Abstract
Conventional biochemical analysis mainly focuses on the expression level of cellular proteins from entire cells. However, it has been increasingly acknowledged that the subcellular location of proteins often carries important information. Analysis of subcellular proteins conventionally requires subcellular fractionation which involves two steps: cell lysis to release proteins and high-speed centrifugation to separate the homogenate. Such approach requires bulky and expensive equipment and is not compatible with processing scarce cell samples of limited volume. In this study, we apply microfluidic flow-through electroporation to breach cell membranes and extract cytosolic proteins selectively in a single step. We demonstrate that this approach allows monitoring the translocation of the transcription factor NF-κB from the cytosol to the nucleus without the need of subcellular fractionation. Our technique is compatible with the processing of samples of various sizes and provides a simple and universal tool for bioanalytical analysis and spatial proteomics.
The 23,000 human protein-coding genes give rise to a far larger number of functional proteins due to alternative splicing and post-translational modification. To further add to the complexity, proteins also vary in their temporal and spatial organization. Regulatory proteins such as kinases, phosphatases and GTPases often exist in low copy numbers and function only at specific subcellular locations. For example, kinases frequently move from one subcellular compartment to another (e.g. from the cytosol to the plasma membrane, or from the cytosol to the nucleus) as a consequence of their phosphorylation and activation1–3. Many transcriptional factors are translocated to the nucleus in response to extracellular stimuli where they bind to DNA and regulate gene transcription4. Thus, the study of cellular proteins in the context of their subcellular locations is important for understanding their cellular functions. Furthermore, studying a specific subcellular proteome is often a practical means to reduce the complexity of the eukaryotic cell proteome allowing the characterization of an entire proteome to become more feasible5, 6. Focusing on proteins from a particular subcellular location ensures that proteins with low copy numbers do not get overshadowed by those of high abundance.
Subcellular fractionation, or the separation of cellular homogenate into fractions representing different subcellular compartments, is the most common method for preparing subsets of proteins from different locations5. Subcellular fractionation involves two steps: disruption of the cellular organization, typically by physical homogenization or chemical lysis using detergents, followed by differential centrifugation7. Physical homogenization methods such as mechanical disruption, liquid homogenization, freeze/thaw cycles and manual grinding lack reproducibility and often result in the incomplete release of proteins. Chemical lysis introduces detergents or reagents that often interfere with downstream analyses by tools such as mass spectrometry. The centrifugation process separates various subcellular fractions based on their physical properties (e.g. the particle size). Centrifugation can be labor-intensive and require bulky and expensive equipment especially when applied to large-volume samples. The process is also difficult to scale down for handling of samples of small volumes.
In this study, we report a simple method to disrupt the cell membranes and release selected intracellular proteins from a specific subcellular location in a single step. We use a high electric field to generate pores in the plasma membrane (i.e., electroporation) and to mobilize intracellular proteins into the surrounding solution. We show that such protein release under the electric field is highly dependent on the protein's subcellular localization: cytosolic proteins are much more readily released than nuclear proteins. We demonstrate using this approach to track the translocation of the transcriptional factor NF-κB from the cytosol to the nucleus over time without subcellular fractionation. Our technique employs a simple microfluidic flow-through electroporation device that offers the capacity to process a wide range of sample sizes and is generally applicable to studies involving subcellular fractions of intracellular proteins.
Electroporation is a simple physical method to breach the cell membrane barrier by applying a strong external electric field8. It is well established that electroporation generates nanoscale pores in the membrane of cells that allow intracellular molecules to be released into the surrounding solution9–13. However, the dependence of such release on subcellular location has only recently started to be understood and appreciated14.
In this study, we examine the relationship between the electroporative release of intracellular molecules and their subcellular locations and how such relationship can be exploited for selective analysis of subcellular proteins. Fig. 1 shows the flow-through electroporation device used in this study. The cell sample flowed through a microfluidic channel with alternating wide (~800 μm wide) and narrow (~100 μm wide) sections while a constant DC voltage was established across the channel. As we demonstrated previously15, 16, in such a flow-through electroporation device, the local field intensity is inversely proportional to the width of the section. Thus, electroporation occurs exclusively in the narrow sections due to the significantly higher field intensity there. Flow-through electroporation has the versatility of handling sample volumes ranging from microliters to liters, which makes it suitable for multiple applications17.
Figure 1.
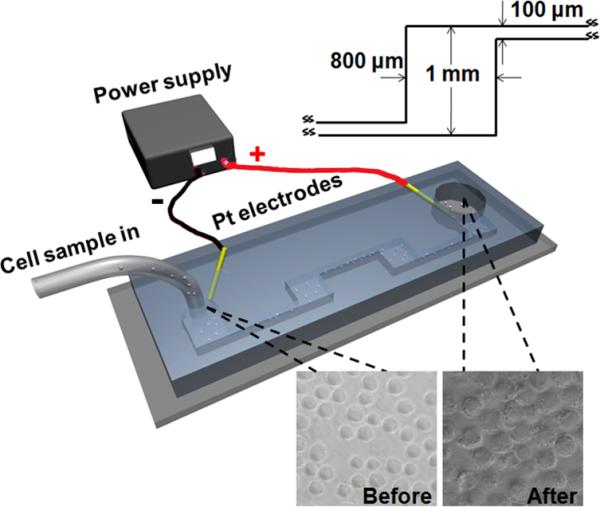
The layout of the electroporation device used for the selective release of intracellular proteins. The geometry of the wide sections is shown in the inset image. Each narrow section is 2.8 mm long and the channel has a depth of 60 μm. The inset images show that cells are mostly in one piece after electroporation.
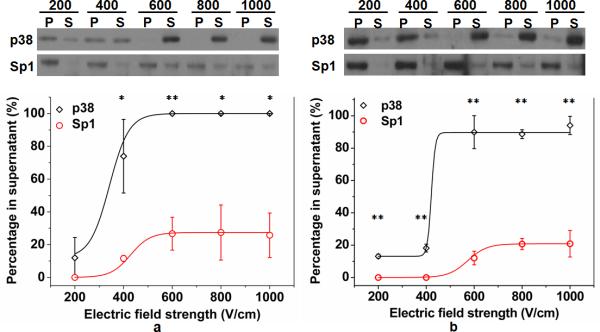
In order to observe release of intracellular proteins from specific subcellular locations, we used p38 as a cytosolic protein marker and the transcription factor Sp1 as a nuclear protein marker. p38 is a small, 38 kDa protein kinase that is localized to the cytoplasm in normal, unstressed cells and Sp1 is an 80 kDa transcription factor that is localized to the nucleus. Both have been used previously as markers of subcellular fractions in DT40 cells3. DT40 B cells suspended in electroporation buffer were flowed through the electroporation device. The solution flowing out of the device was centrifuged to generate separate supernatant and pellet fractions. The supernatant contained the intracellular proteins released into the solution by electroporation and the pellet contained the cellular “remains”. Cells after electroporative release are mostly in one piece with their nuclei enclosed within the plasma membrane, as shown in ESI Fig. S1. We examined each fraction using SDS-PAGE and Western blotting analysis and the percentage of each protein (p38 or Sp1) present in the supernatant was calculated under specific electroporation conditions (by assuming that the protein in the supernatant and the pellet together equaled 100%). As shown in Fig. 2, with an electroporation duration (the total residence time in the narrow sections) of 100 (Fig. 2a) or 50 ms (Fig. 2b), the release of both proteins into the solution increased with the electroporation field intensity (in the narrow sections). p38 was substantially more susceptible to electroporative extraction than Sp1 due to the difference in subcellular location. The data in Figure 2 show that flow-through electroporation provides a significant differentiation in terms of its extraction of cytosolic and nuclear proteins. For example, with field duration of 50 ms and field intensity of 400 V/cm, we were able to release 18% of the cytosolic p38 without extracting the nuclear Sp1. Alternatively, with a field duration of 100 ms and field intensities>600 V/cm, nearly all cytosolic p38 was released into the supernatant and only a small percentage (25%) of the nuclear Sp1 was released. It is worth noting that due to the semi-quantitative nature of Western blotting, the sum of the supernatant and pellet bands can vary up to ~20% among different experiments.
Figure 2.
Intracellular protein release under different electroporation conditions. The levels of p38 and Sp1 in supernatant (S) and pellet (P) fractions from DT40 B cells electroporated at different field strengths (200, 400, 600, 800, 1000 V/cm) for 100 (a) or 50 (b) ms was analyzed by Western blotting (upper panels). The percentage of p38 and Sp1 in the supernatant fraction (calculated based on three trials of Western blot analysis) at different field strengths for electroporation of 100 (a) and 50 (b) ms is shown in the lower panels. The difference between the two data points is statistically significant with P values less than 0.05 (*) and 0.01 (**).
We are able to track intracellular protein translocation using our approach without the need for subcellular fractionation. Protein translocation refers to the change in the subcellular localization of a protein without alteration in its overall expression level. Studies involving protein translocation require analysis and quantification of subcellular protein contents. NF-κB is a family of dimeric transcription factors that regulates cellular stress responses, cell division, apoptosis, and inflammation4, 18, 19. Signals from extracellular stimuli (e.g. TNFα, IL-1, LPS and DNA-damaging agents etc.) induce NF-κB to translocate from the cytoplasm to the nucleus via phosphorylation and degradation of its cytoplasmic inhibitor IκB. Such process was previously studied in microfluidic devices using fluorescence imaging when the protein of interest was tagged with a fluorescent protein marker20, 21. This translocation process has also been routinely studied by the combination of subcellular fractionation and Western blotting. Using our flow-through electroporation technique, we found that in general cells stimulated by IL-1β (that induced NF-κB translocation to the nucleus) retained more NF-κB after electroporation (as shown in ESI Fig. S2 and S3), as expected. The difference in the electroporative release between the stimulated and unstimulated populations was small when the electroporation field intenisty was 600 V/cm or lower but became very significant at field intensities 800 and 1000 V/cm (ESI Fig. S3). We then used 800 V/cm and 50 ms for the electroporation and based on Figure 2b most cytosolic fraction (e.g. ~89% for p38) would be released into the supernatant together with a small percentage (e.g.~21% for Sp1) of nuclear proteins. In this case, the intracellular molecules extracted into the supernatant closely resemble the protein composition in the cytoplasm and the pellet fraction is very similar to the nuclear composition. As shown in Figure 3, by analyzing the supernatant and pellet fractions generated by flow-through electroporation, we clearly observed the progress of NF-κB translocation from the cytosol to the nucleus over time after cell stimulation by IL-1β. This confirms that our approach provides the differential extraction required by subcellular protein analysis.
Figure 3.
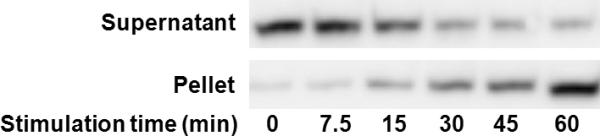
Tracking of NF- κB translocation from the cytosol to the nucleus over time. The intracellular proteins were extracted by flow-through electroporation and the supernatant and pellet fractions were analyzed by Western blotting after stimulation of CHO/GFP-NFκBp65 cells by IL-1β for different periods of time. We used 800 V/cm and 50 ms for the electroporation.
It needs to be noted that centrifugation, although used in the procedure to separate the supernatant and the pellet, is not an essential requirement for our method. Filtration that removes the cellular remains can achieve similar results. Depending on the studies, the supernatant, which has very similar protein composition to that of cytosolic proteins, can be used alone (after removal of cellular remains) for analysis if a simpler procedure is desired. Furthermore, assays other than Western blotting can be coupled with the protein extraction. Finally, the optimal electric parameters for the electroporative extraction are likely specific to the protein under investigation and the cell type. We envision that this technique will provide a simple and general solution to sample preparations involved in subcellular biochemistry and spatial proteomics studies.
Supplementary Material
Acknowledgement
We thank NSF CBET 1016547 and NIH NCI CA037372 for the financial support of this research.
Footnotes
Electronic Supplementary Information (ESI) available: Experimental procedures and supplepmentary data
References
- 1.Carpenter G, Liao HJ. Exp. Cell. Res. 2009;315:1556–1566. doi: 10.1016/j.yexcr.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma H, Yankee TM, Hu JJ, Asai DJ, Harrison ML, Geahlen RL. J. Immunol. 2001;166:1507–1516. doi: 10.4049/jimmunol.166.3.1507. [DOI] [PubMed] [Google Scholar]
- 3.Zhou F, Hu J, Ma H, Harrison ML, Geahlen RL. Mol. Cell. Biol. 2006;26:3478–3491. doi: 10.1128/MCB.26.9.3478-3491.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosh S, May MJ, Kopp EB. Annu. Rev. Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 5.Huber LA, Pfaller K, Vietor I. Circ. Res. 2003;92:962–968. doi: 10.1161/01.RES.0000071748.48338.25. [DOI] [PubMed] [Google Scholar]
- 6.Andersen JS, Mann M. EMBO Rep. 2006;7:874–879. doi: 10.1038/sj.embor.7400780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham JM, Rickwood D. Subcellular Fractionation: a Practical Apprach. Oxford University Press; New York: 1997. [Google Scholar]
- 8.Weaver JC, Chizmadzhev YA. Bioelectroch. Bioener. 1996;41:135–160. [Google Scholar]
- 9.McClain MA, Culbertson CT, Jacobson SC, Allbritton NL, Sims CE, Ramsey JM. Anal. Chem. 2003;75:5646–5655. doi: 10.1021/ac0346510. [DOI] [PubMed] [Google Scholar]
- 10.Lu H, Schmidt MA, Jensen KF. Lab Chip. 2005;5:23–29. doi: 10.1039/b406205a. [DOI] [PubMed] [Google Scholar]
- 11.Wang HY, Lu C. Chem. Commun. 2006:3528–3530. doi: 10.1039/b605722e. [DOI] [PubMed] [Google Scholar]
- 12.Marc PJ, Sims CE, Bachman M, Li GP, Allbritton NL. Lab Chip. 2008;8:710–716. doi: 10.1039/b719301g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valero A, Post JN, van Nieuwkasteele JW, Ter Braak PM, Kruijer W, van den Berg A. Lab Chip. 2008;8:62–67. doi: 10.1039/b713420g. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Bao N, Paris LL, Geahlen RL, Lu C. Anal. Chem. 2008;80:9840–9844. doi: 10.1021/ac801940w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang HY, Lu C. Anal. Chem. 2006;78:5158–5164. doi: 10.1021/ac060733n. [DOI] [PubMed] [Google Scholar]
- 16.Bao N, Le TT, Cheng JX, Lu C. Integr. Biol. 2010;2:113–120. doi: 10.1039/b919820b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geng T, Zhan Y, Wang HY, Witting SR, Cornetta KG, Lu C. J. Control. Release. 2010;144:91–100. doi: 10.1016/j.jconrel.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann A, Baltimore D. Immunol. Rev. 2006;210:171–186. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann A, Natoli G, Ghosh G. Oncogene. 2006;25:6706–6716. doi: 10.1038/sj.onc.1209933. [DOI] [PubMed] [Google Scholar]
- 20.Li PC, de Camprieu L, Cai J, Sangar M. Lab Chip. 2004;4:174–180. doi: 10.1039/b400770k. [DOI] [PubMed] [Google Scholar]
- 21.James CD, Moorman MW, Carson BD, Branda CS, Lantz JW, Manginell RP, Martino A, Singh AK. Biomed. Microdevices. 2009;11:693–700. doi: 10.1007/s10544-008-9281-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.