Abstract
Objectives
Interferon treatment for chronic viral hepatitis C (HCV) has been associated with the development of retinopathy in 19–29% of adults. Our purpose is to describe the ophthalmological complications of pegylated interferon (PEG) α2a with either placebo or ribavirin in children with chronic HCV (THE PEDS-C TRIAL).
Methods
Prospective comprehensive ophthalmologic examinations including slit lamp at enrollment and after 24 and 48 weeks of treatment of 114 children participating in a randomized clinical trial.
Results
128 children were screened for entry of which 123 had an eye exam and no child had existing retinal disease. 114 children were eligible and were treated. 110 children had an eye exam at 24 weeks and 103 at 48 weeks. 3 of 114 subjects (2.6%) developed documented (n=2) or possible (1) serious eye complications: One developed evidence of ischemic retinopathy (cotton wool spots) by week 24, one developed uveitis by week 48, and one reported at week 48 transient (<4 hours) monocular blindness that had occurred at week 36 with a subsequent normal exam at week 48.
Conclusions
Ophthalmologic complications are infrequent in children who are treated with PEG α2a for HCV (2–3%). Because of the potential severity of ischemic retinopathy and uveitis, prospective ocular assessment should remain part of the monitoring strategy for children who are treated with interferon for HCV.
Keywords: retinopathy, hepatitis C, interferon
INTRODUCTION
Chronic hepatitis C infection (HCV) is a global problem with up to 170 million people worldwide infected. (1) In the United States, the prevalence of HCV in children is approximately 0.1–0.5% with an estimated 40,000 children infected (2, 3). The combination of pegylated interferon (PEG) α2a and ribavirin (RV) has been shown to result in sustained virologic response rates of 45–90% in adults dependent on the genotype and other factors (4).
Interferon, whether pegylated or not, can lead to a wide range of side effects. To date, there have been limited data on treatment outcomes or ocular side effects in children treated with PEG. The most common side effects in children are headache, fever, fatigue, anorexia, abdominal pain vomiting, nausea and myalgias (5). Hematologic side effects and neuropsychiatric side effects can also be prevalent in adults and children (5, 6, 7).
Ophthalmologic side effects have been reported with both HCV and interferon in adults (8). Following the initial reports of retinopathy in adults by Ikebe (9) there have been many reports in adults with HCV of the ophthalmologic complications of interferon (8, 9, 10, 11, 12, 13, 14, 15, 16, 17). Common interferon induced ophthalmologic side effects include retinopathy characterized by cotton-wool spots, often with retinal hemorrhage and microaneurysms (8). Optic neuropathy has been described in up to 20% of patients (11). Rarer findings include optic neuritis, papilledema, retinal artery occlusion, retinal vein thrombosis, intraocular hemorrhage and Vogt-Koyanagi-Haradalike disease (idiopathic panuveitis) (8, 12, 15, 16, 17, 18, 19). HCV infection in and of itself may be associated with retinopathy, as the prevalence of retinopathy in adults with HCV who have not received interferon may be higher that adults without HCV (10). The prevalence of interferon-associated ophthalmologic side effects in adults with HCV has varied from 2 to 69% in prospective studies (11, 13, 14, 20) with an increased risk for ischemic retinopathy in those with hypertension, diabetes and those from Japan or individuals who have received PEG compared to standard interferon (13, 14, 20).
There has not been a prospective study of the ophthalmologic complications of interferon in children. In the largest prospective study of children treated with interferon for HCV to date, ophthalmologic assessments were not part of the study protocol (5). Thus there is no information about ophthalmologic complications of interferon in children. Our purpose is to report the prevalence of interferon associated retinopathy and other serious ocular complications in a prospectively studied group of children receiving PEG α2a with or without RV, who underwent ophthalmologic examinations by pediatric ophthalmologists at 11 sites prior to and after 24 and 48 weeks of treatment.
MATERIALS AND METHODS
PEDS C conducted a multicenter randomized placebo controlled trial of PEG α2a with either placebo or RV in children aged 5–17 years of age with chronic HCV confirmed by liver biopsy. The dose of PEG α2a was 180 mcg/1.73 m2 weekly and the dose of RV was 15 mg/kg/day in two divided doses orally. This study was reviewed and approved by all Institutional Review Boards involved. Written informed consent was provided by parents or guardians.
As part of the prospective study, patients had complete ophthalmologic examinations (Visual acuity, ocular motility, external structures, anterior and posterior segments, intraocular pressure when possible, and confrontation visual fields when possible) prior to initiation of treatment and after 24 and 48 weeks of treatment, performed by pediatric ophthalmologists. Presence of any retinopathy at enrollment was an exclusion criterion. Per protocol, patients who developed retinopathy had their treatment discontinued and were followed for resolution of their eye findings.
RESULTS
128 patients were screened for entry into the study, 123 underwent an eye examination and 114 patients enrolled and initiated treatment (59 PEG α2a +RV, 55 PEG α2a + Placebo). The number of children who remained on treatment and underwent ophthalmologic examinations was 110 after 24 weeks and 103 after 48 weeks of treatment.
No patient had ischemic retinopathy at the screening eye exam prior to initiation of treatment. During the course of treatment, one patient (1 %) developed ischemic retinopathy with cotton wool spots and one patient (1%) developed uveitis. One patient complained of transient unilateral blindness which resolved spontaneously. Case summaries are detailed below.
Patient 1: Nine year old male with chronic HCV acquired by vertical transmission. Baseline ophthalmologic exam was normal with 20/25 vision in each eye. The subject received PEG α2a and RV. At 24 weeks he was found to have 20/25 vision in each eye without visual complaints. He had developed a single cotton-wool spot in the papillomacular bundle of the retina in the right eye. Per protocol, treatment was discontinued. Repeat examination one week later demonstrated scattered cotton wool spots in both eyes without any visual symptoms. Follow up exam 6 weeks later demonstrated resolution of the cotton wool spots without sequelae.
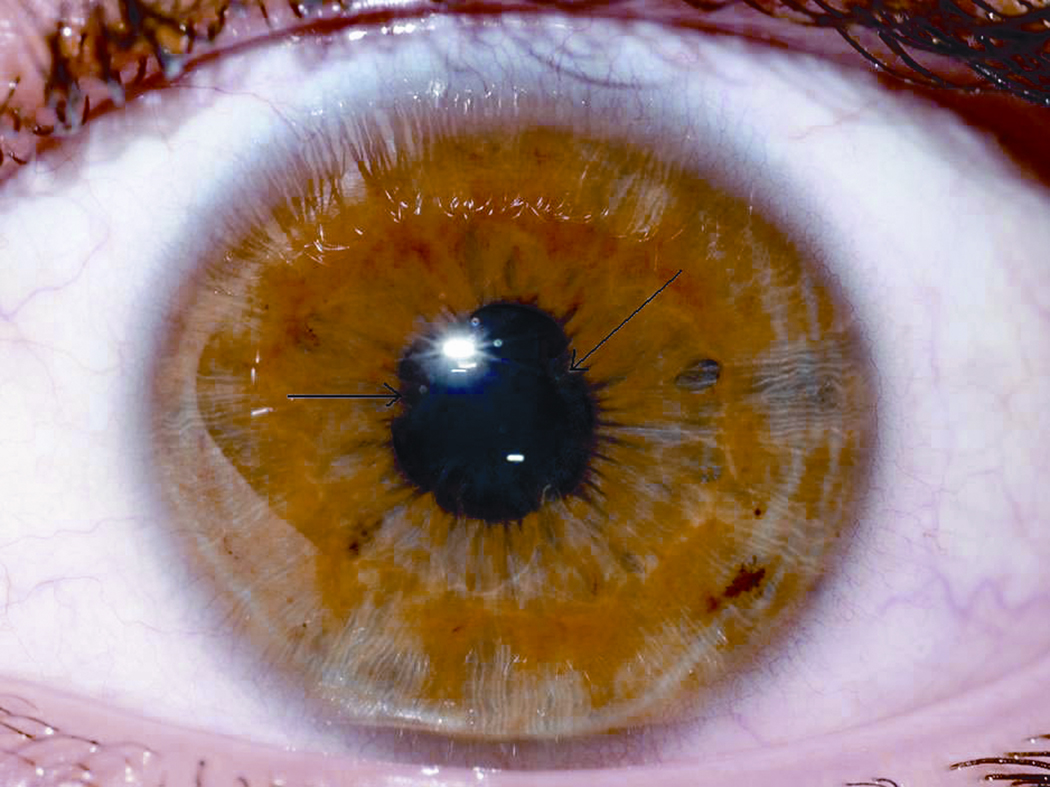
Patient 2: Twelve year old male with HCV. At entry, his eye exam was normal. Exam at 24 weeks was normal. He developed conjunctivitis 40 weeks into treatment that persisted. He completed 48 weeks of treatment with PEG α2a and placebo. At 48 weeks, he was reported to have uveitis which was treated with topical prednisolone acetate 1% and cyclopentylate 1%. The patient was poorly compliant with his medications and returned 4 weeks later with continued bilateral anterior uveitis. More intensive treatment was prescribed, but compliance continued to be poor with persistent uveitis at 16 months post initiation of treatment (5 months off treatment). Visual acuity was 20/60 in the right eye and 20/80 in the left eye. Slit lamp exam demonstrated small keratic precipitates in both eyes, with 1+ cells in the right anterior chamber and 3+ cells in the left anterior chamber. There were extensive synechiae in the left eye. The retinas were normal. Topical prednisolone was again prescribed, but 3 weeks later there were no significant changes. Oral prednisone (40 mg/day) was added to the topical therapy. The uveitis was rapidly controlled and over the next two months the oral prednisone was tapered and then discontinued. Twenty months from the start of treatment for HCV (9 months off treatment), both eyes were completely quiet off of all topical and systemic medications. However, extensive posterior synechiae persisted in the left eye (Figure 1). Final visual acuity was 20/25 in the right eye and 20/20 in the left. Throughout the course of his ocular inflammatory disease he was never noted to have involvement of the vitreous, retina, retinal vessels or choroid of either eye. He was also never noted to have elevated intraocular pressures. In the course of his treatment a systemic workup found no cause for his iritis. Testing included normal ACE, lysozyme, ANA, RF, RPR and urine beta 2 microglobulin and negative serologies for Treponema pallidum and T. gondii.
Figure 1.
Patient 2: Left eye with extensive synechiae (arrows) secondary to uveitis
Patient 3: Nine year old female with chronic HCV. She was treated with PEG α2a and RV. Eye exams at entry and at 24 weeks were normal. 36 weeks after enrollment she awoke complaining of the inability to see from the right eye. Her family chose to observe and not contact the medical team and used prayer therapy and her vision returned in approximately 60 minutes. This possible complication was brought to the attention of the research team 7 weeks later and therapy was discontinued. An ophthalmologic exam conducted 1 week later was normal.
DISCUSSION
In this cohort of 114 children with chronic hepatitis C treated with PEG α2a with or without RV in a prospective randomized clinical trial with serial ophthalmological monitoring, the overall prevalence of ophthalmologic complications is quite low. The prevalence of retinopathy in children with chronic HCV who have not received interferon is low compared to adults. The largest study in adults suggests that abnormal findings may be present in up 31% of adults with chronic HCV prior to any treatment (10). In our study, no child had abnormal retinal findings at entry. This is not unexpected and is likely due to the lack of other comorbidities such as diabetes and hypertension in our study population.
The prevalence of retinopathy in children with HCV treated with PEG α2a was low (1%) compared to adult data (19–29% in the two largest studies) (13, 14, 20). Adults develop the retinopathy at a mean of 12 weeks; (13) however, as many as 67% may resolve even on continued treatment (13). Thus, we may have underestimated the prevalence of retinopathy in children since our eye examinations were at 24 week intervals. However, persistent retinopathy may be the more important issue. If retinopathy is progressive, it could lead to permanent vision loss. There are reasons to believe that children would have a lower prevalence of retinopathy as our patients did not have hypertension, diabetes or renal disease, all of which may be risk factors for retinopathy(13, 14, 20). We do not know if the case of ischemic retinopathy would have resolved on continued treatment as to the protocol stipulated stopping treatment for any child who developed retinopathy. We were cautious in our study design with respect to the development of retinopathy until we have more information on the long term outcome of children treated with PEG α2a for HCV. Until the outcome of retinopathy associated with interferon is clarified in children, if retinopathy develops in children undergoing treatment with interferon, clinicians will need to weigh the risk of vision loss against the possible benefit of viral eradication.
Uveitis has been reported much less frequently with interferon and generally responds to immunosuppressive therapy as in our case and indeed interferon has been used as a treatment for some forms of uveitis. However, uveitis has also been reported to be due to interferon-induced sarcoidosis (21). Visual changes have been reported with interferon and are most commonly associated with retinopathy, ischemic optic nerve injury (22) or retinal vein thrombosis (23). In summary, ocular complications in children treated with PEG α2a for chronic HCV appear to be infrequent. Health care providers who treat children with interferon for chronic HCV should be vigilant for the complication and other potential ophthalmologic complications in children. Because of the potential severity, prospective assessment for ocular complications should remain part of the monitoring strategy for children who are treated with interferon for HCV.
Acknowledgments
Funded by a Grant from the NIH UO1DK067767-01 and UL1 RR 025005 (John Hopkins) and MO1 RR00069 (Colorado), General Clinical Research Centers Program, National Center for Research Resources, NIH
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
REFERENCES
- 1.Szabo E, Lotz G, Paska C, et al. Viral hepatitis: new data on hepatitis C infection. Pathol Oncol Res. 2003;9:215–221. doi: 10.1007/BF02893380. [DOI] [PubMed] [Google Scholar]
- 2.Jhaveri R, Grant W, Kauf TL, et al. The burden of hepatitis C virus infection in children: estimated direct medical costs over a 10-year period. J Pediatr. 2006;148:353–358. doi: 10.1016/j.jpeds.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 3.Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 4.Heathcote EJ. Antiviral therapy: chronic hepatitis C. J Viral Hepat. 2007;14 Suppl 1:82–88. doi: 10.1111/j.1365-2893.2007.00921.x. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Peralta RP, Kelly DA, Haber B, et al. Interferon alfa-2b in combination with ribavirin for the treatment of chronic hepatitis C in children: efficacy, safety, and pharmacokinetics. Hepatology. 2005;42:1010–1018. doi: 10.1002/hep.20884. [DOI] [PubMed] [Google Scholar]
- 6.Pearlman BL. Hepatitis C treatment update. Am J Med. 2004;117:344–352. doi: 10.1016/j.amjmed.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson KR, Murray K, Zellos A, et al. An analysis of published trials of interferon monotherapy in children with chronic hepatitis C. J Pediatr Gastroenterol Nutr. 2002;34:52–58. doi: 10.1097/00005176-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Zegans ME, Anninger W, Chapman C, et al. Ocular manifestations of hepatitis C virus infection. Curr Opin Ophthalmol. 2002;13:423–427. doi: 10.1097/00055735-200212000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Ikebe T, Nakatsuka K, Goto M, et al. A case of retinopathy induced by intravenous administration of interferon. Folia Ophthalmol Jpn (Ganka-Kiyo) 1990;41:2291–2296. [Google Scholar]
- 10.Abe T, Nakajima A, Satoh N, et al. Clinical characteristics of hepatitis C virus-associated retinopathy. Jpn J Ophthalmol. 1995;39:411–419. [PubMed] [Google Scholar]
- 11.Manesis EK, Moschos M, Brouzas D, et al. Neurovisual impairment: a frequent complication of alpha-interferon treatment in chronic viral hepatitis. Hepatology. 1998;27:1421–1427. doi: 10.1002/hep.510270533. [DOI] [PubMed] [Google Scholar]
- 12.Matsuo T, Takabatake R. Multiple sclerosis-like disease secondary to alpha interferon. Ocul Immunol Inflamm. 2002;10:299–304. doi: 10.1076/ocii.10.4.299.15588. [DOI] [PubMed] [Google Scholar]
- 13.Okuse C, Yotsuyanagi H, Nagase Y, et al. Risk factors for retinopathy associated with interferon alpha-2b and ribavirin combination therapy in patients with chronic hepatitis C. World J Gastroenterol. 2006;12:3756–3759. doi: 10.3748/wjg.v12.i23.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulman JA, Liang C, Kooragayala LM, et al. Posterior segment complications in patients with hepatitis C treated with interferon and ribavirin. Ophthalmology. 2003;110:437–442. doi: 10.1016/S0161-6420(02)01741-4. [DOI] [PubMed] [Google Scholar]
- 15.Sene D, Touitou V, Bodaghi B, et al. Intraocular complications of IFN-alpha and ribavirin therapy in patients with chronic viral hepatitis C. World J Gastroenterol. 2007;13:3137–3140. doi: 10.3748/wjg.v13.i22.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vardizer Y, Linhart Y, Loewenstein A, et al. Interferon-alpha-associated bilateral simultaneous ischemic optic neuropathy. J Neuroophthalmol. 2003;23:256–259. doi: 10.1097/00041327-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Zandieh I, Adenwalla M, Cheong-Lee C, et al. Retinal vein thrombosis associated with pegylated-interferon and ribavirin combination therapy for chronic hepatitis C. World J Gastroenterol. 2006;12:4908–4910. doi: 10.3748/wjg.v12.i30.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicolo M, Artioli S, La Mattina GC, et al. Branch retinal artery occlusion combined with branch retinal vein occlusion in a patient with hepatitis C treated with interferon and ribavirin. Eur J Ophthalmol. 2005;15:811–814. [PubMed] [Google Scholar]
- 19.Kuga K, Hasumura S, Nagamori S, et al. Intraocular hemorrhage developing during interferon therapy. Intern Med. 1996;35:15–18. doi: 10.2169/internalmedicine.35.15. [DOI] [PubMed] [Google Scholar]
- 20.d'Alteroche L, Majzoub S, Lecuyer AI, et al. Ophthalmologic side effects during alpha-interferon therapy for viral hepatitis. J Hepatol. 2006;44:56–61. doi: 10.1016/j.jhep.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 21.Yan KK, Dinihan I, Freiman J, et al. Sarcoidosis presenting with granulomatous uveitis induced by pegylated interferon and ribavirin therapy for Hepatitis C. Intern Med J. 2008;38:207–210. doi: 10.1111/j.1445-5994.2007.01625.x. [DOI] [PubMed] [Google Scholar]
- 22.Kiuchi K, Kitagawa C, Miyashiro M. [Serious loss of vision in bilateral anterior ischemic optic neuropathy caused by interferon] Nippon Ganka Gakkai Zasshi. 2009;113:16–23. [PubMed] [Google Scholar]
- 23.Goncalves LL, Farias AQ, Goncalves PL, et al. Branch retinal vein thrombosis and visual loss probably associated with pegylated interferon therapy of chronic hepatitis C. World J Gastroenterol. 2006;12:4602–4603. doi: 10.3748/wjg.v12.i28.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]