The authors describe changes in the extracellular matrix in bovine eyes with elevated intraocular pressure that resemble those seen in human eyes with primary open-angle glaucoma and steroid-induced glaucoma. Therefore, steroid-treated bovine eyes provide the first animal model in which to study the pathophysiology of increased intraocular pressure in glaucoma.
Abstract
Purpose.
To analyze morphologic changes in the trabecular meshwork (TM) of bovine eyes treated with topical prednisolone and exhibiting elevated intraocular pressure for 4 weeks.
Methods.
The TM of four adult Braford cow eyes treated with 0.5% prednisolone eye drops three times daily for 7 weeks and their contralateral eyes treated with artificial tear preparation and that of two adult untreated Braford cows and untreated young calves eyes were analyzed with light and electron microscopy. Increased extracellular matrix (ECM) under the outflow loops was evaluated quantitatively. Additionally, deparaffinized tissue of treated eyes was labeled with an antibody against type VI collagen for immunocytochemistry.
Results.
In steroid-treated eyes ECM (plaques) accumulated under the endothelium of the inner wall of the outflow loops. On electron microscopy, this material contained fine fibrils that labeled for type VI collagen. Plaques were also seen in the contralateral controls of the treated animals but here they were significantly less in amount. In the untreated Braford controls and in untreated calf eyes, plaques were nearly absent. In the TM cells of the treated eyes there was a loss of glycogen from the cytoplasm and an increase in basement membrane-like material. These changes were not seen in contralateral eyes or eyes of untreated animals.
Conclusions.
Accumulations of ECM in the treated eyes resembled morphologic changes in human eyes with primary open-angle glaucoma and steroid-induced glaucoma. This animal model, therefore, provides a good tool in which to further study the pathogenesis of TM changes in glaucoma.
Recently, increased intraocular pressure (IOP) has been reported in normal cattle that had been treated with 0.5% prednisolone three times daily.1 Onset of IOP elevation occurred after approximately 3 weeks and reached a peak 1 week later. IOP increases were reported in the treated eye of every animal, and the IOP differences between treated eyes and fellow eyes reached up to 15 mm Hg. Increased IOP and development of glaucoma after treatment with corticosteroids is well known in humans. Approximately 30% to 40% of patients treated with potent glucocorticoids topically or systemically develop ocular hypertension.2–5 Steroid responders have a higher predisposition to develop glaucoma than nonresponders. Moreover, nearly 90% of patients with preexisting primary open-angle glaucoma (POAG) respond to steroid treatment with further elevation of IOP.5–7
Morphologically, the trabecular meshwork (TM) of human eyes treated long term with steroids showed a marked increase in extracellular material (ECM).8–12 Electron microscopic investigations of eyes with steroid-induced glaucoma (SG) revealed accumulations of fingerprint-like arranged basement membrane material in the outer corneoscleral and inner cribriform regions of the TM.8 Immunohistochemical evaluation showed a marked accumulation of type IV collagen and fibronectin in the TM.13 Additionally a conspicuous deposition of fine fibrillar material morphologically resembling that seen in POAG eyes adhering to the cribriform elastic net was observed under the inner wall of Schlemm's canal (SC).8,14 The biochemical nature of the fine fibrillar material under SC in SG is still unknown.13
In this study we investigated structural changes in the outflow pathways of cow eyes with elevated IOP levels induced by steroid treatment and compared these findings with those previously described in human eyes with POAG and SG.
Materials and Methods
Animal Eyes and Steroid Treatment
All animal experiments were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Eight pairs of eyes derived from Argentinean Braford cows and seven untreated calf eyes that were available at the local abattoir were investigated.
Animal experiments were performed as previously described in detail by Gerometta et al.1 However, experimental work described in this article was performed at a different farm (with different cowboys handling the animals) and at a different time of the year (spring instead of autumn) from that previously reported.1
Briefly, six healthy female cows between 3 and 5 years of age and weighing between 350 and 420 kg were treated with 0.5% prednisolone acetate eye drops in one eye while the contralateral eye received an artificial tear preparation for 49 days. Two drops of prednisolone or tear preparation were instilled three times a day. IOP was measured with a handheld Perkins applanation tonometer. Animals were killed on the last day of treatment. The eyes of two untreated Braford cows (each 3 years old) served as additional controls.
The morphology of the TM of untreated Braford cows was compared with that of seven eyes from untreated calves (6 months old) obtained from the local abattoir because these have previously been used for organ culture studies and their TM morphology has been well described.15–17
Fixation and Preparation of the Eyes
Eyes were enucleated immediately after sacrificing and subdivided at the equator into an anterior and a posterior segment, and the two halves were placed in 300 mL fixative. Two of the treated Braford cow eyes and their contralateral control eyes were fixed in 4% paraformaldehyde (PFA) for 60 to 80 days at 4°C. Anterior segments were subdivided into quadrants after careful removal of the lens. From all quadrants 1 to 2 mm-wide specimens, including TM, outflow loops (Schlemm's canal equivalent in cows), ciliary body, and sclera, were prepared, and the specimens were embedded in paraffin. The paraffin blocks were sent to Erlangen.
Anterior segments of four pairs of Braford cow eyes (four treated and four contralateral controls) and the anterior segments of the untreated Braford cows' eyes were placed in Ito's fixative for 60 to 80 days at 4°C and sent to Erlangen in fixative. In Erlangen, anterior segments were subdivided as described, and the specimens were postfixed in osmium tetroxide and embedded in Epon resin for ultrastructural analysis.
Of the seven calf eyes obtained at the local abattoir, three were placed in PFA and four in Ito's fixative. Fixation time was approximately 60 days at 4°C. The specimens were prepared as described. PFA-fixed specimens were embedded in paraffin, and Ito-fixed specimens were embedded in Epon resin.
Histologic and Ultrastructural Analyses
From all Epon resin–embedded blocks, 1-μm semithin sagittal and serial frontal sections were cut with an ultramicrotome (Ultracut E; Reichert Jung, Vienna, Austria) and subsequently stained with toluidine blue. Histologic sections were viewed with a microscope (Aristoplan; Ernst Leitz, Wetzlar, Germany) and photographed with a camera (DC 500; Leica; Wetzlar, Germany).
Ultrathin sections from the middle portion of the outer TM that included the outflow loops were cut, stained with uranyl acetate and lead citrate, and viewed with an electron microscope (EM 902; Zeiss, Oberkochen, Germany).
Immunohistochemistry
Immunohistochemical staining was performed on paraffin sections of the two steroid-treated eyes, their untreated contralateral controls, and three calf eyes. Unfortunately, fresh material was not available. From the different antibodies used to analyze the composition of the increased ECM in the steroid-treated eyes, only the antibody against type VI collagen (Rockland, Gilbertsville, PA) gave sufficient and repeatable results in this paraffin-embedded material. The secondary antibody was an affinity-purified rabbit monoclonal antibody (anti-rabbit Alexa 488; MobiTec, Göttingen, Germany).
Five-micrometer sagittal sections were cut with a microtome, deparaffinized in xylol, and washed with phosphate-buffered saline (PBS, pH 7.4). The sections were incubated with dry milk solution (Blotto; Santa Cruz Biotechnology, Heidelberg, Germany) for 1 hour at room temperature to prevent nonspecific staining. Afterwards they were rinsed and incubated at room temperature in a moist chamber with the primary antibody (dilution, 1:100) diluted in PBS buffer (PBS with 2% [wt/vol] bovine serum albumin [BSA; Merck, Darmstadt, Germany], and 0.2% [vol/vol] Triton-X-100) overnight. The sections were rinsed in PBS three times for 10 minutes each and then incubated for 1 hour with the secondary antibody (dilution, 1:2000). After another rinse in PBS, the sections were mounted in Kaiser's glycerine jelly (Merck KGaA, Darmstadt, Germany).
The sections were viewed under a fluorescence microscope (Aristoplan; Ernst Leitz) and photographed with a camera (DC 500; Leica). For controls, the primary antibody was omitted. A blocking peptide was not available
Immunoelectron Microscopy
The paraffin-embedded tissue of the two treated eyes and of their contralateral controls was deparaffinized in xylol. Tissue samples of the TM were then immersed in 4% (wt/vol) sucrose in PBS for at least 24 hours. For pre-embedding immunohistochemistry, 25-μm cryostat sections were cut and mounted on coverslips (Thermanox; Nunc, Rochester, NY). Cryosections were blocked (Blotto; Santa Cruz Biotechnology) for 30 minutes at room temperature. The anti-collagen VI antibody (Rockland; dilution 1:100) was applied in PBS/0.2% (wt/vol) BSA and incubated overnight at 4°C. After six rinses with PBS/2% (wt/vol) BSA, the sections were incubated overnight at 4°C with ultrasmall gold-conjugated anti-rabbit F(ab′)2 (Aurion, Wageningen, The Netherlands; dilution 1:100; size <0.8 nm) in PBS/0.2% (wt/vol) BSA. Sections were then rinsed five times with PBS/0.2% (wt/vol) BSA and two times with PBS and were fixed with 2.5% (vol/vol) glutaraldehyde in PBS for 2 hours at 4°C. Silver enhancement was then performed (R-Gent SE-EM; Aurion) for 1.5 hours in darkness, as described in the data sheet. Sections were postfixed with 0.5% (wt/vol) osmium tetroxide in PBS for 15 minutes and embedded in Epon resin. Ultrathin sections were cut and examined with an electron microscope (EM 902; Zeiss). Apart from omission of the primary antibody, control sections were treated the same way. No control section showed labeling with gold particles.
Semiquantitative and Quantitative Analyses
Semiquantitative and quantitative analyses were performed in 1-μm semithin sagittal sections of the Epon resin–embedded material. For semiquantitative analysis, the number of loops was counted in three sections, each taken from the superior, temporal, and nasal quadrants of the eye. Each section and each loop was rated from 0 to 2 with regard to the amount of ECM forming homogeneous plaque-like deposits, termed plaques under the endothelium (see Figs. 2A, 2B, 3A). Loops with no plaques were rated 0, loops with plaques under only parts of the endothelium of the outflow loops were rated 1, and loops with accumulations of plaque under the entire inner wall of the loops were rated 2. Semiquantitative analysis was performed once by three investigators (OYT, CMH, and ELD) who were masked to the analysis.
Figure 2.
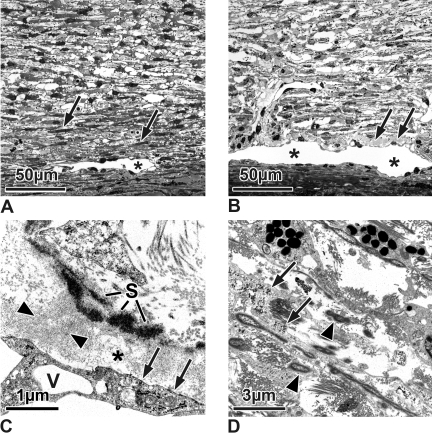
(A, B) Semithin sections of the TM and outflow loops (Schlemm's canal equivalent in the cow; asterisks) of a 6-month-old calf eye (A) and an untreated control Braford cow eye (B). (A) In the calf eye, the inner TM is more reticular than lamellar. The outer trabecular cells are connected to the outflow loops by smaller and more elongated TM cells (arrows) than those of the remaining reticular meshwork. There is no plaque material under the inner wall of the outflow loop (stage 0). (B) In the untreated Braford cow eyes, the TM also is reticular. There are only few elongated cells in the subendothelial region of the loops. Directly under the endothelium are some plaques (stage 1, arrows), but they are sparsely located. (C, D) Electron micrographs of untreated Braford cow control eyes. (C) In the subendothelial region (asterisk), fine fibrils (arrowheads) span the area from the elastic fiber sheath (S) to the endothelium. Accumulations of these connecting fibrils appear as loosely arranged plaques. At places of fibril attachment, the endothelium forms dense bands (arrows). V, giant vacuole. (D) TM cells between the collagen and elastic fibers show nearly no basement membranes. Within their cytoplasm are numerous glycogen granules (arrows). Elastic fibers contain electron light material within the osmiophilic core and a sheath of fine fibrillar material (arrowheads).
Figure 3.
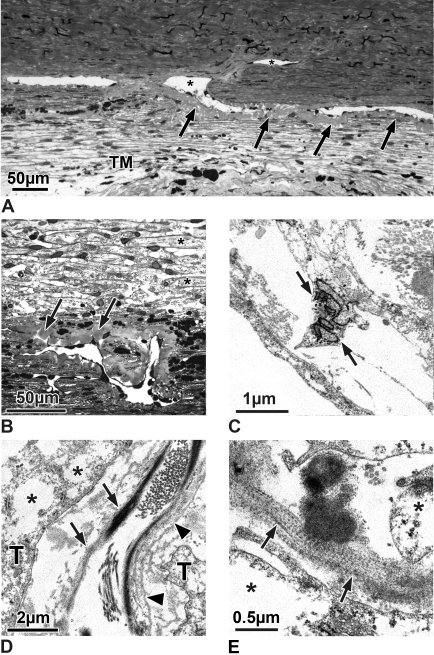
(A) Semithin frontal section through the TM, outflow loops, and a collector channel (asterisks) of an eye treated with steroids for 49 days. Under the entire inner wall endothelium of the outflow loops was homogeneous plaque material (arrows) with nearly no optically empty spaces. The morphology of the plaques appears independent from the vicinity to the collector channel. (B) Semithin sagittal section of an outflow loop and the TM of an eye treated with steroids for 49 days. In this higher magnification, one can see that the TM has a nearly lamellated appearance and that the connective tissue core of the lamellae (asterisks) was nearly completely surrounded by elongated trabecular cells. In the subendothelial region of the outflow loop can be seen a profound increase in homogenous plaque material (arrows) throughout several layers of subendothelial trabecular cells. (C–E) Electron micrographs of the TM of eyes treated with steroids for 49 days. (C) The TM cells show large cisterns of rough endoplasmic reticulum (arrow). (D) The central core of the lamellae contains elastic fibers with a small cross-linked sheath (arrows), collagen fibers, and layers of basement membrane-like material (arrowheads) that separate the core from the overlying trabecular cells (T). Within the cytoplasm of the trabecular cells are empty spaces (asterisks) instead of glycogen. (E) Higher magnification of an elastic fiber sheath showing cross-banding of the fine fibrils (arrows). Asterisks: empty spaces within the cytoplasm of trabecular cells.
For quantitative evaluation, micrographs of the loops were taken at a magnification of 320×, and the plaque areas under the inner wall endothelium of the outflow loops (μm2) was measured per length (μm) of outflow loop endothelium using a PC-based morphometric system (Quantimet 500; Leica, Cambridge, UK). These measurements were taken in three sections each from the superior, temporal, and nasal quadrants. Quantitative analysis was performed by one masked investigator (OYT) three times at intervals of at least 1 week. Intraobserver reproducibility was assessed within these data. Calf eyes contained so little plaque material that only a semiquantitative evaluation was performed.
Statistical Analysis
Scores of semiquantitative analyses in the various groups were compared with goodness of fit χ2 testing. Areas covered by plaques (quantitative analysis) were compared across groups using analysis of variance (ANOVA). P < 0.05 was considered significant.
Results
Intraocular Pressure
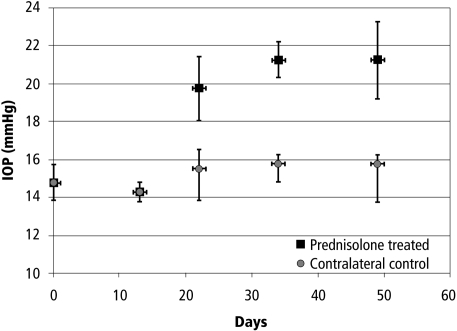
In each of the prednisolone-treated eyes, IOP was increased compared with the contralateral eye after approximately 3 weeks and remained elevated until the end of treatment (Fig. 1). Contralateral control eyes of these animals showed a minimal increase in IOP. Elevation of IOP was lower than that of the previous study1 but was significant compared with the contralateral eyes and compared with baseline during the course of the experiment (P < 0.000002; two-way ANOVA). The IOP difference between contralateral eyes was approximately 30%. IOP in contralateral control eyes was also statistically elevated from baseline over the course of the experiment (P < 0.04; ANOVA), but that change for all eyes was 2 mm Hg or less.
Figure 1.
IOP history (mean ± SD) of four Braford cows treated uniocularly with prednisolone acetate. Treatment started on day 0 and continued for the duration of the experiment. IOP in all prednisolone-treated eyes was elevated 3 weeks after the initiation of treatment. Contralateral (control) eyes show small IOP elevations.
IOP data of the untreated Braford cow eyes and the calf eyes was unavailable. In a previous study,1 however, it was shown that normal IOP in Braford cows ranges between 16 and 17 mm Hg and this was also true for the eyes before treatment in the present study (Fig. 1).
Control Eyes
In semithin sagittal and serial frontal sections, the morphology of the TM and the aqueous humor outflow loops (equivalent of Schlemm's canal in the cow) of the 3-year-old Braford cows in principle resembled that of the 6-month-old calf eyes derived from the local abattoir.15–17 In both groups the TM consisted of elastic and collagen fibers embedded in a ground substance. Bands of these fibers were incompletely separated from each other by trabecular cells, thereby forming a meshwork that was more reticular than lamellated. The subendothelial myofibroblast-like small elongated cells connecting the outflow loops with the ciliary muscle in the calf eyes (Fig. 2A) were not seen in the Braford cow eyes. In addition, occasional accumulations of homogeneous plaques separating the endothelial cells from the first subendothelial layer (Fig. 2B) were found at a few places in the Braford cow TM that were virtually absent in the calf eyes. These plaques were variable in amount interindividually and between the different loops of the same section.
At the electron microscopic level, the TM in both the calf eyes and the Braford cow eyes was connected to the outflow loops of the aqueous plexus by an elastic fiber net. From the sheath of the elastic fibers, fine fibrils spanned toward the endothelium of the loops (Fig. 2C). In some areas, these fibrils were so numerous and densely packed that they had a plaque-like appearance (Fig. 2C). At places of attachment, the endothelium formed dense band-like structures (Fig. 2C). The connecting fibrils were more numerous in the Braford cow than in the calf eyes. The elastic fiber net was also continuous with that of the TM. The elastic fibers in the Braford cow eyes were thicker than in calf eyes and contained more electron light material within the osmiophilic core and a sheath of fine fibrillar material, nearly absent in the calf eyes (Fig. 2D). Together with collagen fibers and ground substance, it formed the central core of the trabecular lamellae. The lamellae were incompletely covered by trabecular cells, and there was little basement membrane material adjacent to the cells (Fig. 2D). Trabecular cells in the Braford cow eyes, as in the calf eyes, were connected to each other and contained rough endoplasmic reticulum, a well-developed Golgi apparatus, single mitochondria, and large amounts of granules that previously had been shown to be glycogen (Fig. 2D).17 In calf eyes the glycogen-rich TM cells were present up to the endothelium of the loop, whereas in Braford cow eyes the subendothelial TM cells rarely showed glycogen inclusions.
Steroid-Treated Eyes
Treated Braford cow eyes differed profoundly from the eyes of untreated Braford animals. Histologically, in the subendothelial region of the loops, the accumulation of plaques was more pronounced than in the untreated Braford control eyes (Figs. 3A, 3B). Plaques were present under the endothelium of all outflow loops. There were nearly no optically empty spaces between the plaques. Furthermore, the plaques at places were also present under the outer wall of the loops, but here they were smaller, fewer, and more unevenly distributed. Frontal sections through the outflow loops showed that the plaques were distributed evenly circumferentially and that there were no differences in plaques under the loop endothelium in the area of the collector channels compared with loops that were not in direct contact with the channels (Fig. 3A). In sagittal sections there were no differences between plaques deposited under the endothelium of loops located in the anterior TM compared with the posterior TM (Fig. 3B).
In the subendothelial regions of all loops, the plaques extended more deeply into the adjacent TM (Fig. 3B). The TM itself had a shape that resembled that of trabecular lamellae in primates, and the central core of the lamellae was lined by a basement membrane-like structure (Fig. 3B).
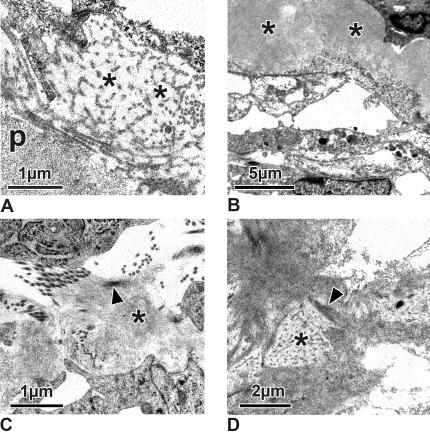
In the TM of the steroid-treated eyes, many of the trabecular cells showed enlarged cisterns of rough endoplasmic reticulum when examined electron microscopically (Fig. 3C). Glycogen was absent in nearly all cells, and most of the cells showed large empty spaces within their cytoplasm (Fig. 3D). The central core of the trabecular lamellae was surrounded by and separated from the trabecular cells by multiple layers of basement membrane material (Fig. 3D). The sheaths of the elastic fibers in the central core appeared thickened, and the fine fibrillar sheaths showed cross-banding (Fig. 3E). Irregularly arranged basement membrane-like material resembling the fingerprint material described in human eyes with steroid-induced glaucoma8,10 was present adjacent to the trabecular lamellae (Fig. 3D) and at places next to the plaque material under the endothelium of the outflow loops (Fig. 4A). The plaques themselves consisted of densely packed fine fibrils embedded in a light osmiophilic ground substance (Fig. 4B). These fibrils often were connected to the sheaths of subendothelial elastic fibers (Fig. 4C). At places these fibrils appeared cross-linked (Fig. 4D). In some areas they were in contact with increased amounts of basement membrane-like material (Fig. 4D).
Figure 4.
Electron micrographs of the subendothelial region of steroid-treated eyes. (A) Irregularly arranged basement membrane-like material (asterisks) can be seen adhering to the fine fibrillar subendothelial plaques (p). (B) Fibrillar material within the plaques (asterisks) is more densely packed than in the controls, and fibrils are embedded in a more osmiophilic ground substance. (C) Fine fibrils (asterisk) of the plaques often adhere to the sheath of the subendothelial elastic fibers (arrowhead). (D) Fibrils of the plaques are cross-linked (arrowhead). Occasionally, they are in contact with basement membrane-like material (asterisk).
Contralateral Control Eyes
In the contralateral controls, the TM was more reticular and less lamellated than in the treated eyes but was similar to that in the untreated controls. Glycogen granules were more numerous in the contralateral controls than in the treated eyes but less numerous than in the untreated Braford cow and calf eyes. In the subendothelial region of the loops, plaques were more numerous than in untreated controls but less numerous than in the treated eyes.
Staining for Type VI Collagen
In the calf eye, staining was present only at some loops, and here only small dots under the inner wall endothelium were stained (Fig. 5A). In the treated eyes, all loops were surrounded by a sheath of type VI immunoreactive plaques (Fig. 5B). A type VI collagen immunoreactive sheath was also seen in the contralateral controls, but not all loops were surrounded (data not shown).
Figure 5.
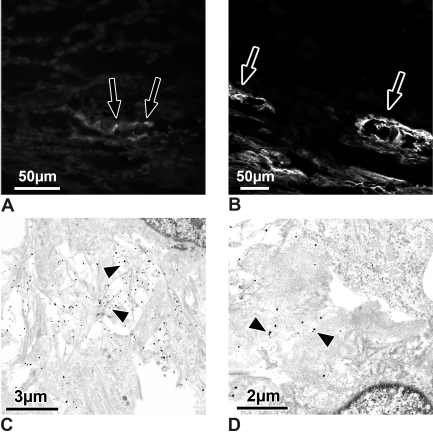
(A, B) Light micrographs of sections through outflow loops, immunohistochemically stained for type VI collagen. (A) Calf eye. There are only small dots (arrows) of type VI collagen staining under the endothelium of the outflow loop's inner wall. (B) Steroid-treated eye. Extracellular material surrounding the loops stains intensely for type VI collagen (arrows) in treated and in contralateral control eyes. (C, D) Electron micrographs of the inner wall of outflow loops immunogold-labeled for type VI collagen. (C) Contralateral control eye. Note that the fine fibrils in the subendothelial region are labeled intensely (arrowheads) for type VI collagen. (D) Steroid-treated eye. Where the plaques contained cross-linked fibrils, only those fibrils that entered the plaques are labeled (arrowheads).
At the ultrastructural level, fine fibrils between the inner wall endothelium and subendothelial trabecular cells were labeled for type VI collagen (Fig. 5C). These fibrils were nicely seen in the contralateral controls of the treated eyes (Fig. 5C). In the treated eyes, such accumulations of labeled fibrils were also present. In addition, in the plaques in which the numerous fibrils appeared cross-linked, only those fibrils entering the plaques were labeled (Fig. 5D).
Semiquantitative and Quantitative Analysis
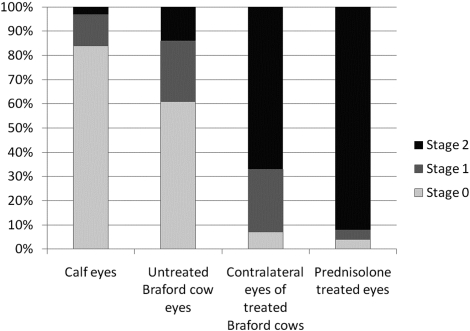
Semiquantitative analysis of the two groups of untreated control eyes showed that 84% of the outflow loops in calf eyes and 61% of the Braford eye loops had no plaques under the inner wall endothelium (stage 0). There was some plaque material under the inner wall endothelium (stage 1) in only 13% of the calf eye loops and 25% of the Braford eye loops. Accumulation of plaque material under the entire inner wall of a loop (stage 2) was observed in only 3% of the calf eye loops and 14% of the Braford eye loops (Fig. 6).
Figure 6.
Diagram showing the results of semiquantitative analysis of the plaque accumulation at the inner wall endothelium of the loops. Results are given as percentages of loops showing the stage 0, 1, and 2 changes.
Semiquantitative analysis of the steroid-treated eyes disclosed severe accumulations (stage 2) of plaque in 92% of the loops. Four percent of the loops showed plaque occasionally in the endothelium (stage 1), and the other 4% of the loops did not show plaque material. In the contralateral control eyes (treated with only artificial tears), stage 2 accumulation was found in 67% of the loops, and stage 1 accumulation was found in 26% of the loops; 7% of the loops were considered unchanged (stage 0; Fig. 6). Semiquantitative scores of plaque accumulation differed significantly among the various groups of eyes (P < 0.00001; χ2 test). Agreement among the three observers was 100% for all specimens (κ = 1 for all pairs).
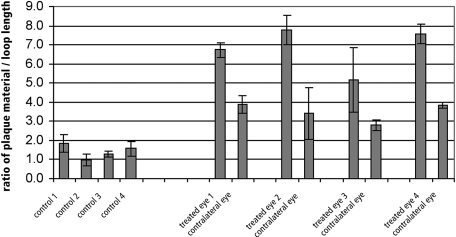
Plaque areas per loop length were significantly (P < 0.0002) larger in treated eyes than in eyes in all other groups (P < 0.05, Bonferroni post hoc comparison). All treated eyes had larger areas of plaque than did their contralateral control eyes (6.73 ± 0.38 μm vs. 3.90 ± 0.47 μm, 7.76 ± 0.76 μm vs. 3.41 ± 0.47 μm, 5.17 ± 2.81 μm vs. 3.94 ± 0.40 μm, and 7.57 ± 0.49 μm vs. 3.85 ± 0.16 μm; Fig. 7). All treated eyes and contralateral eyes had significantly more plaque than did untreated Braford cow eyes (1.86 ± 0.46 μm, 0.97 ± 0.33 μm, 1.27 ± 0.15 μm, and 1.57 ± 0.40 μm; P < 0.05 Bonferroni post hoc comparison; Fig. 7). Triplicate measurements revealed no significant differences (P > 0.89, repeated-measures ANOVA)
Figure 7.
Results of quantitative analysis of the subendothelial plaques. Results are given as a function of area of plaque material (μm2) per length of loop endothelium (μm). Plaque amounts were significantly greater in treated eyes than in contralateral eyes and significantly greater in contralateral control eyes than in the four eyes of untreated Braford cows (P < 0.0002, ANOVA; P < 0.05, Bonferroni post hoc comparison).
Discussion
Steroid treatment in bovine eyes induced increased deposition of ECM under the endothelium of the outflow loops and in the TM. This material resembled changes seen in human eyes with POAG and SG.
As in the human POAG eyes, plaques under the loop endothelium of the steroid-treated bovine eyes were partially connected to the fibrillar sheaths of the elastic fibers and their connecting fibrils to the inner wall endothelium.18 In addition, these fibrils stained for collagen type VI. In human POAG eyes, most of the SD plaques contained cross-linked fibrillar material that was not digestible by proteases, and it was assumed that they might have been formed by activity of the enzyme tissue transglutaminase.19
In addition to these plaque formations, there was an increase in basement membrane-like material. This basement membrane-like material formed multiple layers surrounding the connective tissue lamellae of the TM, giving the meshwork a more lamellar than reticular appearance. At places this material formed fingerprint-like structures resembling those described in human SG8 and juvenile glaucoma eyes.20 Unfortunately, the fixation of our material did not allow staining for type IV collagen or other basement membrane structures. In the steroid-treated cow eyes, this basement membrane-like material was located next to TM cells, and it is tempting to speculate that they were formed by these cells. Morphologically, TM cells in the steroid-treated eyes showed cisterns of rough endoplasmic reticulum and Golgi material—signs of protein biosynthesis.
In fact, in vitro corticosteroid treatment of human TM cells induces the expression and secretion of several ECM proteins,21 including fibronectin,22,23 laminin,24 and collagen type IV.22 In addition, it induces thrombospondin-1 (TSP-1) expression.25 TSP-1 is a multifunctional protein involved in TGF-β activation. It is unknown whether the formation of type VI collagen under the inner wall was caused by a thrombospondin-induced increase in active TGFβ or whether steroid treatment itself induced type VI collagen formation. Furthermore, increases in ECM in the TM could also be attributed to a decrease in ECM degradation. In vitro steroid treatment inhibits the activity of several TM extracellular matrix metalloproteinases (MMPs),26,27 and TGF-β2 induces active plasminogen activator inhibitor-1 (PAI1) expression, thereby inhibiting a variety of MMPs.28
Why the loss of glycogen granules was greater in treated eyes than in controls that were similarly fixed and embedded is unknown. We assume that the empty spaces in the cytoplasm of TM cells were formed during the fixation and embedding process by loss of glycogen granules. In recent studies it has been shown that steroid treatment affects the actin elements within the cytoplasm of the trabecular cells in human and in bovine eyes, finally leading to cross-linked actin network formation.29,30 It is possible that changes in the architecture of the cytoskeleton influence the structural fixation of the glycogen granules.
In our previous report,1 all treated eyes had IOP elevations to approximately 36 mm Hg, whereas the contralateral eyes displayed normal or only slightly increased IOP. In this series of animals, IOP was again consistently elevated in treated eyes, but maximum IOP was approximately 23 mm Hg. The reason for this difference in IOP elevation is unknown. One possible explanation may be the time of the year during which these experiments were performed (spring instead of autumn). IOP can differ at different times of the year in humans.31–33 Environment might also have affected the levels of IOP elevation—the experiments described here were performed at a different farm than the ones reported earlier.
Nonetheless, significant differences (both statistically and clinically) in IOP levels between treated and control eyes were evident in the present study. Significantly more loops with plaques developed in the treated eyes than in the contralateral control eyes.
On the other hand, the contralateral control eyes with nearly normal IOP did have increases in plaque. Why this occurred in the contralateral eyes of these large animals is still unknown. In the past, we had observed effects in the contralateral eyes in all monkeys treated uniocularly with various kinds of antiglaucoma drugs, even if care was taken to avoid a wiping effect.34–37 Therefore, we always used untreated animals as controls.
The treated cow eyes had pronounced changes in the TM that were not present in the contralateral eyes. We assume that these TM changes might be related to the increase in IOP and that a combination of inner wall plaques and TM changes might have been causative for the IOP increases. On the other hand, our findings in contralateral eyes indicate that the formation of plaques can occur even when IOP elevation is minimal and is probably not secondarily induced by elevated IOP.
In summary, steroid treatment of bovine eyes causes ECM changes in the TM that clearly resemble ECM increases in human eyes with POAG, juvenile glaucoma, and SG. This animal model, therefore, provides a good tool to investigate the pathogenesis of IOP increases in glaucoma.
Acknowledgments
The authors thank Anke Fischer, Gertrud Link, and Hong Nguyen for excellent assistance with electron microscopy, immunoelectron microscopy, and immunohistochemistry, and Jörg Pekarsky and Marco Gösswein for preparation of the micrographs.
Footnotes
Supported by National Eye Institute Grants EY00160, EY016050, and EY13749 (OAC), a Secretaría General de Ciencia y Técnica, UNNE, Argentina, and an unrestricted grant from Research to Prevent Blindness, Inc., New York, NY.
Disclosure: O.-Y. Tektas, None; C.M. Hammer, None; J. Danias, None; O.A. Candia, None; R. Gerometta, None; S.M. Podos, None; E. Lütjen-Drecoll, None
References
- 1.Gerometta R, Podos SM, Candia OA, et al. Steroid-induced ocular hypertension in normal cattle. Arch Ophthalmol 2004;122:1492–1497 [DOI] [PubMed] [Google Scholar]
- 2.Hovland KR, Ellis PP. Ocular changes in renal transplant patients. Am J Ophthalmol 1967;63:283–289 [DOI] [PubMed] [Google Scholar]
- 3.Bernstein HN, Schwartz B. Effects of long-term systemic steroids on ocular pressure and tonographic values. Arch Ophthalmol 1962;68:742–753 [DOI] [PubMed] [Google Scholar]
- 4.Becker B, Mills DW. Corticosteroids and intraocular pressure. Arch Ophthalmol 1963;70:500–507 [DOI] [PubMed] [Google Scholar]
- 5.Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics, I: the effect of dexamethasone in the normal eye. Arch Ophthalmol 1963;70:482–491 [DOI] [PubMed] [Google Scholar]
- 6.Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics, II: the effect of dexamethasone in the glaucomatous eye. Arch Ophthalmol 1963;70:492–499 [DOI] [PubMed] [Google Scholar]
- 7.Becker B. Intraocular pressure response to topical corticosteroids. Invest Ophthalmol 1965;4:198–205 [PubMed] [Google Scholar]
- 8.Johnson D, Gottanka J, Flugel C, Hoffmann F, Futa R, Lutjen-Drecoll E. Ultrastructural changes in the trabecular meshwork of human eyes treated with corticosteroids. Arch Ophthalmol 1997;115:375–383 [DOI] [PubMed] [Google Scholar]
- 9.Spaeth GL, Rodrigues MM, Weinreb S. Steroid-induced glaucoma: A. persistent elevation of intraocular pressure B. histopathological aspects. Trans Am Ophthalmol Soc 1977;75:353–381 [PMC free article] [PubMed] [Google Scholar]
- 10.Rohen JW, Linner E, Witmer R. Electron microscopic studies on the trabecular meshwork in two cases of corticosteroid-glaucoma. Exp Eye Res 1973;17:19–31 [DOI] [PubMed] [Google Scholar]
- 11.Kayes J, Becker B. The human trabecular meshwork in corticosteroid-induced glaucoma. Trans Am Ophthalmol Soc 1969;67:339–354 [PMC free article] [PubMed] [Google Scholar]
- 12.Tektas OY, Lutjen-Drecoll E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp Eye Res 2009;88:769–775 [DOI] [PubMed] [Google Scholar]
- 13.Tawara A, Tou N, Kubota T, Harada Y, Yokota K. Immunohistochemical evaluation of the extracellular matrix in trabecular meshwork in steroid-induced glaucoma. Graefes Arch Clin Exp Ophthalmol 2008;246:1021–1028 [DOI] [PubMed] [Google Scholar]
- 14.Rohen JW, Futa R, Lutjen-Drecoll E. The fine structure of the cribriform meshwork in normal and glaucomatous eyes as seen in tangential sections. Invest Ophthalmol Vis Sci 1981;21:574–585 [PubMed] [Google Scholar]
- 15.Rohen JW. Das Auge und seine Hilfsorgane. In: von Möllendorf WaBW, ed. Handbuch der Mikroskopischen Anatomie des Menschen Berlin: Springer Verlag; 1964:189–237 [Google Scholar]
- 16.Erickson-Lamy K, Rohen JW, Grant WM. Outflow facility studies in the perfused bovine aqueous outflow pathways. Curr Eye Res 1988;7:799–807 [DOI] [PubMed] [Google Scholar]
- 17.Flugel C, Tamm E, Lutjen-Drecoll E. Different cell populations in bovine trabecular meshwork: an ultrastructural and immunocytochemical study. Exp Eye Res 1991;52:681–690 [DOI] [PubMed] [Google Scholar]
- 18.Lutjen-Drecoll E, Futa R, Rohen JW. Ultrahistochemical studies on tangential sections of the trabecular meshwork in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci 1981;21:563–573 [PubMed] [Google Scholar]
- 19.Welge-Lussen U, May CA, Lutjen-Drecoll E. Induction of tissue transglutaminase in the trabecular meshwork by TGF-β1 and TGF-β2. Invest Ophthalmol Vis Sci 2000;41:2229–2238 [PubMed] [Google Scholar]
- 20.Furuyoshi N, Furuyoshi M, Futa R, Gottanka J, Lutjen-Drecoll E. Ultrastructural changes in the trabecular meshwork of juvenile glaucoma. Ophthalmologica 1997;211:140–146 [DOI] [PubMed] [Google Scholar]
- 21.Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res 2009;88:752–759 [DOI] [PubMed] [Google Scholar]
- 22.Zhou L, Li Y, Yue BY. Glucocorticoid effects on extracellular matrix proteins and integrins in bovine trabecular meshwork cells in relation to glaucoma. Int J Mol Med 1998;1:339–346 [PubMed] [Google Scholar]
- 23.Steely HT, Browder SL, Julian MB, Miggans ST, Wilson KL, Clark AF. The effects of dexamethasone on fibronectin expression in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci 1992;33:2242–2250 [PubMed] [Google Scholar]
- 24.Dickerson JE, Jr, Steely HT, Jr, English-Wright SL, Clark AF. The effect of dexamethasone on integrin and laminin expression in cultured human trabecular meshwork cells. Exp Eye Res 1998;66:731–738 [DOI] [PubMed] [Google Scholar]
- 25.Flugel-Koch C, Ohlmann A, Fuchshofer R, Welge-Lussen U, Tamm ER. Thrombospondin-1 in the trabecular meshwork: localization in normal and glaucomatous eyes, and induction by TGF-β1 and dexamethasone in vitro. Exp Eye Res 2004;79:649–663 [DOI] [PubMed] [Google Scholar]
- 26.Samples JR, Alexander JP, Acott TS. Regulation of the levels of human trabecular matrix metalloproteinases and inhibitor by interleukin-1 and dexamethasone. Invest Ophthalmol Vis Sci 1993;34:3386–3395 [PubMed] [Google Scholar]
- 27.Snyder RW, Stamer WD, Kramer TR, Seftor RE. Corticosteroid treatment and trabecular meshwork proteases in cell and organ culture supernatants. Exp Eye Res 1993;57:461–468 [DOI] [PubMed] [Google Scholar]
- 28.Fuchshofer R, Welge-Lussen U, Lutjen-Drecoll E. The effect of TGF-β2 on human trabecular meshwork extracellular proteolytic system. Exp Eye Res 2003;77:757–765 [DOI] [PubMed] [Google Scholar]
- 29.Hoare MJ, Grierson I, Brotchie D, Pollock N, Cracknell K, Clark AF. Cross-linked actin networks (CLANs) in the trabecular meshwork of the normal and glaucomatous human eye in situ. Invest Ophthalmol Vis Sci 2009;50:1255–1263 [DOI] [PubMed] [Google Scholar]
- 30.Wade NC, Grierson I, O'Reilly S, et al. Cross-linked actin networks (CLANs) in bovine trabecular meshwork cells. Exp Eye Res 2009;89:648–659 [DOI] [PubMed] [Google Scholar]
- 31.Qureshi IA, Xi XR, Lu HJ, Wu XD, Huang YB, Shiarkar E. Effect of seasons upon intraocular pressure in healthy population of China. Korean J Ophthalmol 1996;10:29–33 [DOI] [PubMed] [Google Scholar]
- 32.Blumenthal M, Blumenthal R, Peritz E, Best M. Seasonal variation in intraocular pressure. Am J Ophthalmol 1970;69:608–610 [DOI] [PubMed] [Google Scholar]
- 33.Qureshi IA, Xiao RX, Yang BH, Zhang J, Xiang DW, Hui JL. Seasonal and diurnal variations of ocular pressure in ocular hypertensive subjects in Pakistan. Singapore Med J 1999;40:345–348 [PubMed] [Google Scholar]
- 34.Lutjen-Drecoll E, Kaufman PL, Eichhorn M. Long-term timolol and epinephrine in monkeys, I: functional morphology of the ciliary processes. Trans Ophthalmol Soc U K 1986;105(pt 2):180–195 [PubMed] [Google Scholar]
- 35.Lutjen-Drecoll E, Kaufman PL. Echothiophate-induced structural alterations in the anterior chamber angle of the cynomolgus monkey. Invest Ophthalmol Vis Sci 1979;18:918–929 [PubMed] [Google Scholar]
- 36.Nilsson SF, Drecoll E, Lutjen-Drecoll E, et al. The prostanoid EP2 receptor agonist butaprost increases uveoscleral outflow in the cynomolgus monkey. Invest Ophthalmol Vis Sci 2006;47:4042–4049 [DOI] [PubMed] [Google Scholar]
- 37.Richter M, Krauss AH, Woodward DF, Lutjen-Drecoll E. Morphological changes in the anterior eye segment after long-term treatment with different receptor selective prostaglandin agonists and a prostamide. Invest Ophthalmol Vis Sci 2003;44:4419–4426 [DOI] [PubMed] [Google Scholar]