Abstract
Sickle cell disease (SCD), a genetically-determined pathology due to an amino acid substitution (i.e., valine for glutamic acid) on the beta-chain of hemoglobin, is characterized by abnormal blood rheology and periods of painful vascular occlusive crises. Sickle cell trait (SCT) is a typically benign variant in which only one beta chain is affected by the mutation. Although both SCD and SCT have been the subject of numerous studies, information related to neurological function and transfusion therapy is still incomplete: an overview of these areas is presented. An initial section provides pertinent background information on the pathology and clinical significance of these diseases. The roles of three factors in the clinical manifestations of the diseases are then discussed: hypoxia, autonomic nervous system regulation and blood rheology. The possibility of a causal relationship between these three factors and sudden death is also examined. It is concluded that further studies in these specific areas are warranted. It is anticipated that the outcome of such research is likely to provide valuable insights into the pathophysiology of SCD and SCT and will lead to improved clinical management and enhanced quality of life.
Keywords: Sickle cell disease, autonomic nervous function, transfusion therapy
1. Introduction
Sickle cell disease (SCD) is one of the most common genetic disorders that affects 1/400 individuals of African descent as well as people of Arab, Indian and Hispanic descents. It was the first genetic disorder in which the molecular abnormality, i.e., a single point mutation, was precisely defined: the normal codon GAG at position beta-6 is replaced by GUG, inserting a hydrophobic valine in place of a glutamic acid. This leads to the synthesis of sickle hemoglobin S (HbS) rather than the normal hemoglobin A (HbA). HbS undergoes solgel transformation under low oxygen tensions, which results in polymer formation within the red blood cells (RBC), with the kinetics of polymerization varying as the 30th power of hemoglobin (Hb) concentration (i.e., MCHC). Gel formation leads to profound hemorheological changes: 1) RBC become distorted into elongated or spiculated shapes, resulting in their characteristic “sickle” appearance; 2) The deformability of sickled cells is greatly reduced such that they are unable to traverse small vessels of the microcirculation; 3) Multiple sickle cells arriving simultaneously at the entrance of a vascular branch may be unable to negotiate the restriction, thus blocking local circulation and causing ischemia.
In addition to the significant hemorheological alterations, homozygous SCD is also characterized by chronic, severe hemolytic anemia with hematocrit (Hct) values as low as 20%. While the low Hct may offset the increase in blood viscosity owing to reduced RBC deformability, significant amounts of Hb are released into the plasma as intravascular hemolysis occurs. Free Hb is known to scavenge nitric oxide (NO), one of the most potent naturally occurring vasodilators, thereby leading to endothelial dysfunction in this patient population.
Endothelial dysfunction, combined with altered hemorheological parameters and inflammation, markedly affects in vivo blood flow, and individuals with homozygous SCD often suffer severe vasoocclusive complications such as repeated periods of painful crises, acute chest syndrome, stroke and priapism. The sickling process is continuous in these patients, even when they are in a symptom free, steady state condition; to date, only a few factors have been identified as triggers for the transition to widespread crisis. These include transient episodes of hypoxia (e.g., sleep apnea) and a decrease in local perfusion, often due to elevated local blood viscosity. Intense research in this area is imperative because vasoocclusive complications significantly reduce the quality of life, impair cognitive function, lead to organ damage and, ultimately, increased mortality. Interestingly, a significant number of premature deaths in SCD patients remain unexplained “sudden deaths”, presumed to be of cardiac origin with no specific cause identified at autopsy. Literature data for patients with congestive heart failure (CHF) often relates sudden death to the loss of beat-to-beat heart rate variability (HRV), a non-invasive measure of autonomic nervous activity. Although cardiovascular autonomic dysfunction has been reported for SCD and sickle cell trait carrier patients (SCT) by several groups [17, 27], its possible relationship to a known risk factor for crisis has not yet been evaluated.
Current treatment strategies for SCD rely on symptomatic patient management, a limited number of drugs that reduce intracellular HbS polymerization upon deoxygenation, and transfusion therapy with compatible HbA red blood cells. Regular transfusions are utilized to 1) Lessen the number of rigid HbS containing red cells in the circulation thereby improving blood viscosity and blood flow; 2) Decrease hemolysis; 3) Suppress production of RBC containing HbS in the bone marrow. Although transfusion therapy does not stop acute pain crisis related to slow blood flow and local ischemia, it is relatively effective in decreasing complications that involve high shear flow regions (e.g., major stroke, acute chest syndrome). Current guidelines for optimal post-transfusion Hct and HbS concentration were not established by controlled clinical trials but rather are based on decades of clinical experience and an in vitro study performed under non-physiological conditions [42]. As detailed below, we have examined the oxygen delivery potential of sickle (SS) and normal (AA) RBC mixtures that closely simulate the in vivo effects of transfusion therapy and have also explored the effects of shear rate on the optimal Hct of these mixtures.
2. Hypersensitivity of heart rate variability to hypoxia in homozygous sickle cell disease
Hypoxia is thought to be one of the most common triggers of sickle crisis. Hargrave et al. have shown that low nocturnal oxygen saturation is associated with higher incidence of painful crisis in childhood (p < 0.0001) [24]. In SCD patients, nocturnal hypoxemia is a good predictor of central nervous system (CNS) complications [31]. Approximately 40% of patients with mean overnight oxygen saturation of 95% or less suffered a CNS event within 5 years of follow-up. In contrast, less than 10% of patients with mean overnight oxygen saturation of 96% or above had any CNS complications during the same time period. To explore the effects of transient hypoxia in SCD, we induced transient hypoxia in subjects with SCD and monitored the physiological responses.
Controls and patients breathed five breaths of 100% nitrogen, which lead to short-term hypoxia and mimic that experienced during sleep. Heart rate variability studies were performed at Childrens Hospital Los Angeles according to the Declaration of Helsinki and the study was approved by the local Institutional Review Board. Vital signs, ECG, and tidal volume were among the parameters continuously monitored and recorded during the study period.
When we analyzed heart rate variability (HRV) from the ECG tracing, significant differences in autonomic nervous system (ANS) activity were seen. It is commonly accepted that parasympathetic (i.e., vagal) activity is the major contributor to the high-frequency (HF, 0.15–0.4 Hz) components of HRV, while both vagal and sympathetic activities contribute to its low-frequency (LF, 0.04–0.15 Hz) components. Thus, the power of HRV in the HF band has widely been used to quantitatively describe vagal activity and the ratio of LF to HF spectral powers have been utilized as a broad index of “sympathovagal balance” [45]. To detect rapid changes in HRV following a hypoxia stimulus, we applied a time-varying modification of the traditional HRV computation, a methodology based on a recursive autoregressive algorithm previously used by our group [11]. In addition, we adapted the technique reported by Khoo et al. [30] to compensate for HRV variability caused by respiration using the continuous tidal volume signal.
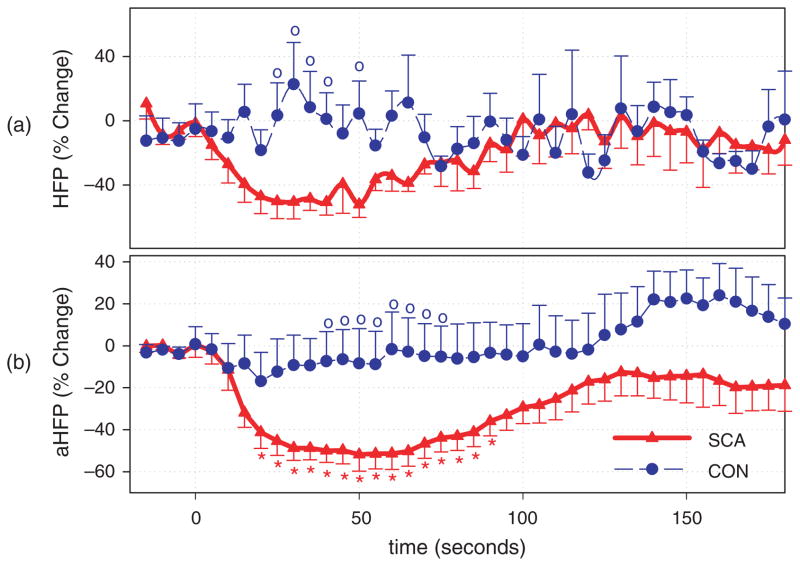
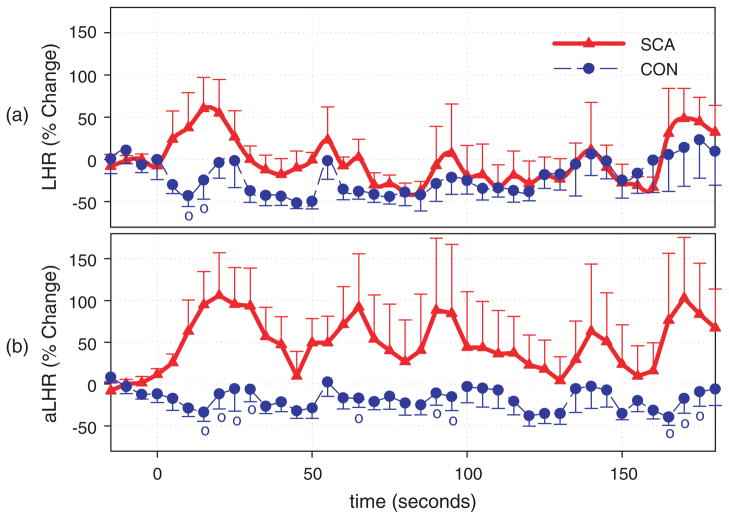
HRV indices related to the parasympathetic activity (0.15–0.4 Hz) decreased significantly in SCD subjects 10 sec following the initial drop in oxygen saturation (SaO2) (t = 0; Fig. 1) while no significant changes were observed in normal controls. Although there was a tendency for indices related to the sympathovagal balance to increase following the stimulus in the patient group, the change was not significant (Fig. 2). Our method adjusting for the effects of ventilation on HRV made possible for a more-detailed study of non-respiratory evoked autonomic changes, thereby rendering all changes in the SCD group more apparent (Figs 1b and 2b). After respiratory adjustment, it is clear that vagal tone is readily reduced in subjects with SCD following transient hypoxia, resulting in the substantial increases of heart rate in this cohort following the stimulus. This finding is consistent with the reports by Pearson et al. that SCD children who exhibit a greater parasympathetic withdrawal during challenges suffered from more clinically severe disease [39].
Fig. 1.
Time course of the parasympathetic HRV indices. o Indicates significant difference between control and SCD subjects ( p < 0.05). * Indicates significant difference from the baseline of the same time-course ( p < 0.05). (a) High frequency power of HRV (HFP), (b) adjusted high frequency power (aHFP).
Fig. 2.
Time course of the sympathovagal balance indices. o Indicates significant difference between control and SCD subjects ( p < 0.05). (a) Ratio between high frequency and low frequency powers (LHR), (b) adjusted ratio between high frequency and low frequency powers (aLHR).
Although the exact pathomechanism for HRV hypersensitivity and its direct link to sickle crisis are not yet known, multiple mechanisms have been proposed: 1) Alterations of blood viscosity: Multiple groups have shown that, due to the sickling of RBC, the viscosity of SS blood increases significantly with decreasing oxygen saturation [35, 49]. Recent evidence also suggests a direct correlation between altered blood rheology and multiple HRV indices [16]; 2) RBC adhesion: Literature data suggests that the adhesion of SS RBC to vascular endothelium increases the response of β2-adrenegic receptors to adrenaline stimulation [23] and hence has a critical role in dilating vessels throughout the circulatory system. Hines et al. have demonstrated that peripheral SS RBC contain significantly higher amounts of cAMP than normal AA cells [26]. The group also reported that exposure to epinephrine further elevates intracellular cAMP and increases SS RBC adhesion to endothelium. These mechanisms might contribute to the initiation and progression of vasoocclusive crisis in SCD as well as to the abnormal ANS responses in this group of patients; 3) Inflammation: A growing number of investigators have suggested inflammation is an essential feature of vasoocclusive crisis. SCD patients have higher than normal white blood cell counts and elevated levels of inflammatory markers [9]. In addition, a close association was suggested between inflammation and altered ANS activity [47] with inflammation being mediated, at least in part, through the parasympathetic nervous system.
Our preliminary work demonstrated a clear causal relationship between transient hypoxia and alterations of HRV in SCD that was not present in normal subjects, and suggests that a heightened hypoxia-induced ANS imbalance is at play in subjects with SCD. The signal processing technique employed herein allowed us to directly study the non-respiratory-derived components of autonomic function that, we believe, may be important in the fundamental pathology of SCD. Further studies are needed and are under way.
3. Autonomic nervous system activity and hemorheological impairment in sickle cell trait carriers
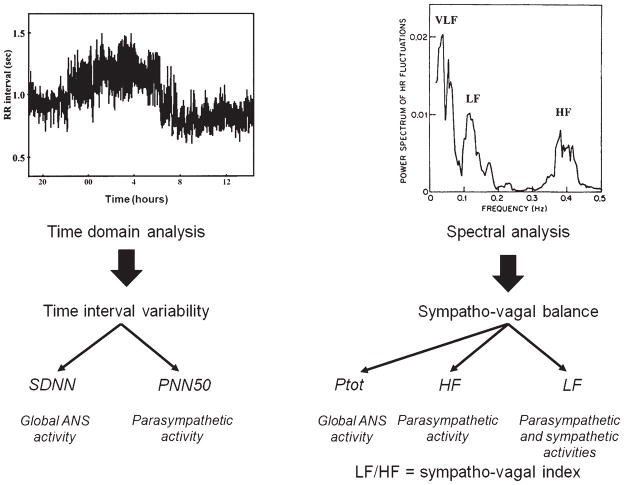
As detailed above, HRV depends primarily on the balanced activity of the autonomic nervous system. After obtaining a baseline electrocardiogram, further data processing may be performed, such as time domain and spectral analyses, that provide valuable information to characterize sympathovagal balance [46] (Fig. 3). A decrease in ANS activity is widely recognized as a predictor for severe cardiac and cerebral complications and death of any cause in the general population [32, 46].
Fig. 3.
Left side: RR interval series. Time domain analysis allows the calculation of indices such as standard deviation of all normal RR intervals (SDNN), known to reflect global ANS activity. The proportion of adjacent normal RR intervals differing more than 50 ms from the preceding RR (PNN50) reflects the parasympathetic activity. Right side: Power spectrum density. Spectral analysis allows us to estimate sympathovagal balance with various calculated indices, such as: Total power of the spectrum (Ptot), High frequency of the spectrum (HF), Low frequency of the spectrum (LF) and LF/HF ratio. These reflect global ANS activity, parasympathetic activity, parasympathetic and sympathetic activities and sympathovagal balance, respectively.
Although sickle cell trait (SCT) is generally considered a benign condition [4, 6], recent evidence suggests that it can be considered a risk factor for cardiovascular complications as a higher prevalence of coronary artery disease [38] and venous thromboembolism [5] have been reported in this population compared to controls. In addition, several studies have shown elevated blood viscosity, decreased RBC deformability and higher plasma concentrations of soluble adhesion molecules in this group of patients [11, 13, 19, 36, 37, 40]. It has been widely debated whether these abnormalities contribute to the higher prevalence of exercise-related sudden death in this population [5, 7, 18, 33] with no direct association established to date. We believe that ANS dysfunction might serve as the common link [20] and thus, we investigated the rheology of blood and the activity of ANS in SCT patients at rest and following strenuous exercise.
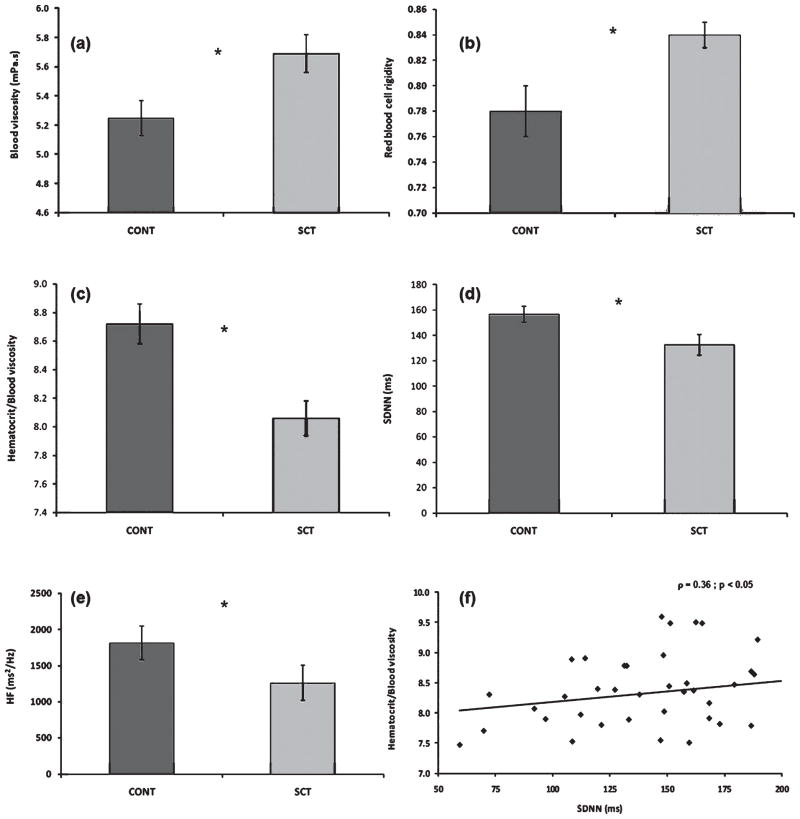
We previously compared resting ANS activity and blood rheology between twenty three SCT patients and seventeen control subjects. We found hemorheological parameters to be markedly different between the two populations [17], with the patient group having elevated blood viscosity, reduced RBC deformability and decreased hematocrit (Hct) to blood viscosity ratio (HVR, often referred to as oxygen transport effectiveness of blood [22]; Fig. 4). We also documented lower ANS activity (i.e., lower standard deviation of all normal RR intervals, i.e., SDNN index) in SCT subjects when compared to the control group. As indicated by the HF component values, low ANS activity was primarily due to the low activity of the parasympathetic system. The positive correlation between HVR and SDNN suggests that global autonomic control might be significantly affected by the hemorheological profile (Fig. 4). The observed negative correlation between RBC rigidity and parasympathetic activity [16] further supports our suggestion. Reduced parasympathetic activity might be considered as a logical physiological adaptation mechanism to compensate for elevated blood viscosity in order to maintain adequate blood flow and tissue oxygen supply. In addition, results by Sangkatumvong et al. [41] (detailed above), showed a direct relationship between hypoxia and altered HRV suggesting that hypoxia, autonomic dysregulation and impaired blood rheology are interacting factors promoting sickle crisis.
Fig. 4.
Hemorheological parameters and ANS activity in controls with HbA (CONT group, black bar) and in SCT carriers (SCT group, white bar); *difference between the two groups ( p < 0.05). (a): Blood viscosity at 225s−1; (b): RBC rigidity index derived from viscometry data obtained at 225s−1; (c): hematocrit-blood viscosity ratio calculated for a shear rate of 225s−1; (d): SDNN; (e): HF. (f) Corresponds to the relationship between the hematocrit-blood viscosity ratio and SDNN. (Modified from Connes et al. [16].)
Medical complications are relatively rare in SCT and mostly develop in response to strenuous physical exercise. To examine this observation in more detail, we compared the changes in hemorheological profile and ANS activity in 7 SCT and 6 healthy controls in response to strenuous exercise [25, 48]. ANS activity and rheological parameters were measured one day prior to the test and one and two days after completing the exercise protocol. Results obtained on the day immediately following the exercise showed that RBC rigidity increased above baseline in both study groups and remained elevated two days after the exercise. However, the increase was significantly higher (approximately two-fold) in SCT carriers than in controls [48]. By analyzing the evolution of the spectral power of heart rate variability (Ptot), an index known to reflect global ANS activity, exercise had similar effects in both groups as ANS activity decreased below baseline the night following the stress test then returned to pre-exercise values by the second day. However, values measured in SCT subjects were always lower than those recorded for controls, thus indicating permanent alterations in their ANS activity. Again, the reduced ANS activity caused by decreased parasympathetic activity might represent a physiologically logical but clinically dangerous ANS response attempting to compensate for the reduced amounts of oxygen delivered by RBC in SCT individuals.
Whether this sympathovagal imbalance itself represents an enhanced risk for cardiovascular complications in SCT subjects remains unclear and further studies are clearly warranted. Fortunately, most individuals with SCT have no major difficulty participating in competitive sports and are without complications; the overall incidence of sudden death in this population is relatively rare. It seems likely that altered ANS activity alone may not explain all exercise-related sudden deaths reported for SCT patients [29]. However, ANS dysfunction might enhance the risk for cardiovascular complications in this population when combined with other risk factors such as altered hemorheological parameters [48] and extreme climatic conditions (e.g., warm and humid environment [29]). Large scale cohort studies are needed to identify sub-profile of ANS activity in SCT subjects.
4. Hemorheological aspects of transfusion therapy in patients with sickle cell disease
Transfusion of normal, AA RBC is commonly utilized in the management of patients with SCD since it is effective for preventing or reversing severe complications of the disease [15, 44, 48, 50, 51]. The primary aims of transfusion therapy in SCD are to improve the reduced oxygen delivery potential of blood [12] due to the patient’s severe anemia and to enhance tissue perfusion by reducing the proportion of rigid, sickled red cells. However, the corresponding increase in hematocrit (Hct) increases blood viscosity [1, 14], which, in situations where hyperviscosity syndrome develops, reduces blood flow, compromises tissue perfusion and prompts severe clinical complications [43].
Current guidelines for optimal post-transfusion Hct and HbS concentration are based on decades of clinical experience and an in vitro study that introduced the concept of “optimal” Hct [42]. This optimum is obtained by examining the ratio of Hct divided by blood viscosity (i.e., the HVR) versus Hct and determining the Hct value where the HVR is the greatest; thus, the HVR is regarded as a reflection of the oxygen transport effectiveness of blood. However, this early in vitro study of optimal Hct was performed with suspensions of RBC in buffer rather than in plasma, thereby excluding physiologically relevant plasma protein-mediated RBC-RBC adhesive interactions. We designed a study to investigate the viscosity and oxygen delivery potential of SS + AA RBC mixtures in autologous sickle plasma, a protocol that closely simulates transfusion therapy and allows examining the effects of shear rate on the optimal Hct.
Blood samples were collected into vacuum tubes containing EDTA (1.5 mg/ml) from adult steady state SCD patients seen regularly at the USC Comprehensive Sickle Cell Center. Patients had not been transfused for at least 90 days prior to enrollment. ABO- and Rh-matched AA RBC were obtained from healthy volunteers and cells were confirmed for compatibility using immediate spin crossmatch. SS RBC were combined with autologous plasma and AA cells to produce suspensions at 25%, 30% and 40% Hct, with each containing 25%, 50%, 75% or 100% SS RBC. All samples were deoxygenated using a humidified mixture of 95% nitrogen and 5% carbon dioxide to an average pO2 of 15–20 mmHg. Viscosity was measured at 37°C over a continuous, physiologically relevant shear rate profile (1 to 1000 s−1) using a computer-controlled tube viscometer system (RheologTM, Rheologics Inc., Exton, PA; [2, 52]).
As anticipated from previous reports, the viscosity of both SS and AA blood exhibited non-Newtonian, Hct-dependent flow behavior: viscosity increased with decreasing shear and increasing Hct, with the effects of Hct most evident at low rates of shear (data not shown). Also consistent with literature data, viscosity values obtained for SS blood were slightly above control values even when oxygenated and, at constant Hct and percent SS RBC, increased significantly under deoxygenated conditions [35, 49]. When reducing the proportion of sickle cells from 100% to 75%, 50% or 25% to simulate the effects of simple or exchange transfusions, there were marked decreases in blood viscosity at all shear rates and Hct values.
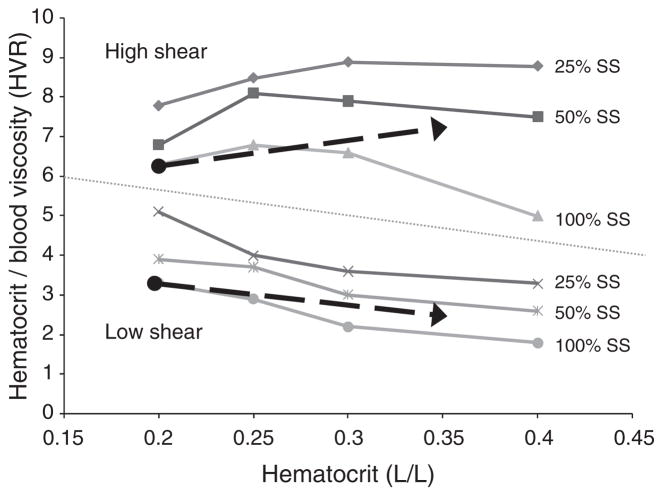
Using our data, we calculated the oxygen transport effectiveness (i.e., HVR) of each sample at a wide range of shear rates. In agreement with prior reports [1], increasing Hct yielded a biphasic relation for HVR at high shear rates (75–1000 s−1): all curves reached a maximum value representing an optimal Hct for HVR (Fig. 5), and the optimal Hct increased progressively with the percentage of AA RBC (data not shown). However, no optimum Hct could be determined at low shear rates (3–11 s−1): regardless of percent AA RBC, increasing Hct led to a progressive decrease of HVR. The influence of Hct and percent SS RBC on HVR were evaluated for a typical, guideline-based transfusion protocol (i.e., increase Hct from 20% to 35%, reduce percent SS RBC from 100% to 50%; see Fig. 5). As demonstrated by the upper arrow, HVR improved by approximately 15% at 300 s−1 while there was an approximately 12% decrease in oxygen transport effectiveness at the lower shear rate (5 s−1; lower arrow). Thus our findings at high shear rates are consistent with previous studies performed in a non-aggregating buffer [28, 42], while no optimum Hct for transfusion appears to exist at low shear rates.
Fig. 5.
Oxygen transport effectiveness (i.e., HVR) of samples at a selected high (300 s−1) and a selected low (5 s−1) shear rate. While at high shear all curves reached a maximum value representing an optimal Hct for HVR, no such optimum Hct could be determined at low shear rates. Arrows represent the influence of Hct and percent SS RBC on HVR for a typical, guideline-based transfusion protocol (i.e., increase Hct from 20% to 35% and reduce proportion of SS RBC from 100% to 50%). Figure modified from Alexy et al. [3].
The differences between the results of the present and previous studies at lower rates of shear are most likely due to the use of different suspending media: our experiments were performed in the sickle patients autologous plasma rather than in a non-physiological buffer. Our approach allows a more realistic modeling of in vivo conditions following transfusion therapy [14], primarily because it considers the non-Newtonian effects caused by plasma-mediated RBC–RBC interactions in low shear regions. Comparable lower shear rates are a normal finding in post-capillary venules [1, 10, 14], and these shear rates may be further reduced by hypotension, shock or vasoocclusive episodes [15, 48, 51]. Thus, the “optimal” Hct may depend not only on the percentage of HbS, but also upon the prevailing shear rates in the relevant organ or tissue (e.g., lung, brain, bone marrow) and the clinical condition of the patient. In summary, our results strongly suggest the need to consider local flow conditions as well as Hct and percent SS RBC when evaluating the specific effects of transfusion therapy in sickle cell disease.
5. Conclusions
The data presented herein suggest that the pathophysiology of SCD is independently related to hypoxia, blood viscosity and autonomic nervous system regulation, and that all these parameters are closely related to each other. Given the high frequency of unexplained sudden deaths in patients with SCD and the known association of autonomic dysregulation with sudden death in patients with cardiovascular disease [21], it is clear that further studies of the relationship between these parameters are needed that will likely provide important insights into the pathophysiology and clinical management of sickle cell disease.
Acknowledgments
Funded in part by NIH Grants HL07180 and HL090511 (TDC); HL15722 and HL70595 (HJM); HL090451 and EB001978 (MCK). MO1 RR00046 (TDC) from the Children’s Hospital Los Angeles General Clinical Research Center; HG003956 (TCF) from NHGRI.
Footnotes
This material is a brief summary of four papers presented at the 13th International Congress on Biorheology and 6th International Conference on Clinical Hemorheology, Pennsylvania State University, State College, PA, July, 2008.
References
- 1.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 2.Alexy T, Wenby RB, Pais E, et al. An automated tube-type blood viscometer: validation studies. Biorheology. 2005;42:237–247. [PubMed] [Google Scholar]
- 3.Alexy T, Pais E, Armstrong JK, Meiselman HJ, Johnson CS, Fisher TC. Rheologic behaviour of sickle and normal red blood cell mixtures in sickle plasma: implications for transfusion therapy. Transfusion. 2006;46:912–918. doi: 10.1111/j.1537-2995.2006.00823.x. [DOI] [PubMed] [Google Scholar]
- 4.Ashcroft MT, Desai P. Mortality and morbidity in Jamaican adults with sickle-cell trait and with normal haemoglobin followed up for twelve years. Lancet. 1976;2:784–786. doi: 10.1016/s0140-6736(76)90612-7. [DOI] [PubMed] [Google Scholar]
- 5.Austin H, Key NS, Benson JM, Lally C, Dowling NF, Whitsett C, Hooper WC. Sickle cell trait and the risk of venous thromboembolism among blacks. Blood. 2007;110:908–912. doi: 10.1182/blood-2006-11-057604. [DOI] [PubMed] [Google Scholar]
- 6.Awodu OA, Famodu AA. Haemostatic variables and their relationship to body mass index and blood pressure in adult Nigerians with the sickle cell trait. Clin Hemorheol Microcirc. 2007;36:89–94. [PubMed] [Google Scholar]
- 7.Baskurt OK, Meiselman HJ, Bergeron MF. Re: Point: Counterpoint: sickle cell trait should/should not be considered asymptomatic and as a benign condition during physical activity. J Appl Physiol. 2007;103:2142. doi: 10.1152/japplphysiol.00886.2007. Author reply 2143–2144. [DOI] [PubMed] [Google Scholar]
- 8.Baskurt OK, Meiselman HJ. Blood rheology and hemodynamics. Semin Thromb Hemost. 2003;29:435–450. doi: 10.1055/s-2003-44551. [DOI] [PubMed] [Google Scholar]
- 9.Belcher JD, Bryant CJ, Nguyen J, Bowlin PR, Kielbik MC, Bischof JC, Hebbel RP, Vercellotti GM. Transgenic sickle mice have vascular inflammation. Blood. 2003;101:3953–3959. doi: 10.1182/blood-2002-10-3313. [DOI] [PubMed] [Google Scholar]
- 10.Bishop JJ, Popel AS, Intaglietta M, et al. Effect of aggregation and shear rate on the dispersion of red blood cells flowing in venules. Am J Physiol Heart Circ Physiol. 2002;283:H1985–1996. doi: 10.1152/ajpheart.00888.2001. [DOI] [PubMed] [Google Scholar]
- 11.Blasi A, Jo J, Valladares E, Morgan BJ, Skatrud JB, Khoo MC. Cardiovascular variability after arousal from sleep: time-varying spectral analysis. J Appl Physiol. 2003;95:1394–1404. doi: 10.1152/japplphysiol.01095.2002. [DOI] [PubMed] [Google Scholar]
- 12.Bowers AS, Pepple DJ, Reid HL. Oxygen delivery index in subjects with normal haemoglobin (HbAA), sickle cell trait (HbAS) and homozygous sickle cell disease (HbSS) Clin Hemorheol Microcirc. 2008;40:303–309. [PubMed] [Google Scholar]
- 13.Brandao MM, Fontes A, Barjas-Castro ML, Barbosa LC, Costa FF, Cesar CL, Saad ST. Optical tweezers for measuring red blood cell elasticity: application to the study of drug response in sickle cell disease. Eur J Haematol. 2003;70:207–211. doi: 10.1034/j.1600-0609.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- 14.Chien S. Biophysical behaviour of red cells in suspension. In: Surgenor DM, editor. The Red Blood Cell. Academic Press; New York: 1975. pp. 1031–1033. [Google Scholar]
- 15.Claster S, Vichinsky EP. Managing sickle cell disease. Br Med J. 2003;327:1151–1155. doi: 10.1136/bmj.327.7424.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connes P, Hue O, Hardy-Dessources MD, Boucher JH, Pichot V, Barthelemy JC. Hemorheology and heart rate variability: Is there a relationship? Clin Hemorheol Microcirc. 2008;38:257–265. [PubMed] [Google Scholar]
- 17.Connes P, Martin C, Barthelemy JC, Monchanin G, Atchou G, Forsuh A, Massarelli R, Wouassi D, Thiriet P, Pichot V. Nocturnal autonomic nervous system activity impairment in sickle cell trait carriers. Clin Physiol Funct Imaging. 2006;26:87–91. doi: 10.1111/j.1475-097X.2006.00655.x. [DOI] [PubMed] [Google Scholar]
- 18.Connes P, Hardy-Dessources MD, Hue O. Counterpoint: Sickle cell trait should not be considered asymptomatic and as a benign condition during physical activity. J Appl Physiol. 2007;103:2138–2140. doi: 10.1152/japplphysiol.00338.2007a. discussion 2140–2141. [DOI] [PubMed] [Google Scholar]
- 19.Connes P, Sara F, Hardy-Dessources MD, Etienne-Julan M, Hue O. Does higher red blood cell (RBC) lactate transporter activity explain impaired RBC deformability in sickle cell trait? Jpn J Physiol. 2005;55:385–387. doi: 10.2170/jjphysiol.S653. [DOI] [PubMed] [Google Scholar]
- 20.Curtis BM, O’Keefe JH., Jr Autonomic tone as a cardiovascular risk factor: the dangers of chronic fight or flight. Mayo Clin Proc. 2002;77:45–54. doi: 10.4065/77.1.45. [DOI] [PubMed] [Google Scholar]
- 21.Darbari DS, Kple-Faget P, Kwagyan J, Rana S, Gordeuk VR, Castro O. Circumstances of death in adult sickle cell disease patients. Am J Hematol. 2006;81:858–863. doi: 10.1002/ajh.20685. [DOI] [PubMed] [Google Scholar]
- 22.Dupuy-Fons C, Brun JF, Pellerin F, Laborde JC, Bardet L, Orsetti A, Janbon C. Relathionships between blood rheology and transcutaneous oxygen pressure in peripheral occlusive arterial disease. Clin Hemorheol. 1995;15:191–199. [Google Scholar]
- 23.Eyler CE, Jackson T, Elliott LE, De Castro LM, Jonassaint J, Ashley-Koch A, Telen MJ. Beta(2) adrenergic receptor and adenylate cyclase gene polymorphisms affect sickle red cell adhesion. Br J Haematol. 2008;141:105–108. doi: 10.1111/j.1365-2141.2008.07008.x. [DOI] [PubMed] [Google Scholar]
- 24.Hargrave DR, Wade A, Evans JP, Hewes DK, Kirkham FJ. Nocturnal oxygen saturation and painful sickle cell crises in children. Blood. 2003;101:846–848. doi: 10.1182/blood-2002-05-1392. [DOI] [PubMed] [Google Scholar]
- 25.Hedreville M, Barthelemy JC, Tripette J, Roche F, Hardy-Dessources MD, Pichot V, Hue O, Connes P. Effects of strenuous exercise on autonomic nervous system activity in sickle cell trait carriers. Auton Neurosci. 2008;143:68–72. doi: 10.1016/j.autneu.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Hines PC, Zen Q, Burney SN, Shea DA, Ataga KI, Orringer EP, Telen MJ, Parise LV. Novel epinephrine and cyclic AMP-mediated activation of Bcam/Lu-dependent sickle (Ss) RBC adhesion. Blood. 2003;101:3281–3287. doi: 10.1182/blood-2001-12-0289. [DOI] [PubMed] [Google Scholar]
- 27.Jaja SI, Kehinde MO, Ogungbemi SI. Cardiac and autonomic responses to change in posture or vitamin C supplementation in sickle cell anemia subjects. Pathophysiology. 2008;15:25–30. doi: 10.1016/j.pathophys.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Jan K, Usami S, Smith JA. Effects of transfusion on rheological properties of blood in sickle cell anemia. Transfusion. 1982;22:17–20. doi: 10.1046/j.1537-2995.1982.22182154208.x. [DOI] [PubMed] [Google Scholar]
- 29.Kark JA, Ward FT. Exercise and hemoglobin S. Semin Hematol. 1994;31:181–225. [PubMed] [Google Scholar]
- 30.Khoo MC, Kim TS, Berry RB. Spectral indices of cardiac autonomic function in obstructive sleep apnea. Sleep. 1999;22:443–451. doi: 10.1093/sleep/22.4.443. [DOI] [PubMed] [Google Scholar]
- 31.Kirkham FJ, Hewes DK, Prengler M, Wade A, Lane R, Evans JP. Nocturnal hypoxaemia and central-nervous-system events in sickle cell disease. Lancet. 2001;357:1656–1659. doi: 10.1016/s0140-6736(00)04821-2. [DOI] [PubMed] [Google Scholar]
- 32.Kleiger RE, Stein PK, Bigger JT., Jr Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol. 2005;10:88–101. doi: 10.1111/j.1542-474X.2005.10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Gallais D, Lonsdorfer J, Bogui P, Fattoum S. Point: Sickle cell trait should be considered asymptomatic and as a benign condition during physical activity. J Appl Physiol. 2007;103:2137–2138. doi: 10.1152/japplphysiol.00338.2007. discussion 2141. [DOI] [PubMed] [Google Scholar]
- 34.Lester LA, Sodt PC, Hutcheon N, Arcilla RA. Cardiac abnormalities in children with sickle cell anemia. Chest. 1990;98:1169–1174. doi: 10.1378/chest.98.5.1169. [DOI] [PubMed] [Google Scholar]
- 35.Mohandas N, Hebbel RP. Erythrocyte deformability, fragility and rheology. In: Embury SH, Hebbel RP, Mohandas N, Steinberg MH, editors. Sickle cell disease. Raven Press; New York: 1994. pp. 205–216. [Google Scholar]
- 36.Monchanin G, Serpero LD, Connes P, Tripette J, Wouassi D, Bezin L, Francina A, Ngongang J, de la Pena M, Massarelli R, Gozal D, Thiriet P, Martin C. Effects of a progressive and maximal exercise on plasma levels of adhesion molecules in athletes with sickle cell trait with or without α-thalassemia. J Appl Physiol. 2007;102:169–173. doi: 10.1152/japplphysiol.00272.2006. [DOI] [PubMed] [Google Scholar]
- 37.Monchanin G, Serpero LD, Connes P, Tripette J, Wouassi D, Francina A, Massarelli R, Gozal D, Thiriet P, Martin C. Plasma levels of adhesion molecules ICAM-1 and VCAM-1 in athletes with sickle cell trait with or without alpha-thalassemia during endurance exercise and recovery. Clin Hemorheol Microcirc. 2008;40:89–97. [PubMed] [Google Scholar]
- 38.Ould Amar AK, Pi Gibert A, Darmon O, Besse P, Cenac A, Cesaire R. AS heterozygote hemoglobinopathy and coronary risk. Arch Mal Coeur Vaiss. 1999;92:1727–1732. [PubMed] [Google Scholar]
- 39.Pearson SR, Alkon A, Treadwell M, Wolff B, Quirolo K, Boyce WT. Autonomic reactivity and clinical severity in children with sickle cell disease. Clin Auton Res. 2005;15:400–407. doi: 10.1007/s10286-005-0300-9. [DOI] [PubMed] [Google Scholar]
- 40.Reid HL, Oli JM. The possible significance of abnormal blood rheology in diabetics with sickle-cell trait (HbAS) West Afr J Med. 1986;5:249–256. [Google Scholar]
- 41.Sangkatumvong S, Coates TD, Khoo MC. Abnormal autonomic cardiac response to transient hypoxia in sickle cell anemia. Physiol Meas. 2008;29:655–668. doi: 10.1088/0967-3334/29/5/010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmalzer EA, Lee JO, Brown AK, et al. Viscosity of mixtures of sickle and normal red cells at varying hematocrit levels, implications for transfusion. Transfusion. 1987;27:228–233. doi: 10.1046/j.1537-2995.1987.27387235626.x. [DOI] [PubMed] [Google Scholar]
- 43.Serjeant G. Blood transfusion in sickle cell disease: a cautionary tale. Lancet. 2003;361:1659–1660. doi: 10.1016/S0140-6736(03)13293-X. [DOI] [PubMed] [Google Scholar]
- 44.Styles LA, Vichinsky E. Effects of long-term transfusion regimen on sickle cell-related illness. J Pediatr. 1994;125:909–911. doi: 10.1016/s0022-3476(05)82006-2. [DOI] [PubMed] [Google Scholar]
- 45.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 46.Task force, heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 47.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 48.Tripette J, Hardy-Dessources MD, Sara F, Montout-Hedreville M, Saint-Martin C, Hue O, Connes P. Does repeated and heavy exercise impair blood rheology in carriers of sickle cell trait? Clin J Sport Med. 2007;17:465–470. doi: 10.1097/JSM.0b013e31815aed23. [DOI] [PubMed] [Google Scholar]
- 49.Usami S, Chien S, Scholtz PM, Bertles JF. Effect of deoxygenation on blood rheology in sickle cell disease. Microvasc Res. 1975;9:324–334. doi: 10.1016/0026-2862(75)90069-2. [DOI] [PubMed] [Google Scholar]
- 50.Vichinsky E. Transfusion therapy. In: Embury SH, Hebbel RP, Mohandas N, Steinberg MH, editors. Sickle Cell Disease: Basic Principles and Clinical Practice. Raven Press; New York: 1994. pp. 781–798. [Google Scholar]
- 51.Vichinsky E. New therapies in sickle cell disease. Lancet. 2002;360:629–631. doi: 10.1016/S0140-6736(02)09776-3. [DOI] [PubMed] [Google Scholar]
- 52.Wang S, Boss AH, Kensey KR, et al. Variations of whole blood viscosity using Rheolog™ – a new scanning capillary viscometer. Clin Chim Acta. 2003;332:79–82. doi: 10.1016/s0009-8981(03)00125-6. [DOI] [PubMed] [Google Scholar]