Abstract
The ubiquitin-proteasome system has been implicated in oxidative stress–induced endothelial dysfunction in cardiovascular diseases. However, the mechanism by which oxidative stress alters the ubiquitin-proteasome system is poorly defined. The present study was conducted to determine whether oxidative modifications of PA700, a 26S proteasome regulatory subunit, contributes to angiotensin II (Ang II)–induced endothelial dysfunction. Exposure of human umbilical vein endothelial cells to low concentrations of Ang II, but not vehicle, for 6 hours significantly decreased the levels of tetrahydro-L-biopterin (BH4), an essential cofactor of endothelial NO synthase, which was accompanied by a decrease in GTP cyclohydrolase I, the rate-limiting enzyme for de novo BH4 synthesis. In addition, Ang II increased both tyrosine nitration of PA700 and the 26S proteasome activity, which were paralleled by increased coimmunoprecipitation of PA700 and the 20S proteasome. Genetic inhibition of NAD(P)H oxidase or administration of uric acid (a peroxynitrite scavenger) or NG-nitro-L-arginine methyl ester (nonselective NO synthase inhibitor) significantly attenuated Ang II–induced PA700 nitration, 26S proteasome activation, and reduction of GTP cyclohydrolase I and BH4. Finally, Ang II infusion in mice decreased the levels of both BH4 and GTP cyclohydrolase I and impaired endothelial-dependent relaxation in isolated aortas, and all of these effects were prevented by the administration of MG132, a potent inhibitor for 26S proteasome. We conclude that Ang II increases tyrosine nitration of PA700 resulting in accelerated GTP cyclohydrolase I degradation, BH4 deficiency, and consequent endothelial dysfunction in hypertension.
Keywords: angiotensin II, proteasome, hypertension, oxidative stress, endothelial NO synthase, tetrahydrobiopterin, GTP cyclohydrolase I
The ubiquitin-proteasome system plays a pivotal role in the degradation of short-lived, regulatory proteins important for a variety of basic cellular processes, including regulation of the cell cycle, modulation of cell-surface receptors and ion channels, and antigen presentation.1 The crucial role of this system in human disease has become increasingly apparent, because its deregulation leads to inappropriate degradation of specific proteins and ensuing pathological consequences.2 Aberrations in the ubiquitin-proteasome system have been implicated in certain cancers and neurodegenerative disorders.3 However, a possible role for this system in the pathogenesis of cardiovascular diseases (CVDs), such as atherosclerosis, has only recently emerged.4 An early feature of CVD is abnormal endothelial function, or so-called endothelial dysfunction, in which the endothelium loses its homeostatic potential to inhibit the disease process.5 Oxidative stress is widely accepted to play a causal role in the pathogenesis of endothelial dysfunction.6 Interestingly, oxidative stress also induces proteasome activation.7,8 Oxidants such as peroxynitrite (ONOO−) enhance proteasomal degradation of GTP cyclohydrolase I (GTPCH I), the rate-limiting enzyme for de novo tetrahydro-L-biopterin (BH4) synthesis.9 Degradation of GTPCH I results in BH4 deficiency and consequent endothelial dysfunction.7,8 However, the mechanism by which oxidants such as ONOO− activate the proteasome remains undefined.
PA700 is a key regulatory complex that associates with the proteolytic core of the 20S proteasome to form the active 26S proteasome. Multiple 26S proteasome activities are associated with PA700, including ATPase activity, polyubiquitin chain-binding activity, deubiquitination activity, chaperone-like activity, and substrate remodeling activity.10 The concerted action of these activities generates efficient degradation of protein substrates by the 26S proteasome. In fact, binding of PA700 to the proteasome greatly enhances the ability of the proteasome to degrade target proteins.11 Recently, PA700 has been shown to undergo several posttranslational modifications, offering the possibility that such alterations modulate 26S proteasome activity.12
Angiotensin II (Ang II) is a potent vasoconstrictor peptide that is endogenously produced. Ang II is one of the key participants in the pathogenesis of hypertension. Overwhelming evidence suggests that Ang II potently induces the formation of oxidants,13 including superoxide (O2•−) anions,14 hydrogen peroxide,15 and ONOO−.16–18. As a consequence, Ang II induces endothelial dysfunction evidenced by impaired aortic endothelium-dependent vas-motion.19–22 Ang II also induces proteasomal degradation of certain proteins through upregulation of the ubiquitin-proteasome system.23 However, the requirement of oxidative stress in Ang II–induced proteasome activation has not been studied. Moreover, whether oxidative modification of PA700 alters proteasome activity and endothelial function in vivo remains unclear. In the present study, we investigated the possible role of oxidative modification to PA700 in Ang II–induced endothelial dysfunction. Our data reveal that Ang II stimulates ONOO−-dependent PA700 nitration, which activates the 26S proteasome, resulting in endothelial dysfunction in vivo.
Materials and Methods
Reagents and Cells
Human GTPCH I antibody was kindly provided by Dr Gabriele Werner-Felmayer (Innsbruck Medical University, Innsbruck, Austria). All of the other antibodies or reagents were obtained from Fisher Scientific except for the following: mouse GTPCH I antibody from Ascenion; ubiquitin antibody from Santa Cruz Biotechnology; mouse PA700 antibody from Abcam; MG132 from BioMol; fluorogenic proteasome substrates from Calbiochem; and BH4 from Cayman. Human umbilical vein endothelial cells (HUVECs) and human microvascular endothelial cells (HMVECs) were obtained from Cascade Biologics and ScienCell, respectively.
Detection of BH4 and Total Biopterins
The levels of BH4 and total biopterins were determined via differential oxidation followed by high-performance liquid chromatography quantification, as described previously.7
26S Proteasome Activity Assay
26S proteasome activity was assayed as described previously by measuring ATP-dependent degradation of proteasome fluorescence substrate.7,24
Cellular Small-Interfering RNA Transfection
HUVECs were transfected according to Ambion’s instructions with either control small-interfering RNA (siRNA) duplex (Ambion) or PA700-specific siRNA duplex (Ambion).
Ang II–Induced Hypertension
Ang II was continuously administered in 10-week–old C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) at a rate of 0.8 mg/kg per day for 14 days using a miniosmotic pump (Alzet), as reported previously.21
Blood Pressure Measurement
Arterial blood pressure was determined using an Institutional Animal Care and Use Committee–approved (No. 08-012H) carotid catheter method, as described previously.25
Organ Chamber Experiments
Aortic rings isolated from the treated mice were subjected to organ chamber assay of endothelium-dependent and -independent vasodilation, as previously described.7
Statistical Analysis
Comparison of vasodilation or data from other experiments involving >2 factors was performed with a 2-way ANOVA, and intergroup differences were determined using the Bonferroni inequality method. All of the other results were analyzed with a 1-way ANOVA. Values are expressed as mean±SEM. P<0.05 was accepted as significant.
Results
Ang II Decreases Both Total Biopterins and BH4 in HUVECs
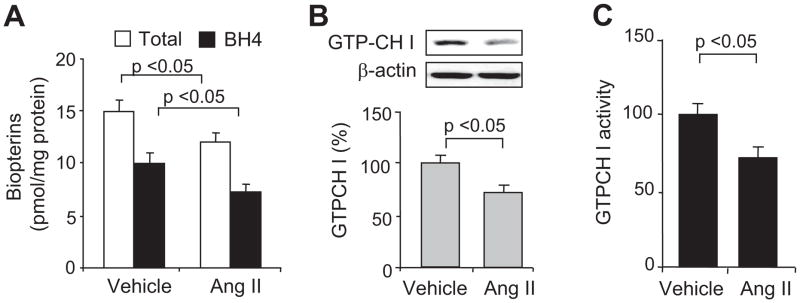
Ang II is known to alter endothelial cell function.26 Therefore, we determined whether Ang II–induced changes in endothelial cell function are accompanied by alterations in total biopterin and BH4 levels. As shown in Figure 1A, exposure of HUVECs to 0.1 μmol/L of Ang II, but not vehicle (culture medium), decreased the levels of both total biopterins (BH4+dihydrobiopterin+other biopterins) and BH4 (−23.0±4.6%; n=3; P<0.05) at 6 hours. BH4 reduction did not occur before this time. Analysis of the ratios of BH4 to oxidized biopterin (dihydrobiopterin+other biopterins) revealed that this index of BH4 oxidation27 did not differ between cells treated with vehicle (2.6±0.5) and Ang II (2.5±0.7), suggesting that BH4 reduction was mainly attributed to a reduction of its de novo synthesis.
Figure 1.
Acute exposure of HUVECs to Ang II selectively decreases GTPCH I activity and reduces levels of total biopterins, BH4, and GTPCH I. A, Levels of total biopterins and BH4 after a 6-hour exposure to Ang II (0.1 μmol/L). Cells were also analyzed for changes in (B) GTPCH I protein levels and (C) total GTPCH I activity. The blot is representative of 3 independent experiments. Results (n=3) were analyzed with a 1-way ANOVA.
Ang II Reduces GTPCH I Levels and Activity in HUVECs
GTPCH I is the rate-limiting enzyme for the de novo synthesis of biopterins,28 including BH4. Evidence suggests that GTPCH I reduction contributes to BH4 deficiency in diabetes mellitus and hypertension.7,29–31 Thus, we investigated whether Ang II–induced BH4 reduction was associated with a reduction of GTPCH I protein and/or activity. A 6-hour exposure of HUVECs to Ang II, but not vehicle, significantly reduced GTPCH I protein levels (Figure 1B), as well as GTPCH I activity (Figure 1C), as assessed by the rate of neopterin conversion from GTP.32 In contrast, expression of dihydrofolate reductase, a protein believed to participate in BH4/dihydrobiopterin recycling,33 was unaffected by Ang II (data not shown), implying that GTPCH I was sensitive to Ang II.
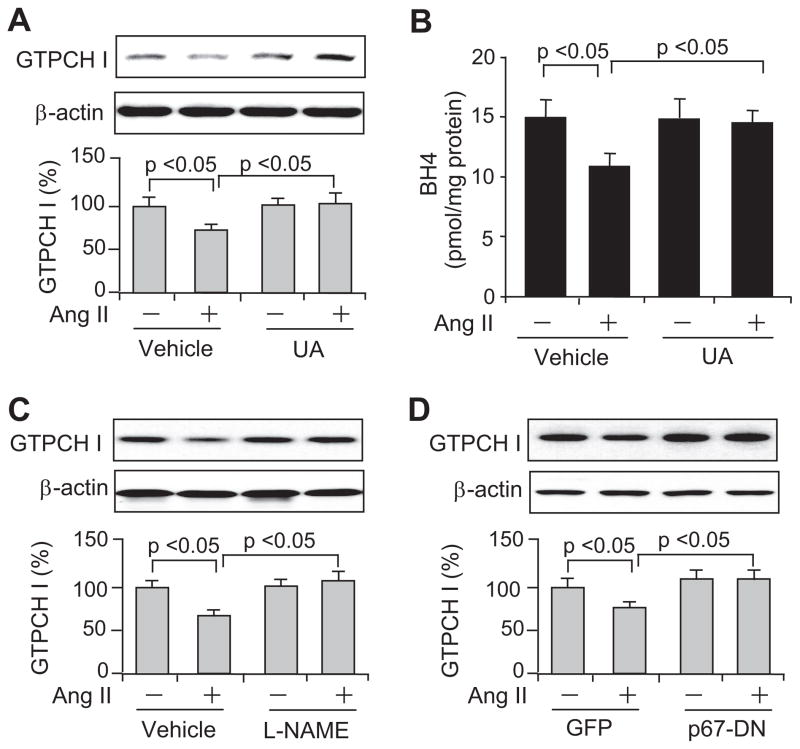
Ang II–Induced GTPCH I Reduction Is ONOO− Dependent
We had shown previously that ONOO− reduces GTPCH I levels in HUVECs.7 To determine whether ONOO− is required for Ang II–induced GTPCH I reduction in HUVECs, we cotreated cells with uric acid (UA), a potent scavenger of ONOO−. In the absence of Ang II, UA (50 μmol/L) did not alter GTPCH I expression (Figure 2A). However, UA abolished Ang II–induced decreases in GTPCH I protein (Figure 2A). Consistent with this result, UA also abolished Ang II–induced decreases in BH4 (Figure 2B). Given the fact that ONOO− is formed from both O2•− and NO34,35 and that Ang II reportedly increases O2•− in vascular cells by activating NAD(P)H oxidase,36 which is subsequently forming ONOO−, it is expected that inhibition of either O2•− or NO attenuates the formation of ONOO−. Either administration of NG-nitro-L-arginine methyl ester (L-NAME; 1 mmol/L), a nonselective NO synthase inhibitor (Figure 2C), or overexpression of a dominant-negative form of the p67phox subunit of NAD(P)H oxidase (p67-DN; Figure 2D) prevented GTPCH I reduction. It is still difficult to estimate precisely how much the uncoupled endothelial NO synthase versus NADPH oxidase (the trigger) will contribute to the observations. Taken together, these results implied that endogenous ONOO− mediates this effect, and NAD(P)H oxidases may be the primary source of O2•− and ONOO− in Ang II–exposed HUVECs.
Figure 2.
Ang II induces ONOO−-dependent GTPCH I reduction in HUVECs. ONOO− scavenging by UA (50 μmol/L of preincubation for 1 hour) block Ang II–induced reduction of (A) GTPCH I protein and (B) BH4 levels. The GTPCH I reduction can be prevented by (C) NO synthase inhibition (1 mmol/L of L-NAME) or (D) adenoviral overexpression of a dominant-negative form of the p67 NADPH oxidase subunit (p67-DN), with a multiplicity of infection of 100. All of the blots shown are representative of 3 independent experiments. All of the results (n=3) were analyzed with a 1-way ANOVA.
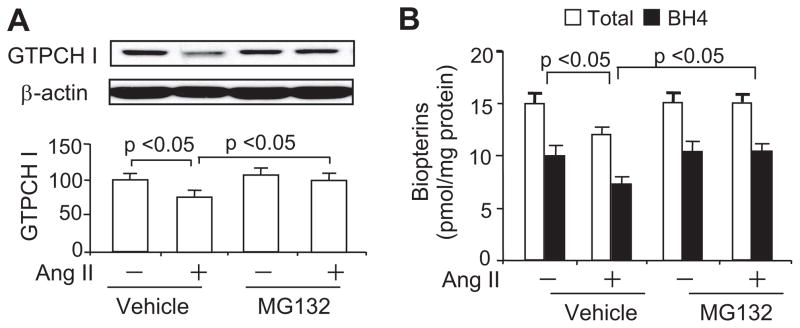
Proteasome Inhibition With MG132 Abolishes the Ang II–Induced Reduction of Both GTPCH I and BH4 in HUVECs
We had found previously that diabetes-induced decreases in BH4 result from increased 26S proteasome–dependent GTPCH I degradation.7 Thus, we tested whether the proteasome participates in Ang II–induced GTPCH I reduction by treating HUVECs with the potent proteasome inhibitor MG132. MG132 (0.5 μmol/L) did not alter the basal levels of GTPCH I (Figure 3A). However, a 1-hour preincubation of HUVECs with MG132 blocked the Ang II–induced reduction of GTPCH I at both 6 hours (Figure 3A) and 24 hours (data not shown). In parallel, MG132 prevented an Ang II–induced reduction of both total biopterins and BH4 (Figure 3B), although it had no effect on the basal levels of total biopterins or BH4 (Figure 3B).
Figure 3.
Inhibition of the 26S proteasome in HUVECs abolishes Ang II–induced reductions in GTPCH I and BH4. Confluent cultures were incubated with Ang II in the presence or absence of the proteasome inhibitor MG132 (0.5 μmol/L). MG132 abolished Ang II–induced reduction in (A) GTPCH I protein levels and (B) total biopterin and BH4 levels. The blots shown are representative of 3 independent experiments. Results (n=3) were analyzed with a 1-way ANOVA.
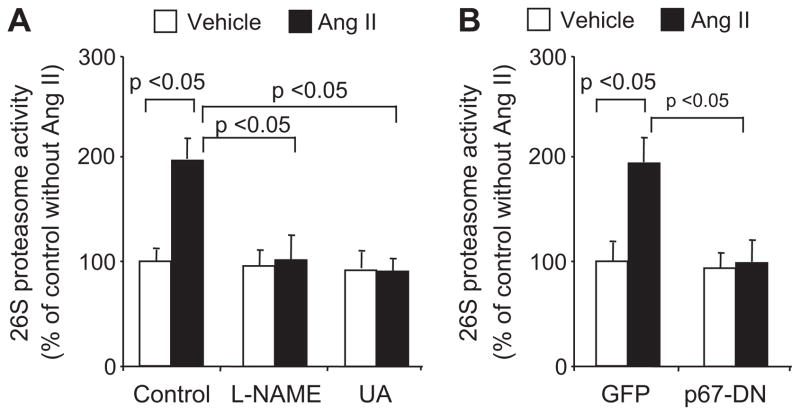
Ang II Activates the 26S Proteasome via ONOO− in HUVECs
Next, the effect of Ang II on total 26S proteasome activity was determined using specific fluorescent proteasome substrates. Exposure of HUVECs to Ang II, but not vehicle, increased proteasome (chymotrypsin-like) activity >2-fold (Figure 4A). Suppression of ONOO− production with L-NAME (1 mmol/L) or the addition of UA, an ONOO− scavenger, abrogated the Ang II–induced increase in 26S proteasome activity (Figure 4A). Genetic inhibition of NADP(H) oxidase by expressing p67-DN had a similar effect (Figure 4B). Altogether, these results indicate that Ang II activates the 26S proteasome via ONOO− in HUVECs.
Figure 4.
Ang II activates the 26S proteasome via ONOO− in HUVECs. HUVECs were preincubated for 1 hour with (A) vehicle (medium), L-NAME (1 mmol/L, NO synthase inhibitor) or UA (50 μmol/L, ONOO− scavenger), or (B) overexpressing green fluorescent protein (GFP) or p67-DN (with MOI of 100), followed by Ang II exposure and proteasome activity assay. All of the results (n=3) were analyzed with a 1-way ANOVA.
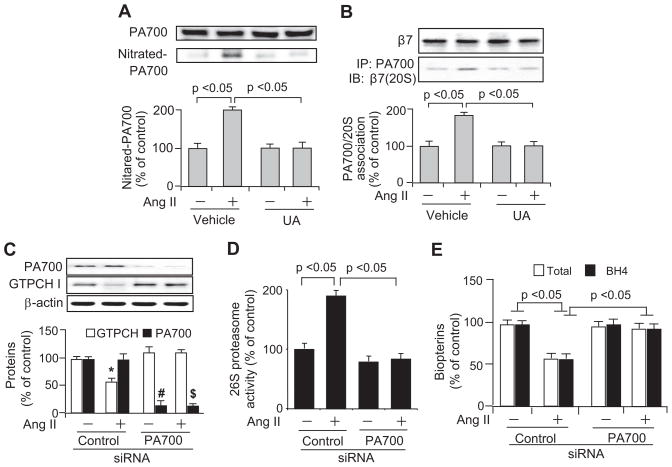
Ang II Increases Tyrosine Nitration of PA700, Which Increased Its Association With 20S Proteasome in HUVECs
Binding of PA700 to the 20S proteolytic core greatly enhances 26S proteasome activity.11 Thus, PA700 might be essential for modulating 26S proteasome activation.12 Our published data demonstrated that ONOO−-mediated 26S proteasome activation is associated with proteasome tyrosine nitration.7 We wondered whether Ang II–enhanced 26S proteasome activation was via increased PA700 nitration. Indeed, compared with their vehicle-exposed counterparts, Ang II significantly increased the detection of nitrated PA700 (Figure 5A). Finally, UA administration abolished the effects of Ang II on PA700 (Figure 5A).
Figure 5.
PA700, through its tyrosine nitration by ONOO−, mediates Ang II–induced proteasome activation and the consequent reduction of GTPCH and BH4 in HUVECs. A and B, HUVECs challenged with Ang II (0.1 μmol/L; 6 hours) with or without UA (50 μmol/L; 1-hour preincubation) were subjected to the analysis of (A) levels of total and nitrated PA700 and (B) the association of components from 19S (PA700) and 20S proteasome (β7). C through E, Effect of siRNA-mediated PA700 knockdown on Ang II–induced (C) GTPCH I protein reduction, (D) 26S proteasome activation, and (E) total pterin and BH4 reduction. All of the results (n=3) were analyzed with a 1-way ANOVA. *, #, $ P<0.05 vs corresponding controls.
We further explored whether PA700 nitration enhances its association with the catalytic complex, the 20S proteasome, a mechanism known to be crucial for 26S proteasome activation.11 Western blot analysis of PA700 immunoprecipitates using 20S proteasome-specific antibody (against its subunit β7) showed greater association of PA700 with 20S proteasome in Ang II–treated cells than in those of the vehicle-treated cells (Figure 5B). However, coincubation with UA prevented the effect of Ang II (Figure 5B). All of these treatments did not alter protein levels of PA700 (Figure 5A) and β7 (Figure 5B). In sum, PA700 nitration is associated with proteasome activation in Ang II–exposed HUVECs.
PA700 Gene Silencing Abolishes Ang II–Induced 26S Proteasome Activation and Consequent Reductions in GTPCH I and BH4
To determine whether PA700 is required for Ang II–induced proteasome activation and consequent GTPCH I reduction, we suppressed the expression of PA700 in HUVECs using PA700-specific siRNA. As expected, transfection of PA700-specific siRNA, but not control siRNA, significantly reduced PA700 protein levels (Figure 5C) and 26S proteasome activity (Figure 5D). Interestingly, Ang II significantly increased 26S proteasome activity (chymotrypsin-like) in control siRNA-transfected cells but not in cells receiving PA700-specific siRNA (Figure 5D). Similarly, Ang II reduced levels of GTPCH I (Figure 5C), total biopterins (Figure 5E), and BH4 (Figure 5E) only in control siRNA-transfected cells. As a whole, these data indicate that PA700 is essential for Ang II–induced 26S proteasome activation and the consequent reduction in GTPCH I and BH4.
In hypertension, endothelial dysfunction occurs in resistance vessels. Although HUVECs share many features of HMVECs, HMVECs might have many unique features in response to Ang II. Thus, we replicated our key findings in HMVECs. As depicted in Figure S1 (please see http://hyper.ahajournals.org for the online Data Supplement), Ang II induced an accelerated proteasomal degradation of GTPCH I (Figure S1A) that led to BH4 reduction (Figure S1B) in HMVECs. In addition, Ang II also enhanced PA700 nitration (Figure S1C) in HMVECs, which was related to Ang II–enhanced 26S proteasome activity (Figure S1D).
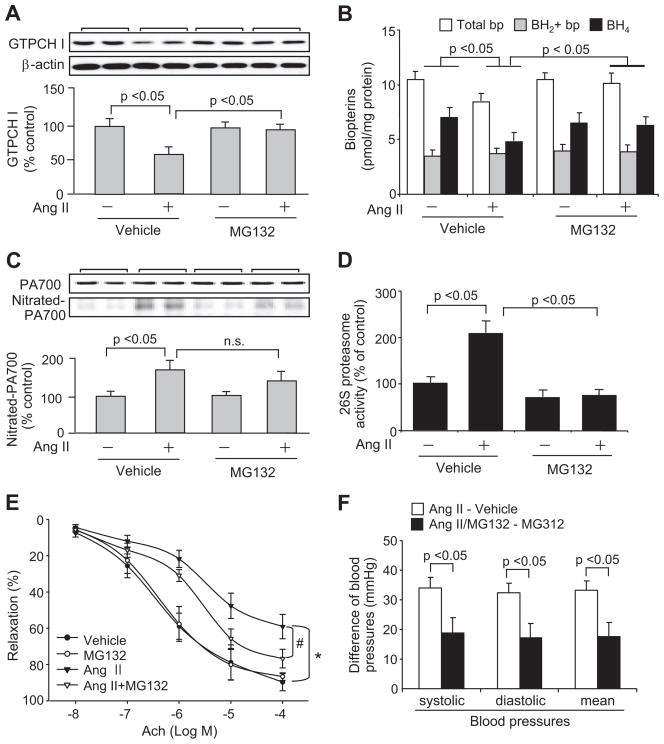
Ang II–Infused Hypertensive Mice Exhibit an MG132-Reversible Reduction of Aortic GTPCH I and BH4
Although resistant vessels are responsible for Ang II–induced elevation of blood pressure, Ang II has been shown to induce endothelial dysfunction manifested as diminished endothelium-dependent relaxation in conduit vessels, such as aortas.19–22 In addition, we also showed in mice that lack of GTPCH I causes BH4 deficiency that results in impaired aortic endothelium-dependent relaxation.7,25 To extend our findings in HUVECs to an in vivo model, we investigated whether GTPCH I and BH4 reduction and the resultant endothelial dysfunction are associated with Ang II–infused hypertensive mice and whether this reduction is sensitive to proteasome inhibition. Blood pressure measurements were performed to confirm that Ang II infusion induced hypertension (Figure S2). In non-Ang II–infused mice, injection of the 26S proteasome inhibitor, MG132, was associated with normal blood pressures (systolic: 114.5±7.3 mm Hg; diastolic: 82.2±6.7 mm Hg; mean: 92.9±6.9 mm Hg; not significant versus vehicle; n=6). No significant difference in body weight was observed between any of these groups (data not shown). However, examination of aortas from these animals revealed that infusion of Ang II, but not vehicle, significantly decreased GTPCH I protein levels (Figure 6A) and BH4 levels (Figure 6B). Administration of MG132 to vehicle-infused mice for 2 days did not alter GTPCH I protein levels (Figure 6A) or BH4 levels (Figure 6B). However, administration of MG132 to Ang II–infused mice abolished the reduction of both GTPCH I protein (Figure 6A) and BH4 (Figure 6B).
Figure 6.
Ang II–induced hypertension enhances 26S proteasome activation, which decreases aortic levels of GTPCH I and BH4 and impairs endothelium-dependent vessel relaxation. Mice were infused with Ang II (0.85 mg/kg per day for 14 days) or saline and also injected with MG132 (IP, 5 mg/kg of body weight for 2 days) or vehicle on the last 2 days of Ang II or saline infusion (n=6 for all 4 groups). Aortas were then collected for analysis of (A) GTPCH I protein levels, (B) total biopterins and BH4 levels, (C) tyrosine-nitrated PA700, (D) 26S proteasome activity, and (E) endothelium-dependent relaxation. F, Blood pressures were measured in conscious animals to compare the differences in the increase of blood pressure by Ang II infusion without MG132 treatment and those with MG132 treatment. Results (n=6) were analyzed with ANOVA. *P<0.05 vs vehicle, #P<0.05 vs Ang II alone. n.s. indicates not significant.
Ang II–Infused Hypertensive Mice Exhibit Increased Aortic PA700 Tyrosine Nitration and 26S Proteasome Activity
Analysis of aortic PA700 nitration in hypertensive animals revealed that infusion of Ang II, but not the vehicle, enhanced tyrosine nitration of PA700 without changing PA700 protein levels (Figure 6C). Importantly, the Ang II–enhanced PA700 nitration was related to the increased aortic 26S proteasome activity, which was ≈2-fold greater than that in the vehicle-infused group (Figure 6D).
Ang II–Infused Hypertensive Mice Exhibit MG132-Reversible Endothelial Dysfunction
Confirmed with others19–22 in our model, acetylcholine-induced, endothelium-dependent relaxation was significantly impaired in the Ang II–treated group compared with the vehicle-treated group (Figure 6E). However, endothelium-independent relaxation was not affected (data not shown), consistent with others.19 Importantly, administration of MG132 to hypertensive mice abolished the impaired endothelium-dependent relaxation (Figure 6E), likely because of a restored GTPCH I (Figure 6A) and BH4 (Figure 6B). Interestingly, coadministration of MG132 for 2 days significantly attenuated the Ang II–induced increase in blood pressures (Figure 6F). Given the emerging role of endothelial GTPCH I in maintaining endothelial function, as well as blood pressure,25,29 such an attenuation in the otherwise increased blood pressure might attribute to the recovered GTPCH I and the consequent well being of endothelial function globally. It warrants further study to decide whether improving endothelial function of conduit vessel tends to be beneficial for those of the resistance vessels and whether proteasome inhibition could be an option to improve high blood pressure.
In summary, all of these in vivo data suggest that Ang II reduces GTPCH I and BH4 by increasing PA700 tyrosine nitration and 26S proteasome activity, which contribute endothelial dysfunction.
Discussion
The data presented here reveal a novel mechanism for Ang II–induced endothelial dysfunction. In this mechanism, tyrosine nitration of PA700 activates the 26S proteasome to accelerate degradation of GTPCH I, a key enzyme in maintaining homeostatic BH4 balance and endothelial function. Importantly, these effects of Ang II could be reversed by proteasome inhibition or knockdown of the proteasome activator PA700, supporting the idea that PA700 nitration is essential for Ang II–induced proteasome activation and endothelial dysfunction in hypertension. These findings suggest that oxidative modification of PA700 is essential for proteasome activation and that PA700-mediated, proteasome-dependent endothelial dysfunction might be a common mechanism among CVDs, including hypertension (Figure S3).
One of the major findings in the current study is that tyrosine nitration of PA700 by reactive nitrogen species causes a functional activation of 26S proteasome, which is manifested in both cultured endothelial cells and Ang II–infused hypertensive mice. As a key regulatory complex, PA700 is crucial in associating with the catalytic 20S proteasome to form the active 26S proteasome.10 As a result, the binding of PA700 to the 20S partner displays greatly increased proteolytic activity of the 26S proteasome.37 In addition, posttranslational modifications of PA700, such as phosphorylation38,39 or glycation,12,40 are reported to modulate 26S proteasome activity. Here, for the first time, we report that tyrosine nitration of PA700 by reactive nitrogen species increases 26S proteasome activation by increasing its association with the 20S proteasome. The evidence can be summarized as described here. First, Ang II–induced ONOO− generation was capable of nitrating the PA700, an event that was associated with increased 26S proteasome activity (Figure 5A and 5B). The finding that ONOO− scavenging in HUVECs prevented not only Ang II–induced PA700 nitration (Figure 5B) but also activation of the 26S proteasome (Figure 4A) and reduction of GTPCH I protein (Figure 2A) and BH4 levels (Figure 2B) is consistent with a role for PA700 nitration in regulating 26S proteasome activity. PA700 was essential for 26S proteasome activity, because siRNA-mediated PA700 knockdown inhibited Ang II–induced activation of the 26S proteasome (Figure 5D) and reduction of GTPCH I (Figure 5C) and BH4 (Figure 5E). Consistent with findings in HUVECs, Ang II infusion in mice induced PA700 nitration in the aorta (Figure 6D). Of note, proteasomal degradation of substrates may require concerted actions other than proteasome activation (eg, substrate modification with ubiquitin). However, the ability of PA700 knockdown to abolish the effects of Ang II suggests that 26S proteasome activation, rather than 20S proteasome alone, is required for these effects.
Oxidative stress is considered as an independent risk factor for CVDs. Oxidative modifications of the ubiquitin-proteasome system might play a causative role in the development and progression of CVDs. Findings from our previous study of a diabetes model,7 as well as this hypertension study, suggest that oxidants such as ONOO− are essential in regulating proteasome function. Indeed, oxidative stress has been shown to stimulate the ubiquitin pathway in macrophages by inducing the expression of its enzymatic components, such as ubiquitin-binding proteins.41 In addition, exposure of isolated proteasome to oxidants, such as ONOO−, alters proteasome activation,42 and exposure of certain cellular proteins to ONOO− increases their oxidation, leading to preferential degradation through the proteasome.43 In models of diabetes mellitus and hypertension, selective or enhanced degradation of targeted proteins, such as insulin receptor substrates44 or angiotensin type 1 receptor,45 occurs through an altered ubiquitin-proteasome system. A randomized, controlled study46 revealed that plaques from diabetic patients contain more ubiquitin, proteasome activity, and markers of oxidative stress (3-nitrotyrosine and O2•− production) than those of control patients. Recently, the atheroprotective effects of hormone replacement therapy in postmenopausal women were linked to inhibition of the ubiquitin-proteasome system through decreased oxidative stress.47 The present findings suggest that this clinically observed upregulation of the ubiquitin-proteasome system by oxidative stress results, at least in part, from a disease-associated modification of PA700, likely through ONOO−-dependent tyrosine nitration.
Perspectives
The essential cofactor BH4 of the vascular protective enzyme endothelial NO synthase has been regarded as crucial in maintaining endothelium homeostasis, because its deficiency could be manifested as CVDs, including hypertension and diabetes mellitus, in animal models and in patients. Increasing evidence from other laboratories, as well as from ours, has demonstrated the essential role of a key BH4-making enzyme, GTPCH I, in maintaining endothelial function and blood pressure. However, how GTPCH I deficiency is developed in hypertension remains elusive. In the current study, we have discovered that Ang II–induced hypertension activated the 26S proteasome via ONOO−-induced tyrosine nitration of PA700 and the consequent enhanced association of PA700 to the catalytic complex 20S proteasome to accelerate GTPCH I degradation and impair endothelial function. The clinical implication of the present study is supported by the emerging recognized roles of the deregulated proteasome in various forms of CVD. Because ONOO− is generated in these forms of CVD, tyrosine nitration of PA700 and consequent GTPCH I degradation might play key roles in the development of these CVDs, including hypertension, diabetes mellitus, and atherosclerosis. Therefore, the beneficial effect of administration of a proteasome inhibitor, which is currently used in cancer chemotherapy, might be useful in treating vascular complications in hypertension or other form of CVDs.
Supplementary Material
Figure S1. Key findings made in HUVECs are reproducible in human arterial originated microvascular endothelial cell (HMVEC). Acute exposure of HMVEC to Ang II induces (A) MG132-inhibitable reduction of GTPCH I protein and (B) total biopterins and BH4 levels; Ang II also increases (C) PA700 nitration without altering its protein levels; and (D) 26S proteasome activity. The blots in (A) and (C) are representatives of three independent experiments, respectively. Results (n=3, respectively) were analyzed with a one-way ANOVA; n.s. for not significant.
Figure S2. Ang II elevates blood pressure in mice. Mice were infused with Ang II (0.85 mg/kg/day for 14 d) or Vehicle (saline) (n = 6/group). Results (n=6) were analyzed with ANOVA.
Figure S3. Proposed mechanism underlying hypertension-induced BH4 deficiency. Hypertension induces formation of ONOO−, in turn triggering proteasome-mediated degradation of GTPCH I, the rate-limiting enzyme for de novo BH4 synthesis. NAD(P)H oxidase (NOX) is likely the initial source of O2−• ONOO- scavenging (with UA) or inhibition of ONOO− formation (with the O2−• scavenger SOD or the NOS inhibitor L-NAME) blocks hypertension-induced GTPCH I breakdown and concomitant BH4 deficiency, which is an important contributor to eNOS uncoupling and resultant endothelial dysfunction. Ang II might increase 26S proteasome activity by enhancing tyrosine nitration of PA700, the regulatory and activating component of the 26S proteasome.
Acknowledgments
We thank Dr Gabriele Werner-Felmayer for providing specific anti-GTPCH I antibody.
Sources of Funding
This work was supported by National Institutes of Health grants (HL079584, HL080499, HL074399, HL089920, and HL096032), a research award from the American Diabetes Association, a research award from the Juvenile Diabetes Research Foundation, a grant from the Oklahoma Center for the Advancement of Science and Technology, and Paul H. Doris Eaton Travis Chair Funds in Endocrinology from the University of Oklahoma Health Sciences Center. M.-H.Z. is a recipient of the National Established Investigator Award of the American Heart Association. J.X. is a recipient of an American Heart Association Postdoctoral Fellowship (0820075Z).
Footnotes
Presented in part at the 2007 Scientific Sessions of the American Heart Association, Orlando, Fla, November 4–7, 2007.
The authors had full access to the data and take full responsibility for the integrity of the data. All of the authors have read and agree to the article as written.
Disclosures
None.
References
- 1.Schwartz AL, Ciechanover A. The ubiquitin-proteasome pathway and pathogenesis of human diseases. Annu Rev Med. 1999;50:57–74. doi: 10.1146/annurev.med.50.1.57. [DOI] [PubMed] [Google Scholar]
- 2.Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol. 2006;17:1807–1819. doi: 10.1681/ASN.2006010083. [DOI] [PubMed] [Google Scholar]
- 3.Ciechanover A, Brundin P. The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron. 2003;40:427–446. doi: 10.1016/s0896-6273(03)00606-8. [DOI] [PubMed] [Google Scholar]
- 4.Herrmann J, Soares SM, Lerman LO, Lerman A. Potential role of the ubiquitin-proteasome system in atherosclerosis: aspects of a protein quality disease. J Am Coll Cardiol. 2008;51:2003–2010. doi: 10.1016/j.jacc.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 5.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 6.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 7.Xu J, Wu Y, Song P, Zhang M, Wang S, Zou MH. Proteasome-dependent degradation of guanosine 5′-triphosphate cyclohydrolase I causes tetrahydrobiopterin deficiency in diabetes mellitus. Circulation. 2007;116:944–953. doi: 10.1161/CIRCULATIONAHA.106.684795. [DOI] [PubMed] [Google Scholar]
- 8.Whitsett J, Picklo MJ, Sr, Vasquez-Vivar J. 4-Hydroxy-2-nonenal increases superoxide anion radical in endothelial cells via stimulated GTP cyclohydrolase proteasomal degradation. Arterioscler Thromb Vasc Biol. 2007;27:2340–2347. doi: 10.1161/ATVBAHA.107.153742. [DOI] [PubMed] [Google Scholar]
- 9.Werner-Felmayer G, Golderer G, Werner ER. Tetrahydrobiopterin biosynthesis, utilization and pharmacological effects. Curr Drug Metab. 2002;3:159–173. doi: 10.2174/1389200024605073. [DOI] [PubMed] [Google Scholar]
- 10.Liu CW, Strickland E, Demartino GN, Thomas PJ. Recognition and processing of misfolded proteins by PA700, the 19S regulatory complex of the 26S proteasome. Methods Mol Biol. 2005;301:71–81. doi: 10.1385/1-59259-895-1:071. [DOI] [PubMed] [Google Scholar]
- 11.DeMartino GN, Proske RJ, Moomaw CR, Strong AA, Song X, Hisamatsu H, Tanaka K, Slaughter CA. Identification, purification, and characterization of a PA700-dependent activator of the proteasome. J Biol Chem. 1996;271:3112–3118. doi: 10.1074/jbc.271.6.3112. [DOI] [PubMed] [Google Scholar]
- 12.Zachara NE, Hart GW. O-GlcNAc modification: a nutritional sensor that modulates proteasome function. Trends Cell Biol. 2004;14:218–221. doi: 10.1016/j.tcb.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Toda N, Ayajiki K, Okamura T. Interaction of endothelial nitric oxide and angiotensin in the circulation. Pharmacol Rev. 2007;59:54–87. doi: 10.1124/pr.59.1.2. [DOI] [PubMed] [Google Scholar]
- 14.Touyz RM, Schiffrin EL. Ang II-stimulated superoxide production is mediated via phospholipase D in human vascular smooth muscle cells. Hypertension. 1999;34:976–982. doi: 10.1161/01.hyp.34.4.976. [DOI] [PubMed] [Google Scholar]
- 15.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 16.Guo W, Adachi T, Matsui R, Xu S, Jiang B, Zou MH, Kirber M, Lieberthal W, Cohen RA. Quantitative assessment of tyrosine nitration of manganese superoxide dismutase in angiotensin II-infused rat kidney. Am J Physiol Heart Circ Physiol. 2003;285:H1396–H1403. doi: 10.1152/ajpheart.00096.2003. [DOI] [PubMed] [Google Scholar]
- 17.Kase H, Hashikabe Y, Uchida K, Nakanishi N, Hattori Y. Supplementation with tetrahydrobiopterin prevents the cardiovascular effects of angiotensin II-induced oxidative and nitrosative stress. J Hypertens. 2005;23:1375–1382. doi: 10.1097/01.hjh.0000173520.13976.7d. [DOI] [PubMed] [Google Scholar]
- 18.Escobales N, Crespo MJ. Oxidative-nitrosative stress in hypertension. Curr Vasc Pharmacol. 2005;3:231–246. doi: 10.2174/1570161054368643. [DOI] [PubMed] [Google Scholar]
- 19.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung O, Schreiber JG, Geiger H, Pedrazzini T, Busse R, Brandes RP. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation. 2004;109:1795–1801. doi: 10.1161/01.CIR.0000124223.00113.A4. [DOI] [PubMed] [Google Scholar]
- 21.Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H, Yabe-Nishimura C. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation. 2005;112:2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- 22.Szabo C, Pacher P, Zsengeller Z, Vaslin A, Komjati K, Benko R, Chen M, Mabley JG, Kollai M. Angiotensin II-mediated endothelial dysfunction: role of poly(ADP-ribose) polymerase activation. Mol Med. 2004;10:28–35. doi: 10.2119/2004-00001.szabo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delafontaine P, Akao M. Angiotensin II as candidate of cardiac cachexia. Curr Opin Clin Nutr Metab Care. 2006;9:220–224. doi: 10.1097/01.mco.0000222103.29009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fekete MR, McBride WH, Pajonk F. Anthracyclines, proteasome activity and multi-drug-resistance. BMC Cancer. 2005;5:114. doi: 10.1186/1471-2407-5-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S, Xu J, Song P, Wu Y, Zhang J, Chul Choi H, Zou MH. Acute inhibition of guanosine triphosphate cyclohydrolase 1 uncouples endothelial nitric oxide synthase and elevates blood pressure. Hypertension. 2008;52:484–490. doi: 10.1161/HYPERTENSIONAHA.108.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev. 2000;52:11–34. [PubMed] [Google Scholar]
- 27.Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:413–420. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
- 28.Nichol CA, Smith GK, Duch DS. Biosynthesis and metabolism of tetrahydrobiopterin and molybdopterin. Annu Rev Biochem. 1985;54:729–764. doi: 10.1146/annurev.bi.54.070185.003501. [DOI] [PubMed] [Google Scholar]
- 29.Du YH, Guan YY, Alp NJ, Channon KM, Chen AF. Endothelium-specific GTP cyclohydrolase I overexpression attenuates blood pressure progression in salt-sensitive low-renin hypertension. Circulation. 2008;117:1045–1054. doi: 10.1161/CIRCULATIONAHA.107.748236. [DOI] [PubMed] [Google Scholar]
- 30.Meininger CJ, Marinos RS, Hatakeyama K, Martinez-Zaguilan R, Rojas JD, Kelly KA, Wu G. Impaired nitric oxide production in coronary endothelial cells of the spontaneously diabetic BB rat is due to tetrahydrobiopterin deficiency. Biochem J. 2000;349:353–356. doi: 10.1042/0264-6021:3490353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pannirselvam M, Verma S, Anderson TJ, Triggle CR. Cellular basis of endothelial dysfunction in small mesenteric arteries from spontaneously diabetic (db/db −/−) mice: role of decreased tetrahydrobiopterin bioavailability. Br J Pharmacol. 2002;136:255–263. doi: 10.1038/sj.bjp.0704683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nar H, Huber R, Auerbach G, Fischer M, Hosl C, Ritz H, Bracher A, Meining W, Eberhardt S, Bacher A. Active site topology and reaction mechanism of GTP cyclohydrolase I. Proc Natl Acad Sci U S A. 1995;92:12120–12125. doi: 10.1073/pnas.92.26.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2005;102:9056–9061. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou MH, Ullrich V. Peroxynitrite formed by simultaneous generation of nitric oxide and superoxide selectively inhibits bovine aortic prostacyclin synthase. FEBS Lett. 1996;382:101–104. doi: 10.1016/0014-5793(96)00160-3. [DOI] [PubMed] [Google Scholar]
- 35.Guzik TJ, West NE, Pillai R, Taggart DP, Channon KM. Nitric oxide modulates superoxide release and peroxynitrite formation in human blood vessels. Hypertension. 2002;39:1088–1094. doi: 10.1161/01.hyp.0000018041.48432.b5. [DOI] [PubMed] [Google Scholar]
- 36.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 37.DeMartino GN, Slaughter CA. Regulatory proteins of the proteasome. Enzyme Protein. 1993;47:314–324. doi: 10.1159/000468689. [DOI] [PubMed] [Google Scholar]
- 38.Lee SW, Dai G, Hu Z, Wang X, Du J, Mitch WE. Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol. 2004;15:1537–1545. doi: 10.1097/01.asn.0000127211.86206.e1. [DOI] [PubMed] [Google Scholar]
- 39.Gomes AV, Zong C, Edmondson RD, Li X, Stefani E, Zhang J, Jones RC, Thyparambil S, Wang GW, Qiao X, Bardag-Gorce F, Ping P. Mapping the murine cardiac 26S proteasome complexes. Circ Res. 2006;99:362–371. doi: 10.1161/01.RES.0000237386.98506.f7. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Dosal R, Sorensen MD, Clark BF, Rattan SI, Kristensen P. Phage-displayed antibodies for the detection of glycated proteasome in aging cells. Ann N Y Acad Sci. 2006;1067:474–478. doi: 10.1196/annals.1354.068. [DOI] [PubMed] [Google Scholar]
- 41.Ishii T, Itoh K, Sato H, Bannai S. Oxidative stress-inducible proteins in macrophages. Free Radic Res. 1999;31:351–355. doi: 10.1080/10715769900300921. [DOI] [PubMed] [Google Scholar]
- 42.Amici M, Lupidi G, Angeletti M, Fioretti E, Eleuteri AM. Peroxynitrite-induced oxidation and its effects on isolated proteasomal systems. Free Radic Biol Med. 2003;34:987–996. doi: 10.1016/s0891-5849(02)01369-2. [DOI] [PubMed] [Google Scholar]
- 43.Grune T, Blasig IE, Sitte N, Roloff B, Haseloff R, Davies KJ. Peroxynitrite increases the degradation of aconitase and other cellular proteins by proteasome. J Biol Chem. 1998;273:10857–10862. doi: 10.1074/jbc.273.18.10857. [DOI] [PubMed] [Google Scholar]
- 44.Taniyama Y, Hitomi H, Shah A, Alexander RW, Griendling KK. Mechanisms of reactive oxygen species-dependent downregulation of insulin receptor substrate-1 by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25:1142–1147. doi: 10.1161/01.ATV.0000164313.17167.df. [DOI] [PubMed] [Google Scholar]
- 45.Gildea JJ, Wang X, Jose PA, Felder RA. Differential D1 and D5 receptor regulation and degradation of the angiotensin type 1 receptor. Hypertension. 2008;51:360–366. doi: 10.1161/HYPERTENSIONAHA.107.100099. [DOI] [PubMed] [Google Scholar]
- 46.Marfella R, D’Amico M, Esposito K, Baldi A, Di Filippo C, Siniscalchi M, Sasso FC, Portoghese M, Cirillo F, Cacciapuoti F, Carbonara O, Crescenzi B, Baldi F, Ceriello A, Nicoletti GF, D’Andrea F, Verza M, Coppola L, Rossi F, Giugliano D. The ubiquitin-proteasome system and inflammatory activity in diabetic atherosclerotic plaques: effects of rosiglitazone treatment. Diabetes. 2006;55:622–632. doi: 10.2337/diabetes.55.03.06.db05-0832. [DOI] [PubMed] [Google Scholar]
- 47.Marfella R, Di Filippo C, Portoghese M, Ferraraccio F, Crescenzi B, Siniscalchi M, Barbieri M, Bologna C, Rizzo MR, Rossi F, D’Amico M, Paolisso G. Proteasome activity as a target of hormone replacement therapy-dependent plaque stabilization in postmenopausal women. Hypertension. 2008;51:1135–1141. doi: 10.1161/HYPERTENSIONAHA.107.105239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Key findings made in HUVECs are reproducible in human arterial originated microvascular endothelial cell (HMVEC). Acute exposure of HMVEC to Ang II induces (A) MG132-inhibitable reduction of GTPCH I protein and (B) total biopterins and BH4 levels; Ang II also increases (C) PA700 nitration without altering its protein levels; and (D) 26S proteasome activity. The blots in (A) and (C) are representatives of three independent experiments, respectively. Results (n=3, respectively) were analyzed with a one-way ANOVA; n.s. for not significant.
Figure S2. Ang II elevates blood pressure in mice. Mice were infused with Ang II (0.85 mg/kg/day for 14 d) or Vehicle (saline) (n = 6/group). Results (n=6) were analyzed with ANOVA.
Figure S3. Proposed mechanism underlying hypertension-induced BH4 deficiency. Hypertension induces formation of ONOO−, in turn triggering proteasome-mediated degradation of GTPCH I, the rate-limiting enzyme for de novo BH4 synthesis. NAD(P)H oxidase (NOX) is likely the initial source of O2−• ONOO- scavenging (with UA) or inhibition of ONOO− formation (with the O2−• scavenger SOD or the NOS inhibitor L-NAME) blocks hypertension-induced GTPCH I breakdown and concomitant BH4 deficiency, which is an important contributor to eNOS uncoupling and resultant endothelial dysfunction. Ang II might increase 26S proteasome activity by enhancing tyrosine nitration of PA700, the regulatory and activating component of the 26S proteasome.