Abstract
This study assessed the relationship between treatment outcome and perceived drug assignment in smokers (nicotine patch (NP) or placebo) using abstinence and relapse status. Smokers (N = 424) were randomly assigned to receive either NP or placebo as part of a study that examined the effects of combining NP with self-help programs. Beliefs about drug assignment, assessed at the 12-month follow-up, were obtained from 384 participants. Beliefs were related to abstinence at the 2-month, p < .05, and 6-month follow-ups, p < .05, for the NP group, but not the placebo. Beliefs were not related to abstinence at 12 months for either group. Survival analysis assessing relapse revealed that beliefs were related to relapse status, regardless of actual group assignment. Our results suggest that there is a relationship between perceived drug assignment and treatment outcome. Future studies using multiple treatment outcome measures and assessments of beliefs over time are warranted.
Keywords: perceived drug assignment, placebo, nicotine patch, smoking abstinence, treatment outcome
1. Introduction
Double-blind, randomized placebo-controlled clinical trials are typically conducted to assess the efficacy of anti-smoking medications. In these trials, both investigators and participants are blinded to treatment assignment in order to limit expectancy and co-intervention biases. However, participants’ perception of medication assignment may be affected by the presence or absence of side effects or treatment effects (Gottlieb, Killen, Marlatt & Taylor, 1987).
Although it is important to determine the success of blinding in clinical trials (Hughes & Krahn, 1985), such data, if collected, are seldom reported. One review of 73 double-blind, placebo controlled clinical trials of nicotine replacement therapies found that only 17 studies assessed blindness integrity (Mooney, White, & Hatsukami, 2004). Of these 17 studies, 12 reported that the odds of participants correctly guessing drug assignment were better than chance. Deficiencies of the double-blind paradigm and/or lack of information on blindness integrity are not unique to clinical trials assessing anti-smoking medications (Kirsch & Weixel, 1988; Quitkin, Rabkin, Gerald, Davis, & Klein, 2000). In a review of the efficacy of double-blind procedures in evaluating psychotropic drugs, Fisher & Greenburg (1993) found that “…the vast majority of studies of psychiatric drug efficacy have simply assumed that the double-blind design is effective and did not test the actuality of blindness by determining whether patients and participating research personnel were able to differentiate active drug from placebo” (Fisher & Greenburg, 1993, p. 346). In studies that did include assessments of blind integrity, participants and clinicians were often able to correctly identify drug condition at odds levels better than chance (Fisher & Greenburg, 1993; Rabkin, Markowitz, Stewart, McGrath, Harrison, Quitkin, et al, 1986).
Data bearing on the relationship between participants’ perceived drug assignment and treatment outcome is seldom presented in reports of smoking cessation treatment research. In one study, Dar and colleagues (Dar et al., 2005) found that regardless of actual treatment assignment, smokers who believed they had received nicotine replacement therapy reported larger reductions in smoking than those who thought they had received a placebo at the 6-month follow-up.
Hall and colleagues (Hall et al., 2007) assessed the relationship between participants’ perceived drug assignment and treatment outcome in two smoking cessation trials examining the efficacy of the antidepressant nortriptyline. Among participants on active medication, those who failed to quit smoking at end of treatment (12 weeks) were more likely to believe they had received placebo than successful quitters who received nortiptyline. However, belief about drug assignment was not associated with abstinence status at end of treatment for participants in the placebo condition. Perceived drug assignment was not associated with abstinence status at final follow-up in either group.
Most recently, two studies examined the relationships between perceived drug assignment and abstinence rates in clinical trials of bupropion for smoking cessation. Schnoll and colleagues (Schnoll et al., 2008) found that the effect of treatment arm guess was most pronounced at the end of treatment for both groups (bupropion and placebo), but that this relationship only remained evident at the 6- and 12-month follow-ups for the bupropion participants. Those participants who correctly guessed that they had received bupropion were more likely to be abstinent at the follow-ups than those who received bupropion but guessed placebo. Analyses conducted to assess whether side effects, withdrawal symptoms, or mood mediated the relationship between perceived drug assignment and actual drug assignment found no evidence of mediation. In the other bupropion study, (Thomas, Guo, Lynam, Powell, Okuyemi, Bronars, et al., 2008), after adjusting for actual treatment assignment, age, and baseline cotinine levels, participants who perceived being assigned to bupropion versus placebo were more likely to be abstinent at end of treatment (week 6) and at the week 26 follow-up.
Although these studies reported significant relationships between perceived drug assignment and treatment outcome, there were conflicting findings regarding the strength of these relationships at various time points and depending on the drug assignment (placebo vs. drug). Several factors could account for these differences. Different medications were examined in most of these trials, and the medications were used for different lengths of time. The length of time between the query about participants’ beliefs and smoking status also was different among the studies. Finally, treatment outcome was measured differently. Hall, Schnoll, and Thomas used biologically-verified abstinence as the primary outcome measure, whereas the Dar analysis was based on self-reported number of cigarettes smoked. The use of different pharmacotherapies and/or dependent variables might explain discrepancies in the results of these studies. To assess these hypotheses regarding the source of variation in results across studies, Hall and colleagues (2007) called for an analysis examining the relationship between participants’ perceived drug assignment and treatment outcome using biologically-verified abstinence among smokers given nicotine replacement therapy versus placebo.
This paper presents the results of secondary analyses of the relationships of actual versus perceived drug assignment and treatment outcome in smokers given nicotine (NP) or placebo patches. Treatment outcome was defined in two ways in the analysis: biologically-verified 7-day point prevalence abstinence and relapse. The examination of multiple outcomes is in keeping with recent recommendations for the analysis of multiple outcomes in smoking cessation clinical trials (Hughes, Keely, Niaura, Ossip-Klein, Richmond, & Swan, 2003). As in previous studies (Dar et al., 2005; Scholl et al., 2008), we also examined potential demographic and tobacco-related factors that could be associated with correct guessing to further understand the processes through which participants construct their beliefs about drug treatment assignment.
To our knowledge, this is the first study to assess the relationships between multiple measures of treatment outcome and participants’ perceived drug assignment using nicotine patches in a randomized clinical trial. This study extends the examination of beliefs about treatment effects from laboratory experiments to more naturalistic settings. Assessing perceived drug assignment in randomized placebo-controlled clinical trials provides information on the relationship between beliefs and treatment outcome over time (Dar et al., 2005). The findings of this study may help to improve our understanding of the relationship between perceived drug assignment and treatment outcome and could potentially influence the design of future treatment studies, such as inclusion of a no medication group. As noted by Perkins and colleagues (Perkins, Sayette, Conklin, & Caggiula, 2003), better understanding of patients’ expectations and/or beliefs could help to determine the therapeutic mechanisms of pharmacotherapy. Further, if expectancies and treatment outcome are associated, providing more information on the likely therapeutic effects of NRT and addressing any negative expectations for treatment response in clinical settings may positively influence treatment responses to pharmacotherapy (Schnoll et al., 2008).
2. Methods
2.1. Recruitment of participants
Program announcements in the San Jose Bay Area were placed in local newspapers. Interested smokers at least 18 years of age were instructed to contact the program office via telephone. A baseline interview was conducted at the time of the initial telephone contact to collect demographic information and determine eligibility. Interested smokers who were pregnant, lactating, or receiving active treatment for cancer or peptic ulcer were excluded from the study. Those who reported a history of heart disease, recent chest pain, diabetes, or thyroid disease were asked to obtain written permission to participate from their personal physician.
2.2. Randomized controlled trial design
In the original study designed to assess the efficacy of the nicotine patch combined with a self-help treatment program, a total of 424 adult smokers (214 males, 210 females) were randomized to a 2 (NP vs. placebo) × 2 (Video + Manual vs. Manual only) fully crossed factorial experiment (Killen, Fortmann, Davis, & Varady, 1997). Half of the participants received an 8-week supply of nicotine patches (21 mg) for the primary treatment phase, and half received placebo patches that appeared identical to active patches. After Week 8, participants receiving NP were titrated to 14 mg for Weeks 9–12 and then 7 mg for Weeks 13–16. Participants in the placebo condition received matching placebo patches throughout the down-titration phase. Assignment to medication condition was double-blind. The self-help program included two levels; all participants received a self-help manual, half of them also received a 20-minute video designed to enhance the efficacy of the manual.
During the 8-week treatment period, participants were contacted by telephone at 24 hours, 1 week, and 1 month after beginning treatment to asses smoking status, side effects, and compliance with patch use. Abstinence and relapse were assessed at 2, 6 and 12-month time-points.
The study was approved by the Stanford University Administrative Panels on Human Subjects in Medical Research. Participants read and signed written consent forms during the initial office visit.
2.3. Measures
2.3.1. Smoking abstinence
Abstinence was defined as not smoking, even a puff, for the 7 consecutive days prior to contact with study staff. This was verified by saliva cotinine levels below 20 ng/ml at the 2, 6, and 12 month follow-ups except for those still wearing patches at the 2-month assessment; abstinence for those participants was assessed by exhaled air carbon monoxide (CO) levels below 9 ppm.
2.3.2. Relapse to smoking
Relapse was defined as smoking even a puff on 7 consecutive days after quitting. The first day of 7 consecutive days smoking was considered the date of relapse (Ossip-Klein, Bigelow, Parker, Curry, Hall, & Kirkland, 1986). Those unable to provide biochemical verification of non-smoking status were re-classified as smokers, with the exception of those who were out of the area at the time of assessment.
2.3.3. Perceived drug assignment
At the 12-month follow-up, participants were asked to guess drug assignment by responding to the following statement: “I’m now going to tell you whether you received patches which contained nicotine, or patches that contained no drug, but first I’d like you to guess which type you had.” The participant provided a guess and then was informed of their true assignment.
2.3.4. Covariates
Demographic characteristics (age, years of education, marital status, ethnicity, gender) and number of cigarettes smoked per day were assessed at the initial baseline telephone interview. Body mass index (BMI), the modified Fagerström Tolerance Questionnaire (mFTQ; Killen, Fortmann, Telch, & Newman, 1988), a measure of nicotine dependence, and the 20-item Center for Epidemiological Studies Depression Scale (CES-D; Radloff, 1977) used to assess depressive symptoms, were evaluated at the initial office visit prior to quit date. A two item craving measure (“Have you felt cravings for a cigarette?” and “Have you felt strong urges to smoke?” was used to assess cravings 24 hours after the quit date (Killen et al., 1988). For each item, participants rated on a 6-point scale how upsetting cravings and urges had been and a craving score was computed by averaging the two items. Withdrawal symptoms (Killen et al., 1988) also were measured 24 hours after the quit date. Compliance with the nicotine patch treatment protocol was measured by telephone at 24 hr, 1 week, 1 month, and 2 months after beginning treatment. Participants were considered to be compliant if, at all four assessments, they responded “yes” to the question, “Are you wearing a patch now?”
2.4. Data analyses
2.4.1. Demographics and comparability of study groups
Differences between treatment groups and differences between participants included in this secondary analysis and those who were not were compared using one-way ANOVA for continuous measures and a Chi-square test for categorical data.
2.4.2. Integrity of the blind
The integrity of the blind was tested using a chi square test comparing the actual drug assignment to the perceived drug assignment.
2.4.3. Relationship between perceived drug assignment and abstinence status
Perceived drug assignment and abstinence status was tested via chi-square analyses using nested guesses within actual drug treatment group (Dar et al., 2005; Hall et al., 2007).
2.4.4. Relationship between perceived drug assignment and relapse status
Differences in relapse curves were examined using Cox proportional hazards survival analysis.
2.5. Correctness of guess
For each drug treatment group, separate one-way ANOVA (for continuous variables) and chi-square tests (for categorical variables) were conducted to compare those who correctly guessed drug assignment with those who guessed incorrectly on the following variables: number of cigarettes per day, the five-question modified Fagerström Tolerance Questionnaire (mFTQ; Killen, Fortmann, Telch, & Newman, 1988), the Center for Epidemiological Studies Depression Scale (CES-D; Radloff, 1977), craving (Killen et al., 1988), withdrawal symptoms (Killen et al., 1988), body mass index (BMI), age, years of education, marital status, ethnicity, gender, and compliance with patch treatment. As appropriate, Cohen’s d (continuous measures) and odds ratios (categorical measures) were computed as effect sizes.
3. Results
3.1. Demographics and comparability of study groups
Of the 424 participants, 384 participants were contacted at 12-month follow-up and provided a guess of drug assignment. Of the 384 participants, 188 were male, less that half were married, and the majority of participants were Caucasian. As in the original study, participants who were self-reported abstainers but were not able to provide biologically-verified confirmation of smoking status due to being out of the area were retained as non-smokers. In the current analysis, this resulted in retaining 2 participants as nonsmokers at 6 months and 4 self-reported abstainers at 12 months. Table 1 describes the demographic characteristics and smoking behavior of the sample by drug assignment (NP vs. placebo; N = 384). There were no significant differences in these variables for those who received placebo versus NP, or for those who provided a guess (N = 384) and those who did not (N = 40) (data not shown).
Table 1.
Demographic characteristics and smoking behavior of the sample by drug assignment (N = 384)
| Variable | Active (N = 196) | Placebo (N = 188) |
|---|---|---|
| M (and SD) | ||
| Cigarettes smoked per day | 24.1 (9.4) | 23.2 (8.8) |
| mFTQa | 16.6 (3.8) | 16.8 (3.4) |
| CES-Db | 12.7 (8.6) | 13.2 (9.8) |
| Age (years) | 46.4 (11.1) | 44.7 (11.6) |
| Education (years) | 14.3 (2.3) | 14.1 (2.4) |
| BMI (kg/m2)c | 26.7 (5.7) | 26.1 (4.9) |
| N (%) | ||
| Gender (male) | 100 (51%) | 88 (47%) |
| Marital Status (married) | 93 (47%) | 84 (45%) |
| Ethnicity (Caucasian) | 166 (85%) | 153 (81%) |
Note. No statistically significant differences between treatment and control or between those who answered questions and those who did not. N = 194 for the mFTQ for the active group; N = 195 for the CES-D for the active group; N = 185 for the CES-D for the placebo group.
mFTQ = modified Fagerström Tolerance Questionnaire.
CES-D = Center for Epidemiological Studies Depression Scale.
BMI = body mass index.
3.2. Integrity of the blind
There was a significant relationship between actual drug treatment condition and perceived drug treatment, χ2 (1, n = 384) = 60.3, p < .0001, OR = 5.4, 95% CI = 3.5 – 8.4; 75% of those on active nicotine patch guessed that they were on active medication and 64% of those in the placebo condition guessed that they were in the placebo group. There were no statistically significant differences in guess of medication group between those in the video plus self-help treatment manual group (56% guessed active) and those in the self-help treatment manual only group (55% guessed active).
3.3. Perceived drug assignment and abstinence status
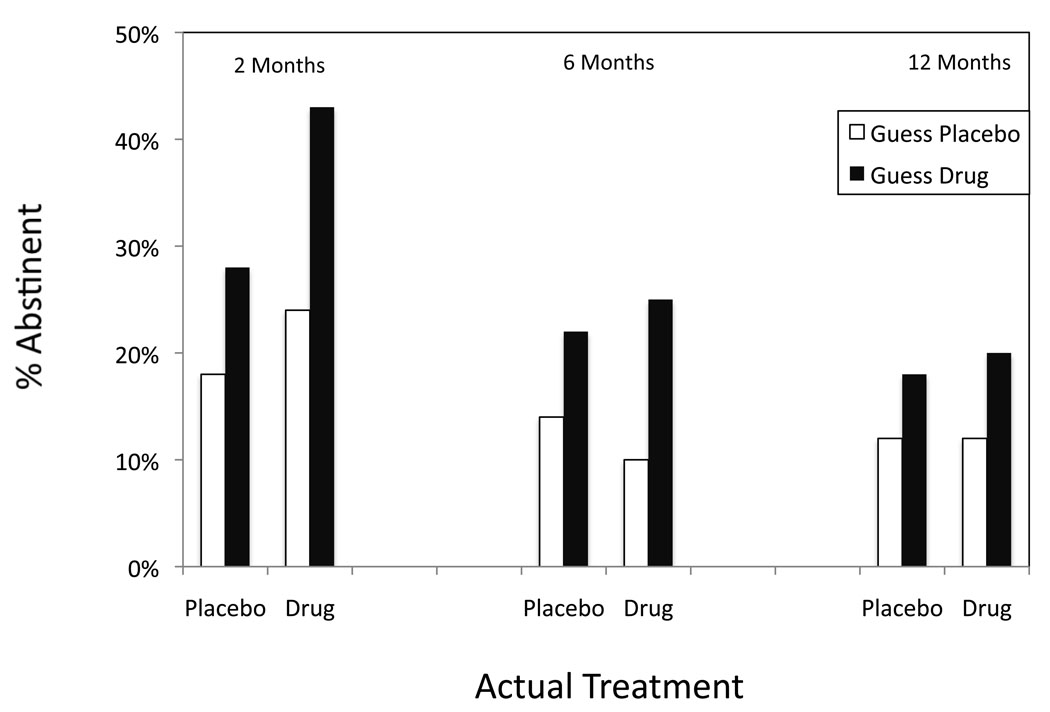
As displayed in Figure 1, perceived drug assignment assessed at 12 months was related to abstinence status at 2 months for those in the NP treatment condition, χ2 (1, n = 196) = 5.2, p = .02, OR = 2.3, 95% CI = 1.1 – 4.8. Those in the NP treatment group who believed they were on active medication were more likely to be abstinent at the 2-month follow-up compared to those who were on NP but believed they were on placebo. This was not the case for those assigned to the placebo. Here, perceived drug assignment was not related to abstinence at the 2-month follow-up, χ2 (1, n = 188) = 2.6, p = .11, OR = 1.8, 95% CI = .9 – 3.6.
Figure 1.
Perceived drug assignment at 12 months and abstinence status at 2-, 6-, and 12-month follow-ups (N = 384).
Similarly, at 6 months, NP participants who later guessed NP had significantly higher rates of abstinence at the 6-month follow-up than those on NP who guessed placebo, χ2 (1, n = 196) = 4.9, p = .03, OR = 3.0, 95% CI = 1.1 – 8.0. As before, among those assigned to placebo, perceived drug assignment was not associated with abstinence status, χ2 (1, n = 188) = 2.1, p = .15, OR = 1.8, 95% CI = .8 – 3.8.
At 12-month follow-up, treatment outcome was not associated with perceived drug assignment for either the NP group, χ2 (1, n = 196) = 1.6, p = .20, OR = 1.8, 95% CI = .7 – 4.7 or the placebo group, χ2 (1, n = 188) = 1.5, p = .23, OR = 1.7, 95% CI = .7 – 3.9.
3.4. Perceived drug assignment and non-relapse status
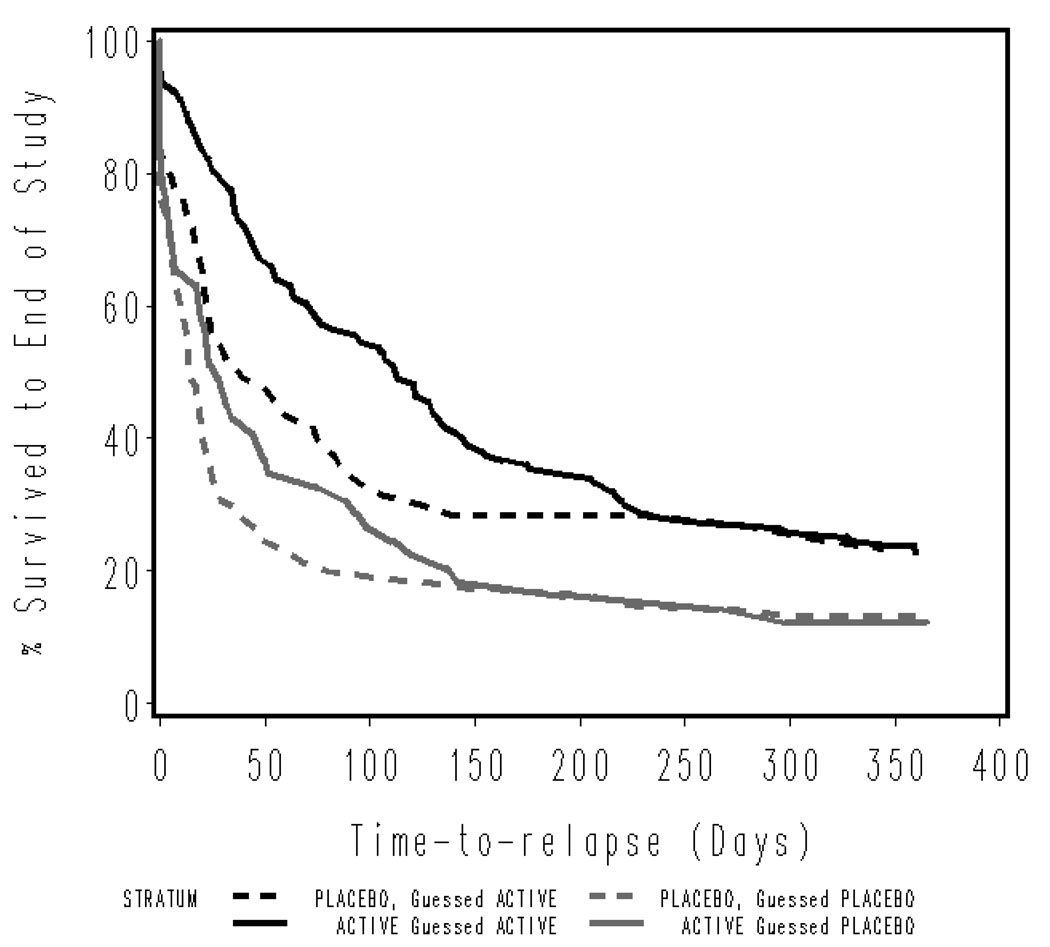
The survival analysis presented in Figure 2 illustrates the differences in relapse rates over time for each of the treatment groups by perceived drug assignment. There was a significant relationship between perceived drug assignment and amount of time to relapse, regardless of actual drug assignment. Among participants who received NP, log-rank χ2 (1, n = 196) = 11.6, p = .0007, and participants who received placebo, log-rank χ2 (1, n = 188) = 6.9, p = .008, those who believed they had received NP avoided relapse longer than those who believed they had received placebo.
Figure 2.
Relapse to regular smoking (daily use for at least 1 week) over 1 year after randomization by actual versus perceived drug treatment assignment (nicotine patch or placebo).
3.5. Correctness of guess
Among active patch users, those who had higher baseline mFTQ scores, F (1, 192) = 7.5, p = .007, Cohen’s d = .46, and those who were more compliant with patch usage, χ2 (1, n = 196) = 18.5, p < .0001, OR = 4.9, 95% CI = 2.3 – 10.6, were more likely to correctly guess that they were receiving NP than those who guessed placebo. In the placebo condition, none of the factors were associated with correctness of guess.
4. Discussion
Perceived drug assignment at the 12-month follow-up was related to abstinence status at both the 2-month and 6-month follow-ups only for those in the NP treatment group. At both follow-ups, NP users who later guessed that they had received NP were more likely to be abstinent than NP users who later guessed that they had been given a placebo. In contrast, perceived drug assignment was not associated with abstinence status for those in the placebo group.
We could find only four other published analyses of the relationships between participants’ perceived drug assignment and treatment outcome in smokers. The findings with respect to abstinence are similar to those reported by Hall and colleagues (2007) and Scholl et al. (2008). In Hall’s studies, beliefs at 12-month follow-up were related to abstinence status for drug treatment group at 6-month follow-up, but not for placebo. Similarly, perceived drug assignment was not associated with abstinence status at the 12-month follow-up. In the Schnoll study (2008), the effect on abstinence status for perceived drug assignment appeared most pronounced at earlier assessments; however, statistical tests of the relationship were not reported. The comparability of these findings across medications is of interest. Previously, Hall et al. (2007) suggested that their findings might have been different from results reported by Dar and colleagues (2005) because the studies examined different medications. Our findings offer evidence against this explanation.
The analysis of relapse tells a somewhat different story and resembles more closely the results reported by Dar and colleagues (2005) and Thomas and colleagues (2008). Perceived drug assignment was related to treatment outcome, regardless of actual treatment assignment. As is evident in Figure 2, those who believed they had received NP were more likely to avoid relapse than those who believed they had received placebo, regardless of whether they actually received NP or placebo patch.
How to account for the discrepancies? The analysis of abstinence is based on a dichotomous dependent variable. In contrast, the analysis of relapse is based on a continuous dependent variable (as was the treatment outcome of cigarette reduction in the Dar et al. study), thus yielding a more powerful test of the relationship. Thomas et al. (2008) assessed abstinence with a dichotomous variable, although with a different sample (primarily African American smokers). As recommended by Hughes et al. (2003), clinical trials should report results from survival analyses in addition to more traditional measures of abstinence. It would be of interest to evaluate the relationship between perceived treatment assignment and relapse in previous studies that have survival data available. It also should be noted that three of the four studies allowed a response option of “not sure” (Schnoll et al., 2008), “did not know” (Dar et al., 2007), or “uncertain” (Thomas et al., 2008) when asked to guess drug assignment. It is unknown if some of the discrepancies between studies may be related to the nature of the treatment arm guess.
Current understanding of the factors that influence smokers’ conclusions about treatment assignment is limited. Dar et al. (2007) found that guess accuracy was related to Fagerström score only in the placebo group. That is, those in the placebo group who falsely believed they had received NP reporting higher Fagerström scores compared with those who correctly guessed that they received placebo. We found that those in the NP group with higher Fagerström scores and higher compliance with patch usage were more likely to guess correctly than those in the NP group who guessed incorrectly. However, none of the putative predictors were related to correctness of guess in the placebo group. None of the variables examined by Hall et al. (2007) predicted correctness of guess; however, the Fagerström was not included in their analyses. Schnoll et al. (2008) assessed side effects, withdrawal symptoms, and mood as potential mechanisms through which participants might guess drug assignment and, although they found that reduced negative mood and increased positive mood were related to a greater likelihood of participants’ guessing bupropion, these factors did not mediate the relationship between perceived and actual drug assignment.
It is of interest that measures of nicotine dependence predicted correctness of guess in our study and in Dar’s study (2005). One interpretation of our findings is that participants with higher levels of nicotine dependence are better able to distinguish whether or not they are receiving active medication because they benefit most from the effects of NRT on withdrawal symptom reduction; however, Dar and colleagues’ findings do not support this. Future studies should assess common correlates of treatment outcome to determine what factors contribute to participants’ ability to correctly guess drug assignment.
In general, our results suggest that there is an association between perceived drug assignment and treatment outcome as assessed by both abstinence and relapse status. Although the effect in the placebo group was detected only in the relapse data, the pattern of results with respect to abstinence was similar for participants in both nicotine patch and placebo conditions. It is especially noteworthy that those in the placebo condition who guessed active medication had lower relapse rates than those participants who were on active medication but guessed placebo. If these findings are replicated, this has implications for clinical trials designed to assess the efficacy of pharmacotherapy for smoking cessation. One reason for utilizing placebo-controlled designs is to limit expectancy bias; however, participants’ expectancies may influence treatment outcome despite use of placebo-controlled designs. To fully assess the placebo effect of pharmacotherapy for nicotine dependence, inclusion of no-treatment control groups or use of active placebos may be warranted (Hróbjartsson & Gøtzsche, 2004). Clinically, if future studies reveal that expectancies of pharmacotherapy can influence treatment outcome, clinicians should discuss both positive and negative expectancies of nicotine replacement with their patients. If patients are informed of the positive effects of nicotine replacement on smoking cessation, these expectations could have a positive impact on treatment outcome.
Our analysis shares several limitations with previous published research in this area (Dar et al., 2005; Hall et al., 2007; Schnoll et al., 2008; Thomas et al., 2008). First, as in the other investigations, there was a significant relationship between actual drug treatment condition and perceived drug assignment (blinding failure). Second, we cannot know if perceived drug assignment influences treatment outcome or vice versa, and beliefs about drug assignment cannot be equated with expectations of treatment because, in each case, participants were asked to guess their treatment assignment only at final follow-up. However, if this were true, one would postulate that most participants who were abstinent at 12-month would guess that they were on active medication; as seen in Figure 1, this clearly is not the case. Although lab-based studies have the advantage of manipulating belief and expectations about drug assignment prior to assessing the effect of nicotine replacement, thus allowing for causal inferences, this is at the expense of assessing these relationships over time within a smoking cessation medication trial (Dar et al, 2005). Future treatment studies should employ multiple assessments of beliefs about drug assignment over time, including expectations regarding treatment to further our understanding of these relationships.
Acknowledgements
This research was funded by Public Health Service Grant #47219 from the National Heart, Lung, and Blood Institute. A version of this study was presented as a poster at the 14th Annual Meeting of the Society for Research on Nicotine & Tobacco, Portland, OR, February, 2008.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Dar R, Stronguin F, Etter J-F. Assigned versus perceived placebo effects in nicotine replacement therapy for smoking reduction in Swiss smokers. Journal of Consulting and Clinical Psychology. 2005;73(2):350–353. doi: 10.1037/0022-006X.73.2.350. [DOI] [PubMed] [Google Scholar]
- Fisher S, Greenberg RP. How sound is the double-blind design for evaluating psychotropic drugs? The Journal of Nervous and Mental Disease. 1993;181:345–350. doi: 10.1097/00005053-199306000-00002. [DOI] [PubMed] [Google Scholar]
- Gottlieb AM, Killen JD, Marlatt GA, Taylor CB. Psychological and pharmacological influences in cigarette smoking withdrawal: Effects of nicotine gum and expectancies on smoking withdrawal symptoms and relapse. Journal of Consulting and Clinical Psychology. 1987;55(4):606–608. doi: 10.1037/0022-006X.55.4.606. [DOI] [PubMed] [Google Scholar]
- Hall SM, Gorecki JA, Reus VI, Humfleet GL, Muñoz RF. Belief about drug assignment and abstinence in treatment of cigarette smoking using nortriptyline. Nicotine and Tobacco Research. 2007;9(4):467–471. doi: 10.1080/14622200701239480. [DOI] [PubMed] [Google Scholar]
- Hróbjartsson A, Gøtzsche PC. Placebo interventions for all clinical conditions. Cochrane Database of Systematic Reviews. 2004:2. doi: 10.1002/14651858.CD003974.pub2. Art. No.: CD003974. DOI: 10.1002/14651858.CD003974.pub2. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine and Tobacco Research. 2003;5(1):13–25. [PubMed] [Google Scholar]
- Hughes JR, Krahn D. Blindness and the validity of the double-blind procedure. Journal of Clinical Psychopharmacology. 1985;5(3):138–142. [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Davis L, Varady A. Nicotine patch and self-help video for cigarette smoking cessation. Journal of Consulting and Clinical Psychology. 1997;65(4):663–672. doi: 10.1037//0022-006x.65.4.663. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Telch MJ, Newman B. Are heavy smokers different from light smokers? A comparison after 48 hours without cigarettes. Journal of the American Medical Association. 1988;260(11):1581–1585. [PubMed] [Google Scholar]
- Kirsch I, Weixel LJ. Double-blind versus deceptive administration of a placebo. Behavioral Neuroscience. 1988;102(2):319–323. doi: 10.1037//0735-7044.102.2.319. [DOI] [PubMed] [Google Scholar]
- Mooney M, White T, Hatsukami D. The blind spot in the nicotine replacement therapy literature: Assessment of the double-blind in clinical trials. Addictive Behaviors. 2004;29(4):673–684. doi: 10.1016/j.addbeh.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Ossip-Klein DJ, Bigelow G, Parker SR, Curry S, Hall S, Kirkland S. Task Force 1: Classification and assessment of smoking behavior, Proceedings of the National Working Conference on Smoking Relapse. Health Psychology. 1986;5 Suppl.:3–11. [PubMed] [Google Scholar]
- Perkins K, Sayette M, Conklin C, Caggiula A. Placebo effects of tobacco smoking and other nicotine intake. Nicotine and Tobacco Research. 2003;5(5):695–709. doi: 10.1080/1462220031000158636. [DOI] [PubMed] [Google Scholar]
- Quitkin FM, Rabkin JG, Gerald J, Davis JM, Klein DF. Validity of clinical trials of antidepressants. American Journal of Pyschiatry. 2000;157(3):327–337. doi: 10.1176/appi.ajp.157.3.327. [DOI] [PubMed] [Google Scholar]
- Rabkin JG, Markowitz JS, Stewart J, McGrath P, Harrison W, Quitkin FM, Klein DF. Psychiatry Research. 1986;19:75–86. doi: 10.1016/0165-1781(86)90094-6. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Schnoll RA, Epstein L, Audrain J, Niaura R, Hawk L, Shields PG, Lerman C, Wileyto EP. Can the blind see? Participant guess about treatment arm assignment may influence outcome in a clinical trial of bupropion for smoking cessation. Journal of Substance Abuse Treatment. 2008;34:234–241. doi: 10.1016/j.jsat.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JL, Guo H, Lynam IM, Powell JN, Okuyemi KS, Bronars CA, Ahluwalia JS. The impact of perceived treatment assignment on smoking cessation outcomes among African-American smokers. Journal of General Internal Medicine. 2008;23:1361–1366. doi: 10.1007/s11606-008-0656-3. [DOI] [PMC free article] [PubMed] [Google Scholar]