Abstract
As part of an ongoing research program to discover natural products that suppress the hypoxia-activated tumor survival pathways, the lipid extract of the Papua New Guinea marine sponge Diacarnus levii was found to suppress hypoxia-induced HIF-1 activation and hypoxic tumor cell survival. Bioassay-guided isolation of D. levii yielded four new norsesterterpene peroxides, diacarnoxides A – D. Diacarnoxide B exhibits a significantly enhanced ability to suppress the growth of tumor cells under hypoxic conditions.
As solid tumors grow, they rapidly outstrip the capacity of their surrounding vasculature to supply oxygen and nutrients.1–2 This produces a tumor mass that is highly oxygen starved (hypoxic) and necrotic in the center. These hypoxic tumor cells are typically resistant to chemotherapy and radiation,3–4 and often exhibit an extremely malignant and highly metastatic phenotype.5 In addition to promote resistance to conventional therapy, tumor hypoxia also selects for tumor cells that have mutated p53-mediated apoptotic pathways.5 Exposure to hypoxic conditions induces the activation of hypoxia-inducible factor-1 (HIF-1), a transcription factor that regulates oxygen homeostasis.6 Overall, hypoxic-activation of HIF-1 increases the expression of genes that enhance tumor cell adaptation and survival under hypoxic conditions.6 Since normal cells are well-oxygenated, this rather unique hypoxic tumor microenvironment has emerged as an exciting new target for anticancer drug discovery.1–2 Several strategies to selectively target hypoxic tumor cells are currently under investigation. These include the discovery of natural products and other compounds that act as HIF-inhibitors that target hypoxia-induced gene expression,7–8 and the development of compounds that function as bioreductive cytotoxins.1–2 Bioreductive cytotoxins are organic compounds that are chemically activated under the reducing conditions that exist within hypoxic tissues. Once activated, these compounds exert hypoxic cell-selective cytotoxic effects by mechanisms such as inducing DNA damage. The most clinically advanced example of bioreductive cytotoxins is the aromatic N-oxide tirapazamine (TPZ) that has reached phase III clinical trials in combination with cisplatin for the treatment of advanced head and neck cancer.9
As part of an ongoing research program to discover natural product-based anticancer agents that target tumor hypoxia, the lipid extract of the Papua New Guinea sponge Diacarnus levii (Latrunculiidae) (NCI Open Repository of marine invertebrates and algae lipid extracts collection #CO18983) was found to inhibit hypoxia-induced HIF-1 activation in a T47D breast tumor cell-based reporter assay10 (73% inhibition at 5 μg mL−1). Bioassay-guided fractionation of the extract yielded four new norsesterterpene peroxides, trivially named diacarnoxides A – D (1 – 4). The genus Diacarnus is known to be a rich source of terpene peroxides.11–14 Marine sponge terpene peroxides are an unusual class of compounds that have shown significant cytotoxic activity against a wide assemblage of human tumor cell lines.11–16 The cytotoxic potency of terpene peroxides vary for specific tumor cell lines examined and are highly influenced by the unique chemical substituent effects that can be attributed to specific peroxide structure classes. While numerous studies have demonstrated the antitumor effects of terpene peroxides, this study represents the first examination of the effect of marine terpene peroxides on hypoxic tumor cell growth/viability. Herein, we describe the isolation, structural elucidation, and unique activities of these new compounds.
Results and Discussion
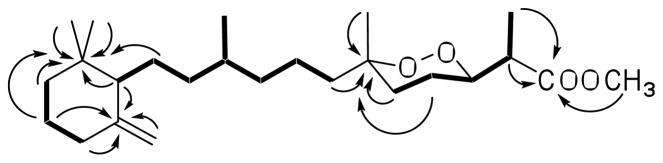
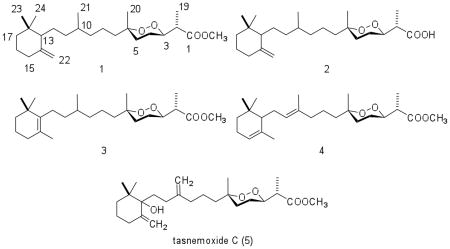
Compound (1) was isolated as colorless oil with the molecular formula C25H44O4, as deduced from HRESIMS spectrometric and 13C NMR spectroscopic data. The 1H NMR spectrum of 1 (Table 1) displayed the characteristic resonances of six methyl groups, of which four were singlet resonances (δ 0.93, 0.87, 1.25, 3.70) and two were doublet resonances (δ 0.75, d, J = 6.5 Hz; δ 1.28, d, J = 6.6 Hz). Two proton resonances of an exomethylene (δ 4.51, 4.87) were observed, as well as one resonance (δ 4.12 ppm) for a proton attached to an oxygenated carbon. Consistent with the 1H NMR data analysis, 25 carbon resonances were observed in the 13C and 13C DEPT NMR spectra of 1 (Table 2), including six methyl resonances (δ 13.5, 18.5, 20.8, 28.6, 28.6, 51.7), one oxygenated quaternary carbon resonance (δ 80.3), an oxygenated methine resonance (δ 81.3), two resonances of a exomethylene (δ 149.3, 109.3, C-14 – C-22), and a ester carbon resonance (δ 174.2, C-1). Accordingly, the structure of 1 could be assigned as that of a norsesterterpene peroxide methyl ester with one exomethylene bond. Analysis of the 1H-1H COSY and 1H-13C HMQC spectra indicated that the structure of 1 was made up of three major 1H-1H spin systems: -CH3(19)-CH(2)-CH(3)-CH2(4)-CH2(5)-, -CH2(7)-CH2(8)-CH2(9)-CH(10)[CH3(21)]-CH2(11)-CH2(12)-CH(13)-, and -CH2(15)-CH2(16)-CH2(17)-. These proton-proton spin systems were readily assembled by analysis of long-range 1H-13C couplings that were observed in the 1H-13C HMBC spectrum between C-1 and H-2, H-19, C-1-OCH3; between C-6 and H-4, H-5, H-7, H-20; between C-14 and H-13, H-15, H-16, H-22; and between C-18 and H-13, H-16, H-17, H-23, H-24 (Fig. 1). The overall spectroscopic data of this compound were similar to those of tasnemoxide C (5), a norsesterterpene peroxide previously isolated from Diacarnus erythraenus.11 The primary differences between the NMR spectra of the two compounds were that the C-21 methylene singlet resonance observed in 5 was replaced by a C-21 methyl group in 1 and that the C-13 carbon in the spectrum of 1 did not bear an oxygen, as in the hydroxyl-substituted C-13 carbon resonance observed in the 13C spectrum of 5. These changes produced corresponding upfield shifts of both the C-10 and C-13 resonances that were observed in the 13C spectrum of 1. Comparison of the spectral data of 1 with those of tasnemoxide C (5) confirmed that they possessed the same cyclic peroxide functionality and relative configuration at C-2, C-3, and C-6. Thus, diacarnoxide A (1) was determined to be a new D. levii norsesterterpene peroxide methyl ester that was structurally related to the D. erythraenus peroxide 5.
Table 1.
1H NMR Spectral Data for 1 – 4 (400 MHz, CDCl3)
| Proton | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 2 | 2.67 m | 2.58 m | 2.54 m | 2.65 m |
| 3 | 4.12 m | 4.12 m | 4.22 m | 4.10 m |
| 4 | 1.70 m | 1.66 m | 1.64 m | 1.70 m |
| 5 | 1.65 m | 1.60 m | 1.60 m | 1.64 m |
| 7 | 1.59 m | 1.59 m | 1.60 m | 1.60 m |
| 8 | 1.45 m | 1.46 m | 1.40 m, | 1.31 m |
| 9 | 1.23m | 1.20 m | 1.23 m | 1.99 m |
| 10 | 1.30 m | 1.24 m | 1.35 m | – |
| 11 | 1.20 m | 1.22 m | 1.26 m | 5.06 m |
| 12 | 1.88 m | 1.88 m | 2.06 m | 2.08 m |
| 13 | 1.58 m | 1.58 m | – | 2.04 m |
| 15 | 2.00 m | 1.99 m | 1.89 m | 5.09 m |
| 16 | 1.35 m | 1.41 m | 1.64 m | 1.96 m |
| 17 | 1.45 m | 1.45 m | 1.48 m | 1.35 m |
| 19 | 1.28 d (6.6) | 1.24 d (6.6) | 1.25 d (6.6) | 1.28 d (6.6) |
| 20 | 1.25 s | 1.22 s | 1.25 s | 1.26 s |
| 21 | 0.75 d (6.5) | 0.76 d (6.1) | 0.77 d (6.6) | 1.69 s |
| 22 | 4.51 s, 4.87 s | 4.51 s, 4.86 s | 1.60 s | 1.61 s |
| 23 | 0.93 s | 0.95 s | 1.00 s | 0.94 s |
| 24 | 0.87 s | 0.87 s | 0.97 s | 0.88 s |
| OCH3 | 3.70 s | – | 3.67 s | 3.71 s |
Table 2.
13C NMR Spectral Data for 1 – 4 (100 MHz, CDCl3)
| Carbon | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 174.2 | 180.1 | 174.2 | 174.4 |
| 2 | 42.9 | 43.0 | 42.7 | 43.1 |
| 3 | 81.3 | 81.1 | 81.3 | 81.6 |
| 4 | 23.5 | 22.9 | 22.9 | 22.7 |
| 5 | 32.0 | 32.4 | 33.0 | 32.5 |
| 6 | 80.3 | 80.6 | 80.5 | 80.3 |
| 7 | 39.5 | 39.0 | 39.2 | 39.8 |
| 8 | 21.7 | 21.7 | 21.8 | 24.8 |
| 9 | 37.3 | 37.3 | 37.5 | 39.8 |
| 10 | 36.4 | 36.4 | 36.6 | 135.6 |
| 11 | 33.0 | 32.4 | 33.2 | 124.5 |
| 12 | 24.1 | 23.5 | 27.6 | 23.5 |
| 13 | 55.1 | 54.5 | 136.9 | 61.0 |
| 14 | 149.3 | 149.3 | 127.3 | 131.5 |
| 15 | 32.5 | 32.5 | 31.8 | 124.1 |
| 16 | 23.7 | 23.6 | 19.9 | 21.6 |
| 17 | 36.6 | 36.6 | 40.1 | 30.1 |
| 18 | 35.5 | 35.5 | 34.9 | 33.9 |
| 19 | 13.5 | 13.5 | 12.9 | 13.7 |
| 20 | 20.8 | 20.8 | 21.0 | 20.3 |
| 21 | 18.5 | 18.2 | 18.5 | 15.9 |
| 22 | 109.3 | 109.3 | 19.7 | 20.3 |
| 23 | 28.6 | 28.8 | 28.7 | 25.6 |
| 24 | 28.6 | 28.8 | 28.8 | 29.9 |
| OCH3 | 51.7 | – | 51.8 | 51.9 |
Figure 1.
Selected 1H-1H COSY (bold solid bars) and 1H-13C HMBC (arrows) correlations of 1.
Compound 2 was obtained as colorless oil with the molecular formula C24H42O4, as deduced from HRESIMS spectrometric and 13C NMR spectroscopic data. The 13C NMR spectrum of 2 (Table 2) exhibits 24 carbon resonances and the DEPT spectrum indicated the presence of five methyl groups, eleven methylene groups, four methine groups, and four quaternary carbon atoms. Comparison of the NMR spectra of 2 (Tables 1 and 2) with those of 1 revealed that 2 possesses a similar structure, except for the absence of the resonances for the C-1 methyl ester observed in 1. The downfield shift in the 13C resonance observed for C-1 in 2 (δ = 180.1 ppm), relative to the δ = 174.2 ppm observed for C-1 in 1, was consistent with a proposed structure that contains a C-1 carboxylic acid. The HRESIMS mass spectrum of 2 displayed a molecular ion that was 14 mass units less than that observed for 1. Therefore, the structure of 2 was deduced to be that of a new norsesterterpene peroxide acid, trivially named diacarnoxide B.
Compound 3 was obtained as colorless oil with the molecular formula C25H44O4, as deduced from HRESIMS spectrometric data. The 1H, 13C and 13C DEPT NMR spectra of 3 (Tables 1, 2) were closely related to those of 1. The 13C NMR spectrum of 3 (Table 2) exhibited seven methyl, ten methylene, three methine, and five quaternary carbon resonances. Moreover, the 1H NMR spectrum (Table 1) exhibited the presence of seven methyl groups, of which five were observed as singlet proton resonances (δ 0.97, 1.00, 1.25, 1.60, 3.67) and two were observed as doublet resonances (δ 0.77, d, J = 6.6 Hz; δ 1.25, d, J = 6.6 Hz). Analysis of the 1H-1H COSY and 1H-13C HMQC spectra indicated the presence of three major 1H-1H spin systems in the structure of 3: –CH3(19)-CH(2)-CH(3)-CH2(4)-CH2(5)-, –CH2(7)-CH2(8)-CH2(9)-CH(10)[CH3(21)]-CH2(11)-CH2(12)-, and CH2(15)-CH2(16)-CH2(17)-. These proton-proton spin systems in 3 could readily be connected through analysis of the long-range heteronuclear correlations that were observed in the 1H-13C HMBC spectrum. Specifically, couplings between C-6 and H-5, H-7, H-20; between C-13 and H-12, H-22, H-23, H-24; between C-14 and H-12, H-15, H-22; and between C-18 and H-12, H-17, H-23, H-24 facilitated assemblage of the complete carbon skeleton. Therefore, the structure of this new D. levii peroxide was deduced to be that of diacarnoxide C (3).
Analysis of the 13C NMR and 13C DEPT spectra indicated that 4 also possessed 25 carbon resonances, for seven methyl groups, eight methylene groups, five methine groups, and five quaternary carbons. Overall, the 1H NMR spectrum of 4 was very similar to those of 1, 2, and 3. The major difference was the presence of two distinct downfield olefinic proton resonances (δ 5.06, 5.09) in the spectrum of 4, rather than the upfield exomethylene resonances observed in 1 and 2. Long-range 13C-1H correlations that were observed between C-10 and H-9, H-11, H-12, H-21; between C-14 and H-13, H-15, H-22; and between C-18 and H-13, H-17, H-23, H-24 allowed the assignment of the norsesterterpene diacarnoxide D (4).
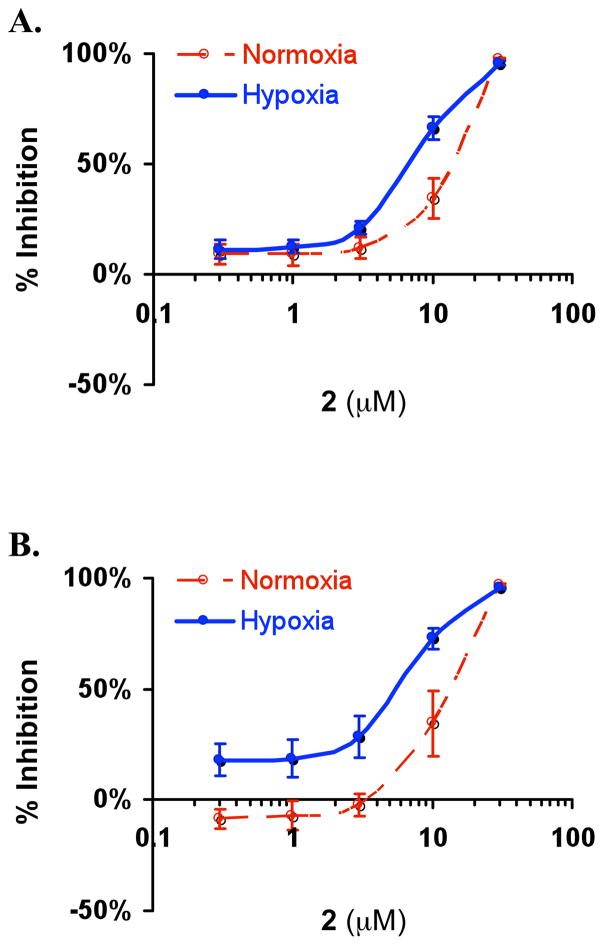
The effects of 1 – 4 on hypoxia (1% O2)-induced HIF-1 activation and tumor cell proliferation/viability under hypoxic conditions were examined in T47D breast carcinoma cells. Initial examination revealed that 2 (12.7 μM) inhibited both hypoxia-induced HIF-1 activation (95%) and tumor cell proliferation/viability (97%) following a 16 h exposure. No inhibition was observed at a lower concentration (1.27 μM). Under experimental conditions, none of the other compounds (1, 3, and 4) achieved greater than 30% inhibition of either hypoxia-induced HIF-1 activation or cell proliferation/viability at the highest concentration tested (12.7 μM). Compounds 1 – 4 were isolated from two pooled chromatographic fractions of D. levii that inhibited hypoxia-activated HIF-1 by 80% and 91% (at 5 μg mL−1), and suppressed tumor cell proliferation/viability by 38% and 34%, respectively. At lower concentration (0.5 μg mL−1), neither fraction inhibited HIF-1 activation by greater than 30%. Since HIF-1 regulates the ability of cells to adapt and survive under hypoxic conditions, inhibition of HIF-1 could directly inhibit the growth/viability of certain tumor cell lines under hypoxic conditions. Alternatively, a significant component of the HIF-1 inhibitory activity of purified diacarnoxides could be attributed to the ability of these compounds to preferentially suppress the overall viability of tumor cells under hypoxic conditions. An expanded concentration-response study was performed to investigate the effects of diacarnoxides on tumor cell proliferation/viability under hypoxic and normoxic conditions. In a panel of human prostate (DU145 and PC-3) and breast (MCF-7, MDA-MB-231, and T47D) carcinoma cell lines examined, the most active compound 2 inhibited cell proliferation/viability under both normoxic and hypoxic conditions (IC50 values shown in Table 3). At lower concentrations, 2 displayed an unusual enhanced growth/viability inhibitory activity that was observed in MCF-7 and MDA-MB-231 cells under hypoxic conditions (Figure 2). The observation that 1 (the methyl ester of 2) is far less active than 2 suggests that the free acid form of specific Diacarnus norsesterterpene peroxides such as 2 is critical for its inhibitory activity on cell proliferation/viability.
Table 3.
IC50 Values (μM) of 1 and 2 on Tumor Cella Proliferation/Viability Under Hypoxic (Hyp)b and Normoxic (Norm)c Conditions.
| 1 | 2 | ||
|---|---|---|---|
| DU145 | Hyp | > 30 | 5.3 |
| Norm | > 30 | 6.6 | |
| PC-3 | Hyp | > 30 | 12.9 |
| Norm | > 30 | 16.7 | |
| MCF-7 | Hyp | > 30 | 6.6 |
| Norm | > 30 | 13.8 | |
| MDA-MB-231 | Hyp | 17.8 | 5.6 |
| Norm | 17.8 | 13.3 | |
| T47D | Hyp | 26.9 | 6.0 |
| Norm | 14.7 | 5.6 |
Cell lines: DU145 and PC-3, prostate cancer; MCF-7, MDA-MB-231, and T47D, breast cancer.
Hypoxic condition: 1% O2:94% N2:5% CO2, 48 h.
Normoxic condition: 95% Air:5% CO2, 48 h. The IC50 values were determined from one experiment performed in quadruplicate, and the standard deviation for each data point is less than 10%.
Figure 2.
At lower concentrations, 2 preferentially suppress MCF-7 (A) and MDA-MB-231 (B) breast tumor cell proliferation/viability under hypoxic conditions. Proliferation/viability of MDA-MB-231 cells was suppressed at the lowest concentration of 2 tested (0.3 μM), relative to MDA-MB-231 hypoxic control cells. The data shown are averages from one experiment performed in quadruplicate and the bars represent standard deviation.
We observed that in the purified state, diacarnoxides are relatively unstable and require chromatographic purification immediately before examination in order to assure reproducible results in cell viability assays. The mechanisms responsible for the cytostatic and/or cytotoxic activities of marine terpene peroxides are unclear. It is possible that reactive peroxide functionalities may be capable of binding to specific enzymes of vital cellular proteins.17 The propensity of hypoxic conditions to specifically enhance the ability of 2 to suppress tumor cell growth/viability may provide direct insight into the possible mechanisms involved in the biological activities attributed to natural product-based peroxides.17 These preliminary findings may lend support to previously proposed mechanisms for antimalarial and antitumor natural product peroxides that involve the generation of particular reactive oxygen species (ROS). Moreover, if hypoxic conditions can enhance the potential antitumor effects of natural product peroxides, it may be possible to develop natural product-based peroxides that are significantly hypoxia-selective to establish a reasonable therapeutic margin.
Experimental Section
General Experimental Procedures
The IR spectrum was obtained using an AATI Mattson genesis Series FTIR. The NMR spectra were recorded in CDCl3 on Bruker AMX-NMR spectrometers operating at either 400 or 500 MHz for 1H and either 100 or 125 MHz for 13C, respectively. The NMR spectra were recorded running gradients and using residual solvent peaks (δ 7.27 for 1H) and (δ 77.0 for 13C) as internal references. The HRESIMS spectra were measured using a Bruker Daltonic micro TOF with electrospray ionization. Silica gel (200–400 mesh) was used for column chromatography. TLCs were run on Merck Si60F254 or Si60RP18F254 plates and visualized under UV at 254 nm or by heating after spraying with a 1% anisaldehyde solution in acetic acid:H2SO4 (50:1).
Sponge Material
The sponge material was obtained from the National Cancer Institute’s Open Repository Program. Diacarnus levii (Latrunculiidae) was collected (# C018983) at a depth of −10 M off the coast of Papua New Guinea on June 6, 1998, frozen at −20 °C, and ground in a meat grinder. A voucher specimen was placed on file with the Department of Invertebrate Zoology, National Museum of Natural History, Smithsonian Institution, Washington, D.C.
Extraction and Isolation
Ground Diacarnus levii material was extracted with water. The residual sample was then lyophilized and extracted with CH2Cl2:MeOH (1:1), residual solvents were removed under vacuum, and the crude extract stored at −20 °C in the NCI repository at the Frederick Cancer Research and Development Center (Frederick, Maryland). Bioassay-guided fractionation (2 g of the crude extract) by CC (Si gel, 30 g), using gradients of EtOAc in hexanes (15:85, 50:50, 100:0) resulted in four fractions. The first two fractions that eluted with 15% EtOAc in hexanes (5 mg mL−1 HIF-1 assay inhibition values 80%, 91%, respectively; 355.5 mg combined mass) were combined and further separated by a second level of CC (Si gel, 5.0 g) with EtOAc in hexanes (1:15, 500 ml) to give 1 (7.0 mg, 0.35% yield), 2 (5.0 mg, 2.5% yield), 3 (2.5 mg, 1.25% yield), 4 (4.2 mg, 0.21% yield).
Diacarnoxide A (1), (2S*)-methyl-2-((3R*,6S*)-6-(6-(2,2-dimethyl-6-methylenecyclohexyl)-4-methylhexyl)-6-methyl-1,2-dioxan-3-yl)propanoate
Colorless oil: [α]D24 +39.5 (c 0.3, CH2Cl2); IR (film) νmax 2935, 1714, 1455, 1213, 1010 cm−1; 1H (Table 1) and 13C NMR (Table 2); HRESIMS: m/z 408.3245 (calcd for C25H44O4 408.3240).
Diacarnoxide B (2), (2S*)-2-((3R*,6S*)-6-(6-(2,2-dimethyl-6-methylenecyclohexyl)-4-methylhexyl)-6-methyl-1,2-dioxan-3-yl)propanoic acid
Colorless oil: [α]D24 +41.5 (c 0.5, CH2Cl2); IR (film) νmax 2935, 1712, 1455, 1213 cm−1; 1H (Table 1) and 13C NMR (Table 2); HRESIMS: m/z 394.3087 (calcd for C24H42O4 394.3083).
Diacarnoxide C (3), (2S*)-methyl-2-((3R*,6S*)-6-methyl-6-(4-methyl-6-(2,6,6-trimethylcyclohex-1-enyl)hexyl )-1,2-dioxan-3-yl)propanoate
Colorless oil: [α]D24 +51.3 (c 0.4, CH2Cl2); IR (film) νmax 2937, 1712, 1450, 1238 cm−1; 1H (Table 1) and 13C NMR (Table 2); HRESIMS: m/z 408.3239 (calcd for C25H44O4 408.3240).
Diacarnoxide D (4), (2S*)-methyl-2-((3R*,6S*)-6-methyl-6-((E)-4-methyl-6-(2,6,6-trimethylcyclohex-2-enyl)h ex-4-enyl)-1,2-dioxan-3-yl)propanoate
Colorless oil: [α]D24 +46.1 (c 0.45, CH2Cl2); IR (film) νmax 2940, 1734, 1453 cm−1; 1H (Table 1) and 13C NMR (Table 2); HRESIMS: m/z 406.3081 (calcd for C25H42O4 406.3083).
Cell Proliferation/Viability Assay
Human breast carcinoma MCF-7, MDA-MB-231, T47D and prostate carcinoma DU145 and PC-3 cells (ATCC) were grown in DMEM/F12 medium with glutamine (Mediatech) supplemented with 10% (v/v) fetal calf serum (FCS, Hyclone), 50 units mL−1 penicillin G (sodium salt) and 50 μg mL−1 streptomycin sulfate (referred to as “Pen/Strep”) (Invitrogen) in a humidified atmosphere (5% CO2:95% air) at 37°C. Exponentially grown cells were plated at the density of 30,000 cells/well into 96-well tissue culture plates (Corning) in a volume of 100 μL DMEM/F12 medium with 10% FCS and Pen/Strep. The cells were incubated at 37 °C overnight. Test compounds diluted in DMEM/F12 medium with Pen/Strep were added in a volume of 100 μL per well. The highest concentration of the solvent (DMSO, Fisher) was 0.6%. After a 30 min incubation, the compound treatment continued for another 48 h at 37 °C under normoxic (5% CO2:95% air) and hypoxic (5% CO2:1% O2:94% N2) conditions. Cell proliferation/viability (performed in quadruplicate) was determined using the neutral red method18 with modifications as previously described. 19 The absorbance at 540 nm was measured on a BioTek Synergy HT microplate reader with correction wavelength at 630 nm. The data were normalized to hypoxic or normoxic controls, respectively. The following formula was used to calculate % inhibition of cell proliferation/viability: % Inhibition = 1 - OD540(treated)/OD540(control).
Cell-Based Reporter Assay for HIF-1 Activity
The transfection, compound treatment, exposure to hypoxic conditions (1% O2:5% CO2:94% N2), normoxic conditions (5% CO2:95% Air), and a hypoxia mimetic (10 μM 1,10-phenanthroline), and luciferase activity determination were performed as previously described.10
Acknowledgments
The authors thank the Natural Products Branch Repository Program at the National Cancer Institute for providing marine extracts from the NCI Open Repository used in these studies, T. Smillie (University of Mississippi) for coordinating sample acquisition from the NCI, D.K. Jones (University of Mississippi) for screening NCI samples in the HIF-1 assay, R.V. Rao (University of Mississippi) for measuring the HRESIMS spectra, S.L. McKnight (University of Texas Southwestern Medical Center at Dallas) for providing the pTK-HRE3-luc construct. This work was supported by the National Institutes of Health-NCI CA 98787 (DGN/YDZ), the DOD-Prostate Cancer Research Program PC040931 (DGN), and NOAA NURP/NIUST NA16RU1496. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Grant No. C06 RR-14503-01 from the National Institutes of Health. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, and Fort Detrick MD 21702-5014 is the awarding and administering acquisition office for the DOD support. The content herein reported does not necessarily reflect the position or the policy of the Government, and no official endorsement should be inferred.
References
- 1.Boyle RG, Travers S. Anti-Cancer Agents Med Chem. 2006;6:281–286. doi: 10.2174/187152006777698169. [DOI] [PubMed] [Google Scholar]
- 2.Brown JM, Wilson WR. Nat Rev Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 3.Brizel DM, Dodge RK, Clough RW, Dewhirst MW. Radiother Oncol. 1999;53:113–117. doi: 10.1016/s0167-8140(99)00102-4. [DOI] [PubMed] [Google Scholar]
- 4.Teicher BA, Lazo JS, Sartorell AC. Cancer Res. 1981;41:73–81. [PubMed] [Google Scholar]
- 5.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 6.(a) Harris AL. Nature Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]; (b) Semenza GL. Nature Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 7.Nagle DG, Zhou YD. Curr Drug Targets. 2006;7:355–369. doi: 10.2174/138945006776054979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powis G, Kirkpatrick L. Mol Cancer Ther. 2004;3:647–654. [PubMed] [Google Scholar]
- 9.von Pawel J, von Roemeling R, Gatzemeier U, Boyer M, Elisson LO, Clark P, Talbot D, Rey A, Butler TW, Hirsh V, Olver I, Bergman B, Ayoub J, Richardson G, Dunlop D, Arcenas A, Vescio R, Viallet J, Treat J. J Clin Oncol. 2000;18:1351–1359. doi: 10.1200/JCO.2000.18.6.1351. [DOI] [PubMed] [Google Scholar]
- 10.Hodges T, Hossain FC, Kim YP, Zhou YD, Nagle DG. J Nat Prod. 2004;67:767–771. doi: 10.1021/np030514m. [DOI] [PubMed] [Google Scholar]
- 11.Youssef DTA. J Nat Prod. 2004;67:112–114. doi: 10.1021/np0340192. [DOI] [PubMed] [Google Scholar]
- 12.Youssef DTA, Yoshida WY, Kelly M, Scheuer PJ. J Nat Prod. 2001;64:1332–1335. doi: 10.1021/np010184a. [DOI] [PubMed] [Google Scholar]
- 13.Sperry S, Valeriote FA, Corbett TH, Crews P. J Nat Prod. 1998;61:241–247. doi: 10.1021/np970467w. [DOI] [PubMed] [Google Scholar]
- 14.EI Sayed KA, Hamann MT, Hashish NE, Shier WT, Kelly M, Khan AA. J Nat Prod. 2001;64:522–524. doi: 10.1021/np000529+. [DOI] [PubMed] [Google Scholar]
- 15.Davidson BS. J Org Chem. 1991;56:6722–6724. [Google Scholar]
- 16.Phuwapraisirisan P, Matsunaga S, Fesetani N, Chaitanawisuti N, Kritsanapuntu S, Menasveta P. J Nat Prod. 2003;66:289–291. doi: 10.1021/np020417d. [DOI] [PubMed] [Google Scholar]
- 17.Efferth T. Curr Drug Targets. 2006;7:407–421. doi: 10.2174/138945006776359412. [DOI] [PubMed] [Google Scholar]
- 18.Borenfreund E, Puerner JA. Toxicol Lett. 1985;24:119–124. doi: 10.1016/0378-4274(85)90046-3. [DOI] [PubMed] [Google Scholar]
- 19.Zhou YD, Kim YP, Mohammed KA, Jones DK, Muhammad I, Dunbar DC, Nagle DG. J Nat Prod. 2005;68:947–950. doi: 10.1021/np050029m. [DOI] [PMC free article] [PubMed] [Google Scholar]