The alternative splice form of VEGF, VEGF-A165b, inhibits choroidal neovascularization at very low doses in mice, indicating that it may be an effective therapy for age-related macular degeneration, comparable with or better than existing anti-VEGF therapy.
Abstract
Purpose.
Vascular endothelial growth factor (VEGF-A) is the principal stimulator of angiogenesis in wet age-related macular degeneration (AMD). However, VEGF-A is generated by alternate splicing into two families, the proangiogenic VEGF-Axxx family and the antiangiogenic VEGF-Axxxb family. It is the proangiogenic family that is responsible for the blood vessel growth seen in AMD.
Methods.
To determine the role of antiangiogenic isoforms of VEGF-A as inhibitors of choroidal neovascularization, the authors used a model of laser-induced choroidal neovascularization in the mouse eye and investigated VEGF-A165b effects on endothelial cells and VEGFRs in vitro.
Results.
VEGF-A165b inhibited VEGF-A165–mediated endothelial cell migration with a dose effect similar to that of ranibizumab and bevacizumab and 200-fold more potent than that of pegaptanib. VEGF-A165b bound both VEGFR1 and VEGFR2 with affinity similar to that of VEGF-A165. After laser injury, mice were injected either intraocularly or subcutaneously with recombinant human VEGF-A165b. Intraocular injection of rhVEGF-A165b gave a pronounced dose-dependent inhibition of fluorescein leakage, with an IC50 of 16 pg/eye, neovascularization (IC50, 0.8 pg/eye), and lesion as assessed by histologic staining (IC50, 8 pg/eye). Subcutaneous administration of 100 μg twice a week also inhibited fluorescein leakage and neovascularization and reduced lesion size.
Conclusions.
These results show that VEGF-A165b is a potent antiangiogenic agent in a mouse model of age-related macular degeneration and suggest that increasing the ratio of antiangiogenic-to-proangiogenic isoforms may be therapeutically effective in this condition.
Ocular neovascularization (ONV) is the leading cause of blindness in the western world. In age-related macular degeneration (AMD), choroidal neovascularization (CNV) affects 10% of all patients and is the most severe and rapidly progressing form of the disease. In “wet” or neovascular AMD, there is rapid, devastating loss of central vision with rarely any symptoms until the later stages. Examination of pathologic specimens has demonstrated that there is an abnormal proliferation of choroidal vessels beneath the retina, which subsequently bleed, resulting in fibrosis and macular scarring. VEGF-A has been shown to be substantially upregulated in AMD,1 particularly in retinal pigment epithelial cells and fibroblasts.2 ONV seen in AMD is associated with increased levels of VEGF. Angiogenesis is a complex process mediated by factors from the VEGF, angiopoietin, and ephrin families.3,4 It is essential in normal physiology, such as in wound healing, endometrial maturation, embryogenesis, and fat deposition. However, it also underlies many disease states in addition to AMD, including retinal and other complications of diabetes, cancer, atherosclerosis,5 rheumatoid arthritis, and psoriasis.6,7 VEGF-A is the dominant proangiogenic factor in AMD,8,9 stimulating endothelial cell proliferation and migration and increased microvascular permeability by activation of its cognate receptors VEGFR1 (flt-1) and VEGFR2 (KDR/flk1).10 Anti-VEGF therapies that influence new vessel formation and that were shown to be effective in animal models11,12 have successfully completed clinical trials in AMD, and three agents have been widely used: pegaptanib (Macugen; Pfizer, New York, NY),13,14 an RNA aptamer that targets the heparin-binding domain of VEGF-A, ranibizumab (Lucentis; Genentech, South San Francisco, CA), an antibody fragment to the VEGFR binding domain of VEGF-A, and bevacizumab (Avastin; Genentech), the full-length antibody equivalent to ranibizumab. Other members of the VEGF family of proteins, (e.g., VEGF-C, VEGF-D) are formed from different gene products and are structurally distinct from VEGF-A (see Ref. 15 for review). Human VEGF-A is differentially spliced from 8 exons to form a variety of different mRNAs encoding at least 14 different proteins in two families, the proangiogenic VEGF-Axxx family and the antiangiogenic VEGF-Axxxb family, where xxx denotes the number of amino acids of the secreted isoform, VEGF-A121, VEGF-A165, (the dominant proangiogenic isoform) VEGF-A165b, and others.15,16 VEGF-Axxxb isoforms are formed by alternate splice acceptor site selection in exon 8, forming an mRNA containing 18 bases coded by exon 8b in place of the 18 bases of exon 8a.17 This alternative splicing produces proteins of the same length as in the VEGF-Axxx family but with a different C-terminal amino acid sequence.18 Exons 8a and 8b both code for six amino acids, exon 8a for CDKPRR and exon 8b for SLTRKD. Therefore, exon 8b lacks the Cys residue that forms the final disulfide bond19 and the terminal two charged Arg residues postulated to be involved with receptor signaling.20,21 Instead, exon 8b codes for Ser instead of Cys and a less basic C-terminal than exon 8a. The receptor binding domains are still present in VEGF-A165b, which acts as a competitive inhibitor of VEGF-A165 (i.e., it binds the receptors but does not stimulate angiogenesis signaling). VEGF-A165b inhibits the proliferative, migratory, and vasodilator effects of VEGF-A165.17 VEGF-A165b is antiangiogenic in the rabbit cornea, chick chorioallantoic membrane, mouse skin,22 lactating mammary gland,23 and rat mesentery, and it inhibits tumor growth in xenotransplanted tumors in mice.24–26 Unlike angiogenic VEGF isoforms, the antiangiogenic isoform VEGF-A165b is downregulated in renal and colorectal carcinoma and malignant prostate tissue,17,25,27 metastatic melanoma,28 diabetic retinopathy,18 and Denys-Drash syndrome.29 We have also identified VEGF-A165b protein expression in many other human tissues, including the eye.18 To determine whether the antiangiogenic activity of VEGF-A165b was sufficient to inhibit CNV, we used a laser-induced photocoagulation model of CNV in the mouse. We show here that VEGF-A165b dose dependently inhibited CNV as assessed by fluorescein angiography (FA), lectin staining, and lesion size.
Materials and Methods
Protein Extraction
Protein was extracted from human eyes obtained from the Bristol Eye Bank (Bristol, UK) with local ethics approval. The tissues were dissected manually and were treated with lysis buffer (50 mM Tris-HCl pH 8, 150 mM NaCl, 1 mM EDTA pH 8, 100 mM NaF, 1.5 mM MgCl2, 10% vol/vol glycerol, 1% vol/vol Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VaO4, and 1 μg/mL of each of the protease inhibitors aprotinin, leupeptin, and pepstatin), and the solution was mechanically homogenized. Lysates were then centrifuged again for 9 minutes at 4°C, 13000 rpm, and supernatants were used for further analysis. Protein was quantified with a Bio-Rad (Hercules, CA) protein assay. Recombinant VEGF-A165 (100 ng/well; R&D Systems, Minneapolis, MN) and VEGF-A165b (50 ng/well, as described in Rennel et al.30) reconstituted in phosphate-buffered saline (PBS) were used as controls on each gel. Samples were adjusted to 150 μg total protein in PBS and were loaded in loading buffer (100 mM Tris HCl pH 6.8, 4% SDS, 20% glycerol [BDH, Poole, UK], 0.2% [wt/vol] bromophenol blue, 5% final concentration β-mercaptoethanol). Samples and recombinant human VEGF (denatured lanes) were heated for 5 minutes at 100°C. For native recombinant human VEGF, the β-mercaptoethanol and heating steps were omitted. Proteins were loaded onto a 10% polyacrylamide gel and run in 25 mM Tris, 250 mM glycine (BDH), 0.1% SDS. The gel was then trimmed, equilibrated, and transferred in 50 mM Tris, 38 mM glycine (BDH), 20% methanol, 0.1% SDS onto a polyvinylidene (PVDF) membrane (Fisher Scientific, Leicestershire, UK) at 60 V for 90 minutes. The PVDF membrane was incubated in 10% low-fat powdered milk/0.05% TBS-Tween blocking solution for 1 hour at room temperature, washed with 0.05% TBS-Tween, incubated with primary antibody, and diluted in 5% low-fat powdered milk/0.05% TBS-Tween overnight at 4°C. For detection of all VEGF-A isoforms, 1.25 μg/mL purified mouse monoclonal IgG2B (MAB293; R&D Systems) was used. For detection of VEGF-Axxxb isoforms, mouse monoclonal antibody, raised to the nine carboxyl-terminal amino acids of VEGF-A165b, was used (MAB3045; R&D Systems). After further washing the membrane was incubated for 1 hour in 1.4 ng/mL horseradish peroxidase (HRP)-goat anti–mouse IgG (Pierce, Cheshire, UK) diluted in 5% low-fat powdered milk/TBS-Tween. The membrane was washed again and developed using an enhanced chemiluminescence (ECL) kit (SuperSignal West Femto Maximum Sensitivity Substrate; Pierce, Cheshire, UK).
Cell Migration Assay
Endothelial cells for migration were used in passages 3 to 6 at 70% to 80% confluence. Human microvascular endothelial cells (HMVECs) were serum starved in endothelial basal media without FBS and supplements (EBM) for 8 to 10 hours. Cells were trypsinized and resuspended in 0.1% vol/vol FBS in EBM, and 150,000 cells in 500 μL medium were seeded on attachment factor (Cascade Biologics, Portland, OR)–coated filter inserts (8 μm, 12 mm; Millipore, Billerica, MA) with the treatment in the bottom well. Each treatment was performed in triplicate. The cells were incubated at 37°C and allowed to migrate overnight. The inserts were washed with PBS, and cells were fixed with 4% PFA/PBS, pH 7.4, for 10 minutes. Nonmigrated cells were removed from the membranes, and the nuclei of migrated cells were stained with Hoechst 33258 (5 μg/mL in 0.5% Triton/PBS). Membranes were cut out of the wells and mounted on microscope slides with mounting medium (Vectashield; Vector Laboratories, Burlingame, CA). Migrated cells were counted in 10 fields per membrane under a fluorescence microscope (DM; Leica, Wetzlar, Germany; 40× objective). Change in migration was expressed relative to the basal migration rate toward zero chemoattractant and was plotted as average ± SEM. The inhibitory effect on migration of VEGF inhibitors over VEGF-A165 was determined by increasing concentrations of inhibitor with 1 nM (40 ng/mL) VEGF-A165. IC50 was calculated from the normalized data using a variable slope sigmoidal fit (Prism 4; GraphPad, San Diego, CA).
Surface Plasmon Resonance
To compare the binding affinities of VEGF-A165 and VEGF-A165b with those of VEGF receptors, Fc-VEGFR1 or Fc-VEGFR2 was amine coupled to a sensor chip (CM5; Biacore AB, Uppsala, Sweden) to an immobilization level of 630 response units (RU) VEGFR1 and 580 RU (VEGFR2). Coupling was performed using EDC/NHS and 1 mol/L ethanolamine (Biacore AB) in accordance with the manufacturer's instructions, with the VEGFR dissolved in 10 mmol/L sodium acetate (pH 4.5). A blank reference cell was formed by the same activation and deactivation process involved in amine coupling without adding antibody. Samples containing VEGF-A165 or VEGF-A165b diluted in HBS-EP sample buffer (HEPES-buffered saline with EDTA and P20 surfactant; Biacore AB) were then run at twofold serial dilutions in random order in duplicate. Injection was performed at 30 μL/min for 3 minutes, followed by 6 minutes of buffer only, for monitoring of dissociation. Regeneration between each interaction was performed by injection of 4 mol/L MgCl2 at 20 μL/min for 40 seconds, followed by a 2-minute period of stabilization before the next injection.
Induction of Choroidal Neovascularization
Thirty 6- to 8-week-old C56Bl/6J male mice (National Institutes of Health, Bethesda, MD) were used in the study. All procedures were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and approved by the University of Southern California Animal Use Committee. The mice were anesthetized for all procedures with intraperitoneal injection of 0.1 mL of a 50:50 mixture of ketamine hydrochloride (20 mg/mL) and xylazine hydrochloride (100 mg/mL; Phoenix Pharmaceutical Inc., St. Joseph, MO). The pupils were dilated with 5% phenylephrine hydrochloride and 0.8% tropicamide. Four photocoagulation lesions were delivered with a diode green laser (150 mW, 0.05 s, 75 μm; 532 nm Oculight GLx Diode laser; Iridex, Mountain View, CA) between the retinal vessels in a peripapillary distribution at a distance of 1 to 2 disc diameters in each eye. Only laser lesions with a subretinal bubble at the time of treatment were included in the study. Two microliters of saline or rhVEGF-A165b at various concentrations (0.01, 0.1, 1, and 10 ng in each eye) was injected into the vitreous immediately after photocoagulation using a 35-gauge needle (NanoFil; World Precession Instruments, Sarasota, FL) or 100 μg rhVEGF-A165b injected subcutaneously twice weekly for 2 weeks. Saline was used because it has previously been shown that proteins such as albumin have no effect on CNV.31 The injections were repeated 7 days later. Fourteen days after laser treatment, FA of the fundi was carried out, and the animals were killed after imaging. Eyes were enucleated and fixed in 4% PFA for further histology processes.
Fluorescein Angiography
Fluorescein angiography was carried out 14 days after photocoagulation. Pupils were dilated using 2.5% phenylephrine hydrochloride and 1% tropicamide eye drops, and 10% fluorescein sodium chloride was injected intraperitoneally into the anesthetized animals. After 180 to 200 seconds, fundus photographs were taken using an angiography camera (VK2e, KD-2UC; Kowa, Nagoya, Japan). Two experienced observers who were blinded to the study processed and scored the images. Scores were given as previously described (0, no staining; 1, slight leakage; 2, moderate leakage; and 3, heavy leakage). Images were standardized for the process.
Whole Mount Staining
Eyes were fixed overnight in 4% paraformaldehyde and washed in PBS overnight at 4°C. After the sclera-choroid-complex was dissected and permeabilized in 1% Triton X-100 for 2 hours, biotinylated isolectin B4 (specific endothelial cell marker, 1:100; Vector Laboratories) was added and incubated at 4°C overnight. Samples were washed and incubated with streptavidin-conjugated Alexa Fluor 594 overnight. The samples were mounted (Vectashield; Vector Laboratories) after a final wash.
Measurement of Lesion Volume and Surface Area
A laser scanning confocal microscope (LSM; model 510; Carl Zeiss Meditec, Dublin, CA) was used to image the CNV lesions. Fluorescence volume measurements were accomplished by creating image stacks of optical slices within lesions. The image stacks were generated in the z-plane by 2 μm optical sectioning, with the confocal microscope set to excite at 594 nm. Images were further processed using the LSM software, by closely circumscribing and digitally extracting the fluorescent lesion areas throughout the entire image stack. The extracted lesion was processed through the LSM topography software to generate a digital topographic image representation of the lesion and an implicit image volume. Units for implicit volume were designated as volume (in cubic micrometers).
Cross-Section and HE-Staining
The cornea and lens were removed immediately after the animals were killed and the eye enucleated. Dissected eyecups were embedded in OCT compound (Tissue-Tek, OCT 4583; Sakura Finetek Inc., Torrance, CA) and frozen in liquid nitrogen. Air-dried and acetone-fixed 8-μm serial cross-sections of the whole specimen were stained with hematoxylin and eosin (H&E staining). The maximum cross-section of each lesion was observed and photographed using a light microscope with a digital camera (DM3000; Leica). The area of each cross-section was measured in ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html).
Statistical Analysis
Binding curves and dose-response curves were fitted by normalization to PBS followed by nonlinear regression analysis modeled with a sigmoid curve with variable slope (Prism 4.0; GraphPad). Results are given as means and SEM. All parameters except H&E staining for the PBS treated mice were normally distributed according to the KS Normality test. Thus parametric statistics (ANOVA followed by Student's Newman-Keuls or unpaired t-test for subcutaneous VEGF-A165b vs. PBS) were used for most parameters, but for the H&E-stained subcutaneous injections, nonparametric Mann-Whitney U test was used.
Results
VEGF-A165b—the Predominant VEGF-Axxxb Isoform in the Eye
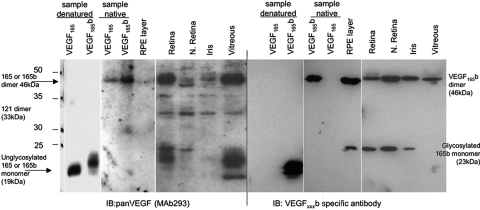
We have previously shown that VEGF-Axxxb isoforms are expressed as a protein in the retina, iris, and vitreous of the eye. To determine whether VEGF-A165b expression was localized to the RPE-choroid or neuronal layers of the retina, the neuronal retina was isolated from the underlying tissue by dissection of the Bruch's membrane or the RPE-choroid was separated from the overlying tissue. Figure 1 (left-hand side) shows an immunoblot of a reducing SDS-PAGE gel probed for all VEGF-A isoforms. Recombinant VEGF-A165b and VEGF-A165, both of which have forms at 19 kDa (unglycosylated) and 21 kDa (partially glycosylated), are detected by this antibody. A strong band at 23 and 46 kDa is consistent with expression of the 165-amino acid isoforms of VEGF-A, VEGF-A165, and VEGF-A165b. Weaker bands are present at 34 kDa, consistent with the 121-amino acid isoforms (VEGF-A121 and VEGF-A121b) as dimers. When a parallel blot was probed with an antibody specific for the VEGF-Axxxb isoforms (Fig. 1, right-hand side), a 46-kDa and a 23-kDa protein was detected, showing that VEGF-A165b is the predominant VEGF-Axxxb isoform and is expressed in the neuronal layer of the retina and in the whole retina, iris, and vitreous. Interestingly, when protein from the RPE-choroid layer was included, there was significant expression in that layer as well, indicating that VEGF165b is expressed in both the neuronal and the RPE-choroid layers of the retina.
Figure 1.
VEGF-A165b is the predominant VEGF-Axxxb isoform in the eye. Protein was extracted from dissected tissues of donor eyes from the Bristol Eye Bank and subjected to SDS-PAGE and immunoblotting using anti-VEGF (left) or anti-VEGFxxxb (right). Protein was extracted from eyes either by dissection of the RPE layer from the rest of the retina or by dissection of the neuronal layer from the rest of the retina or the whole retina. Recombinant human (rh) VEGF165 and VEGF165b were run both as denatured and native to demonstrate monomer and dimers. The expected sizes of VEGF121, VEGF189, and VEGF145 are demonstrated.
VEGF-A165b Inhibition of Endothelial Cell Migration—Similar Efficacy to Bevacizumab
To determine the relative potency of VEGF-A165b as an inhibitor of endothelial cell migration, critical for angiogenesis, we compared the inhibitory effect of VEGF-A165b with ranibizumab, bevacizumab, and pegaptanib. Figure 2A shows that VEGF-A165b inhibits endothelial cell migration induced by 1 nM VEGF-A165 (IC50, 0.28 nM) at the same efficacy on a mole-for-mole basis as ranibizumab (IC50, 0.24 nM) and bevacizumab (IC50, 0.21 nM; P > 0.1 compared with ranibizumab and VEGF-A165b), all of which were 200 times more potent than pegaptanib (IC50, 4.08 nM; P < 0.001 compared with ranibizumab, VEGF-A165b, and bevacizumab). To determine the binding affinity of VEGF-A165b to its receptors, we used biotinylated VEGF-A165b to carry out a binding-affinity experiment. Increasing doses of biotinylated-VEGF-A165b were added to an ELISA plate coated with Fc-VEGFR1. Figure 2B shows that this resulted in an affinity of 3 × 10−11 M. To confirm this we carried out surface plasmon resonance (SPR) on a chip coated with Fc-VEGFR1. Figure 2C shows that this resulted in a binding curve with a similar affinity of 1.9 × 10−11 M. This compares well with previously published figures of 2.2 × 10−11 M for SPR using VEGF-A165.32 We therefore used the same technique to measure binding of VEGF-A165b to VEGFR2. Figure 2D shows that the affinity of VEGF-A165b to VEGFR2, as measured by SPR, was 1.35 × 10−10 M, similar to the published values for VEGF-A165 (2.1 × 10−10 M).33
Figure 2.
VEGF-A165b inhibits VEGF-A165–mediated endothelial cell migration but binds to VEGFR. (A) HMVECs were seeded onto polycarbonate 8-μm pore filters, and migration across the transwell was measured to 1 nM VEGF-A165 with increasing doses of inhibitors. (B) Fc-VEGFR1 was coated onto an ELISA plate, and increasing concentrations of biotinylated VEGF-A165b were added. After development with HRP-SA, the percentage of bound VEGF-A165b was calculated relative to saturated values, and IC50 was calculated using a nonlinear sigmoid variable slope fit. (C) Fc-VEGFR1 was coupled to an SPR sensor chip, and increasing concentrations of VEGF-A165b were allowed to flow over the chip. Response units relative to the reference chip were measured by surface plasmon resonance. (D) Fc-VEGFR2 was coupled to a similar chip, and the experiment was repeated. Affinity and dissociation constants were calculated by 1:1 Langmuir binding models.
Intravitreal VEGF-A165b Inhibition of Choroidal Neovascularization
Mice were subjected to diode laser–induced photocoagulation and were injected with VEGF-A165b on the day of laser procedure and 7 days later. We have previously shown that the intraocular terminal half-life of VEGF-A165b is 62.5 hours. Fourteen days after laser injury, mice were subjected to FA. Figure 3A shows that the laser-induced photocoagulation produced lesions in the retina visible under FA when mice were injected with saline. However, these lesions were progressively smaller with increasing doses of VEGF-A165b. Semiquantitative scoring of lesion size by an observer masked to treatment showed that VEGF-A165b induced a dose-dependent decrease in lesion size, with an IC50 of 18 pg/eye (log IC50, −10.2 ± 0.21; Fig. 3B). To determine whether the increase in FA lesion size was the result of endothelial growth into the lesion, retinas were prepared as whole mounts and stained for endothelial cells by lectin staining. Two-dimensional epifluorescence microscopy was used to determine lesion area. Figure 3C shows a significant dose-dependent reduction in lesion area, quantified by tracing around the lesion and measurment of the area. The IC50 for this was 0.6 pg/eye (log IC50, −12.2 ± 2.3; Fig. 3D). To determine whether VEGF-A165b reduced the effect of laser coagulation on the structure of the retina, the eyes of mice that underwent photocoagulation followed by VEGF-A165b or saline injection were enucleated, fixed, embedded in paraffin, and prepared for histology of 5-μm-thick sections stained with H&E. Figure 3E shows that the lesion size was dose dependently reduced by VEGF-A165b injection. To quantify this, the lesion was traced around and the area was measured. VEGF-A165b induced a significant dose-dependent reduction in lesion cross-sectional area with an IC50 of 8.4 pg (log IC50, −11.08 ± 0.21; Fig. 3F). There was no significant difference in IC50 or Hill slope between the different measurements (F test).
Figure 3.
Intravitreal VEGF-A165b inhibits CNV. Mice underwent retinal laser coagulation in both eyes and intravitreous injection with VEGF-A165b or HBSS directly after laser procedure (day 0) and on day 7. (A) FA on day 14 showed dose-dependent inhibition of leakage (arrows). (B) Quantification using 0 to 3 scoring scheme by three independent masked observers. (C) Staining with isolectin B4 of the RPE-choroid-sclera complex showed a dose-dependent decrease in lesion size. (D) The IC50 for lesion size was 0.78 pg. (E) H&E staining of the middle section of each photocoagulation area showed disrupted RPE layer and lesions. These were dose dependently reduced by VEGF-A165b injection. (F) Measurement of the lesion size.
Subcutaneous VEGF-A165b Reduces Lesion Size
We have previously shown that systemic administration of VEGF-A165b reduces tumor growth in nude mice by inhibiting VEGF-A165–mediated angiogenesis.26 To determine whether VEGF-A165b could inhibit CNV when given systemically, mice were subjected to laser photocoagulation and treated twice weekly with 100 μg VEGF-A165b subcutaneously. Figure 4A shows that this resulted in a reduction of fluorescein leakage as assessed by FA. Retinas were stained for endothelial cells, and, again, systemic VEGF-A165b administration resulted in a significant reduction in lesion size (from 0.43 ± 0.03 mm2 to 0.31 ± 0.27 mm2; P < 0.02, unpaired t-test; Fig. 4B). To determine whether VEGF-A165b also reduced the volume of the endothelial lesion, a stack of images was generated through the lesion using 2-μm optical slicing, and the area of each slice of the stack was calculated. This allowed calculation of the volume of the lesion. Figure 4C shows the mean volumes of up to four lesions for each of the 10 mice in each group. It can be seen that subcutaneous treatment with VEGF-A165b significantly reduced lesion volume. To determine the histologic effect of VEGF-A165b, retinas were cut and sectioned for H&E staining. Figure 4D shows that subcutaneous injection also resulted in reduced lesion size by cross-sectional area.
Figure 4.
Systemic VEGF-A165b inhibits CNV. Mice underwent retinal laser coagulation in both eyes and subcutaneous injection with 100 μg VEGF-A165b or PBS twice weekly. (A) Quantification of FA on day 14 using 0 to 3 scoring scheme by three independent masked observers showed inhibition of leakage. Representative images for each treatment are shown above the bars. (B) Staining with isolectin B4 of the RPE-choroid-sclera complex. (C) Confocal three-dimensional reconstruction was used to calculate volume size. (D) H&E staining of the middle section of each photocoagulation area showed disrupted RPE layer and lesions. These were reduced by VEGF-A165b injection. **P < 0.02 and ***P < 0.001 compared with PBS. Unpaired t-test (A–C). Mann-Whitney U test (D).
Discussion
We show here that of the antiangiogenic isoforms, VEGF165b appears to be the most abundant endogenous isoform in the eye. Interestingly, it is most abundant in the RPE layer and the retinal layer of normal eyes, but its expression in patients with AMD is not yet known, and this is a key area that must be addressed. Our assumption of the predominance of VEGF165b among the antiangiogenic isoforms does depend on the affinity of the antibodies to VEGFxxxb being the same for all the isoforms. Although this has been demonstrated for VEGF121b, it is not yet clear for VEGF189b, VEGF145b, or VEGF183b, and the conclusion that VEGF165b is the most highly expressed must bear that assumption in mind. However, the lack of strong expression of VEGF proteins consistent with those sizes in the pan-VEGF Western blot supports that conclusion. The lack of tools for mouse studies on VEGF165b has so far precluded investigation of the expression of VEGF165b in mouse models of CNV. Antiangiogenic therapy has become a cornerstone of therapy for CNV resulting from AMD. Results of the MARINA34 and ANCHOR35 trials have shown clearly that VEGF antagonism reduces or reverses vision loss in wet AMD. Analysis of the ANCHOR trial shows that 30% of patients experienced improved vision (reversal of vision loss) in response to ranibizumab,35 approximately 30% experienced less vision loss than if they were untreated, and the remainder lost vision similar to control. Thus, though 95% of patients met the trial end point in the treated group compared with 65% in the control group, anti-VEGF therapy by antibodies that target all isoforms of VEGF-A is still not a universally effective treatment for AMD. One possible reason for this may be that VEGF comes in many different isoforms, including the antiangiogenic VEGF-A165b isoforms. Anti-VEGF antibodies have been shown also to affect VEGF-A165b. For instance, tumors expressing VEGF-A165b, while growing more slowly than tumors not expressing VEGF-A165b, are resistant to bevacizumab,25 suggesting that antiangiogenic therapy that does not target VEGF-A165b may be more effective. Here we show that an alternative approach to anti-VEGF antibodies is also effective in animal models of AMD. Treatment of mice with the antiangiogenic isoforms of VEGF by injection of recombinant protein is an effective treatment for CNV in animal models. The dose range of VEGF-A165b used (0.1–10 ng/eye) translates to a concentration in the eye of 0.01 to 1 μg/mL, or an effective dose in humans of 3 μg/eye, 1000- to 10-fold lower than ranibizumab or bevacizumab.35 Ranibizumab and bevacizumab are both human specific, so it is not possible to compare directly the relative efficacies in mice. However, VEGF-A165b did inhibit CNV at a dose 20,000 times lower than that required by pegaptanib in rodents.36 The terminal half-life in the eye of VEGF-A165b is similar to that of ranibizumab,37 so it can be postulated that effective dosing in humans may be monthly or longer.
Intravitreal anti-VEGF treatment in the eye comes with relatively few side effects,38 at least in the short to intermediate term. This low side effect profile is in contrast with the systemic dosing of anti-VEGF agents in which side effects include fatal gastric perforation,39 increased risk for stroke, hypertension, and renal dysfunction and failure.40 Moreover, anti-VEGF agents and other antiangiogenic agents affect a relatively small proportion of patients when given systemically with chemotherapy for cancer. They do not appear to work by themselves,39 and recent studies have shown that bevacizumab does not work in earlier stages of colon cancer even with chemotherapy.41 Moreover, resistance to such therapy is increasingly common42,43 and may depend on the level of VEGF165b present in those tissues. The levels of VEGF165b appear to vary widely from cell type to cell type44 and can be highly regulated.45 It is not widely accepted that systemic administration of anti-VEGF agents can be given for AMD. In contrast, however, VEGF-A165b treatment of mice does not result in hypertension, proteinuria, or any other known side effects to date.30 Although this does not necessarily translate to a lack of side effects in humans, it is possible that the lack of cardiovascular effects of VEGF165b, combined with its demonstrated cytoprotective properties in renal,46 gastrointestinal,25 and ocular epithelial cells,47 will result in VEGF-A165b treatment having a lower side effect profile than systemic treatment with anti-VEGF agents. The finding here that VEGF-A165b was effective when given systemically bodes well for its possible use in humans. It is perhaps surprising that a molecule with a relatively short half-life (tracer plasma clearance, ∼12 minutes) should be effective when given biweekly, but previous studies have shown that though the tracer plasma half-life is short, VEGF-A165b is rapidly taken up into the areas of growing blood vessels, particularly tumors.30 VEGFR2 is upregulated during choroidal angiogenesis; therefore, it is likely that the effect after systemic administration is due to the uptake of VEGF-A165b by VEGFR2 in the choroidal vasculature, although this has not been shown directly.
In summary, we show here that VEGF-A165b, which is equally as potent in blocking endothelial cell migration in vitro as ranibizumab and bevacizumab, potently inhibits CNV in vivo when given intraocularly. This inhibition can also be brought about by systemic administration of VEGF-A165b.
Footnotes
Supported by Wellcome Trust Grants 79736 (JH) and 69029 (YQ), Fight for Sight (ER), British Heart Foundation Grant BS/06/005 (DOB), Cancer Research UK Grant C18064/A5730 (AHV), the National Eye Research Centre, the Richard Bright VEGF Research Trust and the North Bristol NHS Trust Adrian Wright Bequest into Disability in the Elderly (AM), PhiloGene Inc. (SD), National Institutes of Health Core Grant EY03040, and the Arnold and Mabel Beckman Foundation.
Disclosure: J. Hua, PhiloGene Inc. (R); C. Spee, None; S. Kase, None; E.S. Rennel, None; A.L. Magnussen, None; Y. Qiu, None; A. Varey, None; S. Dhayade, None; A.J. Churchill, None; S.J. Harper, PhiloGene Inc. (F, C), P; D.O. Bates, PhiloGene Inc. (F, C, R), P; D.R. Hinton, None
References
- 1.Kliffen M, Sharma HS, Mooy CM, Kerkvliet S, de Jong PT. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol 1997;81:154–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kvanta A, Algvere PV, Berglin L, Seregard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci 1996;37:1929–1934 [PubMed] [Google Scholar]
- 3.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000;407:249–257 [DOI] [PubMed] [Google Scholar]
- 4.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature 2000;407:242–248 [DOI] [PubMed] [Google Scholar]
- 5.Celletti FL, Waugh JM, Amabile PG, Brendolan A, Hilfiker PR, Dake MD. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat Med 2001;7:425–429 [DOI] [PubMed] [Google Scholar]
- 6.Detmar M, Brown LF, Claffey KP, et al. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med 1994;180:1141–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fava RA, Olsen NJ, Spencer-Green G, et al. Vascular permeability factor/endothelial growth factor (VPF/VEGF): accumulation and expression in human synovial fluids and rheumatoid synovial tissue. J Exp Med 1994;180:341–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Usui T, Ishida S, Yamashiro K, et al. VEGF164(165) as the pathological isoform: differential leukocyte and endothelial responses through VEGFR1 and VEGFR2. Invest Ophthalmol Vis Sci 2004;45:368–374 [DOI] [PubMed] [Google Scholar]
- 9.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994;331:1480–1487 [DOI] [PubMed] [Google Scholar]
- 10.Bates DO, Lodwick D, Williams B. Vascular endothelial growth factor and microvascular permeability. Microcirculation 1999;6:83–96 [PubMed] [Google Scholar]
- 11.Lathi KG, Vale PR, Losordo DW, et al. Gene therapy with vascular endothelial growth factor for inoperable coronary artery disease: anesthetic management and results. Anesth Analg 2001;92:19–25 [DOI] [PubMed] [Google Scholar]
- 12.Margolin K, Gordon MS, Holmgren E, et al. Phase Ib trial of intravenous recombinant humanized monoclonal antibody to vascular endothelial growth factor in combination with chemotherapy in patients with advanced cancer: pharmacologic and long-term safety data. J Clin Oncol 2001;19:851–856 [DOI] [PubMed] [Google Scholar]
- 13.Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 2004;351:2805–2816 [DOI] [PubMed] [Google Scholar]
- 14.Eyetech Anti-vascular endothelial growth factor therapy for subfoveal choroidal neovascularization secondary to age-related macular degeneration: phase II study results. Ophthalmology 2003;110:979–986 [DOI] [PubMed] [Google Scholar]
- 15.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 1999;13:9–22 [PubMed] [Google Scholar]
- 16.Harper SJ, Bates DO. VEGF-A splicing: the key to antiangiogenic therapeutics? Nat Rev Cancer 2008;8:880–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bates DO, Cui TG, Doughty JM, et al. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res 2002;62:4123–4131 [PubMed] [Google Scholar]
- 18.Perrin RM, Konopatskaya O, Qiu Y, Harper S, Bates DO, Churchill AJ. Diabetic retinopathy is associated with a switch in splicing from anti- to proangiogenic isoforms of vascular endothelial growth factor. Diabetologia 2005;48:2422–2427 [DOI] [PubMed] [Google Scholar]
- 19.Potgens AJ, Lubsen NH, van Altena MC, et al. Covalent dimerization of vascular permeability factor/vascular endothelial growth factor is essential for its biological activity: evidence from Cys to Ser mutations. J Biol Chem 1994;269:32879–32885 [PubMed] [Google Scholar]
- 20.Keyt BA, Berleau LT, Nguyen HV, et al. The carboxyl-terminal domain (111–165) of vascular endothelial growth factor is critical for its mitogenic potency. J Biol Chem 1996;271:7788–7795 [DOI] [PubMed] [Google Scholar]
- 21.Cebe-Suarez S, Grunewald FS, Jaussi R, et al. Orf virus VEGF-E NZ2 promotes paracellular NRP-1/VEGFR-2 coreceptor assembly via the peptide RPPR. FASEB J 2008;22:3078–3086 [DOI] [PubMed] [Google Scholar]
- 22.Cebe Suarez S, Pieren M, Cariolato L, et al. A VEGF-A splice variant defective for heparan sulfate and neuropilin-1 binding shows attenuated signaling through VEGFR-2. Cell Mol Life Sci 2006;63:2067–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu Y, Bevan H, Weeraperuma S, et al. Mammary alveolar development during lactation is inhibited by the endogenous antiangiogenic growth factor isoform, VEGF165b. FASEB J 2008;22:1104–1112 [DOI] [PubMed] [Google Scholar]
- 24.Woolard J, Wang WY, Bevan HS, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res 2004;64:7822–7835 [DOI] [PubMed] [Google Scholar]
- 25.Varey AH, Rennel ES, Qiu Y, et al. VEGF165b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br J Cancer 2008;98:1366–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rennel E, Waine E, Guan H, et al. The endogenous antiangiogenic VEGF isoform, VEGF165b inhibits human tumour growth in mice. Br J Cancer 2008;98:1250–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz R, Pena C, Silva J, et al. p73 Isoforms affect VEGF, VEGF165b and PEDF expression in human colorectal tumors: VEGF165b downregulation as a marker of poor prognosis. Int J Cancer 2008;123:1060–1067 [DOI] [PubMed] [Google Scholar]
- 28.Pritchard-Jones RO, Dunn DB, Qiu Y, et al. Expression of VEGFxxxb, the inhibitory isoforms of VEGF, in malignant melanoma. Br J Cancer 2007;97:223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schumacher VA, Jeruschke S, Eitner F, et al. Impaired glomerular maturation and lack of VEGF165b in Denys-Drash syndrome. J Am Soc Nephrol 2007;18:719–729 [DOI] [PubMed] [Google Scholar]
- 30.Rennel ES, Hamdollah-Zadeh MA, Wheatley ER, et al. Recombinant human VEGF165b protein is an effective anti-cancer agent in mice. Eur J Cancer 2008;44:1883–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Epstein RJ, Hendricks RL, Stulting RD. Interleukin-2 induces corneal neovascularization in A/J mice. Cornea 1990;9:318–323 [PubMed] [Google Scholar]
- 32.Keyt BA, Nguyen HV, Berleau LT, et al. Identification of vascular endothelial growth factor determinants for binding KDR and FLT-1 receptors: generation of receptor-selective VEGF variants by site-directed mutagenesis. J Biol Chem 1996;271:5638–5646 [DOI] [PubMed] [Google Scholar]
- 33.Cunningham SA, Arrate MP, Brock TA, Waxham MN. Interactions of FLT-1 and KDR with phospholipase C gamma: identification of the phosphotyrosine binding sites. Biochem Biophys Res Commun 1997;240:635–639 [DOI] [PubMed] [Google Scholar]
- 34.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–1431 [DOI] [PubMed] [Google Scholar]
- 35.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006;355:1432–1444 [DOI] [PubMed] [Google Scholar]
- 36.Ishida S, Usui T, Yamashiro K, et al. VEGF164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J Exp Med 2003;198:483–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci 2005;46:726–733 [DOI] [PubMed] [Google Scholar]
- 38.Dafer RM, Schneck M, Friberg TR, Jay WM. Intravitreal ranibizumab and bevacizumab: a review of risk. Semin Ophthalmol 2007;22:201–204 [DOI] [PubMed] [Google Scholar]
- 39.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–2342 [DOI] [PubMed] [Google Scholar]
- 40.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 2008;358:1129–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Genentech Phase III C-08 study of avastin in early-stage colon cancer did not meet primary endpoint. http://www.gene.com/gene/news/press-releases/display.do?method=detail&id=12067; 2009.
- 42.Bergers G, Hanahan D. Modes of resistance to antiangiogenic therapy. Nat Rev Cancer 2008;8:592–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 2005;8:299–309 [DOI] [PubMed] [Google Scholar]
- 44.Woolard J, Bevan HS, Harper SJ, Bates DO. Molecular diversity of VEGF-A as a regulator of its biological activity. Microcirculation 2009;16:572–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nowak DG, Woolard J, Amin EM, et al. Expression of pro- and antiangiogenic isoforms of VEGF is differentially regulated by known splicing and growth factors. J Cell Sci 2008;121:3487–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bevan HS, van den Akker NM, Qiu Y, et al. The alternatively spliced antiangiogenic family of VEGF isoforms VEGFxxxb in human kidney development. Nephron Physiol 2008;110:57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magnussen A, Rennel ES, Hua J, et al. VEGF-A165b is cytoprotective and antiangiogenic in the retina. Invest Ophthalmol Vis Sci 2010;51:4273–4281 [DOI] [PMC free article] [PubMed] [Google Scholar]