Abstract
Aims
We used a novel technique, high definition manometry (HDM) that utilizes 256 tactile sensitive micro-transducers to define the characteristics of vaginal high-pressure zone.
Methods
16 nullipara asymptomatic women were studied using HDM, transperineal 2D dynamic ultrasound and dynamic magnetic resonance (MR) imaging.
Results
Vaginal high-pressure zone revealed higher contact pressures in anterior and posterior compared to lateral directions, both at rest and squeeze. At rest, anterior pressure cluster is located 10 mm cephalad to posterior pressure cluster; with squeeze the latter moves in the cranial direction by 7 mm. Ultrasound and MR images reveal that the anorectal angle moves cephalad and ventrally during squeeze. Cephalad movement of posterior pressure cluster during squeeze is similar to the cranial movement of anorectal angle.
Conclusions
We propose that the vaginal high-pressure zone represents the constrictor function and cranial movement of the posterior pressure cluster represents the elevator function of pelvic floor.
INTRODUCTION
Vaginal pressure is a key measure of the strength of the pelvic floor muscles. Kegel was the first to use a pneumatic resistance chamber to measure vaginal pressure and perform biofeedback therapy using his device to improve the strength of pelvic floor muscle1, 2. Since then several investigators have used various types of devices to measure vaginal pressure/force as a measure of the pelvic floor strength3–10. Pressures in general are directionless and should be symmetrical on all sides. However such is not the case in various sphincters or high pressure zones of body because these are contact and not the cavity or fluid pressures. Using side hole infusion manometery technique that can measure contact pressure at a point location, we found that there is a high pressure zone (HPZ) in the distal part of the vagina which shows axial and circumferential asymmetry11 of the contact pressures at rest and during contraction. Furthermore the contact pressures increase significantly during pelvic floor contraction. Using a water filled bag placed in the vagina and 3 dimensional ultrasound imaging of the pelvic floor, we visualized the vaginal HPZ to be located just above the level of hymen. Shape of the deformed water bag located in the vagina suggests that the forces responsible for genesis of vaginal HPZ are directed in the anterior–posterior direction12. Based on above characteristics, we postulated that the vaginal HPZ is most likely related to the ventral movement of the puborectalis component of pelvic floor muscles.
The goal of our current study was to define the static and dynamic characteristics of vaginal HPZ using a novel, tactile pressure-sensing technology, i.e., the high definition manometry (HDM). The HDM can measure contact pressures at closely spaced intervals and therefore it has the potential to provide information on the direction of forces and specific muscles responsible for the genesis of vaginal HPZ. In order to further understand the characteristics of vaginal HPZ revealed by HDM, we recorded dynamic, two dimensional (2D) ultrasound images of the pelvic floor muscles. Additionally, dynamic magnetic resonance (MR) imaging of the pelvis and pelvic floor muscles was performed to study the anatomical relationship between vaginal HPZ and the adjacent pelvic floor structures.
METHODS
The UCSD Institutional Review Board approved the study protocol and each subject signed an informed consent prior to participation in the study protocol. These subjects responded to an advertisement and were reimbursed nominal amount of money for participation in the study. The study was conducted in 16 nulliparous women with a mean age of 37.4 years (range: 21–61 years). 2D-ultrasound and HDM were performed in 11 subjects and MR imaging was obtained in 5 subjects. Each subject completed medical history and a previously validated urinary incontinence13 and anal incontinence14 scoring questionnaires to confirm the absence of urinary and anal incontinence symptoms. Prior to starting the study, each subject was instructed to contract the pelvic floor by a prompt ‘‘squeeze as if you were trying to stop your stream of urine.’’ A simultaneous digital vaginal examination by the investigator ensured contraction of the pelvic floor muscle.
High Definition Manometry (HDM)
Vaginal pressures were recorded using a newly developed HDM probe by Sierra Scientific Instruments Inc (ManoScan 360 HD™, Los Angles CA.) that has following features; 1) the probe is 10 mm in diameter and the pressure sensitive part of the probe is 64 mm in length, 2) there are 256 transducers on the surface of the HDM probe that form a continuous grid in both the axial and circumferential directions, and 3) each transducer is 4 mm long (axially) and 2 mm wide (circumferentially). HDM probe has following functional characteristics: 1) pressures from all transducers are recorded digitally and displayed on a personal computer as color plots (ManoView HD beta™, Sierra Scientific Instruments Inc), 2) in–vitro testing revealed that an externally applied pressure on each transducer does not influence the output of the adjacent transducer; 3) pressure recordings have an accuracy of 5 mmHg. The HDM probe was placed in the vagina in such a fashion that the entire vaginal HPZ was captured; the most cranial part of the probe recorded abdominal pressure and the most caudal part measured the atmospheric pressures. The circular orientation of the probe in relation to the anterior midline, posterior midline, left lateral and right lateral orientation of the vagina was noted. Measurements were obtained while subject was at rest and then during 3 sustained maximal pelvic floor contractions (squeeze) and relaxations.
Two Dimensional Dynamic Ultrasonography
A 3D-ultrasound system (Philips HD11; Phillips Medical Systems, Bothell, WA) was used to acquire images of pelvic organs and pelvic floor muscles. With the subject in the lithotomy position, 2D-ultrasound dynamic cinematic loops were obtained by placing a 3–9 MHz, transvaginal ultrasound transducer on the perineum. In order to increase the field of view of structures close to the skin, a custom-built standoff, made of agar, was placed on the US transducer. The US probe with the standoff in place was directed cranially to image the pelvic floor hiatus and surrounding muscles. The 2D ultrasound cinematic loops were recorded during pelvic floor contraction; they were archived on compact disk and viewed off-line and analyzed with the Q lab 4.2 software program (Phillips Medical Systems, Bothell, WA).
Magnetic Resonance (MR) Imaging
Dynamic MR imaging of the pelvis was recorded in the mid-sagittal plane with and without a water-filled bag placed in the vagina (Figure 3). The bag, built from a non-compliant polyethylene material was 10 cm long and had a maximal diameter of 3.5 cm when fully distended. MR imaging was performed using 1.5 T super conducting magnet (Symphony; Siemens Medical Systems, Erlangen, Germany) machine. The following MR parameters were used for MR imaging, echo time of 2.52 milliseconds, repetition time of 5.03 milliseconds, slice thickness of 10 mm and slice gap of 20%, yielding an image matrix of 256 × 100. Images were recorded at rest and during contraction of pelvic floor muscles. The MR sequence allowed capturing of 2D MR images in the mid sagittal plane at a temporal resolution of 1 Hz. The bag was filled with 50 cc of water before the acquisition of dynamic MR images. Images were recorded at rest and continuously during a sustained and maximal pelvic floor contraction. MR images were analyzed using Syngo Fast View software (Siemens AG 2004, Munich Germany).
Figure 3.
(panels A and B) Sagittal MR images demonstrating the relationship between pelvic structures at rest and during squeeze. (panel C and D) Sagittal MR images outlining the high pressure zone (HPZ) with the help of water filled bag located in the vagina. White arrow shows the anterior (ventral) and cephlad (superior) motion of the anorectal angle (ARA). EAS-external anal sphincter, PS-Pubic symphsis, PRM-puborectalis muscle.
DATA ANALYSIS
Vaginal High Definition Manometry
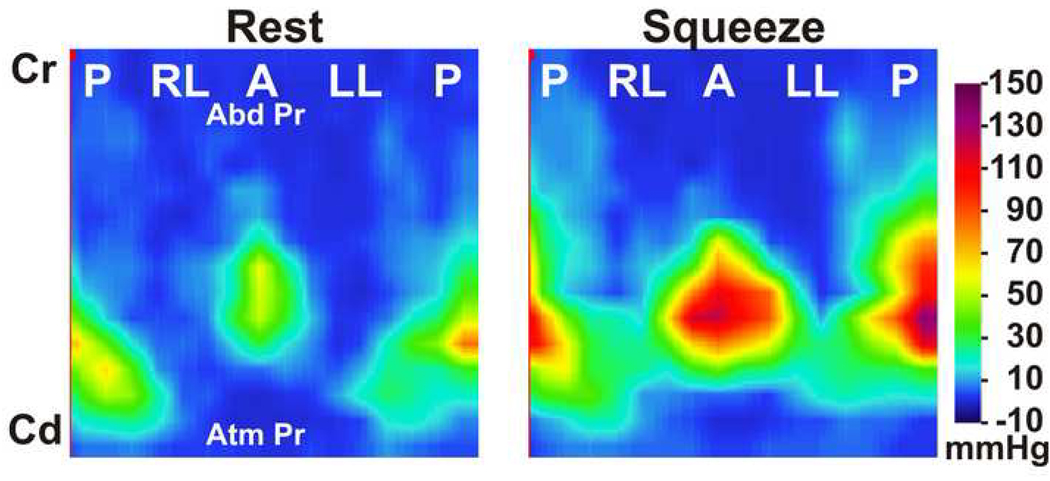
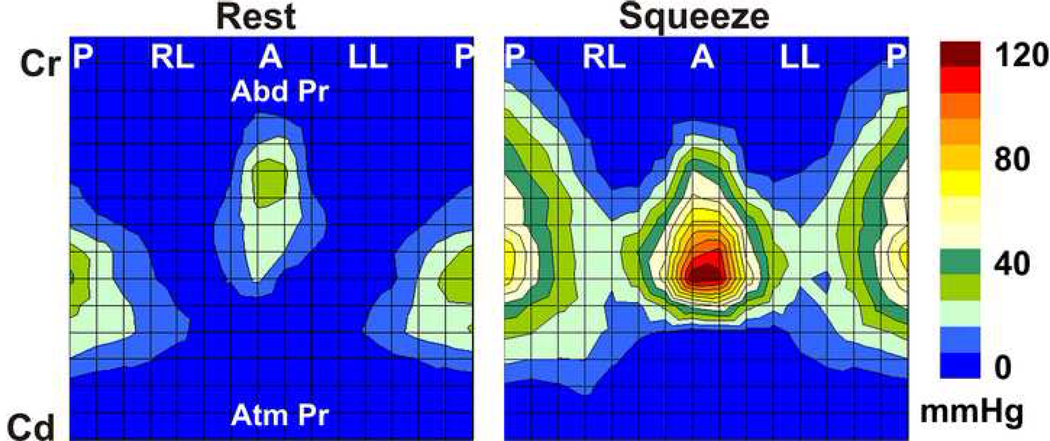
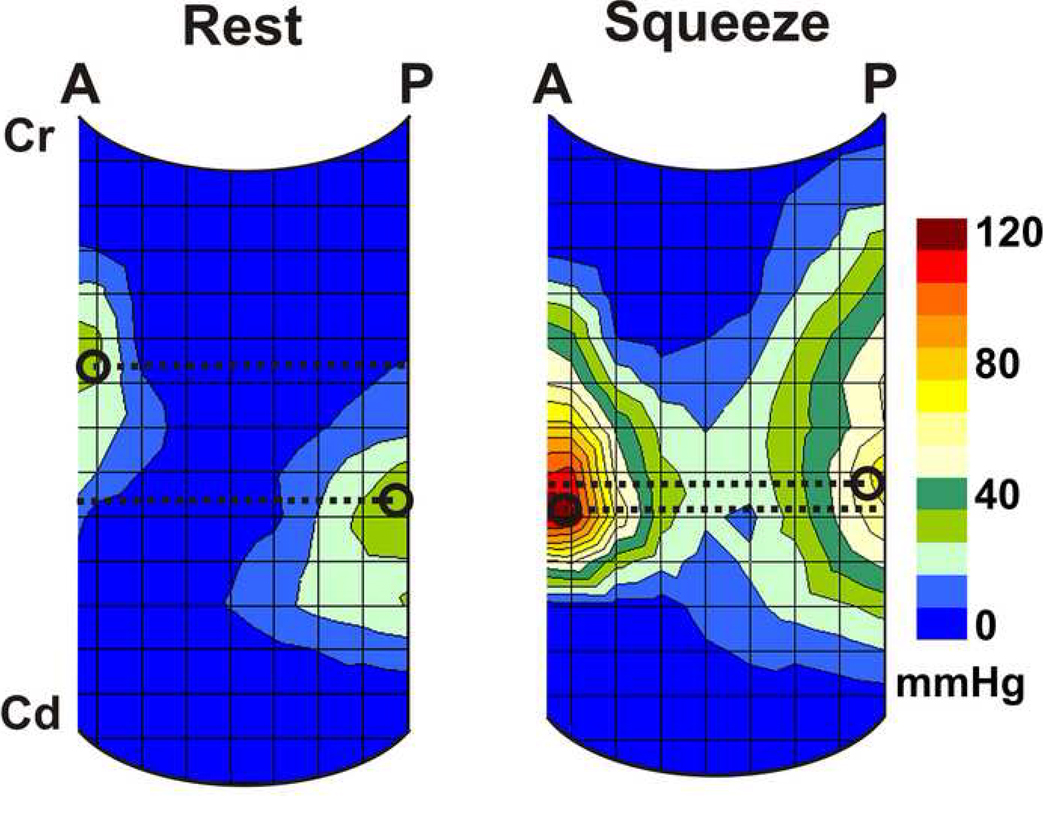
Pressures were displayed as color plots and revealed three distinct pressure zones. The upper zone (distal part of the probe) shows uniform pressures that reflect transmitted abdominal pressure into the vagina; the middle zone represents the axial and circumferential asymmetric contact pressures of the vaginal HPZ and the lower zone (proximal part of the probe) represents pressures from the part of the probe located below the hymen that represents atmospheric pressure (Figure 1). From the first two (proximal) and the last two rows (distal) of pressure sensors, the mean values and standard deviation were computed to determine the mean atmospheric and mean abdominal pressures respectively, which were used to define the caudal and cranial edges of the vaginal HPZ respectively. In order to define the upper and lower borders of vaginal HPZ, the raw data from 256 sensors was exported to an Excel sheet and viewed as a 16 × 16 table. At rest and during squeeze, contact pressures of the vaginal HPZ were averaged over 2 s periods. The cranial edge of the vaginal HPZ was defined at a location where the pressure was noted to be 2 standard deviations higher than the abdominal pressure while the caudal edge was defined where the pressure was noted to be 2 standard deviations higher than the atmospheric pressures. Measurements of the length, peak contact pressures and location of peak contact pressures in the vaginal HPZ were determined from the reconstructed vaginal HPZ. Lastly, contact pressures from the vaginal HPZ of the 11 subjects were averaged in order to obtain a composite vaginal HDM profile at rest and pelvic floor contraction (Figure 2). Mean pressure profile was calculated by optimally aligning the pressure plots of all subjects. The optimal alignment was achieved by calculating the correlation coefficients between the pressure values of the two subjects at a time and then sliding the transducer position axially (± 3 transducer position) and circumferentially (±1 transducer position). The optimal pressure transducer alignment was the one that yielded a maximal correlation coefficient. This method of alignment allowed correction for variations in the depth of probe insertion and possible slight misalignment in the circumferential direction.
Figure 1.
Color high definition manometry plot of the vaginal pressures at rest and pelvic floor contraction. Plot represents pressures from 256 pressure sensors displayed in a single plane. (P-posterior, RL-right lateral, A-anterior and LL-left lateral, Cr-cranial end, Cd-caudal end). Note the axial and circumferential pressure asymmetry of the vaginal high-pressure zone.
Figure 2.
Average color plot of the high definition manometry derived from resting data from 11 subjects. (Abbreviations, same as figure 1).
Two-Dimensional Dynamic Ultrasonography
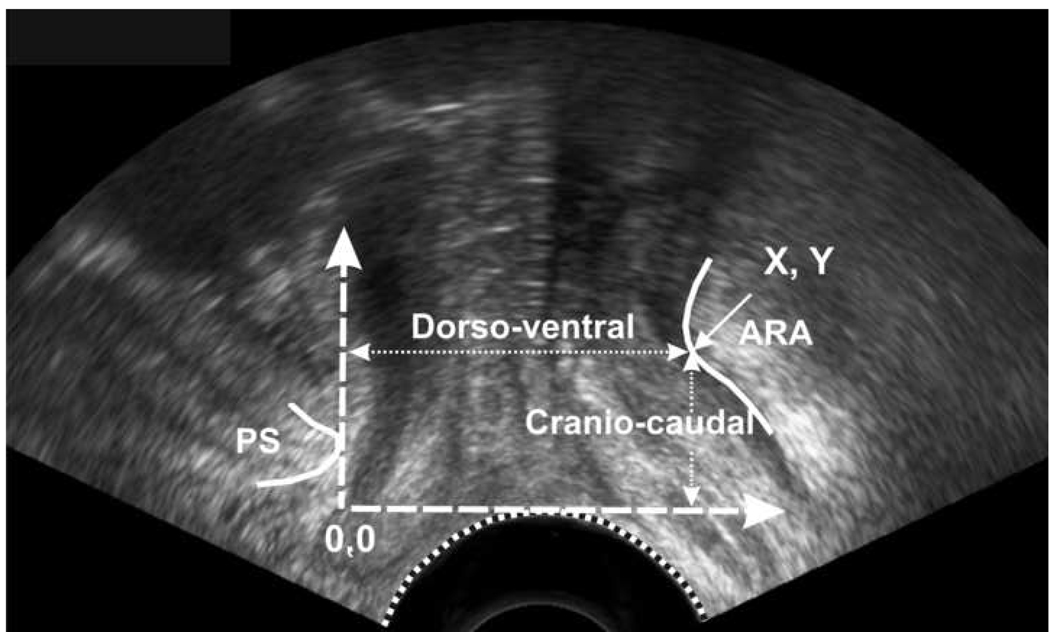
2D- ultrasound images of the pelvic floor, at rest and at peak pelvic floor contraction were extracted from the cinematic loops and analyzed with the help of sigma scan. X-axis (horizontal line) was drawn parallel to the transducer surface where it touched the skin and was used as a reference line against which the cranio-caudal distance moved by the anorectal angle was measured. Y-axis (vertical line) was drawn tangential to the inferior and posterior point of pubic symphysis and perpendicular to the first line and was used as a reference line against which the dorso-ventral distance moved by the anorectal angle was measured. These reference lines were part of the Cartesian coordinate system as described previously15. Our measurement system is slightly different from the one suggested by others16 17 and was designed to measure both the horizontal and vertical movements of anorectal angle on US images.
MR Imaging
The anorectal angle moves in the cranial and ventral direction during pelvic floor contraction and these movements were analyzed using Syngo Fast View (Siemens AG 2004). A horizontal line, passing tangentially through the lower edge of the pubic symphysis was used to measure the cranio-caudal movement of ARA. A vertical line, passing tangentially through the lower edge of the pubic symphysis was used to measure the dorso-vental movement of anorectal angle. Similar to ultrasound image analysis, all measurements were obtained using a Cartesian coordinate system.
Statistical analysis
The vaginal HPZ length, contact pressures and location of peak contact pressure obtained from each quadrant were compared at rest and during contraction. Cranial movement of the location of the peak contact pressure recorded by HDM was compared with the cranial movement of the anorectal angle observed on dynamic ultrasound images. Two tailed paired t-test was used for statistical comparisons and significance was defined as P < 0.05.
RESULTS
Vaginal Pressure at Rest & Pelvic Floor Contraction
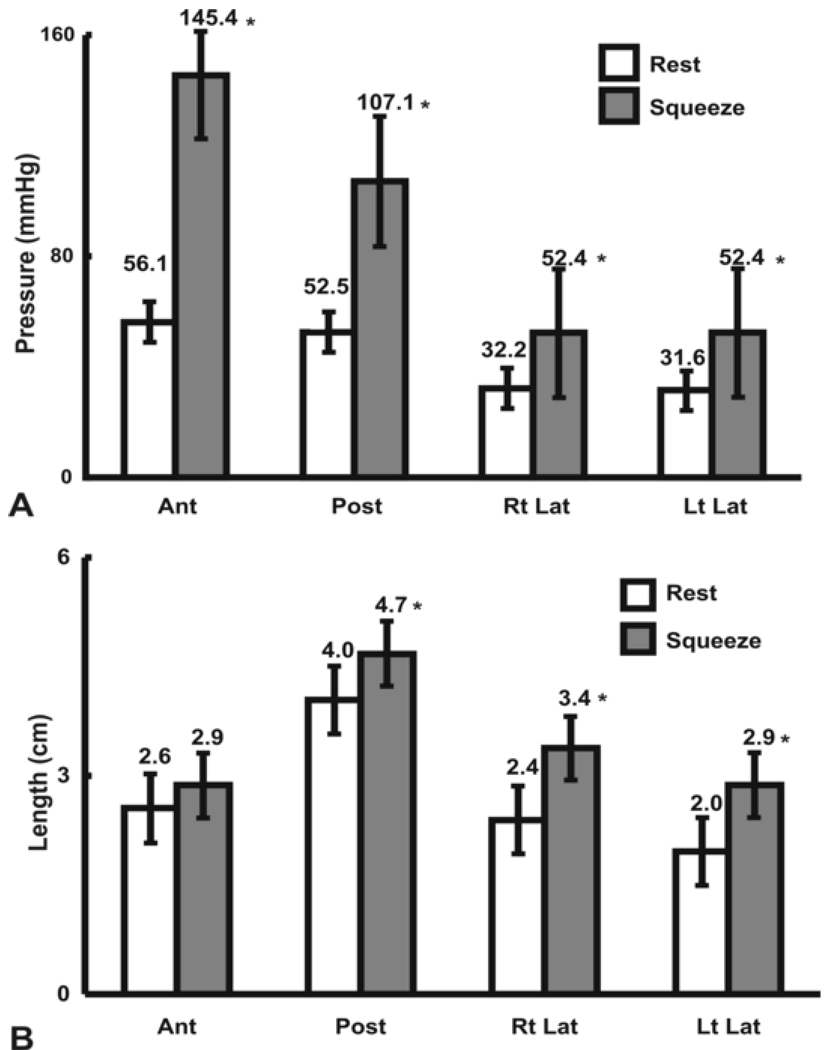
Figure 1 shows the HDM color plots of vaginal pressures at rest and at peak pelvic floor contraction in one subject. These measurements are highly reproducible from one pelvic floor contraction to another in a given subject. Figure 2 shows the HDM plots derived from the mean data obtained from 11 subjects. These plots reveal several important characteristics of the vaginal HPZ at rest and during pelvic floor contraction. At rest and during contraction, the HDM plot shows that the contact pressures are not uniformly distributed in the vaginal HPZ, i.e., there is an asymmetry of contact pressures in both the longitudinal and the circumferential directions. Figure 4 (A) and (B) show mean data from 11 subjects with regards to the peak contract pressure and the lengths of vaginal HPZ in four different directions, i.e., anterior midline, posterior midline, right lateral and left lateral. The contact pressures in the vaginal HPZ are higher in the anterior and posterior direction as compared to lateral directions. Furthermore, the anterior peak contract pressures are greater than the posterior peak contact pressures, especially during pelvic floor contraction. With pelvic floor contraction, the contact pressures increase in all directions in the vaginal HPZ but more so in the anterior and posterior directions compared to lateral directions. Vaginal HPZ is longer in the posterior compared to anterior and lateral directions. Dimension of the vaginal HPZ increases in all directions with pelvic floor contraction and this increase is maximal in the posterior direction.
Figure 4.
(A) Bar graph showing the vaginal peak pressures in the vaginal high pressure zone in each quadrant, at rest and during squeeze (B) Bar graph shows the length of vaginal high pressure zone in four quadrants, at rest and during squeeze (pelvic floor contraction). * = p < 0.05, compared to rest
HDM plots at rest and during pelvic floor contraction reveal another important aspect of the vaginal HPZ, i.e., the relative location of the anterior and posterior contact pressure clusters in the vaginal HPZ. At rest, the anterior contact pressure cluster is located 10 mm cephalad to the posterior contact pressure cluster. With pelvic floor contraction, the posterior but not the anterior contact pressure cluster moves in the cranial direction so that the peak contact pressure points in the anterior and posterior pressure clusters are located nearly at the same axial level on the HDM probe. This phenomenon is exemplified in the averaged plot derived from 11 subjects in figure 5. In fact, in some subjects, the posterior contact pressure cluster relocates to a more cephalad location than the anterior contact pressure cluster. The posterior peak contact pressure point moves by a mean distance of 7 mm (n = 11) in the vertical or cephalad direction during pelvic floor contraction.
Figure 5.
Sagittal view of the high definition manometry plots at rest and during squeeze demonstrating the location of the peak pressure in the anterior (A) and posterior (P) midline. Black circle marks the location and broken line depicts the level of peak pressures. With pelvic floor contraction the distance (Δ = Delta) between the peak pressures decreases from 1.0 to 0.2 cm. Cr-Cranial end, Cd-Caudal end
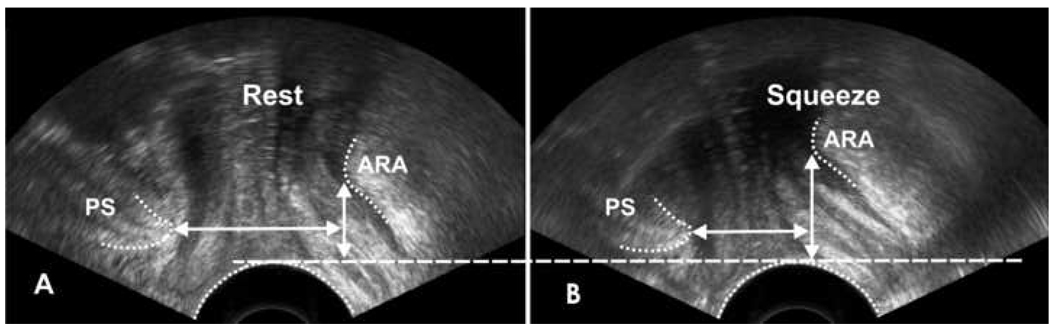
2D-ultrasound cinematic loops of the pelvis show that the anorectal angle moves in the ventral and cranial directions during pelvic floor contraction (Figure 6). The mean anorectal angle displacement in ventral and cranial directions is show in table 1.
Figure 6.
Ultrasound images at rest (A) and during squeeze (B) depicting the movement of anorectal angle (ARA). With squeeze the ARA moves in cranial (vertical white arrow) and ventral (horizontal white arrow) direction.
Table 1.
Location of anorectal angle (ARA) at rest and pelvic floor contraction, HDM-high definition manometry, US-ultrasound, MRI-magnetic resonance imaging (*= No statistical difference between delta cranial movement recorded by HDM & US). MRI also detected cranial and ventral movement of the ARA that were similar to the one made by US.
| Distance (cm) moved by ARA in cranial and ventral directions | |||||
|---|---|---|---|---|---|
| CRANIAL | VENTRAL | ||||
| HDAM | US | MRI | US | MRI | |
| REST | 2.5 | 2.7 | 1.8 | 3.6 | 4.8 |
| CONTRACTION | 3.2 | 3.3 | 2.4 | 2.5 | 3.7 |
| DELTA | 0.7 * | 0.5 | 0.7 | 1.1 | 1.1 |
In order to further our understanding of the dynamic properties of the vaginal HPZ, we performed dynamic MRI images of the pelvis in the mid-sagittal plane. For these MR images, a water-filled bag was placed in the vagina to determine the location of the vaginal HPZ in relation to the other anatomical structures in the pelvis (Figure 3). The vaginal HPZ is seen as an anterior and posterior indentation on the bag in the mid-sagittal image of the pelvis. Several important characteristics of the vaginal HPZ are seen in these images. First, note the anatomical location of the constriction on the bag and its relationship to other anatomical structures, i.e., pubic symphysis, urethra and anal canal. The constriction on the bag is sandwiched between the urethra anteriorly and anal canal posteriorly. The vaginal HPZ is longer in the posterior, as compared to anterior direction. With pelvic floor contraction, similar to US images, the anorectal angle moves ventrally and cephaladly. Interestingly, the cephalad movement of the anorectal angle on MRI and US images is numerically same as the cephalad movement of posterior contact pressure cluster on the HDM plot, (Table 1). Ventral movement of the anorectal angle during pelvic floor contraction, as seen on the MR and ultrasound images are also similar and are shown in Table 1.
DISCUSSION
In brief, our data show the following: 1) HDM plots reveal that in the vaginal HPZ, the contact pressures are distributed asymmetrically in different quadrants, with lateral contact pressures being significantly smaller than the anterior and posterior ones. During pelvic floor contraction there is significant increase in the contact pressure in all quadrants of the vaginal HPZ but the major increase occurs in the anterior and posterior directions. 2) With pelvic floor contraction, there is significant cranial movement of the posterior contact pressure cluster in relationship to the anterior contact pressure cluster. In fact, anterior and posterior contact pressure clusters come axially (along the probe) closer to each other. 3) 2D-ultrasound and MR images show that the anorectal angle moves in the ventral and cranial directions during pelvic floor contraction. The cranial movement of the anorectal angle, as revealed by US images and MR images is of the same magnitude as that of the vertical movement of the posterior contact pressure cluster on the HDM probe. 4) MR images with a collapsible bag in the vagina show the location of vaginal HPZ in relationship to the pubic symphysis, urethra, anal canal and other pelvic floor structures.
At first impression, it does not make sense that pressures in vaginal HPZ are asymmetric in the axial and circumferential direction because pressure is generally considered to be directionless. In fact, ones initial reaction may be that the pressure asymmetry is an artifact of the measuring system. However, an important distinction needs to be made between the cavity pressure and the contact pressures. The pressures in the sphincters or HPZs are contact and not the cavity pressure. Contact pressures may be asymmetric depending upon the anatomical direction of muscle fibers contributing to the HPZ and the surrounding structures. Asymmetry of the contact pressures is well described in the other high pressure zones in the body, i.e., lower esophageal sphincter18, 19, upper esophageal sphincter20 and anal sphincters21. The reason for the pressure asymmetry in these sphincters is related to their unique anatomy. For example the upper esophageal sphincter, which is related to the contraction of cricopharyngeus muscle (a “horse shoe” shaped muscle), reveals that the contact pressures are higher in the anterior and posterior directions as compared to the lateral directions. Similarly, the pressure asymmetry in the anal sphincter is related to the unique anatomy of anal canal, which is thought to be made up of three loops22. We believe that the unique pressure profile of the vaginal HPZ reveals important information as to what muscle is responsible for its genesis. The vagina does not have an intrinsic sphincter within its walls. There is a general consensus that the vaginal HPZ is related to the contraction of the pelvic floor muscles. Pelvic floor muscles are many and can be divided into 2 broad groups, group 1 muscles, pubococcygeus and ileococcygeus, both of which are thin and sheet-like muscles that originate from the ileo-pectinate line located on the superior ramus of pubic bone and arcus tendinus, and group 2 muscles, located caudal to the group 1 muscles and originates from the inferior pubic ramus 23 24. Pubo-uretheralis (also called pubo-vaginalis by Lawson23), puboperinealis and puborectalis are the major components of group 2 muscles.
We believe that the characteristics of vaginal HPZ described in this paper reveal important information with regards to the genesis of vaginal HPZ. Based on the shape of constriction on the bag noted on the US images in our previous study and MR images in the current study and pressure distribution seen on the HDM plots, it may be inferred that the vaginal HPZ is the result of forces directed in a dorso-ventral (posterior-anterior) direction. The anatomical location of the vaginal HPZ suggests that the vaginal wall is compressed between the urethra and anal canal. We propose that the above two features are consistent with the action of puborectalis muscle that moves the anal canal in the ventral or anterior direction and probably compresses urethra, vagina and anal canal against each other and in turn against the back of pubic symphsis. How does such an explanation account for the pressure differences between anterior and posterior direction? The diameter of anal canal is significantly bigger than that of the urethra (see ultrasound and MR images), which likely results in a difference in the contact area of these structures with the HDM probe located inside the vagina. The HDM probe contact area will be expected to be larger in the dorsal/posterior direction where it is in contact with the anal canal compared to the ventral/anterior direction where it is in contract with the urethra. Since pressure is inversely proportional to the area (Pressure = Force /Area) one would expect posterior contact pressures to be lower than the anterior contact pressures, a finding actually seen in our previous and current studies.
Another important feature of vaginal HPZ is that the anterior pressure cluster is located cephalad to the posterior pressure cluster and the latter moves cephalad during pelvic floor contraction. These features can be explained on the basis of unique anatomy of the puborectalis muscle and its movement during pelvic floor contraction. The puborectalis muscle originates from the inferior pubic rami somewhat inferior and lateral to the pubic symphysis. The right and left arms of the puborectalis muscle meet posterior to the anal canal and form a sling around it. During pelvic floor contraction the anorectal angle moves ventrally and cranially25, 26 thereby changing the axis of the puborectalis muscle. We propose that the cranial relocation of the anorectal angle results in the cranial movement of the posterior pressure clusters. Based on the direction of the fibers in the puborectalis muscle, one would expect the anorectal angle to move in the caudal direction (towards the origin of the muscle at the inferior pubic ramus). To the contrary, anorectal angle moves in the cranial direction. We propose that the cranial movement of the anorectal angle is related to the lifting or the elevator action of pubococcygeus and ileococcygeus, as observed by other investigators as well 25, 27, 28.
Pelvic floor muscles are thought to have two important functions, they provide; 1) physical support or floor to the pelvic viscera and 2) constrictor function to the anal canal, vagina and urethra. The vaginal HPZ reflects the constrictor function of the pelvic floor muscle and its characteristics described in this manuscript can be explained on the basis of the action of puborectalis muscle contraction. The cranial movement of the anorectal angle with pelvic floor contraction is thought to reflect the elevator function of pelvic floor muscle. Our observation that the cranial movement of the posterior pressure cluster of the vaginal HPZ is similar to the cranial movement of the anorectal angle observed on the MR and ultrasound images suggests that the HDM may be used to measure the constrictor and the elevator functions of pelvic floor muscle. In our previous studies we found that the 3D- ultrasound imaging is a reproducible method to make various measurements of the puborectalis muscle15, 29. In the current study, we found the distance moved by the anorectal angle, as detected by US and MR images is identical thus further confirming the utility of dynamic ultrasound imaging in studying pelvic floor muscle function29.
Pelvic floor disorders are many and can be broadly divided into disorders of continence, i.e., fecal and urinary incontinence, and disorders of pelvic floor support, i.e., organ prolapse. We propose that the continence function of pelvic floor is related to the constrictor function, which in turn is related to the puborectalis muscle component of the pelvic floor. On the other hand, the support function of pelvic floor is related to the elevator function, which in turn is related to the pubo-ileococcygeus muscle. Future studies using HDM probe may investigate if it is possible to divide pelvic disorders into disorders of elevator and constrictor functions.
Acknowledgments
This research was supported by an NIH RO-1 grant (DK60733).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Kegel AH. Progressive resistance exercise in the functional restoration of the perineal muscles. Am J Obstet Gynecol. 1948;56:238–248. doi: 10.1016/0002-9378(48)90266-x. [DOI] [PubMed] [Google Scholar]
- 2.Kegel AH. Physiologic therapy for urinary stress incontinence. J Am Med Assoc. 1951;146:915–917. doi: 10.1001/jama.1951.03670100035008. [DOI] [PubMed] [Google Scholar]
- 3.Levitt EE, Konovsky M, Freese MP, Thompson JF. Intravaginal pressure assessed by the Kegel perineometer. Arch Sex Behav. 1979;8:425–430. doi: 10.1007/BF01541198. [DOI] [PubMed] [Google Scholar]
- 4.Logan TG. The vaginal clasp. A method of comparing contractions across subjects. J Sex Res. 1975;11:353–358. doi: 10.1080/00224497509550913. [DOI] [PubMed] [Google Scholar]
- 5.Dougherty MC, Abrams R, McKey PL. An instrument to assess the dynamic characteristics of the circumvaginal musculature. Nurs Res. 1986;35:202–206. [PubMed] [Google Scholar]
- 6.Bo K, Raastad R, Finckenhagen HB. Does the size of the vaginal probe affect measurement of pelvic floor muscle strength? Acta obstetricia et gynecologica Scandinavica. 2005;84:129–133. doi: 10.1111/j.0001-6349.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- 7.Guerette N, Neimark M, Kopka SL, Jones JE, Davila GW. Initial experience with a new method for the dynamic assessment of pelvic floor function in women: the Kolpexin Pull Test. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:39–43. doi: 10.1007/s00192-003-1115-7. discussion 43. [DOI] [PubMed] [Google Scholar]
- 8.Dumoulin C, Bourbonnais D, Lemieux MC. Development of a dynamometer for measuring the isometric force of the pelvic floor musculature. Neurourol Urodyn. 2003;22:648–653. doi: 10.1002/nau.10156. [DOI] [PubMed] [Google Scholar]
- 9.Verelst M, Leivseth G. Force-length relationship in the pelvic floor muscles under transverse vaginal distension: a method study in healthy women. Neurourol Urodyn. 2004;23:662–667. doi: 10.1002/nau.20070. [DOI] [PubMed] [Google Scholar]
- 10.Guaderrama NM, Liu J, Nager CW, et al. Evidence for the innervation of pelvic floor muscles by the pudendal nerve. Obstet Gynecol. 2005;106:774–781. doi: 10.1097/01.AOG.0000175165.46481.a8. [DOI] [PubMed] [Google Scholar]
- 11.Guaderrama NM, Nager CW, Liu J, Pretorius DH, Mittal RK. The vaginal pressure profile. Neurourol Urodyn. 2005;24:243–247. doi: 10.1002/nau.20112. [DOI] [PubMed] [Google Scholar]
- 12.Jung SA, Pretorius DH, Padda BS, et al. Vaginal high-pressure zone assessed by dynamic 3-dimensional ultrasound images of the pelvic floor. Am J Obstet Gynecol. 2007;197(52):e1–e7. doi: 10.1016/j.ajog.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Fantl JA. Short forms to assess life quality and symptom distress for urinary incontinence in women: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program for Women Research Group. Neurourol Urodyn. 1995;14:131–139. doi: 10.1002/nau.1930140206. [DOI] [PubMed] [Google Scholar]
- 14.Vaizey CJ, Carapeti E, Cahill JA, Kamm MA. Prospective comparison of faecal incontinence grading systems. Gut. 1999;44:77–80. doi: 10.1136/gut.44.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raizada V, Weinstein MM, Bhargava V, Pretorius DH, Wan J, Mittal RK. Constrictor and Elevator Functions of the Pelvic Floor are Distinct: Evidence from Studies in Patients with Fecal Incontinence. Journal of Pelvic Medicine and Surgery. 2008;14:227–228. [Google Scholar]
- 16.Dietz HP. Ultrasound imaging of the pelvic floor. Part I: two-dimensional aspects. Ultrasound Obstet Gynecol. 2004;23:80–92. doi: 10.1002/uog.939. [DOI] [PubMed] [Google Scholar]
- 17.Tunn R, Schaer G, Peschers U, et al. Updated recommendations on ultrasonography in urogynecology. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:236–241. doi: 10.1007/s00192-004-1228-7. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Parashar VK, Mittal RK. Asymmetry of lower esophageal sphincter pressure: is it related to the muscle thickness or its shape? Am J Physiol. 1997;272:G1509–G1517. doi: 10.1152/ajpgi.1997.272.6.G1509. [DOI] [PubMed] [Google Scholar]
- 19.Winans CS. Manometric asymmetry of the lower-esophageal high-pressure zone. Am J Dig Dis. 1977;22:348–354. doi: 10.1007/BF01072193. [DOI] [PubMed] [Google Scholar]
- 20.Welch RW, Luckmann K, Ricks PM, Drake ST, Gates GA. Manometry of the normal upper esophageal sphincter and its alterations in laryngectomy. J Clin Invest. 1979;63:1036–1041. doi: 10.1172/JCI109372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor BM, Beart RW, Jr, Phillips SF. Longitudinal and radial variations of pressure in the human anal sphincter. Gastroenterology. 1984;86:693–697. [PubMed] [Google Scholar]
- 22.Shafik A. A new concept of the anatomy of the anal sphincter mechanism and the physiology of defecation. The external anal sphincter: a triple-loop system. Invest Urol. 1975;12:412–419. [PubMed] [Google Scholar]
- 23.Lawson JO. Pelvic anatomy. I. Pelvic floor muscles. Ann R Coll Surg Engl. 1974;54:244–252. [PMC free article] [PubMed] [Google Scholar]
- 24.Kearney R, Sawhney R, DeLancey JO. Levator ani muscle anatomy evaluated by origin-insertion pairs. Obstet Gynecol. 2004;104:168–173. doi: 10.1097/01.AOG.0000128906.61529.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh K, Reid WM, Berger LA. Magnetic resonance imaging of normal levator ani anatomy and function. Obstet Gynecol. 2002;99:433–438. doi: 10.1016/s0029-7844(01)01743-4. [DOI] [PubMed] [Google Scholar]
- 26.Bharucha AE, Fletcher JG, Harper CM, et al. Relationship between symptoms and disordered continence mechanisms in women with idiopathic faecal incontinence. Gut. 2005;54:546–555. doi: 10.1136/gut.2004.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hjartardottir S, Nilsson J, Petersen C, Lingman G. The female pelvic floor: a dome--not a basin. Acta Obstet Gynecol Scand. 1997;76:567–571. doi: 10.3109/00016349709024586. [DOI] [PubMed] [Google Scholar]
- 28.Guo M, Li D. Pelvic floor images: anatomy of the levator ani muscle. Dis Colon Rectum. 2007;50:1647–1655. doi: 10.1007/s10350-007-0262-1. [DOI] [PubMed] [Google Scholar]
- 29.Weinstein MM, Jung SA, Pretorius DH, Nager CW, den Boer DJ, Mittal RK. The reliability of puborectalis muscle measurements with 3-dimensional ultrasound imaging. Am J Obstet Gynecol. 2007;197(68):e1–e6. doi: 10.1016/j.ajog.2007.02.041. [DOI] [PubMed] [Google Scholar]