The alternative splice variant of VEGF, VEGF165b, is cytoprotective for endothelial and epithelial cells and is antiangiogenic, making it a new candidate for the treatment of ischemic retinopathies.
Abstract
Purpose.
A number of key ocular diseases, including diabetic retinopathy and age-related macular degeneration, are characterized by localized areas of epithelial or endothelial damage, which can ultimately result in the growth of fragile new blood vessels, vitreous hemorrhage, and retinal detachment. VEGF-A165, the principal neovascular agent in ocular angiogenic conditions, is formed by proximal splice site selection in its terminal exon 8. Alternative splicing of this exon results in an antiangiogenic isoform, VEGF-A165b, which is downregulated in diabetic retinopathy. Here the authors investigate the antiangiogenic activity of VEGF165b and its effect on retinal epithelial and endothelial cell survival.
Methods.
VEGF-A165b was injected intraocularly in a mouse model of retinal neovascularization (oxygen-induced retinopathy [OIR]). Cytotoxicity and cell migration assays were used to determine the effect of VEGF-A165b.
Results.
VEGF-A165b dose dependently inhibited angiogenesis (IC50, 12.6 pg/eye) and retinal endothelial migration induced by 1 nM VEGF-A165 across monolayers in culture (IC50, 1 nM). However, it also acts as a survival factor for endothelial cells and retinal epithelial cells through VEGFR2 and can stimulate downstream signaling. Furthermore, VEGF-A165b injection, while inhibiting neovascular proliferation in the eye, reduced the ischemic insult in OIR (IC50, 2.6 pg/eye). Unlike bevacizumab, pegaptanib did not interact directly with VEGF-A165b.
Conclusions.
The survival effects of VEGF-A165b signaling can protect the retina from ischemic damage. These results suggest that VEGF-A165b may be a useful therapeutic agent in ischemia-induced angiogenesis and a cytoprotective agent for retinal pigment epithelial cells.
Retinal epithelial and endothelial cell loss are key events during the progression of a number of ocular abnormalities. For instance, diabetic retinopathy (DR) is associated with vascular closure and subsequent ischemia, followed by hypoxia-induced proliferative angiogenesis. In advanced retinal neovascularization (RNV), vitreous hemorrhage, fibrosis, and retinal detachment may occur. Severe DR is the most common reason for blindness in the working population of developed countries, despite conventional treatments. Additionally, retinal pigment epithelial (RPE) cell loss in age-related macular degeneration (AMD) can contribute to geographic atrophy and possibly to invasive choroidal angiogenesis as seen it neovascular AMD.1
It is increasingly clear that the inhibition of angiogenesis prevents ocular neovascularization in humans. It can prevent progression in models of proliferative RNV,2 which occurs through hypoxia-driven expression of angiogenic vascular endothelial growth factor (VEGF)3,4 and choroidal neovascularization,5 resulting from metabolic insult to RPE cells, possibly involving excess oxidized cholesterol uptake.1 Inhibitors of VEGF have been shown to be effective in treating the choroidal neovascularization seen in AMD5 by inhibiting angiogenesis and reducing vascular permeability.6 They have also been shown to induce endothelial cell death and vascular regression.7 These latter properties are undesirable in the hypoxic diabetic eye; therefore, their use as a treatment for proliferative diabetic retinopathy is limited.
Inhibitory splice variants of VEGF-A—VEGFxxxb8—block the ability of VEGF to stimulate endothelial proliferation and migration, vasodilatation,8 and tube formation in vitro.9 VEGF-A165b and VEGF-A121b have also been shown to inhibit angiogenesis in rabbit cornea,10 mouse mammary gland11 and skin,12 rat mesentery,10 chick chorioallantoic membrane,12 and five different tumor models.13–15 We have demonstrated the presence of both angiogenic and antiangiogenic isoforms in human retina, vitreous, and iris,16 and others have shown it in rodent eye.17 Furthermore, we have shown that though inhibitory VEGFxxxb isoforms are the most abundant species in normal vitreous, they are relatively downregulated in diabetic vitreous, resulting in a switch to an angiogenic phenotype.16 Moreover, the proangiogenic isoform VEGF-A165 has been shown to act as a neuroprotective agent during retinal ischemia.18 There appears, therefore, to be a contradiction in that endogenously the eye has high levels of VEGF-Axxxb, which is a competitive inhibitor of the actions of VEGF-A165 in normal physiology, and yet it is well vascularized and has healthy neurons. It is conceivable, therefore, that the VEGF-A165b–mediated inhibition of angiogenesis in the eye does not result in vascular regression, endothelial cell death, or neuronal impairment. It may specifically target VEGF-A165–mediated neovascularization, which is the formation of additional new vessels in the retina, rather than revascularization, which is the reformation of existing blood vessels back into previously vascularized areas of the retina. We have previously shown that VEGF-A165b is cytoprotective for epithelial cells of the human glomerulus,19 and we hypothesized that VEGF-A165b may be similarly cytoprotective for retinal epithelial and endothelial cells. We tested this by investigating the effect of VEGF-A165b on endothelial and retinal epithelial survival, neovascularization, and revascularization. To determine whether VEGF-A165b could be a potentially useful agent in vivo, the pharmacodynamic half-life was determined, and the interaction between VEGF and pegaptanib was investigated. We show here that VEGF-A165b inhibits neovascularization but not revascularization and that it is cytoprotective for endothelial cells and epithelial cells in vivo and in vitro. These results indicate that this molecule may be a novel therapy for ischemia-induced angiogenesis.
Materials and Methods
Cell culture details for human microvascular endothelial cells (HMVECs), umbilical vein endothelial cells (HUVECs), retinal microvascular endothelial cells (RECs), RPE cells, and ARPE-19 cells are available in the Supplementary Material, http://www.iovs.org/cgi/content/full/51/8/4273/DC1. VEGF-A165 protein was purchased from R&D Systems (Minneapolis, MN) and kindly provided by Kurt Ballmer-Hofer (Paul Scherrer Institute, Villigen, Switzerland). VEGF165b protein was generated by Cancer Research Technologies (London, UK)9 and purchased from R&D Systems (Cat. no. 3045-VE-025) and PhiloGene Inc. (New York, NY).
Half-Life of Radiolabeled VEGF-A165b in the Eye
Recombinant VEGF-A165b protein was radiolabeled with iodine 125 (125I), as previously described,9 and intraocular injection of 7 kBq 125I-VEGF-A165b (100 ng VEGF-A165b in 5 μL) was given to anesthetized rats. After 4, 12, 24, and 72 hours and 8 and 13 days, rats were culled, and the eyes, urine, and blood samples were counted in a gamma counter. The dose of 125I-VEGF-A165b was calculated based on tissue weight. Terminal half-life was calculated by nonlinear regression analysis using a single-phase exponential decay model, with a positive K constraint, for clearances greater than 4 hours.
Oxygen-Induced Retinopathy
Oxygen-induced retinopathy (OIR) was induced as previously described,20 with slight modification. Experiments adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Briefly, 1-week-old C57/Bl6 pups (postnatal day [P] 7) and their CD1 nursing dams were exposed to 75% oxygen (PRO-OX 110 chamber oxygen controller; Biospherix Ltd., Redfield, NY) for 5 days and were returned to room air on P12. In total, 25 C57/Bl6 pups from several litters were randomized into five groups. The mice underwent intravitreous injection in the right eye on P13. Each of four groups received intravitreous injection of 1 μL VEGF-A165b in Hanks buffered saline solution (HBSS, with CaCl2 and MgCl2; Gibco-Invitrogen, Grand Island, NY) using a 35-gauge beveled needle (NanoFil; World Precision Instruments, Sarasota, FL) at the following concentrations: 10 ng/μL, 1 ng/μL, 0.1 ng/μL, 0.01 ng/μL. The control group received 1 μL HBSS.
Mice were culled on day P17, and both eyes were enucleated and fixed in 4% paraformaldehyde for 4 hours at 4°C, followed by a 4-hour wash in PBS. The retinal whole-mounts were dissected and stained as previous described.21,22 Briefly, retinas were placed in 96-well plates and permeabilized in PBS containing 0.5% Triton X-100, 1% normal fetal calf serum, and 0.1 mM CaCl2 at 4°C overnight. The retinal vasculature was visualized by incubation in biotinylated isolectin B4 (IB4, 20 μg/mL; Sigma-Aldrich, St. Louis, MO) and by Alexa 488-streptavidin (1:100; Molecular Probes, Eugene, OR). The retinas were then flat-mounted in reagent (Vectashield; Vector Laboratories, Peterborough, UK) and imaged with an epifluorescence microscope with a digital camera (Eclipse 400; Nikon, Tokyo, Japan). Images were taken at 4× magnification and processed with image editing software (Photoshop; Adobe, Mountain View, CA). Areas of avascular ischemic retina, normal intraretinal vascularization, and preretinal neovascularization were measured in ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html). Percentages of these areas in total retina were calculated. A trained single-masked observer analyzed all coded and randomized retinal flat-mounts.
Cellular Assays
Cytotoxicity assay, migration assay, immunoblot analysis, cell signaling, and immunocytofluorescence details are available in the Supplementary Material. In brief, cytotoxicity assays were carried out with a lactate dehydrogenase (LDH) cytotoxicity detection kit (Promega, Madison, WI), in accordance with the manufacturer's instructions, that correlated well with trypan blue staining.23,24 Cell viability assays (Cell Proliferation Reagent WST-1; Roche Diagnostics GmbH, Mannheim, Germany) were carried out according to the manufacturer's instructions. Transwell migration assays were performed across 8-μm pore, 12-mm polycarbonate inserts, as previously described.25 Change in migration was expressed relative to the basal migration rate toward zero chemoattractant and was plotted as average ± SEM. The inhibitory effect on migration of VEGF-A-165b over VEGF-A-165 was determined by increasing concentrations of VEGF-A165b (0–2 nM), with or without 1 nM VEGF-A165. IC50 was calculated from the normalized data using a variable slope sigmoidal fit (Prism 4 software; GraphPad, San Diego, CA).
Cell Signaling
Serum-starved human dermal endothelial cells were activated with 1 nM VEGF-A165 or VEGF-A165b. Cell lysates were subjected to SDS-PAGE and were immunoblotted with mouse anti–human phospho-p38 MAP kinase (Thr180/Tyr182) antibody (9216), rabbit anti–human phospho-VEGF receptor 2 (Tyr1175), (2478), rabbit anti–human-VEGF receptor 2 antibody (2479), mouse-anti–human phospho-p44/p42 MAPK (Thr202/Tyr204) antibody (9106), and rabbit anti–human p44/42 MAPK antibody (9102; all from Cell Signaling Technologies, Beverly, MA).
Effect of VEGF on IGFBP3 Expression
Human primary RPE cells at passage 3 to 4 and at 70% to 80% confluence were cultured in serum-free medium in the absence of FBS for 24 hours before treatment. Two milliliters of 1 ng/mL human recombinant VEGF-A165 or VEGF-A165b in serum-free medium was added. Twenty-four hours later, the RPE cells were washed three times with ice-cold PBS and lysed in 200 μL Laemmli buffer for Western blot analysis, as described with mouse anti–human IGFBP3 antibody (2 μg/mL; Sigma).
Pegaptanib Interaction with VEGF Protein
VEGF-A-165 and VEGF-A-165b were incubated with increasing molar ratios (1:0–1:40) of an RNA aptamer (pegaptanib sodium; Macugen; Pfizer, New York, NY) or a scrambled inactive sequence of the same ribonucleotides26 in HBSS + 1 mM CaCl2 and MgCl2 for 30 minutes at room temperature. The samples were separated on a 12% Laemmli acrylamide SDS gel in SDS 0.15 M Tris-HCl buffer, without SDS and mercaptoethanol in nonreducing conditions. For VEGF-A165 detection, membranes were incubated overnight at room temperature with a combination of rabbit anti–VEGF-A antibody A-20 (Santa Cruz Biotechnology, Santa Cruz, CA) and mouse anti–VEGF-A165 antibody (R&D Systems). For VEGF-A165b detection, the membranes were incubated with mouse anti–VEGF-A165b antibody (MAB3045; R&D Systems).
Results
Half-life of VEGF-A165b
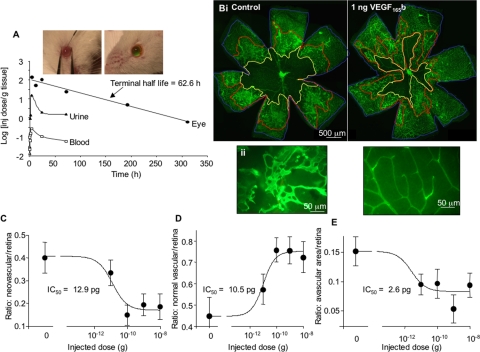
To determine whether VEGF-A165b could be a potential therapeutic agent in retinal neovascular conditions such as proliferative diabetic retinopathy, we determined the half-life of the protein when injected intraocularly. Radiolabeled VEGF-A165b was injected into the eyes of rats, and the animals were killed at intervals after injection. Figure 1A shows intraocular injection of sodium fluorescein (green) to ensure complete injection without leakage. The amount of VEGF-A165b remaining in the enucleated eye is plotted in Figure 1A. Fitting to a monoexponential curve resulted in a predicted time constant of 0.011 ± 0.033 s−1 and a half-life of 62.6 hours.
Figure 1.
VEGF-A165b inhibits neovascularization in the OIR model but does not block revascularization. (A) Intraocular injection of VEGF-A165b has a half-life of 62.6 hours in the eye. 125I-VEGF-A165b was injected into the vitreous, the rats were killed, and the eyes, urine, and blood were assayed using a gamma counter. Biexponential clearance was expressed as gamma counts per gram of tissue; terminal half-life was 2.6 days (62.6 hours). Uptake into the urine and blood was seen within 30 minutes. Fluorescein-dextran did not leak after intraocular injection into mice (insets). (B) Mice were subjected to hyperoxia during postnatal development and were injected with increasing concentrations of VEGF-A165b or HBSS as a control. Retinal vessels were visualized by isolectin B4 staining at low (Bi, 4×) and high (Bii, 40×) power. Left: HBSS-treated (control); right: VEGF-A165b–treated retinas. The central ischemic avascular region (yellow line), preretinal proliferation region (neovascularization, red line), and total vascularized retina (blue line) were measured. (C–E) The area in square micrometers of each defined region was measured in Image J. Neovascularization was significantly reduced by VEGF-A165b injection (C), and the amount of normal vascularization was increased (D). This was partly a result of blood vessels growing into the avascular area, reducing the avascular area (E). Thus, VEGF-A165b is able to maintain normal revascularization while inhibiting neovascularization, making it an ideal agent for ischemia-induced angiogenesis.
Effect of VEGF-A165b on Neovascularization in OIR
To determine the potency of VEGF-A165b in vivo, increasing amounts of VEGF-A165b were injected into the eyes of neonatal mice after removal from high oxygen in the established model of OIR. At day 17 the mice were killed, eyes were enucleated, and retinas were prepared and stained with fluorescent lectin. Examination of the retina enabled areas of ongoing angiogenesis to be delineated (Fig. 1B) and calculated as a percentage of the total area of the retina. Analysis of the retinas revealed that intraocular injection of VEGF-A165b significantly reduced preretinal neovascularization (areas of sprouting endothelial cells) in the mouse retina in a dose-dependent manner (Fig. 1C) with an IC50 of 1.29 × 10−11g (12.9 pg)/eye. The vitreous of the mouse eye measures approximately 5.3 μL; therefore, the IC50 for the protein in OIR is approximately 2.8 ng/mL, or 70 pM.
Effect of VEGF-A165b on the Normally Vascularized Area in OIR
The normally vascularized area (no sprouting or evidence of ongoing angiogenesis) of the peripheral retina in OIR mice was also increased in a dose-dependent manner by treatment with VEGF-A165b, with an IC50 of 10.5 pg/eye (Fig. 1D).
Effect of VEGF-A165b on Revascularization in the Central Retina of OIR Eye
The retinal area lacking blood vessels was measured as described. There was a significant decrease in the ischemic area of the retina and, thus, a corresponding increase in the revascularized area (area of retina that would be ischemic in the control eye but that has normal, nonsprouting blood vessels in treated eyes) of the retina in VEGF-A165b–treated mice with an IC50 of 2.6 pg/eye (Figs. 1D, 1E). The revascularized area differs from the neovascular area in that there is no evidence of sprouting angiogenesis in the revascularized area. The vasculature has a normal network appearance but is present in areas that were not vascularized in control animals.
Effect of VEGF-A165b on Retinal Endothelial Cell Migration
To determine whether VEGF-A165b acted on retinal endothelial cells to prevent migration in a manner similar to that previously shown for microvascular endothelial cells, retinal endothelial cells were exposed to increasing concentrations of recombinant human VEGF-A165b in the presence of 1 nM VEGF-A165. Figure 2A shows that VEGF-A165b causes a dose-dependent inhibition of migration with an IC50 of 1 nM, similar to that previously described for HUVECs.9 For comparison with a known inhibitor of VEGF, we assayed the IC50 concentration against an equal dose of ranibizumab. Figure 2B shows that both 1 nM ranibizumab and 1 nM VEGF-A165b resulted in a 50% inhibition of VEGF-A165–mediated migration.
Figure 2.
VEGF-A165b inhibits human REC migration. (A) Human RECs were seeded onto polycarbonate filters, and migration toward increasing concentrations of VEGF-A165b was measured. (B) Inhibition of REC migration in response to 1 nM VEGF-A165b compared with 1 nM ranibizumab.
Effect of VEGF-A165b on Endothelial Cell Survival
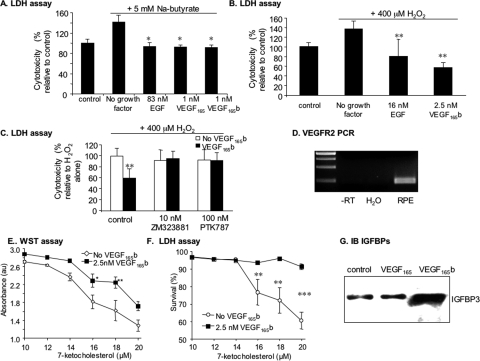
VEGF-A165 acts as both a growth factor and a survival factor for endothelial cells. To determine the effect of VEGF-A165b on endothelial cell survival, HUVECs were incubated with VEGF-A165b, VEGF-A165, or a combination of both and were assayed for LDH release as a measure of cellular cytotoxicity. Figure 3A shows that VEGF-A165b could rescue the endothelial cells (P < 0.001, ANOVA), reducing cytotoxicity from 68% ± 5.2% to 52% ± 1% at 1 nM (P < 0.01, Bonferroni correction), as did 1 nM VEGF-A165 (44% ± 0.2%; P < 0.001, Bonferroni correction). A combination of the two did not further reduce cytotoxicity.
Figure 3.
VEGF-A165b is a survival factor for human endothelial cells. (A) HUVECs were serum starved (0.1% serum, SFM). LDH assay to measure cytotoxicity after 48-hour treatment with VEGF isoforms. VEGF-A165 and VEGF-A165b both inhibited cytotoxicity induced by serum starvation. (B) Cells were incubated either with VEGF-A165b, VEGF-A165, inhibitors, or VEGF-A165b in the presence of VEGFR inhibitors and cytotoxicity determined by LDH activity in the media. Cytotoxicity is expressed relative to the appropriate control (i.e., inhibitor in SFM). The VEGFR inhibitors PTK787 (blocks both VEGFR) and ZM323881 (specific to VEGFR2) inhibited cytotoxicity. (C) Cells were treated with three different signal transduction inhibitors in the presence or absence of VEGF-A165b, SB203580 (which blocks p38MAPK), PD98059 (which blocks p42/p44 MAPK phosphorylation by MEK), and LY294002 (which inhibits PI3K and cytotoxicity measured). MEK and PI3K inhibitors blocked the reduction in cytotoxicity but not the p38MAPK inhibitor. (D) Activation of VEGFR2, Tyr residue 1175 of VEGFR2, Akt, p42p44MAPK, and p38MAPK in endothelial cells by VEGF-A165 and VEGF-A165b. Cells were treated for 10 minutes with VEGFs. **P < 0.01 and ***P < 0.001 compared with control. One-way ANOVA, Student's Newman-Keuls post hoc test.
To determine whether VEGF-A165b acts through the VEGF receptor to reduce cytotoxicity, cells were pretreated with VEGFR tyrosine kinase inhibitors. After treatment with 10 nM ZM323881, previously shown to be a specific VEGFR2 antagonist,27 the reduction in cytotoxicity was abolished. The reduction in cytotoxicity was also abolished by 100 nM PTK787, an inhibitor of both VEGFR1 and VEGFR2 (Fig. 3B; P < 0.01, ANOVA). To identify downstream signaling pathways that may be involved in this cytoprotective effect, cells were treated with inhibitors of three common kinases involved in cytoprotection: p42/p44 mitogen-activated protein kinase (MAPK), p38 MAPK, and PI3-kinase (PI3K). The cytoprotective effects of VEGF-A165b were abolished by inhibitors of MEK and PI3K (PD98059 and LY294002), but not by the p38 MAPK inhibitor (SB203580; Fig. 3C). To determine whether VEGF-A165b stimulated PI3K, p42/p44, and p38MAPK, endothelial cells were serum starved and treated with 1 nM VEGF-A165b or 1 nM VEGF-A165, and protein was extracted and subjected to SDS-PAGE immunoblot analysis. Figure 3D shows that VEGF-A165b activated VEGFR2 phosphorylation along with all three downstream kinases to a degree similar to that for treatment with VEGF-A165. However, in keeping with previous experiments, VEGFR2 was not fully phosphorylated28 because immunoblot analysis using a Tyr1175-specific antibody did not show phosphorylation by VEGF-A165b, indicating that tyrosine residue 1175 was less phosphorylated by VEGF-A165b than by VEGF-A165 in endothelial cells.
Effect of VEGF-A165b on Epithelial Survival
We have previously demonstrated that VEGF-A165b is cytoprotective to renal epithelial cells.19 Therefore, we sought to determine whether VEGF-A165b was a survival factor for RPE cells. RPE cells were serum starved and treated with 5 mM sodium butyrate, which resulted in a small but significant increase in cell death (Fig. 4A; 141% ± 13% of control; P < 0.05 compared with untreated). VEGF-A165b abolished the cytotoxic effect of sodium butyrate, as did VEGF-A165, and the known epithelial growth factor EGF (all three P < 0.05 compared with sodium butyrate; ANOVA, Dunnett's test; Fig. 4A). To confirm that this effect was not specific to sodium butyrate, cells were treated with 400 μM hydrogen peroxide. Treatment with H2O2 resulted in an increase in cytotoxicity to 136% ± 16% of control (P < 0.05, Wilcoxon test). Treatment with either EGF or VEGF-A165b reduced cytotoxicity to 80% ± 35% and 57% ± 10%, respectively (P < 0.01, Dunnett's post hoc test; Fig. 4B).
Figure 4.
VEGF-A165b is a cytoprotective agent for RPE cells. (A–C) ARPE19 cells were treated with either Na butyrate (A) or hydrogen peroxide (B, C). Cells were incubated with VEGF-A165b, VEGF-A165, or EGF, and cytotoxicity was determined by measurement of LDH activity in the media. VEGF-A165b inhibited cytotoxicity induced by Na butyrate (A) and H2O2 (B). Cells were treated with H2O2 and two different inhibitors in the presence or absence of VEGF-A165b (C). PTK787, which blocks both VEGFR1 and VEGFR2, or ZM323881, which is specific for VEGFR2. Both inhibitors blocked the reduction in cytotoxicity induced by VEGF-A165b. (D) RT-PCR of mRNA extracted from RPE cells indicated VEGFR2 expression. (E) VEGF165b reduced loss of cell viability induced by 7-ketocholesterol, as assessed by WST1 assay. (F) VEGF165b reduced LDH release from cells during treatment with 7-ketocholesterol. (G) VEGF-A165b increased IGFBP3 expression in RPE cells, whereas VEGF-A165 did not. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with no growth factor.
To identify the receptor mediating this reduction in cytotoxicity, cells were treated with the VEGFR tyrosine kinase inhibitors PTK787 (100 nM) and ZM323881 (10 nM). Both inhibitors abolished the VEGF-A165b-induced reduction in cytotoxicity (Fig. 4C; P < 0.05, ANOVA). RT-PCR for VEGFR2 demonstrated VEGR2 mRNA expression in RPE cells (Fig. 4D). To confirm that the inhibition of LDH levels resulted in an increase in viable cell number, we repeated the assays using a third inducer of RPE cell death, increasing concentrations of 7-ketocholesterol, which has previously been shown to induce apoptosis in ARPE19 cells.29 Figure 4E shows that treatment with 7-ketocholesterol for 24 hours resulted in a dose-dependent decrease in cell viability, as assessed by accumulation of the colorimetric product of mitochondrial processing of the tetrazolium salt WST1. This reduction in viability was inhibited by coincubation with 2.5 nM VEGF165b (P < 0.01, ANOVA), and Figure 4F shows that this same dose-response curve could be seen when the cell cytotoxicity was assayed by measuring LDH levels (P < 0.0001, ANOVA). Again, this was blocked by VEGF165b treatment.
Insulinlike growth factor (IGF) has previously been shown to downregulate VEGF-A165b in RPE cells,30,31 and IGF binding protein 3 is known to be produced as part of the autocrine IGF signaling pathway in RPE cells.32 To determine whether VEGF-A165b could influence this pathway, IGFBP3 immunoblot analysis was carried out. Surprisingly, there was a significant increase in IGFBP3 production by RPE cells treated with VEGF-A165b, but not with VEGF-A165, indicating that VEGF-A165b had a specific signaling pathway in RPE cells that was not shared by VEGF-A165 (Fig. 4G). We have previously demonstrated by ELISA that RPE cells can produce VEGF-A165b,33 which suggests there may be an autocrine feedback loop in RPE cells involving VEGF splice forms and IGF.
To determine whether VEGF-A165b could therefore act as an autocrine growth factor on RPE cells, being released from the cell and then acting on it, we first confirmed VEGF-A165b expression by immunofluorescence (Fig. 5Ai), immunoblot analysis (Fig. 5Aii), and RT-PCR (Fig. 5Aiii). We then measured the effect of neutralizing antibodies either to VEGF-A165b or to all isoforms (bevacizumab) on cytotoxicity. Addition of the VEGF-A165b-specific antibody MAB3045 attenuated the VEGF165b-mediated inhibition of VEGF165-mediated migration in HUVECs from 44% ± 6% of that without VEGF165b to 83% ± 6.7%, indicating that the MAB3045 antibody inhibited the effect of VEGF165b, and was a neutralizing antibody. Treatment with MAB3045 resulted in a significant increase in cytotoxicity of cells at 500 μg/mL (Fig. 5Aiv) that was not observed with a nonspecific mouse IgG, even at 2.5 mg/mL. Blocking all VEGF isoforms with bevacizumab resulted in a small increase in cytotoxicity at 2.5 mg/mL, in agreement with results previously shown.34
Figure 5.
VEGF-A165b is an endogenous survival factor. (A) Immunofluorescence staining revealed expression of VEGF165b (red) in RPE cells (Ai) that was confirmed by Western blot analysis (Aii) using a VEGFxxxb-specific antibody, and mRNA was confirmed by RT-PCR (Aiii). Inhibition of endogenous VEGFxxxb or all VEGF isoforms by bevacizumab increased cytotoxicity (Aiv). (B) Human endothelial cells show VEGF165b expression (Bi) and inhibition of VEGFxxxb increased cytotoxicity (Bii). ***P < 0.001 compared with control. Actin (green) and nucleus (blue).
Given tha VEGF-A165b also had a cytoprotective effect on endothelial cells and that endogenous endothelial VEGF has been shown to be required for endothelial cell survival,35 we determined whether VEGF-A165b was expressed by endothelial cells and whether it had an autocrine function. Immunofluorescence staining of HMVECs showed strong VEGF-A165b staining in the cytoplasm (Fig. 5Bi, red). Figure 5Bii shows that a VEGF-A165b-specific antibody (MAB3045) at 250 μg/mL induced significant endothelial cytotoxicity (from 61% ± 2% to 99% ± 1%.
VEGF-A165b Interaction with Pegaptanib
We have previously shown that VEGF-A165b binds to bevacizumab with equal affinity to VEGF-A165.14 Because ranibizumab is the variable fragment of bevacizumab, it is reasonable to assume that both of these widely used VEGF antagonists bind VEGF-A165b. However, it is unknown whether the aptamer, pegaptanib sodium (Macugen; Pfizer), also recognizes VEGF-A165b. To determine whether VEGF-A165b and pegaptanib interact directly, we incubated VEGF-A165 and VEGF-A165b with pegaptanib and ran the samples under nondenaturing conditions on an acrylamide gel to determine whether a shift in molecular weight could be observed. Figure 6A shows that although VEGF-A165 binds to pegaptanib (as evidenced by a detection of a shifted band in the VEGF-A165 + pegaptanib lane, but not the VEGF-A165 + scrambled sequence lane), no such shift toward higher molecular weight was seen with VEGF-A165b under the same conditions. To determine whether VEGF-A165b and pegaptanib could interact indirectly, combination experiments were performed. Figure 6B shows that pegaptanib dose dependently inhibited the migration of HMVECs but at a much higher dose than did VEGF-A165b (IC50 for VEGF-A165b in HMVECs, 0.33 nM36). VEGF-A165b at 1 nM pegaptanib gave the same level of inhibition as at 10 nM pegaptanib. To determine any interaction between the two, we incubated pegaptanib with 0.5 nM VEGF-A165b. This resulted in a significantly less potent effect of pegaptanib. Figure 6C shows that the dose-response curve to pegaptanib shifts to the right with 0.5 nM VEGF-A165b. Similarly, when the half-maximal dose of pegaptanib was given with increasing concentrations of VEGF-A165b, the VEGF-A165b effect was significantly blunted (Fig. 6D).
Figure 6.
Pegaptanib binds VEGF-A165 but not VEGF-A165b and is not complementary to VEGF-A165b. (A) VEGF protein (2 pmol) was incubated with 16 pmol pegaptanib or with a nonbinding scrambled aptamer for 30 minutes in HBS + 1 mM Ca2+/Mg2+, subjected to native SDS-PAGE, and probed using an antibody that detects both families of VEGF isoforms. The blot shows a band shift of VEGF-A165 when aptamer was added but not when scrambled RNA or buffer, respectively, was added. VEGF-A165b does not show a band shift when aptamer or when scrambled RNA was added under identical conditions. (B) Maximum inhibition of migration is seen at 10 nM pegaptanib. The same effect was seen with 1 nM VEGF-A165b. The IC50 for pegaptanib is 4 nM. (C) Adding in 0.5 nM VEGF-A165b (close to IC50) does not block the effect of pegaptanib but does cause a significant rightward shift in the dose-response curve, doubling the IC50. (D) Adding 5 nM pegaptanib prevented the VEGF-A165b mediated inhibition of migration induced by VEGF-A165. **P < 0.01 compared with 1 nM VEGF-A165 alone; ++P < 0.01 compared with pegaptanib (ANOVA followed by Newman-Keuls test).
These results indicate that in vitro pegaptanib is a weaker inhibitory agent and that combination therapy with VEGF-A165b removes the benefit of each agent, indicating that though there is no direct interaction, there is no added benefit to combining the two agents.
Discussion
Ischemic retinal disease can lead to hypoxia-induced VEGF production, as it does in diabetes, in which retinal vascular regression is such a key contributor37 that endothelial cell death is considered a hallmark of diabetic retinopathy.38 Impaired blood flow provokes a hypoxic response, which leads to the overproduction of VEGF and ultimately to uncontrolled angiogenesis generating abnormal vessels.39 In proliferative eye disease, the conflict has been highlighted between the desirability of reduced neovascularization by anti–VEGF therapy and the requirement not to harm the normal vasculature or indeed the associated functional supportive epithelial and neuronal cells.
We show here that the in vivo effect of intraocular injected VEGF-A165b in OIR, an animal model for ischemia-induced angiogenesis, effectively reduced the pathologic preretinal proliferation and reduced the ischemic area. The clearance of VEGF-A165b from the rat vitreous was similar to that described in other animal models for ranibizumab.40 Treatment with VEGF-A165b resulted in an increase in the proportion of the normal vascularized peripheral retina in OIR eyes in a dose-dependent manner. Revascularization of the retina may be attributed to intraretinal proliferation and remodeling of retinal vessels into a normal morphologic and functional retinal vessel network. This process usually occurs 17 to 22 days after vaso-obliteration in the OIR model, but it appeared to be enhanced in the VEGF-A165b–treated eyes at 17 days in a dose-dependent manner. Taken together, this means that, in contrast to its potent inhibition of pathologic angiogenesis,9,10,12,13 VEGF-A165b did not prevent physiological revascularization in the central ischemic retina. These results confirm VEGF-A165b as a potent antineovascular agent in the eye in that it can inhibit the formation of abnormal vessels. However, they also show VEGF-A165b to be more than simply an antiangiogenic factor in that it can facilitate the regrowth of blood vessels into previously vascularized areas. We also found that VEGF-A165b is protective for epithelial and endothelial cells in vitro and that it is a potent survival factor when exogenously given or endogenously produced. The regression of vessels resulting from VEGF withdrawal or inhibition was similar to what occurred in hyperoxia. This has been shown experimentally in the mouse tracheal model and in humans with diabetes. The capillaries collapse and blood flow stops, followed by endothelial cell regression, leaving gaps where capillaries once were connected, creating empty “sleeves” of basement membrane.7 Blood vessel “casts,” which closely resemble sleeves, can be seen in the diabetic retina by light and electron microscopy41,42; the basement membrane is left behind, forming an acellular capillary.37 In the tracheal model, these same casts are formed under continuous VEGF-A inhibition, but the endothelial cells grow back along this basement membrane scaffold once VEGF-A is restored.7 It is clear that the regrowth of blood vessels along these casts must be mechanistically different from neovascularization whereby endothelial cells break down the basement membrane and invade the surrounding tissues. It is not yet clear, however, whether this is the mechanism by which VEGF-A165b allows revascularization of an ischemic area. The identification of downstream signaling pathways of PI3K and MEK-p42/p44 (rather than p38MAPK) as responsible for survival signaling and the previously identified upregulation of matrix metalloproteinases by p38MAPK43 are consistent with the invasive phenotype of endothelial cell suppression by VEGF-A165b. Recent studies have suggested that a growing phenotype characterized by tight junctional integrity, lack of invasion, and inhibited sprouting, termed endothelial phalanx cell44 phenotype, may be involved in physiological angiogenesis,45 and it is possible that VEGF-A165b promotes this type rather than the tip cell/stalk cell phenotype induced by VEGF-A165.22
The cytoprotective effects of VEGF-A165b are consistent with those of its sister isoform, VEGF-A165.46 Many studies have shown that VEGF-A165 (or the rodent equivalent, VEGF164) is cytoprotective in vitro to injury induced by a variety of cellular insults to epithelial cells (for a review, see Ref. 47). Usually cytoprotection is mediated by VEGFR2,47 with emerging evidence that PI3K/Akt signal transduction is involved and that p38 MAPK signaling is inhibited.48 VEGF-A165 also enhances neuronal migration, neurite outgrowth, and in vivo postischemic neurogenesis.18 The effect of VEGF-A165b on neuronal cell survival is now under investigation. However, the findings here show that VEGF165b is a potent and significant exogenous and endogenous survival factor for RPE cells. This finding has significant implications for ocular diseases associated with RPE cell loss, particularly AMD. Both the neovascular and the atrophic forms of AMD are associated with RPE cell loss.1 In geographic atrophy, visual impairment results from RPE cell loss and is the most common cause of blindness after wet AMD.49 These results suggest there may be a role for VEGF165b in both forms of this disease. Moreover, RPE cell cytotoxicity, mediated by oxidized lipids,29 may be a contributor to the pathogenesis of neovascular AMD, suggesting that treatment of patients with VEGF165b may not only prevent choroidal neovascularization, it may protect the RPE cell layer from further loss. The finding that ranibizumab inhibits the effects of VEGF165b but that pegaptanib does not also indicates that strategies that specifically target the proangiogenic forms of VEGF, such as exon 8a monoclonal antibodies may be more effective than pan-VEGF antibodies, such as ranibizumab, in the longer term.
In view of the importance of VEGF-dependent neovascularization in the pathophysiology of many conditions, anti–VEGF therapies have entered clinical practice in oncology50 and in AMD.5 Although they are very effective, there is a concern about the safety profile of these strategies in relation to nonendothelial tissues and cell types in which VEGF has been shown to have cytoprotective properties (epithelial cells51 and neurons52). Bevacizumab has been linked with proteinuria in clinical trials53 and has been shown to reduce the viability of RPE cells in culture34 and to affect the ultrastructure of the choriocapillaris and choroidal melanocytes in primates.54 Although the local administration of ranibizumab makes it unlikely to have a clear effect systemically, the effect on local epithelial cell survival may be significant.
The VEGFxxxb isoforms are not simply competitive inhibitors. They have been shown to be weak activators of VEGFR2, resulting in differential tyrosine residue phosphorylation.28 The data presented here demonstrate that VEGF-A165b may play a physiological role through as yet undescribed mechanisms in cytoprotection, endothelial cell survival, and vascular remodeling, and these properties may make it an ideal candidate for treating proliferative ischemia-induced angiogenesis.
Supplementary Material
Footnotes
Supported by Fight for Sight (ER), the National Eye Research Centre, the Richard Bright VEGF Research Trust and the North Bristol NHS Trust Adrian Wright Bequest into Disability in the Elderly (AM), the Skin Cancer Research Fund (MG), Wellcome Trust Grants 79736 (JH, NBL) and 69029 (HSB), and British Heart Foundation Grant BS/06/005 (DOB).
Disclosure: A.L Magnussen, None; E.S. Rennel, None; J. Hua, PhiloGene Inc. (R); H.S. Bevan, None; N.B. Long, None; C. Lehrling, None; M. Gammons, None; J. Floege, None; S.J. Harper, PhiloGene Inc. (F, C), P; H.T. Agostini, None; D.O. Bates, PhiloGene Inc. (F, C, R), P; A.J. Churchill, None
References
- 1.Roth F, Bindewald A, Holz FG. Key pathophysiologic pathways in age-related macular disease. Graefes Arch Clin Exp Ophthalmol 2004;242:710–716 [DOI] [PubMed] [Google Scholar]
- 2.Kinose F, Roscilli G, Lamartina S, et al. Inhibition of retinal and choroidal neovascularization by a novel KDR kinase inhibitor. Mol Vis 2005;11:366–373 [PubMed] [Google Scholar]
- 3.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992;359:843–845 [DOI] [PubMed] [Google Scholar]
- 4.Shima DT, Adamis AP, Ferrara N, et al. Hypoxic induction of endothelial cell growth factors in retinal cells: identification and characterization of vascular endothelial growth factor (VEGF) as the mitogen. Mol Med 1995;1:182–193 [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–1431 [DOI] [PubMed] [Google Scholar]
- 6.Tolentino MJ, Miller JW, Gragoudas ES, et al. Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology 1996;103:1820–1828 [DOI] [PubMed] [Google Scholar]
- 7.Baffert F, Le T, Sennino B, et al. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ 2006;290:H547–H559 [DOI] [PubMed] [Google Scholar]
- 8.Bates DO, Cui TG, Doughty JM, et al. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res 2002;62:4123–4131 [PubMed] [Google Scholar]
- 9.Rennel ES, Hamdollah-Zadeh MA, Wheatley ER, et al. Recombinant human VEGF165b protein is an effective anti–cancer agent in mice. Eur J Cancer 2008;44:1883–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woolard J, Wang WY, Bevan HS, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res 2004;64:7822–7835 [DOI] [PubMed] [Google Scholar]
- 11.Qiu Y, Bevan H, Weeraperuma S, et al. Mammary alveolar development during lactation is inhibited by the endogenous antiangiogenic growth factor isoform, VEGF165b. FASEB J 2008;22:1104–1112 [DOI] [PubMed] [Google Scholar]
- 12.Cebe Suarez S, Pieren M, Cariolato L, et al. A VEGF-A splice variant defective for heparan sulfate and neuropilin-1 binding shows attenuated signaling through VEGFR-2. Cell Mol Life Sci 2006;63:2067–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rennel E, Waine E, Guan H, et al. The endogenous antiangiogenic VEGF isoform, VEGF165b inhibits human tumour growth in mice. Br J Cancer 2008;98:1250–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varey AH, Rennel ES, Qiu Y, et al. VEGF165b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br J Cancer 2008;98:1366–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rennel ES, Varey AH, Churchill AJ, et al. VEGF121b, a new member of the VEGFxxxb family of VEGF-A splice isoforms, inhibits neovascularization and tumor growth in vivo. Br J Cancer 2009;101(7):1183–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perrin RM, Konopatskaya O, Qiu Y, Harper S, Bates DO, Churchill AJ. Diabetic retinopathy is associated with a switch in splicing from anti- to pro-angiogenic isoforms of vascular endothelial growth factor. Diabetologia 2005;48:2422–2427 [DOI] [PubMed] [Google Scholar]
- 17.Ergorul C, Ray A, Huang W, Darland D, Luo ZK, Grosskreutz CL. Levels of vascular endothelial growth factor-A165b (VEGF-A165b) are elevated in experimental glaucoma. Mol Vis 2008;14:1517–1524 [PMC free article] [PubMed] [Google Scholar]
- 18.Nishijima K, Ng YS, Zhong L, et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol 2007;171:53–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bevan HS, van den Akker NM, Qiu Y, et al. The alternatively spliced antiangiogenic family of VEGF isoforms VEGFxxxb in human kidney development. Nephron Physiol 2008;110:57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith LE, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 1994;35:101–111 [PubMed] [Google Scholar]
- 21.Chan-Ling T. Glial, vascular, and neuronal cytogenesis in whole-mounted cat retina. Microsc Res Tech 1997;36:1–16 [DOI] [PubMed] [Google Scholar]
- 22.Gerhardt H, Golding M, Fruttiger M, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 2003;161:1163–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jauregui HO, Hayner NT, Driscoll JL, Williams-Holland R, Lipsky MH, Galletti PM. Trypan blue dye uptake and lactate dehydrogenase in adult rat hepatocytes—freshly isolated cells, cell suspensions, and primary monolayer cultures. In Vitro 1981;17:1100–1110 [DOI] [PubMed] [Google Scholar]
- 24.Krohne TU, Hunt S, Holz FG. Effect of 308 nm excimer laser irradiation on retinal pigment epithelium cell viability in vitro. Br J Ophthalmol 2009;93:91–95 [DOI] [PubMed] [Google Scholar]
- 25.Hamdollah Zadeh MA, Glass CA, Magnussen A, Hancox JC, Bates DO. VEGF-mediated elevated intracellular calcium and angiogenesis in human microvascular endothelial cells in vitro are inhibited by dominant negative TRPC6. Microcirculation 2008;15:605–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ostendorf T, Kunter U, Eitner F, et al. VEGF(165) mediates glomerular endothelial repair. J Clin Invest 1999;104:913–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whittles CE, Pocock TM, Wedge SR, et al. ZM323881, a novel inhibitor of vascular endothelial growth factor-receptor-2 tyrosine kinase activity. Microcirculation 2002;9:513–522 [DOI] [PubMed] [Google Scholar]
- 28.Kawamura H, Li X, Harper SJ, Bates DO, Claesson-Welsh L. Vascular endothelial growth factor (VEGF)-A165b is a weak in vitro agonist for VEGF receptor-2 due to lack of coreceptor binding and deficient regulation of kinase activity. Cancer Res 2008;68:4683–4692 [DOI] [PubMed] [Google Scholar]
- 29.Ong JM, Aoki AM, Seigel GM, et al. Oxysterol-induced toxicity in R28 and ARPE-19 cells. Neurochem Res 2003;28:883–891 [DOI] [PubMed] [Google Scholar]
- 30.Nowak DG, Amin EM, Rennel ES, et al. Regulation of VEGF splicing from pro-angiogenic to antiangiogenic isoforms—a novel therapeutic strategy for angiogenesis. J Biol Chem 2010;285(8):5532–5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nowak DG, Woolard J, Amin EM, et al. Expression of pro- and antiangiogenic isoforms of VEGF is differentially regulated by known splicing and growth factors. J Cell Sci 2008;121:3487–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slomiany MG, Rosenzweig SA. Autocrine effects of IGF-I-induced VEGF and IGFBP-3 secretion in retinal pigment epithelial cell line ARPE-19. Am J Physiol Cell Physiol 2004;287:C746–C753 [DOI] [PubMed] [Google Scholar]
- 33.Konopatskaya O, Churchill AJ, Harper SJ, Bates DO, Gardiner TA. VEGF165b, an endogenous C-terminal splice variant of VEGF, inhibits retinal neovascularization in mice. Mol Vis 2006;12:626–632 [PubMed] [Google Scholar]
- 34.Spitzer MS, Wallenfels-Thilo B, Sierra A, et al. Antiproliferative and cytotoxic properties of bevacizumab on different ocular cells. Br J Ophthalmol 2006;90:1316–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee S, Chen TT, Barber CL, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell 2007;130:691–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hua J, Spee C, Kase S, et al. Recombinant human VEGF165b inhibits experimental choroidal neovascularisation. Invest Ophthalmol Vis Sci 2010;51;4282–4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson HR, Stitt AW, Gardiner TA, Archer DB. Diabetic retinopathy: morphometric analysis of basement membrane thickening of capillaries in different retinal layers within arterial and venous environments. Br J Ophthalmol 1995;79:1120–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol 2001;158:147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol 1994;118:445–450 [DOI] [PubMed] [Google Scholar]
- 40.Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci 2005;46:726–733 [DOI] [PubMed] [Google Scholar]
- 41.Stitt AW, Gardiner TA, Archer DB. Histological and ultrastructural investigation of retinal microaneurysm development in diabetic patients. Br J Ophthalmol 1995;79:362–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest 1996;97:2883–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun HW, Li CJ, Chen HQ, et al. Involvement of integrins, MAPK, and NF-κB in regulation of the shear stress-induced MMP-9 expression in endothelial cells. Biochem Biophys Res Commun 2007;353:152–158 [DOI] [PubMed] [Google Scholar]
- 44.Mazzone M, Dettori D, Leite de Oliveira R, et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 2009;136:839–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benest AV, Augustin HG. Cancer: blood vessels kept quiet. Nature 2009;458:41–42 [DOI] [PubMed] [Google Scholar]
- 46.Saint-Geniez M, Maharaj AS, Walshe TE, et al. Endogenous VEGF is required for visual function: evidence for a survival role on Müller cells and photoreceptors. PLoS One 2008;3:e3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bogaert E, Van Damme P, Van Den Bosch L, Robberecht W. Vascular endothelial growth factor in amyotrophic lateral sclerosis and other neurodegenerative diseases. Muscle Nerve 2006;34:391–405 [DOI] [PubMed] [Google Scholar]
- 48.Tolosa L, Mir M, Asensio VJ, Olmos G, Llado J. Vascular endothelial growth factor protects spinal cord motoneurons against glutamate-induced excitotoxicity via phosphatidylinositol 3-kinase. J Neurochem 2008;105:1080–1090 [DOI] [PubMed] [Google Scholar]
- 49.Holz FG, Pauleikhoff D, Klein R, Bird AC. Pathogenesis of lesions in late age-related macular disease. Am J Ophthalmol 2004;137:504–510 [DOI] [PubMed] [Google Scholar]
- 50.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–2342 [DOI] [PubMed] [Google Scholar]
- 51.Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 2003;21:60–65 [DOI] [PubMed] [Google Scholar]
- 52.Oosthuyse B, Moons L, Storkebaum E, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet 2001;28:131–138 [DOI] [PubMed] [Google Scholar]
- 53.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 2008;358:1129–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peters S, Heiduschka P, Julien S, et al. Ultrastructural findings in the primate eye after intravitreal injection of bevacizumab. Am J Ophthalmol 2007;143:995–1002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.