Abstract
Reactivity to environmental stressors influences vulnerability to neurological and psychiatric illnesses, but little is known about molecular mechanisms that control this reactivity. Since mice with forebrain-specific glucocorticoid receptor overexpression (GRov mice) display anxiety-like behaviors in novel environments and have difficulty adjusting to change in memory tasks, we hypothesized that these may be facets of a broader phenotype of altered reactivity to environmental demands. Male GRov and wild-type mice were tested in a multiple-trial object interaction test comprising environmental and object habituation and spatial and object novelty trials. Half the mice received restraint stress before testing. GRov mice exhibited more locomotor activity and, without stress, more object interaction than wild-type mice. Following acute stress, GRov mice no longer showed increased object exploration. While stress dampened responses to object novelty in both groups, GRov mice were particularly impaired in discrimination of spatial novelty post-stress. These data demonstrate that GRov leads to increased environmental reactivity, responsiveness to salience, and vulnerability to stress-induced cognitive deficits. They implicate forebrain GR in fine-tuning interactions with the environment and the interplay of emotional salience, coping abilities, and cognitive function.
Keywords: object interaction, restraint stress, spatial and object novelty, transgenic mice
An organism's physical and emotional response to environmental challenges determines its ability to adapt and survive. Unexpected or uncontrolled environmental change, especially change that threatens the organism, is considered stressful and different individuals can vary significantly in their ability to cope with stress. Indeed, while stress and “life events” are often cited as proximal precipitating factors in many mood and other psychiatric disorders (McEwen, 2004), it can be argued that it is the individual's biological and psychological response to the stressor that is the key factor, and that this stress reactivity has both genetic and experiential antecedents in its own right. Few molecular targets have been implicated in differential vulnerability to stress in humans, with the notable exception of the serotonin transporter (Caspi and Moffitt, 2006). The present study asks whether the glucocorticoid receptor (GR) might represent another molecule which affects not only the stress response, but modifies several features of environmental reactivity, which can in turn lead to differences in vulnerability to psychiatric and neurological disorders.
Stress leads to activation of the limbic-hypothalamic-pituitary-adrenal (LHPA) axis which leads to the rapid synthesis and release of glucocorticoids that then coordinate neural, immune, and endocrine responses to the stressor. Glucocorticoids modulate a variety of neural functions including neuronal excitability and plasticity, neurogenesis, neuronal death, stress reactivity, emotional behavior, and learning and memory (Akil, 2005; De Kloet et al, 1998). Their actions are mediated by two ligand-dependent transcription factors, the mineralocorticoid receptor (MR) and GR. MR is considered a regulator of the basal, diurnal tone of the LHPA axis (Akil and Morano, 1996), while GR is considered a sensor of stress and a key player in the negative feedback limiting the stress response once it has taken place (Akil, 2005; Caamano et al, 2001; De Kloet et al, 1998; Diorio et al, 1993; Herman et al, 1989; Lopez et al, 1999).
Forebrain areas that are rich in GR include the hippocampus (HPC) and the frontal cortex, both of which have been implicated in the control of the LHPA axis (Diorio et al, 1993; Herman et al, 1989; Morimoto et al, 1996). In particular, beyond its role in negative feedback the HPC is critical in various types of contextual learning and memory (Morris, 2006). While the two functions, stress control and memory, may appear disparate, they are critically involved via the HPC in guiding an animal's behavioral responses to environmental stimuli (O'Keefe and Nadel, 1978) by assessing their novelty, determining their salience, comparing them to previous knowledge, and committing them to memory if highly relevant (Lemaire et al, 1999). However, the role of specific molecules in mediating one or more of these hippocampal functions is far from clear. In particular, while forebrain GR is clearly implicated in the regulation of stress biology, its role in fine-tuning responsiveness to the environment and determining novelty and salience both under normal and stressful conditions remains to be elucidated.
Genetic modification of GR in the brain has been created in several animal models to explore the specific role that this gene plays in stress and affective behavior (see Akil, 2005 for mini-review). In our laboratory, we created transgenic mice with GR overexpression specifically in the forebrain area (GRov), including the HPC and cortex. These animals have normal basal endocrine profiles. Nonetheless, they show increased emotional lability and an aging-like neuroendocrine phenotype. Specifically, GRov mice exhibit a significant increase in anxiety- and depression-like behaviors, yet they are also supersensitive to antidepressants and show enhanced sensitization to cocaine, a set of features seen in human bipolar illness (Wei et al, 2004). Moreover, GRov mice exhibit impaired termination of the stress response following restraint stress, a pattern similar to that found with aging and mood disorders. They also display a mild cognitive deficit during the reversal phase of the Morris water maze and a broad downregulation of glutamate receptor signaling in the HPC (Wei et al, 2007). Thus, these animals exhibit evidence of hippocampal dysfunction mediated by lifelong over-expression of GR in their forebrains.
The combined features of GRov mice—increase in anxiety-like behaviors, difficulty adjusting to a change in a learning and memory task, hyperresponsiveness to various pharmacological challenges, and an aging-like neuroendocrine phenotype—may all be part of a broad alteration in reactivity to environmental stimuli even if these stimuli are not threatening. This is consistent with the view that the LHPA axis, beyond its role in responding to severe stressors, is relevant to monitoring environmental stimuli and assessing salience on an ongoing basis. In this paper, we address this question by asking whether forebrain GR overexpression affects spontaneous exploration and responsiveness to novelty. We also ask whether this environmental monitoring and exploration is altered by a recent stress experience.
Experimental Procedures
Subjects
The generation of GRov mice was described previously (Wei et al, 2004). GRov mice exhibit significantly higher levels of total GR mRNA and approximately 78% more GR protein in the forebrain than WT controls (Wei et al, 2004). The GRov mouse line was established by breeding founders and their progeny to C57BL/6J mice, and all transgenic mice are maintained as hemizygotes. Mice were housed on a 14:10 light/dark cycle (lights on at 5:00 A.M.) with ad libitum access to food and water. Prior to behavioral testing, 2 - 4.5-month-old mice were housed individually for seven days. The mice were handled one time per day (two minutes per mouse) during the five days immediately prior to testing. Male GRov and wild type (WT) littermates were matched and assigned as experimental pairs. One to two WT-GRov pairs per litter were identified from 27 litters. Half of the WT-GRov littermate pairs were randomly assigned to receive 30 minutes of restraint stress, which ended five minutes before behavioral testing. The other half of the WT-GRov littermate pairs did not receive restraint stress and remained in their home cages during the 30-minute period. Paired WT-GRov littermates were always tested simultaneously. All procedures were conducted in accordance with the guidelines outlined in the National Institutes of Health Guide for the Care and Use of Animals and were approved by the University Committee for the Use and Care of Animals at the University of Michigan.
Restraint Stress
The restraint device consists of a 9 × 12 cm piece of flexible Teflon® attached to a 9 × 3 cm platform with Plexiglas® ends containing a tail slot and air holes. The Teflon® was wrapped snugly around the mouse and fastened with velcro straps. Mice were placed in their home cage immediately after 30 minutes of restraint and were then moved into the adjacent testing room to begin the object interaction test five minutes later.
Object Interaction Test
Rodents have a natural tendency to spend more time exploring novel objects and objects in novel locations more than familiar objects and objects in unchanged locations, and these preferences can be used as an index of object and spatial recognition (Mumby et al, 2002). We, therefore, relied on a multiple-trial object interaction test composed of initial object interaction, habituation, spatial novelty, and object novelty trials (Frick and Gresack, 2003; Thinus-Blanc et al, 1996).
The apparatus was a rectangular open field (35.5 cm wide × 38 cm long × 26.5 cm high) made of white plastic. Four open fields were located in a quiet room under fluorescent lighting with a light intensity of 125 lux in the center of each open field. The use of four open fields allowed simultaneous testing of two sets of WT-GRov pairs. Four objects were used for testing from the Lego® Duplo® Dora's Treasure Island™ set: a double layer flower, three Lego® pieces put together in the shape of a boot, a treasure chest on top of a flat rectangle, and a Dora the Explorer™ figurine. The objects were similar in material and size (4-7 cm × 4-7 cm × 4 cm), but were distinctively different shapes and colors. During testing, the objects were placed approximately six cm from the walls of the open fields to allow for exploration of all sides of the objects. The objects were secured to the floor of the open fields with removable velcro.
Each mouse was tested in a series of eight five-minute trials with an inter-trial interval (ITI) of five minutes. Mice were placed in the center of the open field at the beginning of each trial and allowed to freely explore. After completing each trial, mice were removed from the open fields and placed in their respective home cages next to each testing arena for the duration of the ITI. During this ITI and between testing of different mice, the open fields and objects were wiped with 70% ethanol. During Trial 1, the open fields were empty (Fig. 1A). During Trial 2, three objects were placed near the corners of each open field (Fig. 1B). The configuration of the objects remained unchanged for Trials 3 and 4 to allow the mice to habituate to the objects. Response to spatial novelty was examined in Trial 5 by moving Object 1 to a new location in the open field (Fig. 1C). The object configuration remained the same in Trial 6 to permit habituation to the new configuration. Response to object novelty was examined in Trial 7 by replacing one of the familiar objects (3a) with a novel object (3b; Fig. 1D). In Trial 8, the object configuration remained unchanged to allow for habituation.
Figure 1.
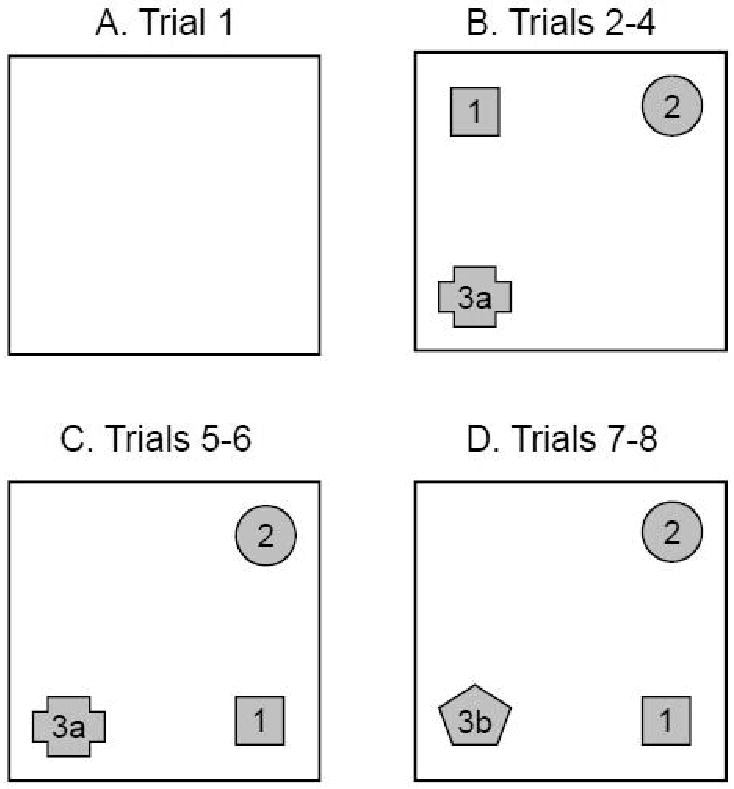
Schematic diagram depicting the object configurations in the object interaction test: open field without objects (A), habituation trials with three stable objects (B), spatial novelty trials with Object 1 moved (C), and object novelty trials with Object 3 replaced (3a vs. 3b) (D).
Data Collection and Analysis
Data were collected and analyzed using Ethovision® (Noldus Technology, Inc.), Observer® (Noldus Technology, Inc.), and SAS statistical software. A total of 80 mice (n=19-20/group) completed all eight behavioral trials. A video camera was mounted on the ceiling above the four open fields and connected to monitors and a computer containing the Ethovision® video tracking system. Ethovision® was used to measure locomotor activity for each trial. Four experimenters using laptop computers containing Observer® software were present at a distance from the open fields to watch the monitors and code exploratory behaviors. Object interaction was defined as contact with an object via a mouse's nose or front paws.
Data were analyzed using linear mixed models (SAS proc mixed) with genotype, stress, trial, and all interactions as factors. Models included random effects components for parents when the covariance parameter estimate was significant and a repeated measures component to take into account the correlation among observations made on the same mouse across trials. Post hoc least-squared means tests with slices were performed to determine effects of genotype and stress in specific groups on any given trial. Post hoc t-tests were performed to determine differences between specific trials. In these cases, significance was determined using Bonferroni-corrected p values to account for multiple comparisons. These linear mixed models were used to examine distance traveled across the eight trials and latency to interact, number of interactions, and time interacting with each object separately and with all three objects together across Trials 2 through 8, including a focus on Trials 2 to 4 to measure the extent of habituation. For object preference determination, object preference was defined as statistically unequal amounts of interaction with the different objects in a given trial, as determined by the post hoc least-squared means test. The object with the highest mean number of interactions was deemed the preferred object.
Results
Behavioral Reactivity
Examining the effects of forebrain GR overexpression and acute stress on behavior during the object interaction test reveals that non-stressed GRov mice were overall more behaviorally active than their WT counterparts. In the presence of objects, forebrain GR overexpression led to more object interactions, increased object interaction time, and shorter latencies to interact with objects over the eight-trial test, along with an increase in locomotor activity. Interestingly, many of these differences disappeared when the mice were subjected to restraint stress immediately prior to behavioral testing, primarily because of the impact of the stressor on the GRov mice.
Locomotor Activity
A linear mixed model analysis of the distance traveled of all mice across the eight-trial object interaction test shows significant main effects for genotype (F1, 76 = 5.26, P < 0.05) and trial (F7, 524 = 40.05, P< 0.001) but not stress group (F1, 76 = 1.68, P = 0.199), indicative of the general trends for GRov mice to be more active than WT mice and for the mice to habituate over time (see Figure 2A). Post hoc tests reveal, however, no significant differences among groups in the distance traveled during the first trial in an empty open field (F1,524 = 2.46, P = 0.12). This is consistent with our previous data showing that GRov mice show levels of locomotor activity similar to WT mice in an empty novel open field (Wei et al, 2004). It was not until Trial 2 of the present study that significant differences among groups became apparent.
Figure 2.
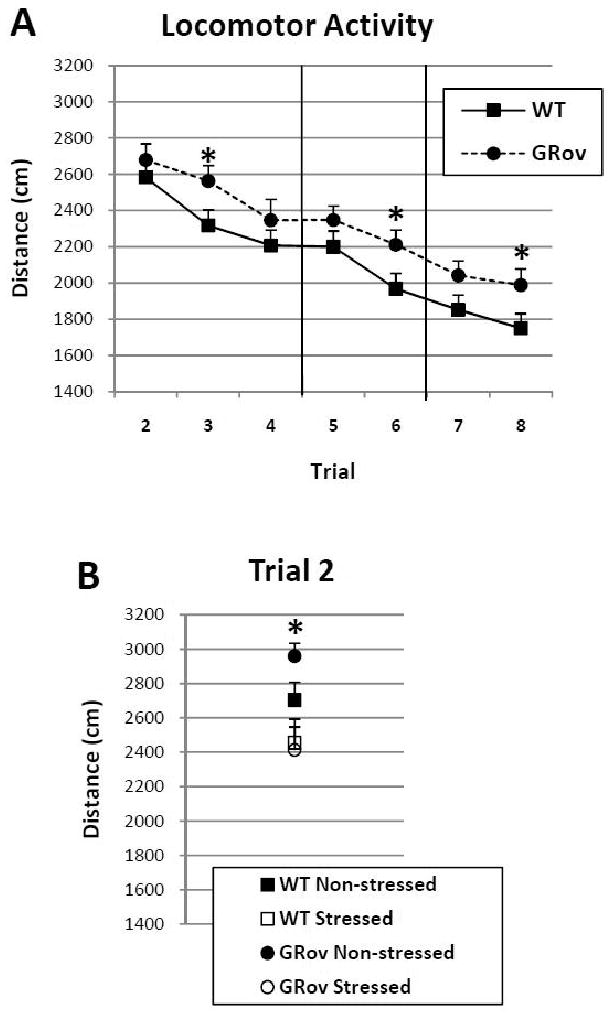
Locomotor activity over the course of the multi-trial experiment for WT and GRov mice (A) and for non-stressed and stressed WT and GRov mice during the first habituation trial (Trial 2; B). Trials 2-4: Habituation trials; Trials 5-6: Spatial novelty trials; Trials 7-8: Object novelty trials. Each point represents the mean ± SEM. Squares and circles represent WT and GRov mice, respectively. [*, P < 0.05 GRov vs. WT (A) and non-stressed GRov vs. stressed WT and GRov (B)].
When the mice first encountered the three objects in Trial 2--but not later trials--non-stressed mice exhibited more locomotor activity than stressed mice (see Figure 2B); thus, lending to the significant stress × trial interaction (F7, 524 = 2.13, P < 0.05). This effect was especially notable among the GRov mice that showed significantly more activity than their stressed counterparts upon first exposure to the novel objects during Trial 2 (posthoc tests: F1,524 = 9.76, P < 0.01). During later trials, all GRov mice were more active than their WT counterparts, especially during Trials 3, 6, and 8 (posthoc tests: Trial 3 - F1,524 = 4.10, P < 0.05; Trial 6 – F1,524 = 4.10, P < 0.05; Trial 8 – F1,524 = 4.39, P < 0.05; see Figure 2A). Thus, these data indicate that GRov mice exhibit more locomotor activity in the presence of objects over repeated exposures. Further, prior experience with acute restraint stress attenuates this increase during the first exposure to novel objects.
Interactions with Objects
Other measures, such as total number of object interactions, latency to first object interaction after being placed in the open field, and total object interaction time, are more specific to assessing object interaction than the general index of locomotor activity. None of the groups of mice showed a preference for any of the three objects during the habituation trials (Trials 2-4) using these measures, with one exception (i.e., stressed GRov mice showed a preference for Object 2 during Trial 3, data not shown). Since these measures all showed similar results, we will only report the total number of object interactions to avoid redundancy. Consistent with locomotor activity, these measures indicate that GRov mice are more reactive than WT mice, with GRov mice displaying increased amounts of object interaction and decreased object interaction latency. This increased GRov reactivity is attenuated when the mice are acutely stressed.
Examining the total number of object interactions reveals that GR overexpression in the forebrain leads to increased reactivity to objects that is clearly influenced by prior acute restraint stress. A linear mixed model analysis of the number of interactions with any of the three objects across Trials 2-8 reveals significant effects for genotype (F1, 76 = 9.18, P < 0.01), stress group (F1, 76 = 6.67, P = 0.01), and trial (F6, 454 = 29.44, P < 0.001). Non-stressed GRov mice exhibited a significantly higher number of object interactions compared to all other groups of mice during their first exposure to novel objects in Trial 2 (posthoc contrasts: non-stressed GRov vs. non-stressed WT-t454 = 2.87, P < 0.01; vs. stressed WT-t454 = 3.64, P < 0.001; vs. stressed GRov-t454 = 3.91, P < 0.001; Fig. 3A and B]. Although non-stressed GRov mice habituated to the objects by decreasing the number of interactions from the first to the last habituation trial (Trial 2 to Trial 4) like that of WT mice (posthoc contrasts: GRov: t454 = 4.87, P < 0.001; WT: t454 = 4.02, P < 0.001), they still interacted significantly more with the objects than their WT counterparts in Trials 2-5 (Trial 2: F1, 454 = 8.21, P < 0.01; Trial 3: F1, 454 = 5.15, P < 0.05; Trial 4: F1, 454 = 3.92, P< 0.05; Trial 5: F1, 454 = 4.75, P < 0.05; Fig. 3A). In fact, non-stressed GRov mice demonstrated a significantly higher number of object interactions averaged over the eight-trial test compared to WT mice (F1, 76 = 8.12, P < 0.01).
Figure 3.
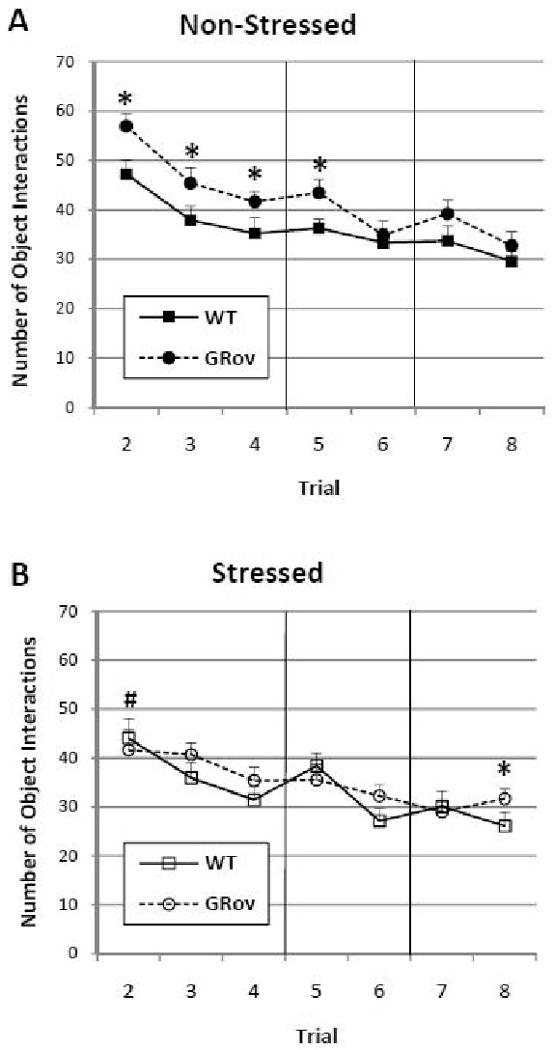
Number of interactions with all objects over the course of the multi-trial experiment for non-stressed (A) and stressed (B) mice. Trials 2-4: Habituation trials; Trials 5-6: Spatial novelty trials; Trials 7-8: Object novelty trials. Each point represents the mean ± SEM. Squares and circles represent WT and GRov mice, respectively. (*, P < 0.05 GRov vs. WT; #, P < 0.05 non-stressed GRov vs. stressed GRov).
Interestingly, this increased number of object interactions was eliminated when GRov mice were stressed, indicating that the significant main effect of genotype was primarily carried by the increased reactivity in the non-stressed GRov mice (Fig. 3B). Following acute restraint stress, GRov mice displayed a similar number of interactions to that of their stressed WT counterparts (F1, 76 = 2.19, P = 0.139; Fig. 3B) and significantly fewer interactions than non-stressed GRov mice across trials (F1, 76 = 6.24, P = 0.02). Thus, the impact of GR overexpression on increasing reactivity to objects, as measured by number of interactions, is most clearly evident in the absence of acute stress.
Spatial Novelty (Trial 5)
Object 1 was moved to a different location for Trials 5 and 6 to assess the animals' ability to discriminate spatial novelty. The number of interactions with each object best illustrated whether the mice in the current study showed preference for the displaced object. Thus, we will rely on this measure to assess group differences in response to spatial novelty.
Examining the number of interactions with each object during the first trial in which the mice experienced spatial novelty (Trial 5) reveals that stress significantly disrupts discrimination of spatial novelty in GRov mice. A linear mixed model analysis for the number of interactions with each object across the multi-trial test shows significant effects for genotype (F1, 76 = 4.07, P < 0.05), stress group (F1, 76 = 5.92, P < 0.05), and trial (F6, 454 = 33.32, P < 0.001), as well as several interactions (genotype × object: F2, 152 = 4.81, P < 0.01; trial × object: F12, 908 = 6.62, P < 0.001; genotype × stress × trial: F6,454 = 2.19, P < 0.05; and genotype × trial × object: F12, 908 = 184, P < 0.05). Post hoc tests reveal that several groups of mice showed a preference for the moved object (i.e., Object 1) during Trial 5 by having the highest number of interactions with that object compared to the other two objects. Specifically, all WT mice, regardless of stress group, interacted most with the displaced object (non-stressed: F2, 908 = 4.51, P = 0.01; stressed: F2, 908 = 11.54, P < 0.001; Fig. 4). Interestingly, non-stressed GRov mice also interacted most with the displaced object (Object 1) (F2, 908 = 3.36, P < 0.05], but their stressed GRov counterparts showed no preference for any of the three objects (F2, 908 = 0.81, P = 0.447). Thus, these data show that unstressed GRov mice, like WT mice, are able to discriminate an object when it is moved. However, unlike WT mice, the GRov mice lose this spatial discrimination ability when they are stressed. In fact, stress sharpened spatial discrimination in WT mice while dampening it in the GRov mice.
Figure 4.
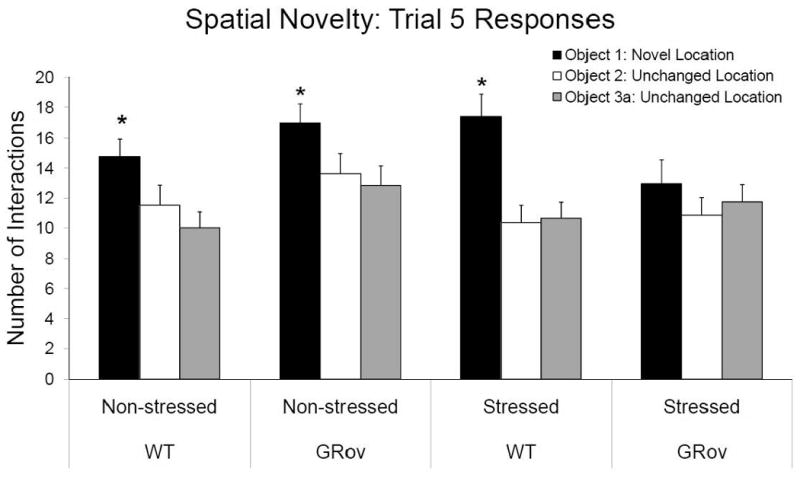
Discrimination of spatial novelty as displayed by the number of interactions with each object in Trial 5. Each bar represents the mean + SEM. (*, P < 0.05 preference for moved object).
Object Novelty (Trial 7)
A novel object (Object 3b) replaced Object 3a for Trials 7 and 8 to assess the animals' ability to discriminate object novelty. Analysis of the number of interactions and time spent interacting with each object provide similar results showing preference for the novel object, but analysis of latency to first object interaction did not clearly reveal object preferences (data not shown). For consistency, only the number of interactions will be reported for object novelty.
In general, mice showed a preference for the replaced object during Trial 7, by exhibiting the highest number of interactions with that object compared to the other two objects. This was demonstrated by post hoc tests from a linear mixed model analysis of the number of interactions with each object across Trials 2-8 (see Spatial Novelty above). Non-stressed WT mice interacted with the novel object more times than the two familiar objects during Trial 7 (F2, 908 = 3.64, P < 0.05; Fig. 5). However, stress attenuated this preference for the novel object such that the preference in stressed WT mice is not significant (F2, 908 = 154, P = 0.215). The non-stressed GRov mice also showed a significant preference for the novel object (F2, 908 = 10.28, P < 0.001). Interestingly, stress did not affect GRov mice as it did the WT mice. The stressed GRov mice also interacted significantly more with the novel object than the two familiar objects (F2, 908 = 4.27, P = 0.01; Fig.5). These data reveal that, in the absence of acute stress, WT and GRov mice discriminate novel objects normally. In addition, acute restraint stress dampens the ability of WT mice to discriminate a novel object from familiar ones. GRov mice, however, retain the ability to discriminate novel objects following restraint stress.
Figure 5.
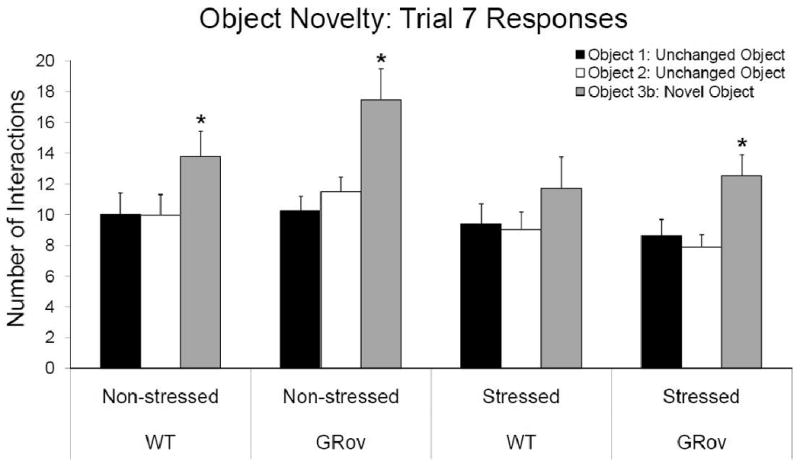
Discrimination of object novelty as displayed by the number of interactions with each object in Trial 7. Each bar represents the mean + SEM. (*, P < 0.05 preference for novel object).
Discussion
The results of the present study demonstrate that overexpression of GR in the forebrain leads to increased spontaneous reactivity to environmental stimuli. This heightened environmental reactivity, however, does not interfere with spatial or object novelty discrimination under normal conditions. Interestingly, acute restraint stress normalizes the elevated exploration of environmental stimuli. Moreover, acute stress has a differential effect on the responses of GRov mice to novelty—stress does not affect discrimination of spatial novelty in the WT animals but hinders it in GRov mice. By contrast, stress interferes with novel object discrimination in the WT but not in GRov mice. Together, these findings suggest that forebrain overexpression of GR leads to increased environmental reactivity as well as qualitative differences in vulnerability to stress-induced cognitive deficits.
General Activity and Habituation
GRov mice are more active and, when not exposed to strong acute stress, show more object exploration than WT mice in the presence of novel stimuli (i.e., objects). Even though locomotor activity and number of object interactions decreased across the habituation trials for all and non-stressed GRov mice, respectively, these behaviors continued at a higher level than for WT mice. Non-stressed GRov mice also showed shorter latencies to interact with objects and spent more time interacting with the objects over the course of the multi-trial test (data not shown). These behaviors show that overexpression of GR in forebrain not only increases general locomotor activity in response to novelty, but also increases exploration of novel stimuli. Since a novel object is both salient and able to draw an animal's attention inducing active exploration, our data suggest that GR overexpression changes responsiveness to saliency. Although these behaviors habituate over time, GRov mice show a continued higher level of interaction with the now familiar objects than do WT mice. Thus, GR overexpression appears to alter the assessment of saliency, leading an animal to respond as if stimuli, even after significant exposure, continue to be highly salient. This increased reactivity to saliency may well interact with the degree of threat in the environment, producing a qualitatively different behavioral pattern, relative to wild types. Thus, at low levels of threat such as novel objects in a contained environment, this can lead to increased exploration on the part of the GRov mouse. However, under more threatening conditions such as in classical tests of anxiety (e.g., Elevated Plus Maze, Light/Dark Box), GR overexpression can lead to increased anxiety-like behavior including decreased exploration of threatening features of the context because the salient features of that situation are amplified and sustained (Wei et al, 2004). Thus, the apparent contradiction--increased exploration of novel objects but increased avoidance in tests of anxiety, can be reconciled by the notion that these animals are more reactive in general to surrounding stimuli, especially salient ones, both in magnitude and duration of response.
The pattern we observe in GRov mice is distinctly different from that described in mice with decreased GR in forebrain (FBGRKO). The FBGRKO mice exhibit increased locomotor activity in all novel environments including typical tests of anxiety-like behavior (i.e., Light/Dark Box and Elevated Plus Maze), reflecting increased agitation and an exaggerated fight-or-flight stress response (Boyle et al, 2006). By contrast, GRov mice show no differences in non-specific motor activity during tests of anxiety, while exhibiting greater anxiety as indexed by their spatial choices.
The notion that GR influences the way the organism assesses external stimuli to guide appropriate behavioral responses is supported by the findings from GR deficient mice. These studies show that impaired GR function results in decreased object exploration and impaired discrimination of object novelty (Steckler et al, 1999). When kept in the testing box until they explore the objects as long as WT mice, GR deficient mice are then able to discriminate object novelty (Steckler et al, 1999). Thus, it is as if the GR deficient animals do not appropriately assess the saliency of the novel object and accord it the necessary level of investigation. By contrast, forebrain overexpression of GR results in a heightened response to saliency as shown by GRov mice in the current study. GRov mice spontaneously exhibited increased amounts of interaction with objects during the habituation trials which likely helped them to later discriminate spatial and object novelty.
We have characterized the behavioral phenotype of GRov animals as “increased emotional lability” (Wei et al, 2004). This is because stimuli trigger affective responses from these animals that are amplifications of normative responses, regardless of valence – e.g., more anxiety- and depressive-like behavior on the one hand, yet greater responsiveness to antidepressants, greater sensitization to psychostimulants, and, as this study shows, more exploration and novelty seeking, on the other. If we consider that increased anxiety- and depressive-like behaviors in animals are models of vulnerability to “internalizing disorders” in humans, and that novelty seeking and increased sensitization to drugs of abuse are models of vulnerability to “externalizing disorders” (Gilpin and Koob, 2008; Tackett et al, 2008), then GRov mice represent a good model of increased vulnerability to both classes of disorders, depending on the nature of the environmental stimuli.
Spatial and Object Discrimination
Interestingly, acute stress hindered discrimination of spatial, but not object, novelty in the GRov mice. Acute stress can either positively or negatively affect cognitive processes, depending on the time point of its occurrence, its relation to the context, and the degree of aversiveness of the task (De Kloet et al, 1999). Given the multi-trial nature of the object interaction test with five-minute inter-trial intervals in this study, it is difficult to distinguish the effects of stress (and thus, glucocorticoids) on acquisition, consolidation, and retrieval of newly learned memories. However, since the effects of glucocorticoids on minimally aversive tasks such as this object interaction test demonstrate an inverted U-shaped function at every stage of memory processing, it is not necessary to separate the effects of glucocorticoids on each stage (Conrad, 2005). Corticosterone levels measured 15 minutes following the last trial (i.e., 2 hours after commencement of restraint stress) in mice of the current study were not different between between GRov and WT mice (data not shown) whether or not they were exposed to restraint stress prior to testing. However, one cannot rule out that corticosterone levels may have been different among groups during testing since we have reported that GRov mice have a slower turn-off of the stress response following restraint stress (Wei et al, 2007). Thus, GRov mice exposed to stress prior to behavioral testing may have shown impaired discrimination of spatial novelty because they may have had corticosterone levels high enough to impair memory, while their WT counterparts may have had corticosterone levels at the top portion of the inverted U that actually enhanced spatial discrimination. Interestingly, stressed GRov mice later discriminated object novelty but WT mice did not, suggesting that corticosterone levels may have dropped to the top and ascending parts of the inverted U-curve for GRov and WT mice, respectively. Discrimination of object novelty in GRov mice might also have been preserved because the HPC is often not thought to be as important in non-spatial memory tasks such as object identity recognition and memory (Mumby et al, 2002; Thinus-Blanc et al, 1996; Save et al, 1992; Winters and Bussey, 2005a and b). In contrast, stressed WT mice may not have considered the novel object change as salient as the preceding spatial novelty trials leading to attenuation of object novelty discrimination.
Underlying Mechanisms
The phenotype of increased responsiveness to salient stimuli and associated increase in lability are likely mediated by changes in expression of several genes in the GRov mice that regulate environmental reactivity (Wei et al, 2004). Indeed, many systems implicated in arousal and environmental reactivity are affected by forebrain GR overexpression. Microarray analyses show a broad downregulation of glutamate receptor signaling in the HPC of GRov mice (Wei et al, 2007). Past work in our lab shows that the hippocampal glutamatergic system is involved in the control of negative feedback of the LHPA axis (Cullinan et al, 1993; Herman and Cullinan, 1997; Herman et al, 1989, 2003) and, thus, an alteration in this system would likely contribute to the delayed negative feedback observed in the GRov mice (Wei et al, 2007). Glutamate signaling is also essential in controlling the structural and functional plasticity of the synapse and plays a critical role in learning and memory mechanisms within the HPC (Kim and Diamond, 2002). MR activity has also been implicated in mediating environmental reactivity (Oitzl et al, 1994). Although MR mRNA expression is not altered in the HPC of GRov mice, altered MR/GR ratios could very well play a role in altered arousal and reactivity levels observed with overexpression of GR in the forebrain.
Changes in the amygdala of GRov mice (i.e., increased corticotropin releasing hormone in the central nucleus of the amygdala) may also contribute to the emotionally labile phenotype and the increased behavioral reactivity to objects observed in the current study. The amygdala has been implicated in object recognition and memory (Clark et al, 2000; Moses et al, 2002; Moses et al, 2005; Rossato et al, 2007; Zola et al, 2000;) as well as in the stress modulation of cognition (Bangasser and Shors, 2007; Roozendaal et al, 2006a; Roozendaal et al, 2006b). Activation of the amygdala via noradrenergic projections from the nucleus of the solitary tract or the locus coeruleus may play a role in stress-induced cognitive modulation (Roozendaal et al, 2006a). Amygdalar activation helps regulate environmental salience and attention, and emotional arousal consistent with amygdalar activation is essential in stress modulation of object recognition memory (Roozendaal et al, 2006a; Roozendaal et al, 2006b; Okuda et al, 2004). Thus, the increased expression of the norepinephrine transporter in the locus coeruleus of GRov mice (Wei et al, 2004) is ideal for contributing to the increased arousal level of the GRov mice seen over the course of the experiment, and it could play a role in their impaired discrimination of spatial novelty following stress.
Alterations in the serotonergic system may also play a role in the increased reactivity of GRov mice to environmental stimuli. Numerous reports indicate how alterations in one or more of its receptors, its transporter, or serotonin itself can modulate locomotor activity or even exploratory activity to environmental stimuli (File and Gonzalez, 1996; Geyer, 1996; Grailhe et al, 1999; Kalueff et al 2007a and b; Malleret et al, 1999; Ramboz et al, 1998). Studies with serotonin (5-HT) knock-out mice have shown that mice lacking the 5-HT1B or 5-HT5A receptor do not differ from WT mice in locomotor activity (5-HT1B knock-outs only) or anxiety-like behavior, but show a higher level object exploratory activity and lack of exploratory habituation (at least 5-HT1B knock-outs) (Grailhe et al, 1999; Malleret et al, 1999). The level of the serotonin metabolite 5HIAA in the prefrontal cortex has also been found to negatively correlate with the latency to approach objects in an open field, suggesting that higher prefrontal serotonin activity dampens the inhibition to approach novel objects (Bowman et al, 2003). More importantly, some studies indicate that serotonin transporter (SERT) expression contributes to locomotor activity as well as novel object and other environmental exploratory behavior (Kalueff et al, 2007b), and hippocampal 5-HT1A receptor activation increases locomotor behavior in some circumstances (File and Gonzalez, 1996). GRov mice have increased SERT mRNA expression in the ventromedial dorsal raphe and increased 5HT1A expression in the HPC (Wei et al, 2004), These serotonergic alterations may contribute to the increased reactivity to environmental stimuli found with forebrain GR overexpression.
Conclusion
Forebrain GR overexpression increases environmental reactivity and leads to impaired discrimination of spatial novelty following acute stress. The findings from this study show that prolonged perturbation of a stress-related gene has far-reaching behavioral consequences that are likely the result of alterations in multiple emotional arousal and memory-related systems. Previously, we have shown that forebrain GR overexpression leads to increased emotional lability and perturbability by pharmacological agents, as well as an aging-like neuroendocrine phenotype with evidence of hippocampal dysfunction and subtle cognitive deficits (Wei et al, 2004, 2007). The current study shows how the increased environmental reactivity of the GRov mice extends to increased reactivity to non-threatening environmental surroundings. Additionally, the modulation of cognitive function following stress is negatively affected in these mice. Thus, the increased vulnerability of the GRov mouse to environmental perturbation makes it an ideal model in which to study the interplay between emotional salience, coping abilities, and cognitive function, all of which likely play a role in a variety of psychiatric, age-related, and neurological illnesses.
Acknowledgments
This research was supported by National Institutes of Health Grants: NIMH PO1MH42251 (S.J.W., H.A.); NIDA RO1DA3386 (H.A); and NIDA 5P01DA021633-02 (H.A., S.J.W).
List of Abbreviations
- GRov
glucocorticoid receptor overexpression
- GR
glucocorticoid receptor
- LHPA
limbic-hypothalamic-pituitary-adrenal
- MR
mineralocorticoid receptor
- HPC
hippocampus
- WT
wild type
- ITI
inter-trial interval
- FBGRKO
forebrain glucocorticoid receptor knockout
- 5-HT
serotonin
- SERT
serotonin transporter
- 5-HT1B, 5-HT5A, 5-HT1A
serotonin receptors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akil H, Morano MI. The Biology of Stress: From Periphery to Brain. In: Watson SJ, editor. Biology of Schizophrenia and Affective Disease (ARNMD) Raven Press; New York: 1996. [Google Scholar]
- Akil H. Stressed and depressed. Nat Med. 2005;11:116–118. doi: 10.1038/nm0205-116. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Shors TJ. The hippocampus is necessary for enhancements and impairments of learning following stress. Nature Neuroscience. 2007;10:1401–1403. doi: 10.1038/nn1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm Behav. 2003;43:48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Boyle MP, Kolber BJ, Vogt SK, Wozniak DF, Muglia LJ. Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J Neurosci. 2006;26:1971–1978. doi: 10.1523/JNEUROSCI.2173-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caamano CA, Morano MI, Akil H. Corticosteroid receptors: a dynamic interplay between protein folding and homeostatic control. Possible implications in psychiatric disorders. Psychopharmacology Bulletin. 2001;35:6–23. [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. The relationship between acute glucocorticoid levels and hippocampal function depends upon task aversiveness and memory processing stage. Nonlinearity Biol Toxicol Med. 2005;3:57–78. doi: 10.2201/nonlin.003.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol. 1993;332:1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocrine Reviews. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. Journal of Neuroscience. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Gonzalez LE. Anxiolytic effects in the plus-maze of 5-HT1A-receptor ligands in dorsal raphe and ventral hippocampus. Pharmacol Biochem Behav. 1996;54:123–128. doi: 10.1016/0091-3057(95)02108-6. [DOI] [PubMed] [Google Scholar]
- Frick KM, Gresack JE. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav Neurosci. 2003;117:1283–1291. doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- Geyer MA. Serotonergic functions in arousal and motor activity. Behav Brain Res. 1996;73:31–35. doi: 10.1016/0166-4328(96)00065-4. [DOI] [PubMed] [Google Scholar]
- Gilpin N, K GF. Neurobiology of alcohol dependence: focus on motivational mechanisms. Alcohol Research & Health. 2008;31:185–195. [PMC free article] [PubMed] [Google Scholar]
- Grailhe R, Waeber C, Dulawa SC, Hornung JP, Zhuang X, Brunner D, Geyer MA, Hen R. Increased exploratory activity and altered response to LSD in mice lacking the 5-HT(5A) receptor. Neuron. 1999;22:581–591. doi: 10.1016/s0896-6273(00)80712-6. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–80. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Herman JP, Schafer MK, Young EA, Thompson R, Douglass J, Akil H, Watson SJ. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J Neurosci. 1989;9:3072–3082. doi: 10.1523/JNEUROSCI.09-09-03072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, Fox MA, Gallagher PS, Murphy DL. Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes Brain Behav. 2007;6:389–400. doi: 10.1111/j.1601-183X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Jensen CL, Murphy DL. Locomotory patterns, spatiotemporal organization of exploration and spatial memory in serotonin transporter knockout mice. Brain Res. 2007;1169:87–97. doi: 10.1016/j.brainres.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Aurousseau C, Le Moal M, Abrous DN. Behavioural trait of reactivity to novelty is related to hippocampal neurogenesis. Eur J Neurosci. 1999;11:4006–4014. doi: 10.1046/j.1460-9568.1999.00833.x. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Akil H, Watson SJ. Neural circuits mediating stress. Biol Psychiatry. 1999;46:1461–1471. doi: 10.1016/s0006-3223(99)00266-8. [DOI] [PubMed] [Google Scholar]
- Malleret G, Hen R, Guillou JL, Segu L, Buhot MC. 5-HT1B receptor knock-out mice exhibit increased exploratory activity and enhanced spatial memory performance in the Morris water maze. J Neurosci. 1999;19:6157–6168. doi: 10.1523/JNEUROSCI.19-14-06157.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neurosci Res. 1996;26:235–269. doi: 10.1016/s0168-0102(96)01105-4. [DOI] [PubMed] [Google Scholar]
- Morris RG. Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging and schemas. Eur J Neurosci. 2006;23:2829–2846. doi: 10.1111/j.1460-9568.2006.04888.x. [DOI] [PubMed] [Google Scholar]
- Moses SN, Cole C, Driscoll I, Ryan JD. Differential contributions of hippocampus, amygdala and perirhinal cortex to recognition of novel objects, contextual stimuli and stimulus relationships. Brain Research Bulletin. 2005;67:62–76. doi: 10.1016/j.brainresbull.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Moses SN, Sutherland RJ, McDonald RJ. Differential involvement of amygdala and hippocampus in responding to novel objects and contexts. Brain Res Bull. 2002;58:517–527. doi: 10.1016/s0361-9230(02)00820-1. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzl MS, Fluttert M, de Kloet ER. The effect of corticosterone on reactivity to spatial novelty is mediated by central mineralocorticosteroid receptors. Eur J Neurosci. 1994;6:1072–1079. doi: 10.1111/j.1460-9568.1994.tb00604.x. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford Unviversity Press; 1978. [Google Scholar]
- Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci U S A. 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Gaylord R, Topczewski F, Girotti M, Rubin B, Spencer RL. Immediate-early gene induction in hippocampus and cortex as a result of novel experience is not directly related to the stressfulness of that experience. Eur J Neurosci. 2005;22:1679–1690. doi: 10.1111/j.1460-9568.2005.04354.x. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci U S A. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, de Quervain DJ, McGaugh JL. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2006;103:6741–6746. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossato JI, Bevilaqua LR, Myskiw JC, Medina JH, Izquierdo I, Cammarota M. On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learn Mem. 2007;14:36–46. doi: 10.1101/lm.422607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Save E, Poucet B, Foreman N, Buhot MC. Object exploration and reactions to spatial and nonspatial changes in hooded rats following damage to parietal cortex or hippocampal formation. Behav Neurosci. 1992;106:447–456. [PubMed] [Google Scholar]
- Steckler T, Weis C, Sauvage M, Mederer A, Holsboer F. Disrupted allocentric but preserved egocentric spatial learning in transgenic mice with impaired glucocorticoid receptor function. Behav Brain Res. 1999;100:77–89. doi: 10.1016/s0166-4328(98)00115-6. [DOI] [PubMed] [Google Scholar]
- Tackett JL, Quilty LC, Sellbom M, Rector NA, Bagby RM. Additional evidence for a quantitative hierarchical model of mood and anxiety disorders for DSM-V: the context of personality structure. J Abnorm Psychol. 2008;117:812–825. doi: 10.1037/a0013795. [DOI] [PubMed] [Google Scholar]
- Thinus-Blanc C, Save E, Rossi-Arnaud C, Tozzi A, Ammassari-Teule M. The differences shown by C57BL/6 and DBA/2 inbred mice in detecting spatial novelty are subserved by a different hippocampal and parietal cortex interplay. Behav Brain Res. 1996;80:33–40. doi: 10.1016/0166-4328(96)00016-2. [DOI] [PubMed] [Google Scholar]
- Wei Q, Hebda-Bauer EK, Pletsch A, Luo J, Hoversten MT, Osetek AJ, Evans SJ, Watson SJ, Seasholtz AF, Akil H. Overexpressing the glucocorticoid receptor in forebrain causes an aging-like neuroendocrine phenotype and mild cognitive dysfunction. J Neurosci. 2007;27:8836–8844. doi: 10.1523/JNEUROSCI.0910-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Lu XY, Liu L, Schafer G, Shieh KR, Burke S, Robinson TE, Watson SJ, Seasholtz AF, Akil H. Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc Natl Acad Sci U S A. 2004;101:11851–11856. doi: 10.1073/pnas.0402208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ. Glutamate receptors in perirhinal cortex mediate encoding, retrieval, and consolidation of object recognition memory. J Neurosci. 2005;25:4243–4251. doi: 10.1523/JNEUROSCI.0480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ. Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. J Neurosci. 2005;25:52–61. doi: 10.1523/JNEUROSCI.3827-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J Neurosci. 2000;20:451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


