Abstract
The availability of HBIG and several oral antiviral therapies have reduced but not eliminated HBV recurrence. We aimed to determine the rate of HBV recurrence after orthotopic liver transplantation (OLT) in relation to virologic breakthrough pre-OLT and HBIG regimens post-OLT. Data from the NIH HBV-OLT database were analyzed. A total of 183 patients transplanted between 2001 and 2007 followed for a median of 42 months (range 1–81) post-OLT were studied. At transplant, 29% were HBeAg (+), 38.5% had HBV DNA >5 log10 copies/mL, 74% were receiving antiviral therapy. Twenty-four patients experienced virologic breakthrough before OLT. Post-OLT, 26%, 22%, 40% and 12% of patients received IV high-dose, IV low-dose, IM low-dose, and a finite duration of HBIG, respectively as maintenance prophylaxis. All but two patients also received antiviral therapy. Cumulative rates of HBV recurrence at 1 and 5 years were 3% and 9%, respectively. Multivariate analysis showed that listing HBeAg status and HBV DNA level at OLT were the only factors associated with HBV recurrence.
Conclusion
Low rates of HBV recurrence can be accomplished with all the HBIG regimens used when combined with antiviral therapy including patients with breakthrough pre-OLT as long as rescue therapy is administered pre- and post- OLT.
Keywords: aAdefovir, antiviral resistance, HBV DNA, hepatitis B e antigen, lamivudine
Introduction
The introduction of high-dose intravenous (IV) hepatitis B immune globulin (HBIG) monotherapy reduced the 3-year hepatitis B virus (HBV) recurrence rate after orthotopic liver transplantation (OLT) from 80% to 36% (1). However, HBIG monotherapy has limited efficacy in patients with high levels of HBV replication pre-transplant, is very expensive, and may select for immune escape mutants (2–4). In the study by Samuel et al. the overall 3-year HBV recurrence rate among hepatitis B e antigen (HBeAg) and HBV DNA positive patients transplanted for cirrhosis was 83% (1). McGory et al. (5) and Dickson et al. (6) observed that the half-life of HBIG was shorter in patients who were HBeAg positive or had detectable serum HBV DNA at the time of transplantation highlighting the importance of suppressing HBV replication prior to OLT.
Addition of oral antiviral agents to HBIG has resulted in a further decrease in HBV recurrence rates to <10% (7–9). Long-term administration of nucleos(t)ide analogs is associated with an increasing risk of antiviral drug resistance prior to OLT (10–13). However, several studies have reported that patients with antiviral resistance prior to OLT can be safely transplanted provided that rescue therapy is administered (14–16).
With the availability of multiple nucleos(t)ide analogs, an important question is how much HBIG is needed to prevent HBV recurrence, when used in combination with antiviral therapy. Many investigators have explored low-dose HBIG regimens or HBIG withdrawal (17–20) but the duration of post-transplant follow-up was short (1–3 years) in many studies and rescue therapy for patients with lamivudine-resistant HBV was not uniformly available in studies conducted prior to 2003.
The National Institutes of Health study on “Prevention of HBV Recurrence after Liver Transplantation” (NIH HBV-OLT Study) is a retrospective-prospective observational study involving 15 liver centers in the United States. The study provided guidelines on the management of patients before and after OLT but each center was allowed to follow its own HBIG protocol. The aims of this study were (i) to determine the HBV recurrence rates in an era when antiviral therapy is used in combination with HBIG and rescue therapy is available for patients with lamivudine resistance, (ii) to analyze the factors associated with HBV recurrence post-OLT, and (iii) to describe the HBIG regimens used in U.S. liver transplant centers and the impact of different HBIG regimens on HBV recurrence post-OLT.
Materials and Methods
Patient Population
The NIH HBV-OLT study enrolled hepatitis B surface antigen (HBsAg) positive patients who were 13 years of age or older from 15 centers in the United States (21). The study was approved by the Institutional Review Board representing each of the participating centers, and written informed consent was obtained from all patients prior to study entry.
A total of 317 patients were enrolled. Patients were listed between 1993 and 2005; 18 were listed prior to approval of lamivudine (December-1998) and 151 were listed after approval of adefovir (September-2002). For this analysis, patients still on the transplant waiting list (n = 109), those listed for re-transplantation (n = 17), and those with less than 1 month of post transplant follow-up (n = 4) were excluded. Demographics, clinical, laboratory (blood counts, creatinine, liver panel, prothrombin time/international normalized ratio [INR], alpha-fetoprotein [AFP], hepatitis B serology and HBV DNA), and radiologic data, as well as start and stop dates of antiviral therapy and HBIG dosing, were recorded. Data were collected at enrollment, transplant listing, and time of transplant, every 6 months while on the transplant waiting list, every 3 months during the first year post-transplant, and every 6 months after the first post-transplant year. Data up to the time of study closure on November 30, 2007 were analyzed.
At each visit, an extra tube of blood was collected for testing at a central laboratory at the University of Michigan.
HBV DNA Assay
All laboratory tests except for HBV DNA, antiviral- and HBIG- resistance mutations were performed at the participating centers. Serum HBV DNA levels were quantified by the Cobas Amplicor HBV Monitor assay (Roche Molecular Systems, Inc., Branchburg, NJ). The lower limit of detection of this assay is 200 copies/mL. Samples with values >100,000 copies/mL were diluted and retested. For patients with missing central laboratory samples, HBV DNA results at the participating centers were used.
HBIG and HBV Antiviral Resistance Mutation Testing
All pre-transplant samples from patients with detectable HBV DNA after ≥6 months of antiviral therapy were tested for antiviral drug-resistance mutations and all post-transplant samples with detectable HBV DNA were tested for both antiviral drug-resistance and HBIG escape mutations by direct sequencing of the HBV polymerase gene which overlaps with the surface gene (22). Antiviral drug-resistance mutations were also tested by a line probe assay (INNO-LiPA DRv2 and DRv3 [Innogenetics, Ghent, Belgium]), which can detect mutations known to be associated with resistance to lamivudine, adefovir or entecavir (23,24).
Definitions and HBIG regimens
Virologic breakthrough during antiviral therapy was defined as ≥1 log increase in serum HBV DNA from nadir or redetection of HBV DNA in serum after its initial disappearance. Genotypic resistance was defined as detection of amino acid substitutions in the HBV polymerase gene or the HBV surface protein that had been documented to be associated with resistance to HBV nucleos(t)ide analogues and HBIG, respectively. Recurrent HBV was defined as the reappearance of HBsAg in serum after the first month post-transplant. Patients were classified into four groups according to the dose and duration of HBIG received: 1) high dose IV HBIG: 10,000 IU during the anhepatic phase, daily for the next 6 days, and monthly thereafter; 2) low dose IV HBIG: 3,000 – 6,000 IU monthly or 10,000 IU every 2–6 months; 3) intramuscular (IM) HBIG: 1,000 – 1500 IU every 1–2 months; and 4) finite duration of HBIG: HBIG discontinued after a varying period.
Statistical analyses
Categorical data were presented as number and percent and compared using chi-square test or Fisher’s exact test as appropriate. Continuous variables were expressed as mean and standard deviation (SD) unless specified otherwise, and were compared using t-test or Mann-Whitney U-test. Serum HBV DNA level was expressed as copies/mL and logarithmically transformed. Continuous variables were dichotomized taking the median as cutoff value, except for serum HBV DNA, where the cutoff used was 5 log10 copies/mL. Univariate analyses of factors associated with HBV recurrence post-transplant were performed using Kaplan-Meier analysis with log rank test. For this purpose patient demographics, OLT indication at the time of transplant, use of antiviral therapy, occurrence of virologic breakthrough and/or presence of genotypic resistance prior to transplant, transplant center, type of HBIG regimen used, HBeAg status and HBV DNA level at listing and at transplant, and OLT date (before or after adefovir approval) were analyzed. Variables that had a p value of <0.2 on univariate analysis were entered into a Cox regression proportional hazards model. Forward and backward logistic regression was performed to determine the independent predictors of HBV recurrence. All statistical analyses were performed using SPSS v. 14.0.8 statistical software (SPSS, Inc., Chicago, IL).
Results
Characteristics of patients
A total of 187 patients transplanted between March 2001 and September 2007 were included in this analysis. Table 1 summarizes the characteristics of these patients at the time of listing and at transplantation. The vast majority (75.4%) of the patients were men and their mean age was 52.4 years. Asians comprised 42.8% of the patient population, Caucasians 41.7%, and African Americans 10.2%. Coinfection with hepatitis C virus (HCV) was diagnosed in 6.1% (10/164) while coinfection with hepatitis D virus (HDV) was diagnosed in 7.5% (4/53) of patients tested. At listing, 30.9% of the patients were HBeAg positive, 48.2% had detectable serum HBV DNA and 30.4% had HBV DNA >5 log10 copies/mL. The median interval between listing and transplant was 3.0 months (range 0.03–97.5). At transplantation, 28.6% of the patients were HBeAg positive, 64.2% had detectable serum HBV DNA and 37.5% had HBV DNA >5 log10 copies/mL.
Table 1.
Characteristics of Ppatients at Llisting and at the Ttime of Ttransplant
| End-stage Cirrhosis | HCC1* | Acute Liver Failure | All | P value | |
|---|---|---|---|---|---|
| No of patients | 73 (39.3) | 97 (51.8) | 17 (8.9) | 187 (100) | |
| Gender, male | 58 (79.5) | 75 (77.3) | 8 (47.1) | 141 (75.4) | 0.013 |
| Age, years | 50.6 ± 8.1 | 55.5 ± 9.6 | 43.0 ± 14.2 | 52.4 ± 10.2 | 0.001 |
| Race | 0.005 | ||||
| Caucasian | 33 (45.2) | 36 (37.1) | 9 (53.0) | 78 (41.7) | |
| Asian | 23 (31.5) | 53 (54.6) | 4 (23.5) | 80 (42.8) | |
| African American | 10 (13.7) | 5 (5.2) | 4 (23.5) | 19 (10.2) | |
| Other | 7 (9.6) | 3 (3.1) | 0(0) | 10 (5.3) | |
| Labs at listing | |||||
| HBeAg (+) | 20/67 (29.8) | 25/84 (29.7) | 6/14 (42.9) | 51/165 (30.9) | 0.600 |
| HBV DNA detectable | 31/66 (47.0) | 42/85 (49.4) | 8/17 (47.1) | 81/168 (48.2) | 0.952 |
| HBV DNA >5 log10 copies/mL | 16/66 (24.2) | 31/85 (36.5) | 4/17 (23.5) | 51/168 (30.4) | 0.218 |
| CTP Score | 9.7 ± 2.2 | 7.7 ± 2.5 | 12.0 ± 1.7 | 9.1 ± 2.6 | <0.001 |
| Lab MELD | 19.7 ± 8.7 | 13.1 ± 7.1 | 30.4 ± 5.9 | 18.4 ± 9.1 | <0.001 |
| Anti-HCV (+) | 5/66 (7.6) | 5/81 (6.2) | 0/17 (0) | 10/164 (6.1) | 0.507 |
| Anti-HDV (+) | 3/25 (12.0) | 1/23 (4.3) | 0/5 (0) | 4/53 (7.5) | 0.483 |
| Time on the waiting list, months | 5 (0.1–84.0) | 3 (0.1–97.5) | 0.06 (0.03–0.27) | 3.0 (0.03–97.5) | 0.034 |
| Labs at transplantation | |||||
| HBeAg (+) | 18/63 (28.6) | 21/79 (26.6) | 5/12 (41.7) | 44/154 (28.6) | 0.559 |
| HBV DNA detectable | 48/68 (70.6) | 45/80 (56.3) | 13/17 (76.5) | 106/165 (64.2) | 0.104 |
| HBV-DNA >5 log10 copies/mL | 22/68 (32.3) | 33/80 (41.3) | 7/17 (41.2) | 62/165 (37.5) | 0..077 |
| HBV DNA >3 log10 copies/mL | 35/68 (51.5) | 39/80 (48.7) | 12/17 (70.5) | 86/165 (52.1) | 0.259 |
| HBV-DNA, log10 copies/mL | 4.0 ± 2.2 | 4.1 ± 2.2 | 4.6 ± 2.1 | 4.1 ± 2.2 | 0.651 |
| Albumin, g/dL | 2.6 ± 0.6 | 3.2 ± 0.7 | 2.7 ± 0.6 | 2.9 ± 0.7 | <0.001 |
| AST, U/L | 337 ± 862 | 154 ± 322 | 1212 ± 2133 | 323 ± 909 | <0.001 |
| ALT, U/L | 258 ± 675 | 108 ± 203 | 1261 ± 2158 | 273 ± 841 | <0.001 |
| Bilirubin, mg/dL | 10.4 ± 12.4 | 2.9 ± 5.4 | 20.7 ± 9.6 | 7.5 ± 10.7 | <0.001 |
| Alkaline phosphatase, U/L | 146 ± 80.5 | 155 ± 111 | 137 ± 56.3 | 150 ± 96.0 | 0.716 |
| INR | 2.1 ± 1.5 | 1.3 ± 0.5 | 3.0 ± 1.7 | 1.8 ± 1.2 | <0.001 |
| CTP Score | 11.8 ± 3.1 | 7.7 ± 2.5 | 13 ± 1.4 | 10.1 ± 3.4 | 0.001 |
| Lab MELD | 24.5 ± 10.1 | 15.4 ± 8.1 | 32.1 ± 5.8 | 24.0 ± 9.5 | <0.001 |
| AFP, ng/mL | 39.7 ± 215 | 453 ± 1538 | 68 ± 98 | 286 ± 1206 | <0.001 |
| Antiviral treatment at transplantation | 56 (76.7) | 75 (77.3) | 7 (41.2) | 138 (73.8) | 0.006 |
| Duration of antiviral treatment prior to OLT, months | 19.8 ± 21.0 | 23.3 ± 21.8 | 0.1 ± 0.3 | 20.7 ± 21.4 | 0.021 |
| Types of treatment | 0.072 | ||||
| LAM | 39 | 48 | 7 | 94 | |
| ADV | 3 | 12 | 0 | 15 | |
| ETV | 0 | 4 | 0 | 4 | |
| TDF | 4 | 0 | 0 | 4 | |
| LAM+ADV | 10 | 6 | 0 | 16 | |
| LAM+TDF | 0 | 2 | 0 | 2 | |
| ETV+ADV | 0 | 2 | 0 | 2 | |
| TDF+ADV | 0 | 1 | 0 | 1 | |
| Virologic breakthrough prior to OLT | 12/56(21.4) | 13/75(17.3) | 0/7(0) | 25/138(18.1) | 0.200 |
| LAM | 11 | 11 | 0 | 22 | |
| ADV | 0 | 1 | 0 | 1 | |
| LAM+ADV | 1 | 1 | 0 | 2 |
Results expressed as number (%) or mean ± SD unless specified otherwise
LAM: Lamivudine, ADV: Adefovir, TDF: Tenofovir, ETV: entecavir
HCC = Hepatocellular carcinoma;, CTP: = Child Turcotte Pugh; MELD = Model for Endstage Liver Disease
AST: aspartate aminotransferase, ALT: alanine aminotransferase
Patients found to have HCC while on the waiting list or at transplant were included in the HCC column
At listing, 98 (52.5%) patients had end-stage cirrhosis, 72 (38.6%) had HCC and 17 (8.9%) had acute liver failure. Twenty-five (25.5%) patients with end-stage cirrhosis were diagnosed to have HCC while on the transplant waiting list or on the explant liver. Thus, a total of 97 (51.8%) patients had HCC while 73 (39.3%) had end-stage cirrhosis with no HCC at the time of transplant (Table 1).
Antiviral therapy and virologic breakthrough prior to transplant
One-hundred and thirty-eight (73.8%) patients were receiving antiviral therapy at the time of transplantation: 117 were receiving nucleos(t)ide monotherapy (including 94 patients on lamivudine monotherapy) and 21 were receiving combination therapy (Table 1). The mean duration of antiviral therapy prior to OLT was 20.7 ± 21.4 months (maximum 94).
Twenty-five (18.1%) patients experienced virologic breakthrough prior to transplantation. Genotypic resistance was confirmed in 14 (58.3%) of 24 patients in whom serum samples prior to initiation of rescue therapy were available for testing. Of the 25 patients with virologic breakthrough, 24 received lamivudine monotherapy and one received adefovir monotherapy as their initial antiviral therapy and breakthrough was diagnosed after a mean of 34.4 ± 18.5 months. Twenty patients received rescue therapy with adefovir (n = 4), lamivudine plus adefovir (n = 12, including 1 patient who had breakthrough while receiving adefovir monotherapy), tenofovir (n= 2), lamivudine plus tenofovir (n = 1), or entecavir (n = 1); 5 patients continued lamivudine monotherapy. At the time of transplantation, serum HBV DNA was detectable in all 24 patients tested and 18 had levels >5 log10 copies/mL.
HBV prophylaxis post-OLT and HBIG regimens
After transplantation, 2 patients received HBIG only, and 4 received antiviral therapy only while the remaining patients received combination prophylaxis with HBIG and antiviral therapy. Of the 185 patients who received antiviral therapy post-OLT, 165 received nucleos(t)ide monotherapy: lamivudine (n = 141 including 4 patients who received antiviral prophylaxis only), ADV (n = 16), TDF (n= 3) or ETV (n = 5). Twenty patients received combination antiviral therapy, including 12 patients who had experienced virologic breakthrough prior to transplantation.
The most common HBIG regimen was IM low dose (39%), followed by IV high dose (25.1%), IV low dose (21.4%) and finite duration of HBIG (12.3%). The last group of patients had received HBIG for a median of 12 months (range, 1 to 48 months) and had been followed for a median of 53 months (range, 6 to 66 months) after HBIG was discontinued. The four groups were comparable regarding HBeAg status, HBV DNA levels, use of antiviral therapy, and virologic breakthrough at the time of transplant (Table 2).
Table 2.
Characteristics of patients according to HBIG regimens
| IV high dose | IV low dose | IM low dose | Finite duration | All | p value | |
|---|---|---|---|---|---|---|
| No of Patients | 47 (25.6) | 40 (21.8) | 73 (39.8) | 23 (12.8) | 183 (100) | |
| Gender, Male | 40 (85) | 26 (65) | 54 (74) | 17 (74) | 137 (74.9) | 0.172 |
| Age, years | 51.7 ± 10.6 | 54.1 ± 9.7 | 53.3 ± 10.3 | 48.8 ± 10.0 | 52.3 ± 10.3 | 0.212 |
| OLT Indication at Transplantation | 0.214 | |||||
| Cirrhosis | 18 (38.3) | 16 (40) | 22 (30) | 14 (60.9) | 70 (38.3) | |
| HCC | 26 (55.3) | 20 (50) | 44 (60.3) | 6 (26.1) | 96 (52.5) | |
| Acute Liver Failure | 3 (6.4) | 4 (10) | 7 (9.6) | 3 (13) | 17 (9.3) | |
| Labs at listing | ||||||
| HBeAg (+) | 16/44 (36.3) | 8/34 (23) | 19/62 (30) | 8/23 (34.8) | 51/163 (31.3) | 0.289 |
| HBV DNA detectable | 26/43 (60.3) | 18/38 (47.4) | 26/61 (42) | 11/23(47.8) | 81/165 (49.1) | 0.418 |
| HBV DNA, log10 copies/mL | 4.0 ± 2.1 | 4.0 ± 2.3 | 3.4 ± 2.1 | 3.4 ± 2.0 | 3.73 ± 2.2 | 0.474 |
| HBV DNA >5 log10 copies/mL | 16/45 (37.2) | 15/38 (39.5) | 17/61 (28) | 3/23(13) | 51/165 (31) | 0.154 |
| Antiviral Rx at Listing | 27 (57.4) | 23 (57.5) | 40 (54.8) | 12 (52.2) | 102 (55.7) | 0.992 |
| Labs at transplantation | ||||||
| HBeAg (+) | 12/41 (25.5) | 9/33 (27) | 16/58 (27.6) | 7/21 (32.3) | 44/153 (28.8) | 0.924 |
| HBV DNA detectable | 28/44 (63.6) | 23/38 (60) | 38/56 (67) | 16/23 (69.6) | 105/161 (65.2) | 0.888 |
| HBV DNA, log10 copies/mL | 4.6 ± 2.4 | 3.8 ± 2.2 | 4.4 ± 2.0 | 3.4 ± 1.8 | 4.2 ± 2.2 | 0.138 |
| HBV DNA >5 log10 copies/mL | 21/44 (47.7) | 12/38 (31.6) | 25/56 (44.6) | 4/23 (17.4) | 62/161 (38.5) | 0.080 |
| Antiviral Rx at transplant | 37 (78.7) | 27 (67.5) | 56 (76.7) | 15 (65.2) | 135(73.8) | 0.387 |
| Breakthrough prior to OLT | 8/37 (21.6) | 5/27(18.5) | 10/56(17.8) | 2/15(13.3) | 25/135 (18.5) | 0.576 |
| Samples Available | 5/8 | 4/5 | 6/10 | 2/2 | 17/25 | |
| Confirmed Genotypic Resistance | 5/5 (100) | 3/4 (75) | 4/6(66) | 2/2(100) | 14/17(82.3) |
Results expressed as number (%) or mean ± SD unless specified otherwise
LAM: Lamivudine, ADV: Adefovir, TDF: Tenofovir, ETV: entecavir, HCC: Hepatocellular carcinoma
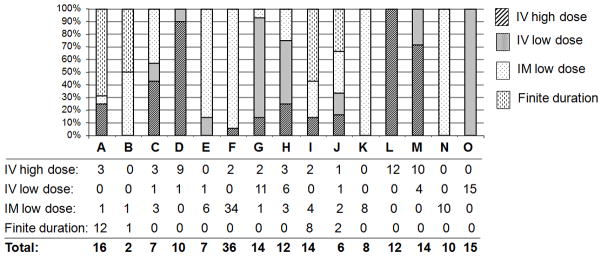
The HBIG regimens used in the 15 participating transplant centers were highly variable not only across the centers but also within the centers (Figure 1). The total HBIG dose in year 1 ranged from 10,000 to 355,000 IU and in each subsequent year from 0 to 200,000 IU.
Figure 1. Distribution of HBIG regimens used in each of the 15 participating centers (A–O). Only 4 centers (K, L, N, O) used one regimen.
Number of patients receiving each HBIG regimen: IV high dose, IV low dose, IM low dose, and finite duration at each center is listed beneath the bars.
Post-transplant outcomes
HBV recurrence
During a median follow-up of 42 months (range 1 to 81 months) post-transplant, 13 (6.9%) patients had HBV recurrence. Ten of the 13 patients had serum samples collected after HBV recurrence was diagnosed and prior to institution of rescue therapy. Of these, 5 had mutations associated with resistance to lamivudine (methionine to valine or isoleucine substitution at position 204 [rtM204V/I]), 1 had mutations associated with HBIG resistance (glycine to arginine substitution at position 145 [sG145R]), 3 had mutations associated with resistance to lamivudine and HBIG (rtM204V/I + sG145R), and 1 had wild type HBV sequence. Among the 5 patients with lamivudine resistance mutations only, 3 received a finite duration of HBIG and HBV recurred 12, 14 and 33 months after discontinuation of HBIG. The other 2 patients received high dose IV HBIG, both had HBV recurrence 1 month after transplant; one was documented to have lamivudine resistance before transplant and had HBV DNA of 8.6 log copies/mL at transplant while the other had undetectable serum HBV DNA 3 months before transplant. Both patients received lamivudine as the only antiviral therapy post-transplant. All 3 patients who had mutations associated with resistance to lamivudine and HBIG were receiving lamivudine and IM HBIG. The patient with HBIG resistance only had HBV DNA of 8.9 log copies/mL at transplantation. This patient had lamivudine resistance prior to transplantation and received prophylaxis with lamivudine, adefovir and IV HBIG but was later found to be non compliant.
The overall probability of HBV recurrence at 1, 3 and 5 years post-transplant was 3%, 7%, and 9%, respectively. The probability of HBV recurrence at 1, 3 and 5 years post-transplant was 1%, 6%, and 9% among patients transplanted for end-stage cirrhosis; and 6%, 8%, and 11% among patients transplanted for HCC; none of the patients transplanted for acute liver failure had HBV recurrence (p = 0.686) (Figure 2A).
Figure 2.
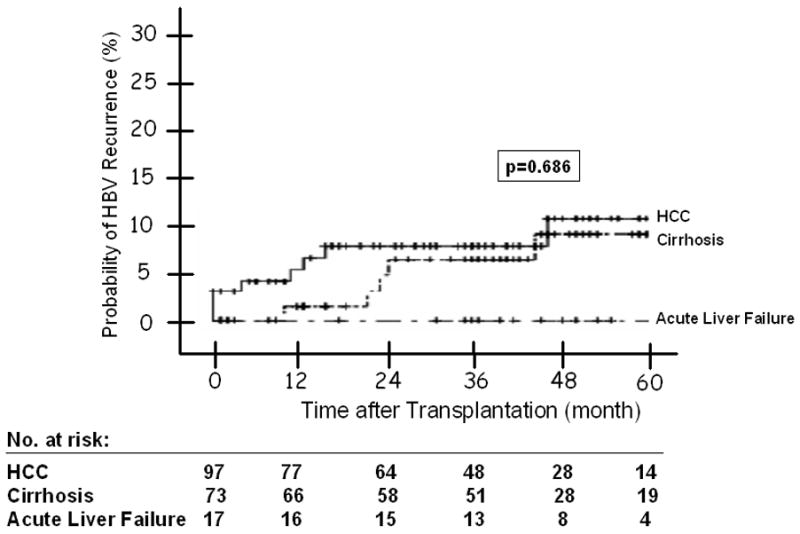
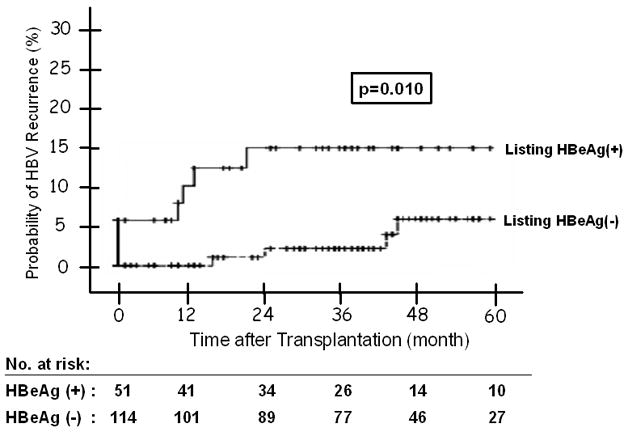
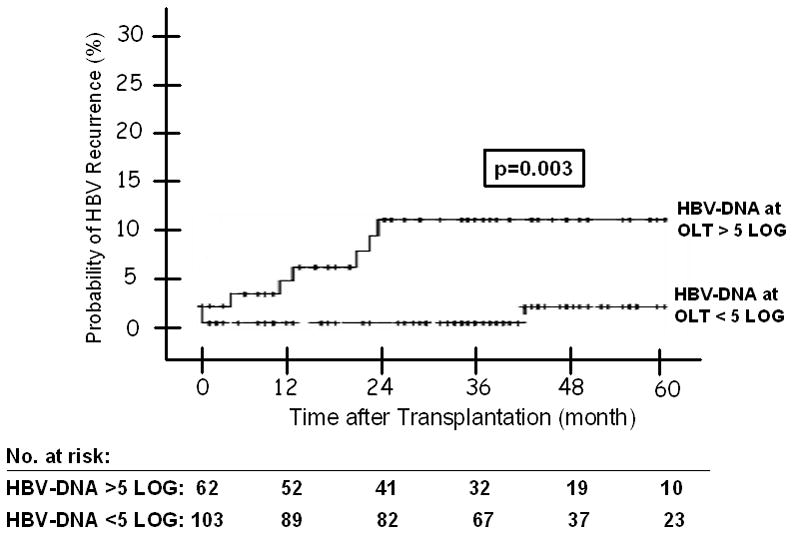
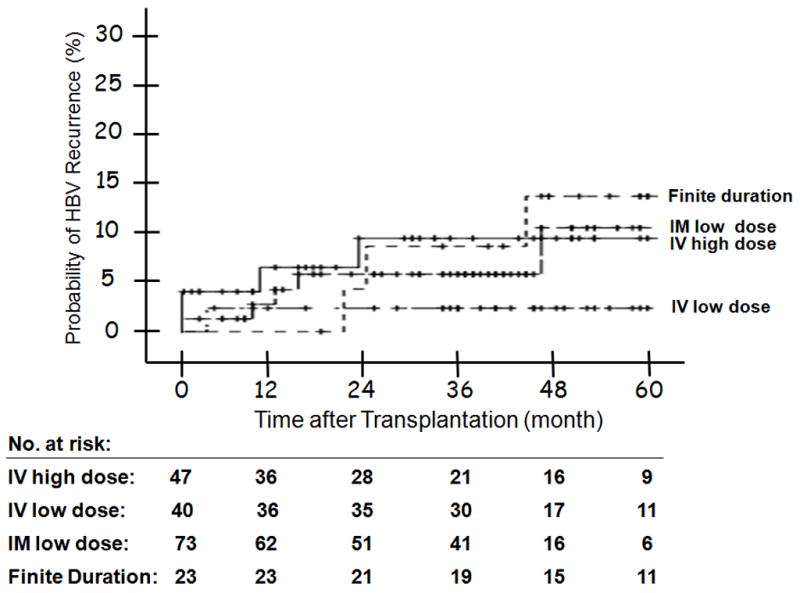
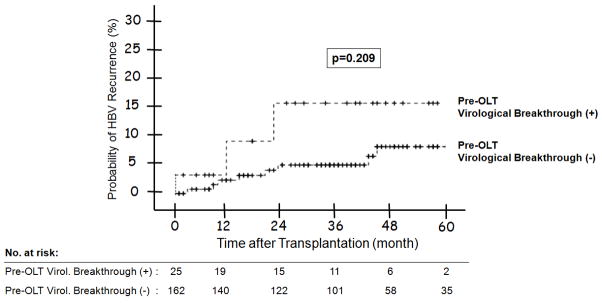
(A) HBV Recurrence in Relation to Indication at Transplantation -- The 1, 3, and 5 year probability of HBV recurrence post-OLT was 1%, 6%, and 9% for patients with end-stage cirrhosis; and 6%, 8% and 11% for patients with HCC; and none for patients with acute liver failure (p = 0.686). Figure 2(B): HBV Recurrence in Patients with and without Virological Breakthrough Pre-Transplant (p = 0.209). Figure 2(C): HBV Recurrence in Relation to HBeAg Status at Listing -- The 1, 3, and 5 year probability of HBV recurrence post-OLT was 10%, 15%, and 15% for patients who were HBeAg positive at listing; and 0, 2%, and 5% for those who were HBeAg negative (p = 0.010). Figure 2(D): HBV Recurrence in Relation to Serum HBV DNA Level at the Time of Transplant -- The 1, 3, and 5 year probability of HBV recurrence post-OLT was 7%, 15%, and 15% for patients with serum HBV DNA >5 log10 copies/mL at transplant; and 1%, 1%, and 3% for those with serum HBV DNA <5 log10 copies/mL (p = 0.003). Figure 2(E): HBV Recurrence in Relation to HBIG Regimens – The 5-year recurrence rates for the groups that received IV high dose, IV low dose, IM low dose, and finite duration of HBIG were 10, 3, 10, and 14, respectively (p = 0.733). The overall 5-year recurrence rate was 9%.
Three (12%) of the 25 patients with virologic breakthrough prior to transplantation had HBV recurrence compared to 8 (7.1%) of 113 patients who received antiviral therapy but did not experience virologic breakthrough, and 2 (4.1%) of 49 patients who did not receive antiviral therapy prior to transplant. The 1-, 3-, and 5-year probability of HBV recurrence was 4%, 15%, and 15% for patients with and 3%, 6%, and 8% for patients without virologic breakthrough prior to transplant (p = 0.209) (Figure 2B).
Factors Associated with HBV Recurrence
Univariate analysis showed that male gender (p = 0.031), Caucasian race (p = 0.034), presence of HBeAg at listing (p = 0.01), serum HBV DNA >5 log10 copies/mL at listing (p = 0.027) and at transplantation (p = 0.003) were associated with post-transplant HBV recurrence while transplant date before ADV approval (p = 0.077) showed a trend (Table 3, Figure 2C and D). Virologic breakthrough prior to transplantation, HBIG regimen, transplant center, duration of steroid use, and treatment for rejection were not associated with HBV recurrence (Figure 2B and E).
Table 3.
Characteristics of patients with and without HBV Recurrence
| HBV Recurrence | No HBV Recurrence | P value | |
|---|---|---|---|
| No. of patients | 13 (6.9) | 174 (93.0) | |
| Gender, male | 13 (100) | 128 (73.6) | 0.031 |
| Age, years | 53.5 ± 9.4 | 52.3 ± 10.3 | 0.708 |
| Race | 0.034 | ||
| Caucasian | 10 (76.9) | 68 (39.2) | |
| Asian | 2 (15.4) | 78 (44.8) | |
| African American | 1 (7.7) | 18 (10.3) | |
| Other | 0 | 10 (5.7) | |
| OLT Indication at transplant | 0.686 | ||
| End-stage Cirrhosis | 5 (38.5) | 68 (39.1) | |
| HCC | 8 (61.5) | 89 (51.1) | |
| Acute Liver Failure | 0 | 17 (9.8) | |
| Labs at listing | |||
| HBeAg (+) | 7/11 (63.6) | 44/154 (28.6) | 0.010 |
| HBV DNA detectable | 8/12 (66.7) | 73/156 (46.8) | 0.177 |
| HBV DNA >5 log10 copies/mL | 7/12 (58.3) | 44/156 (28.2) | 0.027 |
| HBV-DNA log10 copies/mL | 5.3 ± 2.98 | 3.50 ± 2.1 | 0.001 |
| Labs at transplant | |||
| HBeAg (+) | 4/9 (44.4) | 40/145 (27.6) | 0.220 |
| HBV DNA detectable | 9/10 (90) | 97/155 (62.6) | 0.103 |
| HBV DNA >5 log10 copies/mL | 8/10 (80) | 54/155 (34.8) | 0.003 |
| HBV DNA >3 log10 copies/mL | 9/10 (90) | 77/155 (49.7) | 0.017 |
| HBV-DNA log10 copies/mL | 7.0 ± 2.45 | 3.9 ± 2.0 | <0.001 |
| HBIG regimen | 13/13 | 170/174 | 0.733 |
| IV high dose | 4 (30.7) | 43 (25.3) | |
| IV low dose | 1 (7.7) | 39 (22.9) | |
| IM low dose | 5 (38.5) | 68 (40.0) | |
| Finite duration | 3 (23.0) | 20 (11.8) | |
| Antiviral treatment pre-OLT | 11 (84.6) | 127 (73.0) | 0.285 |
| Total duration of treatment before OLT, months | 11.8 ± 9.2 | 21.4 ± 22.0 | 0.153 |
| Treatment at the time of transplant | 0.280 | ||
| LAM | 10/11 (90.9) | 84/127 (66.1) | |
| LAM+ADV/TDF | 1/11 (9.1) | 17/127 (13.5) | |
| Non-LAM | 0 | 26/127 (20.4) | |
| Virologic breakthrough prior to OLT | 3/11 (27.2) | 22/127 (17.3) | 0.209 |
| Post-OLT prophylaxis | 0.898 | ||
| HBIG only | 0 | 2 (1.1) | |
| Antiviral only | 0 | 4 (2.2) | |
| HBIG+antiviral | 13 (100) | 168 (96.5) | |
| Antiviral therapy post-OLT | 13/13 (100) | 172/174 (98.8) | |
| LAM | 12 | 129 | |
| ADV | 0 | 16 | |
| TDF | 0 | 3 | |
| ETV | 0 | 5 | |
| LAM+ADV | 1 | 14 | |
| LAM+TDF | 0 | 3 | |
| ADV+TDF | 0 | 2 | |
| Time of OLT (after 09.2002)1* | 6 (46.2) | 130 (74.7) | 0.077 |
| Duration of Steroid use (month) | 4.42 ± 2.7 | 4.7 ± 5.0 | 0.809 |
| Treatment for Rejection | 1 (7.6) | 26 (14.6) | 0.461 |
Results expressed as number (%) or mean ± SD unless specified otherwise
LAM: Lamivudine, ADV: Adefovir, TDF: Tenofovir, ETV: entecavir, HCC: Hepatocellular carcinoma
Date when adefovir was approved
Of the 97 patients who had HCC, HBV recurrence was observed in 2 of 12 who had HCC recurrence and in 6 of 85 who did not have HCC recurrence.
Among the 25 patients with virologic breakthrough prior to transplantation, the 3 patients who had HBV recurrence differed from the other 22 patients who did not have HBV recurrence in having higher serum HBV DNA at transplantation (p = 0.094). Moreover, only 1 of the 3 patients with HBV recurrence received rescue therapy prior to transplantation compared to 21 of 22 with no recurrence (p = 0.031).
Cox regression analysis found that presence of HBeAg at listing (HR: 11.6, 95% CI 1.36–99.7, p = 0.02) and serum HBV DNA >5 log10 copies/mL at the time of transplant (HR 7.0, 95% CI 1.03–47.28, p = 0.03) were the only factors associated with post-transplant HBV recurrence while race showed a trend (Table 4). The 1-, 3-, and 5-year probability of HBV recurrence was 10%, 15% and 15% for patients who were HBeAg positive at listing and 0, 2%, and 5% for those who were HBeAg negative at listing (Table 5, Figure 2C). The 1-, 3-, and 5-year probability of HBV recurrence was 7%, 15%, and 15% for patients with serum HBV DNA >5 log10 copies/mL at transplant; 0, 0, and 6% for those with HBV DNA 3–5 log10 copies/mL; and 1%, 1%, and 1% for those with HBV DNA<3 log10 copies/mL (Table 5).
Table 4.
Analyses of Predictors for HBV Recurrence
| Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|
| p value | Hazard Ratio (95% CI) | p value | |
| HBeAg positive at listing | 0.010 | 11.6 (1.36–99.7) | 0.025 |
| HBV DNA > 5 log10 c/mL at transplant | 0.003 | 7.00 (1.03–47.28) | 0.038 |
| Race -- non-Caucasian | 0.034 | 0.41 (0.15–1.14) | 0.088 |
| HBV DNA > 5 log10 c/mL at listing | 0.027 | 2.25 (0.39–13.0) | 0.364 |
| Center | 0.202 | 0.98 (0.94–1.02) | 0.387 |
| HBIG Group | 0.733 | 1.24 (0.72–2.13) | 0.816 |
| Transplant date after adefovir approval | 0.077 | 0.84 (0.19–3.71) | 0.818 |
| Male gender | 0.031 | ∞1* | 0.800 |
All patients in the HBV recurrence group were male.
Table 5.
Probability of HBV Recurrence after transplantation
| Probability of HBV Recurrence | |||
|---|---|---|---|
| Year 1 | Year 3 | Year 5 | |
| Gender | |||
| Male | 4 | 9 | 12 |
| Female | 0 | 0 | 0 |
| Race | |||
| Caucasian | 7 | 13 | 15 |
| Asian | 0 | 2 | 4 |
| African American | 6 | 6 | 6 |
| Listing HBeAg Status | |||
| HBeAg (+) | 10 | 15 | 15 |
| HBeAg (−) | 0 | 2 | 5 |
| Serum HBV DNA at OLT (log10 copies/mL) | |||
| >5 | 7 | 15 | 15 |
| 3–5 | 0 | 0 | 6 |
| <3 | 1 | 1 | 1 |
| OLT Date | |||
| Before 09/20021* | 4 | 13 | 15 |
| After 09/2002 | 3 | 4 | 6 |
| Indication at OLT | |||
| Cirrhosis | 1 | 6 | 9 |
| HCC | 6 | 8 | 11 |
| Acute Liver Failure | 0 | 0 | 0 |
| Antiviral breakthrough prior to OLT | |||
| Yes | 4 | 15 | 15 |
| No | 3 | 6 | 8 |
| HBIG Regimen | |||
| IV High Dose | 7 | 10 | 10 |
| IV Low Dose | 3 | 3 | 3 |
| IM Low Dose | 3 | 6 | 10 |
| Finite Duration | 0 | 9 | 14 |
Results expressed as percentage
Date when adefovir was approved.
Patient Survival
A total of 17 (9%) patients (4 of 13 patients with and 13 of 174 patients without HBV recurrence) died: 2 due to complications related to HBV recurrence, 5 due to HCC recurrence, 3 due to other liver causes, 6 due to non-liver causes, and 1 due to an unknown cause. The 1-, 3-, and 5-year probability of post-OLT patient survival for the entire cohort was 95%, 92%, and 90%, respectively. The probability of post-OLT survival was lower among the patients with HBV recurrence (p = 0.028), but the difference was not significant when only liver-related deaths were analyzed (p = 0.209) (Figure 3). Of the 4 patients with HBV recurrence who died, 1 died of liver failure related to recurrent HBV, 1 died due from post-operative complications after retransplantation for recurrent HBV and 2 died of non-liver related causes: brain hemorrhage and primary lung cancer.
Figure 3.
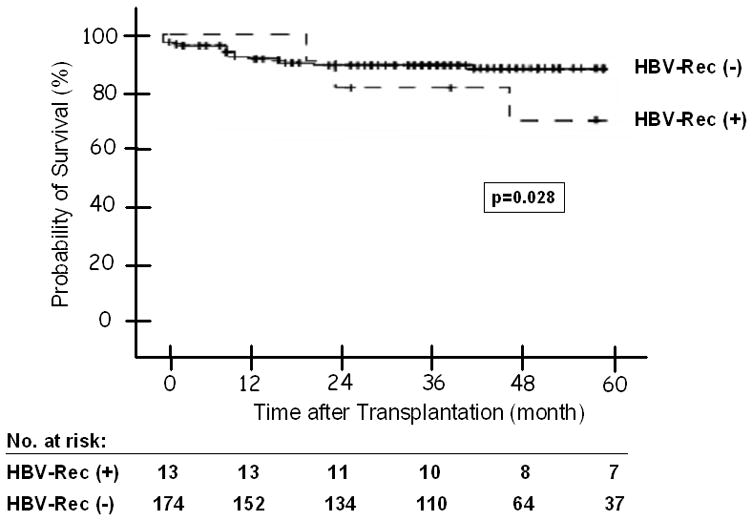
Post-OLT Patient Survival in Patients with and without HBV Recurrence -- The 1, 3, and 5 year probability of post-OLT patient survival was 100%, 85%, and 76% for patients with; and 95%, 93%, and 92% for those without HBV recurrence (p = 0.028).
Discussion
In this study of 187 patients who had liver transplantation for hepatitis B in the U.S. between 2001 and 2007, 6.9% had HBV recurrence after a median follow-up of 42 months. All except 6 patients received combination prophylaxis with HBIG and antiviral therapy. Most (>75%) patients received lamivudine monotherapy while HBIG regimens ranged from indefinite high dose IV to low dose IM or finite duration. The 5-year recurrence rate of 9% is lower than that in previous studies in which HBIG alone or lamivudine alone was used as prophylaxis (1,9,25,26) but higher than the 4% recurrence rate reported by Gane et al. (27) The latter study was unique in that very low dose IM HBIG was used from the time of transplantation (800 IU daily × 1 week then monthly) in combination with lamivudine. Two major differences between these two studies may account for the slightly higher rate of HBV recurrence in our study. Patients in our study had received a longer duration of lamivudine therapy prior to transplant, median 276 vs. 92 days and 25 patients in our study had experienced virologic breakthrough before transplant. Moreover, 64% of our patients vs. none in Gane’s study had detectable HBV DNA at transplantation.
Indeed, high serum HBV DNA (>5 log10 copies/mL) at transplantation and the presence of HBeAg at listing were the only factors associated with HBV recurrence on multivariate analysis. High serum HBV DNA and presence of HBeAg had been shown to decrease the half-life and efficacy of HBIG (5,6). The shortened half-life of HBIG becomes a greater problem in patients who had high serum HBV DNA as a result of lamivudine resistance and who did not receive rescue antiviral therapy post-transplant.
In this study, resistance to lamivudine and/or HBIG was detected in 9 of 10 patients for whom samples at the time of HBV recurrence were available for testing. Mutations associated with lamivudine resistance were detected in 8 patients; of these, 3 also had mutations associated with HBIG resistance. One patient who was noncompliant with antiviral medications had HBIG resistance mutations only. Although antiviral and/or HBIG resistance is the main cause of HBV recurrence, antiviral breakthrough prior to transplant was not a significant predictor of HBV recurrence. Of the 25 patients who were known to have virologic breakthrough prior to transplant, only 3 had HBV recurrence, one due to noncompliance with antiviral medications and two did not receive rescue antiviral therapy. The remaining 22 patients including 2 who discontinued HBIG and 9 who received IM low dose HBIG had no evidence of HBV recurrence up to 61 months post-transplant. Our data confirmed the results of other investigators that patients with antiviral resistance prior to transplant can be safely transplanted provided that rescue therapy is administered (14–16).
The HBIG regimens used in this study varied not only among the centers but also within each center. Many experts had suggested that prophylaxis against HBV recurrence be tailored to HBV replication status prior to transplant (28–30). However, our study found that the type of HBIG regimen used was not related to HBeAg status, serum HBV DNA at transplantation, or antiviral breakthrough prior to transplantation indicating that other factors such as institutional policies may be more important in determining which HBIG regimen was used at each center. The only exception may be in the group that discontinued HBIG after a finite duration. Compared to the other 3 groups, a lower percent of patients in this group had serum HBV DNA >5 log10 copies/mL at transplant: 17.4% vs. 42.0% (p = 0.056) but the percent of patients with virologic breakthrough prior to transplant was similar: 13.3% vs. 19.1% (p = 0.583).
Despite the wide range in HBIG dosing, the HBV recurrence rate was comparable in the 4 groups (p= 0.733). Comparison of the HBV recurrence rate in patients who had stopped HBIG versus the other 3 groups combined also showed no difference: 13% vs. 6.2%, p = 0.439. These data have important implications regarding cost savings. Using pharmacy charges at the University of Michigan and the average amount of HBIG administered in each group, the total charges for HBIG alone in year 1 were $235,692, $191,099, $132,251, and $161,478 for the groups that received indefinite IV high dose, IV low dose, IM low dose, and finite duration, respectively. The total charges for HBIG in each subsequent year were $157,128, $44,712, and $20,424 for the 3 groups that received indefinite HBIG, while the total charges for HBIG decreased from $19,228 in year 2 to $0 in year 5 for the group that received a finite duration of HBIG.
Our study spanned a 6.5 year period during which HBV therapies changed from only 1 drug with a high rate of antiviral resistance (lamivudine) to 3 approved HBV therapies (lamivudine, adefovir and entecavir). Some of our patients were listed for transplantation prior to the approval of lamivudine and many were managed in an era when lamivudine was the only approved HBV therapy. It is likely that use of more potent nucleos(t)ide analogs that have a higher genetic barrier to resistance prior to transplant, close monitoring for virologic response, modification of treatment in patients with suboptimal response, and prompt addition of rescue therapy in patients with virologic breakthrough would result in a higher proportion of patients with undetectable serum HBV DNA at the time of transplant and negligible HBV recurrence rate may be achieved with minimal or no HBIG. Indeed, Angus et al. (17) recently reported that none of 16 patients randomized to switch from lamivudine + HBIG to lamivudine + adefovir had HBV recurrence after a median follow-up of 21 months. In this study, however, HBIG was stopped after a mean of 4.5 years post-transplant. It remains to be determined whether potent nucleos(t)ide analogs that have high genetic barrier to resistance such as entecavir or tenofovir used alone or in combination will completely eliminate the need for HBIG.
The strengths of this study are the large number of patients, the long duration of follow-up, and HBV DNA testing in a central laboratory. However, there are some limitations. Most patients received lamivudine monotherapy as the initial antiviral therapy and rescue therapy was not available during the earlier years. Furthermore, blood samples for central lab testing were missing in some patients.
In summary, in this study involving 187 patients transplanted for HBV we found a wide range in HBIG regimens among the 15 U.S. centers. However, HBV recurrence rate was not related to HBIG regimen or transplant center. The only factors associated with HBV recurrence were HBeAg status at listing and serum HBV DNA level at transplant. Our study showed that if appropriate rescue therapy is started in patients with virologic breakthrough pre-OLT, comparable results can be obtained with IM low dose or finite course of HBIG as IV high dose HBIG. These data suggest that substantially lower doses or a more limited duration of HBIG than is currently used in many U.S. transplant centers is sufficient in preventing HBV recurrence; this is particularly true if more potent nucleos(t)ide analogs with higher genetic barrier to resistance such as entecavir or tenofovir are used.
Acknowledgments
We would like to thank all of the investigators and study staff at the participating sites: California Pacific Medical Center, San Francisco -- Natalie Bzowej, MD, Robert Gish, MD and Jamie Zagorski, RN
Cedars Sinai Medical Center, Los Angeles -- Tram Tran, MD and Amy Crumley, RN
Columbia University, New York -- Paul Gaglio, MD and Maria Martin
Massachusetts General Hospital, Boston -- Raymond T. Chung, MD, Diana Tsui and Marian Bihrle
Mayo Clinic, Rochester -- Michael Ishitani, MD, Heidi Togerson, RN
Mount Sinai University Medical Center, New York -- Sukru Emre, MD, Mark Sturdevant MD and Javaluyas, Aniceto MD
Ochsner Clinic, New Orleans -- Robert Perrillo, MD and Cheryl Denham, LPN
Stanford University, Palo Alto -- Emmet Keeffe, MD and Lucinda Porter, RN
University of California, Los Angeles -- Steve Han, MD, Pearl Kim-Hong, and Val Peacock, RN
University of Florida, Gainesville – Consuelo Soldevila-Pico, MD and Joy Peter, RN, BSN
University of Miami, Miami -- Eugene Schiff, MD and Maria Torres
University of Pennsylvania, Philadelphia -- Rajender Reddy, MD and Timothy Siropaides University of Virginia, Charlottesville --Timothy Pruett, MD
Virginia Commonwealth University, Richmond -- Velimir A.C. Luketic, MD and Stacy McLeod
University of Michigan, Ann Arbor -- Anna Lok, MD, Bulent Degertekin, MD, Terese Howell, Donna Harsh, Munira Hussain, Jim Imus and Morton Brown, PhD
This study was supported by Grant U01 DK57577 (ASL) from the National Institutes of Health. Roche Molecular Diagnostics provided Amplicor kits for HBV DNA assays and Innogenetics provided Inno-LiPA reagents for line probe assays.
Abbreviations
- ADV
adefovir
- AFP
alpha-fetoprotein
- ETV
entecavir
- HBeAg
hepatitis B e antigen
- HBIG
hepatitis B Immune globulin
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- IM
intramuscular
- IV
intravenous
- LAM
lamivudine
- OLT
orthotopic liver transplantation
- TDF
tenofovir
Footnotes
Financial Disclosures:
| Bulent Degertekin | Nothing to disclose |
| Steven-Huy B. Han | Grant support: Gilead, Bristol-Myers Squibb, Roche; Advisory board/consultant: Gilead, Bristol-Myers Squibb, Roche, Schering Plough; Speaker bureau: Gilead, Bristol-Myers Squibb, Roche |
| Emmet B Keeffe | Advisory board/consultant: Abbott Molecular; Speaking and teaching: Bristol-Myers Squibb, Roche Molecular Diagnostics |
| Eugene R Schiff | Grant/research support: Gilead and Bristol Myers Squibb; Scientific Advisory Board: Gilead and Bristol Myers Squibb; Speaker bureau: Bristol-Myers Squibb. |
| Velimir Luketic | Grant support: Schering-Plough, GlaxoSmithKline, Bristol- Myers Squibb, Globimmune, Biolex, ZymoGenetics, Conatus, Vertex, Idenix, Pharmasset, Romark, Human Genome Sciences, Wyeth Ayerst, and Intercept. |
| Robert S. Brown, Jr. | Grant/Research Support; Gilead, Bristol-Myers Squibb; Speaking and Teaching; Gilead. |
| Sukru Emre- | Nothing to disclose |
| Consuelo Soldevila-Pico | Nothing to disclose |
| K. Rajender Reddy | Grant/Research support: GlaxoSmithKline, Schering-Plough, Vertex, Boehringer-Ingelhiem, Gilead; Advisory Board: Gilead, Roche; Schering-Plough, Novartis, Merck. |
| Michael Ishitani | Nothing to disclose |
| Tram Tran | Consultant/Speaker: Gilead Sciences, Bristol Myers Squibb |
| Timothy Pruett | Nothing to disclose |
| Anna SF Lok | Grant/Research Support: Bristol-Myers Squibb, GlaxoSmithKline, Schering-Plough, Roche, Novartis, Gilead, Innogenetics, and Nabi; Consulting: Roche, Gilead, Bristol-Myers Squibb. |
Contributor Information
Bulent Degertekin, Email: degertekinb@hotmail.com.
Steven-Huy B. Han, Email: shbhan@ucla.edu.
Emmet B. Keeffe, Email: ekeeffe@stanford.edu.
Eugene R. Schiff, Email: ESchiff@med.miami.edu.
Velimir A. Luketic, Email: vluketic@vcu.edu.
Robert S. Brown, Jr., Email: rb464@columbia.edu.
Sukru Emre, Email: sukru.emre@yale.edu.
Consuelo Soldevila-Pico, Email: soldevc@medicine.ufl.edu.
K. Rajender Reddy, Email: rajender.reddy@uphs.upenn.edu.
Michael B. Ishitani, Email: Ishitani.Michael@mayo.edu.
Tram T. Tran, Email: tram.tran@cshs.org.
Timothy L. Pruett, Email: tp2w@hscmail.mcc.virginia.edu.
Anna S. F. Lok, Email: aslok@med.umich.edu.
References
- 1.Samuel D, Muller R, Alexander G, Fassati L, Ducot B, Benhamou JP, et al. Liver transplantation in European patients with the hepatitis B surface antigen. The N Engl J Med. 1993 Dec 16;329(25):1842–7. doi: 10.1056/NEJM199312163292503. [DOI] [PubMed] [Google Scholar]
- 2.Ghany MG, Ayola B, Villamil FG, Gish RG, Rojter S, Vierling JM, et al. Hepatitis B virus S mutants in liver transplant recipients who were reinfected despite hepatitis B immune globulin prophylaxis. Hepatology. 1998 Jan;27(1):213–22. doi: 10.1002/hep.510270133. [DOI] [PubMed] [Google Scholar]
- 3.Dan YY, Wai CT, Yeoh KG, Lim SG. Prophylactic strategies for hepatitis B patients undergoing liver transplant: a cost-effectiveness analysis. Liver Transpl. 2006 May;12(5):736–46. doi: 10.1002/lt.20685. [DOI] [PubMed] [Google Scholar]
- 4.Marzano A, Gaia S, Ghisetti V, Carenzi S, Premoli A, Debernardi-Venon W, et al. Viral load at the time of liver transplantation and risk of hepatitis B virus recurrence. Liver Transpl. 2005 Apr;11(4):402–9. doi: 10.1002/lt.20402. [DOI] [PubMed] [Google Scholar]
- 5.McGory RW, Ishitani MB, Oliveira WM, Stevenson WC, McCullough CS, Dickson RC, et al. Improved outcome of orthotopic liver transplantation for chronic hepatitis B cirrhosis with aggressive passive immunization. Transplantation. 1996 May 15;61(9):1358–64. doi: 10.1097/00007890-199605150-00013. [DOI] [PubMed] [Google Scholar]
- 6.Dickson RC, Terrault NA, Ishitani M, Reddy KR, Sheiner P, Luketic V, et al. Protective antibody levels and dose requirements for IV 5% Nabi Hepatitis B immune globulin combined with lamivudine in liver transplantation for hepatitis B-induced end stage liver disease. Liver Transpl. 2006 Jan;12(1):124–33. doi: 10.1002/lt.20582. [DOI] [PubMed] [Google Scholar]
- 7.Gane E, Strasser SI, Patterson S, McCaughan GW, Angus PW. A prospective study on the safety and efficacy of lamivudine and adefovir dipivoxil prophylaxis in HBSAG positive liver transplantation candidates. Hepatology. 2007 Oct;46(4):479a-a. [Google Scholar]
- 8.Angus PW, McCaughan GW, Gane EJ, Crawford DH, Harley H. Combination low-dose hepatitis B immune globulin and lamivudine therapy provides effective prophylaxis against posttransplantation hepatitis B. Liver Transpl. 2000 Jul;6(4):429–33. doi: 10.1053/jlts.2000.8310. [DOI] [PubMed] [Google Scholar]
- 9.Zheng S, Chen Y, Liang T, Lu A, Wang W, Shen Y, et al. Prevention of hepatitis B recurrence after liver transplantation using lamivudine or lamivudine combined with hepatitis B Immunoglobulin prophylaxis. Liver Transpl. 2006 Feb;12(2):253–8. doi: 10.1002/lt.20701. [DOI] [PubMed] [Google Scholar]
- 10.Mutimer D, Pillay D, Dragon E, Tang H, Ahmed M, O’Donnell K, et al. High pre-treatment serum hepatitis B virus titre predicts failure of lamivudine prophylaxis and graft re-infection after liver transplantation. J Hepatol. 1999 Apr;30(4):715–21. doi: 10.1016/s0168-8278(99)80204-9. [DOI] [PubMed] [Google Scholar]
- 11.Mutimer D, Feraz-Neto BH, Harrison R, O’Donnell K, Shaw J, Cane P, et al. Acute liver graft failure due to emergence of lamivudine resistant hepatitis B virus: rapid resolution during treatment with adefovir. Gut. 2001 Dec;49(6):860–3. doi: 10.1136/gut.49.6.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osborn MK, Han SH, Regev A, Bzowej NH, Ishitani MB, Tran TT, et al. Outcomes of patients with hepatitis B who developed antiviral resistance while on the liver transplant waiting list. Clin Gastroenterol Hepatol. 2007 Dec;5(12):1454–61. doi: 10.1016/j.cgh.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fontana RJ, Hann HW, Perrillo RP, Vierling JM, Wright T, Rakela J, et al. Determinants of early mortality in patients with decompensated chronic hepatitis B treated with antiviral therapy. Gastroenterology. 2002 Sep;123(3):719–27. doi: 10.1053/gast.2002.35352. [DOI] [PubMed] [Google Scholar]
- 14.Kamar N, Milioto O, Alric L, El Kahwaji L, Cointault O, Lavayssiere L, et al. Entecavir therapy for adefovir-resistant hepatitis B virus infection in kidney and liver allograft recipients. Transplantation. 2008 Aug 27;86(4):611–4. doi: 10.1097/TP.0b013e3181806c8c. [DOI] [PubMed] [Google Scholar]
- 15.Lo CM, Liu CL, Lau GK, Chan SC, Ng IO, Fan ST. Liver transplantation for chronic hepatitis B with lamivudine-resistant YMDD mutant using add-on adefovir dipivoxil plus lamivudine. Liver Transpl. 2005 Jul;11(7):807–13. doi: 10.1002/lt.20416. [DOI] [PubMed] [Google Scholar]
- 16.Schiff E, Lai CL, Hadziyannis S, Neuhaus P, Terrault N, Colombo M, et al. Adefovir dipivoxil for wait-listed and post-liver transplantation patients with lamivudine-resistant hepatitis B: final long-term results. Liver Transpl. 2007 Mar;13(3):349–60. doi: 10.1002/lt.20981. [DOI] [PubMed] [Google Scholar]
- 17.Angus PW, Patterson SJ, Strasser SI, McCaughan GW, Gane E. A randomized study of adefovir dipivoxil in place of HBIG in combination with lamivudine as post-liver transplantation hepatitis B prophylaxis. Hepatology. 2008 Nov;48(5):1460–6. doi: 10.1002/hep.22524. [DOI] [PubMed] [Google Scholar]
- 18.Dumortier J, Chevallier P, Scoazec JY, Berger F, Boillot O. Combined lamivudine and hepatitis B immunoglobulin for the prevention of hepatitis B recurrence after liver transplantation: lLong-term results. Am J Transplant. 2003 Aug;3(8):999–1002. doi: 10.1034/j.1600-6143.2003.00191.x. [DOI] [PubMed] [Google Scholar]
- 19.Han SH, Martin P, Edelstein M, Hu R, Kunder G, Holt C, et al. Conversion from intravenous to intramuscular hepatitis B immune globulin in combination with lamivudine is safe and cost-effective in patients receiving long-term prophylaxis to prevent hepatitis B recurrence after liver transplantation. Liver Transpl. 2003 Feb;9(2):182–7. doi: 10.1053/jlts.2003.50002. [DOI] [PubMed] [Google Scholar]
- 20.Han SH, Ofman J, Holt C, King K, Kunder G, Chen P, et al. An efficacy and cost-effectiveness analysis of combination hepatitis B immune globulin and lamivudine to prevent recurrent hepatitis B after orthotopic liver transplantation compared with hepatitis B immune globulin monotherapy. Liver Transpl. 2000 Nov;6(6):741–8. doi: 10.1053/jlts.2000.18702. [DOI] [PubMed] [Google Scholar]
- 21.Wong SN, Reddy KR, Keeffe EB, Han SH, Gaglio PJ, Perrillo RP, et al. Comparison of clinical outcomes in chronic hepatitis B liver transplant candidates with and without hepatocellular carcinoma. Liver Transpl. 2007 Mar;13(3):334–42. doi: 10.1002/lt.20959. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Hussain M, Wong S, Fung SK, Yim HJ, Lok AS. A genotype-independent real-time PCR assay for quantification of hepatitis B virus DNA. J Clin Microbiol. 2007 Feb;45(2):553–8. doi: 10.1128/JCM.00709-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Degertekin B, Hussain M, Tan J, Oberhelman K, Lok AS. Sensitivity and accuracy of an updated line probe assay (HBV DR v.3) in detecting mutations associated with hepatitis B antiviral resistance. J Hepatol. 2009 Jan;50(1):42–8. doi: 10.1016/j.jhep.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 24.Hussain M, Fung S, Libbrecht E, Sablon E, Cursaro C, Andreone P, et al. Sensitive line probe assay that simultaneously detects mutations conveying resistance to lamivudine and adefovir. J Clin Microbiol. 2006 Mar;44(3):1094–7. doi: 10.1128/JCM.44.3.1094-1097.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naoumov NV, Lopes AR, Burra P, Caccamo L, Iemmolo RM, de Man RA, et al. Randomized trial of lamivudine versus hepatitis B immunoglobulin for long-term prophylaxis of hepatitis B recurrence after liver transplantation. J Hepatol. 2001 Jun;34(6):888–94. doi: 10.1016/s0168-8278(01)00039-3. [DOI] [PubMed] [Google Scholar]
- 26.Perrillo RP, Wright T, Rakela J, Levy G, Schiff E, Gish R, et al. A multicenter United States-Canadian trial to assess lamivudine monotherapy before and after liver transplantation for chronic hepatitis B. Hepatology. 2001 Feb;33(2):424–32. doi: 10.1053/jhep.2001.21554. [DOI] [PubMed] [Google Scholar]
- 27.Gane EJ, Angus PW, Strasser S, Crawford DH, Ring J, Jeffrey GP, et al. Lamivudine plus low-dose hepatitis B immunoglobulin to prevent recurrent hepatitis B following liver transplantation. Gastroenterology. 2007 Mar;132(3):931–7. doi: 10.1053/j.gastro.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Lok AS. Prevention of recurrent hepatitis B post-liver transplantation. Liver Transpl. 2002 Oct;8(10 Suppl 1):S67–73. doi: 10.1053/jlts.2002.35780. [DOI] [PubMed] [Google Scholar]
- 29.Samuel D. Management of hepatitis B in liver transplantation patients. Semin Liver Dis. 2004;24(Suppl 1):55–62. doi: 10.1055/s-2004-828679. [DOI] [PubMed] [Google Scholar]
- 30.Coffin CS, Terrault NA. Management of hepatitis B in liver transplant recipients. J Viral Hepat. 2007 Nov;14(Suppl 1):37–44. doi: 10.1111/j.1365-2893.2007.00916.x. [DOI] [PubMed] [Google Scholar]