Fd-OCT provides in vivo assessment of retinal structure in retinitis pigmentosa. The relationships between outer nuclear thickness, photoreceptor outer segment thickness, and visual field sensitivity were examined in this study.
Abstract
Purpose.
To explore the relationship between visual field sensitivity and photoreceptor layer thickness in patients with retinitis pigmentosa (RP).
Methods.
Static automated perimetry (central 30-2 threshold program with spot size III; Humphrey Field Analyzer; Carl Zeiss Meditec, Inc., Dublin, CA) and frequency domain optical coherence tomography (Fd-OCT) scans (Spectralis HRA+OCT; Heidelberg Engineering, Vista, CA) were obtained from 10 age-matched normal control subjects and 20 patients with RP who had retained good central vision (better than 20/32). The outer segment (OS+) thickness (the distance between retinal pigment epithelium [RPE])/Bruch's membrane [BM] to the photoreceptor inner–outer segment junction), outer nuclear layer (ONL), and total retinal thickness were measured at locations corresponding to visual field test loci up to 21° eccentricity.
Results.
The average OS+ thickness in the control eyes was 63.1 ± 5.2 μm, varying from approximately 69 μm in the foveal center to 56 μm at 21° eccentricity. In patients with RP, OS+ thickness was below normal limits outside the fovea, and thickness decreased with loss in local field sensitivity, reaching an asymptotic value of 21.5 μm at approximately −10 dB. The ONL thickness also decreased with local field sensitivity loss. Although relative OS thickness was linearly related to visual field loss at all locations examined, a slightly better correlation was found between the product of OS and ONL thickness and visual field loss.
Conclusions.
In patients with RP with good foveal sensitivity, the OS thickness and the product of OS thickness and ONL thickness (assumed to represent the number of photoreceptors) decreases linearly with loss of local field sensitivity. In general, in regions where perimetric sensitivity loss is −10 dB or worse, the OS+ thickness approaches the thickness of the RPE/BM complex.
Retinitis pigmentosa (RP) is a genetic disorder characterized by night blindness, abnormal electroretinograms (ERGs), and visual field constriction.1,2 ERGs and visual fields show exponential decline,3–5 presumably because of constant risk of cell death.6 RP primarily affects the photoreceptor and retinal pigment epithelium complex, whereas the inner retina remains relatively intact. Most gene mutations affect primarily rods that have their highest density at an eccentricity of 18° from the fovea.7 Visual field constriction is typically due to secondary loss of cones, which normally have their highest density in the center of the fovea.7
The relationship between visual function and structure in inherited retinal diseases has been the focus of much research. In RP, both retinal thinning (due to cell loss) and retinal thickening (due to edema) are associated with poor visual acuity.8 Retinal thinning has been associated with elevated rod and cone thresholds.9 The status of the photoreceptor inner segment/outer segment (IS/OS) junction correlates directly with visual acuity in patients with RP.10,11 Jacobson et al.,12 using time-domain optical coherence tomography (OCT), found a satisfactory relationship between local decrease in sensitivity and square of outer nuclear layer (ONL) thickness in patients with RP, Usher syndrome,13,14 and Leber's congenital amaurosis (LCA).15,16
With the introduction of high-resolution frequency domain OCT (Fd-OCT), it has become possible to observe structural changes within individual retinal layers in RP,17–20 including the OS, IS, and the ONL.18 The area of the highly reflective layer, the IS/OS junction, on Fd-OCT correlates with the area of the visual field in patients with RP.17 In patients with Usher syndrome and presumed recessive RP, the area of normal structural retina in the central retina correlates with the degree of normal rod and cone sensitivity.21 In the present study, we were interested in determining the local quantitative relationship between the thicknesses of the photoreceptor OS and ONL and visual field sensitivity.
Methods
Subjects
Static visual field testing and Fd-OCT scanning were performed in 10 eyes of 10 control subjects and 20 eyes of 20 patients with RP. The control subjects had visual acuity of 20/20 or better, ametropia of less than 6.00 D, and no ocular or systemic diseases. Patients were included if they had a visual acuity of 20/32 or better, ametropia of less than 6.00 D, remaining central visual field of at least 20°, foveal sensitivity of 30 dB or better, and absence of present or previous cystoid macular edema. Patients were excluded if they had any other ocular or systemic condition that might affect the retina. Based on family history, the patients were classified as having autosomal dominant RP (n = 4), autosomal recessive RP (n = 4), x-linked RP (n = 3), or isolated RP (n = 9). The mean age of the patients was 37.2 ± 17.9 years, not significantly different (P = 0.7) from that of the normal subjects (34.8 ± 16.7 years). All procedures adhered to the Declaration of Helsinki and were approved by the Institutional Review Board at the University of Texas Southwestern Medical Center. Informed consent was obtained from all subjects after the procedures were completely explained.
Static Automated Perimetry
The central 30-2 threshold program, spot size III, of the Humphrey Field Analyzer (HFA II; Carl Zeiss Meditec, Inc., Dublin, CA) was used in all subjects. This program measures the visual sensitivity of an individual in the central 60° of the visual field. The testing points are 6° from each other, and the sensitivity is expressed in a logarithmic scale of decibels (dB), where a higher dB value corresponds to better visual sensitivity. Total deviation (dB), which represents the difference in the visual sensitivity in dB at any given location for the subject from that of the age-matched normative database, was used in the analysis.
Frequency-Domain Optical Coherence Tomography
Fd-OCT was obtained from all the subjects using the Spectralis HRA+OCT (Heidelberg Engineering, Vista, CA) using the eye-tracking feature (ART; Heidelberg Engineering) with an axial resolution of 5 to 6 μm. Line scans (9 mm in length, average of 100 frames) centered on the fovea and volume scans (31 line scans, each 9 mm in length, and average of 12 frames for each line) were obtained from the central, superior, inferior, temporal, and nasal retina in each subject (Fig. 1). The combination of line scans and volume scans permitted us to compare OCT parameters with field sensitivity at all but the most eccentric test locations of the 30-2 program. Total retinal thickness (distance between the Bruch's membrane [BM] and the inner limiting membrane [ILM]), OS+ thickness (distance between the choroid/Bruch's membrane and the photoreceptor inner–outer segment junction [IS/OS]), and the outer nuclear layer (ONL) thickness (distance between the outer limiting membrane [OLM] and the lower limit of the outer plexiform layer [OPL]) were measured in all subjects, corresponding to the visual field locations along the horizontal and vertical meridians. We measured the thickness of the different layers by using the calibers in the HRA+OCT software (Spectralis; Heidelberg Engineering). All measurements were made with a 1:1-μm setting, at the highest magnification available and were made perpendicular to the RPE.
Figure 1.
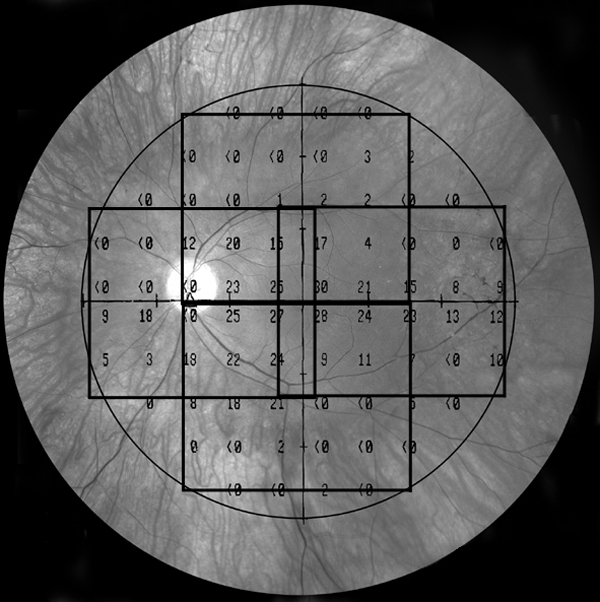
A fundus image overlaid with an example of 30-2 visual field test locations and the extent of the four volume scans of the Fd-OCT.
Assumptions
The OS+ and ONL thicknesses depend on retinal eccentricity in the healthy retina; therefore, we normalized the thicknesses and compared them to visual sensitivity. For OS+, we used the following assumptions.
First, the OS+ thickness that we measured on the OCT scan for any individual at any location is made up of two components. One component, OS, is the thickness due to the OS per se. The other component, the residual or base level b, is everything else. That is,
where the residual b is largely the RPE with small contributions from BM and an even smaller contribution from the distal tips of the IS.
Second, as field sensitivity decreases, the thickness of OS decreases, but the residual level b does not change. This assumption does not hold true in extreme cases of RP where the RPE is also damaged.
Third, the loss in OS thickness is linearly related to the loss in sensitivity (S) on a linear, not dB, scale.
Formally, the OS thickness for an individual at any location is j.
where S is relative sensitivity and is equal to 100.1TD, where TD is the individual's TD in dB from the mean age-matched machine norms; OSoj is the thickness of OS+ in equation 2a when sensitivity is normal (S = 1.0); and bj is the residual thickness.
Rearranging equation 2a, for any location j and individual i,
Substituting 100.1TDi for Si,
We assume that bj is the same at all locations and for all individuals (assumption 2) and is equal to the value of 21.5 μm obtained by taking the median thickness of OS+ in all the patients' points for field losses greater than −20 dB. We estimate the value of OSoi from the mean of the controls at location j. Note that when TD is 0, then [(OS+i) − b]/[OSoi] (the relative thickness of the OS) is 1.0, and when TD is −3 dB, relative thickness is 0.5.
The same linear model can be used to test predictions for ONL thickness and the product of OS and ONL thickness. To fit the linear model for ONL and the product of ONL and OS thickness, we replaced the [(OS+i) − b] in equation 4 with ONLi2 thickness or with (OS*ONL)i thickness and (OS)oi by ONL2oi and (OS*ONL)oi.
Statistics
Student's t-test was used for a planned comparison between the individual retinal layer thickness between control subjects and patients with RP. The Pearson coefficient correlation was used for comparison between linear visual field loss and normalized retinal thickness.
Results
A representative Fd-OCT scan through the fovea from a normal subject is shown in Figure 2A. Retinal thickness in the normal subjects was 225.1 ± 11.5 μm in the fovea, increasing to 350.3 ± 19.4 μm at 3° eccentricity and decreasing to 201.8 ± 11.8 μm at 21° in the inferior retina. The OS+ thickness in the normal subjects was 74.9 ± 4.2 μm, decreasing to 54.6 ± 5.9 μm at 21° eccentricity, and the ONL thickness was 104.9 ± 11.3 μm in the fovea, decreasing to 45.9 ± 5.4 μm at 21° eccentricity. Retinal thickness and OS+ thicknesses obtained in the control subjecrs in this study are comparable to the values obtained previously with manually assisted computer software.18
Figure 2.
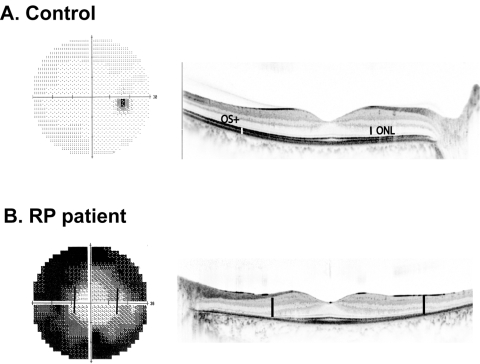
Examples of visual field 30-2 and Fd-OCT line scan through the fovea from a normal subject (A) and a patient with RP (B).
An example of a visual field (left) and Fd-OCT scan through the fovea (right) from a patient with RP is shown in Figure 2B. It can be appreciated from the images in this figure that there is a qualitative agreement between the visual field loss and photoreceptor loss in the patient with RP (as illustrated by the bold lines on the OCT scan and visual field output, which indicate corresponding distances).
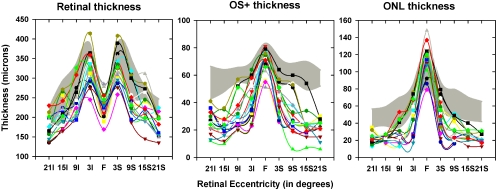
The thickness of the total retina, OS+, and ONL as a function of retinal eccentricity in the vertical meridian is shown in Figure 3. The findings were similar for the horizontal meridian (data not shown). The thicknesses in the individual patients are shown by the different colored symbols and the gray-shaded region shows the mean ± 2 SD of the thickness of the normal subjects for the same eccentricities. Retinal thickness was thinnest in the fovea in the normal subjects as well as the patients with RP. On the other hand, the OS+ and ONL were thickest in the fovea in normal subjects as well as in patients with RP. In the fovea, retinal thickness in patients with RP was not significantly different from that in the normal subjects (P = 0.17), whereas the OS+ and ONL thickness was significantly thinner in patients than in the normal subjects (P = 0.02 for OS+ and P = 0.03 for ONL).
Figure 3.
OCT thickness parameters from the vertical midline as a function of retinal eccentricity, with total retina (left), OS+ (middle), and ONL (right). Shaded region: the mean ±2 SD of normal thickness; colored symbols: the 20 patients with RP.
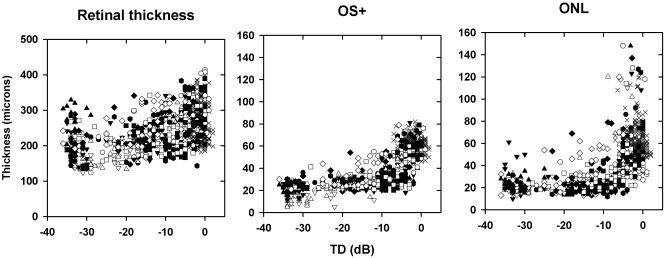
The relationship between retinal thickness parameters and visual field TD combined for all retinal locations on the horizontal and vertical meridians is shown in Figure 4. The X symbols represent the values for the 10 normal subjects, and the open and filled symbols are for the 20 patients with RP. There was no apparent relationship between retinal thickness and TD (left). On the other hand, the OS+ thickness decreased with a worsening of visual field sensitivity (middle), reaching a plateau of ∼20 μm when the visual field sensitivity was 10 dB below maximum. The ONL thickness also decreased as visual field sensitivity decreased in the patients (right). It is clear that there was a range of OS+ thicknesses for a given TD, due in part to our combining all retinal eccentricities in the same plot and plotting the actual thicknesses. A much closer relationship between TD and OCT parameters was found when both were normalized by retinal location (see Figs. 6, 7).
Figure 4.
OCT thickness parameters as a function of TD. Shown are total retina (left), OS+ (middle), and ONL (right) for all retinal locations along the horizontal and vertical meridians. X, normal subjects (n = 10); open and filled symbols: patients with RP (n = 20).
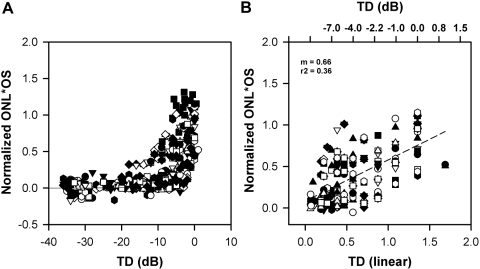
Figure 6.
(A) Plot of product of normalized ONL thickness and OS thickness as a function of TD in dB for all retinal locations. Solid line: the prediction of a linear model, is the same as that shown in Figure 5. (B) The same data as shown in (A), with the TD expressed in linear units; data are shown only up to −10 dB.
Figure 7.
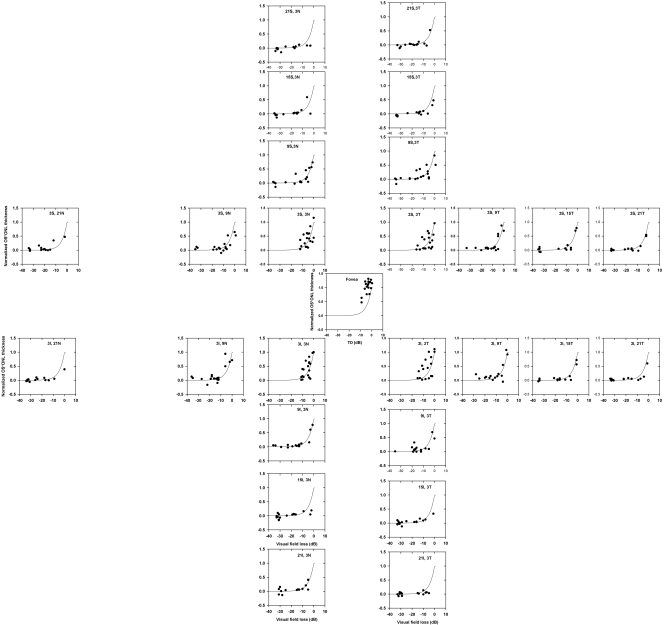
Plot of normalized product of OS and ONL thickness as a function of TD for the different retinal locations, shown separately. Solid line: the prediction of a linear model (the same model is used in all plots and is the same as shown in Figs. 5 and 6).
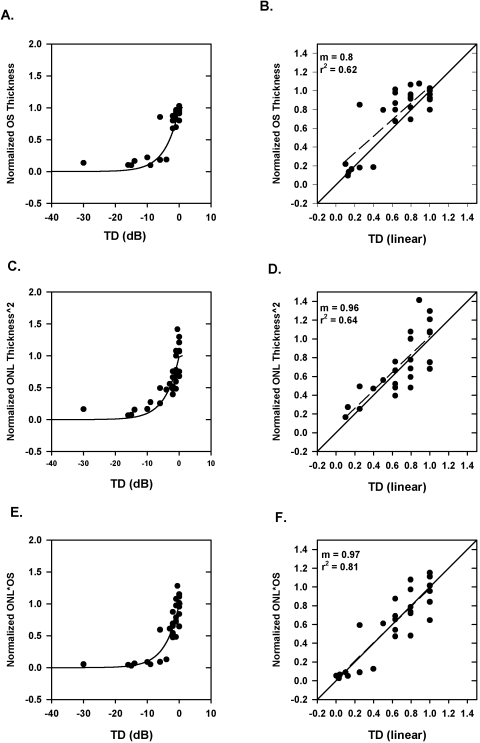
Data from a single patient plotted as (OS+i) − 21.5/OSoi versus TD, are shown in Figure 5A. The smooth curve is the prediction (equation 4) of the simple linear model. It is not a straight line because log values are shown on the x-axis. Figure 5B shows the same data, with the x-axis in linear units with only the data up to −10 dB (0.1 in linear units), which corresponds to the values where most of the change in OS+ occurs. For the data presented in these plots, a simple linear model predicts the relationship between OS thickness and visual field sensitivity well (r2 = 0.62).
Figure 5.
(A) Plot of normalized OS thickness as a function of TD for all retinal locations along the vertical and horizontal meridians from a single patient. (B) The same data as shown in (A), with the TD expressed in linear units and data are shown only up to −10 dB. (C) Plot of normalized ONL thickness squared as a function of TD for all retinal locations along the vertical and horizontal meridians from a single patient. (D) The same data as shown in (C), with the TD expressed in linear units and data are shown only up to −10 dB. (E) Plot of normalized product of OS thickness and ONL thickness as a function of TD for all retinal locations along the vertical and horizontal meridians from a single patient. (F) The same data as shown in (E), with the TD expressed in linear units; data are shown only up to −10 dB. Solid line: the simple linear model; dashed line: linear regression line for the data in the right panels.
We next sought to determine whether the squared ONL thickness model proposed by Jacobson et al.14,16 would fit the sensitivity data. Figure 5C shows the data from the same patient as in Figure 5A, where the y-axis is now the normalized square of the ONL thickness. Again, the fit is reasonably good (r2 = 0.64).
We also determined whether a simple linear model can be applied to the product of ONL and OS thickness versus visual field sensitivity loss. Figure 5E shows the normalized OS*ONL thickness plotted as a function of TD in dB (left) and TD in linear units (right) for the same patient as in Figures 5A and 5C. The data fall along the prediction of a linear model. For this patient, it is evident that the linear fit describes the data well with r2 = 0.81 (better than that for normalized OS thickness and normalized ONL thickness squared).
The normalized OS*ONL thickness as a function of TD in dB (left) and TD in linear units (right) from all patients and all locations is shown in Figure 6. The prediction of a simple linear model fits the data from all patients reasonably well. The r2 values were 0.36 for the normalized OS*ONL thickness versus TD, which was slightly better than the r2 for the normalized OS thickness versus TD of 0.30 (data not shown) or for the normalized squared ONL thickness versus TD (r2 = 0.14; data not shown).
To determine whether the linear relationship holds for all eccentricities measured, the normalized OS*ONL thickness was plotted as a function of TD in dB for all patients and for the different eccentricities (Fig. 7). In this sample of patients and at the eccentricities tested, there did not appear to be any systematic violations from the simple linear model.
Discussion
For the RP patients in this study, the photoreceptor OS thickness and the product of OS and ONL thickness decreased, with a decrease in visual field sensitivity, and this decrease followed the prediction of a simple linear model. As found in a previous study,21 many patients showed normal OS and ONL thickness in the central retina. In extrapolating from our findings, however, one needs to keep in mind that the patients included in this study all had good foveal sensitivity of 30 dB or better.
The thickness of RPE from histologic studies is approximately 12 to 15 μm (Hagerman G, personal communication, April 2009) and this value is smaller than the residual b value of 21.5 μm that we obtained from the Fd-OCT. The difference in the values may be due to contributions from Bruch's membrane, the distal ends of IS, degenerated OS fragments, and possibly RPE thickening. We found that the RPE was intact in most of the patients with RP. There were a few locations in some patients where the RPE appeared to be damaged, but these abnormal RPE regions occurred when the visual field defect was greater than −30 dB.
Previous studies relating structure to function in glaucoma have shown that the relationship between retinal nerve fiber layer thickness and decrease in visual field sensitivity in corresponding regions can be described with a simple linear model.22,23 In this study, we found that a simple linear model fits the relationship between photoreceptor thickness and visual field sensitivity very well. Our results are also consistent with those of previous studies,13,14,16 suggesting that in pure photoreceptor degeneration, the sensitivity to light is proportional to the square of the ONL thickness. ONL thickness squared served as an approximation to the product of ONL and OS thickness.24 In this study, we were able to estimate ONL and OS thickness separately and found that the loss in visual field sensitivity was better fitted by a linear relationship with the product of the ONL and OS thickness than to the square of the ONL thickness. The logic behind using the product of OS and ONL thickness is the following: if the number of receptors is proportional to the ONL thickness and their OS lengths proportional to OS+ thickness, then the product should be a better relative measure of quantum absorption than either alone. Because of spatial summation, the product of the number of photoreceptors in the region (indexed by ONL thickness) and the quantal catch of the photoreceptor (indexed by OS thickness) shows a stronger linear relationship with visual field sensitivity than either OS or ONL thickness alone.
A clear advantage of the visual field is the large dynamic range of over 3 log units. A possible limitation of using OS+ thickness is that most of the decrease in the OS+ thickness occurred over a small range of sensitivity changes. The results of the present study show that by using the product of the OS and ONL thickness, the dynamic range of thicknesses associated with sensitivity changes can be increased. It would also be informative to examine the model in more detail by focusing on the linear range within a more limited region (using, for example, the Humphrey 10-2 protocol).
In conclusion, in patients with RP with good foveal sensitivity, local photoreceptor thickness decreases with loss of local field sensitivity, and the relationship between the product of OS and ONL thickness and OS thickness versus visual field loss is reasonably described by a simple linear model. In general, in regions where perimetric sensitivity loss is −10 dB or worse, the OS+ thickness is reduced to only the RPE/BM complex. It is important to determine whether this relationship holds in patients with specific mutations in the visual cycle and/or phototransduction cascade.
Footnotes
Supported by National Institutes of Health Grant EY09076 and Foundation Fighting Blindness.
Disclosure: N.V. Rangaswamy, None; H.M. Patel, None; K.G. Locke, None; D.C. Hood, None; D.G. Birch, None
References
- 1.Hartong DT, Berson eL, Dryja TP. Retinitis pigmentosa. Lancet 2006;368:1795–1809 [DOI] [PubMed] [Google Scholar]
- 2.Heckenlively JR. Retinitis Pigmentosa Philadelphia: JB Lippincott Co.; 1986. [Google Scholar]
- 3.Berson EL, Sandberg MA, Rosner B, Birch DG, Hanson AH. Natural course of retinitis pigmentosa over a three-year interval. Am J Ophthalmol 1985;99:240–251 [DOI] [PubMed] [Google Scholar]
- 4.Birch DG, Anderson JL, Fish GE. Yearly rates of rod and cone functional loss in retinitis pigmentosa and cone-rod dystrophy. Ophthalmology 1999;106:258–268 [DOI] [PubMed] [Google Scholar]
- 5.Massof RW, Dagnelie G. First order dynamics of visual field loss in retinitis pigmentosa. Clin Vision Sci 1990;5:1–26 [Google Scholar]
- 6.Clarke GLC, McInnes RR. Inherited neurodegenerative diseases: the one-hit model of neurodegeneration. Hum Mol Genet 2001;10:2269–2275 [DOI] [PubMed] [Google Scholar]
- 7.Osterberg Topography of the layer of rods and cones in the human retina. Acta Ophthalmol 1935;(suppl 6):1–103 [Google Scholar]
- 8.Sandberg MA, Brockhurst RJ, Gaudio AR, Berson EL. The association between visual acuity and central retinal thickness in retinitis pigmentosa. Invest Ophthalmol Vis Sci 2005;46:3349–3354 [DOI] [PubMed] [Google Scholar]
- 9.Apushkin MA FG, Alexander KR, Shahidi M. Retinal thickness and visual thresholds measured in patients with retinitis pigmentosa. Retina 2007;27:349–357 [DOI] [PubMed] [Google Scholar]
- 10.Oishi A, Otani A, Sasahara M, et al. Photoreceptor integrity and visual acuity in cystoid macular oedema associated with retinitis pigmentosa. Eye (Lond) 2009;23:1411–1416 [DOI] [PubMed] [Google Scholar]
- 11.Aizawa S, Mitamura Y, Baba T, Hagiwara A, Ogata K, Yamamoto S. Correlation between visual function and photoreceptor inner/outer segment junction in patients with retinitis pigmentosa. Eye (Lond) 2009;23:304–308 [DOI] [PubMed] [Google Scholar]
- 12.Aleman TS, Cideciyan AV, Sumaroka A, et al. Retinal laminar architecture in human retinitis pigmentosa caused by rhodopsin gene mutations. Invest Ophthalmol Vis Sci 2008;49:1580–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson SG, Aleman TS, Sumaroka A, et al. Disease boundaries in the retina of patients with Usher syndrome caused by MYO7A gene mutations. Invest Ophthalmol Vis Sci 2009;50:1886–1894 [DOI] [PubMed] [Google Scholar]
- 14.Jacobson SG, Cideciyan AV, Aleman TS, et al. Usher syndromes due to MYO7A, PCDH15, USH2A or GPR98 mutations share retinal disease mechanism. Hum Mol Genet 2008;17:2405–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson SG, Cideciyan AV, Aleman TS, et al. Photoreceptor layer topography in children with Leber congenital amaurosis caused by RPE65 mutations. Invest Ophthalmol Vis Sci 2008;49:4573–4577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson SG, Aleman TS, Cideciyan AV, et al. Identifying photoreceptors in blind eyes caused by RPE65 mutations: prerequisite for human gene therapy success. Proc Natl Acad Sci U S A 2005;102:6177–6182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer MD, Fleishhauer JC, Gillies MC, Sutter FK, Helbig H, Barthelmes D. A new method to monitor visual field defects caused by photoreceptor degeneration by quantitative optical coherence tomography. Invest Ophthalmol Vis Sci 2008;49:3617–3621 [DOI] [PubMed] [Google Scholar]
- 18.Hood DC, Lin CE, Lazow MA, Locke KG, Zhang X, Birch DG. Thickness of receptor and post-receptor retinal layers in patients with retinitis pigmentosa measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci 2009;50:2328–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murakami TAM, Ooto S, Suzuki T, et al. Association between abnormal autofluorescence and photoreceptor disorganization in retinitis pigmentosa. Am J Ophthalmol 2008;145:687–694 [DOI] [PubMed] [Google Scholar]
- 20.Witkin AJ, Ko TH, Fujimoto JG, et al. Ultra-high resolution optical coherence tomography assessment of photoreceptors in retinitis pigmentosa and related diseases. Am J Ophthalmol 2006;142:945–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobson S, Roman A, Aleman T, et al. Normal central retinal function and structure preserved in retinitis pigmentosa. Invest Ophthalmol Vis Sci 2010;51:1079–1085 [DOI] [PubMed] [Google Scholar]
- 22.Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res 2007;26:688–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hood DC, Anderson SC, Wall M, Raza AS, Kardon RH. A test of a linear model of glaucomatous structure-function loss reveals sources of variability in retinal nerve fiber and visual field measurements. Invest Ophthalmol Vis Sci 2009;50:4254–4266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Machida S, Kondo M, Jamison JA, et al. P23H rhodopsin transgenic rat: correlation of retinal function with histopathology. Invest Ophthalmol Vis Sci 2000;41:3200–3209 [PubMed] [Google Scholar]