Abstract
The intensity of a noise-induced startle response can be reduced by the presentation of an otherwise neutral stimulus immediately before the noise (“prepulse inhibition” or PPI). We used a form of PPI to study the effects of damage to auditory cortex on the discrimination of speech sounds by rats. Subjects underwent control surgery or treatment of the auditory cortex with the vasoconstrictor endothelin-1. This treatment caused damage concentrated in primary auditory cortex (A1). Both before and after lesions, subjects were tested on 5 tasks, most presenting a pair of human speech sounds (consonant-vowel syllables) so that the capacity for discrimination would be evident in the extent of PPI. Group comparisons failed to reveal any consistent lesion effect. At the same time, the analysis of individual differences in performance by multiple regression suggests that some of the temporal processing required to discriminate speech sounds is concentrated anteroventrally in the right A1. These results also confirm that PPI can be adapted to studies of the brain mechanisms involved in the processing of speech and other complex sounds.
Keywords: Auditory cortex, Brain lesions, Prepulse inhibition, Speech perception, Speech sounds, Voice onset time
1. Introduction
The integrity of auditory cortex seems more important for the processing of human speech and other complex sounds than for responses to relatively simple stimuli [31,36]. For example, lesions of auditory cortex in gerbils failed to affect the discrimination of pure tones but disrupted responding to frequency-modulated tones [36]. Likewise, similar lesions in rats disrupted the detection of sounds with rates of amplitude modulation that were relatively high but failed to affect responding to the sounds with the lowest modulation rates [7]. Such lesions also failed to affect the discrimination by rats of pure tones or synthetic vowels incorporating just one formant, but disrupted the discrimination of vowels including 2–4 formants [31].
Though the involvement of auditory cortex in the processing of complex sounds seems widely accepted, it is less clear what specific parts of this cortex contribute to such processing. Some neurophysiological studies document the responsiveness of cells in primary auditory cortex (A1) to acoustic features of speech, suggesting an important role for this cortex in the processing of speech sounds [11–13,46,48,49]. But similar studies comparing A1 and other parts of auditory cortex suggest more selective responses to complex stimuli in nonprimary areas [43,51]. Further, some data suggest that these differences are not simple consequences of hierarchical processing. First, anatomical data show that some nonprimary areas receive direct thalamic inputs suggestive of parallel processing [32,33,42]. Second, consistent with these connections, the inactivation of A1 has been observed to abolish responses to pure tones in some nonprimary areas but not others [42]. Third, whereas some lesion studies suggest that large deficits in responsiveness to complex sounds require damage to A1 (e.g., [24]), others suggest important and possibly independent roles for nonprimary areas. For example, Kudoh et al. [31] recently reported that lesions of A1 in rats did not disrupt the discrimination of 4-formant synthetic vowels whereas lesions dorsal or rostral to this area did.
We have begun to explore the mechanisms for the processing of complex sounds by describing the impact of cortical lesions on the discrimination of human speech sounds by rats. In the process, we have extended a promising method for testing discrimination, one that emphasizes reflexive responses and consequently avoids much of the time and effort of operant training.
In general, studies of “reflex modification” use changes in reflex responses to assess information processing. In one variant, stimuli (“prepulses”) that regularly and immediately precede a loud noise decrease the size of the noise-induced startle response, an effect termed “prepulse inhibition” (PPI) [22]. Despite a variety of previous applications, PPI has been used infrequently to study stimulus discrimination, especially in nonhuman animals. A paradigm permitting this extension was developed by Clark et al. [6], who exposed rats to prepulses consisting of tone pairs that ascended or descended in frequency. For each subject, one “standard” stimulus helped define the acoustic background: It was presented frequently, but rarely preceded a startle stimulus. The other stimulus, or “oddball,” was designed to stand out from the background: It was presented infrequently, and always preceded a startle stimulus. Across a wide range of conditions, startle responses were inhibited more by highly predictive prepulses than by less predictive ones.
These and other results (reviews in [15,16]; also see [17]) suggest that PPI can be used both to study the discrimination of complex sounds and to test the modulation of such discrimination by factors including the integrity of auditory cortex. We have tested this inference by using PPI to monitor the performance of rats on tasks requiring the discrimination of human speech sounds. In recognition of results suggesting that some such discriminations are unaffected by A1 damage (e.g., [31]), these tasks were designed to survey a variety of speech elements or dimensions. We expected that analyses of group or individual responses to lesions would reveal differences across speech elements, with A1 damage disrupting some discriminations and sparing others.
2. Materials and methods
2.1. Animals and test environment
In our study, complete behavioral data were collected from 16 female Sprague-Dawley rats averaging 297 g (SD = 24) at the time of surgery. Except during behavioral tests, each was housed individually in a wire-mesh cage with continuous access to food and water. The colony was maintained at constant temperature and humidity, and on a reversed 12:12 hr light:dark cycle. Housing conditions and treatments were approved by the University of Texas Institutional Animal Care and Use Committee.
During testing, an animal occupied a 20 × 20 × 20 cm wire-mesh cage contained in a 67 × 67 × 67 cm chamber lined with 5 cm acoustic foam. The cage was centered on a startle platform (Lafayette Instrument Co.) that uses a piezoelectric transducer to generate a continuous record of activity in volts. Sounds generated using a real-time processor (RP2.1, Tucker-Davis Technologies) were delivered by a speaker (Optimus Bullet Horn Tweeter) mounted above and to one side of the cage, about 20 cm from its center. Stimuli were adjusted for the speaker’s frequency response using a finite impulse response filter (MATLAB). Sound intensities were measured using an ACO Pacific microphone (PS9200-7016) placed near the center of the test cage.
2.2. Stimuli and startle responses
Startle responses were elicited by 50 ms bursts of white noise at 102.0 dB. The waveform of each response was sampled at 10 kHz using an RP2.1 and the peak to peak voltage generated by the startle platform within 500 ms of the noise was recorded using MATLAB. The peak-to-peak change in voltage observed in the output of the startle platform shortly after presentation of the startle stimulus is taken to indicate the magnitude of the startle response and is the standard index of the extent of startle in studies of prepulse inhibition (PPI; e.g., [22]).
The stimuli included 6 speech sounds that served as prepulses. These were derived from monosyllabic words (consonant-[æ]-[d]) spoken by a female native English speaker. During recording, these were sampled at 10 μs with 16 bit resolution. They then were adjusted to a rat’s hearing range by doubling all frequencies without changing the amplitude envelope [26]. To shorten prepulses and equate them for elements other than the initial consonant, all were truncated at 100 ms into the vowel. The intensity of the loudest 100 ms within each stimulus then was adjusted to approximate 64 dB. Within the pair of stimuli that defined a task, intensity differences averaged 1.9 dB (SD = 1.2).
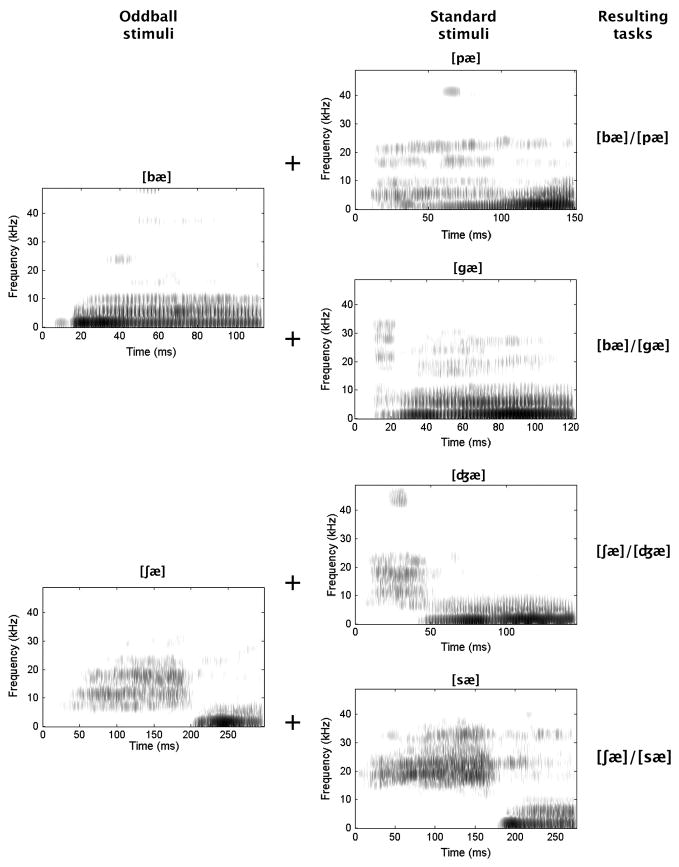
The resulting stimulus set included [bæ], [pæ], [gæ], [ʃ æ], [ʤæ], and [sæ] (derived from English bad, pad, gad, shad, jad and sad, respectively; sound spectrograms in Figure 1). In some behavioral tests, [bæ] was presented in silence to create a detection task. The other tasks required the discrimination of the stimuli within each of the following pairs: [bæ]/[pæ], [bæ]/[gæ], [ʃ æ]/[ʤ æ], [ʃæ]/[sæ].
Figure 1.
Sound spectrograms depicting frequency over time for the 6 speech sounds used as stimuli in this study. The 2 sounds that served as rare stimuli or oddballs, and that defined cued test trials, are described in the left column. The 4 that served as standard stimuli, and that defined uncued trials, are described in the middle column. The combinations that defined the 4 discrimination tasks are specified in the right column.
These tasks were designed to emphasize specific acoustic dimensions. Specifically, [bæ]/[pæ] combines bilabial stop consonants differing in voicing ([b] voiced, [p] voiceless). These should be discriminable on the basis of voice onset time, a temporal difference. The second pair includes consonants differing in place of articulation ([b] bilabial, [g] velar), permitting a spectral discrimination based on the starting points and initial trajectories of the second formant (low then ascending in [b], high then descending in [g]). The third contrasts [ʃ], a voiceless fricative with a relatively long initial noise burst, and [ʤ], a voiced affricative with a shorter burst. This permits a temporal judgment, but one different from that distinguishing [bæ] and [pæ]. The last pair combines voiceless fricatives differing in place of articulation (palatal for [ʃ], alveolar for [s]). These can be discriminated on the basis of the frequency range of the initial noise bursts (relatively narrow and low for [ʃ], broader and higher for [s]), emphasizing a second type of spectral distinction. All of these differences should have been available as potential bases of discrimination. At the same time, natural speech sounds vary in multiple ways [21], so that we cannot be positive how the discriminations here were achieved.
2.3. Standard behavioral test
Each behavioral test included 3 phases. In the detection task, the first phase consisted of 5 min of silence. In the other tasks, it involved 5 min of exposure at 1/s to a “frequent” or standard stimulus (the second of the speech sounds in each of the pairs defined previously). The goal here was to cause this stimulus to recede into the acoustic background.
The second phase introduced the other speech sound (the first in each pair) and the startle stimulus. Each of these was presented 10 times over 5 min, with each of the new speech sounds preceding a noise by a 5 ms interstimulus interval. Such pairings were spaced an average of 30 s (range = 15–45 s) apart. Since the standard stimulus continued to appear at 1/s throughout this phase, it seems reasonable to think of the newly introduced speech sound as a “rare” stimulus or oddball [6]. This phase was designed to highlight the predictive relationship between the oddball and startle stimulus. The interstimulus interval was selected on the basis of pilot testing and is shorter than has been reported to be optimal for PPI using simple sounds (e.g., [22]). This disparity may relate to the fact that much of the information available to support discriminations here was concentrated prior to the vowel, and thus 105 ms or more prior to the startle stimulus.
Each session concluded with a 20 min “test phase” in which animals were exposed to a mixture of “cued” and “uncued” trials, each defined by the presentation of the startle stimulus 5 ms after a prepulse. Specifically, cued trials refer to those on which the rare or oddball stimulus served as the prepulse [6]. In contrast, uncued trials are those on which no stimulus (detection task) or the standard stimulus (discrimination tasks) preceded the startle stimulus. Each test included 10 blocks of 4 trials. Each block included 3 cued trials and 1 uncued trial. These were presented in random order, with successive trials spaced at intervals that averaged 30 s. Between trials, “unreinforced” presentations of the standard stimulus continued at 1/s. Accordingly, uncued trials are those on which there should be no prepulse that stands out from the background. Conversely, cued trials are those on which a prepulse should stand out, though only if the subject can distinguish the standard and oddball prepulses. When discrimination is possible, then, one would expect greater PPI on cued than uncued trials.
2.4. Preoperative testing
The 16 subjects were divided into 3 subsets of 5–6 that progressed through the study separately and on slightly different schedules. However, preliminary analyses showed that these procedural differences had no significant effect on performance, permitting the subsets to be combined. Most subjects experienced 1 day of preoperative (preop) training on the detection task, followed by 5 tests requiring the discrimination of [bæ] and [pæ], then 4 tests on each of the remaining 3 tasks (4 tests on [bæ]/[gæ], then 4 on [ʃ æ]/[ʤæ], then 4 on [ʃæ]/[sæ]), always with just 1 behavioral test per day.
2.5. Lesion surgery
Subjects were assigned to lesion and control groups (of 11 and 5, respectively) so as to roughly match those groups for preop behavior. Each lesion subject was anesthetized with sodium pentobarbital (50 mg/kg, i.p.), supplemented by a local anesthetic. Standard methods then were used to remove the skull and dura overlying the left auditory cortex. Over a period of 12.5–17.5 min, this tissue was exposed to 3–4 μl of a solution containing 0.6–0.8 μg of endothelin-1 (Bachem), a peptide that acts as a potent vasoconstrictor and can cause brain damage resembling that resulting from ischemic strokes [2,18]. Following this treatment, the cortex was left undisturbed for 10 min before the skull fragment was replaced and the wound closed. These steps then were repeated on the right, so as to produce bilateral lesions. On the basis of research on the consequences of “sham” lesions [1], control subjects had the skull overlying each auditory cortex exposed and cleaned but not penetrated.
2.6. Postoperative testing
Each subject was given at least 1 week of recovery before the start of postoperative (postop) testing that extended over 4 weeks, with each week including 1 test on each of the 5 tasks. Regardless of a subject’s schedule of preop testing, postop tests always were run in the following order: detection ([bæ]/silence), [bæ]/[pæ], [bæ]/[gæ], [ʃæ]/[ʤæ], [ʃæ]/[sæ]. Each test was structured as previously described.
2.7. Histological analysis
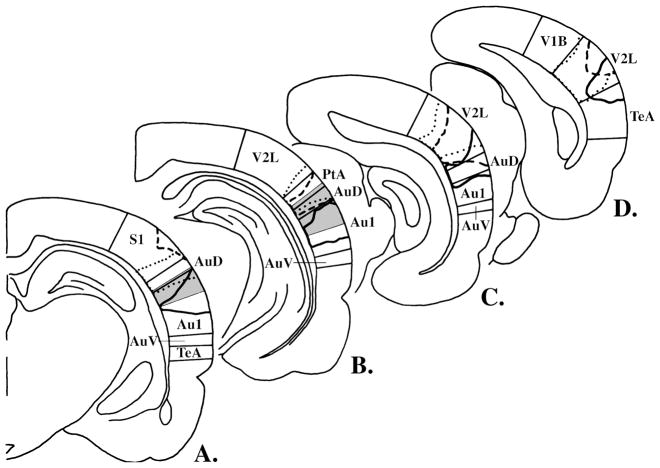
Once testing was complete, each subject was deeply anesthetized and perfused. Frozen 50μ coronal sections through the region of interest were cut and stained with cresyl violet. From all sections for each animal, we selected those most closely matching a series of 6 reference sections spaced at 1 mm intervals in a widely used brain atlas for the rat [39]. These sections were digitized and merged with the reference sections, creating frontal lesion sketches such as presented in Figure 2. In addition, intersections of lesions with the cortical surface were used to “project” each lesion, and A1 as defined by the atlas, onto the cortical surface, as in Figure 3A.
Figure 2.
The lesions in the right hemispheres of 3 representative subjects are outlined on a series of frontal sections running from anterior to posterior at 1 mm intervals (panels A-D depict levels relative to bregma of −4.30, −5.30, −6.30 and −7.30 mm, respectively, in [39]). The lesions represented by the dotted, dashed and solid lines are those closest to the first quartile, median, and third quartile, respectively, in the distribution of total lesion volumes. The figure’s restriction to right-hemisphere lesions is designed to increase clarity and is based on results showing that none of these cortical areas was differentially damaged by lesions on the two sides. The abbreviations here follow those in [39]: Au1, primary auditory cortex; AuD, secondary auditory cortex, dorsal; AuV, secondary auditory cortex, ventral; PtA, parietal association cortex; S1, primary somatosensory cortex; TeA, temporal association cortex; V1B, primary visual cortex, binocular; V2L, secondary visual cortex, lateral area. Elsewhere in this paper, however, we use the more common abbreviation of A1 for primary auditory cortex.
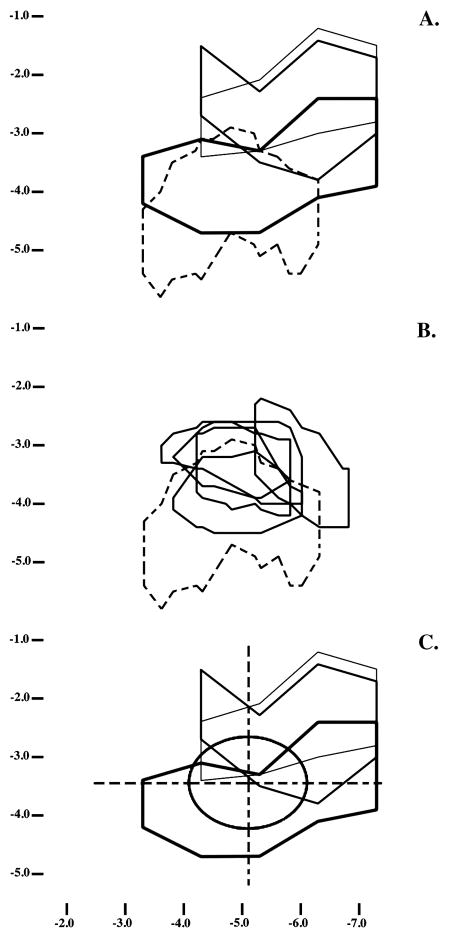
Figure 3.
Each panel shows projections of lesions or A1 maps onto the cortical surface. The vertical axis shows dorsal-ventral coordinates, in mm, relative to the brain’s dorsal surface; the horizontal axis shows anterior-posterior coordinates relative to bregma [39]. In panel A, the representative lesions described in Figure 1 are projected onto the cortical surface, using thin, intermediate and thick lines to depict lesions increasing in total volume. Dashed lines depict the cortical projection of A1, as this area is defined in [39]. Panel B compares A1 as described in [39] (dashed lines) with the boundaries determined in the 5 animals in which this area was mapped electrophysiologically as part of this study (solid lines). In panel C, the lesions from panel A are superimposed on the average location and extent of physiologically-defined A1 (oval). The grid overlaying A1 was used in some analyses to define areas of damage within and immediately adjacent to A1.
The next steps in the histological analysis were dictated by results suggesting that the relevant atlas does not accurately describe the location of physiologically-defined A1 in all rats (e.g., [10]). To properly address this critical issue, the histological analysis was extended to include the electrophysiological mapping of A1 in 5 new subjects. In each, unit activity in the vicinity of A1 in the right hemisphere was recorded from 76–120 electrode penetrations (methods as in [13,28]). Responses were recorded during exposure to 81 pure tone frequencies at 16 intensities. The borders of A1 were then determined relative to vascular landmarks on the basis of continuous tonotopy (with optimal frequency increasing from posterior to anterior) and responses with relatively short latencies, low thresholds and narrow tuning curves. Once these borders were determined and marked, the brains were processed as previously described. This permitted physiologically-defined A1 in each new subject to be compared to the A1 boundaries from the atlas (Figure 3B), in the process suggesting substantial differences with the atlas borders. At the same time, the physiologically-defined boundaries seem to agree better with the A1 placements in other sources, including some older atlases [10,38,40,55]. Finally, the individual A1 maps were averaged, yielding the oval shown in Figure 3C. This served to operationally define A1 in analyses of the distribution of the lesions themselves and their behavioral consequences. Some of these analyses focused on the specific parts of A1 or the adjacent cortex that are defined by the grid that also appears in Figure 3C. The extents of damage to specific cortical areas were measured in Photoshop and taken to be the number of pixels enclosed by a lesion on standardized sketches such as presented in Figure 3.
2.8. Behavioral analysis
The initial statistical assessment of behavior used analysis of variance (ANOVA) to compare average preop and postop levels of startle on the cued and uncued trials included in the final phase of testing in each task. Ratios of responding on cued and uncued trials (cued/uncued ratios) also were calculated for descriptive purposes and use in later analyses. The ANOVAs treated group (control, lesion) as a between-subjects factor and stimulus (standard, oddball) and surgical condition (preop, postop) as repeated factors. Effects with p ≤ .01 were considered to be reliable.
In addition to the group comparisons, we exploited the variability across lesions by using stepwise multiple linear regression to test the relation between patterns of brain damage and postop changes in behavior on each task. The independent variables here included group (a dichotomous variable) and the extents of damage (in pixels, as above) in each hemisphere and in each of 7 cortical areas defined by the average location of A1 and lines extending through its center along the anterior-posterior and dorsal-ventral axes (see Figure 3C; the area immediately anteroventral to A1 experienced insufficient damage for consideration). The dependent variables were the ratios of the average preop and postop ratios of responding on cued and uncued trials (individual ratios = cued/uncued levels of startle; ratio of ratios = average postop ratio/average preop ratio). By this definition, a ratio >1 suggests a postop decline in discrimination whereas a ratio <1 would suggest a postop improvement.
3. Results
3.1. Histology
In relation to physiologically-defined A1 in comparable animals, these lesions damaged an average of 55.3% (SEM = 6.2) of each A1 and 43.9% (SEM = 6.3) of A1 bilaterally (considering just the area of overlap across the hemispheres). As suggested by the results in Figure 3C, this damage was concentrated dorsally within A1: On average, 76.7% (SEM = 5.6) of the dorsal (or medial) half of A1 was damaged, but only 33.4% (SEM = 8.6) of the ventral (or lateral) half. To a lesser extent, the damage also was concentrated posteriorly, with average extents of damage to the anterior and posterior halves of A1 of 48.6% and 61.9%, respectively (SEMs = 6.3 and 7.3). Further, these trends extended to the surrounding tissue, with incidental damage here being greatest dorsally and posteriorly, and less anteriorly and ventrally (Figure 3C).
3.2. Behavior
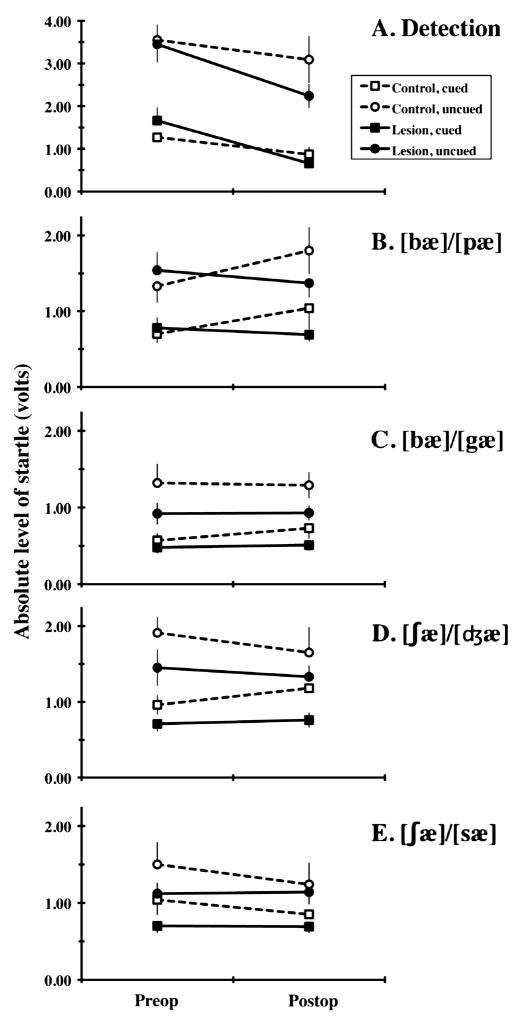
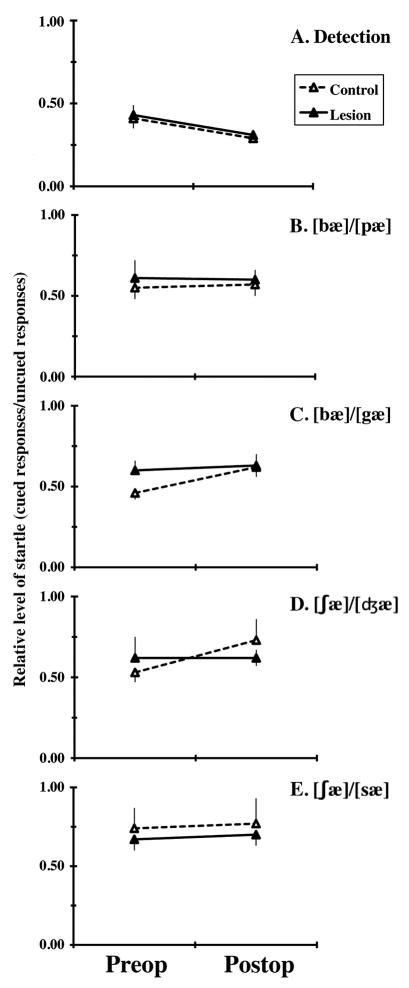
Average preop and postop levels of startle on each task are summarized in Figure 4. The corresponding ratios of responding on cued and uncued trials (cued/uncued ratios) are depicted in Figure 5. Group comparisons of behavior focused on the first of these and used ANOVAs treating group (control, lesion) as a between-subjects factor and stimulus (standard, oddball) and surgical condition (preop, postop) as repeated factors. These analyses failed to reveal any of the 3-way interactions required to document reliable lesion effects on discrimination (Figures 4 and 5). However, the analysis of each task revealed a highly reliable main effect of stimulus (F(1,14) ≥ 16.84, p ≤ .001), reflecting levels of startle on uncued trials that consistently exceeded those on cued trials, documenting successful discrimination of the speech sounds defining that task (Figures 4 and 5, Table 1). In addition, there was a reliable effect of surgery on performance in the detection task (F(1,14) = 10.81, p = .005; Figures 4 and 5). This reflects a significant drop in levels of startle postop. Its restriction to the first postop task suggests that it reflects habituation to the startle stimulus rather than a deficit due to the surgery.
Figure 4.
Mean (and SEM) absolute levels of startle by Control (open symbols connected by dashed lines) and Lesion (filled symbols connected by solid lines) subjects observed before (Preop) and after (Postop) lesion surgery on cued (square symbols) and uncued (circular symbols) trials. Performance on the detection task is described in panel A and that in each of the 4 discrimination tasks is described in panels B-E, as indicated. Note that the scale in panel A differs from that in the remaining panels, reflecting the generally higher levels of startle observed on uncued trials in the detection task.
Figure 5.
Mean (and SEM) relative levels of startle (average response level on cued trials divided by that on uncued trials) are summarized for Control (open symbols connected by dashed lines) and Lesion (filled symbols connected by solid lines) subjects observed before (Preop) and after (Postop) lesion surgery. Performance on the detection task is described in panel A and that in each of the 4 discrimination tasks is described in panels B-E, as indicated.
Table 1.
Startle levels and ratios, averaged across days, groups and subjects
| Task | Absolute levels of startle (volts) | Ratio of responses (cued trials/uncued trials) | ||||
|---|---|---|---|---|---|---|
| Cued trials | Uncued trials | |||||
| Mean | SEM | Mean | SEM | Mean | SEM | |
| Detection | 1.11 | 0.12 | 3.08 | 0.24 | 0.36 | 0.03 |
| [bæ]/[pæ] | 0.80 | 0.10 | 1.51 | 0.14 | 0.58 | 0.06 |
| [bæ]/[gæ] | 0.57 | 0.06 | 1.11 | 0.10 | 0.57 | 0.05 |
| [ʃæ]/[ʤæ] | 0.90 | 0.11 | 1.59 | 0.15 | 0.63 | 0.06 |
| [ʃæ]/[sæ] | 0.82 | 0.09 | 1.25 | 0.14 | 0.72 | 0.06 |
Analyses of individual differences in damage and behavior used stepwise multiple linear regression and independent variables consisting of group and the extents of damage to each of 7 areas defined in each hemisphere by the average location of A1 and lines extending through its center along the anterior-posterior and dorsal-ventral axes (Figure 3C; see above note regarding the sparing of 1 sector defined by this grid). The dependent variables in these analyses were the ratios of the average preop and postop ratios of responding on cued and uncued trials (as noted above, ratios above and below 1 suggest postop deficits and improvements in discrimination, respectively). These analyses revealed a reliable direct relation between postop changes in the discrimination of [bæ] and [pæ] and the extent of damage to the anteroventral quadrant of A1 in the right hemisphere (R2 = 0.45, β = 0.67, F(1,14) = 11.56, p = .004; see Figure 6 for lesions of the animals at the middle and extremes of the distribution of postop changes in the quality of [bæ]/[pæ] discrimination). This correlation suggests that damage to this area disrupts the discrimination of these specific speech sounds. Further support for the specificity of the effect is provided by the fact that a regression excluding the anteroventral quadrant of A1 on the right failed to reveal any other reliable predictor of postop performance.
Figure 6.
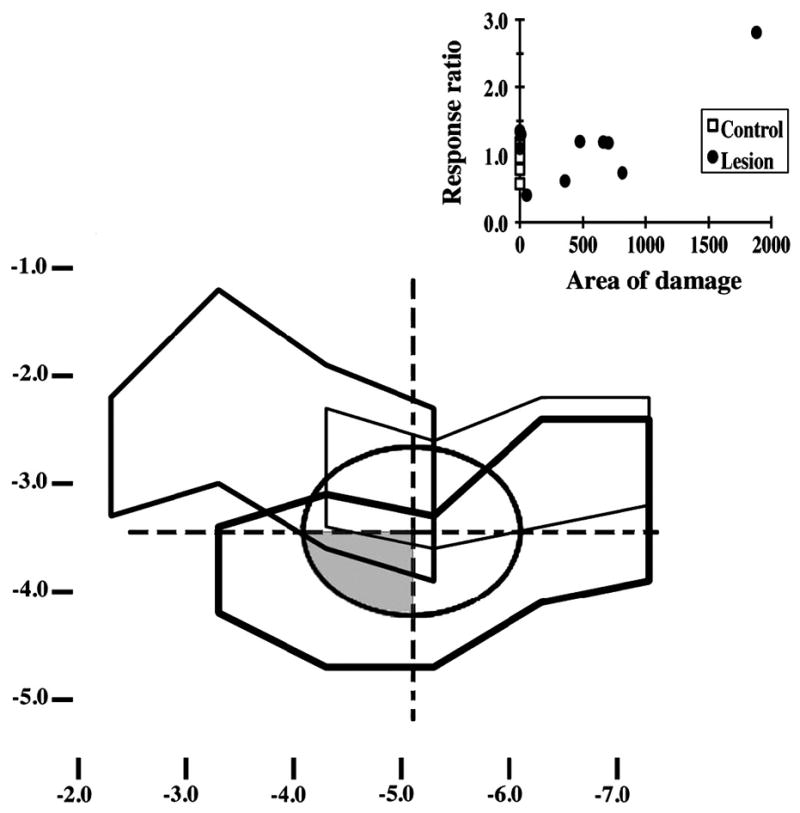
Projections of physiologically-defined A1 and selected lesions onto the cortical surface. The oval at the figure’s center describes the average location and extent of physiologically-defined A1. The grid overlaying this oval was used to define areas within or adjacent to A1 for the purposes of regressions exploring the extent to which patterns of lesion damage predicted aspects of behavioral performance. The shaded area represents the anteroventral quadrant of A1 in the right hemisphere, which these analyses suggested as a possible predictor of decrements in the ability to discriminate stimuli ([bæ] and [pæ]) differing in voice onset time. The 3 irregular figures describe the lesions of the 3 lesion subjects showing the largest postop decrement in [bæ]/[pæ] discrimination (thick lines), the median level of postop change in this discrimination (lines of intermediate thickness), or the smallest postop change on this task (thin lines). The graph in the upper right describes for each lesioned and control subject (filled circles and open squares, respectively) the relation between the [bæ]/[pæ] response ratio (vertical axis) and the extent of damage to the anteroventral quadrant of the right A1 (horizontal axis).
4. Discussion
These results suggest that damage to the anteroventral quadrant of A1 in the right hemisphere can disrupt speech-sound discrimination in rats. The restriction of this effect to the discrimination of [bæ] and [pæ] suggests that the damage in question disrupted the processing of voice onset time (VOT; corresponding to the low-frequency “voice bar” that occupies most of the first 15 ms of [bæ] in Figure 1) without significantly affecting responsiveness to other speech elements. Because these stimuli were natural speech sounds and could differ in multiple ways, we cannot be certain that a change in responsiveness to VOT fully accounts for the lesion effect [21]. However, only one of four discriminations was affected and this was the only one expected to depend primarily or only on VOT. Further, it seems unlikely that incidental differences across stimuli would be unique to any specific pair.
The ability of lesions to affect the discrimination of speech elements selectively is consistent with evidence suggesting that the mechanisms processing these elements are distributed differently within the auditory cortex [35]. Recording evidence from a variety of species including rats confirms that cells in A1 are responsive to VOT [11,13,34,48–50]. Similar but less extensive evidence suggests the responsiveness of A1 cells to other speech cues including the duration of aspiration noise [13,48], the onset spectra of stop consonants [13,49], and the frequency profiles and changes that distinguish vowels [41].
To our knowledge, lesions have not been used to directly test the necessity of cortical activity for the processing of VOT. However, several studies have examined how damage to the auditory cortex affects the detection of gaps in an otherwise continuous sound, a response that resembles that to VOT on its face and that responds to some forms of auditory trauma as does VOT [52]. Lesions concentrated in A1 severely disrupt or eliminate the capacity for gap detection in rats and ferrets [4,27]. In contrast, the same lesions have lesser or no effects on responses to other stimuli (e.g., noise pulses) that may tap the mechanisms that underlie some other forms of temporal processing [4,5].
These results suggest that processing in A1 may be required for the processing of VOT but not for that of other, possibly simpler, temporal cues. Further, even cues that depend on A1 may rely on processing at different points within this area. Electrophysiological data from monkeys suggest that cells responsive to VOT and the duration of aspiration noise are concentrated anteroventrally and posterodorsally, respectively, in A1 [48]. In rats, recent work suggests a tendency for the cells with the shortest onset latencies to be concentrated anteroventrally in A1 and dorsally in the ventral auditory field [40]. Together, these results suggest that the cells best prepared to support discriminations on the basis of VOT are concentrated in or near the anteroventral quadrant of A1, consistent with the present results.
Aside from the suggestion of a lesion effect on the processing of VOT, the most notable outcome of the present study may be the resistance of other discriminations to lesion-induced change. Of course, this must be interpreted very cautiously, since manipulations can fail to affect endpoints for many reasons. An especially salient limiting factor in the present case may be the partial sparing of A1 by most of our lesions, which could be invoked to help explain the scarcity of reliable lesion effects. Even so, the literature suggests that the impact of this limitation varies across tasks.
Of our other tasks, one was a detection task, requiring no discrimination of a specific temporal or spectral feature. The sparing of performance on this task is consistent with previous work showing that the detection of noise pulses or offsets is unaffected by lesions concentrated in A1 [4], and even by much more extensive cortical deactivation [23].
Another of the tasks spared by our lesions was designed to highlight a temporal cue other than VOT, the different durations of the initial noise burst that distinguish [ʃæ] and [ʤæ]. On the basis of previous work, one might expect the processing involved in this discrimination to involve cells in the high-frequency, posterodorsal, segment of A1 [48]. Though this is an area that was damaged in some of our animals, the absence of a lesion effect suggests that these lesions were not optimal in extent or placement, that the difference across stimuli in noise duration was somehow inappropriate, or that some other cue was available to support continued discrimination.
The other tasks included in our study were designed to require spectral discriminations. Past work on such cues has concentrated on FM tones (tone pairs or sweeps changing in frequency) or vowels.
Recording studies suggest the responsiveness of A1 cells to some spectral cues. For example, Steinschneider et al. [49] report the presence in A1 of cells responsive to place of articulation, in turn suggesting responsiveness to onset spectra. Further, a study of cats by Qin et al. [41] suggests the presence in A1 of cells that respond differentially to vowels.
Lesion studies have examined the impact of damage to auditory cortex on responding to FM tones or vowels. Much of this work has focused on the extent to which spectral processing is affected by large lesions of auditory cortex. Such lesions can reduce responsiveness to spectral cues, though their impact seems to depend on other variables, including species, task, and the duration or complexity of the stimuli [3,8,31,37].
Such variability in lesion effects extends to studies of how more specific parts of auditory cortex contribute to vowel discrimination. Early studies suggest that performance is disrupted by A1 damage in nonhuman primates but not cats [8,9]. But the most recent and relevant study seems to be that of vowel discrimination in rats by Kudoh et al. [31]. These results suggest that vowel discrimination is disrupted by lesions anterior or dorsal to A1, but not by damage to A1 itself. These studies raise the possibility that our lesions spared too much of the auditory cortex anterior and dorsal to A1 for any significant impact on responding in the two tasks most likely to depend on spectral processing.
In addition to suggesting impacts of task and lesion site within auditory cortex, our results raise the possibility of a hemispheric asymmetry in the processing of speech sounds by rats. In particular, they suggest that performance on discriminations emphasizing VOT is disrupted only by damage to the auditory cortex of the right hemisphere. This direction of lateralization obviously differs from that suggested by many previous studies of how people process temporal speech elements (e.g., [20]). Even in people, however, the standard pattern of lateralization seems subject to modulation by other variables [20]. In rodents, there is little evidence to dispute the conventional view (e.g., [34]) that the mechanisms for speech processing in nonhuman animals are bilaterally symmetric. Further, the little evidence there is for asymmetry in these mechanisms is inconsistent. Fitch et al. [14] found that male rats discriminated FM tones better when the stimuli were presented to the right ear, and thus presumably processed primarily within the left hemisphere. Though female rats in the same study showed no consistent ear advantage, Geissler and Ehret [19] reported an asymmetry favoring the left in the levels of activity in some auditory fields of female mice exposed to models of ultrasonic vocalizations by mouse pups. In contrast, the female rats studied by Rybalko et al. [45] showed an asymmetry in the opposite direction, experiencing a greater disruption of FM-tone discrimination after lesions of the auditory cortex on the right.
Collectively, these results suggest that the role of auditory cortex in the processing of complex sounds could depend on several variables, including task, hemisphere and gender. Much the same issue has been raised in recent analyses of possible sex differences in the lateralization of mechanisms for language in people. A popular view is that the extent of language lateralization is greater in men than women (reviews in [25,47]). However, this view has been questioned by some recent meta-analyses of neural imaging results [47]. Though this challenge has been disputed even the results offered in support of a sex difference in asymmetry seem to suggest that it is task specific [25,29].
How this particular controversy is resolved may have little bearing on the extent and way in which the mechanisms for the processing of speech and other complex sounds are distributed across the hemispheres in nonhuman animals. Nevertheless, both of the relevant literatures (from humans and nonhumans) agree in suggesting a need for caution in generalizing from the results of any study limited to subjects of one sex. Though our results do suggest hemispheric differences in the processing of VOT by female rats, they cannot tell us if male rats would show a pattern of results that is diminished, increased or altogether different from that reported here.
In addition to making some novel suggestions about how speech sounds are processed by auditory cortex, the present results make several more basic points. First, they confirm that rats can distinguish human speech sounds [13,44,53,54].
Second, they illustrate how effectively PPI can be used to study such processing (also see [6,17] and reviews in [15,16]). In several respects, the results achieved here resemble those from studies using other methods. For instance, the levels of performance described in Table 1 resemble those on operant tasks in the greater difficulty that rats have distinguishing fricatives as opposed to stop consonants [13]. Relative levels of performance here also closely match those predicted by the neural responses to similar stimuli, which themselves correlate highly with operant results [13]. At the same time, operant methods require many days of training to reveal discriminations that often emerge during an initial 30-min test using PPI [16,17].
Third, these data support the sensitivity of PPI to lesion effects on auditory processing. This reinforces previous work by Fitch and colleagues (reviews in [15,16]) and by Bowen et al. [4]. The first group has made extensive and effective use of a form of PPI similar to that used here, focusing on deficits in auditory processing due to neonatal treatments thought to disrupt cortical development, eventually compromising parts of the cortex and the auditory thalamus [6]. These lesions did not affect gap detection but did disrupt some frequency discriminations, specifically when stimuli were brief and incorporated rapid frequency changes. Bowen et al. [4] used a simpler form of PPI to test the effects of damage that was sustained in adulthood and more specific to auditory cortex. They found that such lesions disrupted PPI by noise increments and gaps, but not by noise offsets or pulses. Our results extend these previous observations by illustrating the use of PPI to study brain mechanisms for the discrimination of even more complex stimuli, including human speech sounds. Though lesion studies of such processing clearly can be conducted using other methods (e.g., [3,8,9,13,31]), PPI seems to offer significant practical advantages in speed and ease.
Finally, our results add to evidence suggesting a need to revise current models of PPI. These models place little emphasis on the processing of acoustic prepulses by the auditory cortex (e.g., [30]). But much of the evidence leading to these models reflects the use of relatively simple stimuli as prepulses and forms of PPI emphasizing stimulus detection rather than discrimination. Our results reinforce those of Bowen et al. [4] in suggesting that PPI models should be revised so as to to acknowledge the possible importance of cortical processing, at least when the task or stimuli are relatively complex.
Acknowledgments
This publication was made possible by Grant Number R15DC006624 (Cortical Plasticity and Processing of Speech Sounds) from the National Institute for Deafness and Other Communicative Disorders (NIDCD) and by the Grant Agency of the Czech Republic (309/07/1336). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIDCD or the National Institutes of Health. We thank Chris Skillern, Crystal Engineer, Helen Chen, Chris Heydrick, Dwayne Listhrop and Kevin Chang for their support and assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Owen R. Floody, Department of Psychology, Bucknell University, Lewisburg, Pennsylvania 17837
Ladislav Ouda, Institute of Experimental Medicine, Academy of Sciences of the Czech Republic, Vídenská 1083, 14220 Prague, Czech Republic.
Benjamin A. Porter, Neuroscience Program, School of Behavioral and Brain Sciences, PO Box 830688, GR 41, University of Texas at Dallas, Richardson, Texas 75083-0688
Michael P. Kilgard, Neuroscience Program, School of Behavioral and Brain Sciences, PO Box 830688, GR 41, University of Texas at Dallas, Richardson, Texas 75083-0688
References
- 1.Adams FS, Schwarting RKW, Huston JP. Behavioral and neurochemical asymmetries following unilateral trephination of the rat skull: Is this control operation always appropriate? Physiol Behav. 1994;55:947–952. doi: 10.1016/0031-9384(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 2.Adkins DL, Jones TA. D-amphetamine enhances skilled reaching after ischemic cortical lesions in rats. Neurosci Let. 2005;380:214–218. doi: 10.1016/j.neulet.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 3.Baru AV, Shmigidina GN. Role of the auditory cortex in recognition of synthesized vowels by dogs. Neurosci Behav Physiol. 1977;8:197–204. doi: 10.1007/BF01184058. [DOI] [PubMed] [Google Scholar]
- 4.Bowen GP, Lin D, Taylor MK, Ison JR. Auditory cortex lesions in the rat impair both temporal acuity and noise increment thresholds, revealing a common neural substrate. Cereb Cortex. 2003;13:815–822. doi: 10.1093/cercor/13.8.815. [DOI] [PubMed] [Google Scholar]
- 5.Campeau S, Davis M. Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15:2312–2327. doi: 10.1523/JNEUROSCI.15-03-02312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark MG, Rosen GD, Tallal P, Fitch RH. Impaired processing of complex auditory stimuli in rats with induced cerebrocortical microgyria: An animal model of developmental language disabilities. J Cog Neurosci. 2000;12:828–839. doi: 10.1162/089892900562435. [DOI] [PubMed] [Google Scholar]
- 7.Cooke JE, Zhang H, Kelly JB. Detection of sinusoidal amplitude modulated sounds: Deficits after bilateral lesions of auditory cortex in the rat. Hear Res. 2007;231:90–99. doi: 10.1016/j.heares.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Dewson JH. Speech sound discrimination by cats. Science. 1964;144:555–556. doi: 10.1126/science.144.3618.555. [DOI] [PubMed] [Google Scholar]
- 9.Dewson JH, Pribram KH, Lynch JC. Effects of ablations of temporal cortex upon speech sound discrimination in the monkey. Exptl Neurol. 1969;24:579–591. doi: 10.1016/0014-4886(69)90159-9. [DOI] [PubMed] [Google Scholar]
- 10.Doron NN, LeDoux JE, Semple MN. Redefining the tonotopic core of rat auditory cortex: Physiological evidence for a posterior field. J Comp Neurol. 2002;453:345–360. doi: 10.1002/cne.10412. [DOI] [PubMed] [Google Scholar]
- 11.Eggermont JJ. Representation of a voice onset time continuum in primary auditory cortex of the cat. J Acoust Soc Am. 1995;98:911–920. doi: 10.1121/1.413517. [DOI] [PubMed] [Google Scholar]
- 12.Eggermont JJ. Between sound and perception: reviewing the search for a neural code. Hear Res. 2001;157:1–42. doi: 10.1016/s0378-5955(01)00259-3. [DOI] [PubMed] [Google Scholar]
- 13.Engineer CT, Perez CA, Chen YH, Carraway RS, Reed AC, Shetake JA, Jakkamsetti V, Chang KQ, Kilgard MP. Cortical activity patterns predict speech discrimination ability. Nature Neurosci. 2008;11:603–608. doi: 10.1038/nn.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitch RH, Brown CP, O’Connor K, Tallal P. Functional lateralization for auditory temporal processing in male and female rats. Behav Neurosci. 1993;107:844–850. doi: 10.1037//0735-7044.107.5.844. [DOI] [PubMed] [Google Scholar]
- 15.Fitch RH, Peiffer AM. Behavioral consequences of focal anomalies in the cerebral cortex. In: Rosen GD, editor. The Dyslexic Brain: New Pathways in Neuroscience Discovery. Lawrence Erlbaum Associates; Mahwah, NJ: 2006. pp. 259–288. [Google Scholar]
- 16.Fitch RH, Threlkeld SW, McClure MM, Peiffer AM. Use of a modified prepulse inhibition paradigm to assess complex auditory discrimination in rodents. Brain Res Bull. 2008;76:1–7. doi: 10.1016/j.brainresbull.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Floody OR, Kilgard MP. Differential reductions in acoustic startle document the discrimination of speech sounds in rats. J Acoust Soc Am. 2007;122:1884–1887. doi: 10.1121/1.2770548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuxe K, Bjelke B, Andbjer B, Grahn H, Rimondini R, Agnati LF. Endothelin-1 induced lesions of the frontoparietal cortex of the rat. A possible model of focal cortical ischemia. NeuroReport. 1997;8:2623–2629. doi: 10.1097/00001756-199707280-00040. [DOI] [PubMed] [Google Scholar]
- 19.Geissler DB, Ehret G. Auditory perception vs. recognition: representation of complex communication sounds in the mouse auditory cortical fields. Eur J Neurosci. 2004;19:1027–1040. doi: 10.1111/j.1460-9568.2004.03205.x. [DOI] [PubMed] [Google Scholar]
- 20.Giraud K, Trébuchon-DaFonseca A, Démonet JF, Habib M, Liégeois-Chauvel C. Asymmetry of voice onset time-processing in adult developmental dyslexics. Clin Neurophysiol. 2008;119:1652–1663. doi: 10.1016/j.clinph.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Handel S. Listening: An Introduction to the Perception of Auditory Events. MIT Press; Cambridge, MA: 1993. [Google Scholar]
- 22.Hoffman HS, Searle JL. Acoustic variables in the modification of startle reaction in the rat. J Comp Physiol Psychol. 1965;60:53–58. doi: 10.1037/h0022325. [DOI] [PubMed] [Google Scholar]
- 23.Ison JR, O’Connor K, Bowen GP, Bocirnea A. Temporal resolution of gaps in noise by the rat is lost with functional decortication. Behav Neurosci. 1991;105:33–40. doi: 10.1037//0735-7044.105.1.33. [DOI] [PubMed] [Google Scholar]
- 24.Johnsrude IS, Penhune VB, Zatorre RJ. Functional specificity in the right human auditory cortex for perceiving pitch direction. Brain. 2000;123:155–163. doi: 10.1093/brain/123.1.155. [DOI] [PubMed] [Google Scholar]
- 25.Kansaku K, Kitazawa S. Imaging studies on sex differences in the lateralization of language. Neurosci Res. 2001;41:333–337. doi: 10.1016/s0168-0102(01)00292-9. [DOI] [PubMed] [Google Scholar]
- 26.Kawahara H, de Cheveigne A, Patterson RD. An instantaneous-frequency-based pitch extraction method for high-quality speech transformation: revised TEMPO in the STRAIGHT-suite. Proc. 5th Int. Conf. on Spoken Language Processing (ICSLP’96); Sydney. 1998.12; 1998. [Google Scholar]
- 27.Kelly JB, Rooney BJ, Phillips DP. Effects of bilateral auditory cortical lesions on gap-detection thresholds in the ferret (Mustela putorius) Behav Neurosci. 1996;110:542–550. doi: 10.1037//0735-7044.110.3.542. [DOI] [PubMed] [Google Scholar]
- 28.Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 29.Kitazawa S, Kansaku K. Sex difference in language lateralization may be task-dependent. Brain. 2005;128:E30. doi: 10.1093/brain/awh460. [DOI] [PubMed] [Google Scholar]
- 30.Koch M, Schnitzler H-U. The acoustic startle response in rats-circuits mediating evocation, inhibition and potentiation. Behav Brain Res. 1997;89:35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- 31.Kudoh M, Nakayama Y, Hishida R, Shibuki K. Requirement of the auditory association cortex for discrimination of vowel-like sounds in rats. NeuroReport. 2006;17:1761–1766. doi: 10.1097/WNR.0b013e32800fef9d. [DOI] [PubMed] [Google Scholar]
- 32.Lee CC, Imaizumi K, Schreiner CE, Winer JA. Concurrent tonotopic processing streams in auditory cortex. Cereb Cortex. 2004;14:441–451. doi: 10.1093/cercor/bhh006. [DOI] [PubMed] [Google Scholar]
- 33.Lee CC, Schreiner CE, Imaizumi K, Winer JA. Tonotopic and heterotopic projection systems in physiologically defined auditory cortex. Neurosci. 2004;128:871–887. doi: 10.1016/j.neuroscience.2004.06.062. [DOI] [PubMed] [Google Scholar]
- 34.Liégeois-Chauvel C, de Graaf JB, Laguitton V, Chauvel P. Specialization of left auditory cortex for speech perception in man depends on temporal coding. Cereb Cortex. 1999;9:484–496. doi: 10.1093/cercor/9.5.484. [DOI] [PubMed] [Google Scholar]
- 35.McGee T, Kraus N, King C, Nicol T. Acoustic elements of speechlike stimuli are reflected in surface recorded responses over the guinea pig temporal lobe. J Acoust Soc Am. 1996;99:3606–3614. doi: 10.1121/1.414958. [DOI] [PubMed] [Google Scholar]
- 36.Ohl FW, Wetzel W, Wagner T, Rech A, Scheich H. Bilateral ablation of auditory cortex in Mongolian gerbil affects discrimination of frequency modulated tones but not pure tones. Learn Mem. 1999;6:347–362. [PMC free article] [PubMed] [Google Scholar]
- 37.Ono K, Kudoh M, Shibuki K. Roles of the auditory cortex in discrimination learning by rats. Eur J Neurosci. 2006;23:1623–1632. doi: 10.1111/j.1460-9568.2006.04695.x. [DOI] [PubMed] [Google Scholar]
- 38.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Orlando, FL: 1986. [Google Scholar]
- 39.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 1998. [Google Scholar]
- 40.Polley DB, Read HL, Storace DA, Merzenich MM. Multiparametric auditory receptive field organization across five cortical fields in the albino rat. J Neurophysiol. 2007;97:3621–3638. doi: 10.1152/jn.01298.2006. [DOI] [PubMed] [Google Scholar]
- 41.Qin L, Wang JY, Sato Y. Representations of cat meows and human vowels in the primary auditory cortex of awake cats. J Neurophysiol. 2008;99:2305–2319. doi: 10.1152/jn.01125.2007. [DOI] [PubMed] [Google Scholar]
- 42.Rauschecker JP. Processing of complex sounds in the auditory cortex of cat, monkey, and man. Acta Otolaryngol Suppl. 1997;532:34–38. doi: 10.3109/00016489709126142. [DOI] [PubMed] [Google Scholar]
- 43.Rauschecker JP. Cortical processing of complex sounds. Curr Opin Neurobiol. 1998;8:516–521. doi: 10.1016/s0959-4388(98)80040-8. [DOI] [PubMed] [Google Scholar]
- 44.Reed P, Howell P, Sackin S, Pizzimenti L, Rosen S. Speech perception in rats: Use of duration and rise time cues in labeling of affricative/fricative sounds. J Exptl Anal Behav. 2003;80:205–215. doi: 10.1901/jeab.2003.80-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rybalko N, Suta D, Nwabueze-Ogbo F, Syka J. Effect of auditory cortex lesions on the discimination of frequency-modulated tones in rats. Eur J Neurosci. 2006;23:1614–1622. doi: 10.1111/j.1460-9568.2006.04688.x. [DOI] [PubMed] [Google Scholar]
- 46.Schreiner CE, Wong SW, Dinse HR. Temporal processing in cat primary cortex: Dynamic frequency tuning and spectro-temporal representation of speech sounds. In: Greenberg S, Ainsworth WA, editors. Listening to Speech: An Auditory Perspective. Chap 9. Lawrence Erlbaum Associates; Mahwah, NJ: 2006. pp. 129–141. [Google Scholar]
- 47.Sommer IE, Aleman A, Bouma A, Kahn RS. Do women really have more bilateral language representation than men? A meta-analysis of functional imaging studies. Brain. 2004;127:1845–1852. doi: 10.1093/brain/awh207. [DOI] [PubMed] [Google Scholar]
- 48.Steinschneider M, Fishman YI, Arezzo JC. Representation of the voice onset time (VOT) speech parameter in population responses within primary auditory cortex of the awake monkey. J Acoust Soc Am. 2003;114:307–321. doi: 10.1121/1.1582449. [DOI] [PubMed] [Google Scholar]
- 49.Steinschneider M, Reser D, Schroeder CE, Arezzo JC. Tonotopic organization of responses reflecting stop consonant place of articulation in primary auditory cortex (A1) of the monkey. Brain Res. 1995;674:147–152. doi: 10.1016/0006-8993(95)00008-e. [DOI] [PubMed] [Google Scholar]
- 50.Steinschneider M, Schroeder CE, Arezzo JC, Vaughan HG. Speech-evoked activity in primary auditory cortex: Effects of voice onset time. Electroenceph Clin Neurophysiol: Evoked Poten. 1994;92:30–43. doi: 10.1016/0168-5597(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 51.Suga N. Basic acoustic patterns and neural mechanisms shared by humans and animals for auditory perception. In: Greenberg S, Ainsworth WA, editors. Listening to Speech: An Auditory Perspective. Chap 11. Lawrence Erlbaum Associates; Mahwah, NJ: 2006. pp. 159–181. [Google Scholar]
- 52.Tomita M, Noreña AJ, Eggermont JJ. Effects of an acute acoustic trauma on the representation of a voice onset time continuum in cat primary auditory cortex. Hear Res. 2004;193:39–50. doi: 10.1016/j.heares.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 53.Toro JM, Trobalon JB, Sebastian-Galles N. The use of prosodic cues in language discrimination tasks by rats. Anim Cog. 2003;6:131–136. doi: 10.1007/s10071-003-0172-0. [DOI] [PubMed] [Google Scholar]
- 54.Toro JM, Trobalon JB, Sebastian-Galles N. Effects of backward speech and speaker variability in language discrimination by rats. J Exptl Psychol: Anim Behav Proc. 2005;31:95–100. doi: 10.1037/0097-7403.31.1.95. [DOI] [PubMed] [Google Scholar]
- 55.Zilles KJ. The cortex of the rat: a stereotaxic atlas. Springer-Verlag; Berlin: 1985. [Google Scholar]