Abstract
Chronic pain patients who show aberrant drug-related behavior often are discontinued from treatment when they are noncompliant with their use of opioids for pain. The purpose of this study was to conduct a randomized trial in patients prescribed opioids for noncancer back pain who showed risk potential for or demonstration of opioid misuse to see if close monitoring and cognitive behavioral substance misuse counseling could increase overall compliance with opioids. Forty two patients meeting criteria for high risk for opioid misuse were randomized to either standard control (High-Risk Control; N=21) or experimental compliance treatment consisting of monthly urine screens, compliance checklists, and individual and group motivational counseling (High-Risk Experimental; N=21). Twenty patients who met criteria indicating low potential for misuse were recruited to a low-risk control group (Low-Risk Control). Patients were followed for 6 months and completed pre- and post-study questionnaires and monthly electronic diaries. Outcomes consisted of the percent with a positive Drug Misuse Index (DMI), which was a composite score of self-reported drug misuse (Prescription Drug Use Questionnaire), physician-reported abuse behavior (Addiction Behavior Checklist), and abnormal urine toxicology results. Significant differences were found between groups with 73.7 % of the High-Risk Control patients demonstrating positive scores on the DMI compared with 26.3% from the High-Risk Experimental group and 25.0% from the Low-Risk Controls (p<0.05). The results of this study demonstrate support for the benefits of a brief behavioral intervention in the management of opioid compliance among chronic back pain patient at high-risk for prescription opioid misuse.
Keywords: substance misuse, chronic pain, opioid therapy, motivational counseling, addiction disorder
1. Introduction
There has been a growing use of opioids for the treatment of chronic pain, primarily from providers who prescribe them for chronic noncancer pain [24]. It is also estimated that between 3% and 16% of the general population has an addiction disorder [18,25,31,35], and increasing notice has been given to the abuse of prescription opioid medication [24,32]. Studies also indicate that chronic pain is two to six times greater among patients with a history of substance abuse [20,22,36,42]. Some pain centers, specialty clinics, or primary care practices in which opioids are prescribed for pain are overwhelmed with these challenging patients, and many physicians prescribing pain medication have little training in the assessment and treatment of aberrant medication-related behavior or opioid addiction [45]. Often these physicians prescribe opioids for patients with chronic pain without any assessment of the level of risk for medication abuse [3]. Such an assessment aids the physician in identifying which patients are likely to develop problems, and who can then employ prophylactic measures to improve opioid therapy compliance [5].
While substance misuse is prominent in the chronic pain population there is also a greater potential for inadequate treatment of pain for patients with a history of substance misuse due, in part, to a reluctance of some physicians to address the risks of substance misuse in the context of prescribing opioids [22,29]. Chronic pain patients who show aberrant drug-related behavior often are tapered off opioids and discharged from clinics when they are noncompliant with their use of opioids. Unfortunately, there are few resources for those patients who have chronic pain and a history of prescription opioid misuse.
The Institute of Medicine has directed that persons with chronic pain should be treated in pain programs and opioid use disorders without pain should be treated in addiction treatment centers, however no allowances are made for those with comorbid disorders of pain and substance abuse [27]. Approximately 40% of chronic pain patients have a co-existing, or comorbid, affective disorder, such as a major depression or generalized anxiety disorder [10,14,17]. It is thought that psychiatric comorbidity increases the rate of opioid misuse through inappropriate self-medication of anxious or depressive feelings [19,44]. To our knowledge, no guidelines currently exist for managing opioid misusers with chronic pain and a psychiatric or substance abuse history.
The purpose of this study was to conduct a preliminary randomized trial of patients with back pain who show potential for or demonstration of prescription opioid misuse to see if close monitoring with cognitive behavioral substance misuse counseling can increase overall compliance with opioids and reduce the rate of patients getting dismissed from a treatment center. It was hypothesized that patients at risk of or with signs of misuse of prescription opioids will show a greater incidence of opioid misuse over time, and that frequent urine screen monitoring, monthly self-report compliance checklists, and individual and group substance misuse counseling will result in improved compliance of chronic pain patients at risk for prescription opioid misuse.
2. Methods
Concise definitions of terms are important to minimize confusion and help to clarify the objectives of this study [37]. For purposes of this investigation, substance misuse is defined as the use of any drug in a manner other than how it is indicated or prescribed. Substance abuse is defined as the use of any substance when such use is unlawful, or when such use is detrimental to the user or others. Prescription opioid addiction is a primary, chronic, neurobiologic disease that is characterized by behaviors that include one of more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving [1,46]. Aberrant drug-related behaviors are any behaviors that suggest the presence of substance abuse or addiction. Determining an individual’s potential for aberrant drug behaviors and preventing misuse of prescription opioids is important in the evaluation and management of patients with chronic pain.
2.1 Participants
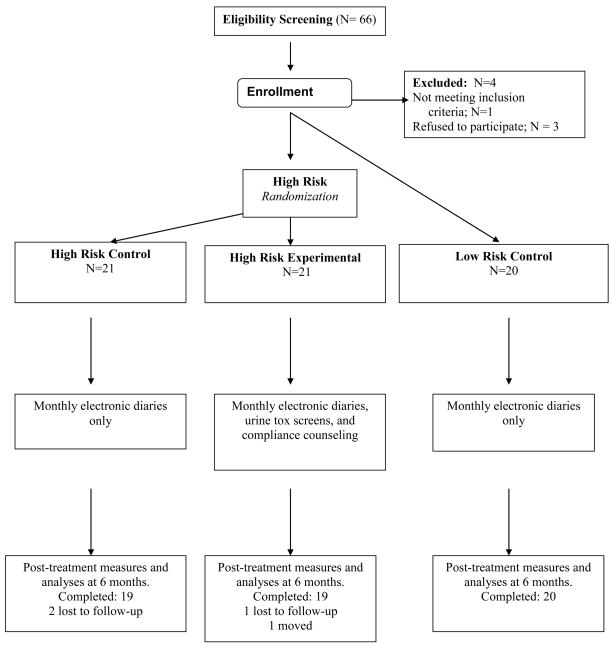
The Human Subjects Committee of Brigham and Women’s Hospital approved this study’s procedures and written informed consent was obtained from every subject. Patients with a diagnosis of back or neck pain with or without radicular symptoms were recruited to participate in this 6-month trial (Fig. 1). Patients were included if they (1) had chronic back or neck pain for > 6 months’ duration, (2) averaged 4 or greater on a pain intensity scale of 0 to 10 with medication, (3) were able to speak and understand English, (4) had been prescribed opioid therapy for pain for > 6 months, and (5) had risk for (Screener for Opioid Abuse for Pain Patients; SOAPP-R > 18) or history of prescription opioid misuse based on past records of abnormal urine screens and physician report (Addiction Behaviors Checklist, ABC > 2).
Fig 1.
Study schema.
Patients were excluded from participation if they met any of the following criteria: (1) current diagnosis of cancer or any other malignant disease, (2) acute osteomyelitis or acute bone disease, (3) present or past DSM-IV diagnosis of schizophrenia, delusional disorder, psychotic disorder, or dissociative disorder, (4) pregnancy, (5) any clinically unstable systemic illness judged to interfere with treatment, (6) an acute condition requiring surgery, and (7) an active addiction disorder, such as IV heroin use within the past year (positive on the Mini International Neuropsychiatric Interview; M.I.N.I. v.5.0 [39]).
All patients were evaluated by a physician and received a complete history and physical examination. Radiological studies were consulted to support the diagnosis. All subjects were maintained on their current opioid medication and asked to remain on a stable dose throughout the study period. All other adjuvant medication remained constant through the course of the 6-month trial. Prescriptions of immediate release (IR) medication for breakthrough pain were based on physician decision. All prescription medications were carefully monitored by the Study Manager through the use of electronic diaries and monthly contacts. Medication was dispensed once per month unless decided otherwise by the treating physician. Patients in the High-Risk Experimental group were offered free individual and group behavioral counseling as part of the study. All subjects received $50 gift cards for completing the baseline and post-treatment measures.
Subjects were determined to be high risk for prescription opioid misuse based on their responses on the SOAPP-R (score > 18), or opioid misuse based on physician report (ABC > 2) and abnormal urine screens. They were invited to participate in the study and were randomly assigned to Control or Experimental treatment arms. Those in the High-Risk Control group were maintained on their current opioid regimen and were seen on a monthly basis at a pain treatment center at a university-based medical center. They completed electronic diaries and had monthly contact with their physician. They represented the usual treatment control condition.
Randomization among the high-risk patients consisted of assignment to treatment group based on a randomized number list created before the start of the study. Subjects were assigned to their group in the order that they entered into the study prior to conducting any data entry. Those high-risk patients randomly assigned to the High-Risk Experimental Group received the same medical treatment as the High-Risk Controls and in addition were asked to participate in a structured cognitive behavioral training program for prevention of substance misuse. As part of the treatment protocol, they (1) participated in one or more group sessions in which risk factors regarding opioid use were discussed, (2) received monthly individual motivational counseling sessions to review compliance issues, (3) were given substance misuse education worksheets, (4) completed a monthly Opioid Compliance Checklist, developed for this study, and (5) had monthly urine screens. The group and individual sessions were designed to offer knowledge and training for substance misuse awareness and recovery. The sessions focused on 1) enhancing and maintaining motivation to abstain from illicit substance use, 2) coping with urges to misuse medication, 3) problem solving (managing thoughts, feelings & behaviors) related to substance misuse, and 4) lifestyle balance (balancing momentary & enduring satisfactions). Additional information on the compliance counseling is presented below.
For comparison purposes, we identified patients with chronic back or neck pain who had been prescribed long-acting opioids for pain for > 6 months and showed no signs of medication misuse. They had SOAPP-R scores of <18 and had a history of compliance with opioid medication based on physician report and ABC scores ≤ 2. These patients, who met criteria for low-risk for substance misuse, completed monthly electronic diaries, were maintained on their opioid therapy regimen, and were followed for a minimum of 6 months, similarly to the High-Risk Controls (Fig. 1).
Data on urine toxicology results and self-report outcome measures from the Current Opioid Misuse Measure (COMM), and Prescription Drug Use Questionnaire (PDUQ) allowed for group comparisons. Outcomes consisted of the percent of patients with a positive Drug Misuse Index (DMI). This is a composite measure triangulating urine screen results, staff ratings of abuse behavior (ABC > 2), and self-report results of the PDUQ (>11) over the course of 6 months. This has been used as an outcome measure by the authors in previous studies [43,44].
2.2 Baseline Measures
2.2.1 Demographic Questionnaire [21]
This questionnaire collected basic demographic information about patients including: 1) age, 2) gender, 3) racial background, 4) education level, 5) marital status, 6) history of medical problems, 7) history of substance abuse (including treatment experience, activity in AA/NA, etc.), and 8) active litigation, disability or worker’s compensation status.
2.2.2 Screening for substance abuse severity
The Mini International Neuropsychiatric Interview (M.I.N.I. v.5.0) [39] was used to screen for active opioid addiction and any other addiction disorder. The MINI was designed as a brief structured interview for the major Axis I psychiatric disorders in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV)[2]. It has demonstrated adequate reliability and validity. We used section K to assess the presence of a current non-alcohol psychoactive substance use disorder. It is designed to identify the use and frequency of 1) stimulants, 2) cocaine, 3) non-prescription opioids, 4) hallucinogens, 5) heroin, 6) inhalants, 7) marijuana, 8) non-prescription tranquilizers, and 9) other substances of abuse. During the screening session, participants were shown a list of street drugs or medications and for each of the drug groups was asked “How many days in the past thirty have you used _____?” Any nonprescription and/or illicit substance used within the past month would trigger further questioning to determine if he/she met criteria for current substance dependence and/or abuse. Active opioid addiction (e.g., IV heroin use) were grounds for exclusion from this study. Patients with a substance use disorder with other substances were excluded based on how recently the abuse occurred (within the past year) and the severity of the disorder (e.g., admitted interference with daily activities, failed attempts to reduce or stop, continued to use despite health, mental, or legal problems). These individuals were referred to a local addiction treatment facility.
2.2.3. Screener and Opioid Assessment for Pain Patients- Revised (SOAPP-R)[5,6,8]
The SOAPP-R is a 24-item, self-administered screening instrument used to assess suitability of long-term opioid therapy for chronic pain patients to help determine risk potential for future aberrant drug-related behavior. Items are rated from 0=never to 4=very often, (e.g., How often have others expressed concern over your use of medication?) and their sum is the total SOAPP-R score with a maximum possible score of 96. Test-retest reliability was .71 with a coefficient alpha of 0.74 [8]. The SOAPP-R has been shown to have good predictive validity, with an area under the curve ratio of 0.88 (95% confidence interval [CI], .81–.95). A cutoff score of 18 shows adequate sensitivity (.86) and specificity (.73) for predicting prescription opioid misuse. Preliminary support has been found for the internal reliability and predictive validity of the SOAPP-R [6]. An accumulated score of 18 or higher is considered positive.
2.2.4. Addiction Behaviors Checklist (ABC) [48]
This is a 20-item instrument designed to track behaviors characteristic of addiction related to prescription opioid medications in chronic pain populations. All items are rated yes, no, or not assessed (e.g., Patient ran out of medications early). Items are focused on observable behaviors during and between clinic visits. This checklist was found to have adequate validity and reliability. A cut-off score of 3 or greater showed optimal sensitivity and specificity in determining whether a patient is displaying inappropriate opioid use.
2.2.5. The Brief Pain Inventory (BPI) [11]
This self-report questionnaire, formerly the Wisconsin Brief Pain Questionnaire [13], is a well-known measure of clinical pain and evidences sufficient reliability and validity. The questionnaire provides information about pain history, intensity, and location as well as the degree to which the pain interferes with daily activities, mood, and enjoyment of life. Scales (rated from 1 to 10) indicate the intensity of pain in general, at its worst, at its least, average pain, and pain “right now.” A figure representing the body is provided for the patient to shade the area corresponding to his or her pain. Test-retest reliability for the BPI reveals correlations of .93 for worst pain, .78 for usual pain, and .59 for pain now. Research suggests the BPI has adequate validity. BPI scores correspond with clinical judgments of pain as reflected in pain medication use and the amount of patient-reported activity interference.
2.2.6. The Hospital Anxiety and Depression Scale (HADS) [49]
The HADS is a 14-item scale designed to assess the presence and severity of anxious and depressive symptoms. Seven items assess anxiety and seven items measure depression, each coded from 0 to 3 with different descriptive anchors. The HADS has been used extensively in clinics and has adequate reliability (Cronbach’s Alpha = .83) and validity, with optimal balance between sensitivity and specificity to predict the presence of a DSM-IV major depression or generalized anxiety disorder [4]. It has been translated into many languages and is widely used around the world in clinical and research settings.
2.2.7. The Pain Disability Index (PDI) [40]
This inventory consists of seven questions designed to measure the degree to which patients believe that their pain interferes with their functioning in family/home responsibilities, recreation, social activities, occupation, sexual behavior, self-care, and life-support (eating, sleeping, and breathing) activity. Patients respond to each item on 0- to 10-point scales anchored with descriptors ranging from “no disability” to “total disability.” The score is the total number for each item divided by 7. This measure has adequate internal consistency (Cronbach alpha = .86) and test-retest reliability (0.91) and is a valid measure of disability [41].
2.2.8. Current Medication Misuse Measure (COMM) [7]
The COMM is a 17-item self-report questionnaire that helps to track current aberrant medication-related behaviors during opioid treatment. All items are rated from 0=never to 4=very often (e.g., In the past 30 days, how often have you needed to take pain medications belonging to someone else?) with a total maximum score of 68. Construct validity has been shown to be adequate with positive correlates with urine toxicology results (p<0.05). Test-retest reliability was .86 with a 95% CI ranging from .77 to .92. The overall accuracy of the COMM for predicting current aberrant drug-related behavior, as measured by the area under the curve ratio, was .81 (95% CI, .74–.86; p < .001) and coefficient α (.86) for the 17 items suggests adequate reliability. A cutoff score of 8 yielded a sensitivity of 0.75 and specificity of 0.65. An accumulated cutoff score of 9 or higher is considered positive.
2.2.9. Electronic diaries [26]
All patients monitored their progress with the use of electronic diaries at the start of the study and once a month during each clinic visit. The pain electronic calendar [26] comprises a comprehensive set of 25 items, incorporating key questions from the Brief Pain Inventory (severity, activity, function and mood), medication questions, and location of pain (pain diagram). The devices consisted of a Hewlett Packard © IPAQ personal digital assistant (PDA). Diary data was downloaded and saved as part of each individual’s study file. The data was used to summarize changes in level of pain, pain description, activity interference, mood, and side effects. Scores for level of pain and interference with activities range from 0 (no pain/does not interfere) to 10 (pain as bad as you can imagine/completely interferes), are recorded to the nearest 1/10 for the electronic diary data, and are stored on a 0–100 scale. An average score for each variable is computed for each participant. Data were also saved for final analyses on number of pain descriptors, number of pixels in the pain diagrams (front/back, left/right), medication use, and side effects. Only those patients who completed 4 monthly diary entries or more over the course of the study were included in the final analyses.
2.2.10. Opioid Compliance Checklist [23]
High-risk patients assigned to the experimental condition were asked to complete a compliance checklist once a month. This is a 12-item yes/no questionnaire developed for this study that addresses the main components of the opioid therapy agreement signed by the patient and treating physician at the time of beginning opioid therapy. The items focus on responsible use of opioid medication including taking medication as prescribed, using only one pharmacy, having only one provider, not running out of medication early, not missing appointments, not borrowing medication from others, not using illegal substances, and taking precaution not to lose the medication.
2.2.11. Urine toxicology screens
Each study participant was asked for a urine sample during their clinic visit at baseline and at the conclusion of the 6-month trial. Those subjects assigned to the High-Risk Experimental Group were asked for monthly urine screens during the 6-month study. The following guidelines were used for the collection and preparation of urine drug screen specimens: 1) At the time of the urine sample, the current medications taken within the past 24 hours were documented; 2) the patient was given a 30-ml labeled container and asked for a urine sample; 3) the subject gave an unobserved urine sample in the clinic bathroom while the researcher assistant waited; 4) the research assistant was sure that the cap was securely screwed onto the container and placed it in a marked pouch; 5) the pouch included a completed ID label with patient ID number, date, and time of the urine sample; and 6) the pouch was sent for analysis. The urine samples were analyzed by Mayo Medical Laboratories of New England, Andover, Massachusetts, using a comprehensive gas chromatography/mass spectroscopy (GC/MS) screen. The urine toxicology report included evidence of 6-MAM (heroin), codeine, dihydrocodeine, morphine, oxycodone, oxymorphone, hydrocodone, hydromorphone, meperidine, methadone, propoxyphene, buprenophine, fentanyl, tramadol, amphetamines, barbiturates, benzodiazepines, cannabinoids, cocaine, phencyclidine, and ethyl alcohol. Results were grouped as 1) negative (i.e., normal urine or equivocal results), 2) positive for illicit substances or alcohol (evidence of marijuana, cocaine, ethanol, phencyclidine), 3) positive for a prescription opioid not prescribed (We recognize that some false negatives are possible based on detected metabolites, e.g., oxycodone is metabolized into oxymorphone.), and/or 4) negative for an opioid that was prescribed.
2.3. Post-Study 6-Month Assessment
At the end of the study, all patients repeated all of the baseline questionnaires listed above except the demographic questionnaire, participated in a structured interview using the Prescription Drug Use Questionnaire (PDUQ), and gave a urine sample for a toxicology screen. We compared secondary outcome differences (pain, activity interference, mood, side effects, retention in the program, treatment satisfaction and helpfulness) between the High-Risk Experimental patients who participated in compliance treatment vs. the High-Risk and Low-Risk Controls.
2.3.1. Treatment Helpfulness Questionnaire (THQ) [9]
This rating scale was completed by the patients at the end of the study. The items are rated from 0 = extremely harmful to 10 = extremely helpful; additional items, which contain statements about issues related to the treatment rated from 1 = Strongly Agree to 5 = Strongly Disagree (e.g., “My participation in this study helped me to comply with my medication use.”) were also included. The THQ has been shown to have good test-retest reliability and validity and assesses how helpful the treatments for pain have been.
2.3.2. Prescription Drug Use Questionnaire (PDUQ) [12]
Self-report of patient status at follow-up was obtained using the PDUQ. This 42-item structured interview is probably the most well-developed abuse assessment instrument for pain patients at this time [38]. The PDUQ is a 20-minute interview during which the patient is asked about his or her pain condition, opioid use patterns, social and family factors, family history of pain and substance abuse, and psychiatric history. In an initial test of the psychometric properties of the PDUQ, the standardized Cronbach’s alpha was 0.79, suggesting acceptable internal consistency. Compton and her colleagues suggested that subjects who scored below 11 did not meet criteria for a substance use disorder, while whose with a score of 11 or greater showed signs of a substance use disorder. For purposes of this study we used scores greater than 11 on the PDUQ as a positive indicator for the Drug Misuse Index.
2.3.3. Drug Misuse Index (DMI)
At post-treatment, after 6 months, patients were categorized on the DMI, which relates positively to opioid medication misuse. The DMI is based on positive scores on the self-reported PDUQ, the physician-reported ABC, and the urine toxicology results. A positive rating on the PDUQ is an accumulated score higher than 11. A positive rating on the ABC is given to anyone who has two or more physician-rated aberrant behaviors [5]. A positive rating from the urine screens is given to anyone with evidence of having taken an illicit substance (e.g., cocaine) or an additional opioid medication that was not prescribed. We chose not to count the omission of a prescribed opioid medication from the urine screen results as a positive rating because of multiple factors that can contribute to this result (e.g., subject appropriately ran out the medication before the urine screen). We also did not classify urines that were rejected by the lab. Urine screen results were confirmed based on chart review of prescription history and a comparison between self-report at the time of the urine screen and the toxicology report. Those with positive scores on the PDUQ were given a positive DMI. If this score (PDUQ < 11) was negative, then positive scores on both the urine toxicology screen and on the ABC (≥2) was necessary to achieve a positive DMI. Thus, this classification method allowed for triangulation of data to identify those patients who admitted to medication misuse, and those who may underreport misuse (e.g., low PDUQ scores), but still presented with a drug misuse profile, including positive ABC and abnormal urine screen results [8]. Those with negative scores on all three scales (minimal risks or indicators of misuse) were given a negative DMI. In using this scoring plan for the DMI in a previous study [5] (previously called aberrant drug-related behavior Index, or ADBI), it was found that 32% met criteria for aberrant drug-related behavior based on the results of self-report (PDUQ), physician ratings of aberrant behavior, and abnormal urine toxicology results. Forty-three percent of the patients followed for over 6 months [5] were shown to have aberrant drug-related behavior.
There is no gold standard for identifying which patients are and which are not misusing their prescription medication. We believe that self-report, when positive, is the most direct measure of a substance use disorder, since false positives (i.e., patients reporting the presence of a substance use disorder when none is present) are presumably quite rare. Thus, those participants who met criteria for a substance use disorder based on the Prescription Drug Use Questionnaire (PDUQ > 11) were given a positive score on the DMI. If not, then we scored the DMI as positive if there were positive urine screen results and 2 or more physician-rated aberrant behaviors (ABC > 1). We decided this after considerable discussion because urine screens can be problematic (e.g., mistakes can be made with urine screen results based on variable drug metabolites and different cutoffs used in detecting a drug). We also know from experience that physician ratings of aberrant behavior can be unreliable [30,33]. An analysis of past data found that those with the most problematic urine screen results (e.g., tested positive for cocaine) also were positive on the PDUQ, which lent support for the reliability of this classification method [43].
2.4. Experimental Group Intervention
The experimental treatment condition consisted of five components: 1) completion of monthly electronic diaries; 2) monthly urine screens for 6 months; 3) monthly completion of the Opioid Compliance Checklist; 4) monthly group education sessions (led by a psychiatrist [ADW] trained in pain and addiction medicine) with worksheet handouts on topics related to substance misuse; and 5) participation in individual motivational compliance counseling (led by a clinical psychologist [RNJ] trained in pain and behavioral medicine). Patients in the experimental condition were told that they should demonstrate that they accomplished each of their assigned tasks before being given their next opioid prescription. Patients randomly assigned to the High-Risk Experimental Group were asked to participate in monthly individual counseling and at least one monthly group session designed to offer knowledge and training for substance misuse awareness and recovery.
The program focused on enhancing and maintaining motivation for abstaining from illicit substance use, coping with urges, problem solving (managing thoughts, feelings and behaviors), and balancing momentary and enduring satisfactions. The focus of the group session was on 1) opioid addiction risks and medication compliance, 2) education regarding misuse and relapse, 3) making lifestyle changes, 4) avoiding drug use triggers, and 5) responsible attendance (at clinic appointments and self-help programs).
Content of the individual sessions included 1) review of medication adherence and substance use since prior visit, 2) review of response to medication, 3) review components of the compliance checklist, 4) advice concerning abstinence from illicit substances, 5) support for patients’ efforts, 6) education on pain management and drug misuse, 7) discussion of noncompliance, and 8) discussion of the educational handouts. Brief notes were placed in the electronic medical record following each individual session. Handouts adopted and revised from the Manual for Individual Drug Counseling for Opioid Dependent Patients [28,47] were used as part of the group and individual sessions. The topics of the educational worksheets were (1) Opioid therapy for pain, (2) How to handle triggers of drug misuse, (3) Relationships and support systems, (4) Managing feelings and coping with drug-misuse situations, (5) Warning signs of recurrent misuse, and (6) Maintaining opioid use compliance.
2.5. Statistical Analyses
All data were analyzed with SPSS (Statistical Package for the Social Sciences; Chicago, IL) v.17.0. The null hypotheses were that 1) no differences would be found between the high-risk experimental and control groups and 2) no differences would be found between the high-risk and low-risk groups. The main analyses were conducted according to an intent-to-treat principle using multiple imputations; that is, patients would be included in the group to which they were originally randomized regardless of whether they completed the intervention assigned and regardless of whether they had missing data as a result of missed visits. In addition, we examined differences between low-risk and high-risk groups, and between the experimental vs. control high-risk groups taking into consideration any covariate factors associated with the outcomes. Power calculations were completed using SAS Version 9.1 (SAS Institute, Inc., Cary, NC). These calculations were based on an assumption of 21 subjects in each cell in a 1×2 factorial design at a significance level of 0.05. With these assumptions we had adequate power (0.748) to detect the difference between the high-risk vs. low-risk groups, and adequate power (0.886) to detect the difference between the high-risk experimental treatment vs. high-risk standard treatment (control) groups. We initially anticipated an attrition rate of 15%.
Relations among demographic data, interview items, questionnaire data, physician ratings, and urine toxicology results were analyzed using Pearson product moment correlations, Chi-square, and analysis of variance (ANOVA) analyses depending on whether the variables were ordinal or numerical. ANOVA with repeated measures were used to investigate the effect of high-risk vs. low-risk groups on the percentages of patients exhibiting drug misuse behavior. Finally, discriminant function analyses were conducted with specified variables using Wilks’ Lambda and canonical correlations in order to identify those items that were best in classifying high- and low-risk subjects.
3. Results
3.1. Baseline data
We recruited a total of 66 subjects for this trial. None of the participants were found to have an active addiction disorder based on results of the M.I.N.I. One subject did not meet inclusion criteria. Two patients withdrew their consent before starting the trial, and one withdrew after completing the initial questionnaires. Thus, 62 patients were entered into the study. Twenty one were randomized to the High-Risk Control, 21 to the High-Risk Experimental treatment arm, and 20 subjects met criteria for the Low-Risk Control group. Three subjects were lost to contact by the end of the study (two High Risk Control and one Low Risk Control) and one subject (High-Risk Experimental) moved and transferred care to another clinic. One subject in the High-Risk Experimental treatment group was voluntarily treated at a detox center, but eventually returned to the Pain Management Center for continued treatment of his pain.
The average age of the patients was 47.7 years (SD=7.14; range 24–63), 43.5% were women, 45.2% were married, and 75.8% were Caucasian. All patients had either chronic neck or back pain with or without other pain sites. Sixty one percent described having low back pain as their primary pain site. The average pain duration was 9.02 years (SD=7.59; median=9.0; range 6 months to 34 years). Their average pain rating was 5.60±2.55 (least=4.04±2.62; worst = 7.81±3.36; now = 6.00±2.15). The patients were prescribed immediate-release opioids (40.5% oxycodone; 29.7% oxycodone with acetaminophen; 8.1% hydrocodone; 8.1% morphine; 8.1% hydromorphine; 5.4% fentanyl), and sustained-release opioids (30.8% methadone; 25.0% oxycodone; 21.1% morphine; 17.3% transdermal fentanyl; and 5.8% hydromorphone) for pain. Forty eight percent were taking both long- and short-acting opioids for pain.
Differences among the three groups on demographic data, pain intensity and interference items from the BPI, and scores on the HADS are presented in Table 1. Even though members of the Low Risk Control group showed lower ratings of disability on the PDI, no significant differences were found between the Low Risk Controls and the High Risk patients at baseline. Also, no descriptive differences between the High-Risk groups were found at baseline suggesting that the randomization process was successful. Thus, level of risk of misuse of opioids was not accounted for by descriptive factors of age, gender, pain intensity, pain interference, level of disability, or mood.
Table 1.
Baseline comparisons of groups on demographic, pain intensity, disability, and mood.
| Variable | High Risk Control (N=21) | High Risk Experimental (N=21) | Low Risk Control (N=20) | P |
|---|---|---|---|---|
| Age | 46.57±6.78 | 47.00±7.75 | 49.55±6.80 | NS |
| Gender (%male) | 57.1 | 47.6 | 65.0 | NS |
| PDI | 6.44±1.84 | 6.25±1.99 | 5.20±1.93 | NS |
| Worst pain+ | 8.00±1.95 | 7.95±7.83 | 7.65±1.42 | NS |
| Least pain+ | 4.71±2.26 | 4.38±2.33 | 4.45±2.13 | NS |
| Ave pain + | 6.24±2.07 | 5.86±1.89 | 5.85±1.76 | NS |
| Now pain+ | 6.14±2.63 | 6.00±2.47 | 6.25±2.45 | NS |
| % pain relief+ | 60.24±25.42 | 57.00±25.57 | 55.79±24.57 | NS |
| Pain Interference: | ||||
| Activity | 6.86±2.67 | 6.52±2.58 | 5.75±1.73 | NS |
| Mood | 4.95±3.29 | 5.71±2.61 | 4.55±2.42 | NS |
| Walking | 6.62±3.14 | 5.14±3.43 | 5.79±2.35 | NS |
| Work | 7.48±3.04 | 6.76±2.98 | 6.83±2.07 | NS |
| Relations with others | 4.24±3.53 | 4.38±2.87 | 3.95±2.46 | NS |
| Sleep | 6.14±3.47 | 6.29±3.18 | 6.45±2.19 | NS |
| Enjoy Life | 6.05±3.03 | 5.81±2.91 | 5.95±2.83 | NS |
| Pain Meds Beneficial | 6.31±2.80 | 6.69±2.43 | 6.58±2.52 | NS |
| HADS-Anxiety | 8.10±3.48 | 7.43±3.84 | 6.40±3.35 | NS |
| HADS-Depression | 8.43±3.61 | 7.14±3.97 | 6.15±3.95 | NS |
Brief Pain Inventory
Subjects were assigned to the high and low risk groups based on SOAPP-R scores and/or physician referrals due to past behavior (e.g., abnormal urine screens). As expected, the Low-Risk Controls had significantly lower SOAPP-R scores (mean = 13.3 ±6.77) than the High Risk Control (mean = 23.1±9.3) and High Risk Experimental (mean = 18.6 ±9.3) groups (F = 6.64; p<0.01; Table 2). Seventeen patients were classified as High Risk due to past behavior even though they had scores below 18 on the SOAPP-R. Significant differences were found between groups on the COMM (p<0.05), ABC (p<0.05), and percent of past abnormal urine screens (p<0.001) at baseline in the predicted direction. This confirmed the relationship between SOAPP-R scores and self-reported current opioid misuse (COMM), physician ratings of aberrant drug-related behavior (ABC), and abnormal results from urine toxicology screens at baseline. From chart review data, those categorized as at high risk for medication misuse at baseline were requested by their treating physician to give urine samples more often than the low-risk controls over the past 2 years (High-Risk Controls mean = 6.05 ±3.51; High-Risk Experimental mean = 6.33 ±3.40; Low-Risk Control 3.40±1.34; df=2; F=6.15; p<.01). This lends further validation to the high and low risk classification of the subjects at baseline.
Table 2.
Baseline SOAPP-R, COMM, ABC, and previous urine screen results
| Variable | High Risk Control (N=21) | High Risk Experimental (N=21) | Low Risk Control (N=20) | F and X2 values |
|---|---|---|---|---|
| SOAPP-R | 23.14±9.63a | 18.57±9.31a | 13.25±6.77b | F=6.64** |
| COMM | 13.80±8.27a | 9.86±6.42 | 7.20±5.14b | F=4.88* |
| ABC | 2.60±3.28 | 2.52±3.43 | 0.70±1.72 | F=3.03* |
| # Urine screens | 6.05±3.51a | 6.33±3.40a | 3.40±1.34b | F=6.15** |
| Abn urines (%) | 39.1 | 37.0 | 5.5 | X2=9.38*** |
p<0.05;
p<0.01;
p<0.001
p<0.05 Bonferroni
3.2. Electronic diary data
Pain intensity and pain description
All subjects were asked to complete electronic diaries [26] once a month upon their return to the clinic. Fifty-six subjects completed four or more monthly diaries and were included in the analyses (21 High-Risk Control; 17 High-Risk Experimental; 18 Low-Risk Control). Reasons for missing diary data included missed appointments, time contraints, and unavailability of the research assistants or the PDAs. Altogether there were 365 diary entries over the 6-month trial (High-Risk Controls = 134; High-Risk Experimental = 110; Low-Risk Control = 121). The average number of per subject completions was 6.55 ±1.25; range 4–9. The results of mean pain intensity ratings over six months are presented in Table 3. No differences were found between groups in how typical they thought their pain was over the course of the trial (from 0=not at all typical to 100=very typical). Significant differences were found between the High Risk Controls and the Low Risk Controls, with the High Risk subjects reporting greater pain intensity (p<0.001). Of note, the High-Risk Experimental subjects reported significantly lower average pain intensity ratings over the course of the trial than the High-Risk Controls (p<0.05). Very few differences were noted in the variables used to describe the pain among group members. The only significant difference found was the use of ‘burning’ in describing the pain among the High-Risk Experimental group compared with the other two groups (p<0.05). Most participants chose an average of four symptoms in describing their pain. No differences were found in the total number of symptoms chosen by members of the three groups.
Table 3.
In-clinic electronic diary ratings of mean pain intensity, mood, and activity interference over six months (0–100).
| Variable | High-Risk Control | High-Risk Experimental | Low-Risk Control | F-values |
|---|---|---|---|---|
| Pain# | ||||
| Now pain | 63.79 (22.06)a | 57.86 (23.77) | 53.82 (21.38)b | 6.43** |
| Worst pain | 76.06 (18.58)a | 76.87 (17.25)a | 67.53 (22.03)b | 8.54*** |
| Least pain | 53.44 (24.31)a | 46.62 (23.84) | 42.43 (21.21)b | 7.35*** |
| Average pain | 65.32 (20.80)a | 57.45 (20.42)b | 56.06 (20.49)b | 7.56*** |
| How typical† | 70.90 (24.99) | 70.51 (27.17) | 67.45 (25.08) | NS |
| Mood | ||||
| Depressed/discouraged+ | 49.94 (31.40)a | 47.59 (33.35)a | 37.71 (26.89)b | 5.48** |
| Tense/anxious+ | 53.78 (29.23)a | 44.44 (30.50)b | 37.43 (26.05)b | 10.37*** |
| Irritable/angry+ | 36.38 (30.16) | 40.21 (31.18) | 31.72 (22.22) | NS |
| How typical† | 64.47 (30.32) | 68.22 (29.37) | 60.82 (29.16) | NS |
| Activity interference‡ | ||||
| Daily routine | 67.00 (28.66)a | 63.73 (26.02) | 59.03 (20.75)b | 3.18* |
| Social | 63.20 (31.29)a | 63.10 (28.69)a | 54.15 (24.00)b | 4.20* |
| Sex | 54.14 (37.11) | 61.77 (36.94) | 59.90 (30.19) | NS |
| Sleep | 64.74 (28.84) | 65.79 (27.26) | 62.64 (26.38) | NS |
| Work | 79.48 (27.71)a | 71.30 (30.45) | 69.21 (26.06)b | 4.89* |
| Outdoor/rec | 73.58 (29.12)a | 73.90 (24.62)a | 66.53 (22.61)b | 3.24* |
| Appetite | 51.81 (31.34)a | 50.27(30.30)a | 40.33 (24.68)b | 5.80** |
Visual analogue scale of 0 = none, 10 = worst pain possible, converted to 0–100.
Over the past 24 hours: 0=not much; 10=very much, converted to 0–100.
Over the past month, 0 = not at all typical; 10 = very typical; converted to 0–100.
Over the past 24 hours, how much has the pain interfered with your: 0=not much; 10=very much.
Bonferroni p<0.05
p<0.05;
p<0.01;
p<0.001
3.2.1. Mood ratings
Monthly ratings of mood (depression, anxiety, irritability) averaged over time between the three groups are presented in Table 3. Most of the responders felt that their mood was typical and not out of the ordinary over the course of the study. The High-Risk groups reported significantly higher levels of depression than the Low-Risk controls (p<0.01) and, notably, the High-Risk Controls reported more tension and anxiety than the High-Risk Experimental and Low-Risk Control subjects. No differences were found between groups on reported irritability.
3.2.2. Side effects
Each of the participants was asked to identify any side effects that they had been experiencing during every diary entry. The most frequently reported side effects were dry mouth (44.9%), constipation (38.4%), sweating (37.5%), memory lapse (28.4%), weakness (24.1%), itching (23.9%) and headaches (18.5%). The results showed that the High-Risk Experimental subjects reported the presence of constipation and itching less and vision problems more than those in the other two groups (p<0.05), while the High-Risk Controls reported more severity of constipation, sneezing and nightmares than those in the other two groups. The Low-Risk Controls reported greater severity in itching than those in the High-Risk groups, while the High-Risk groups reported more severe confusion than the Low-Risk subjects (p<0.05).
3.2.3. Activity interference
All participants rated the extent to which their pain interfered with activities. The results, presented in Table 3, show that the subjects described their pain as interfering the most with working (mean = 73.20 ±28.51) and interfering the least with appetite (mean = 47.08 ±29.23). Those in the High-Risk groups reported more activity interference with daily routine, social activities, working, outdoor activities, and appetite compared to those in the Low-Risk group (p<0.05).
3.2.4. Discriminant function analyses
Discriminant analyses were performed incorporating variables from Table 3 in order to determine which variables over the course of the study were best able to predict group membership. First, from the electronic diary study variables, 11 scores that appeared to be most useful in distinguishing the groups were included in the stepwise procedure. These included (1) pain now, (2) worst pain, (3) least pain, (4) average pain, (5) depression, (6) tension and anxiety, (7) daily routine interference, (8) interference with social activities, (9) interference with work, (10) interferences with outdoor activities, and (11) interference with appetite. These analyses correctly classified 51.8% % of the patients in the high and low risk groups with a combined Wilks’ Lambda was 0.93 (X2 = 27.23; p<.01) and a canonical correlation was 0.27. Next, a stepwise discriminant analysis of those variables from Table 3 showed that three variables, (1) tense and anxious, (2) worse pain intensity, and (3) average pain intensity, correctly classified 47.1% of the patient groups, with a Wilks Lambda = 0.96 (X2 = 27.21; p<0.01) and a Canonical correlation = 0.28.
3.3. End of study data
Three subjects were lost to contact at the close of the 6-month trial (two from the High-Risk Control group and one from the High-Risk Experimental group) and one subject moved and transferred care to another clinic (High-Risk Experimental). Thus 58 subjects were followed and successfully completed post-treatment questionnaires (High Risk Control, N=19; High Risk Experimental, N=19; Low Risk Control, N=20).
No differences were found among the groups on the PDI, although the High-Risk Controls had significantly higher ratings of anxiety (High-Risk Control = 9.00 ±3.39; High-Risk Experimental = 6.38 ±3.78; Low-Risk Control = 6.21 ±3.32; F=3.39; p<0.05) and depression (High-Risk Control = 9.06 ±4.11; High-Risk Experimental = 6.06 ±3.55; Low-Risk Control = 5.55 ±4.10; F=3.92; p<0.05) on the HADS. Surprisingly, no differences were also found among the groups on the THQ. The subjects rated drug prescriptions (mean = 8.33 ±1.55), the whole program (mean = 8.19 ±1.77) and visits with their physicians (mean = 8.02 ±1.87) as most helpful and physical therapy (mean = 6.51 ±1.98), diagnostic tests (mean = 6.75 ±2.42) and procedures (6.83 ±2.38) as least helpful. Overall, among those in the High-Risk Experimental group, the compliance interventions were rated as generally helpful. The subjects rated the individual counseling sessions (mean = 8.61 ±1.24) and compliance checklists (mean = 8.13 ±2.03) as slightly more helpful compared with the electronic diaries (mean = 8.00 ±1.94) and the group sessions (mean = 7.09 ±2.05).
The additional questions administered at the end of the trial did not reveal any significant group differences. In general, the subjects did not feel that participation in the study improved their pain (28.1 % agreed), however they believed that all patients on opioids should be carefully monitored (76.4% agree) and that the eDiaries were particularly helpful in maintaining compliance (59.6%).
Scores on the PDUQ averaged 10.27 ±3.98 (range 4–20), and physicians’ ratings of drug misuse behavior on the ABC averaged 1.00 ±2.15 (range 0–10). Nineteen percent of the patients (N = 10) had positive scores of three or more on the ABC while 38.5% (N = 20) scored higher than 11 on the PDUQ. Fifty three of the subjects (94.6%) had results from urine toxicology screens over the 6-month trial, and 22.6% of these (N = 12) had abnormal urines. Five (9.4%) of the urine toxicology screens were positive for THC; no other illicit substances were detected at the end of the trial. Six of the screens (11.3%) were missing a prescribed opioid medication, and two (3.8%) had evidence of an additional opioid that had not been prescribed. Two of the subjects had more than one abnormal finding. No relation was found between the number of sessions attended by the members of the High-Risk Experimental group and PDUQ or ABD scores or urine screen results.
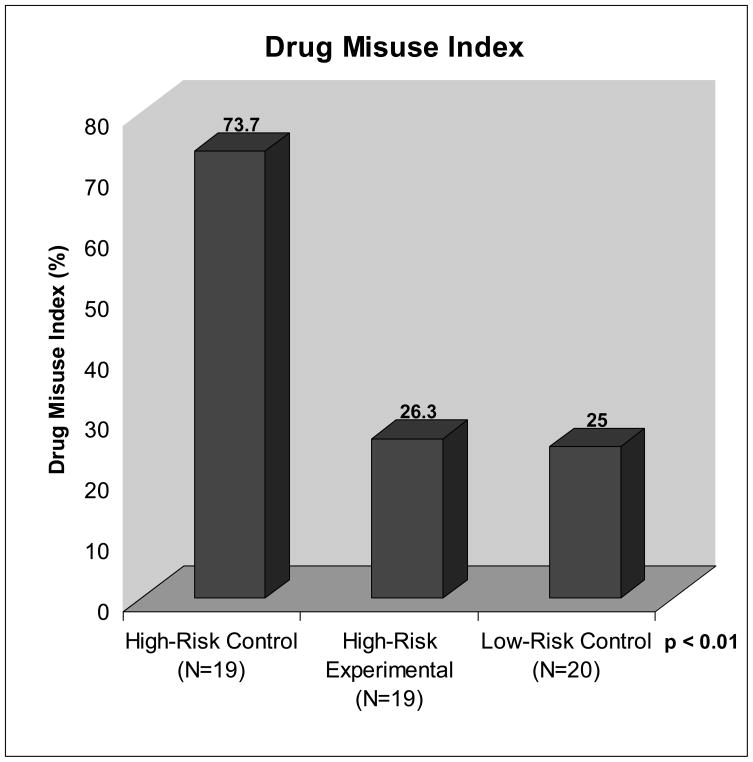
Twenty four (41.1%) of the 58 subjects were positive on the DMI based on results of the PDUQ (>11), ABC (>2) and positive urine screens. Significant differences were found between groups with 73.7 % of the High-Risk Control patients demonstrating positive scores on the DMI compared with 26.3% from the High-Risk Experimental group and 25.0% from the Low-Risk Controls (Table 4; Fig. 2). Secondary analyses of the baseline and demographic factors revealed few significant relationships with the DMI. Being positively identified for drug misuse at the end of the study was unrelated to age, gender, race, marital status, employment status, pain duration, pain intensity, anxiety or depressions (HADS) or disability level (PDI). Baseline scores on the SOAPP-R (15.24 ±7.94 vs. 22.75 ±10.51; t=3.10, p<0.01) and COMM (7.50 ±4.80 vs. 13.65 ±8.41; t=3.51, p>0.001) were predictive of having a positive DMI classification. A significantly positive relationship between having a prior psychiatric history (e.g., major depression, anxiety, or personality disorder) and being positive on the DMI (X2=4.85; p<0.05) was also found. None of the subjects who participated in this study were dismissed from the clinic by their treating physician because of misuse behavior.
Table 4.
End of study scores on the PDUQ, ABC, urine screens, and Drug Misuse Index.
| Variable VISIT 7 | High-Risk Control (N=19) | High-Risk Experimental (N=19) | Low-Risk Control (N=20) | p-values |
|---|---|---|---|---|
| PDUQ | 12.53 (3.38)a | 9.67 (4.11)b | 8.80 (3.61)b | F=4.95* |
| PDUQ >11 (%) | 70.6 | 26.7 | 20.0 | X2=11.18** |
| ABC | 1.50 (2.64) | 1.19 (2.14) | .40 (1.57) | NS |
| ABC > 2 (%) | 33.3 | 18.8 | 5.0 | NS |
|
Urine Screens: Normal (%) |
88.2 | 64.7 | 75.0 | NS |
| THC | 0 | 23.5 | 12.5 | NS |
| Another opioid | 0 | 5.9 | 0 | NS |
| Missing drug | 11.5 | 5.9 | 12.5 | NS |
| Drug Misuse Index‡ (%) | 73.7 | 26.3 | 25.0 | X2=12.16** |
p<0.05;
p<0.01;
NS=nonsignificant
Bonferroni p<0.05
PDUQ > 11; ABC > 2 and Abnormal urine
Fig. 2.
Positive Drug Misuse Index (DMI) percentage among subjects in the three study arms.
4. Discussion
The results of this study demonstrate support for the benefits of a brief behavioral intervention in the management of opioid compliance among chronic back pain patients at high-risk for prescription opioid misuse. No differences were found between the high-risk and low-risk groups on a number of demographic and descriptive variables at baseline, yet significant differences were found between groups by the end of the 6-month trial. Those participants who were labeled as high risk for opioid misuse in the experimental condition were found to have fewer signs of opioid misuse (positive on the DMI) than high-risk subjects in the control condition. In fact, those in the High-Risk Experimental condition demonstrated similar findings on the DMI as the Low-Risk controls. A 6-month follow-up also showed that none of the subjects was dismissed from the clinic due to aberrant drug behavior, which was possibly a partial effect of the attention from being in a study and completing monthly electronic diaries. Overall, however, this study demonstrated a positive effect of improving opioid compliance, particularly among those patients at high risk for misuse of opioids.
No significant demographic differences were found between groups at baseline, and this eliminated the need to control for other factors accounting for group differences through covariate analyses. The High-Risk patients demonstrated significantly higher ratings on the self-reported COMM, the physician-rated ABC, and in the percentage of abnormal urine screens, as expected. A review of the medical records at baseline also showed that those in the High-Risk groups had been asked to give a urine toxicology screen more often then the Low-Risk group. Thus, support was found for the accurate identification of substance misuse risk in predicting future behavior of patients taking oral opioids for pain.
As expected, those patients identified as Low-Risk demonstrated lower scores on the PDUQ (self-reported misuse) and a lower percentage on the combined Drug Misuse Index compared with the High-Risk Controls (p<0.05). On key indicators, the High-Risk Experimental patients also showed improved compliance with opioids. They demonstrated lower scores on the PDUQ, which is a self-report of abuse-like behaviors. At the end of the study, the High-Risk Experimental patients reported less time thinking about their medication and less of an urge to take more medication. These were targeted behaviors from the individual and groups counseling sessions. In this regard, the High-Risk Experimental subjects were similar to the Low-Risk subjects.
Interestingly, the High-Risk Experimental subjects demonstrated more abnormal urine screens than the High-Risk or Low-Risk Controls. Some of this could be explained by the fact that those in the High-Risk Experimental group were specifically asked to give a urine screen every month. Although some physicians asked those patients assigned to the High-Risk Control group for urine screens during the trial, this was not requested consistently for all of the subjects in that group. Thus, if regularly taken, monthly urine screens may have detected more abnormal results in the High-Risk Controls. The individual and group sessions also focused on the results of the urine screens, so that the frequent screens were useful in addressing aberrant drug-related behavior among the High-Risk Experimental patients in order to correct further drug misuse.
Even though few felt that participation in the study improved their pain (28.1%), the majority (71.6%) were satisfied with their treatment from the end-of-study interviews. Half of the subjects felt that craving their medication played a role in the way they took their medication. Over half of all of the study participants (51.8%) felt that their participation in the study helped them to comply with taking their medication. Also, most (59.6%) felt that completing the electronic diaries was useful, and most of the subjects (76.4%) believed that all patients taking opioids for pain should be carefully monitored with urine screens, checklists, and diaries. Also, the participants in the High-Risk Experimental group suggested that changes in craving of the opioids played a role in compliance, in agreement with past research [43]. Thus, targeting this topic in future motivational counseling sessions may be useful in improving compliance. Given a great individual variability in response to behavioral interventions, quite possibly the “package” of compliance measures offered to the High-Risk Experimental subjects accounted for the positive results.
This study was also concerned with the identification of other factors that might be useful in the prediction of opioid misuse. Secondary analyses of the baseline and demographic data as predictors of drug misuse revealed few significant findings; this lack of significant findings may be due, in part, to the small numbers in the study. Importantly, a positive relationship was found between the baseline measures of the SOAPP-R and COMM and independent classifications of drug misuse. Also, in keeping with past research, psychiatric history was an important predictor of drug misuse [44]. Thus, support was found for the relationship between chronic pain, psychiatric diagnosis, and substance misuse.
We were encouraged with the low attrition rate, since we anticipated a 15% dropout. All but four subjects who entered the study were successfully followed for the required 6-month period. No differences were found between those who dropped out and those who completed the study. This is encouraging for future studies designed to examine the efficacy of the study intervention. We were concerned that some patients would feel that opioid compliance treatment would not necessarily apply to them and that they would resent having to complete opioid compliance worksheets and participate with others who have a history of abusing drugs or alcohol. Although the group sessions were not rated as most helpful compared to the other interventions, we were surprised that many high-risk patients without a history of addiction or abuse did not seem to mind participating in a group with others who admitted to a history of misusing opioid medication. This encouraging finding suggests that the high-risk patients would be willing to participate in compliance training even though they may not have a history of misuse.
The High-Risk patients had significantly higher ratings of pain intensity, anxiety, depression and activity interference than the Low-Risk subjects. It is interesting to speculate that there are certain predisposing factors, such as mood disorders, among the High-Risk subjects that contribute to their risk of misuse of opioids. Future studies are needed using quantitative sensory testing and daily monitoring of mood and opioid use to help explain these findings. The identification of genetic markers to help in understanding these differences may also be useful [15,16].
There are a number of limitations of this study that deserve mention. First, this study had a limited number of subjects in each treatment arm. The small group numbers decreased the power of the analyses. Future studies with greater numbers of subjects in each cell are needed. Second, subjects were followed for only six months and longer follow-up is needed. Although we excluded those with an active substance use disorder using the M.I.N.I., some had an addiction history and might be at greater risk for misuse of opioids in the future. A longer follow-up time might reveal differences among those with a history of addiction and those with misuse behavior without an addiction disorder history. Third, the inclusion/exclusion criteria may have omitted some subjects from this study who might be at risk for misuse of opioids. This is a limitation of any controlled trial where selection criteria and signing an informed consent is needed. Thus, the clinical usefulness of these results may not necessary apply to all patients. Also, the subjects were recruited from a university-based pain management center, and a similar study with subjects from an independent primary care practice may have revealed different results. Fourth, not all subjects in the Experimental condition fully participated in all aspects of the intervention. Although no differences were found between those who participated fully in the study and those who did not (e.g., attended each of the monthly individual and group sessions), the importance of uniformity of treatment and full participation could not be assessed. Future studies would benefit from a detailed treatment manual of the interventions and a more stringent requirement to participate in all aspects of the compliance training. Finally, based on the preliminary nature of this study, it could not be determined what treatments were most effective in improving compliance with opioids among chronic pain patients. Additional studies are needed with more subjects followed for longer periods of time to help determine individual difference factors (e.g., gender, age, etc.) and what interventions are most useful.
Despite these limitations, the results of this study are encouraging and suggest that compliance training and very careful monitoring of those patients determined to be at high risk for opioid misuse can be incorporated as part of an anesthesia-based multidisciplinary pain program to help improve compliance with opioids and to reduce the number of those individuals who are discharged from treatment because of aberrant drug-related behavior. Although further research is needed, this trial demonstrates that substantial improvement in compliance with prescription opioids for many high-risk pain patients is possible within a pain management center.
Acknowledgments
This study was supported in part by an investigator-initiated grant from Endo Pharmaceuticals, Chadds Ford, PA, and grants (R21 DA024298, Jamison, PI; K23 DA020682, Wasan, PI) from the National Institute on Drug Abuse (NIDA) of the National Institutes of Health, Bethesda, MD, and the Arthritis Foundation (Investigator Award; Wasan, PI). The authors would like to especially thank Robert Edwards, Kathleen Howard, David Janfaza, Sanjeet Narang, Heather Thomson, Assia Valovska and staff and patients of Brigham and Women’s Hospital Pain Management Center.
Footnotes
There are no conflicts of interest to declare associated with this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Academy of Pain Medicine, The American Pain Society, The American Society of Addiction Medicine. Definitions related to the use of opioids for the treatment of pain: a consensus document from the American Academy of Pain Medicine, the American Pain Society, and the American Society of Addiction Medicine. ASAM; Chevy Chase, MD: 2001. [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 3.Ballantyne JC, Mao J. Opioid Therapy for Chronic Pain. NEJM. 2003;349:1943–53. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- 4.Bjelland I, Dahl AA, Huag TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 5.Butler SF, Budman SH, Fernandez K, Jamison RN. Validation of a Screener and Opioid Assessment Measure for Patients with Chronic Pain. Pain. 2004;112:65–75. doi: 10.1016/j.pain.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 6.Butler SF, Budman SH, Fernandez KC, Fanciullo GJ, Jamison RN. Cross-validation of a screener to predict opioid misuse in chronic pain patients (SOAPP-R) J Addict Med. 2009;3:66–73. doi: 10.1097/ADM.0b013e31818e41da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler SF, Budman SH, Fernandez KC, Houle B, Benoit CM, Katz N, Jamison RN. Development and validation of the current opioid misuse measure. Pain. 2007;130:144–56. doi: 10.1016/j.pain.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butler SF, Fernandez K, Benoit C, Budman SH, Jamison RN. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPP-R) J Pain. 2008;9:360–72. doi: 10.1016/j.jpain.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman SL, Jamison RN, Sanders SH. Treatment helpfulness questionnaire: a measure of patient satisfaction with treatment modalities provided in chronic pain management programs. Pain. 1996;68:349–61. doi: 10.1016/s0304-3959(96)03217-4. [DOI] [PubMed] [Google Scholar]
- 10.Cicero T, Lynskey M, Todorov A, Inciardi JA, Surratt HL. Comorbid pain and psychopathology in males and females admitted to treatment for opioid analgesic abuse. Pain. 2008;139:127–35. doi: 10.1016/j.pain.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 12.Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluations of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355–63. doi: 10.1016/s0885-3924(98)00110-9. [DOI] [PubMed] [Google Scholar]
- 13.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 14.Dersh J, Gatchel R, Polatin P, Mayer T. Prevalence of Psychiatric Disorders in Patients with Chronic Work-Related Musculoskeletal Pain and Disability. J Occ Envir Med. 2002;44:459–68. doi: 10.1097/00043764-200205000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Edwards R, Doleys DM, Lowery D, Fillingim RB. Pain tolerance as a predictor of outcome following multidisciplinary treatment for chronic pain: differential effects as a function of sex. Pain. 2003;106:419–26. doi: 10.1016/j.pain.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Fillingim R, Doleys D, Edwards R, Lowery D. Clinical Characteristics of Chronic back Pain as a Function of Gender and Oral Opioid Use. Spine. 2003;28:143–50. doi: 10.1097/00007632-200301150-00010. [DOI] [PubMed] [Google Scholar]
- 17.Fishbain DA. Approaches to Treatment Decisions for Psychiatric Comorbidity in the Management of the Chronic Pain Patient. Med Clin North Amer. 1999;83:737–60. doi: 10.1016/s0025-7125(05)70132-2. [DOI] [PubMed] [Google Scholar]
- 18.Fishbain DA, Rosomoff HL, Rosomoff RS. Drug Abuse, Dependence, and Addiction in Chronic Pain Patients. Clin J Pain. 1992;8:77–85. doi: 10.1097/00002508-199206000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Geisser ME, Cano A, Foran H. Psychometric properties of the mood and anxiety symptom questionnaire in patients with chronic pain. Clin J Pain. 2006;22:1–9. doi: 10.1097/01.ajp.0000146180.55778.4d. [DOI] [PubMed] [Google Scholar]
- 20.Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: a World Health Organization study in the primary care. JAMA. 1998;280:147–51. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- 21.Jamison RN. Mastering Chronic Pain: A Professional’s Guide To Behavioral Treatment. Sarasota, FL: Professional Resource Press; 1996. [Google Scholar]
- 22.Jamison RN, Kauffman J, Katz NP. Characteristics of methadone maintenance patients with chronic pain. J Pain Symptom Manage. 2000;19:53–62. doi: 10.1016/s0885-3924(99)00144-x. [DOI] [PubMed] [Google Scholar]
- 23.Jamison RN, Wasan AD, Michna E, Ross RL, Chen LQ, Holcomb C, Edwards RR. Substance abuse treatment for high risk chronic pain patients on opioid therapy. American Pain Society; San Diego, CA: 2009. [Google Scholar]
- 24.Joranson DE, Ryan KM, Gilson AM, Dahl JL. Trends in medical use and abuse of opioid analgesics. JAMA. 2000;283:1710–4. doi: 10.1001/jama.283.13.1710. [DOI] [PubMed] [Google Scholar]
- 25.Katon W, Egan K, Miller D. Chronic pain: lifetime psychiatric diagnoses and family history. Am J Psychiatry. 1985;142:1156–60. doi: 10.1176/ajp.142.10.1156. [DOI] [PubMed] [Google Scholar]
- 26.Marceau L, Carolan S, Schuth B, Jamison RN. Pain electronic calendars for pain monitoring: perceived helpfulness by patients and physicians. Pain Med. 2007;8:S101–9. doi: 10.1111/j.1526-4637.2007.00374.x. [DOI] [PubMed] [Google Scholar]
- 27.Institute of Medicine. For the Public Good: Highlights of the Institute of Medicine. National Academy Press; 1995. [PubMed] [Google Scholar]
- 28.Mercer D, Carpenter G, Daley D, Petterson C, Volpicelli J. Group drug counseling manual. University of Pennsylvania; 1992. [Google Scholar]
- 29.Michna E, Ross EL, Hynes WL, Nedeljkovic SS, Soumekh MD, Janfaza D, Palombi D, Jamison RN. Predicting aberrant drug behavior in patients treated for chronic pain: importance of abuse history. J Pain Symptom Manage. 2004;28:250–8. doi: 10.1016/j.jpainsymman.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Michna E, Jamison RN, Pham LD, Ross EL, Janfaza D, Nedeljkovic SS, Narang S, Palombi D, Wasan AD. Urine toxicology screening among chronic pain patients on opioid therapy: frequency and predictability of abnormal findings. Clin J Pain. 2007;23:173–9. doi: 10.1097/AJP.0b013e31802b4f95. [DOI] [PubMed] [Google Scholar]
- 31.Nedeljkovic SS, Wasan AD, Jamison RN. Assessment of Efficacy of Long-Term Opioid Therapy in Pain Patients with Substance Abuse Potential. Clin J Pain. 2002;18:S39–51. doi: 10.1097/00002508-200207001-00005. [DOI] [PubMed] [Google Scholar]
- 32.NIDA. Overview of findings from the 2002 National Survey on Drug Use and Health. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2003. Report nr DHHS publication No. SMA 03–3774. [PubMed] [Google Scholar]
- 33.Passik S, Kirsh K. The need to identify predictors of aberrant drug-related behavior and addiction in patients being treated with opioids for pain. Pain Med. 2003;4:186–9. doi: 10.1046/j.1526-4637.2003.03018.x. [DOI] [PubMed] [Google Scholar]
- 34.Pengel HM, Maher CG, Refshauge KM. Systematic review of conservative interventions for subacute low back pain. Clin Rehabil. 2002;16:811–20. doi: 10.1191/0269215502cr562oa. [DOI] [PubMed] [Google Scholar]
- 35.Portenoy RK, Payne R. Acute and chronic pain. In: Lowinson J, Ruiz P, Millman R, Langrod JG, editors. Substance Abuse: A Comprehensive Textbook. 3. Baltimore: Williams and Wilkin; 1997. pp. 563–90. [Google Scholar]
- 36.Rosenblum A, Joseph H, Fong C, Kipnis S, Cleeland C, Portenoy R. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA. 2003;289:2370–8. doi: 10.1001/jama.289.18.2370. [DOI] [PubMed] [Google Scholar]
- 37.Savage S, Covington EC, Heit HA, Hunt J, Joranson D, Schnoll SH. [Accessed July 21, 2009.];Definitions related to the use of opioids for the treatment of pain: A consensus document from the American Academy of Pain Medicine, the American Pain Society, and the American Society of Addiction Medicine. 2001 Available at: http://www.ampainsoc.org/advocacy/opioids2.htm.
- 38.Savage SR. Assessment for addiction in pain treatment settings. Clin J Pain. 2002;18:S28–38. doi: 10.1097/00002508-200207001-00004. [DOI] [PubMed] [Google Scholar]
- 39.Sheehan D. The Mini-International Neuropsychiatric Interview (M.I.N.I.); the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiat. 1998;59:S22–33. [PubMed] [Google Scholar]
- 40.Tait RC, Pollard CA, Margolis RB, Duckro PN, Krause SJ. The Pain Disability Index: psychometric and validity data. Arch Phys Med Rehab. 1987;68:138–441. [PubMed] [Google Scholar]
- 41.Turk DC, Melzack R. Handbook of Pain Assessment. 2. New York: The Guilford Press; 2001. [Google Scholar]
- 42.Verhaak PFM, Kerssens JJ, Dekker J, Sorbi MJ, Bensing JM. Prevalence of chronic benign pain disorder among adults: a review of the literature. Pain. 1998;77:231–9. doi: 10.1016/S0304-3959(98)00117-1. [DOI] [PubMed] [Google Scholar]
- 43.Wasan A, Butler SF, Budman SH, Fernandez K, Weiss RD, Greenfield S, Jamison RN. Does report of craving opioid medication predict aberrant drug behavior among chronic pain patients? Clin J Pain. 2009;25:193–8. doi: 10.1097/AJP.0b013e318193a6c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wasan AD, Butler SF, Budman SH, Benoit C, Fernandez K, Jamison RN. Psychiatric history and psychological adjustment as risk factors for aberrant drug-related behavior among patients with chronic pain. Clin J Pain. 2007;23:307–15. doi: 10.1097/AJP.0b013e3180330dc5. [DOI] [PubMed] [Google Scholar]
- 45.Wasan AD, Davar G, Jamison RN. The association between negative affect and opioid analgesia in patients with discogenic low back pain. Pain. 2005;117:450–61. doi: 10.1016/j.pain.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 46.Webster LR, Dove B. Avoiding Opioid Abuse While Managing Pain: A Guide for Practitioners. North Branch, MN: Sunrise River Press; 2007. [Google Scholar]
- 47.Woody GE, Crits-Christoph P. Individual Therapy for Substance Abuse Disorders. In: Gabbard GO, editor. Gabbard’s Treatments of Psychiatric Disorders. 4. Arlington, VA: American Psychiatric Publishing, Inc; 2007. pp. 285–303. [Google Scholar]
- 48.Wu SM, Compton P, Bolus R, Schieffer B, Pham Q, Baria A, Van Vort W, Davis F, Shekelle P, Naliboff B. The Addiction Behaviors Checklist: validation of a new clinician-based measure of inappropriate opioid use in chronic pain. J Pain SymptomManage. 2006;32:342–52. doi: 10.1016/j.jpainsymman.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 49.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica. 1983;37:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]