Abstract
Background/Aims
Evidence demonstrates that obesity is associated with progression of chronic hepatitis C virus (HCV) infection and poor response to interferon therapy among HCV-infected adults. However, this evidence has been confounded by multiple comorbidities present in adult cohorts and the use of single adult doses.
Methods
We performed a retrospective investigation to evaluate the role of body mass index (BMI) in chronic HCV progression and response to therapy in the children. One hundred twenty-three children and teenagers followed at Children's Hospital Boston for HCV infection between 1998 and 2007 were included. Patients' weight and height at the time of liver biopsy or before and after HCV therapy were obtained and body mass index (BMI) calculated.
Results
The presence of steatosis was statistically associated with higher mean (±SE) BMI percentiles (72nd (± 5.8) vs. 58th (± 3.5) percentile) F(1,101)=4.2, p=0.04). Non-responders to treatment had a higher mean (±SE) BMI percentile (70th ±7.4) when compared to responders (50th ±6.5) in univariate and multivariate analyses (p=0.04, p=0.02 respectively). Using a multivariate model, it was calculated that one standard deviation (one Z score unit) increase in baseline BMI Z score is associated with a 12% decrease in the probability of sustained virologic response (SVR).
Conclusion
Overweight adversely affects the progression of chronic HCV liver disease and is associated with diminished response to antiviral therapy using weight-based dosing in a cohort with minimal comorbidities.
Keywords: pediatric, obesity, steatosis, fibrosis, interferon
Introduction
In 2004, approximately 34% of children in the United States were overweight or obese (1, 2). Overweight and obesity are among the most important public health concerns of our time, with the prevalence of these conditions increasing dramatically over the past several decades worldwide (3-5). As a result, a rising incidence of associated comorbidities has negatively affected the health of children and young adults globally (6, 7). In addition, overweight may negatively impact the natural history of other conditions through its effects on multiple organ systems. HCV infection is one of the most common chronic liver diseases, affecting approximately 170 million people worldwide (8, 9). Overweight and obesity have been associated with greater degrees of steatosis and fibrosis in HCV-infected adults (9-13). However, the precise role of overweight in the progression of pediatric chronic HCV liver disease remains to be established.
In the United States, approximately 0.2% of 6 to 12-year olds and 0.4% of 13 to 19-year olds, or approximately 174,000 children, are anti-HCV positive (14). Extrapolation from adult data, in which 75% of HCV antibody positive subjects are HCV ribonucleic acid (RNA) positive, leads to an estimate of 130,500 chronically infected children in the U.S. Although treatment of HCV has improved in recent years, a significant portion of individuals (up to 40 to 50% of pediatric patients carrying HCV genotypes 1 or 4) still fail to respond to therapy for unclear reasons (15, 16). A number of determinants associated with diminished response to therapy have been identified. Some of these determinants include older age, longer duration of infection, African American race, infection with HCV genotype 1 or 4, high serum HCV RNA, advanced fibrosis, presence of steatosis and insulin resistance, and possibly overweight (17-20). However, of these, only overweight is potentially modifiable.
The role of overweight in antiviral treatment response for hepatitis C has been previously evaluated in adults (21-23), but the data has been confounded by the frequent use of standard adult doses of antiviral medications which are not adjusted for weight (21-23). Few published reports have evaluated the effect of BMI on response to HCV-treatment using weight-based dosing. Among these, Tarantino et al. (24) noted a higher mean BMI in non-responders as compared to responders, but in multivariate analysis BMI was not independently associated with response to therapy. Gheorghe et al. (25) also evaluated weight-based dosing and found that overweight was not associated with diminished response to therapy. They concluded that the negative impact of increasing weight on virological response can be overcome by dosing pegylated interferon and ribavirin according to body weight. Whether higher BMI is independently associated with lower response to HCV treatment and whether this can be overcome by adjustment of medication dose remains to be definitively established.
Children offer a unique opportunity to evaluate the independent effects of overweight in the setting of chronic HCV infection. Children and young adults infected with HCV tend to have lower degrees of fibrosis, minimal comorbidities, and shorter duration of infection when compared to older adults. In addition, the universal use of weight-based dosing in pediatric medicine precludes confounding due to relative underdosing. The question of whether overweight or obesity decreases response to HCV therapy in youth has relevance for the HCV-infected population at large. Therefore, we performed a two part retrospective investigation to evaluate the role of BMI in HCV progression and response to therapy in the young.
Experimental Procedures
Patient Population
All patients with HCV infection evaluated at Children's Hospital Boston between June 1, 1993 and June 1, 2007 were considered as possible study subjects. All participants had a history of established HCV infection confirmed by HCV RNA testing.
Subjects in the first part of the study (STUDY 1) were selected to evaluate whether increased BMI is associated with more advanced steatosis or fibrosis in chronic HCV infection. Patients who underwent a liver biopsy during the study period and who were <=20 years of age at the time of biopsy were included. Patients with missing height or weight information, and patients who had liver disorders in addition to HCV were excluded. A total of 102 subjects met inclusion criteria for STUDY 1. Ninety three percent of subjects were non-Hispanic Caucasians. Fifty one percent of patients were male. Routes of HCV acquisition were perinatal (42%), via transfusion (41%), from illegal substance abuse (2%) or unknown (15%). Median age was 14.8 years with a range of 4.6 to 19.8 years. Four subjects carried the genotype 2, seven subjects carried genotype 3 and one carried genotype 4, the rest carried genotype 1.
The second part of the study (STUDY 2) consisted of subjects who were selected to determine the association between BMI and response to HCV-therapy. Subjects were included if they underwent treatment for HCV infection during the study period and if they were <=20 years of age at completion of their treatment. Dosages of medications used were as follows: interferon (IFN) (3 MU/m2 to a maximum of 5 MU subcutaneously three times weekly) or pegylated interferon (PEG IFN alfa 2a) (180 mcg/1.73 m2 to a maximum of 180 mcg subcutaneously once per week), with or without oral ribavirin (15 mg/kg/day). Subjects with missing weight or height at the start or end of therapy, and patients with liver disorders in addition to hepatitis C were excluded. Sixty two patients met inclusion and exclusion criteria for STUDY 2. Compliance with treatment was assessed by patient and family interviews at each visit. Routes of HCV acquisition were perinatal (47%), via transfusion (47%), or unknown (15%). Four patients acquired the infection by substance abuse, and these were excluded because of concerns with HCV treatment compliance. The final group consisted of 58 subjects, and all had >90% compliance rates. The vast majority (95%) were non-Hispanic Caucasian. The median age of subjects in STUDY 2 was 13.0 years with a range between 5.9 to 19.2 years, and 51% were male. HCV RNA levels prior to therapy ranged from 2,654 IU/mL to 7,866,000 IU/mL with a mean of 1,158,611 IU/mL.
Patient characteristics and laboratory determinations
For STUDY 1, patients' demographic characteristics included gender, age, and race/ethnicity. Method of HCV contraction, infection duration, and HCV genotype were assessed. HCV infection was confirmed by qualitative and quantitative measurements of HCV RNA using reverse-transcription-polymerase chain reaction. All liver biopsies were evaluated by a single pathologist, who was blinded to response to therapy and body mass index. Fibrosis was scored using the METAVIR scoring system (26). Steatosis was categorized as none, mild, moderate and severe. Anthropometrical data, including patients' weight and height, were measured at the time of biopsy. In addition, available weights and height one year (+/- 3 months) prior to biopsy were reviewed. For STUDY 2, demographic characteristics and laboratory determinations were the same as in STUDY 1 with the exception of liver histology data, which was not collected. In STUDY 2, anthropometrical data, including patients' weight and height, were measured at the start and at the end of antiviral therapy. Available weights and heights one year (+/- 3 months) prior to initiation of treatment were reviewed.
Calculations
Body mass index was calculated as weight (Kg) divided by height squared (m2). BMI percentile for age and gender as well as Z scores were obtained from CDC smoothed percentile curves of the National Health and Nutrition Examination Survey (NHANES) data. For the purposes of this investigation, overweight was defined as BMI > 85 percentile for equivalent age and gender. Infection duration was estimated based on subjects' past medical history. For subjects with vertically acquired HCV infection, age was used as duration. In STUDY 2, change in BMI Z score was calculated from the difference in BMI Z score at the start and at the end of therapy.
Statistical Analysis
Descriptive statistics for continuous variables were expressed as either means with standard deviations or medians with ranges. For descriptive purposes and for identification of potential confounding factors in STUDY 1, an initial univariate analysis was performed on the simple associations between steatosis and fibrosis categories with age at the time of liver biopsy, gender, and infection duration. Stability of BMI was assessed by evaluating the correlation between BMI Z scores at time of biopsy and available BMI Z scores from one year prior. In order to evaluate the association between body mass index and steatosis as well as fibrosis, a general linear model was constructed in which BMI Z scores were regressed on steatosis and fibrosis categories. Age at the time of liver biopsy, gender, race/ethnicity, history of alcohol or illegal substance use, infection duration, and HCV genotype, were also considered in the model. Due to the sparseness of their distributions, METAVIR fibrosis scores 3 and 4 were collapsed into a single category and steatosis was dichotomized as absent or present.
In STUDY 2, subjects were classified into sustained virologic responders (SVR) or non-responders (no SVR). Based on standard definitions, patients were considered SVR if they had undetectable HCV RNA levels 24 weeks following the end of HCV treatment and non-responders if their HCV RNA was detectable at that time. Univariate analysis was performed comparing sustained virologic responders and non-responders with regards to gender, age at the start of therapy, infection duration, HCV genotype, frequency of overweight, and therapy regimen. Stability of BMI was assessed by evaluating the correlation between baseline BMI Z score and available BMI Z scores from one year prior. A multivariate model was then created using logistic regression to evaluate the effect of baseline BMI Z score and possible confounding factors on response to therapy. Change in BMI Z score was included in the model to account for the possibility that this variable could be independently associated with the outcome. Possible confounding variables were evaluated, and the final model was chosen in consideration of relevant confounders.
JMP Statistical Software (SAS Institute, Cary, NC) was used for all statistical analyses.
Results
STUDY 1: Association of BMI with disease stage
Steatosis was distributed as follows: none (73.5%), mild (21.5%), moderate (5%), severe (0%). Steatosis categories were collapsed into absent (73.5%) or present (26.5%). Fibrosis was distributed as follows: METAVIR 0 (8.8%), 1 (52%), 2 (15.7%), 3 (20.6%), and 4 (2.9%). Fibrosis stages 3 and 4 were collapsed into METAVIR 3-4 (23.5%). Mean age and mean infection duration by steatosis and fibrosis categories are given in Tables 1a and 1b. No statistically significant differences in age or infection duration by steatosis and fibrosis levels were noted (p values >0.32). Gender distribution was similar among patients with and without steatosis (χ2 (1) = 0.31, p = 0.58) as well as across the different fibrosis categories (χ2 (3) = 3.8, p = 0.29). Among the 31 subjects with available BMI Z scores one year prior to biopsy, a strong correlation was noted between that measure and BMI Z score at the time of biopsy (R=0.83, p<0.0001) illustrating the stability of BMI.
TABLE 1. TABLES 1A, 1B. Mean Age and Mean Infection Duration by Steatosis and Fibrosis Categories (STUDY 1).
| A. | ||||
|---|---|---|---|---|
| Mean (SD) | Steatosis | |||
| Absent | Present | |||
| Age (yr) | 13.9 (4.2) | 14.1 (3.4) | ||
| Infection Duration (yr) | 11.5 (5.3) | 12.9 (4.5) | ||
| B. | ||||
| Mean (SD) |
Fibrosis Metavir Score |
|||
| 0 | 1 | 2 | 3-4 | |
| Age (yr) | 15.4 (3.0) | 14.1 (3.9) | 13.2 (4.6) | 13.4 (4.3) |
| Infection Duration (yr) | 13.6 (3.3) | 11.9 (5.2) | 11.4 (1.3) | 11.6 (5.6) |
Overweight is associated with progression of chronic HCV infection in the young
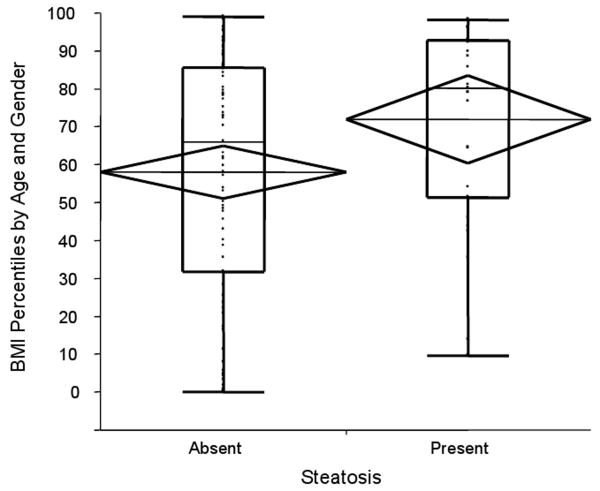
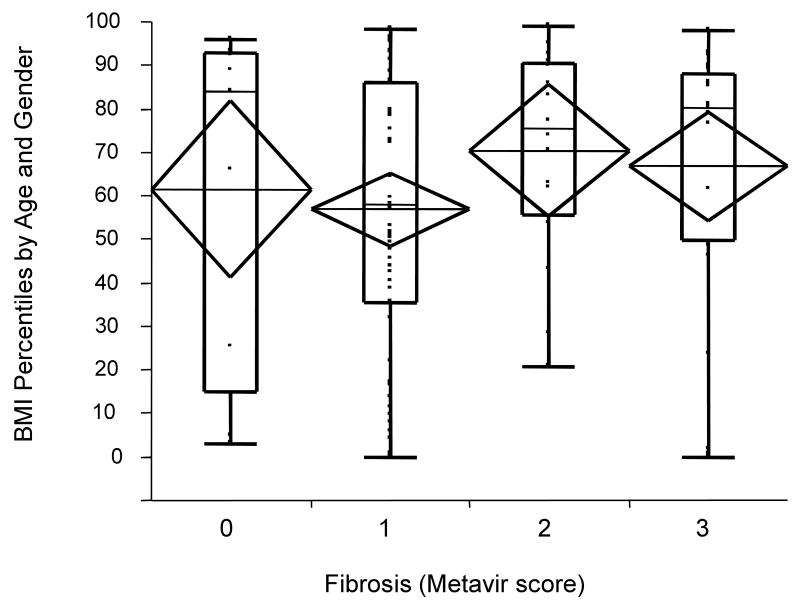
In univariate analysis within STUDY 1, the presence of steatosis was statistically associated with higher mean (±SE) BMI percentiles (72nd (± 5.8) vs. 58th (± 3.5) percentile) F(1,101)=4.2, p=0.04) corresponding to mean BMI Z scores of 0.79 vs. 0.17 (Figure 1). Variables including age, gender, infection duration, and fibrosis were considered in a multivariate model as possible confounders but the results were unchanged (data not shown). Exclusion of subjects with genotype 3 did not alter the results. Among the seven subjects with genotype 3, mean BMI for those mean BMI for subjects with steatosis was 75th percentile (mean BMI Z score = 0.92) while for those without steatosis was 44th percentile (mean BMI Z score= - 0.26), although this was not statistically significant possibly secondary to the small sample size (p=0.36). Mean (±SE) BMI percentiles across the four categories of fibrosis were relatively constant (METAVIR 0 = 61st ± 10.3, METAVIR 1 = 57th ± 4.2, METAVIR 2 = 70th ± 7.7, METAVIR 3-4 = 67th ± 6.3), and not statistically different (F(3,98)=1.1, p =0.36) (Figure 2). In a similar analysis, fibrosis score was then dichotomized as “low” (METAVIR 0-1) or “high” (METAVIR 2-4), and subjects were classified based on their BMI Z scores as either “overweight” or “lean” (using the cutoff point of BMI Z score > 1 which corresponds to > 85th percentile for age and gender). Although not statistically significant (χ2 =1.32, p=0.25), compared to lean subjects, overweight patients included a higher percentage of individuals with “high” fibrosis (47% vs. 35%).
Figure 1.
BMI percentile scores by steatosis category. The presence of steatosis was significantly associated with greater mean (±SE) BMI percentile scores (72nd ± 5.8 vs. 58th ± 3.5 percentile) F(1,101)=4.2, p=0.04). Diamonds represent 95% confidence intervals around the mean. Box plots represent subject to subject variation (box ends represent 25th to 75th percentiles).
Figure 2.
BMI percentile scores by fibrosis stage. Mean (±SE) BMI percentile scores across the four categories of fibrosis were relatively constant (METAVIR 0 = 61st ± 10.3, METAVIR 1 = 57th ± 4.2, METAVIR 2 = 70th ± 7.7, METAVIR 3-4 = 67th ± 6.3), and not statistically different (F(3,98)=1.1, p =0.36). Diamonds represent 95% confidence intervals around the mean. Box plots represent subject to subject variation (box ends represent 25th to 75th percentiles).
STUDY 2: Association of BMI with response to therapy
There were 32 sustained virologic responders (SVR) and 26 non-responders to antiviral therapy. Demographic characteristics by response category are summarized in Table 2. When comparing sustained virologic responders with nonresponders, there were no statistically significant differences in gender, age or infection duration. Not surprisingly, all but one nonresponder harbored HCV genotype 1, while sustained responders harbored genotypes 2, 3, and 4 in addition to 1. Forty five percent of patients were treated with PEG IFN and ribavirin, 22% with standard interferon and ribavirin, while the remainder received either standard interferon (14%) or pegylated interferon alone (19%). Although a larger percentage of responders than non-responders had been treated with the combination of PEG IFN and ribavirin (53% vs. 35%), there were no statistically significant differences between the two comparison groups with regard to regimen received (testing the frequency of use of PEG IFN/ribavirin vs. other treatment by response to therapy: (χ2 (1) = 1.99, p=0.16). There were 27 subjects with available BMI Z score one year prior to treatment. Among these, a strong correlation was also noted between baseline BMI Z score and BMI Z score from one year prior (R=0.975, p<0.0001).
TABLE 2. Demographic Characteristics by Response to Therapy (STUDY 2).
| Covariate (frequency or median, range) | SVR n = 32 (55%) | No SVR n = 26 (45%) | P value |
|---|---|---|---|
| Gender (Male) | (n=17) 53% | (n=15) 58% | 0.72 |
| Age (yr) | 12.8 (7.2 –18.3) | 13.0 (5.9 – 19.2) | 0.94 |
| Infection Duration (yr) | 12.3 (0.6 – 18) | 10.7 (2.8 – 19.2) | 0.86 |
| Genotypes | 1 (n=20) 63% | 1 (n=25) 96% | 0.004† |
| 2 (n=6) | 2 (n=1) | ||
| 3 (n=5) | |||
| 4 (n=1) | |||
| Overweight (BMI >85%ile) | (n=6) 19% | (n=11) 42% | 0.05 |
Testing frequency of genotype 1. SVR, sustained virologic response;
Diminished response to antiviral therapy with increased BMI in HCV infection
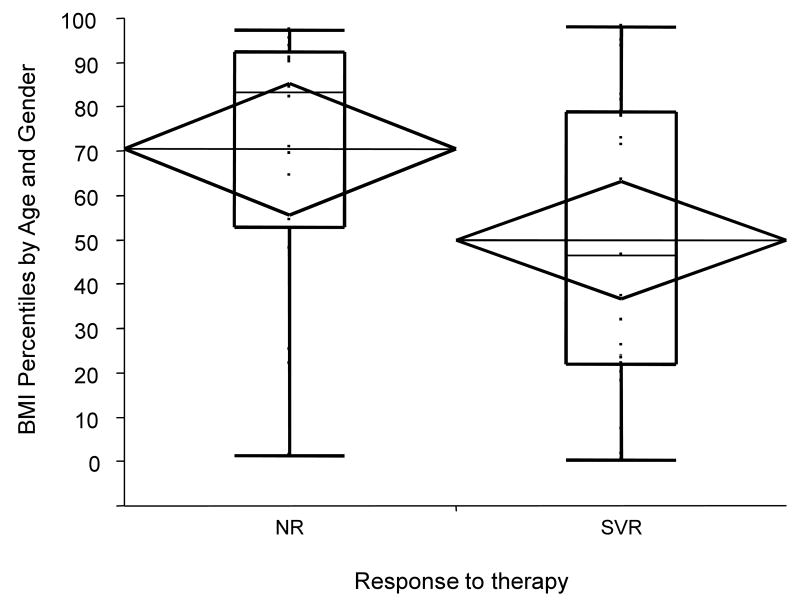
There was a greater percentage of overweight subjects among non-responders (42%) compared to responders (19%), which was statistically significant (χ2 (1) = 3.84, p = 0.05). In addition, non-responders to treatment had a higher mean (±SE) BMI percentile (70th ±7.4) when compared to responders (50th ±6.5) (p=0.04) (Figure 3). Thus, there was a univariate association between baseline BMI and response to therapy.
Figure 3.
BMI percentile scores by response to therapy. Mean (±SE) BMI percentile scores were higher for HCV treatment non-responders (NR) than for sustained virologic responders (SVR) (70th (±7.4) versus 50th (±6.5) respectively, p=0.04).
Association between higher baseline BMI and response to therapy is not explained by confounders
In order to explore the possibility of confounding by other variables, a multivariable model was constructed. A logistic regression model in STUDY 2 evaluated SVR (yes/no) as the dependent variable and BMI Z score, change in BMI Z score during the course of therapy, HCV genotype (1 vs. other), and use of ribavirin as independent variables. Change in BMI Z score during therapy was initially included as a potentially relevant variable given the fluctuations in weight that patients treated for HCV infection often experience. Inclusion of this variable was noted to be necessary because of the phenomenon of cooperative suppression, by which both higher baseline BMI Z score and increase in BMI Z score during therapy were negatively associated with the outcome (SVR), but inversely associated with each other. HCV genotype was included in the model because it is one of the most important determinants of response to therapy. The possibility of confounding by treatment modality was explored. The use of standard versus pegylated interferon did not have a statistically significant impact on response to therapy (data not shown), however, use of ribavirin with any form of interferon was associated with a greater likelihood of response (χ2 (1) = 3.8, p = 0.05). There was no actual bias in this study given that treatment was not chosen based on subjects BMI's. Interestingly, we found a greater BMI z score among subjects that used ribavirin versus those that did not and this was at the borderline of significance. Mean BMI z score for subjects who were not treated with ribavirin was -0.1 while mean BMI z score for those who were treated with ribavirin was +0.6, p =0.048. In order to evaluate the independent effects of treatment modality from BMI, use of ribavirin was included in the multivariate model.
In the final model we found that increasing baseline BMI Z score was independently associated with lower odds of response to therapy (Table 3). From this model it was calculated that one standard deviation (one Z score unit) increase in baseline BMI Z score is associated with a 12% decrease in the probability of SVR (Figure 4).
TABLE 3. Logistic Regression Results (STUDY 2).
| Independent Variables | Estimate* | Std Error | Chi Squared | P value |
|---|---|---|---|---|
| Intercept | 1.56 | 1.19 | 1.72 | 0.19 |
| BMI z score | - 0.75 | 0.33 | 5.09 | 0.02 |
| ΔBMI z score | - 0.44 | 0.56 | 0.62 | 0.43 |
| Genotype (1,4 vs. other) | - 2.65 | 1.19 | 4.96 | 0.03 |
| Ribavirin use | 1.70 | 0.81 | 4.41 | 0.04 |
Modeling log odds [SVR(=1)]. R Squared (U) for full model: 0.22, χ2 (4) = 17.9, p = 0.0013.
Std Error, standard error; BMI, body mass index
Figure 4.
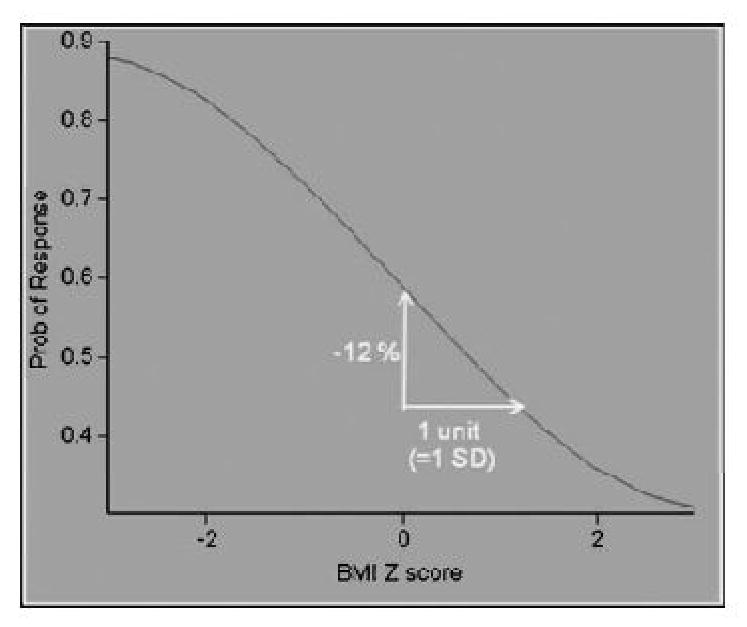
Logistic Response Curve for the Independent Effect of Baseline BMI Z-score on Sustained Virologic Response. Increasing baseline BMI Z score was independently associated with lower odds of response to therapy. One unit increase in baseline BMI Z score is associated with 12% decrease in response to therapy.
Discussion
It is now clear that HCV has a number of unique metabolic effects on the host that, in turn, render the infected person more vulnerable to adverse health outcomes. These effects, including hepatic steatosis, insulin resistance, and lipid changes, seem to be additionally aggravated by the presence of overweight and obesity. Compounding matters, there is evidence that steatosis and insulin resistance also adversely affect the success of antiviral therapy for HCV (24, 27-29). The proposed mechanisms linking obesity to diminished response to HCV therapy include: decreased interferon bioavailability, altered cytokine function, and insulin resistance (8). However, definitive conclusions about the independent contribution of increased BMI to HCV progression and response to therapy have not been reached.
In this investigation, we selected a pediatric cohort to ensure the use of weight-based dosing in antiviral regimens and to minimize comorbidities and potential confounders. Two groups with unique inclusion and exclusion criteria were selected in order to address the primary questions of interest: (1) what is the role of overweight in progression of HCV liver disease? and (2) what is the effect of overweight on response to antiviral therapy?
In STUDY 1, we found that overweight is associated with the development of steatosis in HCV infection. This finding could not be accounted for by varying age or infection duration among our subjects. Although the role of race or ethnicity could not be formally evaluated in our study, having a relatively uniform cohort precludes possible confounding based on racial differences. Though statistical significance was not demonstrated, there was a trend towards more extensive fibrosis among those who were overweight. A retrospective investigation by Giannattasio et al. evaluated 64 children from Naples, Italy, undergoing liver biopsy (30). Their study population carried mostly HCV genotype 1, and the distribution of steatosis was similar to that in our investigation, namely, 25% of children with either mild or moderate steatosis and 75% without steatosis. Giannattasio et al. did not find an association between BMI and steatosis in their cohort. The reason for the discrepancy in this regard with our results is unclear but might be explained by geographic and environmental differences. Interestingly, Giannattasio found an association between steatosis and greater fibrosis.
A more recent prospective multicenter trial, the PEDS-C Trial, demonstrated results more consistent with our findings. This trial investigated a total of 121 children from 11 United States Centers (31). They found that 32% of their subjects had minimal and 9% had mild steatosis, while severe steatosis was not observed. There was a statistically significant correlation between presence of steatosis and overweight in accordance with our data. Similarly, they did not find a statistically significant correlation between fibrosis and BMI. However, when categorizing subjects as lean vs. overweight they found significantly greater fibrosis in overweight children. In our investigation we observed a trend towards greater fibrosis in overweight children but this did not achieve statistical significance. We believe that a stronger association between increasing BMI and fibrosis in our cohort would likely have been manifest with longer-term followup. In addition, it is also possible that newer methods that assess fibrosis in a more comprehensive manner, such as elastography, could yield more informative results. Nevertheless, in light of the evidence supporting a positive correlation between steatosis and fibrosis (10, 12, 32), it seems clear that overweight poses a risk of progression to steatosis and possibly future fibrosis in youth chronically infected with HCV.
In STUDY 2, we noted a univariate association between overweight and higher BMI percentile with non-response to HCV therapy. A statistically higher percentage of therapy nonresponders were overweight when compared to responders, and nonresponders had higher baseline mean BMI percentiles for equivalent age and gender. When evaluating additional variables, we observed that use of ribavirin was associated with improved SVR, while genotype 1 or 4 were associated with diminished SVR. Increasing BMI Z score during the course of therapy was also associated with diminished response to therapy, although this was not statistically significant. More importantly, a higher baseline BMI Z score was independently associated with diminished response in multivariate analysis. In other words, the association noted in univariate analysis became stronger when accounting for important potential confounders. Based on the multivariate model one unit increase in baseline BMI Z score was associated with a 12% decrease in the probability of SVR. These results could not be explained by differences in infection duration, genotype, treatment used or any of the other variables analyzed. It is noteworthy that in both STUDY 1 and STUDY 2, BMI proved to be stable as there was a strong and statistically significant correlation in BMI within one year period. Stability of BMI for longer periods of time has also been previously demonstrated in both children and adults (33, 34).
Although weight-based dosing was used in this study, the upper limits set on dosages are a potential limitation. However, review of BMI levels demonstrated that there were no morbidly obese patients in our cohort and there were no statistically significant differences in raw BMI values between the two comparison groups. Thus, it is unlikely that our findings could be explained by relative underdosing of heavier patients. Another potential limitation of this investigation is its retrospective nature. As a consequence, more than one treatment modality was included in this investigation. However, there was no selection bias as treatment was not chosen based on subjects' BMI values. In addition, careful evaluation of treatment modality revealed that this was not a confounder in the association between BMI and response to therapy. Use of ribavirin was associated with improved response, but subjects who received ribavirin had higher mean BMI z scores and still had lower percentages of eradication of the virus which does not support confounding by treatment. Through multivariate analyses, the independent effects of treatment and BMI were simultaneously evaluated in the final model and reported in Table 3. Because of the retrospective nature of this investigation there were no sample size calculations obtained a priori. Nevertheless, it is unlikely that a larger sample size would have significantly altered our results. Post-hoc calculations of frequency of overweight among patients with significant fibrosis suggest that approximately 560 subjects (280 subjects in the “high fibrosis” and 280 subjects in the “low fibrosis” groups) would be needed to statistically detect a 12% difference (47% versus 35%) at a power of 80%, based on a two tailed test with an alpha level of .05. The clinical significance of this difference in fibrosis is unclear, but it is possible that different methods of assessing fibrosis would be more appropriate and might lead to more meaningful results. Based on our results, controlling overweight prior to therapy should improve the probability of eradication of HCV infection. Despite the apparent protective effect of even very low BMI's from our results, at this point we would not recommend weight reduction below a BMI of 50th percentile for age and gender. Prospective studies may provide a more accurate assessment of the actual effect of change in BMI prior to initiation of treatment on response to therapy through the evaluation of individual fluctuations in BMI. We postulate that such prospective studies may reveal stronger effects than those noted here given the evidence available with regards to the effects of even moderate weight loss on abnormal liver enzymes or metabolic risk factors and other conditions (35, 36).
In summary, our study suggests that overweight adversely affects the progression of chronic HCV liver disease by way of steatosis and demonstrates an association between overweight prior to therapy with diminished response to antiviral therapy using weight-based dosing in a cohort with minimal confounding variables. We believe our results are generalizable to the HCV-infected population at large, including children and adults. Prospective studies to evaluate the effect of weight control on response to HCV therapy will be critical in an attempt to halt the progression and improve the odds of sustained clearance of an otherwise progressive disease of young people.
Acknowledgments
This work was supported by: NIH DK070022 (ADB), DK78772 (RTC), and the Robert Wood Johnson Foundation (ADB)
List of Abreviations
- HCV
hepatitis C virus
- BMI
body mass index
- SVR
sustained virologic response
- RNA
ribonucleic acid
- IFN
interferon
- PEG IFN
pegylated interferon
- NHANES
National Health and Nutrition Examination Survey
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Strauss RS, Pollack HA. Epidemic increase in childhood overweight, 1986-1998. JAMA. 2001;286:2845–2848. doi: 10.1001/jama.286.22.2845. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes. 2006;1:11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 4.Sabin MA, Shield JP. Childhood obesity. Front Horm Res. 2008;36:85–96. doi: 10.1159/000115356. [DOI] [PubMed] [Google Scholar]
- 5.Lob-Corzilius T. Overweight and obesity in childhood--a special challenge for public health. Int J Hyg Environ Health. 2007;210:585–589. doi: 10.1016/j.ijheh.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 6.Lee WW. An overview of pediatric obesity. Pediatr Diabetes. 2007;8 9:76–87. doi: 10.1111/j.1399-5448.2007.00337.x. [DOI] [PubMed] [Google Scholar]
- 7.Lobstein T, Millstone E. Context for the PorGrow study: Europe's obesity crisis. Obes Rev. 2007;8 2:7–16. doi: 10.1111/j.1467-789X.2007.00354.x. [DOI] [PubMed] [Google Scholar]
- 8.Charlton MR, Pockros PJ, Harrison SA. Impact of obesity on treatment of chronic hepatitis C. Hepatology. 2006;43:1177–1186. doi: 10.1002/hep.21239. [DOI] [PubMed] [Google Scholar]
- 9.Younossi ZM, McCullough AJ, Ong JP, Barnes DS, Post A, Tavill A, Bringman D, et al. Obesity and non-alcoholic fatty liver disease in chronic hepatitis C. J Clin Gastroenterol. 2004;38:705–709. doi: 10.1097/01.mcg.0000135372.10846.2a. [DOI] [PubMed] [Google Scholar]
- 10.Hu KQ, Kyulo NL, Esrailian E, Thompson K, Chase R, Hillebrand DJ, Runyon BA. Overweight and obesity, hepatic steatosis, and progression of chronic hepatitis C: a retrospective study on a large cohort of patients in the United States. J Hepatol. 2004;40:147–154. doi: 10.1016/s0168-8278(03)00479-3. [DOI] [PubMed] [Google Scholar]
- 11.Ortiz V, Berenguer M, Rayon JM, Carrasco D, Berenguer J. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol. 2002;97:2408–2414. doi: 10.1111/j.1572-0241.2002.05995.x. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh MH, Lee LP, Hsieh MY, Tsai KB, Huang JF, Hou NJ, Chen SC, et al. Hepatic steatosis and fibrosis in chronic hepatitis C in Taiwan. Jpn J Infect Dis. 2007;60:377–381. [PubMed] [Google Scholar]
- 13.Patton HM, Patel K, Behling C, Bylund D, Blatt LM, Vallee M, Heaton S, et al. The impact of steatosis on disease progression and early and sustained treatment response in chronic hepatitis C patients. J Hepatol. 2004;40:484–490. doi: 10.1016/j.jhep.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, Kaslow RA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Peralta RP. Treatment of chronic hepatitis C in children. Pediatr Transplant. 2004;8:639–643. doi: 10.1111/j.1399-3046.2004.00250.x. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Peralta RP, Kelly DA, Haber B, Molleston J, Murray KF, Jonas MM, Shelton M, et al. Interferon alfa-2b in combination with ribavirin for the treatment of chronic hepatitis C in children: efficacy, safety, and pharmacokinetics. Hepatology. 2005;42:1010–1018. doi: 10.1002/hep.20884. [DOI] [PubMed] [Google Scholar]
- 17.Seeff LB, Hoofnagle JH. National Institutes of Health Consensus Development Conference: management of hepatitis C: 2002. Hepatology. 2002;36:S1–2. doi: 10.1053/jhep.2002.36992. [DOI] [PubMed] [Google Scholar]
- 18.Seeff LB, Hoofnagle JH. Appendix: The National Institutes of Health Consensus Development Conference Management of Hepatitis C 2002. Clin Liver Dis. 2003;7:261–287. doi: 10.1016/s1089-3261(02)00078-8. [DOI] [PubMed] [Google Scholar]
- 19.Harrison SA, Brunt EM, Qazi RA, Oliver DA, Neuschwander-Tetri BA, Di Bisceglie AM, Bacon BR. Effect of significant histologic steatosis or steatohepatitis on response to antiviral therapy in patients with chronic hepatitis C. Clin Gastroenterol Hepatol. 2005;3:604–609. doi: 10.1016/s1542-3565(05)00246-6. [DOI] [PubMed] [Google Scholar]
- 20.Romero-Gomez M, Del Mar Viloria M, Andrade RJ, Salmeron J, Diago M, Fernandez-Rodriguez CM, Corpas R, et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636–641. doi: 10.1053/j.gastro.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 21.Lo Iacono O, Venezia G, Petta S, Mineo C, De Lisi S, Di Marco V, Rodolico V, et al. The impact of insulin resistance, serum adipocytokines and visceral obesity on steatosis and fibrosis in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2007;25:1181–1191. doi: 10.1111/j.1365-2036.2007.03309.x. [DOI] [PubMed] [Google Scholar]
- 22.Bressler BL, Guindi M, Tomlinson G, Heathcote J. High body mass index is an independent risk factor for nonresponse to antiviral treatment in chronic hepatitis C. Hepatology. 2003;38:639–644. doi: 10.1053/jhep.2003.50350. [DOI] [PubMed] [Google Scholar]
- 23.Walsh MJ, Jonsson JR, Richardson MM, Lipka GM, Purdie DM, Clouston AD, Powell EE. Non-response to antiviral therapy is associated with obesity and increased hepatic expression of suppressor of cytokine signalling 3 (SOCS-3) in patients with chronic hepatitis C, viral genotype 1. Gut. 2006;55:529–535. doi: 10.1136/gut.2005.069674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarantino G, Conca P, Sorrentino P, Ariello M. Metabolic factors involved in the therapeutic response of patients with hepatitis C virus-related chronic hepatitis. J Gastroenterol Hepatol. 2006;21:1266–1268. doi: 10.1111/j.1440-1746.2006.04394.x. [DOI] [PubMed] [Google Scholar]
- 25.Gheorghe L, Iacob S, Sporea I, Grigorescu M, Sirli R, Damian D, Gheorghe C, et al. Efficacy, tolerability and predictive factors for early and sustained virologic response in patients treated with weight-based dosing regimen of PegIFN alpha-2b ribavirin in real-life healthcare setting. J Gastrointestin Liver Dis. 2007;16:23–29. doi: 10.1007/s11749-007-0047-9. [DOI] [PubMed] [Google Scholar]
- 26.Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15–20. [PubMed] [Google Scholar]
- 27.Blonsky JJ, Harrison SA. Review article: nonalcoholic fatty liver disease and hepatitis C virus--partners in crime. Aliment Pharmacol Ther. 2008;27:855–865. doi: 10.1111/j.1365-2036.2008.03672.x. [DOI] [PubMed] [Google Scholar]
- 28.Poustchi H, Negro F, Hui J, Cua IH, Brandt LR, Kench JG, George J. Insulin resistance and response to therapy in patients infected with chronic hepatitis C virus genotypes 2 and 3. J Hepatol. 2008;48:28–34. doi: 10.1016/j.jhep.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 29.Soresi M, Tripi S, Franco V, Giannitrapani L, Alessandri A, Rappa F, Vuturo O, et al. Impact of liver steatosis on the antiviral response in the hepatitis C virus-associated chronic hepatitis. Liver Int. 2006;26:1119–1125. doi: 10.1111/j.1478-3231.2006.01347.x. [DOI] [PubMed] [Google Scholar]
- 30.Giannattasio A, Spagnuolo MI, Sepe A, Valerio G, Vecchione R, Vegnente A, Iorio R. Is HCV infection associated with liver steatosis also in children? J Hepatol. 2006;45:350–354. doi: 10.1016/j.jhep.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Goodman ZD, Makhlouf HR, Liu L, Balistreri W, Gonzalez-Peralta RP, Haber B, Jonas MM, et al. Pathology of chronic hepatitis C in children: liver biopsy findings in the Peds-C Trial. Hepatology. 2008;47:836–843. doi: 10.1002/hep.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westin J, Nordlinder H, Lagging M, Norkrans G, Wejstal R. Steatosis accelerates fibrosis development over time in hepatitis C virus genotype 3 infected patients. J Hepatol. 2002;37:837–842. doi: 10.1016/s0168-8278(02)00299-4. [DOI] [PubMed] [Google Scholar]
- 33.Hesketh K. Stability of body mass index in Australian children: a prospective cohort study across the middle childhood years. Public Health Nutrition. 2003;7:303–309. doi: 10.1079/phn2003537. [DOI] [PubMed] [Google Scholar]
- 34.Katzmarzyk PT. Seven-year stability of indicators of obesity and adipose tissue distribution in the Canadian population. The American Journal of Clinical Nutrition. 1999;69:1123–1129. doi: 10.1093/ajcn/69.6.1123. [DOI] [PubMed] [Google Scholar]
- 35.St George A, Bauman A, Johnston A, Farrell G, Chey T, George J. Effect of a lifestyle intervention in patients with abnormal liver enzymes and metabolic risk factors. J Gastroenterol Hepatol. 2009;24:399–407. doi: 10.1111/j.1440-1746.2008.05694.x. [DOI] [PubMed] [Google Scholar]
- 36.Vitola BE, Deivanayagam S, Stein RI, Mohammed BS, Magkos F, Kirk EP, Klein S. Weight Loss Reduces Liver Fat and Improves Hepatic and Skeletal Muscle Insulin Sensitivity in Obese Adolescents. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]