Abstract
Juvenile primary fibromyalgia syndrome (JPFS) is a chronic pain condition associated with significant impairment in physical functioning but no studies have used newer technologies such as actigraphy to document objective physical activity levels in JPFS. This is the first study to objectively describe physical activity in JPFS patients, and examine the relationship of pain, perceived functional impairment, and depressive symptoms on physical activity. One hundred and four clinically referred adolescents with JPFS (ages 11–18) wore a hip-mounted actigraph for one week. Data on pain intensity, functional disability, depressive symptoms, and psychiatric diagnoses were obtained using self- and parent-report measures and a standardized psychiatric interview. Results showed that younger patients were more active. Pain intensity was not significantly associated with physical activity levels overall, but the most highly active group of adolescents reported lower levels of pain and disability than the least active. Parent report of adolescents’ physical functioning and depressive symptoms were significantly correlated with adolescents’ physical activity levels. Actigraphy provides a unique source of information about physical functioning which is distinct from adolescents’ self-report of physical functioning in JPFS. Preliminary findings suggest that further study of factors that predict perceived and actual physical functioning in JPFS is warranted.
Keywords: juvenile fibromyalgia, actigraphy, pediatric pain, physical functioning, depression
Introduction
Juvenile primary fibromyalgia syndrome (JPFS) is a pediatric chronic pain condition typically first diagnosed in the early adolescent years, and affecting mostly females 35. Symptoms of JPFS include widespread musculoskeletal pain, multiple painful tender points, disrupted sleep, chronic fatigue, and other associated features 35. Chronic pain conditions such as JPFS can affect numerous domains of functioning including physical 16, 33, social 17 and psychological functioning 1, 14, 20. Assessments of physical and psychosocial functioning in JPFS, as in most studies of chronic pain, have relied almost exclusively on self-report 7, 19. Two published comprehensive reviews found that studies measuring pain-related disability, including physical functioning, relied solely on self-report and to a lesser extent, parent- or teacher-report 8, 9. While the experience of pain is by definition a subjective experience, the impact of pain on physical function has both subjective (i.e., perceived ability to engage in activity) and objective (i.e., actual engagement in activity) components. Yet, objective physical functioning in patients with JPFS has not been documented.
Use of objective methods, such as actigraphy, to assess physical functioning is important for at least two reasons. First, it is unknown whether self-report instruments reliably reflect the impact of pain on actual physical functioning because adolescents’ self-report of physical functioning may be inconsistent with actual activity. This could be due to retrospective recall bias 31, 32, social-desirability bias 30 or an inaccurate understanding of what constitutes levels of activity (e.g., reporting “shopping at the mall” as a vigorous activity). Second, it is observed that some JPFS patients remain vigorously active despite significant pain, and more research is needed to understand the factors that are associated with their ability to maintain high levels of activity.
Three published studies have demonstrated the utility of actigraphic measurement in individuals with chronic pain. The first study examined physical activity in 22 adult fibromyalgia (FMS) patients, 9 patients with depression and 28 healthy controls 23. Results showed that FMS patients with co-morbid depression demonstrated significantly lower daytime activity as compared to those with FMS only and controls. The second study compared physical activity between 29 adult FMS patients, 9 patients with Chronic Fatigue Syndrome (CFS), and 27 healthy controls 22. Results indicated that FMS and CFS patients had lower levels of vigorous physical activity and that higher levels of pain and fatigue were associated with lower physical activity. In the third study, physical activity levels were compared between 20 adolescents with pain conditions such as chronic head/neck pain, limb pain, back pain etc., (but not specifically JPFS) and 20 healthy controls 25. Results demonstrated that adolescents with chronic pain had greater activity limitations than controls, and spent significantly less time in moderate and vigorous activity. Higher activity levels were associated with younger age, lower pain ratings and fewer depressive symptoms. The current study sought to extend the findings from these early studies by examining physical activity in a relatively large sample of adolescent patients with JPFS.
The specific aims of this study were to 1) describe physical activity levels in adolescents with JPFS, 2) examine differences between high-active and low-active adolescents in terms of age, pain intensity, depressive symptoms, and functional impairment and 3) explore the impact of psychiatric disorders on physical activity. It was hypothesized that the most active adolescents would be younger, and would have lower levels of pain, depressive symptoms and functional impairment, compared to the least active adolescents. It was expected that those who met criteria for a depressive disorder diagnosis would show significantly lower vigorous activity levels. Results of a portion of this study were previously published in abstract form11.
Materials and Methods
Participants
Participants were 104 adolescents between the ages of 11 and 18 years with JPFS (89.4% female) who were screened for a larger ongoing clinical trial on behavioral treatment for juvenile fibromyalgia, and their parents. A description of a subset (N=76) of this JPFS sample was previously published in a study of psychiatric comorbidities in adolescents diagnosed with JPFS18. Adolescent patients were recruited from four pediatric rheumatology clinics from hospitals in the Ohio and Kentucky region and the study was approved by each hospital’s Institutional Review Board. All participants met Yunus and Masi criteria 35 for JPFS classification including: generalized musculoskeletal aching for greater than three months, the presence of at least 5 out of 18 tender points, and at least three associated symptoms such as poor sleep quality, fatigue, chronic anxiety, irritable bowel syndrome or chronic headaches. Exclusion criteria included individuals with other chronic rheumatic diseases such as juvenile idiopathic arthritis or systemic lupus erythematosus, individuals with a documented developmental delay or impairment such as autism, cerebral palsy or mental retardation, and those known to be taking opioid medications for the treatment of fibromyalgia pain. Participants were excluded if they were taking opioid medications due to the requirement in the larger clinical trial for patients to be receiving “usual medical care” for JPFS.
Procedure
Eligible participants were introduced to the study by their primary rheumatologist. If they were interested in participating, they were contacted by the research assistant to provide a detailed description of the study. Written informed consent was obtained from parents and written and verbal assent were obtained from adolescents prior to study participation. Measures in this study were administered as part of the comprehensive screening for the larger clinical trial. Once the evaluation was scheduled, a research assistant mailed daily pain diaries and an actigraph 15 to the patients’ homes. They were instructed to begin keeping daily pain diaries and to wear the hip-mounted actigraph at all times (except while showering or bathing) for one full week prior to the evaluation visit at the clinic. Participants were asked to return the daily diaries and actigraph at the study visit. During the evaluation, parent/s and adolescents completed questionnaires separately, and a trained doctoral level psychologist or psychology fellow conducted a standardized psychiatric interview with the parent/s and adolescent together. Upon completion, participants were given a gift card to compensate them for their time and effort.
Measures
Demographic Information
Parents completed a demographic information form including the adolescents’ age, sex, race and ethnicity, as well as information about socio-economic status. Information about when the adolescents’ JPFS symptoms first began was also obtained.
Physical Activity Monitoring
Small, hip-mounted, battery powered actigraphy monitors 15 were utilized for continuous activity monitoring over a period of one week. The actigraph was worn unobtrusively using a waist belt that fit snugly under clothing. The hip-mounted monitor is considered the most accurate device for measurement of physical activity and energy expenditure, as compared to wrist- or ankle-mounted monitors 27. Because monitors were worn for 7 days, data was collected across weekdays and weekends. The 5 most complete days of data were used for all analyses so that days with 3 or more continuous hours of no activity (consecutive zeros) during daytime hours (6 am–10 pm) were not included, as this was taken to indicate that the monitor had been removed.
Actigraphy monitors are equipped with an omni-directional accelerometer that measures physical activity including the amount and intensity of activity. The monitors contain a sensor that generates a voltage each time there is a change in acceleration in any direction, and its intensity. The signal is amplified and digitized and stored in the device in the form of activity counts. Activity counts were recorded in 15 second epochs and we chose to express activity counts in 1 minute epochs for ease of description and analysis. Average activity counts per minute during daytime hours (6am–10pm), peak activity (highest per minute level of activity in a 5-minute period), and average time (in minutes) spent in sedentary, light, moderate and vigorous activity per day during daytime hours were calculated for each participant using Actical Analysis Software 2.015. Daytime hours were selected based on a previous study 29, which examined activity in 9–11 year olds using daytime hours of 6 am–9 pm. We decided to extend daytime hours by an hour (to 10 pm) to account for the population of adolescents who typically go to bed later. We recognize that some of the adolescents may take naps during the daytime due to their poor sleep. However, for the purposes of this paper we were interested in activity levels of JPFS patients during typical waking hours. Examples of sedentary, light, moderate, and vigorous physical activity levels based upon actigraphy-based data collection are provided in the software manual and a validation study in children 15, 27. Examples of sedentary activity include sleep, rest, or sitting and watching television; light activity - playing a video game, writing a letter, or typing on a computer; moderate activity - light chores (vacuuming), playing with a hula hoop, or playing catch; vigorous activity - walking, jogging, skipping, or jumping rope.
Pain Rating
Adolescents rated average pain intensity on daily diaries (for one week) using a Visual Analog Scale (10 cm horizontal line with no numerical markings) anchored with the descriptors “no pain” and “worst possible pain.” Participants were instructed to mark their pain at the end of the day based on their average pain during the day. VAS scales are well-validated, widely used in pain research 10 and recommended for clinical outcome measurement in pediatric pain research 26. In this study, the average pain rating over the period of one week of daily diaries was used as a measure of pain intensity. The daily diaries corresponded to the week that the participant wore the activity monitoring device. Parents were asked to provide a rating of the adolescents’ average pain intensity during the past week using a single VAS.
Functional Disability Inventory (FDI): is a 15-item inventory that assesses perceived difficulty in performing a variety of daily activities such as “walking up stairs” or “walking the length of a football field.” Items are rated on a 5-point Likert scale, ranging from 0 to 4 (“No Trouble” to “Impossible”). The FDI has sound psychometric properties 34 and has been used for children with recurrent abdominal pain and in studies of pediatric fibromyalgia 6, 19. In a recently published consensus statement, the FDI was recommended for use as the primary outcome measure for physical functioning in pediatric pain research 26. The parent version of the FDI consists of the same 15 items as the FDI-child version, worded so that parents can provide their perception of the adolescent’s difficulty performing activities. The parent version of the FDI is a reliable and valid instrument 34. The FDI total score by adolescent self-report and by parent-report were used in this study. FDI total scores range from 0–60 with higher scores indicating greater impairment. The typical FDI score for a healthy child is 3.5, and average scores in pediatric pain populations range from 17.2 – 26.313, 19, 34.
Children’s Depression Inventory (CDI): is one of the most widely used self-report measures of childhood depressive symptoms and is a 27-item scale 24. It yields a total score and subscale scores on negative mood, interpersonal problems, ineffectiveness, anhedonia and negative self esteem. Separate norms are available for males and females ages 7–12 and 13–17. The CDI provides both raw and T-scores to indicate severity of depressive symptoms. Raw scores were used for the purposes of data analysis. A raw score above 15 indicates clinically significant depressive symptoms and is equivalent to a T-score of 60.
Adolescent Symptom Inventory-4 (ASI-4, Depression Subscale)
The ASI-4 is a parent completed checklist of symptoms referenced to the DSM-IV, assessing for a variety of mental disorders that may occur during adolescence. The Depression subscale is an 11-item section of the checklist in which the parent reported their adolescent’s depressive symptoms on a four-point Likert rating scale for each item ranging from 0 “Never” to 3 “Very Often.” The ASI-4 has established reliability and validity 12. In this study the raw score on the depression subscale of the ASI-4 score was used. A raw score of 5 is equivalent to a T-score of 62 and indicates the presence of clinically significant depressive symptoms.
The Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (KSADS-PL), is a structured psychiatric interview that is the most widely used and standardized measure used in research to diagnose DSM-IV referenced conditions in children and adolescents 21. Adolescents received a complete assessment for DSM-IV psychiatric disorders, as described in detail in a previous publication 18. For the purposes of this study on physical activity, we were specifically interested in examining differences in adolescents who met criteria for anxiety or depressive disorders (i.e., the most commonly present conditions in JPFS) versus those who did not. Therefore, adolescents were categorized into whether or not they had a current DSM-IV anxiety disorder (which included Panic Disorder, Generalized Anxiety Disorder, Obsessive Compulsive Disorder, Post-Traumatic Stress Disorder, Separation Anxiety, Agoraphobia, or Social Phobia) and whether or not they had a current DSM-IV depressive disorder (which included Major Depressive Disorder, Dysthymia or Unspecified Depressive Disorder). Adolescents who only reported having Specific Phobia, such as a fear of needles or insects, without any other psychiatric diagnosis, were not included in the category of those with anxiety disorders because specific phobias are common and are not expected to be disabling in most situations.
Statistical Analyses
All data were analyzed using SPSS Version 15.0 software. First, descriptive data on physical activity, pain, functional disability, and psychological functioning were computed. A series of correlations were then performed to assess relationships between age, duration of pain, average time spent in vigorous physical activity, peak activity (per minute), activity count (per minute), and adolescent and parent-report of pain, depression (CDI/ASI), and functional impairment (FDI). A multivariate analysis of co-variance (MANCOVA) was conducted to explore differences in pain, depression, and functional disability between the most highly active (Q1-top quartile of vigorous activity) versus the least active (Q4-lowest quartile of vigorous activity) adolescents. Given that age was found to be significantly correlated with activity levels and adolescents’ self-report of disability, it was entered as a covariate into the analysis to control for age effects. Finally, T-tests were performed to examine whether there were significant differences in vigorous physical activity among adolescents with and without a current diagnosis of depressive disorder, and adolescents with and without a current diagnosis of anxiety disorder.
Results
The majority of participants in this study were female (89.4%) and Caucasian (88.5%; African American-7.7%; Asian American-1%; American Indian-1%; and Biracial-1.8%), with a mean age of 14.9 years (SD = 1.8). This is consistent with the demographic characteristics of adolescents with JPFS in prior studies 19, 28, 35. In addition to the cardinal features of JPFS (widespread pain and multiple tender points), 91% of adolescents reported fatigue, 89% reported difficulty with sleep, 75% reported chronic headaches, 24% reported chronic anxiety, and 17% reported Irritable Bowel Syndrome.
Descriptive data on Physical Activity
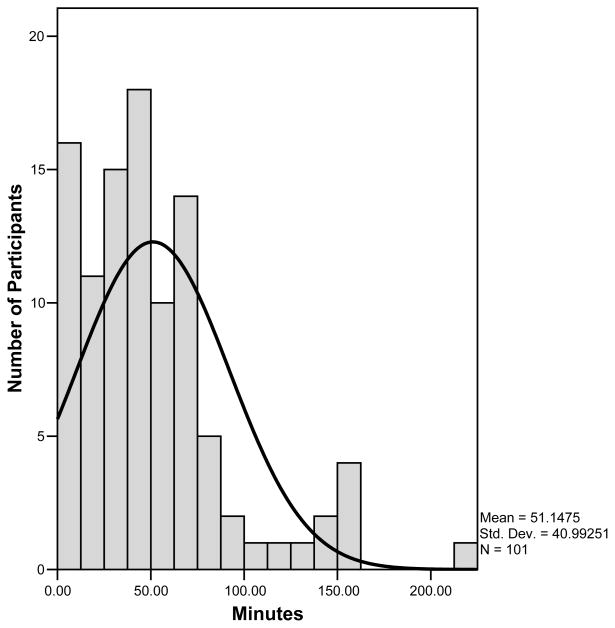
Adolescents had a mean activity count of 268 per minute during daytime hours (6 am–10 pm) and a mean peak activity count of 4980 per minute (across 5 minutes of highest sustained physical activity). Adolescents spent the majority of daytime hours in sedentary activity, which accounted for an average of 537 minutes (Table 1). Adolescents spent an average of 249 minutes of daytime hours in light physical activity, 122 minutes in moderate physical activity, and 51 minutes in vigorous physical activity per day. Of note, the minutes in each level of activity denote the total number of minutes as determined by the device and do not represent sustained activity. Therefore, the average of 51 minutes of “vigorous activity” during daytime hours does not mean that the adolescent maintained a high level of activity for a sustained period. In fact, only 24 participants (23%) met typical rheumatology recommendations for 30 minutes of sustained moderate to vigorous physical activity per day. Younger adolescents engaged in more vigorous activity and had higher activity counts. Although there were relatively few males in this study, males were also more vigorously active (Table 1). Upon closer examination of vigorous physical activity, it was found that the majority of adolescents spent less than 60 minutes per day engaging in vigorous activity. While the distribution of the data on vigorous activity was skewed towards the low end, there was a subset of patients (about 15%) that maintained relatively high levels of vigorous activity (Figure 1). Therefore, the data suggested variability within the sample with regard to physical activity that deserved further investigation.
Table 1.
Average Time (in minutes) Spent in Sedentary, Light, Moderate, and Vigorous Activity, Peak Activity (per min), and Average Activity Counts Per Minute During Daytime Hours (6am–10pm)
| Sedentary* | Light* | Moderate* | Vigorous* | Peak Activity** | Activity Counts*** | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | |
| All Adolescents | 537 | 119.9 | 249 | 54.3 | 122 | 44.2 | 51 | 40.6 | 4980 | 328.5 | 268 | 172.5 |
| Younger (11–14) | 509 | 131.4 | 259 | 58.6 | 129 | 45.7 | 60 | 46.4 | 5174 | 3388.8 | 300 | 207.1 |
| Older (15–18) | 556 | 108.4 | 243 | 50.6 | 117 | 43.0 | 45 | 35.2 | 4847 | 3234.1 | 245 | 141.5 |
| Females | 539 | 115.4 | 248 | 53.7 | 122 | 43.3 | 49 | 36.6 | 4896 | 3224.4 | 257 | 142.2 |
| Males | 514 | 157.4 | 253 | 61.4 | 124 | 53.7 | 69 | 65.0 | 5686 | 3856.9 | 355 | 332.2 |
Average minutes per day (total minutes in each sedentary, light, moderate, and vigorous physical activity across five days, then divided by five) to give the average minutes per day
Peak activity calculated by obtaining the highest sustained activity count during a five minute interval and dividing by five. Average peak activity per minute across one consecutive 5 minute interval
Average activity count per minute during daytime hours
Figure 1.
Distribution of time spent in vigorous physical activity
Descriptive Data on Pain, Functional Disability and Psychiatric Diagnoses
Descriptive data on pain, functional disability, and psychological symptoms based upon parent and adolescent self-report are presented in Table 2. Adolescents reported an average pain rating of 5.3 cm (SD = 1.87) on the VAS, with a mean pain duration (months since onset of pain symptoms) of 34.7 months (SD = 30.5). Adolescents reported a mean score on the FDI of 18.9 (SD = 8.6), which is within the typical range of scores for pediatric chronic pain populations, but slightly lower than our previous published studies in JPFS19, 20 and may be attributed to the much larger sample size of JPFS patients in this study and recruitment from a wider geographic area. Parents reported adolescents’ average pain rating as 5.6 cm (SD = 1.9), and rated their functional disability similarly to adolescents’ reports with a mean score of 19.0 (SD = 9.6). Based on the diagnostic interview (KSADS-PL), 20 adolescents (19.4%) were found to meet DSM-IV criteria for a current depressive disorder and 36 adolescents (35.0%) met criteria for a current anxiety disorder.
Table 2.
Report of Pain, Functional Disability and Psychological Symptoms by Adolescent and Parent-Report
| M | SD | |
|---|---|---|
| Adolescent Report: | ||
| Pain Rating (0–10 cm VAS) | 5.3 | 1.9 |
| Pain Duration (in months) | 34.7 | 30.5 |
| Functional Disability (FDI; 0–60) | 18.9 | 8.6 |
| Depressive Symptoms (CDI; 0–81) | 13.0 | 7.2 |
| Parent Report: | ||
| Pain Rating (0–10 cm VAS) | 5.6 | 1.9 |
| Functional Disability (FDI; 0–60) | 19.0 | 9.6 |
| Depressive Symptoms (ASI-4; 0–25) | 4.6 | 5.8 |
| N | % | |
| Psychiatric Diagnoses*: | ||
| Depressive Disorder | 20 | 19.4 |
| Depressive Disorder NOS | 5 | |
| Dysthymia | 8 | |
| Major Depressive Disorder | 7 | |
| Anxiety Disorder** | 36 | 35.0 |
| Agoraphobia | 4 | |
| Generalized Anxiety Disorder | 17 | |
| Obsessive-Compulsive Disorder | 3 | |
| Panic Disorder | 6 | |
| Post-Traumatic Stress Disorder | 5 | |
| Separation Anxiety | 3 | |
| Social Phobia | 11 | |
13 adolescents were diagnosed with a depressive and comorbid anxiety disorder
10 individuals were diagnosed with multiple (2–4) anxiety disorders
Relationship between Activity Levels, Age, Pain, Depressive Symptoms, Functional Disability, and Psychiatric Diagnoses
Older age was significantly correlated with less time spent in vigorous activity (r = −.32; p<.01) and fewer activity counts (r=−.29; p<.01). Adolescents’ self-report of pain (r = −.13), depressive symptoms (r = −.12), and functional disability (r = −.17) were not significantly correlated with time spent in vigorous physical activity or activity counts. Parent-report of pain was also not significantly correlated with time spent in vigorous physical activity (r = −.14) or activity counts (r =−.13). However, parent-report of depressive symptoms (r = −.26; p<.05) and functional disability (r = −.32; p < .01) were significantly correlated with time spent in vigorous physical activity. Parent-report of depressive symptoms (r = −.23; p<.05) and functional disability (r = −.33; p<.01) were also significantly correlated with activity counts. Peak activity was not significantly correlated with either adolescent or parent reports of pain, depressive symptoms, or functional disability. Complete correlational data is presented in Table 3. T-tests showed no significant differences in participation in vigorous physical activity among adolescents with a comorbid depressive (t=.07) or anxiety (t=.83) disorder, when compared to those without depressive or anxiety disorders.
Table 3.
Correlation Table
| Age | Duration | Pain (Adol.) | Pain (Parent) | CDI | ASI | FDI (Adol.) | FDI (Parent) | Vigorous Activity | Peak Activity | Activity Count | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | -- | .24* | .13 | .18 | .11 | .14 | .21* | .13 | −.32** | −.10 | −.29** |
| 2. Duration of Pain (in months) | -- | .11 | .02 | .05 | .04 | −.03 | .06 | −.08 | −.12 | −.04 | |
| 3. Pain Rating (Adolescent) | -- | .51** | .28** | .14 | .41** | .29** | −.13 | −.10 | −.12 | ||
| 4. Pain Rating (Parent) | -- | .33** | .15 | .46** | .48** | −.14 | −.06 | −.13 | |||
| 5. CDI Raw Score | -- | .30** | .45** | .24* | −.12 | −.07 | −.12 | ||||
| 6. ASI Raw Score (depression subscale) | -- | .20 | .25* | −.26* | −.01 | −.23* | |||||
| 7. FDI (Adolescent) | -- | .52** | −.17 | −.04 | −.16 | ||||||
| 8. FDI (Parent) | -- | −.32** | −.19 | −.33** | |||||||
| 9. Time (in minutes) spent in Vigorous Activity | -- | .56** | .96** | ||||||||
| 10. Peak Activity | -- | .64** | |||||||||
| 11. Activity Counts | -- |
p<.05
p<.01
Comparison of Adolescents Engaging in High versus Low Levels of Vigorous Activity
Due to the variability observed in the amount of time spent in vigorous physical activity (range: 0–175 minutes) and the fact that a subset of adolescents maintained high levels of activity despite their symptoms, we selected a subset of the data for further analysis. Time spent in vigorous activity data was divided into quartiles so that differences between the 26 most active (Q1) and the 26 least active adolescents (Q4) could be compared after removing one outlier; an adolescent who engaged in 223 minutes (nearly 4 standard deviations above the mean) of vigorous physical activity per day. A one-way MANCOVA was then performed to explore differences between the most active (Q1) and least active (Q4) adolescents. After controlling for age, the two groups were compared on adolescent and parent reports of pain, depressive symptoms, and functional disability. There was a statistically significant difference between low-active and high-active adolescents for the omnibus MANCOVA on the combined dependent variables of pain, depressive symptoms and functional disability (F = 2.96, p=.016; Hotelling’s t=.40). When the results for the dependent variables were considered separately, adolescent (p=.013) and parent-reported pain intensity (p=.045) and parent-reported depressive symptoms (p=.007) and functional disability (p=.020) were significantly different between the groups. The most highly active adolescents had lower perceived pain by both adolescent and parent report, and parents perceived the highly active adolescents to have less depressive symptoms and functional disability than the low active adolescents. Adolescents’ reports of depressive symptoms and functional disability were higher in the low-active group, but the difference did not achieve statistical significance.
Discussion
This is the first study to demonstrate the feasibility and utility of actigraphy-based physical activity monitoring as an objective indicator of physical functioning in adolescents with JPFS. Adolescents wore hip-mounted actigraphs for one week of continuous activity monitoring, and adolescents and parents provided information about the adolescents’ pain intensity, psychological symptoms and perceived disability using validated, self-report measures. Although hip-mounted actigraphs are somewhat less convenient than ankle or wrist placements, they provide an accurate indication of whole body movement. It was not uncommon for adolescents to miss one or two days of wearing the monitor for the week, but we were able to obtain at least 5 days of complete data for all participants.
Results showed that adolescents with JPFS spent relatively little time in moderate or vigorous physical activity despite receiving the usual medical recommendations at all study sites for increased aerobic exercise. In fact, only 23% of adolescents met rheumatology recommendations for 30 minutes of moderate to vigorous physical activity and only one met national guidelines of 60 minutes of moderate to vigorous physical activity daily 4. These results were not unexpected, given the decreased activity levels found in children and adolescents with chronic pain 25. National statistics also show that physical activity levels in adolescents in general are quite low 3. The Youth Risk Behavior Surveillance System reported that only 34.7% of adolescents met recommended levels of physical activity 5 and 22.6% of youth did not engage in any type of vigorous physical activity 2. Nevertheless, the low overall levels of activity in JPFS patients are concerning because increased exercise is generally viewed as a key component for improved pain management in JPFS.
Higher pain intensity ratings were not significantly associated with lower levels of vigorous activity or activity counts in the overall sample, suggesting that pain by itself may not be directly related to whether or not adolescents with JPFS are able to engage in higher activity levels. This is contrary to what most adolescents with JPFS and parents typically report, as pain is routinely cited as the primary reason for inability to exercise. This is also different from the results of the Long et al. pediatric study 25 which found that adolescent report of functional impairment, depression and pain were significantly (inversely) associated with activity levels. The results of our study did not replicate these findings, possibly due to differences in actigraphy methodology, or the characteristics of the pain population - which included only adolescents with JPFS in this study. Aside from chronic pain, JPFS patients have a number of associated symptoms such as fatigue, sleep difficulties etc. which may affect activity levels as well. In the current study, it appeared that parents were more likely than adolescents to recognize that psychological factors, such as depressive symptoms, may affect their adolescent’s activity. According to parent reports, higher levels of depressive symptoms and functional disability were significantly correlated with lower engagement in vigorous activity and fewer activity counts whereas correlations between adolescents’ responses on self-report measures and objective measurement of physical activity were very low. The fact that there was essentially no relationship between adolescents’ perception of their functional disability and actual levels of physical activity was notable. Together, these findings suggest that activity monitoring provides a unique source of information which has the potential to be very helpful in educating and monitoring adolescents’ engagement in physical activity. This finding also underscores the importance of multi-method approaches to measurement of physical impairment in adolescents with chronic pain.
There were a small number of adolescents with JPFS who maintained high levels of vigorous activity, which is consistent with our clinical knowledge of this population. Clinicians often report that a subset of patients with JPFS make a concerted effort to remain involved in sports or other vigorous activities despite their symptoms. This group is particularly interesting from the point of view of understanding how some adolescents adaptively cope with their condition and show relatively little physical impairment. Significant differences emerged when we looked more closely at differences between adolescents who were in the most active and least active quartiles of vigorous activity. After controlling for the known effects of age, the subset of the sample that included the most active adolescents reported lower levels of pain, and their parents reported that they had significantly lower depressive symptoms and disability than the least active group. It should be noted that causal connections cannot be made in this cross sectional study. Therefore, higher pain levels in the least active group may be related to their decreased activity or vice versa. Also, the results of this subgroup analysis may not be generalizable to all adolescents with JPFS. Nevertheless, it is clear that patients with JPFS who were highly active were qualitatively different and appeared to have lower levels of physical and psychological impairment.
In contrast to the study in adults with fibromyalgia, there were no significant differences in vigorous activity among adolescents who were diagnosed with a depressive disorder and those who were not. This might be due to the differences in the manifestation of clinical depression in adults (who may have more vegetative symptoms) compared to children, so that a DSM-IV diagnosis of depression in adolescents may not have the same implications for their activity levels. On the other hand, parent-report of the severity of the adolescents’ depressive symptoms appeared to be a more sensitive indicator because it was shown to be significantly associated with activity levels. The presence of anxiety disorders did not appear to have any significant bearing on physical activity.
The findings of this study should be interpreted in light of several limitations. Because this is the first study to use actigraphy in the JPFS population, there are no established norms or comparable studies using the same methodology in other pediatric populations. Hence, replication of the findings is needed to confirm the results of this study. Additionally, there was no control group in this study vigorous physical activity levels of adolescents with JPFS could not be compared to “typical” adolescents. We suspect that the wider availability of newer technologies such as actigraphy will make it more likely that such objective and ecologically valid (real-world setting) measures will be increasingly integrated into chronic pain research. A second limitation is that the cross-sectional nature of the study precludes making any causal connections beyond demonstrating that adolescents who (objectively) remain highly active tend to have fewer depressive symptoms and are seen as less disabled by their parents. Third, we examined activity levels during daytime hours, which may have overlapped with time spent sleeping for those adolescents who napped during the day. Additionally, we did not measure fear or avoidance of activity related to anticipatory anxiety which may partially explain the lack of relationship between adolescents’ reported pain and decreased vigorous physical activity. Finally, there appear to be sex differences in physical activity but these could not be sufficiently examined in this study because JPFS is prevalent mainly in adolescent girls, and more studies with pediatric pain populations are needed to explore how sex differences in physical activity might influence clinical outcomes.
Future studies are needed to investigate whether exercise-based programs and/or cognitive-behavioral approaches that include recommendations for greater physical activity levels have an effect on objectively measured physical activity. It would also be beneficial to explore whether increased physical activity levels might be related to better prognosis and long-term outcomes in those diagnosed with JPFS. Objective information regarding an individual’s engagement in physical activity may be eventually used as an effective tool to increase the effectiveness of treatment approaches to reduce disability and enhance quality of life in adolescent JPFS patients.
Table 4.
MANCOVA Analysis of Differences between High-Active (Q1) and Low-Active (Q4) Adolescents
| High-Active Adolescents (n=26) | Low-Active Adolescents (n=26) | |||
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | F | P | |
| Adolescent-Report | ||||
| Pain Intensity | 4.82 (1.95) | 5.88 (1.45) | 6.59 | .013* |
| Depression Score (CDI) | 12.44 (7.54) | 14.81 (6.51) | 2.46 | .123 |
| Functional Disability (FDI) | 17.84 (8.00) | 20.96 (8.24) | 1.63 | .208 |
| Parent-Report | ||||
| Pain Intensity | 5.0 (2.04) | 6.18 (1.94) | 4.23 | .045* |
| Depression Score (ASI-4) | 2.44 (4.56) | 6.58 (5.54) | 7.95 | .007* |
| Functional Disability (FDI) | 15.40 (8.96) | 21.38 (10.03) | 5.80 | .020* |
Statistically significant at .05 or below
Acknowledgments
This study was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS Grant # R01 AR050028)
Footnotes
Perspective
This study presents the results of physical activity monitoring in adolescents with JPFS using actigraphy. Results indicate that actigraphy provides a unique source of objective information which can advance our understanding of physical disability in JPFS, and the factors associated with physical impairment.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anthony KK, Schanberg LE. Juvenile primary fibromyalgia syndrome. Curr Rheumatol Rep. 2001;3:165–171. doi: 10.1007/s11926-001-0012-7. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention: Physical activity levels among children aged 9–13 years-United States, 2002. Morbidity & Mortality Weekly Report. 2003;52:785–788. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. National Center for Health Statistics, National Health and Nutrition Examination Survey United States, 1995–2005. National Center for Health Statistics, Vital and Health Statistics; 2005. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Division of Nutrition, Physical Activity and Obesity. National Center for Chronic Disease Prevention and Health Promotion; 2008. Physical Activity Guidelines For Americans. < http://www.cdc.gov/nccdphp/dnpa/index.htm>. [Google Scholar]
- 5.Centers for Disease Control and Prevention: Youth Risk Behavior Surveillance -United States, 2007. Morbidity & Mortality Weekly Report. 2008;57:1–131. [PubMed] [Google Scholar]
- 6.Claar RL, Walker LS. Functional assessment of pediatric pain patients: psychometric properties of the functional disability inventory. Pain. 2006;121:77–84. doi: 10.1016/j.pain.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Degotardi PJ, Klass ES, Rosenberg BS, Fox DG, Gallelli KA, Gottlieb BS. Development and evaluation of a cognitive-behavioral intervention for juvenile fibromyalgia. Journal of Pediatric Psychology. 2006;31:714–723. doi: 10.1093/jpepsy/jsj064. [DOI] [PubMed] [Google Scholar]
- 8.Eccleston C, Jordan AL, Crombez G. The impact of chronic pain on adolescents: a review of previously used measures. J Pediatr Psychol. 2006;31:684–697. doi: 10.1093/jpepsy/jsj061. [DOI] [PubMed] [Google Scholar]
- 9.Eccleston C, Morley S, Williams A, Yorke L, Mastroyannopoulou K. Systematic review of randomised controlled trials of psychological therapy for chronic pain in children and adolescents, with a subset meta-analysis of pain relief. Pain. 2002;99:157–165. doi: 10.1016/s0304-3959(02)00072-6. [DOI] [PubMed] [Google Scholar]
- 10.Finley AG, McGrath PJ. Progress in pain research and management. Vol. 10. Seattle, WA: IASP Press; 1998. [Google Scholar]
- 11.Flowers SR, Kashikar-Zuck S, Johnson M, Verkamp E, Lynch-Jordan A, Ting T, Graham B, Schikler K, Spalding S, Hashkes P, Richards M, Banez G. Physical activity monitoring in children and adolescents with juvenile primary fibromyaglia syndrome. The Journal of Pain. 2009;10:S62. doi: 10.1016/j.jpain.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gadow K, Sprafkin J. The Symptom Inventories: An annotated bibliography. Stony Brook, NY: Checkmate Plus; 2005. [Google Scholar]
- 13.Gauntlett-Gilbert J, Eccleston C. Disability in adolescents with chronic pain: Patterns and predictors across different domains of functioning. Pain. 2007;131:132–141. doi: 10.1016/j.pain.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 14.Goldenberg DL. The Interface of Pain and Mood Disturbances in the Rheumatic Diseases. Semin Arthritis Rheum. 2009 doi: 10.1016/j.semarthrit.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Actical Software Instruction Manual [computer program]. Version 2.0. Bend; OR: 2002. [Google Scholar]
- 16.Kashikar-Zuck S, Goldschneider KR, Powers SW, Vaught MH, Hershey AD. Depression and functional disability in chronic pediatric pain. Clinical Journal of Pain. 2001;17:341–349. doi: 10.1097/00002508-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Kashikar-Zuck S, Lynch AM, Graham TB, Swain NF, Mullen SM, Noll RB. Social functioning and peer relationships of adolescents with juvenile fibromyalgia syndrome. Arthritis and Rheumatism. 2007;57:474–480. doi: 10.1002/art.22615. [DOI] [PubMed] [Google Scholar]
- 18.Kashikar-Zuck S, Parkins IS, Graham TB, Lynch AM, Passo M, Johnston M, Schikler KN, Hashkes PJ, Banez G, Richards MM. Anxiety, mood, and behavioral disorders among pediatric patients with juvenile fibromyalgia syndrome. Clinical Journal of Pain. 2008;24:620–626. doi: 10.1097/AJP.0b013e31816d7d23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kashikar-Zuck S, Swain NF, Jones BA, Graham TB. Efficacy of cognitive-behavioral intervention for juvenile primary fibromyalgia syndrome. Journal of Rheumatology. 2005;32:1594–1602. [PubMed] [Google Scholar]
- 20.Kashikar-Zuck S, Vaught MH, Goldschneider KR, Graham TB, Miller JC. Depression, coping and functional disability in juvenile primary fibromyalgia syndrome. Journal of Pain. 2002;3:412–419. doi: 10.1054/jpai.2002.126786. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Kop WJ, Lyden A, Berlin AA, Ambrose K, Olsen C, Gracely RH, Williams DA, Clauw DJ. Ambulatory monitoring of physical activity and symptoms in fibromyalgia and chronic fatigue syndrome. Arthritis Rheum. 2005;52:296–303. doi: 10.1002/art.20779. [DOI] [PubMed] [Google Scholar]
- 23.Korszun A, Young EA, Engleberg NC, Brucksch CB, Greden JF, Crofford LA. Use of actigraphy for monitoring sleep and activity levels in patients with fibromyalgia and depression. J Psychosom Res. 2002;52:439–443. doi: 10.1016/s0022-3999(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 24.Kovacs M. Children’s Depression Inventory. Multi-Health systems, Inc; 908 Niagara Falls Blvd., North Tonawanda, N.Y: 1992. pp. 14120–2060. [Google Scholar]
- 25.Long AC, Palermo TM, Manees AM. Brief report: using actigraphy to compare physical activity levels in adolescents with chronic pain and healthy adolescents. J Pediatr Psychol. 2008;33:660–665. doi: 10.1093/jpepsy/jsm136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, Eccleston C, Finley GA, Goldschneider K, Haverkos L, Hertz SH, Ljungman G, Palermo T, Rappaport BA, Rhodes T, Schechter N, Scott J, Sethna N, Svensson OK, Stinson J, von Baeyer CL, Walker L, Weisman S, White RE, Zajicek A, Zeltzer L. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. Journal of Pain. 2008;9:771–783. doi: 10.1016/j.jpain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Puyau MR, Adolph AL, Vohra FA, Butte NF. Validation and calibration of physical activity monitors in children. Obes Res. 2002;10:150–157. doi: 10.1038/oby.2002.24. [DOI] [PubMed] [Google Scholar]
- 28.Reid GJ, Lang BA, McGrath PJ. Primary juvenile fibromyalgia: psychological adjustment, family functioning, coping, and functional disability. Arthritis and Rheumatism. 1997;40:752–760. doi: 10.1002/art.1780400423. [DOI] [PubMed] [Google Scholar]
- 29.Rowlands AV, Pilgrim EL, Eston RG. Patterns of habitual activity across weekdays and weekend days in 9–11-year-old children. Prev Med. 2008;46:317–324. doi: 10.1016/j.ypmed.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71:S1–14. [PubMed] [Google Scholar]
- 31.Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient compliance with paper and electronic diaries. Control Clin Trials. 2003;24:182–199. doi: 10.1016/s0197-2456(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 32.Stone AA, Turkkan JS, Bachrach CA, Jobe JB, Kurtzman HS, Cain VS, editors. The science of self-report: Implications for research and practice. Mahwah, NY: Lawrence Earlbaum Associates; 1999. [Google Scholar]
- 33.Varni JW, Burwinkle TM, Limbers CA, Szer IS. The PedsQL as a patient-reported outcome in children and adolescents with fibromyalgia: an analysis of OMERACT domains. Health Qual Life Outcomes. 2007;5:9. doi: 10.1186/1477-7525-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. Journal of Pediatric Psychology. 1991;16:39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- 35.Yunus MB, Masi AT. Juvenile primary fibromyalgia syndrome. A clinical study of thirty-three patients and matched normal controls. Arthritis & Rheumatism. 1985;28:138–145. doi: 10.1002/art.1780280205. [DOI] [PubMed] [Google Scholar]