Abstract
Objective
Conventional anticonvulsants reduce neuronal excitability through effects on ion channels and synaptic function. Anticonvulsant mechanisms of the ketogenic diet remain incompletely understood. Since carbohydrates are restricted in patients on the ketogenic diet, we evaluated the effects of limiting carbohydrate availability by reducing glycolysis using the glycolytic inhibitor 2-deoxy-D-glucose (2DG) in experimental models of seizures and epilepsy.
Methods
Acute anticonvulsant actions of 2DG were assessed in vitro in rat hippocampal slices perfused with 7.5mM [K+]o, 4-aminopyridine (4-AP), or bicuculline and in vivo against seizures evoked by 6 Hz stimulation in mice, audiogenic stimulation in Fring’s mice, and maximal electroshock and subcutaneous Metrazol in rats. Chronic antiepileptic effects of 2DG were evaluated in rats kindled from olfactory bulb or perforant path.
Results
2DG (10mM) reduced interictal epileptiform bursts induced by high [K+]o, 4-AP and bicuculline, and electrographic seizures induced by high [K+]o in CA3 of hippocampus. 2DG reduced seizures evoked by 6 Hz stimulation in mice (ED50 = 79.7 mg/kg) and audiogenic stimulation in Fring’s mice (ED50 = 206.4 mg/kg). 2DG exerted chronic antiepileptic action by increasing afterdischarge thresholds in perforant path (but not olfactory bulb) kindling and caused a 2-fold slowing in progression of kindled seizures at both stimulation sites. 2DG did not protect against maximal electroshock or Metrazol seizures.
Interpretation
The glycolytic inhibitor 2DG exerts acute anticonvulsant and chronic antiepileptic actions and has a novel pattern of effectiveness in preclinical screening models. These results identify metabolic regulation as a potential therapeutic target for seizure suppression and modification of epileptogenesis.
Keywords: seizures, kindling, glycolysis, 2-deoxy-D-glucose, ketogenic diet, NRSF, CtBP
INTRODUCTION
Approximately 0.5-1% of the population is afflicted with epilepsy, and as many as one-third of patients with epilepsy are refractory to pharmacological therapy, including the most recent generation of anticonvulsants. The high-fat, low-carbohydrate, adequate-protein ketogenic diet (KD) has proven efficacy in reducing seizures in up to half of drug-refractory patients.1 The mechanisms by which the KD suppresses seizures are largely unknown.2, 3 A remarkable feature of the KD is that ingestion of even a small amount of carbohydrate by patients who have achieved seizure control on the diet can rapidly reduce the diet’s effectiveness and result in seizure recurrence.4 This clinical observation suggests that glycolysis and carbohydrate metabolism might promote seizure susceptibility and that inhibition or reduction of glycolysis might have anticonvulsant effects. In support of this hypothesis, preliminary in vitro observations demonstrated that isomolar substitution of glucose by alternative energy sources such as pyruvate and lactate in hippocampal slices exposed to 7.5 mM [K+]o reduced interictal epileptic burst discharges.5 Furthermore, administration of 2-deoxy-D-glucose (2DG), an inhibitor of glycolysis, had in vivo anticonvulsant effects and reduced the progression of kindling evoked by perforant path stimulation in adult rats.6
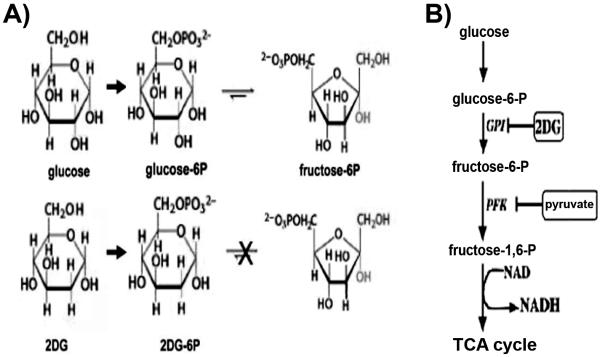
2DG differs from normal glucose only by removal of an oxygen atom from the hydroxyl group at the 2 position (Fig 1A). When provided exogenously, 2DG is taken up by glucose transporters and is subsequently phosphorylated to 2-deoxy-D-glucose 6-phosphate (2DG-6P), which cannot be converted to fructose-6-phosphate by phosphoglucose isomerase, thereby preventing metabolism through subsequent steps of glycolysis (Fig 1B). The trapping of 2DG-6P in cells after uptake through glucose transporters has enabled the use of 2DG as a metabolic tracer for glucose utilization and its adaptation in positron emission tomography (PET) scanning using 18F- 2DG in clinical imaging.7
FIGURE 1.
(A) Chemical structures of glucose, 2DG, and intermediates of the initial steps of glycolysis. Phosphorylation of 2DG yields 2DG-6P, which cannot undergo isomerization by glucose-6P-isomerase to fructose-6P, thereby preventing subsequent steps of glycolysis. (B) Schematic diagram of key steps of glycolysis illustrating the rate-limiting step involving phosphofructokinase which is inhibited by pyruvate, the end-product of the pathway. Oxidation of phosphoenolpyruvate (structure not shown) to pyruvate generates NADH prior to entry into the TCA cycle. Abbreviations: Glu, glucose; G-6-P, glucose-6-phosphate; GPI, glucose-6-phosphate isomerase; 2DG, 2-deoxyglucose; F-6-P, fructose-6-phosphate; NADH, nicotinamide adenine dinucleotide; TCA, tricarboxylic acid.
In our previous study, intraperitoneal administration of 2DG at a dose of 250 mg/kg in rats impaired the development of kindled seizures in response to stimulation of the perforant path to the dentate gyrus and hippocampus.6 The antiepileptic effects of 2DG against kindling were associated with reduction of seizure-induced increases in brain-derived neurotrophic factor (BDNF) and its receptor trkB, which are required for kindling progression.8 Seizure-induced increases in BDNF and trkB were prevented by 2DG through a mechanism of transcriptional repression dependent on Neuron Restrictive Silencing Factor (NRSF) and its NADH sensitive cofactor Carboxy-terminal Binding Protein (CtBP), which maintain a repressive chromatin environment at the BDNF and trkB promoter regions.6 These observations suggest that reducing glycolytic flux by glycolytic inhibitors such as 2DG might have anticonvulsant and antiepileptic effects.
In this study, we further evaluated the acute anticonvulsant properties of 2DG against epileptic burst discharges evoked in vitro in rat hippocampal slices and in in vivo models including seizures evoked by maximal electroshock (MES), subcutaneous Metrazol, 6 Hz stimulation in mice, and audiogenic stimuli in Fring’s mice. The chronic effects of 2DG against epilepsy progression were also examined by investigating the effects of 2DG on development of kindled seizures evoked by stimulation of a different neural structure, the olfactory bulb.
METHODS
Protocols for the experiments reported here were approved by the University of Wisconsin Research Animal Resources Center (RARC) and conform to animal care guidelines of the National Institutes of Health (NIH).
Hippocampal Slice Experiments
Sprague-Dawley male rats (10-13 or 28-120 days old) were deeply anesthetized with isoflurane. After decapitation, brains were quickly removed and placed in ice cold artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl 124; KCl 3.75; NaH2PO4 1.25; MgSO4 2; NaHCO3 26; glucose 10; CaCl2 2 saturated with a mixture of 95% oxygen and 5% CO2. The pH of all solutions was adjusted to 7.4. Horizontal hippocampal slices (500 μm) were cut using a Vibratome series 1000 tissue slicer and were maintained at room temperature for at least 1 hour, after which they were transferred to an interface recording chamber with ACSF maintained at 32° C for electrophysiological recordings.
Extracellular field potentials were recorded in stratum pyramidale of CA3 using a glass pipette filled with 2 M NaCl (impedances of 5–10 MΩ). Spontaneous activity was recorded, amplified, stored using a DIGIDATA 1200 AD converter and Axoclamp 1B amplifier, and analyzed with pCLAMP 6.02. After baseline recording, epileptiform burst discharges were induced by increasing [K+]o to 7.5 mM9, 10 or bath addition of the convulsant agents 4-aminopyridine (4AP, 50-100 μM) or bicuculline (10 μM). There is a long controversy regarding the semantics and phenomenology of seizure activity in in vitro brain slices.11 Strictly speaking, behavioral seizures (the defining feature of clinical epilepsy) cannot occur in a brain slice. Here we use the terminology outlined in comprehensive literature reviews11, 12, defining brief (typically <100 msec), interictal-like discharges as epileptiforms, epileptiform discharges, or epileptiform bursts, and longer (several sec, presumably ictal) seizure-like discharges as electrographic seizures. 9, 10 13, 14
After sufficient recording to determine that the rate of spontaneous epileptic discharges was at steady state (usually about 30 min), effects of alterations in the concentration of glucose, bath additions of alternative energy sources such as pyruvate or lactate, or effects of bath addition of 2DG were evaluated.
Seizures Evoked by Maximal Electroshock in Rats
Maximal electroshock seizures (MES convulsions) were evoked in rats (adult Sprague-Dawley males weighing 100-150 g) by 60 Hz alternating current (150 mA) delivered for 2 sec by corneal electrodes primed with an electrolyte solution containing an anesthetic agent (0.5% tetracaine HCl). Effects of 50-200 mg/kg 2DG p.o. against MES convulsions were evaluated at 15 min - 4 hrs after administration. Animals were considered protected from MES-induced seizures upon abolition of the hindlimb tonic extensor component of the seizure.15
Seizures Evoked by Subcutaneous Metrazol in Rats
Subcutaneous administration of 70 mg/kg of pentylenetetrazole (Metrazol) reliably induces convulsions with features of clonic spasms of limbs, jaws, and vibrissae in 97% of rats (adult Sprague-Dawley males weighing 100-150 g) (convulsive dose or CD97: 70 mg/kg). Clonic spasms of ~ 3-5 sec of the fore and/or hind limbs, jaws, or vibrissae were the endpoints, and animals which did not meet this endpoint criterion were considered protected. Rats were pretreated with 200-400 mg/kg of 2DG i.p. or 50-200 mg/kg of 2DG p.o. 30 minutes prior to administration of Metrazol for assessment of dose response. Rats received 50-200 mg/kg of 2DG i.p. for determination of time course of action against Metrazol-induced seizures at 1-24 hours after administration of 2DG. The time course of action of 30 mg/kg 2DG p.o. was evaluated at 30 minutes and 1 hour after administration.
Audiogenic Seizures in Fring’s Mice
Fring’s mice reliably exhibit seizures with tonic limb extension after exposure to a 110 dB, 11 kHz sound stimulus. Adult Fring’s mice (18-25 g) were placed into a round Plexiglas sound chamber and exposed to 110 dB, 11 kHz sound for 20 sec or until full tonic extension was noted. Animals not demonstrating tonic extension were considered protected. Dose response was determined by exposure to the sound stimulus at 15 minutes after administration of 2DG at a dose of 125-250 mg/kg i.p. Time to peak effect was determined by exposure to the sound stimulus at 15 min to 2 hr after administration of 2DG at a dose of 125-250 mg/kg i.p.
6 Hz Seizures in Mice
Seizures were induced in male albino mice via corneal stimulation (6 Hz, 0.2 ms rectangular pulse width, 3 sec duration at an intensity of 22mA) applied with 0.5% tetracaine corneal anesthesia.16 Seizures were characterized by stun, forelimb clonus, twitching of the vibrissae, and Straub-tail. Protection was defined as the absence of a seizure without toxicity. Dose response was determined by assessment of protection at 15 min after administration of 2DG at a dose of 15-200 mg/kg i.p. Time to peak effect was determined by assessing protection at 15 min - 4 hrs after administration of 2DG at a dose of 75-100 mg/kg i.p.
Kindling Procedures in Rats
Kindled seizures were evoked by stimulation of the olfactory bulb in Sprague-Dawley male rats (250-350 g). Rats were anesthetized with ketamine (80 mg/kg i.p.) and xylazine (10 mg/kg i.m.), and implanted with an insulated stainless steel bipolar electrode for stimulation and recording. The electrode was implanted in the olfactory bulb (coordinates in relation to bregma: 0.9 cm anterior, 0.12 cm lateral, 0.18 cm ventral) and was fixed to the skull with acrylic. A screw inserted into the skull served as ground. These rats were compared with another group of rats reported previously6 that was implanted with electrodes in the perforant path (coordinates in relation to bregma: 0.81 cm posterior, 0.44 cm lateral, 0.35 cm ventral) and otherwise treated identically. After a 1-week recovery period, both groups of unrestrained, awake, implanted rats received twice daily kindling stimulations (5 days per week) with a one-second train of 60-Hz biphasic constant current 1-msec square wave pulses as described below. The EEG was recorded from the bipolar electrode, which was switched to the stimulator for the delivery of kindling stimulations.
On the first day of stimulation, the afterdischarge threshold (ADT) was determined in each rat by delivery of a series of stimulus trains ranging from 100-1100 μA. If the initial stimulus intensity (100 μA) evoked an AD, that intensity was used in subsequent twice daily stimulations. If an AD was not evoked by 100 μA, the stimulation current was increased sequentially to 200, 300, 400, 500, 700, 900, 1000, or 1100 μA, until an AD was evoked. If 1100 μA failed to evoke an AD, stimulation was continued on subsequent days at 1100 μA. The current intensity that initially evoked an AD was used for subsequent twice daily stimulations and was systematically modified according to the following protocol to deliver stimulation at the lowest intensity required to evoke an AD. If an AD was evoked by 3 consecutive stimulations at a given intensity, the stimulation was decreased in 100 μA decrements. If an AD was not evoked after 3 stimulations, the stimulation was increased by 100 μA increments to a maximum of 1500 μA. If 3 stimulations at 100 μA caused an AD, the stimulation was decreased to 70 μA and then to 50 μA.
Evoked behavioral seizures were classified according to standard criteria ranging from class I (behavioral arrest) to class V seizures (bilateral tonic-clonic motor activity with rearing and loss of postural tone),17 which are comparable to human complex partial seizures with secondary generalization. The initial ADT, determined as above, served as the baseline for comparison of the effects of repeated kindling stimulations on ADT and the effect of 2DG treatment. After the 3rd evoked AD, rats were randomized to receive either 2DG 250 mg/kg i.p. or an equal volume of normal saline i.p. 30 min prior to each kindling stimulation.
Analysis and Statistical Procedures
To examine the time course of 2DG effects on the kindling ADT and to allow inter-group comparisons, stimulation intensities for each rat were divided by the intensity required to evoke the baseline ADs and were plotted as a function of AD number. Rats received twice daily kindling stimulations until at least 3 tonic clonic seizures with secondary generalization (Class V) were evoked. All data are presented as the mean ± standard error of the mean (S.E.M.). Differences across groups were analyzed with the Tukey and Holm-Sidak tests for analysis of variance (ANOVA) or the Student’s t-test when appropriate. Differences with confidence levels p < 0.05 were considered significant.
RESULTS
Anticonvulsant Effects of Glycolytic Inhibition on Epileptiform Bursting in Hippocampal Slices
To investigate the possibility that glucose and glycolysis contribute to the maintenance of epileptiform activity, the effect of glucose withdrawal on interictal burst frequency was evaluated in the CA3 region of rat hippocampus. Hippocampal slices bathed in 7.5 mM [K+]o developed brief epileptiform bursts consisting of spontaneous rhythmic high amplitude extracellular positive potentials with superimposed bursts of negative population spikes followed by a prolonged extracellular negativity consistent with interictal discharges (Fig 2A, also inset). These bursts are defined here as interictal based on terminology used in previous literature.10, 11 After establishing the baseline burst firing rate during a period of 30 min of recording in 10 mM glucose (~24.0 ± 1.8 bursts per min), the bathing solution was altered by removal of glucose and isomolar substitution of 10 mM lactate as an alternative energy source (Fig 2A) for the tricarboxylic acid cycle via pyruvate. After 30 min exposure to ACSF containing 10 mM lactate with no added glucose, there was a 60% decrease in burst discharge rate to 9.2 ± 1.8 per min (Fig 2B, n = 21 slices from 8 rats). Removal of lactate and return to 10 mM glucose in the ACSF restored the initial burst frequency, confirming that the reduction in burst discharges in 10 mM lactate was not caused by reduced viability of the slices. When glucose was again removed from the ACSF and replaced with 10 mM pyruvate, which directly inhibits phosphofructokinase and subsequent steps of glycolysis (Fig 1B), burst frequency after 30 minutes decreased to 10.0 ± 3.3 per min, and burst frequency again recovered after washout of pyruvate and re-introduction of 10 mM glucose (Fig 2A,B, n = 17 slices from 8 rats). These data indicate that reducing glycolysis has an anticonvulsant effect via decreased neuronal excitability by reducing high [K+]o–induced interictal epileptiform bursts. These results imply that compounds that inhibit glycolysis might also have an anticonvulsant effect.
FIGURE 2.
Anticonvulsant effects of removal of glucose and substitution with alternative energy sources lactate or pyruvate on interictal burst firing induced by 7.5 mM [K+]O. (A) Extracellular recording of spontaneous interictal discharges in hippocampal area CA3 (1 to 4 month old rats) in standard ACSF with 10 mM glucose. Faster sweep speed in the inset demonstrates that the discharges consisted of spontaneous extracellular depolarizations with superimposed population spikes. Removal of glucose and substitution with 10 mM lactate reduced the frequency of interictal discharges. Washout of lactate and return to standard ACSF containing 10 mM glucose increased interictal discharges, which were subsequently reduced by removal of glucose and substitution with 10 mM pyruvate, a direct inhibitor of glycolysis. (B) Effects of removal of glucose and isomolar substitution of 10 mM lactate or 10 mM pyruvate as illustrated in (A) from recordings in 21 hippocampal slices from 8 rats. Asterisks indicate significant differences.
To test this hypothesis, the glycolytic inhibitor 2DG was added to hippocampal slices bathed in 7.5 mM [K+]o and 10 mM glucose. An example of bath application of 10 mM 2DG is provided in Fig 3. Fig 3A shows an example of spontaneous epileptiform field bursts recorded in CA3 in 7.5 mM [K+]o and10 mM glucose. Burst frequency was reduced at 30 minutes after addition of 10 mM 2DG. At 30 minutes after washout (return to normal ACSF with 10 mM glucose; bottom trace), the effects of 2DG are reversed. The overall effect of 2DG on interictal burst frequency is illustrated in Fig 3B. Burst frequency was reduced by ~ 40% after bath application of 10 mM 2DG (26.5 ± 3.5 to 15.3 ± 2.3 per min; n = 8 slices from 5 rats). While partial washout was observed after removal of 2DG from the ACSF, burst frequency remained reduced at 60 minutes after bath application suggesting that the effects of 10 mM 2DG might be long-lasting. Bath application of lower concentrations of 2DG (1 mM, 5 mM) had no effect on interictal burst frequency, even 1 hour after bath application (data not shown). To determine if the reduction in interictal burst frequency was caused by an action of 2DG, not simply by the effects of increased extracellular osmolality, the bath concentration of glucose was increased to 20 mM for 30 minutes; there was no effect on burst frequency (Fig 3C, n = 5 slices from 3 rats; p=0.966 by t-test), excluding a role of increased osmolality in the reduction of burst frequency.
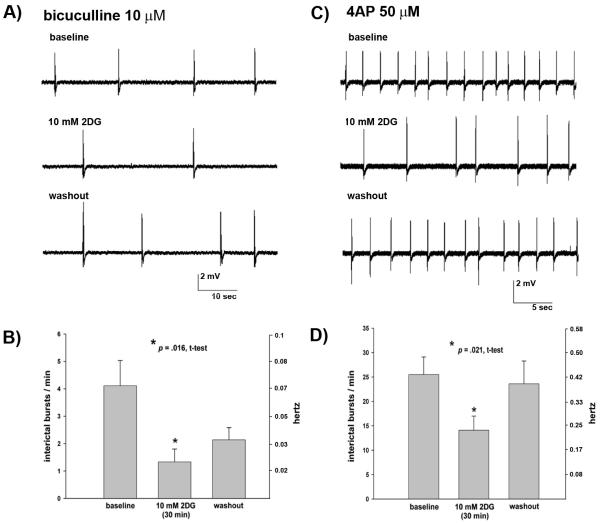
FIGURE 3.
Actions of 2DG on spontaneous interictal and ictal epileptiform bursts induced in hippocampal area CA3 by 7.5 mM [K+]O. (A) Representative example of reversible effects of 10 mM 2DG in reducing frequency of spontaneous interictal discharges. The inset shows an interictal burst at faster sweep speed. (B) Effects of 30 min of bath application of 10 mM 2DG in 8 hippocampal slices from 5 rats. There was partial washout after return to standard ACSF. (C) Effect of increasing bath concentration of glucose from 10 to 20 mM. After 30 minutes of application of 20 mM glucose, there was no change in interictal burst frequency (n = 5 slices from 3 rats). (D) Examples of spontaneous ictal discharges in CA3 consisting of a prolonged extracellular DC shift with superimposed high frequency spike discharges which were observed in a subset of hippocampal slices exposed to 7.5 mM [K+]O. Slower sweep speed in lower trace demonstrates rhythmicity of the spontaneous ictal discharges. (E) Anticonvulsant effect of 30 min bath application of 10 mM 2DG on ictal discharges (n = 7 hippocampal slices from 5 rats). Interictal data (A, B, C above) are from 1 to 4 month old rats. Ictal data (D, E above) are from 10 to 13 day old rats.
In the CA3 region of hippocampal slices from young rats, elevated [K+]o produces longer spontaneous events of neural firing defined as electrographic seizures.10, 11 In our experiments, these ictal-like discharges occurred in a subset of slices exposed to 7.5 mM [K+]o from 10-13 day old rats, and consisted of a prolonged high frequency discharges superimposed on an extracellular field negativity. They developed after a series of briefer interictal epileptiform burst discharges increased in amplitude. An example of an ictal burst discharge recorded from CA3 in a hippocampal slice exposed to 7.5 mM [K+]o (from a 10 day old rat) is illustrated in Fig 3D. Ictal discharges were reliably reduced by bath exposure to 10 mM 2DG (0.5 ± 0.07 per min to 0.2 ± 0.02 per min in 10 mM 2DG, p = 0.002, Fig 3E, n = 7 slices from 5 rats). Although CA3 ictal discharges can decrease over time (“run down”),18 our observations are sufficiently numerous and stable to suggest a reproducible effect. Washout did not abate ictal discharges.
Bath application of 10 mM 2DG also reduced interictal burst discharges in CA3 of hippocampal slices (from 1 to 4 month old rats) exposed to the GABAA receptor antagonist, bicuculline (10μM). Interictal epileptiform burst discharges were reduced by ~ 60% after 30 min bath application of 10 mM 2DG, and suppressive effects of 2DG persisted after washout (Fig 4A,B, n = 9 slices from 4 rats). Interictal bursts in CA3 in hippocampal slices (from 1 to 4 month old rats) exposed to the potassium channel blocker 4-aminopyridine (4-AP) (50-100 μM) were also reversibly decreased by 30 min of bath application of 10 mM 2DG (Fig 4C,D, n = 13 slices from 6 rats).
FIGURE 4.
Effects of 10 mM 2DG on CA3 interictal bursts induced in hippocampal slices (from 1 to 4 month old rats) by 10 μM bicuculline or 50-100 μM 4AP. (A) Bath application of 10mM 2DG for 30 min reduced interictal burst discharges (middle trace) compared to baseline (upper trace). Bursts persisted after return to normal ACSF (lower trace). (B) Effects of 30 min bath application of 10 mM 2DG on interictal bursts induced by 10 μM bicuculline (n = 9 hippocampal slices from 4 rats). (C) Effects of 30 min bath application of 10 mM 2DG on interictal bursts induced by 50 μM 4AP. Interictal burst frequency increased after washout of 2DG and return to normal ACSF (lower trace). (D). Overall effects of 30 min bath application of 10 mM 2DG on interictal bursts induced by 50-100 μM 4AP (n = 13 hippocampal slices from 6 rats).
Overall, these results demonstrate that 2DG suppresses interictal epileptiform discharges and ictal electrographic burst discharges induced in vitro in the hippocampal CA3 region by a variety of convulsant mechanisms. While some observations have suggested that reduced activity in CA3 is associated with ictal activity in the disrupted circuitry of parahippocampal slices,19, 20 the suppressive effects of 2DG against both interictal and ictal local network synchronization in CA3 are consistent with an overall anticonvulsant effect in this region.21
Effects of 2DG on Seizures Evoked by 6 Hz Stimulation in Mice
Protection against 6 Hz evoked seizures was observed at 15 minutes after administration of 2DG at doses of 75 mg/kg i.p. in six of eight mice, after a dose of 200 mg/kg i.p. in five of eight mice, and after a dose of 300 mg/kg six of eight mice (Figure 5A). The calculated dose of 2DG administered i.p. that resulted in protection for 50% of rats (ED50) was 79.7 mg/kg. The time to peak action of 2DG was 15 minutes at a dose of 75 mg/kg i.p. and 1 hr at a dose of 100 mg/kg i.p.
FIGURE 5.
(A) Effects of 2DG on 6-Hz seizures in mice. Percent of mice protected at a stimulus of 22 mA is shown as a function of intraperitoneal 2DG dose. The ED50 is 79.5 mg/kg. (B) Effects of 2DG on audiogenic seizures in Fring’s mice. Percent of mice protected 2 hrs after 2DG administration is shown as a function of intraperitoneal 2DG dose. The ED50 is 206.4 mg/kg.
Effects of 2DG on Audiogenic Seizures in Fring’s Mice
2DG protected against audiogenic seizures in four of eight mice at 2 hr after administration of 220 mg/kg i.p. and in seven of eight mice after 250 mg/kg for a calculated ED50 of 206.4 mg/kg (Figure 5B). The time to peak effect was 2 hr at both of these doses.
Effects of 2DG on Seizures Evoked by Metrazol in rats
There was also some evidence suggestive of anticonvulsant activity of 2DG against seizures evoked by Metrazol (Table 1). Two of eight rats were protected against Metrazol evoked seizures at 30 min after a 2-DG dose of 200 mg/kg i.p., and three of eight were protected after administration of 2-DG 400 mg/kg i.p. Four of twelve rats were protected at 30 min after a dose of 50 mg/kg p.o., but only two of eight were protected at a dose of 100 mg/kg p.o. and only one of eight after a dose of 200 mg/kg p.o. While suggesting that 2DG might have some anticonvulsant activity against seizures evoked by Metrazol, the overall results were not sufficient to calculate an ED50 or a time to peak effect.
TABLE 1.
Metrazol Seizures
| Subcutaneous Metrazol: dose response at 30 min after oral 2DG | |||
|---|---|---|---|
| 2DG dose | 50 mg/kg PO |
100 mg/kg PO |
200 mg/kg PO |
| rats protected |
4/12 | 2/8 | 1/8 |
| Subcutaneous Metrazol: time to peak effect after oral 2DG | ||
|---|---|---|
| time of administration of 50 mg/kg 2DG |
30 min | 1 hr |
| rats protected | 0/8 | 0/8 |
| Subcutaneous Metrazol: dose response at 30 min after intraperitoneal 2DG | ||
|---|---|---|
| 2DG dose | 200 mg/kg IP | 400 mg/kg IP |
| rats protected |
2/8 | 3/8 |
| Subcutaneous Metrazol: time course of action of intraperitoneal 2DG | |||||
|---|---|---|---|---|---|
| time of administration of 2DG |
2 hr | 4 hr | 6 hr | 8hr | 24 hr |
| rats protected (50 mg/kg IP) |
0/4 | 1/4 | 0/4 | 0/4 | 2/4 |
| rats protected (100 mg/kg IP) |
0/4 | 2/4 | 1/4 | 1/4 | 1/4 |
| rats protected (200 mg/kg IP) |
- | 1/8 | - | - | - |
Effects of 2DG on Seizures Evoked by Maximal Electroshock (MES) in Rats
There were no protective effects against MES in rats at 15 min to 4 hrs after 2DG administered at doses of 100-200 mg/kg p.o. (Table 2).
TABLE 2.
Maximal Electroshock Seizures (MES)
| MES: Dose response and time course of action | |||||
|---|---|---|---|---|---|
| 15 min | 30 min | 1 hr | 2 hr | 4 hr | |
| 100 mg/kg PO | 0/4 | 0/4 | 1/4 | 0/4 | 0/4 |
| 200 mg/kg PO | - | - | 0/8 | - | - |
Anticonvulsant and Antiepileptic Effects of 2DG on Seizures Evoked by Kindling
In a previous report,6 administration of 2DG at a dose of 250 mg/kg i.p. 30 min prior to stimulation of the perforant path had anticonvulsant effects manifested by increases in the ADT. This finding contrasts with the gradual decrease in ADT in untreated animals. The anticonvulsant effects of 2DG were further assessed here by examining the effects of 2DG on the ADTs and progression of seizures evoked by kindling of the olfactory bulb. After initial determination of the ADT using the standardized protocol described in Methods, trains of 60 Hz stimulation were delivered twice daily to the olfactory bulb. After 3 ADs were evoked, the rats were randomized to groups that received either 2DG (250 mg/kg i.p.) or saline i.p. 30 min prior to each kindling stimulation. The effects of 2DG were compared to the saline-injected controls and to the previously reported groups of rats that experienced seizures evoked by kindling of the perforant path and treatment with 2DG using the same protocols. The ADTs across animals and groups were normalized by expressing the current required to evoke each AD as a percentage of the initial average current required to evoke ADs in each animal prior to randomization.
The mean ADTs for each group are plotted as a function of the number of evoked seizures in Fig 6. The mean initial ADT for the first 3 evoked ADs for rats treated with 2DG was not significantly different than the ADT for saline treated rats (766.7 ± 105.4 μA vs. 516.7 ± 155.8 μA for olfactory bulb, p = 0.19, t-test; 693.3 ± 85.9 vs. 866.7 ± 88.2 for perforant path, p = 0.176, t-test). The mean ADT of rats treated with saline prior to delivery of kindling stimulation to the perforant path or olfactory bulb exhibited a gradual and persistent reduction as a function of the number of evoked seizures as expected during the progression of evoked kindled seizures. There was no effect of 2DG on the ADT of rats receiving kindling stimulation to the olfactory bulb, which contrasts with the increase in ADT in rats treated with 2DG on experiencing seizures evoked by kindling of the perforant path (Fig 6). These results indicate that the anticonvulsant effect of 2DG on the ADT appears to be specific to the site of stimulation and suggest that the anticonvulsant mechanisms of 2DG may be differentially effective in particular regions of limbic circuitry.
FIGURE 6.
Effects of 2DG on afterdischarge (AD) threshold in rats experiencing kindled seizures evoked by stimulation of the olfactory bulb and perforant path. 2DG (250 mg/kg i.p.) was administered 30 min prior to delivery of stimulation (see text for details of normalization and stimulation methods). Treatment with 2DG increased the normalized mean AD threshold (filled triangles) in rats receiving perforant path stimulation compared to saline treated controls, which demonstrated a reduction in AD threshold (filled circles, reported previously6). In contrast, there were no effects of 2DG on AD threshold of rats receiving stimulation of the olfactory bulb (open triangles) compared to saline treated controls (open circles).
Our previous study also showed that 2DG slowed of the rate of perforant path kindled seizure progression to the milestone of secondary generalized tonic clonic (Class V) seizures.6 The number of evoked ADs required to reach the milestones of class III, IV and V seizures is plotted in Fig 7 for both the previous perforant path data6 and for olfactory bulb stimulations. Rats treated with 2DG required ~50% more ADs evoked by stimulation of the olfactory bulb to reach the first class III or IV seizure or the 1st, 2nd , or 3rd class V seizure (p=0.019, n = 9 2DG, n = 6 Sal). In rats treated with 2DG prior to kindling stimulation of the perforant path, the rate of achieving the milestones of Class III, IV, and V seizures was similarly slowed as indicated by ~50% more ADs required to reach each of these stages (n = 15 2DG, n = 12 Sal). There was no difference in the mean AD duration between 2DG-treated rats and saline-injected controls in either the olfactory bulb or perforant path groups (data not shown), which implies that the reduced progression of seizures in the 2DG treated groups was not merely a result of cumulative anticonvulsant effects of 2DG on ADT.
FIGURE 7.
Antiepileptic effects of 2DG (250 mg/kg i.p.) on kindling progression in rats experiencing seizures evoked by perforant path or olfactory bulb stimulation. Number of ADs required to achieve behavioral seizure stages of class III, class IV, and the first to third class V seizures in response to kindling of the olfactory bulb (A) or perforant path (B). There was a ~ 2-fold slowing in the rate of number of AD required to reach each stage in animals treated with 2DG for both olfactory bulb and perforant path stimulation.
DISCUSSION
These experiments demonstrate that 2DG, a glucose analogue used for decades as the positron emitting tracer 18F-2DG for examination of regional glucose uptake, has acute anticonvulsant and chronic antiepileptic effects in a variety of in vitro and in vivo models of seizures and epilepsy. 2DG reduced the frequency of both interictal epileptiform bursts and ictal electrographic seizures induced by 7.5mM [K+]o in the CA3 region of hippocampal slices, and also reduced interictal epileptiform bursts in CA3 evoked by bath application of 4-AP, a K+ channel antagonist, and bicuculline, a GABAA receptor antagonist.
Inhibition of glycolysis may account for at least some of the reduction in neuronal excitability underlying the acute anticonvulsant action of 2DG in hippocampal slices, since blocking glycolysis by isomolar replacement of glucose with alternative energy sources such as pyruvate or lactate also suppressed epileptic discharges evoked by 7.5 mM [K+]o.
In vivo, 2DG demonstrated chronic antiepileptic effects against kindling progression in response to stimulation of the olfactory bulb, confirming a previous observation of action against seizure progression by stimulation of the perforant path.6 2DG also acutely protected against seizures evoked in vivo by 6 Hz stimulation in mice and against audiogenic seizures in Fring’s mice. It showed only minimal evidence of anticonvulsant activity against seizures evoked by Metrazol and had no protective effects against MES seizures. These results provide evidence that 2DG has acute and chronic anticonvulsant and antiepileptic properties in several preclinical models. The spectrum of 2DG’s effectiveness against seizures evoked by 6 Hz, audiogenic, and kindling stimulation is distinctive relative to other available anticonvulsants. Although the suppression of seizures by 2DG in these models was not complete, many well established anticonvulsants do not confer complete protection or demonstrate effectiveness in all preclinical screening models. Partial but significant protection in various subsets of models implies potential for effectiveness in clinical trials in patients. While none of the currently used screening models have been formally validated as reliable predictors of anticonvulsant action in people, there is consensus that identification of compounds with distinctive profiles of action in the models compared to available drugs is desirable and may be valuable for developing new drugs that are more effective than the currently marketed agents.22 23 24 25 With this unique spectrum of protection in these animal models of epilepsy, 2DG has potential as a therapy for epilepsy with novel mechanisms of action compared to currently available anticonvulsants.
Acute In Vivo Anticonvulsant Actions of 2DG
Protection against minimal clonic seizures evoked by 6 Hz stimulation in mice, regarded as a model of “psychomotor” seizures,16 was observed at 15 min - 1 hr after intraperitoneal administration. Anticonvulsant effects against audiogenic seizures in Fring’s mice, which are generated in part by auditory circuitry in the brainstem, were observed at 1- 2 hr after intraperitoneal administration. Anticonvulsant properties at these short term intervals after in vivo administration are unlikely to be dependent on altered neuronal gene expression. Furthermore, 2DG had rapid acute onset of anticonvulsant action against interictal and ictal discharges in CA3 within minutes after bath application, suggesting that its acute anticonvulsant effects are likely to be operating at the membrane or synaptic levels, or possibly through alterations in second messenger pathways influenced acutely by metabolic effects of 2DG such as inhibition of glycolysis. As 2DG suppressed interictal and ictal events induced by distinctive mechanisms such as depolarization by elevated [K+]o, blockade of potassium channels by 4-AP, and antagonism of GABAA receptors by bicuculline, its acute anticonvulsant actions appear to be broadly suppressive against a variety of cellular and membrane processes generating network synchronization.
Chronic In Vivo Effects Against Progression of Kindled Seizures
These experiments confirmed that the previously reported antiepileptic effect of 2DG against progression of kindled seizures evoked by perforant path stimulation is also observed when kindled seizures are evoked by stimulation of the olfactory bulb. The rate of progression to the first evoked Class V seizure was approximately doubled when 2DG was administered at 30 min prior to stimulation of either the perforant path or the olfactory bulb, indicating that 2DG interferes with neuronal mechanisms underlying seizure progression in limbic circuitry. As temporal lobe epilepsy is the most common form of intractable drug-resistant epilepsy, 2DG could potentially modify the progressive course of seizure-induced alterations in limbic circuitry in that disorder.
While protective effects of 2DG against progression of kindled seizures were observed with either perforant path or olfactory bulb stimulation, differences were observed as a function of the site of seizure induction. ADT was increased by 2DG in perforant path kindling but not in olfactory bulb kindling. The increase in the ADT in response to perforant path stimulation induced by 2DG is an anticonvulsant effect, as elevation of the ADT indicates that seizure induction requires stimulation of increased intensity.6 The ADT typically decreases as kindling progresses, so the observation of an increase in the ADT in perforant path kindling indicates that 2DG modifies progressive seizure-induced plasticity that is normally a part of the kindling process. However, the anticonvulsant effect against ADT was not observed in the olfactory bulb, indicating that at least some of the effects of 2DG are region-specific. The reasons for this difference are uncertain, but one possibility is that 2DG might have different effects on excitability or seizure-induced plasticity in the distinctive circuitries of the perforant path and olfactory bulb. None of the currently available conventional anticonvulsants demonstrates such regionally specific properties.
Possible Mechanisms Underlying the Acute Anticonvulsant and Chronic Antiepileptic Actions of 2DG
The observations that 2DG acutely protects against seizures evoked by 6 Hz stimulation in mice and audiogenic seizures in Fring’s mice and rapidly suppresses hippocampal interictal epileptiform bursts and electrographic seizures in vitro, suggest that 2DG has acute anticonvulsant properties in addition to its chronic antiepileptic effects against kindling progression. These two actions could involve different cellular and molecular mechanisms.
The chronic antiepileptic effects of 2DG have been associated with repression of BDNF and trkB expression. Conditional knockout of BDNF slowed kindling and conditional knockout of trkB blocked kindling progression.8 The repression by 2DG of seizure-induced increases in BDNF and trkB is mediated by the transcriptional repressor NRSF and its NADH sensitive co-repressor CtBP acting at the promoter regions of BDNF and its receptor, trkB.6 In pathophysiological conditions of high neuronal activity such as seizures, which increase glucose uptake and glycolysis, increases in NADH dissociate CtBP from NRSF and decrease repression, resulting in increased expression of BDNF and trkB. In the presence of 2DG, which reduces NADH levels as a consequence of glycolytic inhibition, the NRSF-CtBP complex maintains repression of BDNF and trkB, and kindling progression is slowed.
The rapid onset of anticonvulsant effects of 2DG suggest that this compound may be acting via different mechanisms, such as effects at the synaptic or membrane levels. Alternatively, the acute anticonvulsant actions of 2DG may be a result of unrecognized metabolic effects associated with glycolytic inhibition. For example, reduction of glycolysis might be accompanied by increases in systemic lipid metabolism and alterations in activity of the Kreb’s cycle and mitochondrial metabolism that could influence neuronal hyperexcitability. 2DG has been shown to increase neuronal resistance to oxidative and metabolic insults in cultured hippocampal neurons26 and to increase epileptic tolerance evoked by cerebral ischemia in mice.27 Glycolytic enzymes such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH) maintain GABAA responses by phosphorylation of the α1 subunits of GABAA receptors.28 While GAPDH and glycolytic intermediates can influence GABAA receptors, the suppressive effects of 2DG on burst discharges in vitro and seizures in vivo suggest that glycolysis must have other acute effects on cellular and synaptic mechanisms contributing to network synchronization.
2DG, the Ketogenic Diet, and Potential Anticonvulsant Effects of Altering Brain Energy Metabolism
This study was not intended to investigate cellular mechanisms underlying the KD, but rather to mimic some the diet’s seizure suppressive effects through modulation of glycolysis. Nevertheless, some of these findings may have relevance for the anticonvulsant action of the KD. Hypotheses for the KD mechanism of action include a direct or indirect effect of ketosis,29 improvement in neuronal energy reserves, 30, 31 enhancement of GABAergic inhibition,32 alteration of mitochondrial metabolism,33 upregulation of gene transcripts encoding energy metabolism enzymes,34 and effects of lipids on neuronal excitability.35, 36 Clinical evidence suggests that carbohydrate restriction has beneficial effects and that ingestion of even small amounts of carbohydrate can result in recurrence of seizures in patients who are well controlled on the KD.4 The possibility that decreased carbohydrate (glucose) availability plays a role in the effects of the KD has also been considered 37
Glucose is an obligate energy source for the brain under normal conditions, but in situations of low glucose availability (e.g., fasting or the high-fat, low-carbohydrate KD), other substances can provide the brain’s energy requirements. Alternative energy sources that can maintain brain function and synaptic activity include lactate, pyruvate and β-hydroxybutyrate.38-42 Pyruvate is also a potent inhibitor of glycolysis by direct feedback inhibition of phosphofructokinase (PFK), the rate limiting enzyme of glycolysis. In subjects on the KD, fatty acids are converted into ketone bodies (acetoacetate, β-hydroxybutyrate, acetone), and enter the brain via a monocarboxylate transporter.43 The current results and other studies implicate the reduction in carbohydrate availability or metabolism as feasible mechanisms for decreased neuronal excitability and enhanced seizure control.37, 44, 45 The ability of calorie restriction to afford seizure protection in EL mice supports the hypothesis that reduction in carbohydrate metabolism may play a beneficial role in the KD.46
2DG does not model the KD since 2DG does not produce ketosis. However, 2DG and the KD do share some anticonvulsant effects. Both the KD and 2DG reduce audiogenic seizures in Fring’s mice and in the 6 Hz model.47 The KD is effective in MES whereas 2DG is not. 2DG and the KD are both effective in kindling although experimental studies have utilized different protocols. In our experiments, ADT was determined by stimulation of the perforant path or olfactory bulb, followed by i.p. administration of 2DG 30 minutes before each kindling stimulation. Another study used a “rapid” amygdala kindling protocol and monitored ADT in rats already fully kindled on either KD or standard diet.48 While the KD and 2DG are clearly different therapies, these results support carbohydrate restriction as a potential anticonvulsant and antiepileptic strategy.
2DG Uptake, Metabolism and Storage: Implications for Anticonvulsant Development
Glucose is taken up by neurons and glia, both of which possess uptake transporters and glycolytic enzymes that metabolize glucose for energy. 49-51 As an analogue of glucose, 2DG enters cells through glucose transporters and its uptake is preferentially increased in cells with increased energy consumption and metabolic demands. This aspect of 2DG may be advantageous for enhanced delivery of 2DG in the specific regions of the brain that generate seizures. The activity-dependent uptake of 2DG in regions of brain with increased energy metabolism also implies that pharmacodynamic actions (including anticonvulsant actions) and potential toxicity are likely to be nonlinear in relation to serum levels.
Although 2DG-6P does not undergo isomerization and progress through subsequent steps of glycolysis, glycogen becomes radiolabeled after injection of radiolabeled 2DG through isomerization to 2DG-1P and conjugation to UDP-2DG, 2DG glycogen, and 2DG glycoproteins.52 As 2DG is incorporated into glycogen and glycosylated macromolecules presumably stored in astrocytes, it might be released when astrocytic glycogen is metabolized during states of high energy demand such as seizures. The possibility that 2DG may be stored for long periods in glycogen also raises questions about its potential for chronic toxicity.53 However, 2DG has been administrated safely to humans for decades as the PET imaging tracer 18F-2DG and has been administered to humans in doses as high as 1 gm/kg as adjuvant therapy for cancer,54, 55 suggesting that it has a relatively favorable preliminary toxicity profile for development as an anticonvulsant.
Conclusion
In summary, the experiments reported here demonstrate that 2DG has distinctive acute and chronic anticonvulsant therapeutic effects in preclinical in vivo models of seizures. The rapid onset of anticonvulsant suppressant action against burst discharges evoked in vitro by a variety of induction methods suggests that its anticonvulsant actions may be broad spectrum against a variety of mechanisms of network synchronization. The activity-dependent delivery of 2DG to brain regions with high metabolic activity, as occurs during seizures, further supports the clinical potential of this compound. Experiments are underway to further characterize the dose response and time course of anticonvulsant and antiepileptic actions of 2DG, and its effects on synaptic properties. Future experiments addressing the effects of 2DG on ATP-dependent K+ currents or other ion channels influencing neuronal excitability are of potential interest for understanding how glycolytic metabolism affects seizures and may contribute to the therapeutic actions of the KD.
Acknowledgements
This work was supported by grants from the NIH (RO1-25020, T.P.S.), Wisconsin Alumni Research Foundation, The Charlie Foundation (C.E.S.), and the NINDS Antiepileptic Screening Program. We gratefully acknowledge the contributions of Steven M. Kriegler, Ph.D. to some of the data presented in Figure 2, and to James Stables, MS, RPh of the NIH Antiepileptic Screening Program for assistance with the data presented in Figure 5 and Tables 1 and 2.
Footnotes
Statement of conflict of interest: C.E. Stafstrom, S.M. Kriegler, A. Roopra, and T.P. Sutula are inventors on a patent application on this work through the Wisconsin Alumni Research Foundation. T.P. Sutula has an equity interest in Neurogenomex, Inc., for preclinical development of 2-deoxy-D-glucose.
REFERENCES
- 1.Vining EPG. Clinical efficacy of the ketogenic diet. Epilepsy Res. 1999;37:181–190. doi: 10.1016/s0920-1211(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 2.Stafstrom CE, Bough KJ. The ketogenic diet for the treatment of epilepsy: a challenge for nutritional neuroscientists. Nutr Neurosci. 2003;6:67–79. doi: 10.1080/1028415031000084427. [DOI] [PubMed] [Google Scholar]
- 3.Bough KJ, Rho JM. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia. 2007;48:43–58. doi: 10.1111/j.1528-1167.2007.00915.x. [DOI] [PubMed] [Google Scholar]
- 4.Huttenlocher PR. Ketonemia and seizures: Metabolic and anticonvulsant effects of two ketogenic diets in childhood epilepsy. Pediatr Res. 1976;10:536–540. doi: 10.1203/00006450-197605000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Sutula TP, Ockuly J, Stafstrom CE, Roopra A. Novel anticonvulsant, antiepileptic properties and favorable toxicology profile of 2-deoxy-D-glucose (2DG) in experimental models of epilepsy. Epilepsia. 2006 [Google Scholar]
- 6.Garriga-Canut M, Schoenike B, Qazi R, et al. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nature Neurosci. 2006;9:1382–1387. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- 7.Wree A. Principles of the 2-deoxyglucose method for the determination of the local cerebral glucose utilization. Eur J Morphol. 1990;28:132–138. [PubMed] [Google Scholar]
- 8.He XP, Kotloski R, Nef S, et al. Conditional deletion of TrkB but not BDNF prevents epileptogenesis in the kindling model. Neuron. 2004;43:31–42. doi: 10.1016/j.neuron.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Rutecki PA, Lebeda FJ, Johnston D. Epileptiform activity induced by changes in extracellular potassium in hippocampus. J Neurophysiol. 1985;54:1363–1374. doi: 10.1152/jn.1985.54.5.1363. [DOI] [PubMed] [Google Scholar]
- 10.Traynelis S, Dingledine R. Potassium-induced spontaneous electrographic seizures in the rat hippocampal slice. J Neurophysiol. 1988;59:259–276. doi: 10.1152/jn.1988.59.1.259. [DOI] [PubMed] [Google Scholar]
- 11.Clark S, Wilson WA. Mechanisms of epileptogenesis. Adv Neurol. 1999;79:607–630. [PubMed] [Google Scholar]
- 12.McBain CJ, Traynelis SF, Dingledine R. High potassium-induced synchronous bursts and electrographic seizures. In: Schwartzkroin PA, editor. Epilepsy: models, mechanisms, and concepts. Cambridge University Press; Cambridge, U.K.: 1993. pp. 437–461. [Google Scholar]
- 13.Chamberlin NL, Dingledine R. Control of epileptiform burst rate by CA3 hippocampal cell afterhyperpolarizations in vitro. Brain Res. 1989;492:337–346. doi: 10.1016/0006-8993(89)90917-7. [DOI] [PubMed] [Google Scholar]
- 14.Traynelis SF, Dingledine R, McNamara JO, et al. Effect of kindling on potassium-induced electrographic seizures in vitro. Neurosci Lett. 1989;105:326–332. doi: 10.1016/0304-3940(89)90642-3. [DOI] [PubMed] [Google Scholar]
- 15.White HS, Johnson M, Wolf H, Kupferberg H. The early identification of anticonvulsant activity: role of the maximal electroshock and subcutaneous pentylenetetrazole models. Ital J Sci. 1995;16:73–77. doi: 10.1007/BF02229077. [DOI] [PubMed] [Google Scholar]
- 16.Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001;47:217–227. doi: 10.1016/s0920-1211(01)00302-3. [DOI] [PubMed] [Google Scholar]
- 17.Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 18.Dzhala VI, Staley KJ. Transition from interictal to ictal activity in limbic networks in vitro. J Neurosci. 2003;23:7873–7880. doi: 10.1523/JNEUROSCI.23-21-07873.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biagini G, D’Arcangelo G, Baldelli E, et al. Impaired activation of CA3 pyramidal neurons in the epileptic hippocampus. Neuromolec Med. 2005;7:325–342. doi: 10.1385/NMM:7:4:325. [DOI] [PubMed] [Google Scholar]
- 20.Biagini G, Baldelli E, Longo D, et al. Proepileptic influence of a focal vascular lesion affecting entorhinal cortex-CA3 connections after status epilepticus. J Neuropath Exp Neurol. 2008;67:687–701. doi: 10.1097/NEN.0b013e318181b8ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yee AS, Longacher M, Staley KJ. Convulsant and anticonvulsant effects of spontaneous CA3 populaiton bursts. J Neurophysiol. 2003;89:427–441. doi: 10.1152/jn.00594.2002. [DOI] [PubMed] [Google Scholar]
- 22.Klitgaard H, Matagne A, Gobert J, Wulfert E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur J Pharmacol. 1998;353:191–206. doi: 10.1016/s0014-2999(98)00410-5. [DOI] [PubMed] [Google Scholar]
- 23.Stables JP, Bertram EH, White HS, et al. Models for epilepsy and epileptogenesis: report from the NIH workshop, Bethesda, Maryland. Epilepsia. 2002;42:1410–1420. doi: 10.1046/j.1528-1157.2002.06702.x. [DOI] [PubMed] [Google Scholar]
- 24.White HS. Preclinical development of antiepileptic drugs: past, present, and future directions. Epilepsia. 2003;44(Suppl 7):2. doi: 10.1046/j.1528-1157.44.s7.10.x. [DOI] [PubMed] [Google Scholar]
- 25.Holmes GL, Zhao Q. Choosing the correct antiepileptic drugs: from animal studies to the clinic. Pediatr Neurol. 2008;38:151–162. doi: 10.1016/j.pediatrneurol.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J, Bruce-Keller AJ, Kruman Y, et al. 2-Deoxy-D-glucose protects hippocampal neurons against excitotoxic and oxidative injury: evidence for the involvement of stress proteins. J Neurosci Res. 1999;57:48–61. doi: 10.1002/(SICI)1097-4547(19990701)57:1<48::AID-JNR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 27.Rejdak K, Rejdak R, Sieklucka-Dziuba M, et al. 2-Deoxyglucose enhances epileptic tolerance evoked by transient incomplete brain ischemia in mice. Epilepsy Res. 2001;43:271–278. doi: 10.1016/s0920-1211(01)00184-x. [DOI] [PubMed] [Google Scholar]
- 28.Laschet JJ, Minier F, Kurcewicz I, et al. Glyceraldehyde-3-phosphate dehydrogenase is a GABA-A receptor kinase linking glycolysis to neuronal inhibition. J Neurosci. 2004;24:7614–7622. doi: 10.1523/JNEUROSCI.0868-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostagl Leukotr Essen Fatty Acids. 2004;70:309–319. doi: 10.1016/j.plefa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 30.DeVivo DC, Leckie MP, Ferrendelli JS, McDougal DB. Chronic ketosis and cerebral metabolism. Ann Neurol. 1978;3:331–337. doi: 10.1002/ana.410030410. [DOI] [PubMed] [Google Scholar]
- 31.Gasior M, Rogawski MA, Hartman AL. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav Pharmacol. 2006;17:431–439. doi: 10.1097/00008877-200609000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yudkoff M, Daikhin Y, Nissim I, Nissim I. The ketogenic diet: interactions with brain amino acid handling. In: Stafstrom CE, Rho JM, editors. Epilepsy and the Ketogenic Diet. Humana Press; Totowa, NJ: 2004. pp. 185–199. [Google Scholar]
- 33.Sullivan PG, Rippy NA, Dorenbos K, et al. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann Neurol. 2004;55:576–580. doi: 10.1002/ana.20062. [DOI] [PubMed] [Google Scholar]
- 34.Bough KJ, J. W, Hassel B, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–235. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- 35.Cunnane SC, Musa K, Ryan MA, et al. Potential role of polyunsaturates in seizure protection achieved with the ketogenic diet. Prostagl Leukotr Essen Fatty Acids. 2002;67:131–135. doi: 10.1054/plef.2002.0409. [DOI] [PubMed] [Google Scholar]
- 36.Cullingford TE. The ketogenic diet; fatty acids, fatty acid-activated receptors and neurological disorders. Prostagl Leukotr Essen Fatty Acids. 2004;70:253–264. doi: 10.1016/j.plefa.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Greene AE, Todorova MT, Seyfried TN. Perspectives on the metabolic management of epilepsy through dietary reduction of glucose and elevation of ketone bodies. J Neurochem. 2003;86:529–537. doi: 10.1046/j.1471-4159.2003.01862.x. [DOI] [PubMed] [Google Scholar]
- 38.Schurr A, West CA, Rigor BM. Lactate-supported synaptic function in the rat hippocampal slice. Science. 1988;240:1326–1328. doi: 10.1126/science.3375817. [DOI] [PubMed] [Google Scholar]
- 39.Arakawa T, Goto T, Okada Y. Effect of ketone body (D-3-hydroxybutyrate) on neural activity and energy metabolism in hippocampal slices of the adult guinea pig. Neurosci Lett. 1991;130:53–56. doi: 10.1016/0304-3940(91)90225-i. [DOI] [PubMed] [Google Scholar]
- 40.Wada H, Okada Y, Nabetani M, Nakamura H. The effects of lactate and beta-hydroxybutyrate on the energy metabolism and neural activity of hippocampal slices from adult and immature rat. Dev Brain Res. 1997;101:1–7. doi: 10.1016/s0165-3806(97)00007-2. [DOI] [PubMed] [Google Scholar]
- 41.Izumi Y, Ishii K, Katsuki H, et al. Beta-hydroxybutyrate fuels synaptic function during development: histological and physiological evidence in rat hippocampal slices. J Clin Invest. 1998;101:1121–1132. doi: 10.1172/JCI1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erecinska M, Cherian S, Silver IA. Energy metabolism in mammalian brain during development. Prog Neurobiol. 2004;73:397–445. doi: 10.1016/j.pneurobio.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Leino RL, Gerhart DZ, Duelli R, et al. Diet-induced ketosis increases monocarboxylate transporter (MCT1) levels in rat brain. Neurochem Int. 2001;38:519–527. doi: 10.1016/s0197-0186(00)00102-9. [DOI] [PubMed] [Google Scholar]
- 44.Kirchner A, Veliskova J, Velisek L. Differential effects of low glucose concentrations on seizures and epileptiform activity in vivo and in vitro. Eur J Neurosci. 2006;23:1512–1522. doi: 10.1111/j.1460-9568.2006.04665.x. [DOI] [PubMed] [Google Scholar]
- 45.Melo TM, Nehlig A, Sonnewald U. Neuronal-glial interactions in rats fed a ketogenic diet. Neurochem Int. 2006;48:498–507. doi: 10.1016/j.neuint.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 46.Todorova MT, Tandon P, Madore RA, et al. The ketogenic diet inhibits epileptogenesis in EL mice: a genetic model for idiopathic epilepsy. Epilepsia. 2000;41:933–940. doi: 10.1111/j.1528-1157.2000.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 47.Hartman AL, Lyle M, Rogawski MA, Gasior M. Efficacy of the ketogenic diet in the 6-Hz seizure test. Epilepsia. 2008;49:334–339. doi: 10.1111/j.1528-1167.2007.01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hori A, Tandon P, Holmes GL, Stafstrom CE. Ketogenic diet: effects on expression of kindled seizures and behavior in adult rats. Epilepsia. 1997;38:750–758. doi: 10.1111/j.1528-1157.1997.tb01461.x. [DOI] [PubMed] [Google Scholar]
- 49.Itoh Y, Abe T, Takaoka R, Tanahashi N. Fluorometric determination of glucose utilization in neurons in vitro and in vivo. J Cereb Blood Flow Metab. 2004;24:993–1003. doi: 10.1097/01.WCB.0000127661.07591.DE. [DOI] [PubMed] [Google Scholar]
- 50.Nehlig A, Coles JA. Cellular pathways of energy metabolism in the brain: is glucose used by neurons or astrocytes? Glia. 2007;55:1238–1250. doi: 10.1002/glia.20376. [DOI] [PubMed] [Google Scholar]
- 51.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newman G, Hospod F, Patlak C. Kinetic model of 2-deoxyglucose metabolism using brain slices. J Cereb Blood Flow Metab. 1990;10:510–526. doi: 10.1038/jcbfm.1990.93. [DOI] [PubMed] [Google Scholar]
- 53.Gruetter R. Glycogen: the forgotten cerebral energy store. J Neurosci Res. 2003;74:179–183. doi: 10.1002/jnr.10785. [DOI] [PubMed] [Google Scholar]
- 54.Aft RL, Zhang FW, Gius D. Evaluation of 2-deoxy-D-glucose as a chemotherapeutic agent: mechanism of cell death. Brit J Cancer. 2002;87:805–812. doi: 10.1038/sj.bjc.6600547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mohanti BK, Rath GK, Anantha N, et al. Improving cancer radiotherapy with 2-deoxy-D-glucose: phase I/II clinical trials on human cerebral gliomas. Int J Radiat Oncol Biol Phys. 1996;35:103–111. doi: 10.1016/s0360-3016(96)85017-6. [DOI] [PubMed] [Google Scholar]