Abstract
A form of aversively motivated learning called fear conditioning occurs when a neutral conditioned stimulus (CS) is paired with an aversive unconditioned stimulus (US). US-evoked depolarization of amygdala neurons may instruct Hebbian plasticity that stores memories of the CS-US association, but the origin of US inputs to the amygdala is unknown. Theory and evidence suggest that instructive US inputs to the amygdala will be inhibited when the US is expected, but this has not been demonstrated during fear conditioning. Here we investigated neural pathways that relay US information to the amygdala by recording neurons in the amygdala and periaqueductal gray (PAG) during fear conditioning. US-evoked responses in both amygdala and PAG were inhibited by expectation. Pharmacological inactivation of the PAG attenuated US-evoked responses in the amygdala and impaired acquisition of fear conditioning, indicating that PAG may be an important part of the pathway that relays instructive signals to the amygdala.
INTRODUCTION
The amygdala is an important site of neural plasticity where memories of the CS-US association are stored during fear conditioning 1–5. Evidence suggests that storage of fear memories requires Hebbian long-term potentiation (LTP) at CS input synapses onto neurons in the lateral nucleus of the amygdala (LA) 1,2,6–11. This Hebbian plasticity is thought to be triggered by the US, which causes postsynaptic depolarization of LA neurons in conjunction with presynaptic activation of CS inputs 1,2,5,6. If so, then afferent pathways that transmit US information to the amygdala can be regarded as “teaching inputs” that instruct associative plasticity at CS input synapses.
A number of studies have attempted to identify the teaching input pathways that convey US information to the amygdala during fear learning 12–16, but it remains unclear which neural circuits mediate this function. Behavioral evidence from “blocking” experiments suggests that fear conditioning may be instructed not by a simple sensory representation of the US, but instead by a US signal which is inhibited by expectation 17–21. Modulation of neural signals by expectation has been seen in many learning systems22–25, and recent studies provide neurophysiological evidence that responses of amygdala neurons to aversive (or appetitive) stimuli are also modulated by expectation 26,27. However, it is not clear whether this occurs during Pavlovian fear conditioning at sites of associative plasticity (such as the LA), or in brain regions which participate in relaying US information to the LA.
In the present study, a series of experiments investigated how US information was processed by neurons in the amygdala and PAG during fear conditioning and examined whether the PAG is part of the US pathway. These results show that US processing is modulated by expectation in both the LA and PAG, and suggest that PAG may play an essential role in relaying expectancy-modulated US information to instruct associative plasticity in the LA and regulate fear memory formation.
RESULTS
US processing by amygdala neurons is modulated by expectation
To investigate whether US processing in LA is modulated by expectation during fear conditioning, rats (n=14) were implanted with recording electrodes targeted at the LA nucleus, and with periorbital wires for delivering the shock US to the skin above one eyelid. When well-isolated units were encountered in LA, a pre-conditioning session consisting of 6 test trials (CS alone) was given to assess baseline responses to context (CX) and CS, followed immediately by a conditioning session consisting of 16 CS-US pairings. After a one hour delay, conditioned responding to the CS was assessed during a post-conditioning test session consisting of 6 CS alone presentations. Most rats underwent additional recording sessions on subsequent days to further assess US-evoked responses of amygdala neurons (see below).
Rats acquired conditioned freezing responses to the CS (Fig. 1a). A 2×2 repeated measures ANOVA of freezing scores revealed a significant interaction (F1,12=9.3, p=.01) between stimulus condition (CX vs. CS) and session (pre vs. post conditioning; see Fig. 1a for posthoc comparisons). Freezing levels observed here were similar to those observed in prior studies using the same fear conditioning paradigm28, which are lower than in fear conditioning studies using a standard footshock US (see Methods).
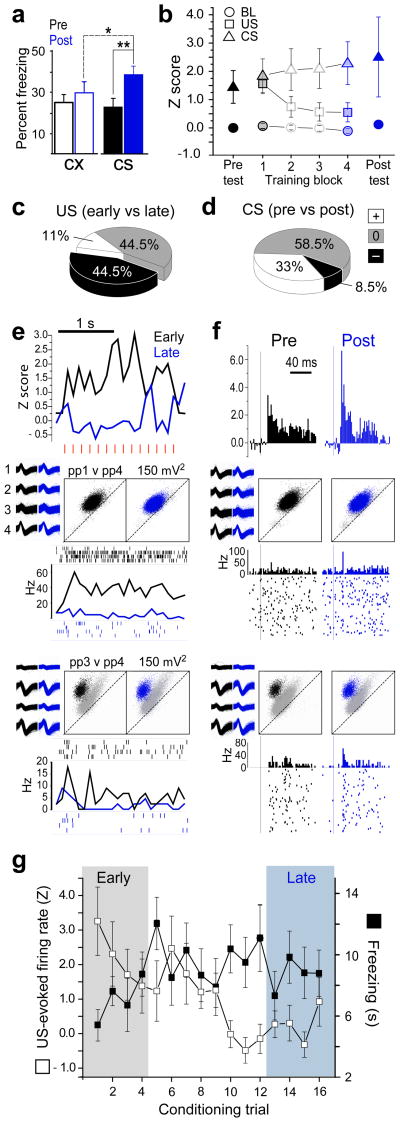
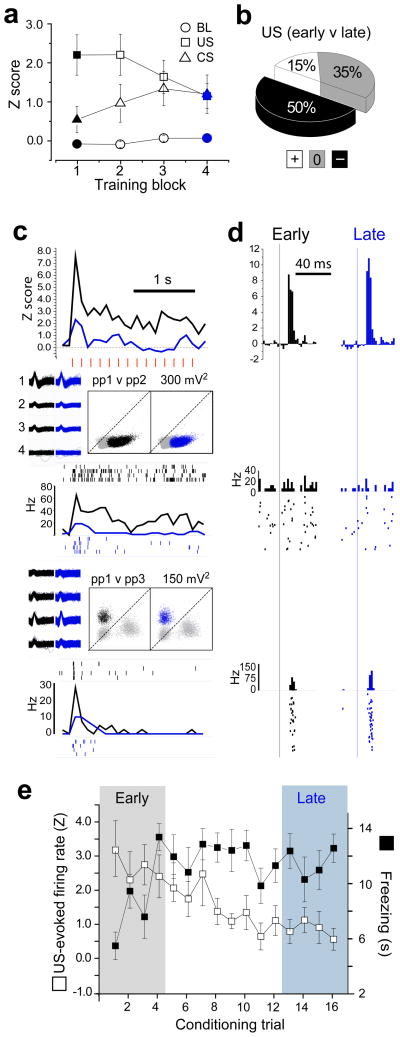
Figure 1.
Activity of LA neurons during acquisition of fear conditioning. (a) freezing behaviour during the 20 s CX (open white bars) and CS (shaded bars) periods is graphed on the Y-axis before (pre) and after (post) fear conditioning (*p =.004, **p=.0007 for Newman-Keuls posthoc tests). (b) Normalized stimulus-evoked responses (y-axis) averaged over the population of shock responsive LA neurons (n=27) for each of the four conditioning trial blocks (4 trials/block) and for the first four trials of the pre- and post-conditioning test sessions. (c) Pie chart showing the percentage of shock-responsive LA cells that significantly reduced (−), increased (+) or did not change (0) their US-evoked responses between the first (early) and last (late) conditioning trial block. (d) Pie chart showing the percentage of shock responsive LA cells that significantly changed their CS-evoked responses between the pre- and post-conditioning test sessions. (e) top graph, PSTH (bin size=100 ms) shows normalized activity during shock trains (individual shock pulses indicated by red hash marks) for early versus late conditioning trials, averaged over the subpopulation of LA neurons that significantly reduced their shock evoked response (n=12); bottom graphs, PSTHs and spike rasters show US-evoked responses for two example neurons from different rats, along with waveforms and cluster plots for spikes fired during the session (‘ppX v ppY’ above scatter plots denotes channel numbers for peak-to-peak spike voltages plotted on the X and Y axes, respectively). (f) top graph, PSTH (bin size = 2 ms) shows CS-evoked responses (onset of white noise pip indicated by vertical line) during the pre- versus post-conditioning test sessions for the subpopulation of LA cells shown in ‘E’; bottom graphs, CS-evoked responses for the same two example neurons shown in ‘E’. (g) Responses to the US (left y-axis) on each of the 16 conditioning trials (averaged over the subpopulation of LA cells that significantly reduced their US responsiveness during conditioning) is graphed alongside average freezing scores (right y-axis) during the CS period on each trial.
Changes in US-evoked responses during conditioning
If US processing is modulated by expectation, then US-evoked responses should become attenuated during training as animals are learning that the CS predicts the US. To test this, a total of 35 well-isolated LA neurons were successfully recorded from 11 of the 14 rats during the initial training session (recording sites shown in Supplementary Fig. 1). Shock-evoked responses were observed in 77% (27/35) of the neurons that were recorded during training. Of these 27 shock-responsive neurons, 24 were held throughout the pre- and post-conditioning test sessions as well as the training session. To analyze how the activity of these shock-responsive neurons changed during conditioning, the 16 conditioning trials were subdivided into four consecutive blocks of four trials each (Fig. 1b). A 4×2 ANOVA revealed a significant interaction (F3,78=3.64, p=.016) between trial block and stimulus. Post-hoc comparisons showed that US-evoked responses during trial block 1 were significantly greater than during blocks 2–4 (p<.0006 for every comparison). The CX baseline did not differ significantly between any pair of trial blocks (p>.67 for every comparison), indicating that baseline firing rates remained stable throughout the conditioning session. These findings indicate that US-evoked responses of LA neurons decreased during early conditioning trials (block 1), and then remained attenuated throughout the remainder of the session (blocks 2–4).
US-evoked responses of individual neurons were analyzed to determine which cells changed their responses to the shock US between the first versus last conditioning trial blocks (see Methods). Fig. 1c shows that 44.5% (12/27) of shock-responsive cells reduced their shock responsiveness over the course of training (see Fig. 1e for population average and individual cell examples), while 11% (3/27) increased their shock responsiveness and 44.5% (12/27) showed no significant change. Hence, individual LA neurons were four times more likely to decrease (n=12) than increase (n=3) their response to the shock US during the training session, and this was a statistically significant bias toward diminution of US-evoked responses (two-tailed binomial test, p=.035). Analysis of baseline firing rates suggested that diminution of US-evoked responding during training did not occur preferentially in principle cells versus interneurons (see Supplementary Figure 2).
Changes in CS-evoked responses during conditioning
Prior studies have reported that auditory fear conditioning enhances CS-evoked responses of LA neurons at short time latencies after CS onset, and these findings have been taken as evidence for conditioning-induced potentiation of input pathways that relay the auditory CS to LA from the thalamus or cortex 9,10. To examine this in the present study, short latency CS-evoked responses were compared from the pre- versus post-conditioning test sessions. Auditory responses were larger after than before conditioning (Fig. 1f), but this enhancement did not reach statistical significance in the population of shock-responsive cells (paired t23= 1.46, p=0.16). However, analysis of individual neurons showed that short latency CS-evoked responses were significantly enhanced in 33% (8/24) of the cells, whereas 58.5% (14/24) of the cells showed no significant change in their firing to the CS after conditioning, and 8.5% (2/24) of the cells showed a decrease in CS-evoked responses (Fig. 1d). These results are consistent with previous electrophysiological, cellular and molecular evidence indicating that fear conditioning enhances short-latency CS responses within a subset of amygdala neurons, but not in all neurons7–10,27,29,30.
A 2×2 ANOVA compared US-evoked responses for cells which exhibited conditioned enhancement of CS-evoked responding (n=8) versus those that did not (n=16). There was a significant main effect of trial block (block 1 vs. 4, F1,22=6.35, p=.019), but no effect of cell type (CS enhanced vs. non-enhanced, F1,22=0.12, p=.73) and no interaction between trial block and cell type (F1,22=0.56, p=.46). Hence, diminution of US-evoked responses in individual cells did not depend upon whether the cell showed conditioned enhancement of its CS-evoked responses (see Discussion).
Modulation of US processing by the predictive CS
Expectation of an aversive stimulus can attenuate the responsiveness of amygdala neurons to that stimulus 27, so US-evoked responses of LA neurons may have decreased during conditioning as rats learned to expect the shock to follow the predictive CS. Supporting this interpretation, freezing behaviour during the CS increased during early conditioning trials and remained elevated for the remainder of the trials (Fig. 1g). For cells that diminished their responses to shock (n=12), the magnitude of US-evoked responses was inversely correlated with CS-evoked freezing responses across conditioning trials (r16=−.53; p=.03), suggesting that diminution of shock responses was related to the rats’ acquired expectation of the shock.
To further investigate whether diminution of shock responses was attributable to a learned expectation of the shock, additional recording sessions were conducted after the initial conditioning session in 9 of the 14 rats. In these later sessions, previously conditioned rats were given 16 presentations of the shock US, of which 8 were preceded by a previously trained CS (signaled US trials) and 8 were not (unsignaled US trials), given in random order. In some rats, electrodes advanced below the LA nucleus and into the B nucleus during later recording sessions (Supplementary Fig. 1). Neurons from both nuclei were combined in the analyses reported below.
A total of 70 well-isolated LA and B neurons were recorded from 9 rats during 24 signaled-unsignaled shock sessions that followed conditioning. Significant shock-evoked responses were observed in 45/70 (64%) of these neurons, and in this population, responses to the unsignaled US were significantly larger than to the signaled US (t44=2.9, p=.006). Analysis of individual neuron responses revealed that LA/B neurons were five times more likely to respond preferentially to the unsignaled US (n=15) than to the signaled US (n=3; see Fig. 2a and b for population and example PSTHs and Fig. 2c), which was a significant bias for preferential responding to the unsignaled US (two-tailed binomial test, p=0.0075) Differential responses of LA/B neurons to predicted versus unpredicted shocks were not attributable to differences in motor responses to the shocks (Supplementary Fig. 3).
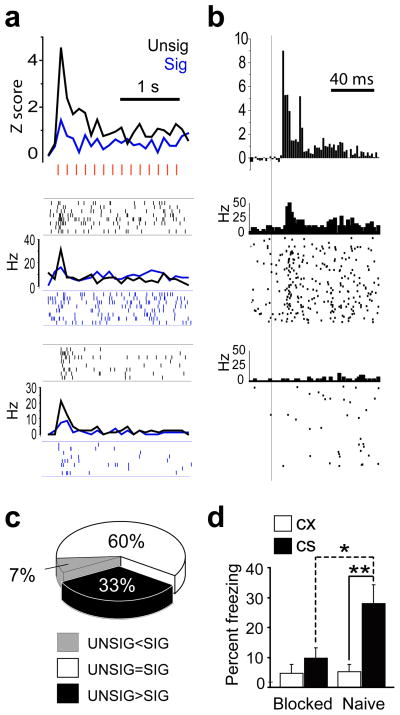
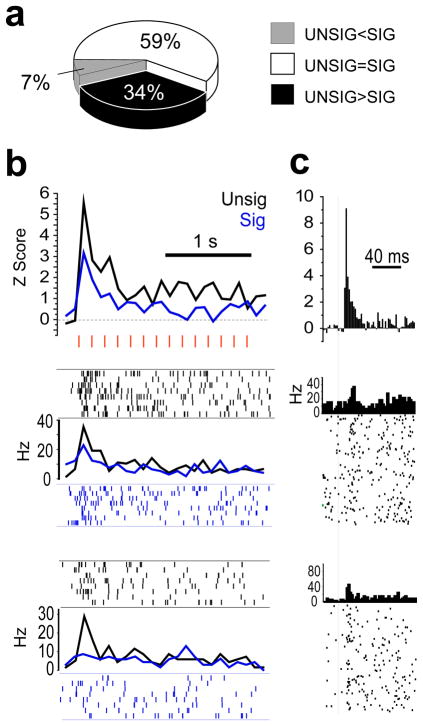
Figure 2.
LA/B neurons responded more to unsignalled (UNSIG) than signalled (SIG) shocks. (a) top graph, PSTHs (bin size=100 ms) shows normalized activity during SIG and UNSIG shock trains (individual shock pulses indicated by red hash marks) for the subpopulation of LA/B neurons (n=15) which responded significantly more to UNSIG than SIG shocks. bottom graphs, PSTH and spike rasters show responses to SIG and UNSIG shocks for two example neurons from different rats. (b) top graph, PSTH (bin size = 2 ms) shows normalized CS-evoked responses (onset of white noise pip indicated by vertical line) during SIG trials for the subpopulation of LA/B neurons shown in ‘b’. bottom graphs, PSTHs and spike rasters show CS-evoked responses for the same two example neurons shown in ‘b’. (c) Pie chart showing the percentage of shock responsive LA/B cells that responded significantly more to UNSIG than SIG shocks (black), significantly more to SIG than UNSIG shocks (white), or the same to both types of shock (gray). (d) Freezing during the final test session of the blocking experiment (*p =.004, **p=.002 for Newman-Keuls posthoc tests).
If fear conditioning is instructed by US-evoked depolarization in the amygdala, then the reduction in US-evoked responses of LA/B neurons that we observed following a predictive CS should be accompanied by a reduction in the ability of the US to produce fear conditioning. To test this, we conducted a blocking experiment in which all rats first received pre-exposure to the auditory pip CS and a flashing light CS and then 8 rats (blocked group) received 16 pairings of the pip CS with the eyelid shock US, and another 8 rats (naive group) were not trained. Both groups then received 16 pairings of a compound CS—consisting of the auditory pips combined with a flashing light—with the eyelid shock US. 24 h later, the flashing light CS by itself evoked less freezing from blocked than naive rats, as revealed by a significant 2×2 interaction effect between stimulus (CX vs. visual CS) and treatment group (blocked vs. control; F1,14=6.1, p=.03, see Fig. 2d for posthoc comparisons). These results suggest that in the presence of the predictive CS, both US-evoked responses of amygdala neurons and the ability of the US to instruct fear conditioning were similarly reduced.
PAG inactivation impairs US processing in LA neurons
Blocking of fear conditioning by a predictive CS may be mediated by conditioned analgesia, whereby the CS activates outputs from amygdala to PAG, which in turn inhibits nociception (and thus blocks US processing) at the level of the spinal and trigeminal dorsal horn18–20,31. If so, then PAG inactivation should prevent antinociception from occurring in the dorsal horn, and thereby prevent the CS from inhibiting US-evoked responses in amygdala (without affecting responses to the US in the absence of the CS). To test this, PAG was inactivated with muscimol while responses to signaled and unsignaled shocks were recorded from LA neurons in previously fear conditioned rats. A total of 15 well isolated LA neurons were recorded from 3 rats before and after muscimol (0.4 ul/side, 0.25 mg/ml) was microinjected into the PAG (see Supplementary Fig. 4), of which 13/15 (87%) were responsive to the eyelid shock US. Of these shock responsive neurons, 7/13 (54%) responded preferentially to unsignaled USs (see Fig. 3a, c and d).
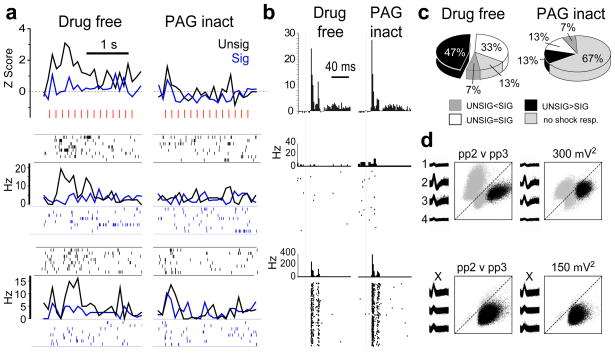
Figure 3.
Attenuation of shock-evoked responding in LA neurons by PAG inactivation. (a) top graphs, PSTHs (bin size=100ms) show normalized activity during SIG and UNSIG shock trains (individual shock pulses indicated by red hash marks) averaged over LA cells (n=7) that responded significantly more to UNSIG than SIG shocks; bottom graphs, PSTHs and spike rasters show responses for two example neurons from different rats. (b) top graphs, PSTHs (bin size = 2 ms) show normalized CS-evoked responses during SIG trials before versus after infusions of muscimol into PAG for the same neurons shown in ‘A’. bottom graphs, PSTHs and spike rasters show CS-evoked responses for the same example neurons as in ‘A’. (c) Pie chart summarizes how US-evoked responses of LA cells that responded to shock before PAG inactivation (n=13) changed after inactivation. (d) Spike waveforms and cluster plots for the example cells in ‘A’ before (left) and after (right) PAG inactivation.
A 2×2 ANOVA revealed a significant interaction between inactivation (pre vs. post) and shock type (signalled vs. unsignalled; F1,12=9.48, p=0.009). Posthoc comparison tests confirmed that prior to inactivation, amygdala neurons responded significantly more to unsignaled than signaled shocks (p=0.0008), but after PAG inactivation the cells no longer responded differently to predicted versus unpredicted shocks (p=0.86), mainly because amygdala neurons no longer responded to shocks of either type (see Fig. 3a and c). There was a significant reduction in the population response to both signaled (p=.006) and unsignaled (p=.0002) shocks after PAG inactivation. However, PAG inactivation did not affect CS-evoked responses (paired t12=1.23, p=0.24; see Fig. 3b) or baseline firing rates (paired t12=0.96, p=0.35) of amygdala neurons. These data provide evidence that in addition to its role as an output structure for mediating conditioned fear responses such as freezing and analgesia, the PAG may also participate in relaying US information to the LA.
PAG inactivation impairs acquisition of conditioned freezing
If PAG participates in relaying aversive US information to the amygdala to instruct associative plasticity, then the acquisition of fear conditioning should require the PAG, and some prior evidence suggests that this is true (Bellgowan, P.S.F., Helmstetter, F.J. & Bailey, D.J. Society for Neuroscience Abstract, 1996.). To investigate whether the PAG was necessary for acquisition of fear conditioning, muscimol (MUS, 0.4 ul/side, 0.25 mg/ml) or vehicle (VEH) was microinjected into the PAG prior to training. Conditioned freezing was assessed during a drug-free test session given six days later (see Supplementary Fig. 5 for injection site locations).
Prior to fear conditioning, rats did not exhibit freezing to the CX or CS (Fig. 4a). During the post-conditioning test session, VEH rats (n=11) froze to the CS while MUS rats (n=12) did not (see Fig. 4b), as revealed by a significant interaction (F1,21=11.180, p=0.003) between drug treatment (MUS vs. VEH) and stimulus condition (CX vs. CS). During conditioning, MUS rats exhibited reduced unconditioned reflex responses (head movement) to the shock (Supplementary Fig 6). Impairment of fear learning with PAG infusions was not attributable to muscimol spreading into brain regions lateral to the PAG, because another group of control (CTL) rats which received pre-training muscimol into sites lateral to the PAG acquired conditioned freezing responses (Supplementary Fig. 7). Impaired fear learning in MUS rats was also not caused by permanent damage to PAG, because impaired rats learned normally when retrained drug-free (Supplementary Fig. 8). We also found (Supplementary Fig. 9) that in well trained animals PAG inactivation reduced expression of conditioned freezing, replicating prior findings 31–34. Thus, in addition to its known role as an output structure for conditioned fear responses, the PAG also appears to be necessary for the acquisition of fear memory formation.
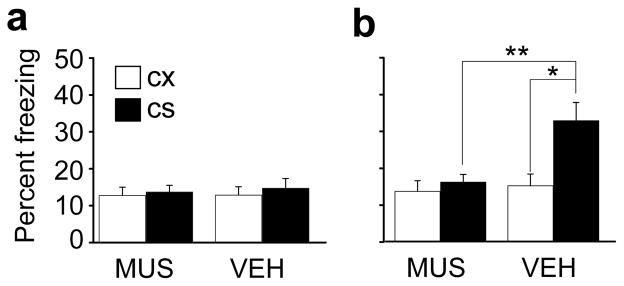
Figure 4.
Effects of PAG inactivation on fear conditioning. (a) Pre-training freezing levels in animals that subsequently received PAG microinjections of MUS or VEH prior to conditioning. (b) Freezing during a test session given 6 days after conditioning. * denotes p=0.0002 and ** denotes p=0.002.
US processing in PAG neurons is modulated by expectation
The above findings suggest that the PAG participates in relaying US signals to the amygdala. Since US-evoked responses in the amygdala were modulated by expectation, and PAG inactivation reduced US-evoked responses in LA neurons, we next examined whether US-evoked responses of PAG neurons were also modulated by expectation. Rats (n=13) were implanted with recording electrodes targeted to pass through the dorsal, lateral, and ventral columns of PAG (see Supplementary Fig. 10) and recording and behavioural procedures were as described above for the amygdala recording experiments.
Changes in US-evoked activity during conditioning
A total of 21 well-isolated PAG neurons were recorded from 8 rats during their initial conditioning session, and 95% (20/21) of these cells were significantly responsive to shocks (see Supplementary Fig. 11 for baseline firing rate distribution). Figure 5a plots the population-averaged firing rates of all shock-responsive cells during the US, CS, and CX baseline over the course of the initial training session (binned into 4 trial blocks). A 4×2 ANOVA revealed an interaction between stimulus (CX vs. CS) and trial block (F3,57=5.78, p=.002), and post-hoc comparisons showed that compared with block 1, US-evoked responses were significantly reduced during blocks 3 (p=.03) and 4 (p=.003). The CX baseline did not differ significantly between any pair of trial blocks (p>.55 for every comparison), indicating that baseline firing rates remained stable throughout the conditioning session. These findings indicate that US-evoked responses of PAG neurons decreased during conditioning.
Figure 5.
Activity of PAG neurons during acquisition of fear conditioning. (a) Normalized stimulus-evoked responses (y-axis) averaged over the population shock responsive PAG neurons (n=20) for each of the four conditioning trial blocks (4 trials/block, 16 trials total). (b) Pie chart shows percentage of shock-responsive PAG cells that significantly reduced (−), increased (+) or did not change (0) their CS-evoked responses between the first (early) and last (late) conditioning trial block. (c) top graph, PSTH (bin size=100 ms) shows normalized activity during shock trains (individual shock pulses indicated by red hash marks) for early versus late conditioning trials, averaged over the subpopulation of PAG neurons that significantly reduced their shock evoked response (n=10); bottom graphs, PSTHs and spike rasters show US-evoked responses for two example neurons from different rats, along with waveforms and cluster plots as in Fig. 1e. (d) top graph, PSTHs (bin size = 2 ms) show CS-evoked responses (onset of white noise pip indicated by vertical line) during the pre- versus post-conditioning test sessions for the subpopulation of PAG cells shown in ‘D’; bottom graphs, PSTHs and spike rasters show CS-evoked responses for the same two example neurons shown in ‘D’. (e) Responses to the US (left y-axis) on each of the 16 conditioning trials (averaged over the subpopulation of PAG cells that significantly reduced their US responsiveness during conditioning) is graphed alongside average freezing scores (right y-axis) during the CS period on each trial.
To analyze how individual PAG neurons changed their responses to the US during conditioning, a statistical comparison was made between each cell’s responses during the first versus last trial block. US-evoked responses decreased significantly in 50% (10/20) of the cells (see Figure 5b and 5c & d for population and individual cell example PSTHs), increased in 15% (3/20) of the cells, and remained unchanged in 35% (7/20) of the cells (Figure 5b). Thus, individual PAG cells were more likely to decrease than increase their responsiveness to the US during conditioning, although this tendency did not reach statistical significance (two-tailed binomial test, p=.09). As in the amygdala, the averaged response of these cells was inversely correlated with freezing to the CS across conditioning trials (r16=−.49; p=.05), suggesting that attenuation of US-evoked responses in PAG emerged as rats learned to expect the US (Figure 5e).
Modulation of US processing by the predictive CS
After the initial conditioning session, responses to signaled and unsignaled shocks were recorded exactly as described above for the amygdala recording experiments. A total of 93 PAG neurons were recorded from 13 rats with at least one shock-responsive cell recorded from each rat. Significant shock-evoked responses were observed in 61/93 (66%) of these neurons, and shock-evoked responses of these cells were larger to unsignaled than signaled shocks (t60=3.34, p=.001). Analysis of individual neuron responses revealed that 34.5% (21/61) of the cells exhibited a larger response to the unsignaled US (see Figure 6a and 6b & c for US and CS-evoked population and individual cell PSTHs), whereas only 8% (4/61) of the cells exhibited a larger response to the signaled US; the remaining 54.5% (36/61) of the neurons responded similarly to signaled versus unsignaled delivery of the shock US (Figure 6a). Hence, PAG neurons were about four times more likely to respond preferentially to the unsignaled US (n=21) than to the signaled US (n=5), and this was a significant bias for preferential responding to unsignaled shocks (two-tailed binomial test, p=.0025). Differential responses of LA/B neurons to predicted versus unpredicted shocks were not attributable to differences in motor responses to the shocks (Supplementary Fig. 12). These results indicate that, like amygdala neurons, shock evoked responding in PAG neurons is modulated by expectation.
Figure 6.
PAG neurons responded more to UNSIG than SIG shocks. (a) Pie chart showing the percentage of shock responsive PAG cells that responded significantly more to UNSIG than SIG shocks (black), significantly more to SIG than UNSIG shocks (white), or the same to both types of shock (gray). (b) top graph, PSTHs (bin size=100 ms) shows normalized activity during SIG and UNSIG shock trains (individual shock pulses indicated by red hash marks) for the subpopulation of PAG neurons (n=21) which responded significantly more to UNSIG then SIG shocks. bottom graphs, PSTH and spike rasters show responses to SIG and UNSIG shocks for two example neurons from different rats. (c) top graph, PSTH (bin size = 2 ms) shows normalized CS-evoked responses (onset of white noise pip indicated by vertical line) during SIG trials for the subpopulation of PAG neurons shown in ‘b’. bottom graphs, PSTHs and spike rasters show CS-evoked responses for the same two example neurons shown in ‘b’.
DISCUSSION
Theory and evidence suggest that fear conditioning is instructed by a teaching signal which diminishes in intensity as expectation of the US increases 17,18,20,21,26,27,35,36. Depolarization of LA neurons by an aversive US is thought to serve as the teaching signal that strengthens CS inputs onto amygdala neurons during fear learning 1,2,6, and here we have presented evidence that US-evoked responses of neurons in both LA and PAG are inhibited by expectation of the US during fear conditioning in rats.
We found that US-evoked responses in LA and PAG decreased over the course of training in a manner that was inversely correlated with increased freezing behaviour (Fig. 1 and 5) and that this training regimen produced a reduction in the ability of a predicted US to support further fear conditioning (Fig. 2d). Following conditioning, LA/B and PAG neurons responded more robustly to shocks when they were presented unexpectedly than when they were signaled by the predictive CS (Figs. 2 and 6). Finally, pharmacological inactivation of the PAG attenuated US-evoked responses in LA neurons (Fig. 3) and impaired acquisition of fear conditioning (Fig. 4). These data provide evidence that LA and PAG neuronal responses to shock USs are negatively modulated by expectation and suggest that the PAG may relay expectancy-modulated shock information to LA neurons to instruct associative neural plasticity and support fear learning (for discussion on mechanisms of expectation modulation of US processing in the fear circuit and PAG involvement in these processes, see Supplementary Discussion section)
We found that some LA neurons exhibited conditioned enhancement of their CS-evoked responses and others did not, but both kinds of neurons were equally likely to exhibit inhibition of their US-evoked responses by expectation. This pattern of results is consistent with learning theories which postulate that a primary function of an expectation-modulated teaching signal is to regulate competition among associative learning elements (in this case, LA neurons) 17,37 (see Supplementary Discussion section for further discussion on the present results and learning models). The opportunity to gain in associative strength may be lost once the US becomes expected, so only those LA neurons which strengthen their CS inputs early in conditioning (while the US is still unexpected) should ever be able to do so. A number of different factors may influence which subset of LA neurons succeed in strengthening their CS inputs, such as the availability of specific intracellular signalling molecules 30, or whether the US-responsive neurons receive convergent inputs from sensory neurons that encode the CS (LA neurons receive inputs from multiple sensory modalities, and many LA cells respond exclusively to one modality38). To serve as a general-purpose teaching signal for fear learning over a wide range of CS modalities and learning contexts, the expectancy-modulated US signal should be broadly distributed throughout the network of amygdala neurons, so that it can instruct plasticity in whichever subset of neurons are best suited for storing the CS-US association in a given learning situation. Hence, enhancement of CS-evoked responses should be observed only in a subset of a larger population of neurons that exhibit inhibition of US processing by expectation, as we have seen here.
Much evidence implicates PAG as an output structure for various fear-conditioned responses including freezing, analgesia and vocalization, as well as unconditioned reactions to aversive USs such as shock 31,32,34,39. Consistent with these prior results, we observed here that PAG inactivation reduced the expression of both conditioned fear responses and unconditioned reflex responses to the shock US (Supplementary Fig. 9 and 6). However, if PAG serves only as an output pathway for fear conditioning, then PAG inactivation should only impair expression but not acquisition of fear conditioning. Contradicting this, we observed here that fear acquisition was impaired by pretraining inactivation of PAG (Fig. 4). Pre-training PAG inactivation may have reduced fear learning by blocking local plasticity in the PAG--a possibility which is suggested by prior evidence 40--but this would not explain why this manipulation reduced shock-evoked responding in LA neurons. A parsimonious interpretation of the data is that the PAG may serve multiple functions during fear conditioning, mediating both the expression of fear responses and the transmission of teaching signals to the amygdala to regulate LA plasticity and the resultant acquisition of fear learning.
The PAG is anatomically well-positioned to receive afferent sensory information about the shock US, since it receives major input from nociceptive and somatosensory neurons in the medullary and spinal dorsal horn 41,42. Moreover, stimulation of PAG neurons can substitute for a noxious US to support fear conditioning 43, suggesting that output from PAG is sufficient to generate an aversive teaching signal that instructs associative plasticity in the amygdala. Direct projections from PAG to LA are sparse44, but there are a number of nociceptive and neuromodulatory brain regions that receive PAG afferents and project to the LA, such as the intralaminar thalamic nuclei, anterior cingulate cortex, hypothalamus, locus coeruleus and the ventral tegmental area45–49. Some of these PAG targets are involved in the acquisition of fear conditioning 12–16,50, so PAG may participate in relaying US signals to the amygdala indirectly via one or more of these brain regions. A more detailed understanding of these aversive teaching signal pathways will be important for guiding future investigations of the neural circuitry mediating fear conditioning, as well as other forms of learning that are instructed by aversive events.
METHODS
Subjects and surgery
Male Long-Evans rats weighing 350–400 g were housed singly and reduced to 85% of ad-lib weight through limited daily feeding. Under deep isoflourane anesthesia, rats were implanted with a pair of insulated stainless steel wires (75 μm diameter) beneath the skin of each eyelid for delivering the periorbital shock US. In addition, rats were implanted with recording electrodes, intracranial infusion cannulae, or both (see below). Electrodes and cannulae were fixed in place by securing screws and bone cement, and rats were given at least five days to recover after surgery before experiments began.
Single-unit recording electrodes
The left or right hemisphere (counterbalanced) of either LA or PAG was implanted with a microdrive containing six tetrode bundles made from .0007″ formvar-insulated nichrome wire (Kanthal Corporation, Palm Coast, FL). For LA recordings, electrode tips were placed just above the lateral tip of LA at 3.0 mm posterior, 5.3 mm lateral, and 7.0 mm ventral to bregma. For PAG recordings, tips were placed just above the dorsal column of PAG at 7.65 mm posterior, 0.75 mm lateral, and 3.5 mm ventral to bregma.
Intracranial infusion cannula
Rats in PAG inactivation experiments were bilaterally implanted with a pair of stainless steel 26G guide cannulae filled with 33G dummies projecting 0.5 mm from the guide tips. Guide tips were placed at 7.8 mm posterior, ±0.75 mm lateral, and 4.3 mm ventral to bregma, so that injectors protruding 1.5 mm from the guide would later deliver infusions into the ventrolateral PAG at 5.8 mm ventral to bregma.
Fear conditioning
Throughout experimental sessions (except for the ‘blocking’ experiment, see below), rats foraged freely for 20 mg purified food pellets (Bioserv, Frenchtown, NJ) dropped randomly from an overhead dispenser. Three different contexts were used in the experiment: A) circular platform made of black plastic cleaned with lemon-scented solution and surrounded by 3 white walls and a white curtain (70 cm diameter), B) square platform (70 × 70 cm) made of gray-painted wood cleaned with mint-scented solution and surrounded by 3 black walls and a black curtain, C) square platform (same as in B) cleaned with Windex and surrounded by 3 gray walls and an open side with no curtain. For all electrophysiology experiments, rats were run on the square platform described in C. For PAG behavioural experiments (Fig. 4), rats were randomly assigned to a platform at the start of the experiment, where they learned to pellet chase over 5 days of pre-exposure. Rats in PAG behavioural experiments began fear conditioning on the first day after pre-exposure, whereas rats in neural recording experiments began fear conditioning on the first day that well-isolated neurons were encountered in the target area. In all experiments, the CS was a train of 70 dB white noise pips, each lasting 250 ms, delivered at 1 Hz for 20 s through an overhead speaker. The US was a train of 2.0 mA shock pulses, each lasting 2.0 ms, delivered to the eyelid contralateral from the recording hemisphere at a rate of 6.66 Hz for 2 s. During CS-US pairing trials, the first shock pulse was delivered 300 ms after the offset of the final (20th) CS pip. The inter-trial interval was uniformly random between 180 and 240 s.
For PAG behavioural experiments, rats were placed on their assigned platform and presented with 6 CS’s alone on the first day after pre-exposure, to measure baseline freezing responses to the CX and CS. 24 h later, rats received either muscimol (MUS) or vehicle (VEH) (0.4 ul, 0.25 mg/ml over a period of 100 s) microinjections into the PAG or the lateral off-site control site. 20 min after microinjection, animals received 16 CS-US pairings on the same previously assigned platform and were then returned to their homecages. Six days later (to allow drug effects to wear off), animals were placed on a novel platform for the first time and presented with 6 test presentations of the CS alone to assess conditioned freezing responses..
Fear conditioning for electrophysiology studies was as described above, except that the pre-training CS session, fear conditioning session, and post-training CS session all occurred on the same day. The pre-training CS session was followed immediately by the fear conditioning session, after which rats rested in their homecages for 1 h before returning to the platform for the post-training CS session. On subsequent days, electrodes were advanced and until new cells were isolated, at which time rats were given a ‘signaled-unsignaled’ session in which 8 unsignaled shocks (not preceded by the CS) and 8 signaled shocks (preceded by the CS) were presented in random order. Between the original conditioning session and all ‘signaled-unsignaled’ sessions animals were retrained with the standard fear conditioning session (16 CS-US pairings) Electrode advancement and recording sessions continued in this manner until electrodes were no longer in the amygdala.
For the blocking experiment, rats were conditioned in a sound isolating chamber and did not chase food pellets. On day 1, rats were given 6 presentations of a flashing light CS (at the same frequency and duration as the auditory CS pips described above) alone followed by 6 presentations of the auditory CS alone. On day 2, the ‘blocking’ group received 16 pairings of the auditory CS and eyelid shock US (identical to prior experiments), while the ‘naïve’ group received no treatment. On day 3, all animals received 16 pairings of a compound CS consisting of the auditory CS and a new flashing light CS (at the same frequency and duration as the auditory pip CS) and the eyelid shock US. 24 h later, all animals were given 6 presentations of the light CS alone followed by 6 presentations of the visual CS alone.
Behavior tracking
Freezing and movement (except in the ‘blocking’ experiment, see below) were measured by an overhead video tracking system which has been described elsewhere28.. Briefly, the video tracker sampled (at 30 Hz) the position of two colored light-emitting diodes (LEDs) attached to the rat’s headstage throughout the experiments, and movement data was extracted by taking the time derivative of the sampled position data. Custom software written in MATLAB was used to analyze the movement data and extract freezing and head-jerking responses. Freezing on each trial was computed as the summed duration of episodes lasting at least 300 ms during which the rat’s movement speed was less than 10 cm/s. Our fear conditioning protocol yields lower freezing scores than other protocols for a variety of reasons, including the fact that the shock is localized to a small somatic area and that there are competing behavioural responses (ie. pellet chasing). Unconditioned head-jerking responses were quantified by averaging the rat’s head velocity (in cm/s) during the first 1.5 s of the shock train.
For the blocking experiment, the animals’ behavior was recorded on video, and a rater who was blind with respect to the treatment group scored animals’ behavioral freezing during the pre CS period (20 sec. preceding CS onset) and during the 20 light CS period offline using a digital stopwatch. Freezing was defined as the cessation of all bodily movement with the exception of respiration related movement.
Single-unit recording
Screening for shock responsive neurons
After rats had recovered from electrode implantation surgery, daily screening sessions were conducted in which electrode tips were slowly advanced (<200 μm per day) into the targeted brain area (LA/B complex or PAG). Neurons were tested for contralateral eyelid evoked shock responsiveness using mild single-shock pulses. If no shock responsive neurons were encountered, the electrodes were advanced.
Cluster analysis
Single-unit recordings were obtained using a 32-channel data acquisition system (Neuralynx, Bozeman, MT). Offline cluster cutting was performed manually using Neuralynx SpikeSort 3D software. To be included in the study, spike trains had to exhibit a refractory period of at least 1 ms and a mean spike amplitude of at least 70μV. Spike waveforms and cluster boundaries were inspected to make sure that they remained stable throughout the recording session (which lasted between 0.75–2.5 hours) for cells included in the data analysis.
Recording and inactivation experiments
To assess effects of PAG inactivation upon amygdala responses, bilateral muscimol infusions were delivered into PAG (0.4 ul, 0.25 mg/ml over a period of 100 s) immediately after a drug-free signaled-unsignaled shock session. The rat was placed back in its home cage for 20 min before returning to the platform for a second signaled-unsignaled shock session, to examine the effects of PAG inactivation on amygdala activity.
Data analysis
Peristimulus time histograms (PSTHs)
The value of each PSTH bin was computed Si = Ci/N, where N is the number of trigger events (trials or stimuli) for the PSTH and Ci is the cumulative number of spikes in the ith bin across trigger events. Prior to population averaging, the PSTH of each cell was normalized by converting Si values to Z-scores using the formula Zi= (Si − μ)/σ, where μ and σ are the mean and standard deviation, respectively, of all Si values within a set of baseline bins. PSTHs that were triggered once per trial had a bin width of 100 ms, and the baseline bins for normalization were the 200 Si values from the 20 s pre-CS period on signalled shock trials, or the 20 s period prior to when the omitted CS would have occurred for unsignaled shock trials. PSTHs that were triggered once per CS pip stimulus had a bin width of 2 ms, and the baseline bins for normalization were the 250 Si values from the 0.5 s period preceding the onset of each pip. Baseline PSTHs were computed from all trials in the session for normalization of PSTHs that included only subsets of trials within a session (such as early conditioning trials, unsignaled shock trials, etc.).
Shock responsiveness of individual neurons
A neuron was considered responsive to shocks during a block of trials if the 20 Zi values from bins in the 2 s shock train period of its normalized PSTH met one of three criteria: 1) at least one bin with Zi >3, 2) two or more consecutive bins with Zi >2, or 3) three or more consecutive bins with Zi > 1. For conditioning sessions, a cell was considered shock responsive if one of the criteria was met by the normalized PSTH for any trial block in the session (N=4 trials per block). For signalled-unsignalled sessions, a cell was considered shock responsive if one of the criteria was met by the normalized PSTH of either signaled (N=8) or unsignaled (N=8) shock trials. All bins meeting one of the three response criteria in a neuron’s PSTH from any trial block within a conditioning session, or either trial type within a signalled-unsignalled session, were combined to define a region of interest (ROI) window for inferential comparisons of the neuron’s responses to stimuli under differing conditions.
Inferential statistics for individual neurons
To determine whether an individual neuron’s shock responsiveness differed between two conditions (early vs. late conditioning trials, or signalled vs. unsignalled shock trials), spikes were counted in a 100 ms window following each shock pulse whose onset occurred within in the neurons’ ROI window (see above), and an independent t-test compared spike counts from the two conditions (so the degrees of freedom for the t-test were df= 2 T (×P) − 2, where T is the number of trials per condition, and P is the number of pulses per trial occurring within the ROI). The neuron was considered to respond differentlyto the stimulus in each condition if p<.05 (two-tailed). To compare pip responsiveness of amygdala neurons before and after conditioning, the ROI was the 10–30 ms time window following the onset of each white noise pip.
Inferential statistics for neural populations
To determine whether a population of neurons changed their responsiveness to a stimulus between two conditions, the Zi values of each neuron in the population was averaged within a specified time window (see Results) of the normalized PSTH to derive the response in each of the two conditions, and a paired t-test or repeated measures ANOVA was performed (with N=number of cells) to compare the responses during different conditions.
Supplementary Material
Acknowledgments
This work was supported by NSF-GRF to JPJ, and a NARSAD Young Investigator Award and NIH grant R01 MH073700-01 to HTB. We are grateful to Saleem Nicola and Adam Welday, for comments on an earlier version of the manuscript and Michael Fanselow, Dean Buonomano, Richard Thompson, Daniela Schiller and Yael Niv for valuable discussions.
Footnotes
Author Contributions: All authors contributed to the planning and design of the study. Data collection was performed by J.P.J. and J.W.T. Data analysis and writing of the manuscript were performed by J.P.J, J.W.T., and H.T.B. All work was conducted in the laboratories of H.T.B. and J.E.L., who supervised the studies
References
- 1.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–52. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 2.Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn Mem. 2001;8:229–42. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- 3.Lang PJ, Davis M. Emotion, motivation, and the brain: reflex foundations in animal and human research. Prog Brain Res. 2006;156:3–29. doi: 10.1016/S0079-6123(06)56001-7. [DOI] [PubMed] [Google Scholar]
- 4.Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol. 2005;56:207–34. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- 5.Sah P, Westbrook RF, Luthi A. Fear conditioning and long-term potentiation in the amygdala: what really is the connection? Ann N Y Acad Sci. 2008;1129:88–95. doi: 10.1196/annals.1417.020. [DOI] [PubMed] [Google Scholar]
- 6.Rosenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature. 2002;417:282–7. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- 7.Collins DR, Pare D. Differential fear conditioning induces reciprocal changes in the sensory responses of lateral amygdala neurons to the CS(+) and CS(−) Learn Mem. 2000;7:97–103. doi: 10.1101/lm.7.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goosens KA, Hobin JA, Maren S. Auditory-evoked spike firing in the lateral amygdala and Pavlovian fear conditioning: mnemonic code or fear bias? Neuron. 2003;40:1013–22. doi: 10.1016/s0896-6273(03)00728-1. [DOI] [PubMed] [Google Scholar]
- 9.Repa JC, et al. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat Neurosci. 2001;4:724–31. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- 10.Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–39. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 11.Romanski LM, Clugnet MC, Bordi F, LeDoux JE. Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behav Neurosci. 1993;107:444–50. doi: 10.1037//0735-7044.107.3.444. [DOI] [PubMed] [Google Scholar]
- 12.Brunzell DH, Kim JJ. Fear conditioning to tone, but not to context, is attenuated by lesions of the insular cortex and posterior extension of the intralaminar complex in rats. Behav Neurosci. 2001;115:365–75. [PubMed] [Google Scholar]
- 13.Shi C, Davis M. Pain pathways involved in fear conditioning measured with fear-potentiated startle: lesion studies. J Neurosci. 1999;19:420–30. doi: 10.1523/JNEUROSCI.19-01-00420.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanuza E, Nader K, Ledoux JE. Unconditioned stimulus pathways to the amygdala: effects of posterior thalamic and cortical lesions on fear conditioning. Neuroscience. 2004;125:305–15. doi: 10.1016/j.neuroscience.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 15.Borszcz GS. Contribution of the ventromedial hypothalamus to generation of the affective dimension of pain. Pain. 2006;123:155–68. doi: 10.1016/j.pain.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang J, et al. Pavlovian fear memory induced by activation in the anterior cingulate cortex. Mol Pain. 2005;1:6. doi: 10.1186/1744-8069-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rescorla RAWAR. A theory of pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Prokasy AHBWF, editor. Classical conditioning II: Current research and theory. Appleton-Century-Crofts; New York: 1972. [Google Scholar]
- 18.Fanselow MS. Pavlovian conditioning, negative feedback, and blocking: mechanisms that regulate association formation. Neuron. 1998;20:625–7. doi: 10.1016/s0896-6273(00)81002-8. [DOI] [PubMed] [Google Scholar]
- 19.Bolles RC, Fanselow MS. A perceptual-defensive-recuperative model of fear and pain. The Behavioral and Brain Sciences. 1980;3:291–323. [Google Scholar]
- 20.McNally GP, Westbrook RF. Predicting danger: the nature, consequences, and neural mechanisms of predictive fear learning. Learn Mem. 2006;13:245–53. doi: 10.1101/lm.196606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNally GP, Cole S. Opioid receptors in the midbrain periaqueductal gray regulate prediction errors during pavlovian fear conditioning. Behav Neurosci. 2006;120:313–23. doi: 10.1037/0735-7044.120.2.313. [DOI] [PubMed] [Google Scholar]
- 22.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Thompson RF, Thompson JK, Kim JJ, Krupa DJ, Shinkman PG. The nature of reinforcement in cerebellar learning. Neurobiol Learn Mem. 1998;70:150–76. doi: 10.1006/nlme.1998.3845. [DOI] [PubMed] [Google Scholar]
- 24.Knudsen EI. Instructed learning in the auditory localization pathway of the barn owl. Nature. 2002;417:322–8. doi: 10.1038/417322a. [DOI] [PubMed] [Google Scholar]
- 25.Herry C, et al. Processing of temporal unpredictability in human and animal amygdala. J Neurosci. 2007;27:5958–66. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yacubian J, et al. Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. J Neurosci. 2006;26:9530–7. doi: 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55:970–84. doi: 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blair HT, et al. Unilateral storage of fear memories by the amygdala. J Neurosci. 2005;25:4198–205. doi: 10.1523/JNEUROSCI.0674-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–8. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 30.Han JH, et al. Neuronal competition and selection during memory formation. Science. 2007;316:457–60. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- 31.Helmstetter FJ, Tershner SA. Lesions of the periaqueductal gray and rostral ventromedial medulla disrupt antinociceptive but not cardiovascular aversive conditional responses. J Neurosci. 1994;14:7099–108. doi: 10.1523/JNEUROSCI.14-11-07099.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–29. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neurosci. 1993;107:1093–8. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Z, Davis M. Fear-potentiated startle in rats is mediated by neurons in the deep layers of the superior colliculus/deep mesencephalic nucleus of the rostral midbrain through the glutamate non-NMDA receptors. J Neurosci. 2004;24:10326–34. doi: 10.1523/JNEUROSCI.2758-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamin LJ. Attention-like processes in classical conditioning. In: Jones MR, editor. Miami Symp Predictability, Behavior and Aversive Stimulation. Univ. Miami Press; Miami: 1968. pp. 9–32. [Google Scholar]
- 36.Young SL, Fanselow MS. Associative regulation of Pavlovian fear conditioning: unconditional stimulus intensity, incentive shifts, and latent inhibition. J Exp Psychol Anim Behav Process. 1992;18:400–13. doi: 10.1037//0097-7403.18.4.400. [DOI] [PubMed] [Google Scholar]
- 37.Sutton RS, Barto AG. Reinforcement Learning. The MIT Press; 1998. [Google Scholar]
- 38.Uwano T, Nishijo H, Ono T, Tamura R. Neuronal responsiveness to various sensory stimuli, and associative learning in the rat amygdala. Neuroscience. 1995;68:339–61. doi: 10.1016/0306-4522(95)00125-3. [DOI] [PubMed] [Google Scholar]
- 39.Fanselow MS. The midbrain periaqueductal gray as a coordinator of action in response to fear and anxiety. In: Depaulis A, Bandler R, editors. The midbrain periaqueductal gray matter. Plenum; New York: 1991. [Google Scholar]
- 40.Helmstetter FJ, Parsons RG, Gafford GM. Macromolecular synthesis, distributed synaptic plasticity, and fear conditioning. Neurobiol Learn Mem. 2008;89:324–37. doi: 10.1016/j.nlm.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keay KA, Feil K, Gordon BD, Herbert H, Bandler R. Spinal afferents to functionally distinct periaqueductal gray columns in the rat: an anterograde and retrograde tracing study. J Comp Neurol. 1997;385:207–29. doi: 10.1002/(sici)1096-9861(19970825)385:2<207::aid-cne3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 42.Gauriau C, Bernard JF. A comparative reappraisal of projections from the superficial laminae of the dorsal horn in the rat: the forebrain. J Comp Neurol. 2004;468:24–56. doi: 10.1002/cne.10873. [DOI] [PubMed] [Google Scholar]
- 43.Di Scala G, Mana MJ, Jacobs WJ, Phillips AG. Evidence of Pavlovian conditioned fear following electrical stimulation of the periaqueductal grey in the rat. Physiol Behav. 1987;40:55–63. doi: 10.1016/0031-9384(87)90185-5. [DOI] [PubMed] [Google Scholar]
- 44.Ottersen OP. Afferent connections to the amygdaloid complex of the rat with some observations in the cat. III. Afferents from the lower brain stem. J Comp Neurol. 1981;202:335–56. doi: 10.1002/cne.902020304. [DOI] [PubMed] [Google Scholar]
- 45.Herrero MT, Insausti R, Gonzalo LM. Cortically projecting cells in the periaqueductal gray matter of the rat. A retrograde fluorescent tracer study. Brain Res. 1991;543:201–12. doi: 10.1016/0006-8993(91)90029-u. [DOI] [PubMed] [Google Scholar]
- 46.Cassell MD, Wright DJ. Topography of projections from the medial prefrontal cortex to the amygdala in the rat. Brain Res Bull. 1986;17:321–33. doi: 10.1016/0361-9230(86)90237-6. [DOI] [PubMed] [Google Scholar]
- 47.Aston-Jones G, et al. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Prog Brain Res. 1991;88:47–75. doi: 10.1016/s0079-6123(08)63799-1. [DOI] [PubMed] [Google Scholar]
- 48.Ennis M, Behbehani M, Shipley MT, Van Bockstaele EJ, Aston-Jones G. Projections from the periaqueductal gray to the rostromedial pericoerulear region and nucleus locus coeruleus: anatomic and physiologic studies. J Comp Neurol. 1991;306:480–94. doi: 10.1002/cne.903060311. [DOI] [PubMed] [Google Scholar]
- 49.Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–53. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 50.Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat Neurosci. 2004;7:398–403. doi: 10.1038/nn1207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.