Abstract
Aims
We sought to assess the relation between time from myocardial infarction (MI) to enrolment and patient outcomes and to examine the association between these outcomes and implantable cardioverter defibrillator (ICD) therapy.
Methods and results
We analysed the Multicenter UnSustained Tachycardia Trial database (n = 1650). In examining all endpoints, Cox proportional hazards models were used to adjust for potential confounders. There was no significant association between time from MI to enrolment and any of the outcomes (P > 0.1). Inducibility by an electrophysiology study (EPS) was associated with a higher risk of arrhythmic death or cardiac arrest [adjusted hazard ratio (HR) 2.51; 95% confidence interval (CI) 1.64–3.84] and all-cause death (adjusted HR 1.45; 95% CI 1.04–2.03) only in patients who had an MI ≤6 months prior to enrolment. ICD therapy was associated with improved survival in patients who had an MI ≤6 months (adjusted HR 0.35; 95% CI 0.17–0.74) and >6 months before enrolment (adjusted HR 0.34; 95% CI 0.21–0.54).
Conclusion
The risk of arrhythmic death or cardiac arrest and all-cause death did not vary as a function of time from the most recent MI to enrolment. Inducibility by an EPS was associated with worse outcomes only in patients with an MI ≤6 months prior to enrolment. Although ICD therapy was associated with improved survival regardless of the time from MI to enrolment, this finding needs to be verified by a randomized clinical trial.
Keywords: Implantable cardioverter defibrillator, Myocardial infarction, Arrhythmic death, Cardiac arrest, MUSTT
Introduction
Although mortality from cardiovascular disease has diminished in recent years, survivors of a myocardial infarction (MI) remain at an increased risk of sudden cardiac death.1 A secondary analysis of the VALsartan In Acute myocardial iNfarcTion Trial (VALIANT) showed that the risk of sudden cardiac death was highest in the first 30 days after MI with 19% of all sudden cardiac death and survived cardiac arrest events occurring in that period.2 This observation suggests that earlier implementation of strategies to prevent sudden cardiac death may be warranted in such patients. However, this suggestion is not supported by data from randomized clinical trials of implantable cardioverter defibrillator (ICD) therapy or the current practice guidelines for primary prevention ICDs that mandate at least a 40-day period after an MI before ICD implantation.3–9
Although clinical trials of ICD therapy have demonstrated survival benefit with an ICD in patients with significant left ventricular (LV) dysfunction due to an MI, the majority of these trials excluded patients who were in the early phase of recovery from an acute MI.3,5,6 Except for the Multicenter UnSustained Tachycardia Trial (MUSTT), the Defibrillator in Acute MI Trial (DINAMIT), and the Immediate Risk Stratification Improves Survival (IRIS) trial, clinical trials of ICD therapy mandated a recovery period of 3–4 weeks following an acute MI before enrolling patients.3–7,9 DINAMIT and IRIS, the two clinical trials that specifically targeted patients recovering from an acute MI, showed no significant improvement in survival with an ICD.7,9
MUSTT, a randomized comparison of electrophysiologically guided antiarrhythmic therapy in patients with LV dysfunction and asymptomatic non-sustained ventricular tachycardia due to coronary artery disease, provides an opportunity for us to assess the association of time from MI to enrolment with the risk of arrhythmic death or cardiac arrest and the risk of all-cause death to determine the prognostic significance of inducibility by an electrophysiology study (EPS) and to examine the association between ICD therapy and these outcomes based on time from MI to enrolment.4
Methods
Patient population
Details of MUSTT were published previously.4 In brief, MUSTT was a randomized trial conducted at 85 clinical sites in the USA and Canada. It evaluated the ability of electrophysiologically guided antiarrhythmic therapy to reduce arrhythmic death or cardiac arrest in patients with an left ventricular ejection fraction (LVEF) ≤40%, coronary artery disease, and spontaneous non-sustained ventricular tachycardia (occurring ≥4 days after an MI and ≤6 months prior to enrolment). The protocol required a cardiac catheterization or an exercise test within 6 months before entry into the trial. Patients with exercise-induced ischaemia had to receive appropriate treatment for ischaemia before enrolment in MUSTT.4
Patients were excluded from MUSTT if they had a history of syncope or sustained ventricular arrhythmias >48 h after an MI and if the non-sustained ventricular tachycardia occurred in the setting of a reversible cause such as ischaemia, metabolic disturbance, or drug toxicity.4 Although a history of MI was not mandatory for enrolment in MUSTT, the majority of patients in this study (90%) had an MI.4 These patients constitute the study population for this analysis. We specified a priori a 6-month from MI to enrolment cut-off in presenting the data (≤6 months vs. >6 months). This cut-off was chosen for descriptive purposes and was felt to be reasonable clinically. Although a 3-month cut-off is also reasonable, the number of patients enrolled within 3 months after an MI in MUSTT was too small to allow any meaningful conclusions.
The institutional review board at each site approved the protocol, and all patients provided written informed consent.4
Treatment
Patients were randomly assigned to receive electrophysiologically guided antiarrhythmic therapy (n = 351) or no antiarrhythmic therapy (n = 353) if the baseline EPS demonstrated inducible sustained ventricular tachycardia. Patients randomized to antiarrhythmic therapy underwent serial testing with antiarrhythmic medications. An ICD could be chosen after at least one failed medication test. Patients in whom sustained ventricular tachycardia could not be induced (n = 1435) by the baseline EPS and patients who had inducible ventricular tachycardia by the baseline EPS but refused randomization (n = 63) were followed in a registry. Only a small number of these patients (n = 45) were treated with antiarrhythmic therapy. The protocol strongly recommended that all patients receive a beta-blocker and an angiotensin-converting enzyme inhibitor. The use of other cardiac medications was left to the discretion of the treating physician.4
Endpoints
The primary endpoint of MUSTT was arrhythmic death or cardiac arrest and one of its secondary endpoints was all-cause death.4 The current analysis focuses on these endpoints within 2 and 5 years and examines the impact of inducibility by the baseline EPS on these endpoints.
Statistics
All analyses were performed using SAS software (SAS Institute Inc., Cary, NC, USA). Continuous variables are presented as medians with 25th and 75th percentiles and categorical variables as frequencies. In analyses of survival from arrhythmic death or cardiac arrest and all-cause death and analyses that stratify these outcomes by inducibility by an EPS, we only included patients who did not receive an ICD within 90 days after enrolment and were discharged following the index hospitalization on no antiarrhythmic medications. Cox proportional hazards models were examined to assess for associations between time from the most recent MI to enrolment and arrhythmic death or cardiac arrest, and between time from the most recent MI to enrolment and all-cause death. Variables adjusted for in these models were age, LVEF, history of coronary artery bypass grafting, presence of three-vessel coronary artery disease, and induction of sustained monomorphic ventricular tachycardia. We studied the association between inducibility by an EPS and all study outcomes. These models were adjusted for age, LVEF, history of coronary artery bypass grafting, and the presence of three-vessel coronary artery disease. Including beta-blocker use at baseline in the model did not change the results. We also studied the association between ICD therapy and all study outcomes based on time from MI to enrolment by comparing patients randomized to electrophysiologically guided therapy who received an ICD with patients randomized to no antiarrhythmic therapy. These models adjusted for age, sex, and LVEF, treating ICD as a time-dependent covariate. Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) are reported. Results were declared significant at P < 0.05.
Results
Patient characteristics
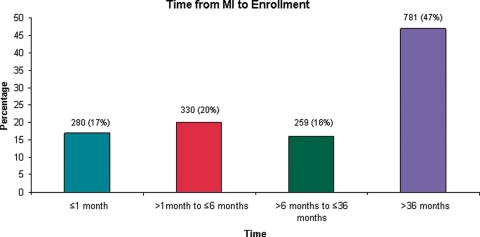
Of patients enrolled in MUSTT, 1974 qualified for this analysis on the basis of history of MI. We excluded 324 patients for lack of data on date of MI. Thus, 1650 patients were analysed. The distribution of time from the most recent MI to enrolment in this trial is shown in Figure 1. The baseline characteristics of these patients are shown in Table 1. Compared with patients who had an MI >6 months before enrolment in the trial, patients who had an MI ≤6 months before enrolment were younger and were less likely to be male. Patients who had an MI ≤6 months before enrolment had a higher LVEF, were less likely to have had coronary artery bypass grafting, were more likely to have had percutaneous coronary intervention, and more likely to have had New York Heart Association (NYHA) class I symptoms at randomization. Patients who had an MI ≤6 months before enrolment were also more likely to receive beta-blockers and aspirin at hospital discharge than patients who had an MI >6 months before enrolment.
Figure 1.
Distribution of time from myocardial infarction to enrolment (≤1 month, >1 month to ≤6 months, >6 months to ≤36 months, >36 months).
Table 1.
Baseline characteristics by time from myocardial infarction to enrolment
| Characteristics | ≤6 months (n = 610) | >6 months (n = 1040) | P-value |
|---|---|---|---|
| Age (years) | 66 (56, 71) | 66 (59, 72) | 0.011 |
| Male | 83 | 89 | <0.001 |
| LVEF (%) | 30 (24, 35) | 28 (20, 35) | <0.001 |
| Previous CABG | 53 | 64 | <0.001 |
| Previous PTCA | 31 | 23 | <0.001 |
| Three-vessel CAD | 36 | 41 | 0.064 |
| NYHA classa | n = 317 | n = 498 | 0.041 |
| I | 45 | 39 | |
| II | 31 | 39 | |
| III | 24 | 22 | |
| Non-sustained VT | |||
| Mean duration (beats) | 5 (4, 8) | 5 (4, 8) | 0.159 |
| Mean cycle length (ms) | 412 (367, 462) | 420 (380, 463) | 0.050 |
| Inducible, randomizable, sustained VT | 36 | 39 | 0.210 |
| Monomorphic | 33 | 38 | |
| Polymorphic with one or two extrastimuli | 5 | 3 | |
| Cycle length of induced monomorphic VT (ms) | 245 (223, 275) | 245 (228, 268) | 0.960 |
| Medications at hospital discharge | |||
| Beta-blockers | 49 | 32 | <0.001 |
| ACE inhibitor | 71 | 72 | 0.825 |
| Aspirin | 69 | 59 | <0.001 |
ACE, angiotensin-converting enzyme; CABG, coronary artery bypass grafting; CAD, coronary artery disease; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association class; PTCA, percutaneous transluminal coronary angiography; VT, ventricular tachycardia.
aData on NYHA class were only available on 50% of the patients. Continuous variables are presented as median (25th, 75th); all others are presented as percentages.
Of the 1650 patients included in this analysis and among patients randomized to electrophysiologically guided therapy, ICD therapy was received at some time during follow-up by 50 patients who had an MI ≤6 months before enrolment and by 110 patients who had an MI >6 months before enrolment.
Inducibility by an EPS
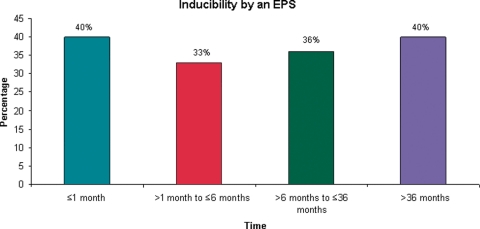
Inducibility by an EPS was defined as having sustained monomorphic ventricular tachycardia with single, double, or triple extrastimuli or having sustained polymorphic ventricular tachycardia with single or double extrastimuli. The distribution of inducibility by an EPS by time from MI to enrolment is shown in Figure 2.
Figure 2.
Inducibility by an electrophysiology study by time from myocardial infarction to enrolment (≤1 month, >1 month to ≤6 months, >6 months to ≤36 months, >36 months).
Outcomes
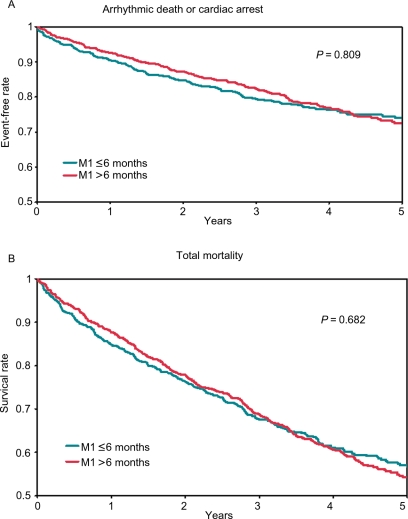
The rates of 2- and 5-year survival from arrhythmic death or cardiac arrest and from all-cause death are shown in Table 2. In the adjusted models, we found no significant association between time from most recent MI to enrolment and any of these outcomes (P > 0.1) when we treated time from most recent MI to enrolment as a categorical variable (Table 2) and as a continuous variable (Figure 3A and B).
Table 2.
Outcomes by time to enrolment
| n | Arrhythmic death or cardiac arrest |
All-cause death |
|||
|---|---|---|---|---|---|
| 2-year survival | 5-year survival | 2-year survival | 5-year survival | ||
| ≤1 month | 227 | 0.86 | 0.79 | 0.80 | 0.63 |
| >1 month to ≤6 months | 262 | 0.88 | 0.79 | 0.78 | 0.60 |
| >6 months to ≤36 months | 214 | 0.88 | 0.77 | 0.77 | 0.59 |
| >36 months | 618 | 0.87 | 0.71 | 0.78 | 0.52 |
| P = 0.995* | P = 0.723* | ||||
| ≤6 months | 489 | 0.87 | 0.79 | 0.79 | 0.62 |
| >6 months | 832 | 0.87 | 0.73 | 0.78 | 0.54 |
| P = 0.809* | P = 0.682* | ||||
This analysis only included patients who did not receive an ICD within 90 days after enrolment and were discharged following the index hospitalization on no antiarrhythmic medications.
*P-values in this table are for comparisons of arrhythmic death or cardiac arrest and of all-cause death among the different groups of time from MI to enrolment. These P-values were adjusted for age, LVEF, prior CABG, presence of three-vessel coronary artery disease, and induction of monomorphic sustained ventricular tachycardia.
Figure 3.
(A) Unadjusted arrhythmic death or cardiac arrest by time from myocardial infarction to enrolment. (B) Unadjusted all-cause death by time from myocardial infarction to enrolment. This analysis only included patients who did not receive an implantable cardioverter defibrillator within 90 days after enrolment and were discharged following the index hospitalization on no antiarrhythmic medications.
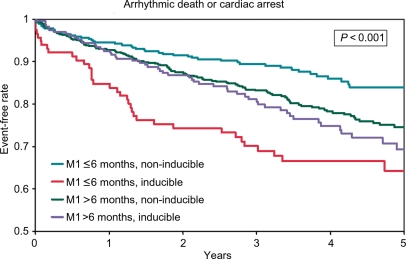
The 2- and 5-year rates of survival from arrhythmic death or cardiac arrest and from all-cause death stratified by inducibility by an EPS are shown in Table 3 and Figure 4. In patients who had an MI ≤6 months before enrolment in the trial, inducibility by an EPS was associated with a higher risk of arrhythmic death or cardiac arrest (adjusted HR 2.51; 95% CI 1.64, 3.84; P < 0.001) and a higher risk of all-cause death (adjusted HR 1.45; 95% CI 1.04, 2.03; P = 0.028). These relationships were not observed in patients with MI >6 months before enrolment (adjusted HR for arrhythmic death or cardiac arrest 1.19; 95% CI 0.87, 1.63; P = 0.273; adjusted HR for all-cause death 1.03; 95% CI 0.82, 1.30; P = 0.807).
Table 3.
Outcomes by time from myocardial infarction to enrolment stratification by inducibility by an electrophysiology study
| Non-inducible |
Inducible |
Adjusted P-value* | |||||
|---|---|---|---|---|---|---|---|
| n | 2-year survival | 5-year survival | n | 2-year survival | 5-year survival | ||
| Arrhythmic death or cardiac arrest | |||||||
| ≤6 months | 374 | 0.91 | 0.83 | 115 | 0.74 | 0.64 | <0.001 |
| >6 months | 595 | 0.87 | 0.74 | 237 | 0.87 | 0.69 | 0.273 |
| All-cause death | |||||||
| ≤6 months | 374 | 0.82 | 0.64 | 115 | 0.69 | 0.55 | 0.028 |
| >6 months | 595 | 0.79 | 0.54 | 237 | 0.75 | 0.53 | 0.807 |
This analysis only included patients who did not receive an ICD within 90 days after enrolment and were discharged following the index hospitalization on no antiarrhythmic medications.
*P-values in this table are for comparisons of arrhythmic death or cardiac arrest and of all-cause death between the group with ≤6 months from MI to enrolment and the group with >6 months from MI to enrolment stratified by inducibility by an EPS.
Figure 4.
Unadjusted arrhythmic death or cardiac arrest by time from myocardial infarction to enrolment and inducibility by an electrophysiology study. This analysis only included patients who did not receive an implantable cardioverter defibrillator within 90 days after enrolment and were discharged following the index hospitalization on no antiarrhythmic medications.
ICD therapy was associated with improved survival from arrhythmic death or cardiac arrest in patients who had an MI ≤6 months before enrolment (adjusted HR 0.23; 95% CI 0.08, 0.65) and in patients who had an MI >6 months before enrolment (HR 0.21; 95% CI 0.10, 0.47). Likewise, ICD therapy was associated with a significant improvement in the overall survival of patients who had an MI ≤6 months before enrolment (HR 0.35; 95% CI 0.17, 0.74) as well as patients who had an MI >6 months before enrolment (HR 0.34; 95% CI 0.21, 0.54). As shown in Table 4, similar trends were observed when the cut-off for time from MI to enrolment was changed from 6 months to 12 months and from 6 months to 18 months.
Table 4.
The association of implantable cardioverter defibrillator therapy with arrhythmic death or cardiac arrest and with all-cause death by time from myocardial infarction to enrolment
| Electrophysiologically guided therapy (ICD only) vs. no AA therapy | ||
|---|---|---|
| na | HR (95% CI) | |
| Arrhythmic death or cardiac arrest | ||
| ≤6 months | 135 | 0.23 (0.08, 0.65) |
| >6 months | 290 | 0.21 (0.10, 0.47) |
| ≤12 months | 157 | 0.23 (0.08, 0.64) |
| >12 months | 268 | 0.21 (0.09, 0.47) |
| ≤18 months | 170 | 0.21 (0.07, 0.58) |
| >18 months | 255 | 0.22 (0.10, 0.49) |
| All-cause death | ||
| ≤6 months | 135 | 0.35 (0.17, 0.74) |
| >6 months | 290 | 0.34 (0.21, 0.54) |
| ≤12 months | 157 | 0.36 (0.18, 0.72) |
| >12 months | 268 | 0.31 (0.20, 0.51) |
| ≤18 months | 170 | 0.35 (0.18, 0.69) |
| >18 months | 255 | 0.32 (0.20, 0.52) |
aThese analyses included patients who were randomized in the MUSTT trial and who had a prior MI and known MI date. AA, antiarrhythmic; CI, confidence interval; HR, hazard ratio; ICD, implantable cardioverter defibrillator.
Discussion
We found that the risk of 2- and 5-year arrhythmic death, cardiac arrest, and all-cause death did not vary as a function of time from the most recent MI to enrolment in the trial. We also found that inducibility by an EPS was associated with a significant increase in the risk of arrhythmic death or cardiac arrest and the risk of all-cause death in patients who had an MI ≤6 months before enrolment, but not in patients who had an MI >6 months before enrolment. These relationships were observed even after adjustment for potential confounders. Finally, ICD therapy was associated with improved survival from arrhythmic death or cardiac arrest and from all-cause death in post-MI patients regardless of time from MI to enrolment.
The lack of variation in the risk of arrhythmic death and cardiac arrest as a function of time from the most recent MI to enrolment in the trial could be explained by the fact that all patients enrolled in this trial had a low LVEF (median LVEF in MUSTT was 30%), suggesting that a low LVEF may be a stronger determinant of long-term arrhythmic death and cardiac arrest than time from the most recent MI.
Our study is the first to show that inducibility by an EPS is associated with a higher risk of arrhythmic death or cardiac arrest and all-cause death only in patients who had an MI ≤6 months prior to enrolment. This finding may be due to the significantly higher LVEF in patients with an MI ≤6 months before enrolment in our study. A MADIT-II substudy showed no association between a positive EPS and the risk of sudden cardiac death and only a weak association between a positive EPS and future occurrence of ventricular tachycardia. Based on these results, the investigators concluded that an EPS may not have appreciable utility in patients with an LVEF <30%.10 Future studies need to verify our finding that inducibility by an EPS is associated with worse outcomes only in patients who had an MI ≤6 months prior to enrolment, as this finding, if verified, may have some clinical implications. Because most patients in the major primary prevention ICD clinical trials were enrolled >6 months after MI, it has been argued that a gap in data on ICD therapy exists from the immediate to the chronic setting after an MI.11 Our results suggest that performing an EPS in patients who are <6 months post-MI may help clinicians and patients make a more informed decision about ICD implantation during that period.
Given that the risk of 2- and 5-year arrhythmic death and cardiac arrest did not vary as a function of time from the most recent MI to enrolment in the trial, it is not surprising that the ICD was found to be beneficial both in patients who had an MI ≤6 months before enrolment in the trial and in those who had an MI >6 months before enrolment. These findings were consistent when the cut-off for time from MI to enrolment was changed from 6 months to 12 months and to 18 months. However, our findings are not concordant with the results of a MADIT-II substudy that showed a significant survival benefit from an ICD in patients ≥18 months after an MI, but not in patients who had an MI <18 months before enrolment. Reasons for this disparity are uncertain but given the relatively small number of patients in our study, we cannot infer an evidence of effectiveness of ICD therapy early after an MI.12
Whether ICD therapy within 40 days after an MI improves survival was examined both by DINAMIT and the IRIS trial. These trials clearly showed no survival benefit with an ICD implanted early after an MI.7 Our findings do not refute or dismiss the findings of these clinical trials. Given the limitations of our analysis, our findings do not prove that ICD therapy confers survival benefit shortly after an MI.
Limitations
Our study has some limitations. First, although data for this study were collected prospectively, this analysis was done retrospectively. Despite our use of rigorous statistical methods to adjust for important confounders, we likely did not measure all factors that may be potential confounders in this analysis. Second, we did not have enough patients with an MI <1 month before enrolment to allow us to accurately determine the rate of arrhythmic death and cardiac arrest during this time period. In addition, only 21 patients received an ICD within 40 days after an MI. As such, we did not have any statistical power to try to support or refute the findings of DINAMIT and the IRIS trial. Third, the number of patients with an ICD in each studied group was relatively small, so all results related to ICD therapy should be interpreted with this limitation in mind. Fourth, a significant limitation is the low use (35%) of beta-blockers in the non-inducible patients. Finally, we did not have data on heart failure and/or NYHA class on a substantial number of patients, so we could not adjust for this variable in the models.
Conclusion
In this study, we demonstrated that the risk of long-term arrhythmic death or cardiac arrest and all-cause death did not vary as a function of time from the most recent MI to enrolment in the trial. We also showed that inducibility by an EPS was associated with worse outcomes only in patients with an MI ≤6 months prior to enrolment. Although ICD therapy was associated with improved survival regardless of the time from MI to enrolment, this finding needs to be verified by a randomized clinical trial.
Conflicts of interest: S.M.A. receives research funding and speaking fees from Medtronic and research funding from Biotronik. G.H. has nothing to report. K.L.L. receives research funding, consulting, and speaking fees from Medtronic. A.E.B. receives honoraria from Medtronic and his institution receives fellowship support from Medtronic and Boston Scientific.
Funding
This work was supported by grants from the National Heart, Lung, and Blood Institute (UO1 HIA5700 and U01 HTA5726); C.R. Bard; Berlex Laboratories; Boehringer Ingelheim Pharmaceuticals; Guidant Cardiac Pacemakers; Knoll Pharmaceutical; Medtronic; Searle; Ventritex-St Jude Medical; and Wyeth-Ayerst Laboratories.
References
- 1.Huikuri HV, Tapanainen JM, Lindgren K, Raatikainen P, Mäkikallio TH, Juhani Airaksinen KE, et al. Prediction of sudden cardiac death after myocardial infarction in the beta-blocking era. J Am Coll Cardiol. 2003;42:652–8. doi: 10.1016/s0735-1097(03)00783-6. doi:10.1016/S0735-1097(03)00783-6. [DOI] [PubMed] [Google Scholar]
- 2.Solomon S, Zelenkofske S, McMurray JJ, Finn PV, Velazquez E, Ertl G, et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005;352:2581–8. doi: 10.1056/NEJMoa043938. doi:10.1056/NEJMoa043938. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmias. N Engl J Med. 1996;335:1933–40. doi: 10.1056/NEJM199612263352601. doi:10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 4.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med. 1999;341:1882–90. doi: 10.1056/NEJM199912163412503. doi:10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 5.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. doi:10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 6.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. doi:10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 7.Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–8. doi: 10.1056/NEJMoa041489. doi:10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 8.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, III, Freedman RA, Gettes LS, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. Circulation. 2008;117:e350–408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 9.Steinbeck G, Andresen D, Seidl K, Brachmann J, Hoffmann E, Wojciechowski D, et al. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009;361:1427–36. doi: 10.1056/NEJMoa0901889. doi:10.1056/NEJMoa0901889. [DOI] [PubMed] [Google Scholar]
- 10.Daubert JP, Zareba W, Hall WJ, Schuger C, Corsello A, Leon AR, et al. Predictive value of ventricular arrhythmia inducibility for subsequent ventricular tachycardia or ventricular fibrillation in Multicenter Automatic Defibrillator Implantation Trial (MADIT) II patients. J Am Coll Cardiol. 2006;47:98–107. doi: 10.1016/j.jacc.2005.08.049. doi:10.1016/j.jacc.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 11.Tung R, Zimetbaum P, Josephson ME. A critical appraisal of implantable cardioverter-defibrillator therapy for the prevention of sudden cardiac death. J Am Coll Cardiol. 2008;52:1111–21. doi: 10.1016/j.jacc.2008.05.058. doi:10.1016/j.jacc.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 12.Wilber DJ, Zareba W, Hall WJ, Brown MW, Lin AC, Andrews ML, et al. Time dependence of mortality risk and defibrillator benefit after myocardial infarction. Circulation. 2004;109:1082–4. doi: 10.1161/01.CIR.0000121328.12536.07. doi:10.1161/01.CIR.0000121328.12536.07. [DOI] [PubMed] [Google Scholar]