C, Sicuro F, Wiernsperger N, Bottino D, Bouskela E. Metformin improves endothelial vascular reactivity in first-degree relatives of type 2 diabetic patients with metabolic syndrome and normal glucose tolerance. Diabetes Care. 2006;29:1083–1089. doi: 10.2337/diacare.2951083. [DOI] [PubMed] [Google Scholar]
- 200.Vitale C, Mercuro G, Cornoldi A, Fini M, Volterrani M, Rosano GM. Metformin improves endothelial function in patients with metabolic syndrome. J Intern Med. 2005;258:250–256. doi: 10.1111/j.1365-2796.2005.01531.x. [DOI] [PubMed] [Google Scholar]
- 201.Natali A, Baldeweg S, Toschi E, Capaldo B, Barbaro D, Gastaldelli A, Yudkin JS, Ferrannini E. Vascular effects of improving metabolic control with metformin or rosiglitazone in type 2 diabetes. Diabetes Care. 2004;27:1349–1357. doi: 10.2337/diacare.27.6.1349. [DOI] [PubMed] [Google Scholar]
- 202.Kautzky-Willer A, Tura A, Winzer C, Wagner OF, Ludvik B, Hanusch-Enserer U, Prager R, Pacini G. Insulin sensitivity during oral glucose tolerance test and its relations to parameters of glucose metabolism and endothelial function in type 2 diabetic subjects under metformin and thiazolidinedione. Diabetes Obes Metab. 2006;8:561–567. doi: 10.1111/j.1463-1326.2005.00568.x. [DOI] [PubMed] [Google Scholar]
- 203.Hardie DG. AMPK and Raptor: matching cell growth to energy supply. Mol Cell. 2008;30:263–265. doi: 10.1016/j.molcel.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 204.Li C. Genetics and molecular biology–protein kinase C-zeta as an AMP-activated protein kinase kinase kinase: the protein kinase C-zeta-LKB1-AMP-activated protein kinase pathway. Curr Opin Lipidol. 2008;19:541–542. doi: 10.1097/MOL.0b013e32830f4a44. [DOI] [PubMed] [Google Scholar]
- 205.Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes (Lond) 2008;32(suppl 4):S7–S12. doi: 10.1038/ijo.2008.116. [DOI] [PubMed] [Google Scholar]
- 206.Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 207.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55:496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- 209.Zou MH, Hou XY, Shi CM, Kirkpatick S, Liu F, Goldman MH, Cohen RA. Activation of 5′-AMP-activated kinase is mediated through c-Src and phosphoinositide 3-kinase activity during hypoxia-reoxygenation of bovine aortic endothelial cells: role of peroxynitrite. J Biol Chem. 2003;278:34003–34010. doi: 10.1074/jbc.M300215200. [DOI] [PubMed] [Google Scholar]
- 210.Zou MH, Hou XY, Shi CM, Nagata D, Walsh K, Cohen RA. Modulation by peroxynitrite of Akt- and AMP-activated kinase-dependent Ser1179 phosphorylation of endothelial nitric oxide synthase. J Biol Chem. 2002;277:32552–32557. doi: 10.1074/jbc.M204512200. [DOI] [PubMed] [Google Scholar]
- 211.Wang S, Xu J, Song P, Viollet B, Zou MH. In vivo activation of AMP-activated protein kinase attenuates diabetes-enhanced degradation of GTP cyclohydrolase I. Diabetes. 2009;58:1893–1901. doi: 10.2337/db09-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Schulz E, Anter E, Zou MH, Keaney JF., Jr Estradiol-mediated endothelial nitric oxide synthase association with heat shock protein 90 requires adenosine monophosphate-dependent protein kinase. Circulation. 2005;111:3473–3480. doi: 10.1161/CIRCULATIONAHA.105.546812. [DOI] [PubMed] [Google Scholar]
- 213.Suzuki K, Uchida K, Nakanishi N, Hattori Y. Cilostazol activates AMP-activated protein kinase and restores endothelial function in diabetes. Am J Hypertens. 2008;21:451–457. doi: 10.1038/ajh.2008.6. [DOI] [PubMed] [Google Scholar]
- 214.Schulz E, Dopheide J, Schuhmacher S, Thomas SR, Chen K, Daiber A, Wenzel P, Munzel T, Keaney JF., Jr Suppression of the JNK pathway by induction of a metabolic stress response prevents vascular injury and dysfunction. Circulation. 2008;118:1347–1357. doi: 10.1161/CIRCULATIONAHA.108.784298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sasaki H, Asanuma H, Fujita M, Takahama H, Wakeno M, Ito S, Ogai A, Asakura M, Kim J, Minamino T, Takashima S, Sanada S, Sugimachi M, Komamura K, Mochizuki N, Kitakaze M. Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation. 2009;119:2568–2577. doi: 10.1161/CIRCULATIONAHA.108.798561. [DOI] [PubMed] [Google Scholar]
- 216.Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem. 2003;278:31629–31639. doi: 10.1074/jbc.M212831200. [DOI] [PubMed] [Google Scholar]
- 217.Gonon AT, Widegren U, Bulhak A, Salehzadeh F, Persson J, Sjoquist PO, Pernow J. Adiponectin protects against myocardial ischaemia-reperfusion injury via AMP-activated protein kinase, Akt, and nitric oxide. Cardiovasc Res. 2008;78:116–122. doi: 10.1093/cvr/cvn017. [DOI] [PubMed] [Google Scholar]
- 218.Xu X, Jhun BS, Ha CH, Jin ZG. Molecular mechanisms of ghrelin-mediated endothelial nitric oxide synthase activation. Endocrinology. 2008;149:4183–4192. doi: 10.1210/en.2008-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Reihill JA, Ewart MA, Hardie DG, Salt IP. AMP-activated protein kinase mediates VEGF-stimulated endothelial NO production. Biochem Biophys Res Commun. 2007;354:1084–1088. doi: 10.1016/j.bbrc.2007.01.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhang Y, Lee TS, Kolb EM, Sun K, Lu X, Sladek FM, Kassab GS, Garland T, Jr, Shyy JY. AMP-activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler Thromb Vasc Biol. 2006;26:1281–1287. doi: 10.1161/01.ATV.0000221230.08596.98. [DOI] [PubMed] [Google Scholar]
- 221.Fleming I, Schulz C, Fichtlscherer B, Kemp BE, Fisslthaler B, Busse R. AMP-activated protein kinase (AMPK) regulates the insulin-induced activation of the nitric oxide synthase in human platelets. Thromb Haemost. 2003;90:863–871. doi: 10.1160/TH03-04-0228. [DOI] [PubMed] [Google Scholar]
- 222.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–8641. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cacicedo JM, Yagihashi N, Keaney JF, Jr, Ruderman NB, Ido Y. AMPK inhibits fatty acid-induced increases in NF-kappaB transactivation in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004;324:1204–1209. doi: 10.1016/j.bbrc.2004.09.177. [DOI] [PubMed] [Google Scholar]
- 224.Prasad R, Giri S, Nath N, Singh I, Singh AK. 5-Aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside attenuates experimental autoimmune encephalomyelitis via modulation of endothelial-monocyte interaction. J Neurosci Res. 2006;84:614–625. doi: 10.1002/jnr.20953. [DOI] [PubMed] [Google Scholar]
- 225.Pilon G, Dallaire P, Marette A. Inhibition of inducible nitric-oxide synthase by activators of AMP-activated protein kinase: a new mechanism of action of insulin-sensitizing drugs. J Biol Chem. 2004;279:20767–20774. doi: 10.1074/jbc.M401390200. [DOI] [PubMed] [Google Scholar]
- 226.Giri S, Nath N, Smith B, Viollet B, Singh AK, Singh I. 5-Aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase. J Neurosci. 2004;24:479–487. doi: 10.1523/JNEUROSCI.4288-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47:1183–1188. doi: 10.1161/01.HYP.0000221429.94591.72. [DOI] [PubMed] [Google Scholar]
- 228.An Z, Wang H, Song P, Zhang M, Geng X, Zou MH. Nicotine-induced activation of AMP-activated protein kinase inhibits fatty acid synthase in 3T3L1 adipocytes: a role for oxidant stress. J Biol Chem. 2007;282:26793–26801. doi: 10.1074/jbc.M703701200. [DOI] [PubMed] [Google Scholar]
- 229.Xie Z, Dong Y, Zhang M, Cui MZ, Cohen RA, Riek U, Neumann D, Schlattner U, Zou MH. Activation of protein kinase C zeta by peroxynitrite regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells. J Biol Chem. 2006;281:6366–6375. doi: 10.1074/jbc.M511178200. [DOI] [PubMed] [Google Scholar]
- 230.Irrcher I, Ljubicic V, Hood DA. Interactions between ROS and AMP kinase activity in the regulation of PGC-1α transcription in skeletal muscle cells. Am J Physiol Cell Physiol. 2009;296:C116–C123. doi: 10.1152/ajpcell.00267.2007. [DOI] [PubMed] [Google Scholar]
- 231.Park IJ, Hwang JT, Kim YM, Ha J, Park OJ. Differential modulation of AMPK signaling pathways by low or high levels of exogenous reactive oxygen species in colon cancer cells. Ann N Y Acad Sci. 2006;1091:102–109. doi: 10.1196/annals.1378.059. [DOI] [PubMed] [Google Scholar]
- 232.Ceolotto G, Gallo A, Papparella I, Franco L, Murphy E, Iori E, Pagnin E, Fadini GP, Albiero M, Semplicini A, Avogaro A. Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD(P)H oxidase via AMPK-dependent mechanism. Arterioscler Thromb Vasc Biol. 2007;27:2627–2633. doi: 10.1161/ATVBAHA.107.155762. [DOI] [PubMed] [Google Scholar]
- 233.Xie Z, Zhang J, Wu J, Viollet B, Zou MH. Upregulation of mitochondrial uncoupling protein-2 by the AMP-activated protein kinase in endothelial cells attenuates oxidative stress in diabetes. Diabetes. 2008;57:3222–3230. doi: 10.2337/db08-0610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 234.Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, Motoshima H, Taguchi T, Matsumura T, Araki E. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 2006;55:120–127. [PubMed] [Google Scholar]
- 235.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]