Abstract
Objectives
Growth hormone (GH) may reduce symptoms and improve growth in Crohn's Disease (CD). The effect upon mucosal inflammation is not known. We hypothesized that GH would improve both clinical and mucosal disease activity, and stimulate linear growth, in pediatric CD.
Methods
Twenty patients aged 7-18 receiving corticosteroids (CTX) for active CD were randomized to begin GH, 0.075 mg/kg/day (group A), or continue CTX alone (group B). Clinical and endoscopic disease activity were assessed after 12 weeks. Group B began GH at 12 weeks and clinical disease activity was assessed at 24 weeks. Subjects who experienced a clinical response after 12 weeks of GH therapy continued treatment for an additional 52 weeks, and linear growth was assessed.
Results
65% of patients receiving GH achieved clinical remission, compared to 20% treated with CTX alone (p=0.03). While endoscopic disease activity trended towards an improvement at week 12 in group A, this did not differ between the groups. 61% of week 12 GH responders maintained their clinical response through week 64. Mean (95th CI) height Z score on GH increased from -1.1 (-1.6,-0.6) to -0.4 (-1,0.2), p=0.004 during this 52 week extension phase. GH was well tolerated with no unexpected safety signals.
Conclusions
The addition of GH to CTX therapy did not induce a reduction in mucosal inflammation, relative to CTX alone. However, GH was safe and effective as an adjunct to CTX for treatment of clinical disease activity and growth failure in pediatric CD.
Keywords: inflammatory bowel disease, human growth hormone, linear growth
INTRODUCTION
Growth failure is present at diagnosis in up to 80% of children with Crohn's disease (CD), and persists into adulthood in 30% 1. Therapies which achieve intestinal healing are associated with a more durable clinical response, and may be more likely to optimize growth. The rate of mucosal healing ranges from approximately 20% for corticosteroids (CTX) to approximately 50% for azathiopurine/6-mercaptopurine (AZA/6-MP), infliximab, or enteral nutrition 2-6. However, 6-MP did not increase height velocity relative to prednisone alone in a randomized pediatric study, while adherence limits long term use of enteral nutrition 7. Infliximab promoted catch-up growth in a recent randomized trial 8. Concerns regarding rare but serious infectious and malignant adverse events limit its widespread use. Therefore, new approaches are needed to enhance linear growth and mucosal healing in children with CD.
Three previous studies have demonstrated benefits of GH therapy in CD. In a 4 month randomized placebo controlled trial in adults with moderate to severely active disease, the primary end point, improvement in the Crohn's Disease Activity Index (CDAI) by 70 points, was met by 74% in the GH group 9. An open label study demonstrated improvements in linear growth, lean body mass (LBM), and bone calcium accretion in Tanner stage I and II children with mild disease activity treated with GH for 6-12 months in addition to low dose corticosteroids 10. A second open label study in pediatric CD has confirmed these beneficial effects of GH upon linear growth and body composition 11. In contrast, an increase in height was not observed over one year in a recent randomized, controlled trial of GH in pediatric CD 12. Mucosal healing was not assessed in these prior studies. These preliminary trials warranted the randomized controlled trial reported herein, designed to determine the effect of GH upon clinical and mucosal disease activity, and linear growth, in children with moderately active CD.
MATERIALS and METHODS
Subject Selection
Eligibility criteria included: age ≥ 5 years, CD diagnosed by standard criteria, and moderate activity defined by a PCDAI ≥ 30. Use of corticosteroids (CTX) as prescribed by the referring gastroenterologist, and stable doses of azathiopurine/6-mercaptopurine (AZA/6-MP), methotrexate (MTX), and/or mesalamine were permitted. To qualify for GH treatment during the 52 week extension phase, the subject was required to either have achieved remission (PCDAI ≤ 10) or to have mild activity (PCDAI < 30). Exclusion criteria included: acute critical illness, active neoplasia, diabetes mellitus, history of intracranial lesion and/or neoplasia, severe CD requiring hospitalization, and current therapy with infliximab.
Study Design
Subjects were randomized using a block design by the CCHMC Investigational Drug Pharmacy to GH (Nutropin AQ, 0.075 mg/kg/day SC daily) + CTX (group A) or CTX alone (group B) for 12 weeks. Subjects in group B did not receive a placebo injection. Group B subjects who completed week 12 assessments were offered GH for a period of 12 weeks. CTX were tapered by the primary gastroenterologist. After 28 days on CTX, subjects could, at the discretion of their treating physician, begin mesalamine at a dose of 50 mg/kg/day (maximum of 4 gm/day). Subjects not already on 6-MP/AZA or MTX could not begin these during the initial 12 weeks. Colonoscopy at entry and week 12 was performed to determine the primary endpoint, mucosal inflammation as measured by the CD histologic index of severity (CDHIS). Secondary endpoints included: CD Endoscopic Index of Severity (CDEIS), colon Ki-67 labeling index, Pediatric Crohn's Disease Activity Index (PCDAI), IMPACT III score (health related quality of life), CTX use, and serum IGF-1/IGFBP-3. Subjects who achieved remission or mild disease activity after 12 weeks of GH therapy were offered continued GH for 52 weeks. During this phase, the dose of Nutropin was adjusted to maintain serum IGF-1 ≤ +2SD for age; all subjects who entered this phase had open epiphyses. The endpoints for this phase were fecal calprotectin and HTV and height z score (HTVz and HTz).
Sample Size
We estimated that the mean ± SD CDHIS value at baseline would be 9±3, and anticipated a 67% decrease in group A, and a 22% decrease in group B 13. A sample size of 12 per group would have 80% power to detect such a difference with □=0.05. Similarly, using a paired t test at □=.05 to compare the pre- and post-treatment values of the CDHIS in group A, the sample size would have more than 80% power to detect the difference. Sample size estimations were obtained using nQuery Advisor Version 5.0.
Materials and Methods
Nutropin AQ™, recombinant human growth hormone (rhGH), was provided by Genentech, Inc. Serum IGF-1, IGFBP-3, and insulin were measured in the Cincinnati Children's Hospital Medical Center (CCHMC) Endocrinology Core Clinical Laboratory; confirmatory serum IGF-1 measurements were also performed by Esoterix Laboratory Services (Austin, TX). Serum bone specific alkaline phosphatase (BSAP) was measured in the CCHMC General Clinical Research Center (GCRC) Core Clinical Laboratory. Serum IL-6 and growth hormone binding protein (GHBP) were measured using a commercial ELISA. Fecal calprotectin was measured by Genova Diagnostics (Asheville, NC).
The CDHIS and CDEIS, which have been validated for use in clinical trials in adult CD, and used to test the effect of infliximab upon mucosal inflammation in pediatric CD, were used to test for an effect of GH upon mucosal disease activity 13. The CDEIS was computed by a gastroenterologist (RB or BO) blinded to the treatment group 5. Six biopsy specimens submitted for routine clinical histology were used to score the CDHIS by two pathologists (EB and MC) blinded to the treatment assignment 14. The intra-class correlation coefficient (ICC) for the CDHIS was calculated using generalized linear model analysis (GLM). The mean (95%CI) ICC was equal to 0.8395 (0.69,0.94). The CDHIS determined by EB was used in the analysis.
Colon biopsies were sectioned and stained to determine the Ki-67 labeling index 15. Images were captured using a Zeiss microscope and Axioviewer image-analysis software (Carl Zeiss, Jena, Germany). All colon crypts in each biopsy were used to quantify colon epithelial cell (CEC) proliferation (Ki-67) under 400× power magnification. For each, a labeling index was calculated as the number of positive CECs per number of CEC nuclei counted.
The PCDAI was determined by the PI or co-PI (LD or MSM) 16. The partial Harvey Bradshaw (pHB) score was computed by the study coordinator (RB) by telephone interview 7.The IMPACT III questionnaire is a self-report measure developed to assess health related quality of life in children with IBD 17. Protein and calorie intake were assessed at baseline and 4 weeks after beginning GH, using three day food records computed by the CCHMC GCRC Clinical Nutrition Core. Subjects were provided with dietary counseling regarding goals for intake. However, supplemental nutrition was not provided.
Analysis
Descriptive procedures were used to summarize the safety data; pre-defined safety endpoints included serum glucose and insulin, arthralgia, peripheral edema, and headache. Subjects withdrawn for worsening of CD were included as efficacy evaluable subjects. Mixed effects model analysis was performed to test for differences between groups A and B as a function of time and treatment arm. Compound symmetry structure was used to fit the within pair dependency. Data were transformed when the normality assumption of the mixed effects model was violated. When the data was transformed, the delta method was used to obtain the 95% confidence interval at the original scale. The comparison was statistically significant only if the interaction of treatment arm by time was significant (p<0.05). Differences within the groups were tested with a paired t test. Mixed effect model analysis was performed with time as a continuous variable to test differential longitudinal trajectory by treatment arm for HTVz and HTz during the extension phase.
RESULTS
Study Subjects and Flow
We enrolled 22 males and females aged 7-18 (Table I). 21 had open epiphyses. Three subjects in each group were newly diagnosed. All subjects had colonic involvement; four in group A and one in group B had colon-only involvement. One subject in group B had had a previous ileo-cecal resection. The dose and duration of corticosteroid, immunomodulator and 5-ASA therapy did not differ between the groups (Table I). Participant flow is illustrated in Fig. 1. 98 subjects were screened. Of these, 55 did not meet inclusion criteria, while 21 were eligible but declined participation. 22 subjects were eligible and enrolled. Two withdrew consent prior to randomization or intervention; 10 were randomized to group A (GH+CTX) and 10 to group B (CTX). Two subjects in group B withdrew because of active disease requiring infliximab or surgery. 18 subjects who responded to GH in groups A and B entered the 52 week extension phase (Table II). During this phase 7 subjects discontinued the intervention because of active disease.
Table I.
Clinical and Demographic Characteristics at Study Entry.
| Group | n | Age (years) |
Gender (male) |
Tanner Stage |
Disease Duration (years) |
Disease Location |
Pred. Dose (mg/kg) |
Bud. Dose (mg/kg) |
6-MP Dose (mg/kg) |
6-MP Duration (months) |
5-ASA Dose (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 10 | 12 (7,15) |
80% | 2.6 (1,5) |
1.8 (0.1,6) |
L2B1:4 L3B1:6 |
70% 0.9 (0.5,1.3) |
30% 0.3 (9,9) |
80% 1.2 (1,1.7) |
16 (1,63) |
50% 65 (24,167) |
| B | 10 | 13 (10,18) |
80% | 2.7 (1,5) |
1.6 (0.1,4) |
L2B1:1 L3B1:8 L3B2:1 |
60% 1.1 (0.6,2) |
40% 0.2 (6,9) |
70% 1.2 (0.8,1.9) |
18 (4,32) |
60% 92 (45,173) |
Parameters are shown as the mean (range), with medication dosages at entry in mg/kg/day. Disease location and behavior were determined using the Montreal Classification (L2: colon-only location, L3: ileo-colonic location, B1: inflammatory behavior, B2: stricturing behavior). The frequency of baseline prednisone (pred.), budesonide (bud.), 6-mercaptopurine (6-MP), and mesalamine (5-ASA) usage is shown. One subject in group B was receiving methotrexate, 0.6 mg/kg SC once weekly at entry, with a duration of 52 months.
Figure 1. Participant Flow Through the Study.
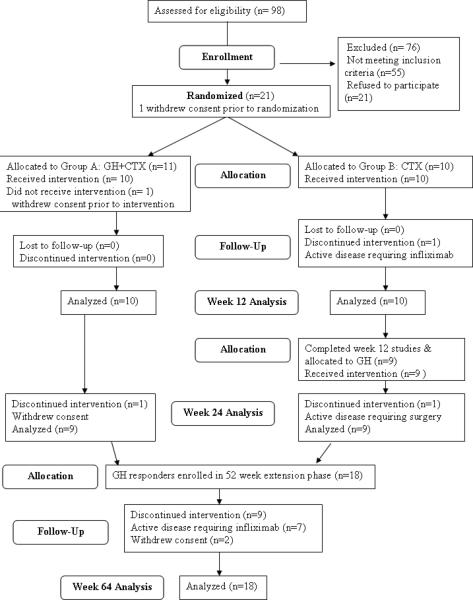
The flow of participants through the study, and the number included for the analysis of each phase, is shown.
Table II.
Clinical and Demographic Characteristics at Entry to 52 Week Extension Phase.
| Group | n | Age (years) |
Gender (male) |
Tanner Stage |
PCDAI | Fecal Calprotectin |
Pred. Dose (mg/kg) |
Bud. Dose (mg/kg) |
6-MP Dose (mg/kg) |
6-MP Duration (months) |
5-ASA Dose (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 10 | 13 (7,15) |
80% | 2.6 (1,5) |
8 (0,23) |
868 (56,1984) |
20% 0.1 (0.1,0.1) |
20% 0.2 (0.2,0.2) |
80% 1.2 (1,1.7) |
19 (4,66) |
50% 65 (24,167) |
| B | 8 | 13 (11,17) |
75% | 2.4 (1,5) |
6 (0,18) |
924 (126,2500) |
10% 0.01 |
40% 0.1 (0.1,0.1) |
63% 1.2 (0.8,1.9) |
20 (10,34) |
50% 107 (45,173) |
Parameters are shown as the mean (range), with medication dosages at entry in mg/kg/day. The median Tanner Stage for both groups was equal to 2. PCDAI: Pediatric Crohn's Disease Activity Index. The frequency of prednisone (pred.), budesonide (bud.), 6-mercaptopurine (6-MP), and mesalamine (5-ASA) usage is shown.
Mucosal Disease Activity and Colon Epithelial Cell (CEC) Proliferation
The mean (95th CI) week 12 CDEIS trended towards a reduction in group A, from 9 (-1,18) to 3 (1,6), compared to group B, 8 (2,13) to 6 (1,12) (Fig. 2A). However, these differences did not reach significance. Neither the CDHIS (group A: 7(5,9) vs. 6(4,8), group B: 8(6,10) vs. 8(5,10)) (Fig. 2B) nor fecal calprotectin (group A: 863(517,1209) vs. 868(523,1214), group B: 904(540,1269) vs. 656(310,1002)) differed within or between groups. The frequency of inactive mucosal disease at week 12 based upon CDEIS (≤ 3, 70% vs. 50%), CDHIS (≤ 3, 20% vs. 10%), or fecal calprotectin (≤ 250 mcg/gm, 20% vs. 20%) did not differ between the groups. We observed no differences in CEC proliferation, as measured by the Ki-67 labeling index, either within or between the groups (data not shown).
Figure 2. Mucosal Disease Activity.
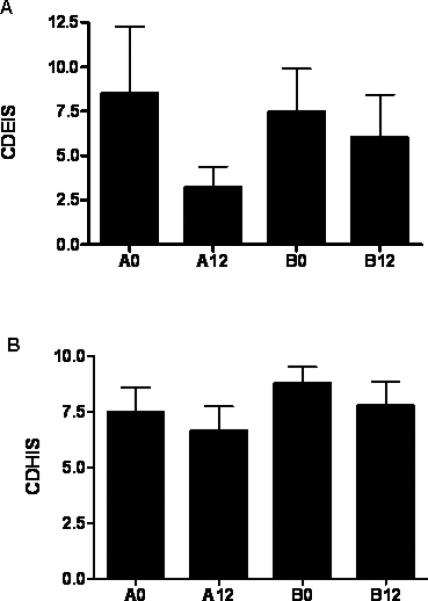
Mucosal disease activity was determined at the time points shown using the Crohn's Disease Histological Index of Severity (CDHIS) and the Crohn's Disease Endoscopic Index of Severity (CDEIS). A) The mean(SEM) CDEIS is shown. B) The mean(SEM) CDHIS is shown. n=10 in each group except for n=6 in group A and n=7 B at week 0 for the CDEIS. A0,A12: group A at weeks 0 or 12; B0,B12: group B at weeks 0 or 12
Clinical Disease Activity and Quality of Life
The mean (95thCI) week 12 PCDAI decreased in group A, from 32 (20,43) to 8 (2,14) (p=0.0004, Fig. 3A). This persisted at week 24. The week 12 PCDAI trended towards a decrease in group B, from 33 (25,40) to 22 (14,30) (p=0.1). The partial Harvey-Bradshaw (pHB) score was computed at week 4, and again at week 12. At week 4, 70% of the subjects in both groups had achieved inactive disease, as defined by a pHB score < 3. At week 12, 70% of group A subjects remained inactive with a pHB < 3, while this had decreased to 40% in group B. Consistent with this, the week 12 PCDAI was lower in group A than group B (p=0.02). ESR (erythrocyte sedimentation rate) did not vary within or between the groups (data not shown). Nine subjects in group B began GH at week 12. The week 24 PCDAI decreased in this group to 6 (1,10) (p=0.002). All subjects in group A achieved a clinical response (reduction in the PCDAI ≥ 15) at week 12, with 60% achieving clinical remission (PCDAI ≤ 10). Results were less satisfactory in Group B, who had a 50 percent response rate, with 20 percent achieving remission (p=0.02 and 0.08, respectively, Figs. 3B and 3C). An additional 78 percent of subjects in group B who received GH achieved clinical remission by week 24 (p=0.02). Quality of life (QOL) was assessed using the IMPACT questionnaire 17. The mean (95thCI) week 12 IMPACT score increased in group A, from 134 (120,148) to 143 (129,157), and in group B, from 132 (119,146) to 136 (123,150), p=0.03. However, the week 12 score did not differ between the groups, and the week 24 score did not change within group B.
Figure 3. Clinical Disease Activity.
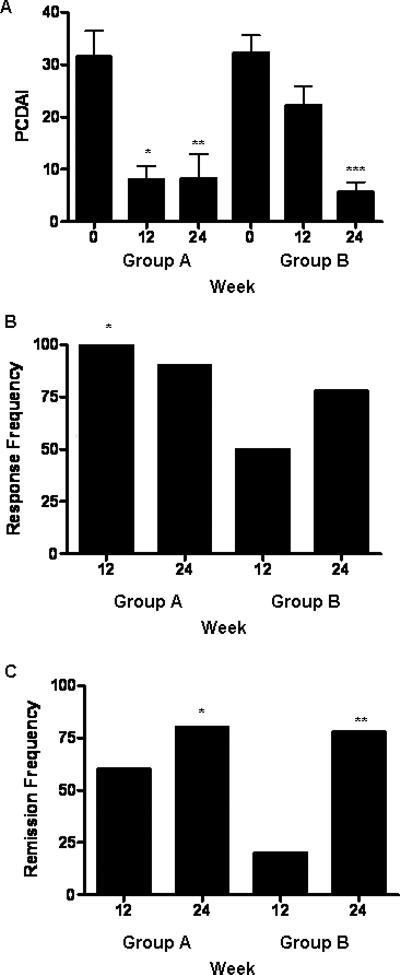
Clinical disease activity was determined at the time points shown using the Pediatric Crohn's Disease Activity Index (PCDAI). A) The mean(SEM) PCDAI is shown. *p= 0.0004 vs. group A at baseline, **p= 0.003 vs. group A at baseline, ***p= 0.002 vs. group B at week 12. The frequency of B) response (decrease in PCDAI ≥ 15) and C) remission (PCDAI ≤ 10) is shown. B) *p= 0.02 for group A at week 12 vs. group B at week 12 by Fisher's Exact test. C) *p= 0.01 for group A at week 24 and **p= 0.02 for group B at week 24 vs. group B at week 12 by Fisher's Exact test. n=10 in each group except for n=9 in groups A and B at week 24.
Corticosteroid Exposure
The frequency of subjects receiving corticosteroids in group A decreased to 30% between weeks 12-24 (p=0.001), and remained lower at 50% during the extension phase (p=0.01) (see Fig. 4A). Similarly, the frequency of subjects exposed to corticosteroids decreased to 50% in group B after receiving GH between weeks 12-24 (p=0.01). Between entry and week 12, total prednisone or budesonide exposure did not differ between groups A and B (see Figs. 4B and 4C). However, total prednisone exposure was significantly reduced in both groups during the period following the initial 12 weeks of GH administration (see Fig. 4B). At week 12, 40% of the subjects in group A had achieved a steroid-free remission, compared to 20% of the subjects in group B. Similarly, 40% of the subjects in group B achieved a steroid-free remission at week 24, although these differences did not reach significance. Collectively, these data demonstrated a steroid-sparing effect of GH administration.
Figure 4. Corticosteroid Exposure.
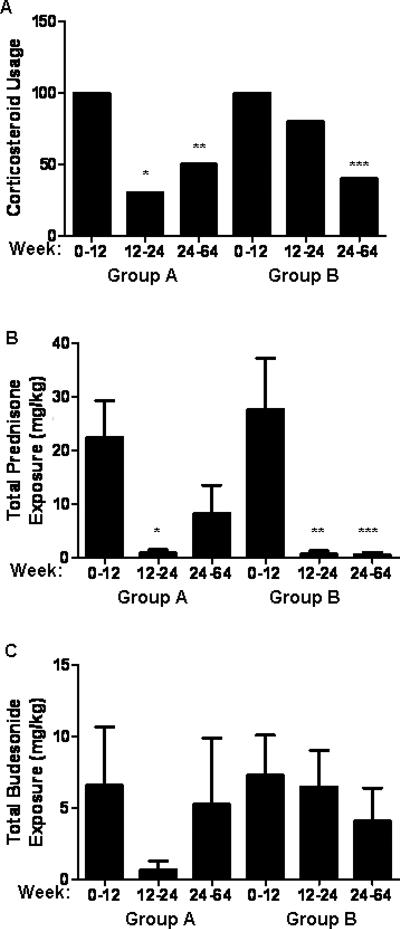
A) The frequency of corticosteroid usage during each phase of the study is shown. *p=0.001, **p=0.01 versus group A between weeks 0-12; ***p=0.01 versus group B between weeks 0-12. The total exposure in mg/kg to B) prednisone and C) budesonide was determined during each phase of the study and is shown as the mean (SEM). *p=0.03 versus group A between weeks 0-12; **p=0.01, ***p=0.01 versus group B between weeks 0-12.
Nutritional Intake and Status
At baseline, mean(SEM) caloric intake of group A was 75(9) kcal/kg/day, compared to 45(7) kcal/kg/day in group B (p=0.02). Daily protein intake was 2.7(0.4) gm/kg in group A, compared to 1.5(0.2) gm/kg in group B (p=0.007). Neither protein nor calorie intake changed in group A or B four weeks after beginning GH (data not shown). Circulating hemoglobin and albumin concentration did not differ between the groups, and did not change with the interventions (data not shown). Weight z score did not differ between the groups. However, the mean(SEM) body mass index z score (BMIz) was reduced in group A compared to group B at baseline (p=0.02). Neither WTz nor BMIz changed significantly within either group at weeks 12 or 24, although there was a trend towards an increase in group A.
Circulating Growth Factors and Linear Growth
The IGF-1 z score was inversely related to serum IL-6 concentration (r=-0.5, p=0.0002, Fig. 5A). Serum IL-6 did not change over the first 12 weeks. However, by week 24, it had decreased in both groups (Fig. 5B). GH therapy reduced week 12 circulating GH binding protein (GHBP) in group A, with a similar trend observed for group B at week 24 (Fig. 5C). The mean (SEM) serum IGF-1 z score increased in group A, from -0.4 (0.6) at baseline to 1.8 (1) at week 12 and 3.3 (1.5) at week 24 (p=0.02) (Fig. 5D). The IGF-1 z score also increased in group B after receiving GH, from -0.7 (0.3) at baseline to 3.8 (1) at week 24 (p=0.001). Similarly, serum IGFBP-3 increased at week 12 in group A, and at week 24 in group B (Fig. 5E). Bone-specific alkaline phosphatase (BSAP) increased from 50 (6) U/L at baseline to 80 (8) U/L following 12 weeks of GH therapy, and did not change in group B prior to GH (p=0.0006). Mean (SEM) height velocity (HTV) increased from 5 (1) cm/yr at baseline to 8 (1) cm/yr at week 12 (p=0.01) and 9 (1) cm/yr at week 24 (p=0.0007) in group A (n=9). HTV did not change in Group B prior to receiving GH. Therefore, the week 12 HTV was significantly higher in group A than group B (p=0.001). HTV in group B increased from 3 (1) cm/yr at week 12 to 7(1) cm/yr at week 24 (p=0.02) (n=8). Similar results were observed for HTV z scores, which exceeded normal for age on GH treatment (Fig. 5F).
Figure 5. Circulating Growth Factors, IL-6, and Linear Growth Velocity.
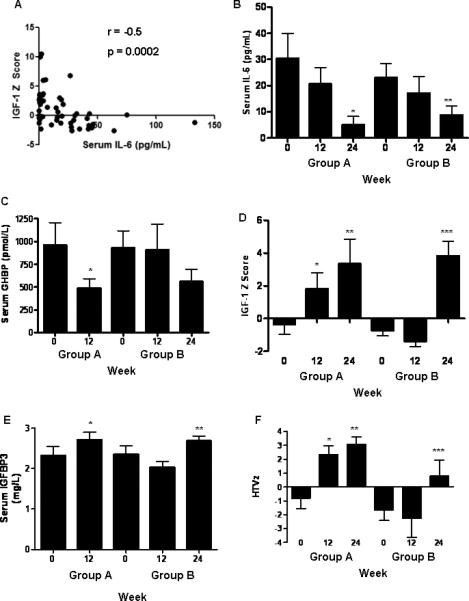
Serum IL-6, GHBP, IGF-1, and IGFBP3 were determined by ELISA at the times shown and are given as the mean (SEM). A) The Spearman correlation was determined for the association between serum IL-6 and IGF-1 z score as shown. B) *p= 0.03 vs. group A at baseline, **p= 0.03 vs. group B at baseline. C) *p= 0.05 vs. group A at baseline. D) *p= 0.05 vs. group A at baseline, **p= 0.02 vs. group A at baseline, ***p= 0.001 vs. group B at week 12. E) *p= 0.03 vs. group A at baseline, **p= 0.003 vs. group B at week 12. F) height velocity (HTV) z scores were determined at the times shown and are given as the mean(SEM). *p= 0.02 vs. group A at baseline, **p= 0.002 vs. group A at baseline, ***p= 0.004 vs. group B at week 12. n=10 in each group except for n=9 in groups A and B at week 24.
Extension Phase
Eighteen subjects who showed a clinical response to GH defined as remission or mild disease activity (PCDAI < 30) after the first 12 weeks of GH administration continued to receive the drug during the 52 week extension phase. As shown in Table II, the subjects from Groups A and B who entered the extension phase did not differ based upon age, gender, pubertal stage, clinical or mucosal disease activity, or medication exposures. The median Tanner stage was equal to II in both groups. Overall, 72% were in clinical remission, and 61% were no longer receiving corticosteroids. During this phase, the GH dose was adjusted to maintain serum IGF-1 within +2 SD of the normal for age. The mean (range) GH dose was 0.05 (0.01-0.1) mg/kg/day, with a mean (SEM) serum IGF-1 z score of 1.8 (0.3). Seven subjects withdrew because of worsening disease activity, and three withdrew while in clinical remission in order to stop daily GH injections. Eight subjects remained in remission on GH through week 64. Fecal calprotectin did not vary within the group between entry and week 64.
The mean (SEM) bone age increased from 11.6 (1.1) years to 12.4 (1.1) years by week 48, p=0.02. Mean (range) Tanner stage increased from II(I,V) to III(I,V), p=0.003. HTV increased to 7(0.6) cm/yr, compared to 4(0.6) cm/yr at entry (p=0.0008). Mixed effect model analysis was performed to test for a differential longitudinal trajectory for HTVz or HTz betweens groups A and B. HTVz and HTz increased to the same degree in both groups. Overall HTVz score increased to 2.4 (0.8), compared to -1.3(0.8) prior to beginning GH (p=0.0002, Fig. 6A). HTz increased from -1.1(0.2) at baseline to -0.4(0.3), p=0.004 (Fig. 6B). Within the group, HTVz trended towards an increase in subjects who remained in clinical remission through-out follow-up (3(1), n=11), compared to those who experienced a flare (0.8(0.7), n=7, p=0.08). HTVz also trended towards an increase in subjects who remained steroid-free through-out follow-up (3(1), n=9), compared to those who received prednisone or budesonide (1,3(0.7), n=9, p=0.2). There was no difference in HTVz between subjects relative to fecal calprotectin at entry (data not shown).
Figure 6. Linear Growth During the Extension Phase.
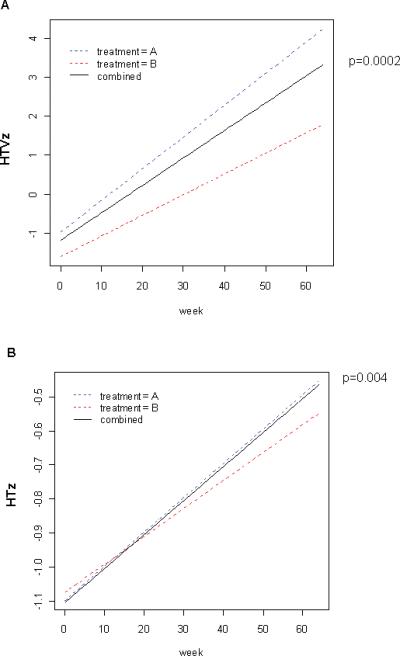
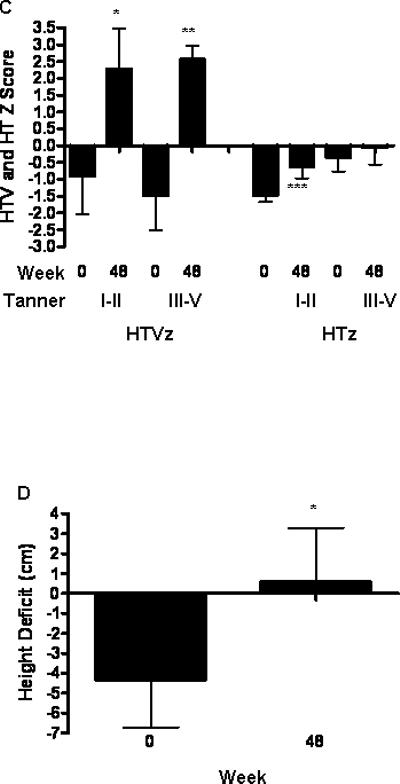
Mixed effect model analysis was performed with time in weeks as a continuous variable to test for a differential longitudinal trajectory for A) HTVz or B) HTz. The baseline HTVz was computed relative to a height measurement obtained at least six months prior to enrollment. The extension phase HTVz was computed relative to the HTVz obtained at the study visit prior to beginning GH. The extension phase HTz was computed at baseline and at the most recent study visit. C) Mean (SEM) HTVz and HTz at baseline and follow-up during the extension phase are shown for subjects stratified by Tanner Stage at entry (I-II vs III-V). *p=0.03 vs. baseline, **p=0.01 vs. baseline, ***p=0.04 vs. baseline. D) Height deficit was estimated for each subject at baseline and week 48 as the difference between the predicated adult height and the mid-parental height, and is shown as the mean(SEM). *p= 0.01 vs. baseline.
As shown in Fig. 6C, the increase in HTVz during the extension phase did not vary by Tanner Stage at entry (I-II, n=11, vs. III-V, n=9). However, only patients who were Tanner Stage I-II at entry achieved a statistically significant increase in HTz by week 48. We estimated the relative height deficit for each subject by determining the difference between predicted adult height (PAH) derived from the mid-parental height and that derived from the bone age determination at baseline 18 19. The value for this parameter at baseline and week 48 is shown in Fig. 6D, for those subjects who were continued on GH treatment through week 48 (n=11). The mean (SEM) relative height deficit at week 48 was equal to +0.6 (2.7) cm, compared to -4.3 (2.3) cm at baseline (p=0.01). At baseline, 8 out of 11 of subjects had a PAH less than mid-parental height. By week 48, only 1 out of 11 of subjects had a PAH less than mid-parental height (p=0.003). These data suggested that the majority of subjects achieved an increase in linear growth consistent with their genetic potential.
Safety Endpoints
Fasting glucose did not change in either group. Fasting insulin did not change between baseline and week 12. However, mean (SEM) fasting insulin did increase during the extension phase, from 9.7 (1.8) microU/mL to 21 (4) microU/mL, p=0.03. No subjects experienced severe headache, hypertension, or peripheral edema. Mild arthralgia was reported by six subjects in group A compared to one subject in group B (p=0.06 by Fisher's Exact Test). This did not require dose reduction. Two subjects reported discomfort at injection sites; one each withdrew at weeks 16, 36, and 60 despite clinical remission in order to discontinue injections. Neither the frequency nor severity of other adverse events differed between groups A and B.
DISCUSSION
We found that the addition of GH to CTX therapy for active pediatric CD led to an improvement in clinical disease activity, compared to CTX alone. Importantly, we observed a significant steroid-sparing effect of GH administration. These observations are in agreement with the randomized placebo controlled trial of GH previously performed in adults with CD9. The week 12 rate of clinical remission in the group treated with CTX alone was lower than would be anticipated based upon prior reports 20. This may have been because this was a relatively refractory population which had flared while receiving immunomodulator therapy, and so was more likely to experience symptoms during corticosteroid tapering.
Previous GH studies did not evaluate quality of life. A recent randomized trial of infliximab in pediatric CD demonstrated a mean improvement in the total IMPACT score of 24, as compared to a mean increase of 9 with GH in the current study 21. The majority of patients who achieved an initial response with GH maintained this over the following year; the 61% rate of sustained response was comparable to that reported in the pediatric infliximab study. We did not observe baseline differences in clinical or demographic parameters between patients who did or did not relapse during the extension phase; this will be important to examine in a future larger study.
Prior studies of GH in CD have not evaluated mucosal healing. We did not observe a difference in mucosal healing between patients treated with combined GH/CTX and those treated with CTX alone. However, we may have been underpowered to detect this difference, as we did observe a numerical trend towards reduced endoscopic severity in the group which received GH. Based upon our results, at least 25 subjects in each group would have been required to test the difference observed. By comparison, induction therapy with exclusive enteral nutrition or infliximab has been shown to induce mucosal healing. Patients with prior CTX exposure have a lower rate of mucosal healing with subsequent infliximab, suggesting that CTX may inhibit mucosal healing in response to other agents 22. A larger study with GH mono-therapy would be required to test this.
Genetic variation in IL-6 production may contribute to linear growth failure in CD 23. We found that serum IL-6 was reduced by week 24 on GH. We observed an increase in linear growth quite similar to prior studies of GH in pediatric CD 10 11. The increase in linear growth velocity was sustained through a median (IQ range) of 60(30,64) weeks of follow-up, with a mean increase in height SDS of 0.7. The increase in HTV and HT SDS (+0.5) reported in the recent pediatric infliximab trial were quite similar {Walters, 2007 #1079}. We observed a trend towards a reduction in HTVz in patients with active disease, and in those receiving corticosteroids, during the extension phase. We did not observe a difference in HTVz in association with differences in mucosal inflammation as measured by fecal calprotectin. However, we were not powered to test for these potential modifiers of the effect of GH upon linear growth. Taken together, these data confirm that GH therapy will promote catch-up growth in pediatric CD.
The most frequent adverse event was mild injection site discomfort. Three subjects discontinued GH for this reason. Consistent with prior reports, we observed no differences in fasting glucose with GH. However, fasting insulin did increase during the extension phase. Intracranial hypertension, peripheral edema, and arthralgias have been reported in association with GH therapy. However, we observed no difference in these between the groups. In summary, we found that GH therapy was well tolerated in the setting of moderately active pediatric CD.
Our study had several strengths, but also some limitations. We did not provide a daily placebo injection to the subjects randomized to group B. It is possible that a placebo effect of the daily GH injection may have contributed to the improvement in clinical disease activity. However, the sixty-five percent remission rate observed was higher than would be anticipated with a placebo effect in CD, and the use of injectable medications would be expected to yield a lower placebo remission rate 24. Moreover, the sustained improvements in disease activity and linear growth in a majority of subjects suggest that there was likely a biologic effect of the GH medication. The majority of the subjects who enrolled were male, and had moderately active disease. Therefore, our results may not be generalizable to females, or to patients with more severe disease activity.
First-line therapy for pediatric CD in most cases includes induction of remission with CTX or enteral nutrition followed by maintenance of remission with 6-MP/AZA or mesalamine 2 7. With this approach, a sustained steroid-free remission with normalization of growth is often not achieved 1 25. We found that GH therapy yielded improvements in clinical disease activity, steroid exposure, and linear growth. A larger randomized trial, potentially with GH mono-therapy, will be required to determine its ability to promote endoscopic healing, maintain suppression of disease activity, and increase final adult height.
ACKNOWLEDGEMENTS
This work was supported by Genentech, Inc., the Children's Hospital Research Foundation (CHRF) Translational Research Institute, the National Institutes of Health (NIH)-supported CHRF Digestive Health Center Integrative Morphology, Microarray, and Bioinformatics Cores (1P30DK078392-01), United States Public Health Service grant M01RR008084, General Clinical Research Centers Program, National Center for Research Resources, NIH, and NIH grant R01 DK058259 (LD). We thank Dr. Barbara Lippe (Genentech, Inc.) for critical reading of the manuscript, Jeanie Bailey and Rubina Dosani for coordinating regulatory aspects of the studies, and Anne Ryan for technical assistance.
Grant Support & Financial Disclosure: The study was supported by a grant from Genentech, Inc. for Investigator-Initiated research projects, the Cincinnati Children's Hospital Research Foundation Translational Research Institute, the National Institutes of Health (NIH)-supported Cincinnati Children's Hospital Research Foundation Digestive Health Center (1P30DK078392-01), United States Public Health Service grant M01RR008084, General Clinical Research Centers Program, National Center for Research Resources, NIH, and NIH grant R01 DK058259 (LD).
Abbreviations
- AZA
azathioprine
- BSAP
bone specific alkaline phosphatase
- CCHMC
Cincinnati Children's Hospital Medical Center
- CD
Crohn's Disease
- CDEIS
CD endoscopic index of severity
- CDHIS
CD histologic index of severity
- CTX
corticosteroids
- GCRC
General Clinical Research Center
- GH
growth hormone
- GHBP
growth hormone binding protein
- GHR
growth hormone receptor
- HT
height
- HTV
height velocity
- IBD
Inflammatory Bowel Disease
- IGF-I
insulin-like growth factor 1
- IGFBP-3
insulin-like growth factor binding protein 3
- IL
interleukin
- MTX
methotrexate
- pHB
partial Harvey-Bradshaw
- PCDAI
Pediatric Crohn's Disease Activity Index
- rhGH
recombinant human GH
- 6-MP
6-mercaptopurine
- STAT
signals transducers and activators of transcription
- SOCS
suppressors of cytokine signaling
- UC
Ulcerative Colitis
Footnotes
Financial disclosures: Lee Denson, MD and David Klein, MD received grant support and study drug from Genentech, Inc. to perform the clinical trial via the Investigator-Initiated research program. Otherwise, the authors have no financial arrangement(s) with a company whose product figures prominently in the submitted manuscript or with a company making a competing product.
Writing assistance: not applicable
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sawczenko A, Ballinger AB, Croft NM, Sanderson IR, Savage MO. Adult height in patients with early onset of Crohn's disease. Gut. 2003;52(3):454–5. doi: 10.1136/gut.52.3.454. author reply 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borrelli O, Cordischi L, Cirulli M, Paganelli M, Labalestra V, Uccini S, et al. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn's disease: a randomized controlled open-label trial. Clin Gastroenterol Hepatol. 2006;4(6):744–53. doi: 10.1016/j.cgh.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 3.D'Haens G, Geboes K, Rutgeerts P. Endoscopic and histologic healing of Crohn's (ileo-) colitis with azathioprine. Gastrointest Endosc. 1999;50(5):667–71. doi: 10.1016/s0016-5107(99)80017-0. [DOI] [PubMed] [Google Scholar]
- 4.D'Haens G, Van Deventer S, Van Hogezand R, Chalmers D, Kothe C, Baert F, et al. Endoscopic and histological healing with infliximab anti-tumor necrosis factor antibodies in Crohn's disease: A European multicenter trial. Gastroenterology. 1999;116(5):1029–34. doi: 10.1016/s0016-5085(99)70005-3. [DOI] [PubMed] [Google Scholar]
- 5.Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn's disease: a prospective multicentre study. Groupe d'Etudes Therapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut. 1989;30(7):983–9. doi: 10.1136/gut.30.7.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Modigliani R, Mary JY, Simon JF, Cortot A, Soule JC, Gendre JP, et al. Clinical, biological, and endoscopic picture of attacks of Crohn's disease. Evolution on prednisolone. Groupe d'Etude Therapeutique des Affections Inflammatoires Digestives. Gastroenterology. 1990;98(4):811–8. doi: 10.1016/0016-5085(90)90002-i. [DOI] [PubMed] [Google Scholar]
- 7.Markowitz J, Grancher K, Kohn N, Lesser M, Daum F. A multicenter trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn's disease. Gastroenterology. 2000;119(4):895–902. doi: 10.1053/gast.2000.18144. [DOI] [PubMed] [Google Scholar]
- 8.Walters TD, Gilman AR, Griffiths AM. Linear growth improves during infliximab therapy in children with chronically active severe Crohn's disease. Inflamm Bowel Dis. 2007;13(4):424–30. doi: 10.1002/ibd.20069. [DOI] [PubMed] [Google Scholar]
- 9.Slonim AE, Bulone L, Damore MB, Goldberg T, Wingertzahn MA, McKinley MJ. A preliminary study of growth hormone therapy for Crohn's disease. N Engl J Med. 2000;342(22):1633–7. doi: 10.1056/NEJM200006013422203. [DOI] [PubMed] [Google Scholar]
- 10.Mauras N, George D, Evans J, Milov D, Abrams S, Rini A, et al. Growth hormone has anabolic effects in glucocorticosteroid-dependent children with inflammatory bowel disease: a pilot study. Metabolism. 2002;51(1):127–35. doi: 10.1053/meta.2002.28972. [DOI] [PubMed] [Google Scholar]
- 11.Heyman MB, Garnett EA, Wojcicki J, Gupta N, Davis C, Cohen SA, et al. Growth hormone treatment for growth failure in pediatric patients with Crohn's disease. J Pediatr. 2008;153(5):651–8. 658, e1–3. doi: 10.1016/j.jpeds.2008.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calenda KA, Schornagel IL, Sadeghi-Nejad A, Grand RJ. Effect of recombinant growth hormone treatment on children with Crohn's disease and short stature: a pilot study. Inflamm Bowel Dis. 2005;11(5):435–41. doi: 10.1097/01.mib.0000159321.58773.a6. [DOI] [PubMed] [Google Scholar]
- 13.Borrelli O, Bascietto C, Viola F, Bueno de Mesquita M, Barbato M, Mancini V, et al. Infliximab heals intestinal inflammatory lesions and restores growth in children with Crohn's disease. Dig Liver Dis. 2004;36(5):342–7. doi: 10.1016/j.dld.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 14.D'Haens GR, Geboes K, Peeters M, Baert F, Penninckx F, Rutgeerts P. Early lesions of recurrent Crohn's disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology. 1998;114(2):262–7. doi: 10.1016/s0016-5085(98)70476-7. [DOI] [PubMed] [Google Scholar]
- 15.Han X, Sosnowska D, Bonkowski E, Denson L. Growth hormone reduces STAT3 activation and improves disease activity in murine colitis. Gastroenterology. 2005;129:185–203. doi: 10.1053/j.gastro.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Hyams JS, Ferry GD, Mandel FS, Gryboski JD, Kibort PM, Kirschner BS, et al. Development and validation of a pediatric Crohn's disease activity index. J Pediatr Gastroenterol Nutr. 1991;12(4):439–47. [PubMed] [Google Scholar]
- 17.Otley A, Smith C, Nicholas D, Munk M, Avolio J, Sherman PM, et al. The IMPACT questionnaire: a valid measure of health-related quality of life in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2002;35(4):557–63. doi: 10.1097/00005176-200210000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: revised for use with the Greulich-Pyle hand standards. J Pediatr. 1952;40(4):423–41. doi: 10.1016/s0022-3476(52)80205-7. [DOI] [PubMed] [Google Scholar]
- 19.Post EM, Richman RA. A condensed table for predicting adult stature. J Pediatr. 1981;98(3):440–2. doi: 10.1016/s0022-3476(81)80718-4. [DOI] [PubMed] [Google Scholar]
- 20.Tung J, Loftus EV, Jr., Freese DK, El-Youssef M, Zinsmeister AR, Melton LJ, 3rd, et al. A population-based study of the frequency of corticosteroid resistance and dependence in pediatric patients with Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2006;12(12):1093–100. doi: 10.1097/01.mib.0000235835.32176.85. [DOI] [PubMed] [Google Scholar]
- 21.Hyams J, Crandall W, Kugathasan S, Griffiths A, Olson A, Johanns J, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology. 2007;132(3):863–73. doi: 10.1053/j.gastro.2006.12.003. quiz 1165-6. [DOI] [PubMed] [Google Scholar]
- 22.D'Haens G, Baert F, van Assche G, Caenepeel P, Vergauwe P, Tuynman H, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet. 2008;371(9613):660–7. doi: 10.1016/S0140-6736(08)60304-9. [DOI] [PubMed] [Google Scholar]
- 23.Sawczenko A, Azooz O, Paraszczuk J, Idestrom M, Croft NM, Savage MO, et al. Intestinal inflammation-induced growth retardation acts through IL-6 in rats and depends on the -174 IL-6 G/C polymorphism in children. Proc Natl Acad Sci U S A. 2005;102(37):13260–5. doi: 10.1073/pnas.0503589102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su C, Lichtenstein GR, Krok K, Brensinger CM, Lewis JD. A meta-analysis of the placebo rates of remission and response in clinical trials of active Crohn's disease. Gastroenterology. 2004;126(5):1257–69. doi: 10.1053/j.gastro.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 25.Iofel E, Grancher K, Markowitz J, Levine J. Permanent Growth Failure (GF) in Pediatric Crohn's Disease (CD). J Pediatric Gastroenterology, Hepatology, & Nutrition. 2003;37(3):372, A138. [Google Scholar]


