Abstract
Objective
We evaluated variation in outpatient antibiotic utilization among U.S. commercial health plans and the implications of this variation on cost and quality.
Study Design and Methods
We measured antibiotic utilization rates among 229 U.S. commercial health plans that participated in the 2005 Healthcare Effectiveness Data and Information Set. Rates were adjusted to account for the age and sex distribution of the health plans. To estimate antibiotic costs, we multiplied utilization data for each drug class by national estimates of intra-class distribution of drugs, duration of therapy, and median Average Wholesale Price.
Results
Antibiotic utilization rates varied markedly among plans, ranging from 0.64 antibiotic fills per member per year (PMPY) at the 5th percentile of plans to 1.08 at the 95th percentile, with a mean of 0.88 (SD +/− 0.15) antibiotic fills PMPY. U.S. census region was the strongest predictor of antibiotic utilization. Antibiotic costs averaged $49 PMPY, and ranged from $34 to $63 PMPY among plans at the 5th and 95th percentiles of cost, respectively. If a health plan with 250,000 members at the 90th percentile of antibiotic costs were to reduce its costs to the 25th percentile, annual drug cost savings would be approximately $4.1 million.
Conclusions
Antibiotic utilization varies substantially among commercial health plans, and is not accounted for by differences in the age and sex distribution of plan members. Since reducing rates of antibiotic utilization is likely to lower costs and improve quality, high-utilizing plans may reap considerable rewards from investing in programs to reduce the overuse of antibiotics.
Keywords: ambulatory care, antibiotic use, benchmarking, quality of care
Introduction
Antibiotics are widely overused in ambulatory practice, particularly in the management of acute respiratory tract infections.1, 2 Although bacterial infections cause a small minority of these illnesses, it is estimated that 40 to 50 percent of all patients in the United States who seek medical attention due to these conditions receive antibiotics.3 The consequences of this overuse are striking. Every year, millions of people are directly exposed to the side effects of antibiotics, ranging from common, bothersome symptoms to infrequent but devastating complications such as Clostridium difficile colitis and anaphylaxis.4 Moreover, research suggests that community levels of bacterial resistance occur in proportion to the volume of community antibiotic use.5–7 Thus, perpetuation of antibiotic overuse promotes the continued evolution of ominous resistance profiles.
Since early this decade, the European Surveillance of Antimicrobial Consumption (ESAC) project has been collecting country-level data on antibiotic utilization and has documented large differences in per capita antibiotic utilization between counties in Europe. 8, 9 Because the ESAC measures are not based on a particular clinical condition, an optimal rate of overall antibiotic utilization is difficult to establish. Nonetheless, clinical evidence and expert opinion strongly suggest that prescribing is most appropriate in countries at the lower end of the spectrum, and that the difference between low-and high -utilizing countries to a considerable extent represents potentially unnecessary and avoidable prescriptions.8, 10, 11
Recently, the United States adopted a similar (albeit more limited) approach to track and report antibiotic utilization. In 2005, the National Committee for Quality Assurance (NCQA) developed and implemented a new measure to compare overall rates of antibiotic utilization between U.S health plans. By evaluating variation in antibiotic use within a country, these data can improve understanding of factors that contribute to variation in overall antibiotic utilization without the confounding effects of different countries’ health care systems. In addition, implementation of this measure in an established program to compare and improve health care quality can directly facilitate efforts to improve quality of care by identifying high-prescribing plans and stimulating them to investigate the factors that contribute to potentially excessive antibiotic use.7, 8, 12–15
In this study, we analyze antibiotic utilization rates among non-elderly members of commercial health maintenance organizations and point-of-service health plans in the United States participating in the National Committee for Quality Assurance’s Healthcare Effectiveness Data and Information Set (HEDIS®) program. We quantify the degree of variation in antibiotic utilization rates across health plans, controlling for member characteristics, and estimate the cost implications of this variation.
Methods
Data for this study were collected by commercial health maintenance organizations (HMO) and point-of-service (POS) health plans in the United States who participate in NCQA’s Healthcare Effectiveness Data and Information Set (HEDIS) program.16 HEDIS is a voluntary program that collects data from health plans on various domains of effectiveness and utilization. These data are used to benchmark and compare the quality of care across health plans. Participating health plans account for over 85 percent of individuals enrolled in U.S. commercial plans. We report data from 2005, the first year plans reported data on overall antibiotic utilization for a new HEDIS measure.
We received a core set of data from 248 commercial health plans, representing 83% of commercial plans in established parts of the HEDIS program. The antibiotic utilization measure was a first-year measure. All first-year measures in HEDIS are not publicly reported in order to evaluate the measure, and, as is common, some plans choose not to report during this first year of data collection. We were able to obtain information on approximately 80% of the plans not contributing core data. Plans which did not report data had fewer members than those which did report (55% of non-reporting plans had fewer than 10,000 members vs. 7% of reporting plans, P<.001).
Among 248 plans contributing core data, we excluded 4 health plans that did not provide basic data necessary for our analyses, 14 health plans whose rate of overall antibiotic utilization was extreme enough to suggest discrepancies in the way these data were collected or reported, and one plan that did not employ a HMO or POS model of care. Our final analytic dataset thus comprised 229 health plans.
Our main outcome variable was each plan’s rate of antibiotic utilization per-member-per year (PMPY), as assessed by pharmacy claims billed. In calculating this rate, we counted each antibiotic prescription fill equally, regardless of drug dose or duration. Our analyses focused on people age 0–64 years, as most people 65 years and older are enrolled in government-sponsored Medicare plans and those remaining in commercial plans may not be representative of the larger population of elders.
Each plan reported antibiotic utilization data for enrollees stratified into age-sex groups. To calculate standardized rates of antibiotic utilization, for each plan we multiplied the PMPY utilization rates for each of these age-sex strata by the proportion of the overall study cohort within each of these strata. Next, we summed each of these weighted rates to create an overall antibiotic utilization rate for all plan members age 0–64 years. This approach yields a PMPY antibiotic utilization rate that adjusts for the age and sex distribution of each plan’s members. This standardized rate yielded similar figures as a crude rate that did not adjust for differences in the age and sex distribution of plans (see technical appendix).
Next, we evaluated variation in use of specific types of antibiotics. Health plans provided utilization information for each of 15 classes of antibiotics, following a categorization scheme defined by a multi-stakeholder expert panel working with NCQA to arrive at a consensus definition that was both clinically relevant and feasible to implement. This panel further grouped each of the antibiotic classes into two categories: “antibiotics of concern,” agents generally considered to have broad-spectrum activity; and all other agents (“other antibiotics”), generally considered to have a narrow spectrum of antimicrobial activity (see Table 2). For purposes of clarity, we will use the terms “broad-spectrum” and “narrow-spectrum” to refer to these categories. We identified potential discrepancies in class-level utilization data for 20 plans. These discrepancies were most likely due to rounding errors, as plan-level data on utilization rates were provided to only 2 decimal places (for example, if the reported utilization rate for an antibiotic class was 0.04 antibiotic fills PMPY, the actual rate could be anywhere from 0.0351 to 0.0449, an error rate of up to +/−12%.)We excluded these 20 plans from our analyses of antibiotic classes, plus an additional 3 plans that did not report data necessary to complete these analyses, leaving 206 plans with usable data. Since these discrepancies could also affect estimates of drug expenditures, we also excluded these plans from our cost analyses, described below.
Table 2.
Use of antibiotic classes by health plans, as a percentage of total antibiotic use
| Percent of all antibiotics dispenseda | |||
|---|---|---|---|
| Mean (±SD) across plans | |||
| All ages | Children (0–17 years) | Adults (18–64 years) | |
| “Narrow-spectrum” antibiotics | 53% (± 8%) | 56% (± 8%) | 51% (± 8%) |
| Penicillins other than Amoxicillin/Clavulanate | 25% (± 4%) | 38% (± 5%) | 21% (± 3%) |
| Cephalosporins, 1st generation | 8% (± 2%) | 6% (± 2%) | 9% (± 2%) |
| Tetracyclines | 8% (± 2%) | 6% (± 2%) | 9% (± 2%) |
| Sulfonamides | 5% (± 2%) | 4% (± 1%) | 5% (± 2%) |
| Miscellaneous narrow spectrum antibiotics | 4% (± 1%) | 1% (± <1%) | 6% (± 1%) |
| Macrolides, 1st generation | 1% (± 1%) | 1% (± 1%) | 1% (± 1%) |
| Aminoglycosides | <1% (± <1%) | <1% (± <1%) | <1% (± <1%) |
| Lincomycins | 0% (± 0%) | 0% (± 0%) | 0% (± 0%) |
| “Broad-spectrum” antibiotics | 47% (± 8%) | 44% (± 8%) | 49% (± 8%) |
| Azithromycin & clarithromycin | 18% (± 3%) | 18% (± 3%) | 18% (± 3%) |
| Fluoroquinolones | 11% (± 2%) | 1% (± <1%) | 15% (± 3%) |
| Amoxicillin/Clavulanate | 9% (± 2%) | 13% (± 3%) | 8% (± 1%) |
| Cephalosporins, 2nd–4th gen. | 5% (± 2%) | 11% (± 4%) | 3% (± 1%) |
| Clindamycin | 2% (± <1%) | 1% (± <1%) | 3% (± 1%) |
| Ketolides | 1% (± 1%) | <1% (± <1%) | 2% (± 1%) |
| Miscellaneous broad-spectrum antibiotics | <1% (± <1%) | <1% (± <1%) | <1% (± <1%) |
Use of each antibiotic class as a percentage of a plan’s total antibiotic use. Values shown are the mean percentage and standard deviation across health plans.
Next, we evaluated plan characteristics associated with the overall rate of antibiotic utilization. First, we conducted bivariate analyses using linear regression, where the outcome of interest was the plan’s age-sex adjusted rate of antibiotic utilization, and the predictor variables were plan characteristics available from HEDIS that we hypothesized might be associated with antibiotic utilization. Next, we entered all variables into a multivariable linear regression model, on which further diagnostic testing confirmed the adequacy of model fit.
To estimate the cost of antibiotic utilization for each plan, we used several steps (see technical appendix for details). Health plans provided data on utilization of antibiotic classes but not specific drugs within those classes. To compensate, we used data from the 2004 and 2005 National Ambulatory and National Hospital Ambulatory Medical Care Surveys (NAMCS/NHAMCS)to estimate the frequency of use of specific antibiotics within each antibiotic class. Next, we estimated the typical course of therapy for each antibiotic, including the most commonly-used formulation, dose, dosing frequency, and duration of therapy, repeating this process separately for persons age 0–3 years, 4–9 years, and 10–64 years. For each antibiotic, we used data from the 2005 Red Book to assess the median price per pill (or other formulation), averaging across all manufacturers of a given drug and all bottle sizes.17 For multisource (generically-available) drugs, we evaluated only the price for the generic versions. Finally, we calculated the estimated cost of a typical course of therapy for each antibiotic and then integrated this cost at the level of the antibiotic class and ultimately at the level of the health plan.
This research was approved by the Committee on Human Research at the University of California, San Francisco and the Research and Development Committee at the San Francisco VA Medical Center. We submitted an advance copy of this paper to NCQA for review and comments, but that institution had no control over the analyses and interpretation of data or over the decision to publish this work. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Results
Characteristics of the 229 health plans are shown in Table 1. Plans were distributed across the United States and varied widely in the size of their enrolled population. Together, these plans accounted for 42.9 million enrollees age 0–64 years. Children ages 0–17 comprised 27% of the overall member population.
Table 1.
Characteristics of health plans
| N of plans (%) | |
|---|---|
| Mean % (± SD) across plans | |
| Plan type | |
| HMO | 61 (27%) |
| HMO/POS | 162 (71%) |
| POS | 6 (3%) |
| Plan locationa | |
| Northeast | 29% |
| Midwest | 29% |
| South | 27% |
| West | 16% |
| Number of enrollees | |
| <10,000 | 12 (5%) |
| 10,000 –50,000 | 48 (21%) |
| 50,000 –200,000 | 103 (45%) |
| 200,000 –1 million | 61 (27%) |
| > 1 million | 5 (2%) |
| Percent of plan’s physicians in different specialties | |
| Primary care adultb | 43% (±8%) |
| Pediatrics | 5% (±4%) |
| Other specialists | 53% (±7%) |
| % of physicians in plan who are board certified | 84% (± 6%) |
| For-profit status | |
| For profit | 161 (70%) |
| Non profit | 63 (28%) |
| Not reported or missing | 5 (2%) |
| Accreditation status | |
| Excellent | 170 (74%) |
| Commendable | 16 (7%) |
| Other | 43 (19%) |
Data on plan location and the percentage of physicians who are board certified were not available for 11 plans. Some numbers do not add to 100% because of rounding.
Denotes the mean percentage of each plan’s population within a given region
Includes adult primary care physicians, geriatricians, and obstetricians/gynecologists
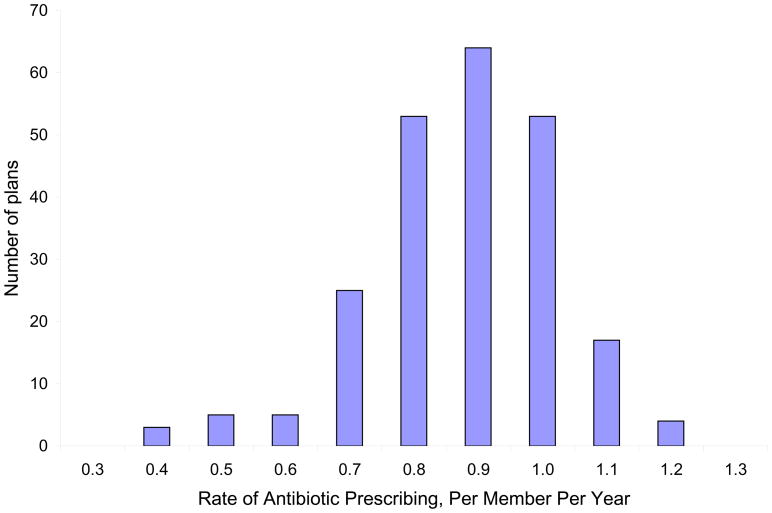
Across all plans, the mean rate of antibiotic utilization was 0.88 prescription fills per member per year (PMPY). Rates of antibiotic utilization varied widely among plans (Figure 1), with standard deviation of 0.15 fills PMPY, and 1.7-fold variation in rates of antibiotic utilization between the plans at the 5th and 95th percentiles of utilization (0.64 and 1.08 prescription fills PMPY, respectively). For a plan with 250,000 members, 89,000 fewer antibiotics would be dispensed each year if plan doctors prescribed antibiotics at the 10th percentile rate compared with the 90th percentile rate. If a similarly-sized plan were to reduce its utilization from the 75th to 25th percentile, 47,000 fewer antibiotic prescriptions would be filled.
Figure 1. Health plans’ antibiotic utilization rate per-member-per-year, age 0–64.
Per-member-per-year antibiotic utilization rates across health plans. Labels on the X-axis represent the mid-point of a range; for example, plans in the 0.90 category dispensed between 0.85 – 0.94 prescriptions PMPY.
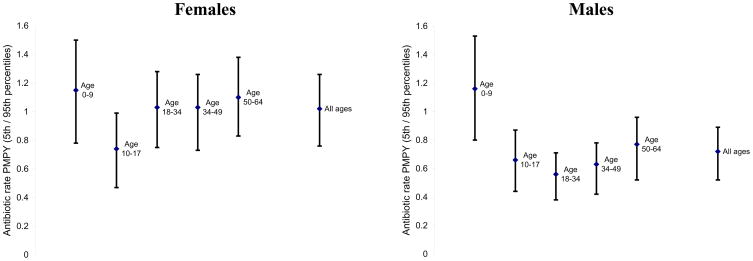
Variation in rates of antibiotic utilization persisted among age and sex groups (Figure 2). Mean rates of antibiotic utilization differed across age-sex groups, and there was considerable variation in utilization rates within each group (standard deviations for each group ranged from 0.10 fills PMPY for males 18–34 to 0.24 fills PMPY for males 0–9).
Figure 2. Health plans’ antibiotic utilization rate per -member-per-year, by age and sex groups.
For each age-sex category, the center dot represents the mean rate of antibiotic utilization across health plans. The bars show the range of antibiotic utilization rates for plans in the 5th to 95th percentile of utilization. Differences in mean utilization rates between adult females and males are likely due in part to differences in disease distribution. For example, urinary tract infections are a common source of antibiotic prescribing in women but not in men.
Table 2 shows variation in the distribution of types of antibiotics used among health plans. Across plans, a mean of 47% of antibiotics dispensed were broad-spectrum agents (“antibiotics of concern”). The proportion of antibiotics that were broad-spectrum varied among plans, with standard deviation of 8%, and ranged from 34% of antibiotic prescription fills for plans at the 5th percentile of broad-spectrum utilization to 59% for plans at the 95th percentile. There was little corrrelation between plans’ overall rate of antibiotic utilization and the proportion of antibiotics that were for broad-spectrum agents (Pearson r = 0.12, P=0.08).
We next evaluated factors associated with a plan’s rate of overall antibiotic utilization. On bivariate analyses, plans with “excellent” accreditation status (the highest achievable rank) used more antibiotics than plans with “commendable” status (the second-highest rank), and plans with a smaller percentage of physicians who were board-certified used more antibiotics than plans with a larger percentage of physicians who were board-certified (Table 3). In addition, national region was strongly associated with rates of antibiotic utilization. Compared to plans in the West, plans in the South used a mean of 0.16 more antibiotic prescription fills PMPY, plans in the Midwest used 0.12 more fills per year, and plans in the Northeast used 0.07 more fills per year. As the mean rate of antibiotic utilization in the West was 0.78 fills PMPY, these differences correspond to a 21% higher rate of antibiotic utilization in the South, 15% higher rate in the Midwest, and 9% higher rate in the Northeast (see Table 3 footnote for further explanation of how these percents were derived). On multivariable analysis, geographic region remained the most notable predictor of antibiotic utilization rates. Thirteen percent of the total variance in antibiotic utilization rates was explained by national region, while multivariable adjustment for all variables shown in Table 3 explained 26% of the total variance.
Table 3.
Health plan characteristics associated with rates of antibiotic utilization
| Characteristic | Bivariate results | Multivariable results |
|---|---|---|
| Mean rate of antibiotic utilization PMPYa | Beta coefficient (95% CI)b | |
| Plan type | ||
| HMO, HMO/POS combined | 0.79 | -- |
| POS | 0.88 | .08 (−0.11 to 0.27) |
| Profit status | ||
| For profit | 0.90 | 0.06 (0.01 to 0.10) |
| Non-profit | 0.84 | -- |
| Accreditation status | ||
| Excellent | 0.89a | -- |
| Commendable | 0.78 | −0.08 (−0.15 to −0.01) |
| Other | 0.88 | 0.01 (−0.06 to 0.04) |
| Plan size | ||
| <100,000 enrollees | 0.88 | −0.02 (−0.06 to 0.02) |
| ≥ 100,000 enrollees | 0.88 | -- |
| Beta coefficient | ||
| % of plan physicians in primary care specialtiesc | 0.21 | 0.44 (0.16 to 0.71) |
| % of plan physicians board certified | −0.43 | −0.56 (−0.89 to −0.23) |
| Regione | ||
| Northeast | 0.07a | 0.09 (0.03 to 0.15) |
| Midwest | 0.12 | 0.13 (0.07 to 0.19) |
| South | 0.16 | 0.16 (0.11 to 0.22) |
| West | Referent | -- |
Rate of utilization for plan enrollees age 0–64, adjusted for the age and sex distribution of plans
Significant at P < 0.05 on bivariate analysis
For variables entered as categories, the beta -coefficient in the multivariable analysis corresponds to the adjusted difference in number of prescriptions PMPY compared to the reference group. For example, for-profit plans utilized 0.06 more antibiotics PMPY than did non-profit plans. For variables entered as a continuous value, the beta-coefficient corresponds to the adjusted difference in the number of antibiotic fills PMPY if a plan had 0% of that characteristic vs. 100% of that characteristic. For example, if a plan had 100% primary care physicians, it would dispense 0.44 more antibiotics PMPY than if that plan had 0% primary care physicians. Similarly, if a plan had 50% primary care physicians, it would dispense 0.044 more fills PMPY than a plan with 40% primary care physicians (e.g., 10% of 0.44).
Primary care specialties include adult primary care physicians, geriatrics, obstetrics-gynecology, and pediatrics
Data for analyses of primary care specialties, board certification, and national region were available for 218 of 229 plans. In the multivariable analysis, complete data were available for 209 of 229 plans
In the Western United States, the mean rate of antibiotic utilization was 0.78 fills PMPY. Thus, on bivariate analyses, plans in the South had a 21% higher rate of antibiotic utilization than plans in the West, calculated as (0.78 + 0.16) divided by 0.78. Regions included the following states: Northeast (CT, ME, MA, NH, NJ, NY, PA, RI, VT); Midwest (IL, IN, IA, KS, MI, MN, MO, NE, ND, OH, SD, WI); South (AL, AR, DE, DC, FL, GA, KY, LA, MD, MS, NC, OK, SC, TN, TX, VA, WV); West (AK, AZ, CA, CO, HI, ID, MT, NV, NM, OR, UT, WA, WY).
Finally, we estimated the cost implications of variation in antibiotic utilization. Across 206 plans with 38 million enrollees available for this analysis, total antibiotic expenditures totaled $1.90 billion, corresponding to a mean antibiotic cost of $49 PMPY. The standard deviation of PMPY antibiotic costs was $9, and costs ranged from $34P MPY for plans at the 5th percentile of costs to $63 PMPY for plans at the 95th percentile of costs. If all plans above the 25th percentile of antibiotic costs PMPY reduced their costs to this level, the mean (SD) drug cost savings would be $10 (+/− $6) PMPY. Across the 38 million enrollees of the plans we studied, this corresponds to $305 million in savings per year. If plans reduced their utilization to the 10th percentile, the drug cost savings would be greater, with mean (SD) savings $14 (+/−$7) PMPY, and total savings of $492 million per year.
Discussion
In this study of 229 commercial health plans in the US, we found substantial variation in the rate of antibiotic utilization, with high-utilizing plans dispensing nearly 70% more antibiotics per capita than low-utilizing plans. There was similarly large variation in the proportion of broad-spectrum antibiotics used by health plans, ranging from 34% of all antibiotics in plans at the lower end of the spectrum to 59% in plans at the higher end. Geographic region was strongly associated with health plans’ rate of antibiotic utilization: after controlling for other factors, plans in the Southern U.S. used 0.16 more antibiotics PMPY than plans in the Western U.S., a difference larger than 1 standard deviation of utilization rates nationally.
This variation in antibiotic utilization has substantial implications for health and health care costs in the United States. Overprescribing of antibiotics results in unnecessary drug side effects and promotes population-level resistance to antibiotics, which - consistent with our data - is greater in Southern than in Western states.18–20 While resistance to any antibiotic is promoted by high-volume use, excessive prescribing of broad-spectrum agents is of particular concern, since this promotes resistance to agents that are commonly used to treat serious or complicated infections where the consequences of treatment failure can be severe.2, 21 Finally, unnecessary antibiotic use has important cost implications.3 Of an estimated $1.9 billion spent on antibiotics per year by health plans in our study cohort, direct drug costs could be reduced by 16% if health plans reduced their antibiotic utilization to the current 25th percentile of costs, and by 26% if plans reduced their utilization to the current 10th percentile of costs.
As with European antibiotic studies and other investigations into variation in the delivery of health care, it is difficult to determine the “correct” amount of antibiotic utilization. Nonetheless, understanding variation can provide valuable information to health plans and policymakers seeking to improve care quality and reduce unnecessary spending.12, 14 Wennberg and colleagues were among the first to highlight the importance of reporting variation, pointing to large geographic differences within the U.S. in the use of health care interventions such as surgical procedures.22 For interventions where an optimal rate of utilization is known, these data can stimulate benchmarking, allowing health plans and institutions to set achievable goals for their practice and to monitor progress toward these goals.23 Where an optimal rate of utilization is not known, reports of variation can help health plans and institutions understand their performance relative to their peers. This can prompt plans with relatively high rates of utilization to examine why their delivery of services varies substantially from the norm, to determine whether this represents a remediable problem in quality, and if so to investigate how to improve their care quality.24
Our results suggest that overall rates of antibiotic utilization are well-suited to a variation-centered approach. A number of commercial health plans are achieving far lower rates of antibiotic utilization than others. Of course, not all health plans are comparable; for example, plans whose enrollees have greater illness severity and barriers to accessing care may have legitimate reasons for prescribing more antibiotics than others.25 Thus, a high observed rate of antibiotic utilization should not be an end unto itself, but should prompt in-depth analysis to identify non-clinical factors that promote increased antibiotic use. Such analyses can be further guided by evaluation of prescribing rates within age-and sex strata to evaluate whether certain patient subgroups have disproportionately high antibiotic utilization rates relative to a health plan’s peers. Where appropriate, local initiatives can be crafted to address the factors that promote increased antibiotic use, preferably borrowing from previous research to employ active forms of clinician education and other methods proven to reduce unnecessary antibiotic prescribing 26–28 This is the approach taken by the National Committee for Quality Assurance in creating this measure for the HEDIS program, in which overall antibiotic utilization has been included in the “use of services” domain which tracks utilization and can be useful for comparison and identification of achievable goals without any specific performance targets.
Although we cannot definitively establish a “correct” rate of antibiotic utilization in commercial health plans, a variety of data suggest opportunities for improvement in the U.S. Numerous studies have documented substantial overuse of antibiotics in the U.S, and cross-national comparisons have found that Americans receive approximately 20% more antibiotics per capita than Europeans, with only 3 of 27 European countries having higher rates of antibiotic dispensing than the U.S. 1–3, 10 Patients in the Netherlands, the lowest-prescribing European country, receive 60% fewer antibiotics than patients in the United States.10, 29 Reducing antibiotic utilization is a complex endeavor and requires attention not only to clinical efficacy but to patient satisfaction and downstream health services utilization. Nonetheless, controlled trials to reduce inappropriate antibiotic use in the outpatient setting found no increase in subsequent health services utilization (e.g., office visits or telephone calls) and little to no adverse impacts on patient satisfaction.28
Our study has several limitations. The data collected were from the first year in which this measure was implemented by HEDIS. Although plans were given detailed instructions for complying with this measure, it is possible that certain plans had not perfected their data-collection and reporting processes. In addition, data is not publicly reported for the first year of any HEDIS measure. As is common, some plans chose not to report their results during this first year, and it is difficult to know whether these plans did not participate because they expected their performance to be poor or because of other factors. (For example, over half of non-reporting plans had fewer than 10,000 members, compared with 7% of reporting plans, suggesting the possibility that smaller plans might have had fewer resources available to put towards a first-year, non-publicly reported measure). Nonetheless, we did receive data from 83% of plans, and all HEDIS data are audited, suggesting that our results are representative of the target population.
Other characteristics of our methods merit consideration in interpreting this study’s findings. First, the manner in which data was reported by health plans may result in slight imprecision in our calculation of overall antibiotic utilization rates. However, this imprecision is likely to be small in relation to the large variation in rates among plans. Second, our estimates of drug cost data were based on extrapolations from another national data source (NAMCS) combined with utilization data from the study plans and do not precisely reflect the actual drug costs incurred by plans (which may be influenced by negotiated deals with drug suppliers, and so forth). Thus, our drug cost analyses should be interpreted as reasonable estimates rather than a precise accounting of real drug costs, and do not account for downstream cost expenditures or savings associated with reduced antibiotic use. Third, we did not have access to clinical data such as comorbid conditions, and thus we could not control for inter-plan differences in members’ health beyond that which is correlated with patient age and sex. Finally, we collected data only on HMOs and POS plans participating in the HEDIS program. While the strong majority of eligible commercial health plans participate in the HEDIS program, we cannot know the generalizability of our results to plans not participating in HEDIS or to persons with public insurance (such as Medicaid), other forms of commercial insurance, or no insurance at all,
It is difficult to improve health care quality unless it can be measured. The substantial unexplained variation in antibiotic utilization across U.S. health plans suggests opportunities to improve the quality and costs of antibiotic prescribing. We believe the NCQA antibiotic utilization measure should stimulate additional efforts to understand and improve antibiotic utilization at the health plan level--particularly for health plans in the higher range of antibiotic use. If successful, these efforts are likely to improve quality of care and to generate meaningful cost savings from reduced antibiotic costs—a “win-win” for patients, payors, and the public health.
Take-Away Points.
Outpatient antibiotic utilization varies substantially among commercial health plans in ways not explained by patient case mix. As a result:
Antibiotic utilization is likely to be a valuable marker of prescribing quality for health plans.
Health plans with high rates of antibiotic utilization may benefit from targeted quality improvement programs to reduce unnecessary antibiotic use.
Cost savings from reducing unnecessary antibiotic use are substantial.
Acknowledgments
Funding: This work was supported by Career Development Transition Award 01-013 from the VA Health Services Research and Development Service (Dr. Steinman), by K23 -AG030999 from the National Institute on Aging and the American Federation for Aging Research (Dr. Steinman), the Agency for Healthcare Research and Quality Translating Research into Practice Program (R01 HS13915; Dr Gonzales and Ms Maselli), and by Grant Number KL2 RR024130 from the National Center for Research Resources (Dr. Yang). The content is solely the responsibility of the authors and does not necessarily represent the official view of the Department of Veterans Affairs, the National Center for Research Resources, or the National Institutes of Health.
The authors thank Min Gayles, formerly at the National Committee for Quality Assurance, for her assistance obtaining the data and facilitating this research, and Saunak Sen, PhD and John Boscardin, PhD for their assistance with data analysis.
Technical appendix
Age-sex standardized rates vs crude rates of antibiotic utilization
To calculate the age-sex standardized per-member-per-year antibiotic utilization rate, we used the following equation:
| Males 0–9 | Females 0–9 |
| Males 10–17 | Females 10–17 |
| Males 18–34 | Females 18–34 |
| Males 35–49 | Females 35–49 |
| Males 50–64 | Females 50–64 |
To calculate the crude per-member-per-year antibiotic utilization rate, we used the following equation:
To calculate the difference between the standardized rate and the crude rate, we subtracted one from the other. The mean difference was −0.001 antibiotic fills PMPY, with standard deviation +/− 0.011 fills PMPY. Differences ranged from −0.043 to + 0.040 antibiotic fills PMPY. Thus, for almost all plans the difference between the age-sex standardized PMPY rate and the crude PMPY rate was small, and considerably smaller than the degree of variation in PMPY rates among plans.
Derivation of antibiotic cost estimates
A. Identification of specific antibiotics
For our cost analyses, we used data provided by health plans that reported antibiotic utilization at the level of antibiotic class. Specifically, for each of 15 antibiotic classes listed in Table 2, plans provided per-member-per-year prescribing rates for each of 10 age-sex strata. To determine the cost of prescribing for each antibiotic class, we first needed to estimate the relative frequency of use of specific antibiotics within a given class. To do this, we used data from the 2004 and 2005 National Ambulatory and National Hospital Ambulatory Medical Care Surveys (NAMCS/NHAMCS), a group of surveys that provide nationally-representative estimates of medications used in outpatient and emergency room settings. Using data from NAMCS and NHAMCS, we determined the relative proportion of specific antibiotics used within each of 15 antibiotic classes. In doing so, we separately evaluated these proportions within 3 age groups (0–3 years, 4–9 years, and 10–64 years) to account for differences in antibiotic prescribing for infants and toddlers, young children, and older children and adults (as described in further detail below).
This method is best illustrated by example. Take the example of fluoroquinolones. Using data from NAMCS and NHAMCS, we determined that among all prescriptions for fluoroquinolones in persons age 10–64 years, ciprofloxacin accounted for 34% of prescriptions, levofloxacin accounted for 37% of prescriptions, moxifloxacin for 15% of prescriptions, and so forth. Thus, if a plan in our dataset reported 0.10 fluoroquinolone fills per member per year among enrollees age 10–64 years, we estimated that 34% of those fluoroquinolone fills were for ciprofloxacin, 37% were for levofloxacin, etc. Thus, the estimated rate of ciprofloxacin utilization for 10–64 year-old members of that plan would be 0.034 ciprofloxacin fills per member per year (i.e., 0.10 * 0.34), the estimated rate of levofloxacin utilization would be 0.37 levofloxacin fills PMPY (i.e., 0.10 * .37), and so forth. We repeated this process for each age group (0–3 years, 4–9 years, 10–64 years) and antibiotic class.
B. Estimating costs based on a typical course of therapy
For each antibiotic, we based cost data on a typical course of therapy. To derive a “typical” course, we used the methods described below. Estimates of typical drug doses, dosing frequencies, and durations were made by one of us (KY), and subsequently reviewed by two other authors (MAS and RG), with disagreements resolved by consensus.
Drug formulation
For persons age 10 and older, we assumed that most drugs were dispensed in tablet or capsule form. Several drugs are available only in intravenous or intramuscular form – for example, as might be used for home IV therapy in the outpatient setting – and in these cases, we priced the parenteral form. For children age 0–9, we assumed that all children ages 0–3 were dispensed elixir or other liquid formulations of drugs taken orally, whereas for children ages 4–9, we assumed that half of children received elixir preparations and the other half received chewable tablets (if available).
Drug dose and frequency
To determine average dose and dosing frequency of each drug, we used clinical judgment to determine a typical dose and dosing frequency for the infections for which a given antibiotic would commonly be used. For example, for adults we assigned trimethoprim-sulfamethoxazole a dose of 160mg/800mg twice a day on the basis of this being a typical dose for urinary tract infections, and cephalexin a dose of 500 mg four times per day on the basis of this being a typical dose for skin and soft tissue infections. For children, we used standard weight tables to assume a weight of 11.4 kg for children age 0–3 and 22.4 kg for children age 4–9, with drug doses weighted accordingly.
Duration of therapy
For each drug, we estimated an average duration of therapy based on clinical practice patterns. Among adults and children, for most drugs we estimated a mean duration of therapy of 10 days, which accounts for some courses of therapy being shorter but occasional courses much longer. For drugs commonly used to treat urinary tract infections in adults, including ciprofloxacin, norfloxacin, and trimethoprim-sulfamethoxazole, we estimated a 5-day duration of therapy. For cefixime, ceftriaxone, and benzathine penicillin, we estimated a single dose of therapy, since these drugs are often used as a one-time therapy for sexually transmitted diseases. Finally, we estimated a 1-year course for sulfadiazine and sulfasalazine on the basis of their presumed use for Toxoplasmosis encephalitis and ulcerative colitis, respectively.
Other considerations
For drugs dispensed as capsules or tablets, we multiplied the average cost per pill by the number of pills needed to complete a typical course of therapy. For drugs dispensed as liquids (or equivalents), we used the cost of smallest vial size needed to complete a course of therapy.
Footnotes
Publisher's Disclaimer: “This is the pre-publication version of a manuscript that has been accepted for publication in The American Journal of Managed Care (AJMC). This version does not include post-acceptance editing and formatting. The editors and publisher of AJMC are not responsible for the content or presentation of the prepublication version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to it (eg, correspondence, corrections, editorials, etc) should go to www.ajmc.com or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.”
Contributor Information
Katherine Y. Yang, Email: yangk@pharmacy.ucsf.edu.
Sepheen C. Byron, Email: Byron@ncqa.org.
Judith H. Maselli, Email: jmaselli@medicine.ucsf.edu.
Ralph Gonzales, Email: rgmexico@medicine.ucsf.edu.
References
- 1.Linder JA, Stafford RS. Antibiotic treatment of adults with sore throat by community primary care physicians: a national survey, 1989–1999. Jama. 2001;286(10):1181–1186. doi: 10.1001/jama.286.10.1181. [DOI] [PubMed] [Google Scholar]
- 2.Steinman MA, Gonzales R, Linder JA, Landefeld CS. Changing use of antibiotics in community-based outpatient practice, 1991–1999. Ann Intern Med. 2003;138(7):525–533. doi: 10.7326/0003-4819-138-7-200304010-00008. [DOI] [PubMed] [Google Scholar]
- 3.Gonzales R, Malone DC, Maselli JH, Sande MA. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757–762. doi: 10.1086/322627. [DOI] [PubMed] [Google Scholar]
- 4.Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47(6):735–743. doi: 10.1086/591126. [DOI] [PubMed] [Google Scholar]
- 5.Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. New England Journal of Medicine. 1997;337(7):441–446. doi: 10.1056/NEJM199708143370701. [DOI] [PubMed] [Google Scholar]
- 6.Austin DJ, Kristinsson KG, Anderson RM. The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(3):1152–1156. doi: 10.1073/pnas.96.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goossens H, Ferech M, Vander Stichele R, Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365(9459):579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 8.Elseviers MM, Ferech M, Vander Stichele RH, Goossens H. Antibiotic use in ambulatory care in Europe (ESAC data 1997–2002): trends, regional differences and seasonal fluctuations. Pharmacoepidemiol Drug Saf. 2007;16(1):115–123. doi: 10.1002/pds.1244. [DOI] [PubMed] [Google Scholar]
- 9.Vander Stichele RH, Elseviers MM, Ferech M, Blot S, Goossens H. European surveillance of antimicrobial consumption (ESAC): data collection performance and methodological approach. Br J Clin Pharmacol. 2004;58(4):419–428. doi: 10.1111/j.1365-2125.2004.02164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goossens H, Ferech M, Coenen S, Stephens P. Comparison of outpatient systemic antibacterial use in 2004 in the United States and 27 European countries. Clin Infect Dis. 2007;44(8):1091–1095. doi: 10.1086/512810. [DOI] [PubMed] [Google Scholar]
- 11.Coenen S, Ferech M, Haaijer-Ruskamp FM, et al. European Surveillance of Antimicrobial Consumption (ESAC): quality indicators for outpatient antibiotic use in Europe. Qual Saf Health Care. 2007;16(6):440–445. doi: 10.1136/qshc.2006.021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aspinall SL, Berlin JA, Zhang Y, Metlay JP. Facility-level variation in antibiotic prescriptions for veterans with upper respiratory infections. Clin Ther. 2005;27(2):258–262. doi: 10.1016/j.clinthera.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Smith S, Smith GE, Heatlie H, et al. Reducing variation in antibacterial prescribing rates for ‘cough/cold’ and sore throat between 1993 and 2001: regional analyses using the general practice research database. Public Health. 2006;120(8):752–759. doi: 10.1016/j.puhe.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor GT, Quinton HB, Traven ND, et al. Geographic variation in the treatment of acute myocardial infarction: the Cooperative Cardiovascular Project. JAMA. 1999;281(7):627–633. doi: 10.1001/jama.281.7.627. [DOI] [PubMed] [Google Scholar]
- 15.The Dartmouth Atlas of Health Care. [Accessed June 11, 2008.]; http://www.dartmouthatlas.org/
- 16.National Committee for Quality Assurance. [Accessed June 11, 2008.];HEDIS & quality measurement. http://www.ncqa.org/tabid/59/Default.aspx.
- 17.Fleming T, editor. Red Book Drug Topics. Vol. 109. Thomson Healthcare; May 30, 2005. 2005 Redbook: Pharmacy’s Fundamental Reference. Rev Ed. [Google Scholar]
- 18.Denys GA, Koch KM, Dowzicky MJ. Distribution of resistant gram-positive organisms across the census regions of the United States and in vitro activity of tigecycline, a new glycylcycline antimicrobial. Am J Infect Control. 2007;35(8):521–526. doi: 10.1016/j.ajic.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Whitney CG, Farley MM, Hadler J, et al. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Engl J Med. 2000;343(26):1917–1924. doi: 10.1056/NEJM200012283432603. [DOI] [PubMed] [Google Scholar]
- 20.Geographic variation in penicillin resistance in Streptococcus pneumoniae--selected sites, United States, 1997. Mmwr. Morbidity and Mortality Weekly Report. 1999;48(30):656–661. [PubMed] [Google Scholar]
- 21.Mainous AG, 3rd, Hueston WJ, Davis MP, Pearson WS. Trends in antimicrobial prescribing for bronchitis and upper respiratory infections among adults and children. Am J Public Health. 2003;93(11):1910–1914. doi: 10.2105/ajph.93.11.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu-Yao GL, McLerran D, Wasson J, Wennberg JE. An assessment of radical prostatectomy. Time trends, geographic variation, and outcomes. The Prostate Patient Outcomes Research Team. JAMA. 1993;269(20):2633–2636. doi: 10.1001/jama.269.20.2633. [DOI] [PubMed] [Google Scholar]
- 23.Kiefe CI, Weissman NW, Allison JJ, Farmer R, Weaver M, Williams OD. Identifying achievable benchmarks of care: concepts and methodology. Int J Qual Health Care. 1998;10(5):443–447. doi: 10.1093/intqhc/10.5.443. [DOI] [PubMed] [Google Scholar]
- 24.Wennberg DE. Variation in the delivery of health care: the stakes are high. Ann Intern Med. 1998;128(10):866–868. doi: 10.7326/0003-4819-128-10-199805150-00012. [DOI] [PubMed] [Google Scholar]
- 25.Zaslavsky AM, Hochheimer JN, Schneider EC, et al. Impact of sociodemographic case mix on the HEDIS measures of health plan quality. Med Care. 2000;38(10):981–992. doi: 10.1097/00005650-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Butler CC, Rollnick S, Pill R, Maggs-Rapport F, Stott N. Understanding the culture of prescribing: qualitative study of general practitioners’ and patients’ perceptions of antibiotics for sore throats. BMJ. 1998;317(7159):637–642. doi: 10.1136/bmj.317.7159.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poses RM, Wigton RS, Cebul RD, Centor RM, Collins M, Fleischli GJ. Practice variation in the management of pharyngitis: the importance of variability in patients’ clinical characteristics and in physicians’ responses to them. Med Decis Making. 1993;13(4):293–301. doi: 10.1177/0272989X9301300405. [DOI] [PubMed] [Google Scholar]
- 28.Ranji SR, Steinman MA, Shojania KG, Gonzales R. Interventions to reduce unnecessary antibiotic prescribing: a systematic review and quantitative analysis. Med Care. 2008;46(8):847–862. doi: 10.1097/MLR.0b013e318178eabd. [DOI] [PubMed] [Google Scholar]
- 29.Cars O, Molstad S, Melander A. Variation in antibiotic use in the European Union. Lancet. 2001;357(9271):1851–1853. doi: 10.1016/S0140-6736(00)04972-2. [DOI] [PubMed] [Google Scholar]