Abstract
Objectives
Previous studies have evaluated rheumatoid arthritis (RA) risk according to pregnancy history with conflicting results. Fetal cells acquired during pregnancy provide a potential explanation for modulation of RA risk by pregnancy.
Methods
We examined parity and RA risk in results from a population-based prospective study in the Seattle, Washington area, comparing women with newly diagnosed RA (n=310) to control women (n=1418). We also evaluated the distribution of parity in cases according to HLA-genotype.
Results
We found a significant reduction of RA risk associated with parity, relative risk (RR) 0.61, 95% confidence interval 0.43-0.86 (p=0.005). RA risk reduction in parous women was strongest for younger women. Most striking was that RA risk reduction correlated with time elapsed since a woman’s last birth. RA risk was lowest among women 1 to 5 years from their last birth (RR 0.29) with risk reduction progressively lessening with increasing time (5-15 years, 0.51 and >15 years, 0.76), compared to nulliparous women (p=0.007 for trend). No correlation was observed with age at first birth or total number of births. Among cases with the highest genetic risk of RA (2 copies of RA-associated HLA alleles) a significant under-representation of parous vs. nulliparous women was observed (p=0.02).
Conclusions
We found a significantly lower risk of RA in parous women which correlated strongly with time elapsed from a woman’s last birth. While the explanation for our findings is not known, HLA-disparate fetal microchimerism can persist many years after a birth and could confer temporary protection against RA.
Keywords: rheumatoid arthritis, parity, pregnancy, HLA
Pregnancy and childbirth have been suspected of influencing the risk of developing rheumatoid arthritis (RA), but the epidemiologic findings are inconsistent. Both case-control studies and cohort studies [1-11] have evaluated gravidity and/or parity as etiologic factors, with some finding a reduced risk of RA associated with pregnancy or childbirth and others showing no significant association (Table 1). Elevations in sex hormones during pregnancy revert very rapidly after parturition and it would be difficult to explain a protective effect of parity on this basis. Feto-maternal cell trafficking during pregnancy has recently been recognized to result in the long-term persistence of small numbers of cells in mothers and children, referred to as fetal and maternal microchimerism respectively [12,13]. Fetal microchimerism that persists in the mother offers a potential explanation for an effect of parity on RA risk in women.
Table 1.
Studies of gravidity and/or parity and risk of RA
| First author Year | Study design | Inclusion criteria | Population |
|---|---|---|---|
| Del Junco* 1989 | Retrospective case-control, 324 cases, 324 controls | 1958 ARA, probable, definite or classical | USA (Minnesota), ages 30-79 at time of study |
| Hernandez 1990 | Prospective cohort, 121,700 with 115 cases | 1987 ACR | USA Nurses Health Study, ages 30-55 at recruitment |
| Hazes* 1990 | Retrospective case-control, 135 cases, 378 controls | 1958 ARA, definite or classical | Netherlands, outpatient clinics, ages 16-50 at onset |
| Spector* 1990 | Retrospective case-control, 150 cases, 337 controls | 1958 “ARA criteria” | UK (London), Rheumatology clinics, ages 35-70 at onset |
| Brun 1995 | Prospective cohort, 25,783 with 355 cases | No ACR criteria, ICD codesˆ | Norway (Bergen), screening for breast cancer, ages 20-69, RA on death certificate |
| Heliövaara 1995 | Retrospective cohort, 15,441 with 269 cases (176 RF+) | Not given | Finland (Helsinki), ages ≥30 at time of study |
| Pope 1999 | Retrospective case-control, 34 cases, 68 controls | 1987 ACR | Canada, clinics, ages 18-44 at recruitment, <3 years duration |
| Reckner* 2001 | Retrospective case-control, 179 cases, 259 controls | 1987 ACR | Sweden (Linkoping), ages 25-75 at recruitment |
| Merlino 2003 | Prospective cohort, 31,336 with 158 cases | 1987 ACR | USA, Iowa Women’s Health Study, ages 55-69 at recruitment |
| Karlson 2004 | Prospective cohort, 104,642 with 674 cases | 1987 ACR | USA, Nurse Health Study, ages 30-79 at onset |
| Jorgensen 2009 | Retrospective cohort, 2,140,056 with 7017 cases | No ACR criteria, ICD codesˆ | Danish national registry, ages 15-69 at first inpatient hospitalization for RA |
Study found a statistically significant association between parity and/or gravidity and RA risk.
No RA onset dates.
We analyzed pregnancy history and risk of RA in detailed data from a prospective case-control study of newly diagnosed RA in women in Seattle, Washington and the surrounding area. We reasoned that a lesser effect of parity might be observed among women who were older and that contradictory results of prior studies might be explained by considering a woman’s age and time elapsed since prior pregnancies. We also asked whether parity might be especially beneficial for women at greater risk of RA because they carried two copies of RA-associated HLA risk alleles. Thus the underlying hypothesis leading to the current study was that pregnancy could provide protection against RA but that this effect, like that of a vaccine, diminishes over time.
Patients and Methods
Data collection
The current study derives from a prospective population-based case-control study of newly diagnosed RA in women living in King County (Seattle area), Washington or receiving medical care at Group Health Cooperative, a large, Seattle-based prepaid health plan. This study recruited women aged 18 through 64 years of age through a surveillance system involving rheumatologists, family physicians and internists to identify RA cases diagnosed from November 1986 through February 1991. The study was designed to evaluate risk of RA in relation to prior or current oral contraceptive use. Each potential case was evaluated in person by a board-certified study rheumatologist, who conducted a standardized clinical history and joint examination. A rheumatoid factor test was performed by the University of Washington Clinical Immunology Laboratory, and a review of outpatient medical records was completed for each participant. Of potential cases identified 93% agreed to the study examination and personal interview, and of these 87% were found to be eligible. The initial study identified 349 women who met the previous American College of Rheumatology criteria for definite or probable RA. The current study was limited to 319 of these women who met the revised American College of Rheumatology criteria for RA [14] following a review of the rheumatologist’s physical examination, rheumatoid factor test result and medical record abstracts.
Control subjects in King County were identified using the Mitofsky-Waksberg method of random digit telephone dialing [15]. Screening for eligibility was successfully completed for 96% of the households contacted. Group Health Cooperative control subjects were selected using age-stratified random sampling from the plan’s master enrollment file. Participation rates among controls were 78% both in King County and at Group Health, and 1457 of 1541 willing participants met the eligibility criteria.
Each subject was interviewed in person about events prior to a specified reference date that was defined for cases as the date of the first physician visit for symptoms ultimately diagnosed as RA. The first visit to a physician for joint symptoms was utilized as the reference date for RA onset because the diagnosis of RA is sometimes delayed and conversely patient recall of first joint symptoms may precede RA onset. Each control subject was assigned a reference date that was selected at random from the distribution of reference dates among the cases. Detailed information regarding demographic information and reproductive history were obtained by trained study interviewers. Four cases and 38 control subjects who were pregnant at reference date, and 5 cases and 1 control missing relevant data points were excluded from the present analysis, yielding 310 cases and 1418 controls for analysis.
HLA-genotyping of RA cases
DNA was extracted either from whole heparinized blood or from peripheral blood mononuclear cells isolated after Ficoll-Hypaque gradient centrifugation. For a few cases DNA was extracted from a mouthswish sample or from serum. DNA-based typing was used to identify specific HLA DRB1 alleles as previously described [16].
Statistical analysis
The primary outcome for analysis was disease status (RA case vs. control). Logistic regression models were used to estimate the association between parity and disease status, while adjusting for confounding factors. The resulting odds ratio estimates can be interpreted as relative risks because of the rarity of RA in the population from which cases and controls were drawn. Gravidity was defined as number of pregnancies prior to reference date, and parity was defined as the number of those pregnancies that lasted at least 20 weeks and resulted in a live birth or stillbirth.
Factors examined as potential confounders included age at reference date, race, education level, marital status, body mass index, smoking status, oral contraceptive use (ever or never), history of spontaneous or induced abortion, and menopausal status. A factor was included as a confounder if there was a difference of 10% or more in any of the estimated coefficients of interest between the multivariable model including the factor and the model without it. The following characteristics were considered as predictors of RA risk: number of children, age at first birth and time since last birth, while adjusting for other confounders as needed. Two-sided p-values from regression models were derived from the Wald test. The distribution of the RA-associated HLA alleles (shared epitope) according to copy number was compared across parity status within RA cases via the chi-square test for trend. Analyses were performed on SAS software version 8 (SAS Institute, Inc., Cary, NC).
Results
Characteristics of the cases and control participants are summarized in Table 2. The groups were similar with respect to age; the median age of the cases and the controls was 43 years. Women diagnosed with RA were less likely to be white, to have been married, and to have attended college than control subjects.
Table 2.
Subject characteristics
| Controls (N = 1418) N (%) |
Cases (N = 310) N (%) |
|
|---|---|---|
| Age in years | ||
| 15 – 24 | 69 (5) | 24 (8) |
| 25 – 34 | 218 (15) | 63 (20) |
| 35 – 44 | 481 (34) | 80 (26) |
| 45 – 54 | 298 (21) | 67 (22) |
| 55 – 64 | 352 (25) | 76 (25) |
| Race | ||
| White | 1343 (95) | 273 (88) |
| Black | 28 (2) | 9 (3) |
| Asian | 41 (3) | 16 (5) |
| Other | 6 (1) | 12 (4) |
| Marital status | ||
| Married or living as married | 1005 (71) | 201 (65) |
| Never married | 149 (11) | 50 (16) |
| Divorced or separated | 224 (16) | 52 (17) |
| Widowed | 40 (3) | 7 (2) |
| Highest level education | ||
| Grade school | 91 (6) | 29 (9) |
| High school | 449 (32) | 117 (38) |
| Technical school | 253 (18) | 49 (16) |
| College or graduate school | 625 (44) | 115 (37) |
Parity of any number, compared to women who were never pregnant, was significantly associated with a lower risk of RA (adjusted RR 0.61, 95% CI 0.43 – 0.86, p=0.005, Table 3). Gravidity without parity conferred no advantage as RA risk among women who were gravid but nulliparous did not differ significantly from nulligravid women. The associations of gravidity with case status were similarly negative when gravidity was sub-categorized according to history of induced or spontaneous abortion (data not shown). Thus all further analyses compared parous subjects to the combined group of nulliparous subjects, regardless of gravidity.
Table 3.
Adjusted relative risks of RA by gravidity and parity, and by parity within age subgroups
| Age (years) |
Parity | Controls (N = 1418) N (%) |
Cases (N = 310) N (%) |
RR* (95% CI) |
|---|---|---|---|---|
| All | Nulligravid | 228 (16) | 68 (22) | 1.0 |
| Gravid, nulliparous | 81 (6) | 29 (9) | 1.20 (0.72 – 2.00) | |
| Parous | 1109 (78) | 213 (69) | 0.61 (0.43 – 0.86) | |
| < 35 | Nulliparous | 151 (53) | 58 (67) | 1.0 |
| Parous | 136 (47) | 29 (33) | 0.54 (0.33 – 0.90) | |
| 35 – 44 | Nulliparous | 98 (20) | 24 (30) | 1.0 |
| Parous | 383 (80) | 56 (70) | 0.52 (0.30 – 0.90) | |
| 45 – 54 | Nulliparous | 36 (12) | 8 (12) | 1.0 |
| Parous | 262 (88) | 59 (88) | 1.00 (0.44 – 2.29) | |
| 55 – 64 | Nulliparous | 24 (7) | 7 (9) | 1.0 |
| Parous | 328 (93) | 69 (91) | 0.79 (0.32 – 1.96) |
for all ages, adjusted for age, race (white vs. non-white) and oral contraceptive use (ever vs. never); within age subgroups, adjusted for race and education level (college or more vs. less than college).
We next asked whether the reduction in RA risk among parous vs. nulliparous women differed according to whether disease onset was in reproductive or post-reproductive years. RA risk was essentially halved for parous women less than 45 years old whereas there was no significant association of parity for women 45 years and older (Table 3). The difference was not simply chronological with advancing age; a test for a linear trend in the association of parity and RA risk over age gave p=0.12. There was limited variability in parity status among older women, as less than 10% of women 45 years and older were nulliparous.
Of special interest in our study was the evaluation of RA risk in women according to the amount of time that had elapsed from a woman’s last childbirth. We found that RA risk was lowest among women who were 1 to 5 years from their last birth with risk reduction progressively lessening with increasing time from last birth. For women who were 1 to 5 years from their last birth the proportion of cases was 11 in 120 subjects (9%), RR 0.29, p<0.001; for 5 to 15 years there were 49 cases in 345 subjects (14%), RR 0.51, p<0.001; and for more than 15 years there were 140 cases in 805 subjects (17%), RR 0.76, p=0.18, relative to nulliparous women in whom the proportion of cases was 97 of 406 subjects (24%) (Figure 1). A test for trend across these levels gave p=0.007 in an adjusted model.
Figure 1. Adjusted relative risks* of RA in parous subjects compared to nulliparous subjects, by time since last birth.
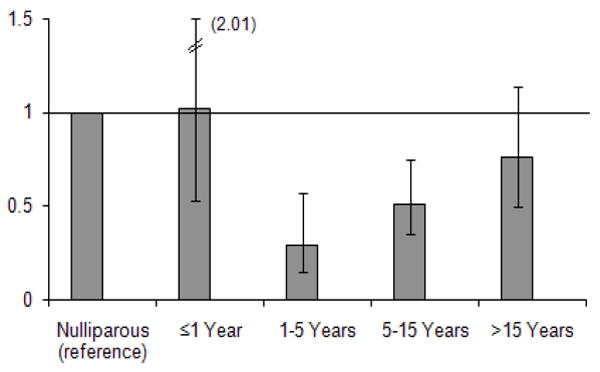
* adjusted for age, race (white vs. non-white) and education level (college or more vs. less than college). A test for trend gave p=0.007 in the adjusted model.
Although some studies have found an increased risk of RA postpartum [17], in our study population overall there was no significant increase or decrease risk in the year following delivery (Figure 1). Using the postpartum year as a reference and restricting analysis to parous women, gave a similar pattern of attenuating relative risk with increasing years from last pregnancy: RR 0.28 for 1-5 years, p=0.006, RR 0.45 for 5-15 years, p=0.03, RR 0.59 for >15 years, p=0.23.
While subject age and time since last childbirth were correlated, the association of RA risk with time since last birth persisted after adjusting for age. Moreover, the pattern among women less than 35 years old was similar to the pattern seen in the entire cohort; the association of parity and risk of RA was strongest in those 1 to 5 years since last birth (RR 0.27, 95% CI 0.11 – 0.67, p=0.005) and weaker in those who were more than 5 years postpartum (RR 0.59, 95% CI 0.30 – 1.17, p=0.13). In contrast, RA-risk was not significantly associated with age at first birth or total number of births (Table 4).
Table 4.
Adjusted relative risks of RA by parity characteristics in parous subjects
| Controls (N = 1109) N (%) |
Cases (N = 213) N (%) |
RR* (95% CI) | Trend p-value* |
|
|---|---|---|---|---|
| Number of births | ||||
| 1 | 190 (17) | 42 (20) | 1.0 | |
| 2 | 426 (38) | 70 (33) | 0.70 (0.45 – 1.09) | |
| 3 | 249 (22) | 52 (24) | 0.81 (0.50 – 1.31) | |
| ≥ 4 | 244 (22) | 49 (23) | 0.77 (0.46 – 1.29) | 0.57 |
| Age at first birth | ||||
| < 20 years | 236 (21) | 50 (23) | 1.0 | |
| 20 – 22 years | 306 (28) | 57 (27) | 0.92 (0.60 – 1.41) | |
| 23 – 25 years | 256 (23) | 57 (27) | 1.14 (0.73 – 1.78) | |
| ≥ 26 years | 311 (28) | 49 (23) | 0.78 (0.48 – 1.28) | 0.55 |
adjusted for age, race (white vs. non-white), number of births, age at first birth and time since last birth.
While samples were not available from children of these study subjects, we were able to also examine the hypothesis that parity is especially beneficial for women who are at greatest risk of RA by conducting HLA-genotyping of all RA cases and analyzing results according to parity. HLA-genotyping results were evaluated for women with RA according to the number of copies of an RA-associated HLA allele, whether 2, 1 or 0 copies. A similar sequence of the HLA-DRβ1 molecule is thought to underlie HLA-associated RA risk, referred to as the “shared epitope” [18], with two copies of the shared epitope conferring the greatest risk of RA. Consistent with the hypothesis, among women with RA who had two copies of the shared epitope, fewer were parous than nulliparous (Table 5). The converse was observed for women with RA who were negative for the shared epitope. Among RA cases with two copies of the shared epitope 18% were parous compared to 29% nulliparous; in those negative for the shared epitope 36% were parous compared to 27% nulliparous (p=0.02 for trend in association of shared epitope copy number and parity). Moreover, mirroring the overall study findings, the highest risk HLA-genotype was most underrepresented in parous women who were within 1 to 5 years of a birth with progressive attenuation with increasing time from last birth. Evaluating the number of RA cases with 2 copies of the shared epitope according to parity and time from last birth, 0% were 1 to 5 years since last birth, 14% were 5 to 15 years from birth and 21% were more than 15 years (Table 5).
Table 5.
Distribution of the RA-associated HLA shared epitope by copy number, parity and time since last childbirth in RA cases
| SE copies | Nulliparous (N = 97) N (%) |
All Parous* (N = 213) N (%) |
Years since last birth in parous cases |
|||
|---|---|---|---|---|---|---|
| ≤ 1 (N = 13) N (%) |
1 – 5 (N = 11) N (%) |
5 – 15 (N = 49) N (%) |
> 15 (N = 140) N (%) |
|||
| 2 | 28 (29) | 38 (18) | 1 (8) | 0 | 7 (14) | 30 (21) |
| 1 | 43 (44) | 98 (46) | 6 (46) | 6 (55) | 23 (47) | 63 (45) |
| 0 | 26 (27) | 77 (36) | 6 (46) | 5 (45) | 19 (39) | 47 (34) |
A test for trend in association of shared epitope copy number and parity gave p=0.02.
Discussion
A beneficial role of pregnancy in RA was initially described over seventy years ago when PS Hench reported that most women with RA who become pregnant experience improvement of their arthritis during pregnancy [19]. Many years later the first studies were reported that addressed the question whether a woman’s prior pregnancy history affects her risk of developing RA. Three studies reported in 1989 and 1990 found a significant difference in RA risk according to a woman’s pregnancy history. Results were expressed as an increased risk among nulliparous women but could also have been expressed as reduced risk in parous women. These case-control studies were carried out in The Netherlands, the UK and the USA and were retrospective in design [1,3,4]. A later study from Sweden also found a significant association [8]. However, other retrospective and prospective studies have found no significant association [2,5-7,9-11]. In addition to differences in the study populations and analyses that were conducted, some studies could not assure ACR (or ARA) criteria and others were not able to obtain information regarding date of RA onset so that pregnancies could have occurred before or after RA, and incident RA cases might not have been identified. Some studies conducted analyses according to parity and others gravidity.
We found that parous women overall were about 40% less likely to have been diagnosed with RA than nulliparous women. The inverse association with prior pregnancy was only observed for parity, as gravidity without parity did not impact RA risk. We reasoned that age for adult women is distinguished by reproductive and post-reproductive years and is not simply chronological as in males, and that contradictory results of prior studies might be explained by considering a woman’s age and time elapsed since prior pregnancies. We found that the lower risk of RA among parous women was most evident in younger women, less than 45 years of age, with no significant reduction of RA risk in women older than 45. More striking was the finding that time since last childbirth strongly and significantly correlated with RA risk, even after controlling for the mother’s age. RA risk was lowest among women 1 to 5 years from their last birth with risk reduction progressively lessening with increasing time from last birth. In contrast, no correlation was observed with a woman’s age at first birth or total number of births. Moreover, these relationships of time and risk held up even among the subset of women less than 35 years old.
It is difficult to envision a mechanism by which hormonal changes could explain an effect of prior pregnancies on RA risk many years later. However, recently it has become known that trafficking of fetal cells to the mother during pregnancy results in the long term persistence of a small number of fetal cells in the mother (as well as maternal cells in her offspring). Both adverse and beneficial effects of fetal microchimerism are currently an active area of investigation [12,13,20]. While the explanation for our study results is not known, persistent fetal microchimerism could potentially explain the protective effect of parity on RA-risk. The absence of association with gravidity alone could also be accounted for on this basis because the types of fetal cells transferred in early vs. late gestation have been found to differ [21,22] and/or the duration of exposure differs for gravidity without parity.
There are a number of known and putative risk factors for RA including smoking and obesity [23], prior use of oral contraceptives, breast-feeding and irregular periods [10]. HLA class II alleles encoding a similar sequence of the DRβ1 chain, referred to as the shared epitope, are known to increase RA risk [18]. As recently reported [24], if a woman acquires microchimerism that carries the shared epitope, whether fetal, maternal or of iatrogenic origin, it might be expected to convey increased risk of RA. The results of the current study suggest the converse may also occur, i.e. we hypothesize that acquisition of fetal microchimerism with RA-protective HLA alleles could reduce RA risk. Protection from RA has been proposed for HLA-molecules that carry the sequence “DERAA” instead of the shared epitope at the same location of the DRβ1 chain. Interestingly, a recent study found that the ”DERAA” sequence was significantly underrepresented as a non-inherited maternal HLA antigen among RA patients [25], an observation that also lends support to the hypothesis that microchimerism with RA-protective HLA-alleles (maternal or fetal) could impact RA risk.
In conclusion, we observed a lower risk of RA among parous women and found a significant correlation of time elapsed since last birth with reduction in RA risk. While any disease-protective mechanism underlying our findings is unknown an attractive explanation is a protective effect of HLA-disparate fetal microchimerism. The additional finding that parous women were underrepresented among those with two copies of the RA-associated shared epitope, and thus at higher genetic risk of RA, is also consistent with this explanation. Diminishing strength of protection over time could result from changes in maternal immunity as the mother ages, but could also occur due to aging of the “fetal” microchimerism as time progresses. RA is a common disease that causes substantial morbidity and disability, and the possibility that pregnancy might act like a vaccine in conferring temporary protection against RA merits further investigation.
Acknowledgments
Funded by Contract NO1-HD-62914 from the National Institute of Child Health and Human Development and NIH grants AI 45659 and AI 41721 (to JLN)
Abbreviations
- RA
rheumatoid arthritis
References
- 1.Del Junco DJ, Annegers JF, Coulam CB, Luthra HS. The relationship between rheumatoid arthritis and reproductive function. Br J Rheumatol. 1989;28(Suppl 1):33. doi: 10.1093/rheumatology/xxviii.suppl_1.33. discussion 42-5. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez Avila M, Liang MH, Willett WC, Stampfer MJ, Colditz GA, Rosner B, et al. Reproductive factors, smoking, and the risk for rheumatoid arthritis. Epidemiology. 1990;1(4):285–91. doi: 10.1097/00001648-199007000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Hazes JM, Dijkmans BA, Vandenbroucke JP, de Vries RR, Cats A. Pregnancy and the risk of developing rheumatoid arthritis. Arthritis Rheum. 1990;33(12):1770–5. doi: 10.1002/art.1780331203. [DOI] [PubMed] [Google Scholar]
- 4.Spector TD, Roman E, Silman AJ. The pill, parity, and rheumatoid arthritis. Arthritis Rheum. 1990;33(6):782–9. doi: 10.1002/art.1780330604. [DOI] [PubMed] [Google Scholar]
- 5.Brun JG, Nilssen S, Kvale G. Breast feeding, other reproductive factors and rheumatoid arthritis. A prospective study. Br J Rheumatol. 1995;34(6):542–46. doi: 10.1093/rheumatology/34.6.542. [DOI] [PubMed] [Google Scholar]
- 6.Heliövaara M, Aho K, Reunanen A, Knekt P, Aromaa A. Parity and risk of rheumatoid arthritis in Finnish women. Br J Rheumatol. 1995;34(7):625–8. doi: 10.1093/rheumatology/34.7.625. [DOI] [PubMed] [Google Scholar]
- 7.Pope JE, Bellamy N, Stevens A. The lack of associations between rheumatoid arthritis and both nulliparity and infertility. Semin Arthritis Rheum. 1999;28(5):342–50. doi: 10.1016/s0049-0172(99)80019-5. [DOI] [PubMed] [Google Scholar]
- 8.Reckner Olsson Ǻ, Skogh T, Wingren G. Comorbidity and lifestyle, reproductive factors, and environmental exposures associated with rheumatoid arthritis. Ann Rheum Dis. 2001;60:934–9. doi: 10.1136/ard.60.10.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merlino LA, Cerhan JR, Criswell LA, Mikuls TR, Saag KG. Estrogen and other female reproductive factors are not strongly associated with the development of rheumatoid arthritis in elderly women. Semin Arthritis Rheum. 2003;33:72–82. doi: 10.1016/s0049-0172(03)00084-2. [DOI] [PubMed] [Google Scholar]
- 10.Karlson EW, Mandl LA, Hankinson SE, Grodstein F. Do breast-feeding and other reproductive factors influence future risk of rheumatoid arthritis? Results from the Nurses’ Health Study. Arthritis Rheum. 2004;50(11):3458–67. doi: 10.1002/art.20621. [DOI] [PubMed] [Google Scholar]
- 11.Jorgensen KT, Pedersen BV, Jacobsen S, Biggar RJ, Frisch M. National cohort study of reproductive risk factors for rheumatoid arthritis in Denmark – a role for hyperemesis, gestational hypertension, and pre-eclampsia? Ann Rheum Dis. 2009 doi: 10.1136/ard.2008.099945. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen Huu S, Dubernard G, Aractingi S, Khosrotehrani K. Feto-maternal cell trafficking: a transfer of pregnancy associated progenitor cells. Stem Cell Rev. 2006;2:111–6. doi: 10.1007/s12015-006-0017-8. [DOI] [PubMed] [Google Scholar]
- 13.Gammill H, Nelson JL. Naturally acquired microchimerism. Int J Dev Biol. doi: 10.1387/ijdb.082767hg. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnett C, Edworthy S, Bloch D, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 15.Waksberg J. Sampling methods for random digit dialing. J Am Stat Assoc. 1978;73:40–46. [Google Scholar]
- 16.Yan Z, Ostensen M, Lambert NC, Guthrie KA, Nelson JL. Prospective study of cell-free fetal DNA and disease activity during pregnancy in women with inflammatory arthritis. Arthritis Rheum. 2006;54:2069–73. doi: 10.1002/art.21966. [DOI] [PubMed] [Google Scholar]
- 17.Silman A, Kay A, Brennan P. Timing of pregnancy in relation to the onset of rheumatoid arthritis. Arthritis Rheum. 1992;35(2):152–5. doi: 10.1002/art.1780350205. [DOI] [PubMed] [Google Scholar]
- 18.Winchester R. The molecular basis of susceptibility to rheumatoid arthritis. Adv Immunol. 1994;56:389–466. doi: 10.1016/s0065-2776(08)60456-3. [DOI] [PubMed] [Google Scholar]
- 19.Hench PS. The ameliorating effect of pregnancy on chronic atrophic (infectious rheumatoid) arthritis, fibrositis, and intermittent hydrarthrosis. Mayo Clin Proc. 1938;13:161–7. [Google Scholar]
- 20.Nelson JL. Naturally acquired microchimerism: For better or for worse. Arthritis Rheum. 2009;60:5–7. doi: 10.1002/art.24217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shields LE, Andrews RG. Gestational age changes in circulating CD34+ hematopoietic stem/progenitor cells in fetal cord blood. Am J Obstet Gynecol. 1998;178:931–7. doi: 10.1016/s0002-9378(98)70526-5. [DOI] [PubMed] [Google Scholar]
- 22.Pahal G, Jauniaux E, Kinnon C, Thrasher A, Rodeck C. Normal development of human fetal hematopoiesis between eight and seventeen weeks’ gestation. Am J Obstet Gynecol. 2000;183:1029–34. doi: 10.1067/mob.2000.106976. [DOI] [PubMed] [Google Scholar]
- 23.Voigt LF, Koepsell TD, Nelson JL, Dugowson CE, Daling JR. Smoking, obesity, alcohol consumption, and the risk of rheumatoid arthritis. Epidemiology. 1994;5(5):525–32. [PubMed] [Google Scholar]
- 24.Rak JM, Maestroni L, Balandraud N, Guis S, Boudinet H, Guzian MC, et al. Transfer of the shared epitope through microchimerism in women with rheumatoid arthritis. Arthritis Rheum. 2009;60(1):73–80. doi: 10.1002/art.24224. [DOI] [PubMed] [Google Scholar]
- 25.Feitsma AL, Worthington J, van der Helm-van Mil AHM, Plant D, Thomson W, Ursum J, et al. Protective effect of noninherited maternal HLA-DR antigens on rheumatoid arthritis development. PNAS. 2007;104:19966–70. doi: 10.1073/pnas.0710260104. [DOI] [PMC free article] [PubMed] [Google Scholar]


