Abstract
Objective
Maternal diabetes adversely impacts embryonic development. We test the hypothesis that hyperglycemia-induced JNK1/2 activation mediates iNOS induction.
Study Design
Levels of iNOS mRNA and nitrosylated protein were determined in cultured C57BL/6J conceptuses exposed to hyperglycemia (300 mg/dl glucose) and C57BL/6J embryos exposed to streptozotocin-induced diabetes. iNOS-luciferase activity and endogenous reactive nitrogen species were determined in transfected PYS-2 (mouse teratocarcinoma) cells exposed to hyperglycemia (450 mg/dl glucose).
Results
Hyperglycemia increased iNOS mRNA and SP600125, a potent JNK1/2 inhibitor, abolished this effect. Hyperglycemia increased iNOS-luciferase activities and SP600125 blocked this effect. Diabetes increased iNOS mRNA and jnk2 gene deletion abrogated this effect. Correlated with iNOS gene induction, both hyperglycemia in vitro and diabetes in vivo enhanced the production of reactive nitrogen species and increased protein nitrosylation. jnk2 gene deletion blocked diabetes-induced protein nitrosylation.
Conclusion
JNK1/2 activation mediates hyperglycemia-induced iNOS gene expression and consequent nitrosative stress in diabetic embryopathy.
Keywords: iNOS gene expression, nitrosative stress, diabetic embryopathy, JNK activation, JNK inhibitor
INTRODUCTION
Maternal diabetes is a significant risk factor for adverse pregnancy outcomes including miscarriages and congenital malformations. These adverse pregnancy outcomes that are associated with diabetic women are significant clinical problems. The recent rise of diabetes in reproductive age women 1 makes these pregnancy complications a continuing issue 2. Because glycemic control in diabetic women is difficult to control and maintain 3 and malformation rates of children of diabetic women are at least six times higher than those in non-diabetic women 1, therapeutic interventions in addition to insulin are needed for diabetes-associated adverse pregnancy outcomes. Mechanistic studies are essential steps for the development of accessible, convenient and effective prevention strategies. Hyperglycemia resulting from diabetes mellitus adversely impacts embryonic development through induction of apoptosis in embryonic tissues. The mechanisms underlying hyperglycemia-induced apoptosis are not completely understood. We have found that the pro-apoptotic c-Jun N-terminal kinases 1 and 2 (JNK1/2) is activated in embryonic tissues exposed to maternal diabetes in vivo and hyperglycemic embryo cultures of both Sprague–Dawley rats and C57BL/B6J mice in vitro 4–6. JNK1/2 agonist mimics the teratogenic effect of hyperglycemia to induce embryonic malformations whereas targeted deletion of jnk2 gene significantly ameliorates diabetes-induced malformations 4. Thus, JNK1/2 plays a causative role in the induction of diabetic embryopathy.
JNK has three isoforms (JNK1, JNK2, and JNK3) encoded by three different genes. The jnk1 and jnk2 genes are ubiquitously expressed. The specific molecular targets of JNK include transcription factor AP-1 (mainly c-Jun, JunB, and ATF-2), Foxo factors 7, 8, as well as many other non-transcription factors such as Bcl-2 proteins 9. The JNK pathway specifically responds to stress-induced signals that drive apoptosis 10, 11. Activation of transcription factors downstream of JNK1/2 activation relays the pro-apoptotic signals to the nucleus thus inducing apoptotic gene expression that leads to apoptosis. Mice having null mutations in a single JNK gene develop normally. However, the double JNK1/JNK2 null mutants are embryonic lethal due to abnormal apoptosis in the brain 12. The single JNK gene null mice including jnk1−/− (JNK1KO) and jnk2−/− (JNK2KO) mice are useful models for delineating pro-apoptotic effects emanating from JNK1/2 activation.
Increased levels of nitric oxide (NO) are associated with the adverse impact of maternal diabetes on embryonic development 13. NO is synthesized from oxidation of L-arginine by three distinct NO synthases (NOS): neuronal (nNOS), endothelial (eNOS), and inducible (iNOS) 14, 15. nNOS and eNOS are constitutively expressed at low levels. When iNOS is induced, it generates very high concentrations of NO 16. NO has been shown to be involved in cell differentiation, proliferation and apoptosis 17–19. Although NO is of physiological importance, it can also be cytotoxic. iNOS and eNOS are expressed during early embryonic development 13. Hyperglycemia increases NO production in embryonic tissues 13 inducing the production of reactive nitrogen species that leads to nitrosative stress.
We hypothesize that hyperglycemia-induced JNK1/2 activation mediates iNOS induction. To test this hypothesis, we investigated the relationship between JNK1/2 activation and iNOS gene expression in diabetic embryopathy. By using pharmacological inhibitors of JNK1/2 activation (SP600125) in vitro and target deletion of jnk2 in mice, we have demonstrated that JNK1/2 activation is responsible for hyperglycemia-induced iNOS gene expression and consequent nitrosative stress.
Material and methods
Animals and reagents
C57BL/6J mice (median body weight 22 g) and jnk2−/− (JNK2KO) mice on the same background were purchased from Jackson Laboratory (Bar Harbor, Maine). Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA). PYS-2 cell line was purchased from ATCC (Cat# CRL-2745). Streptozotocin (STZ) from Sigma was dissolved in sterile 0.1 M citrate buffer (pH 4.5). Sustained-release insulin pellets were purchased from Linplant (Linshin, Canada).
Whole-conceptus culture
C57BL/6J mice were paired overnight. The next morning was designated embryonic day (E) 0 if a vaginal plug was present. Mouse conceptuses at E8 were dissected out of the uteri in PBS (Invitrogen, La Jolla, CA). The parietal yolk sac was removed using a pair of fine forceps and the visceral yolk sac was left intact. Conceptuses (4/bottle) were cultured in 4 ml rat serum at 38°C in 30 rev/min rotation in the roller bottle system. For the initial 24 hr of culture, the bottles were gassed with 5% O2/5% CO2/90% N2. For the following 12 hr, the bottles were gassed with 20% O2/5% CO2/75% N2, and in the last 12 hr, 40% O2/5% CO2/75% N2 were applied. Conceptuses were cultured under euglycemic (150 mg/dl glucose, a value close to the blood glucose level of non-diabetic mice) and hyperglycemic (300 mg/dl glucose) conditions in the presence and absence of SP600125, a pharmacological JNK1/2 inhibitor.
Real-time PCR (RT-PCR)
Total RNA was isolated from embryonic tissues of cultured conceptuses or conceptuses retrieved from non-diabetic or diabetic mice using an RNeasy Mini Kit (Qiagen, Valencia, CA). RT-PCR for iNOS, eNOS and β-actin were performed using ABI TaqMan Gene Expression Assays (assay ID: Mm01309897_m1, Mm01164908_m1 and Mm00607939_s1, respectively, Applied Biosystems, Foster City, CA). Briefly, RNA was reverse transcribed by using the high-capacity cDNA archive kit (Applied Biosystems). RT-PCR and subsequent calculations were performed by a 7700 ABI PRISM sequence detector system (Applied Biosystems), which detected the signal emitted from fluorogenic probes during PCR.
Cell culture, transient transfection and luciferase assay
PYS-2 cells were cultured in DMEM (Invitrogen) plus 2% FBS. Cells were plated overnight to reach about 80% confluency and were transfected with 0.8 μg mouse iNOS promoter luciferase constructor (iNOS-luc) using Lipofectamine™ 2000 (Invitrogen). The iNOS-luc that contains the mouse iNOS promoter from −1588 to +165 plus the luciferase coding sequence was provided by Dr. Sang Geon Kim, Seoul National University, South Korea. After co-transfection with Renilla-luc (normalization control, Promega), PYS-2 cells were incubated for 24h under euglycemic (90 mg/dl glucose, a value commonly used in cell culture studies) or hyperglycemic (300, 450, 800 or 1000 mg/dl glucose) conditions in the presence or absence of 400 nM SP600125. Osmotic controls were not used in this study. Luciferase activities were measured by a dual luciferase kit (Promega) according to the manufacturer’s instructions.
Mouse models of diabetic embryopathy
The procedures for animal use were approved by the Institutional Animal Care and Use Committee of University of Maryland School of Medicine. Eight-week old JNK2KO mice on C57BL/6J background and wild-type (WT) mice of the same strain were intravenously (iv) injected daily with 75 mg/kg STZ over three days to induce diabetes. Insulin pellets were subcutaneously implanted in diabetic mice to restore euglycemia prior to mating. WT and JNK2KO homozygous female mice were mated with male mice of the same respective genotype. On Day 5 of pregnancy (E5), insulin pellets were removed to permit frank hyperglycemia (>250 mg/dl glucose level), so the developing conceptuses would be exposed to a hyperglycemic environment during organogenesis (E7–11). Non-diabetic WT mice with vehicle injections were served as non-diabetic WT controls. On E9, mice were euthanized, and conceptuses were dissected out of the uteri for analysis.
Detection of nitrosylated proteins using Western Blotting
Western Blotting was performed as described by Yang, at el. 20. Briefly, embryonic samples were sonicated in 80 μl ice-cold lysis buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 10 mM NaF, 2 mM Na orthovanadate, 1 mM PMSF and 1% Triton 100) containing a protease inhibitor cocktail (Sigma, St. Louis, MO). Equal amounts of protein (50 μg) were resolved by SDS-PAGE and transferred onto a nitrocellulose membrane (Schleicher & Schuell). Membranes were incubated for 18 h at 4 °C with the following primary antibodies at 1:1000 to 1:2000 dilutions in 5% nonfat milk: rabbit anti-nitrotyrosine (CHEMICON). Membranes were exposed to goat anti-rabbit, anti-mouse (Jackson ImmunoResearch Laboratories, West Grove, PA) secondary antibodies. To ensure that equivalent amounts of protein were loaded among samples; membranes were stripped and probed with a mouse antibody against β-actin (Abcam, Cambridge, MA). Signals were detected using an Amersham ECL Advance Detection Kit (GE Healthcare, Piscataway, NJ) and chemiluminescence emitted from the bands was directly captured using a UVP Bioimage EC3 system (UVP, Upland, CA). Densitometric analysis of chemiluminescence signals were performed by VisionWorks LS software (UVP, Upland, CA). Images of representative immunoblots were arranged using Adobe Photoshop and Microsoft PowerPoint software. All experiments were repeated three times with the use of independently prepared tissue lysates.
Detection of endogenous reactive nitrogen species by immunofluorescence
Reactive nitrogen species production was measured by 2 μM DAF-FM diacetate (4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate, Molecular Probes, Eugene, OR). PYS-2 cells after different treatments were washed with PBS and subsequently incubated with DAF-FM diacetate in PBS at 37 °C for 30 min. After washing PYS-2 cells with PBS, chamber slide containing cells were mounted in DAPI/Antifade Solution (CHEMICON) and the fluorescence was visualized under an Olympus BX41 microscope (Optical Elements Corp.) equipped with an Olympus DP70 digital camera with FITC, TRITC and FITC/TRITC filter sets. Cell nuclei were labeled as blue and DAF-FM diacetate emitted green signals.
Statistics
Data are presented as means ± SE (standard error). One way ANOVA or t-test were performed using SigmaStat 3.5 software. In ANOVA analysis, Tukey-test was used to estimate the significance of the results. Statistical significance was accepted at p< 0.05.
Results
Hyperglycemia increases iNOS but not eNOS mRNA, and pharmacological inhibition of JNK1/2 blocks this effect
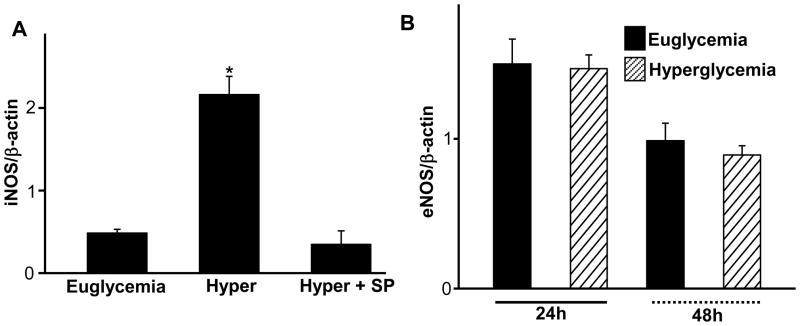
Since hyperglycemia increased embryonic levels of NO [10], we tested if hyperglycemia affected iNOS expression and JNK1/2 activation played a role. E7 mouse conceptuses were cultured under euglycemic and hyperglycemic conditions in the presence or absence of 800 nM SP600125 for 24h. Yolk sacs were harvested for assessment of iNOS mRNA levels. Hyperglycemia significantly increased iNOS mRNA expression in yolk sacs and inhibition of JNK1/2 activation by SP600125 blocked hyperglycemia-induced iNOS expression (Fig. 1A). eNOS is another major NOS that can dramatically modulate NO levels. To determine if hyperglycemia altered eNOS mRNA expression, E7 mouse conceptuses were cultured under euglycemic and hyperglycemic conditions for 24h and 48h. Yolk sacs were harvested for assessment of eNOS mRNA levels. Hyperglycemia did not alter eNOS mRNA levels (Fig. 1B).
Figure 1.
JNK1/2 inhibition blocks hyperglycemia-induced iNOS mRNA expression. iNOS or eNOS mRNA levels were determined by RT-PCR and normalized by β-actin mRNA. A) E7 mouse conceptuses were cultured under euglycemic (150 mg/dl glucose) and hyperglycemic (300 mg/dl) conditions in the presence or absence of 800 nM SP600125 for 24h. Levels of iNOS mRNA were determined in yolk sacs of cultured conceptuses. Hyper: hyperglycemia; Hyper+SP: hyperglycemia plus SP600125. B) E7 mouse conceptuses were cultured under euglycemic (150 mg/dl glucose) and hyperglycemic (300 mg/dl) conditions for 24h and 48h. eNOS mRNA were determined in yolk sacs. Data expressed as Mean ± standard errors (SE) (n=3). * denotes significant difference (p<0.05) with other groups when analyzed by one-way ANOVA and Tukey test.
Hyperglycemia significantly induces iNOS-luc activities, reactive nitrogen species and protein nitrosylation, and pharmacological inhibition of JNK1/2 blocks hyperglycemia-induced iNOS activities
To determine if hyperglycemia induces iNOS gene expression via a transcription mechanism, PYS-2 cell line was used. PYS-2 cells transfected by iNOS-luc and luciferase activities were monitored as indices of iNOS transcription. Cells cultured under euglycemia (90mg/dl glucose) had lower iNOS-luc activities whereas hyperglycemia (450 mg/dl) significantly increased iNOS-luc activities when compared to those in the euglycemia group (Fig. 2B). 450 mg/dl glucose was chosen because a dose-dependent study using 300, 450, 800 and 1000 mg/dl glucose showed that the induction of iNOS-luc activities reaches plateau at 450 mg/dl glucose (Fig. 2A). 450 mg/dl glucose was similar to the blood glucose levels in our diabetic mice. Treatment with 400 nM SP600125 blocked hyperglycemia-increased iNOS-luc activities (Fig. 2B), implicating that JNK1/2 activation mediates hyperglycemia-induced iNOS transcription. Hyperglycemia increased ROS production and elevated iNOS expression enhancing NO production. Because the interaction of ROS and NO results in enhanced production of reactive nitrogen species, we reasoned that hyperglycemia increased reactive nitrogen species production and consequently induced nitrosative stress. To determine if hyperglycemia increased reactive nitrogen species production, fluorescence signals emitted from cells treated with DAF-FM diacetate were used as indices of reactive nitrogen species production. Fluorescence signals in cells of the hyperglycemia group (450 mg/dl glucose) were robust whereas fluorescence signals in cells of the euglycemia group (90mg/dl glucose) were negligible (Fig. 2C), implicating that hyperglycemia potently increased reactive nitrogen species production. Levels of nitrosylated proteins were measured because nitrosylated proteins are prominent markers for nitrosative stress resulting from reactive nitrogen species. Cells treated with 300 or 450 mg/dl glucose gradually increased levels of nitrosylated proteins, especially a band at about 35 kDa, than that of cells cultured in euglycemia (Fig. 2D), demonstrating that hyperglycemia increased protein nitrosylation thus induces nitrosative stress.
Figure 2.
Hyperglycemia increases iNOS transcription and induces nitrosative stress. PYS-2 cells were transfected with iNOS-luc and cultured under euglycemic and hyperglycemic conditions in the presence or absence of 400 nM SP600125. In A, high glucose dose-dependently increased iNOS-luc activities. In B, iNOS-luc activities were significantly increased in hyperglycemia group (Hyper: 450 mg/dl glucose) group than that in the euglycemia group (90 mg/dl glucose). SP600125 blocked hyperglycemia-induced iNOS-luc activities. C showed representative images of DAF-FM diacetate immunofluorescence. Bar represented 0.1 mm. Hyper: hyperglycemia (450 mg/dl glucose). In D, representative images of nitrosylated proteins were shown. The arrow pointed the most elevated band of nitrosylated protein by hyperglycemia. Experiments were repeated three times and same results were obtained. Data expressed as Mean ± SE (n = 3). * denotes significant difference (p<0.05) compared to other groups when analyzed by one-way ANOVA and Tukey test.
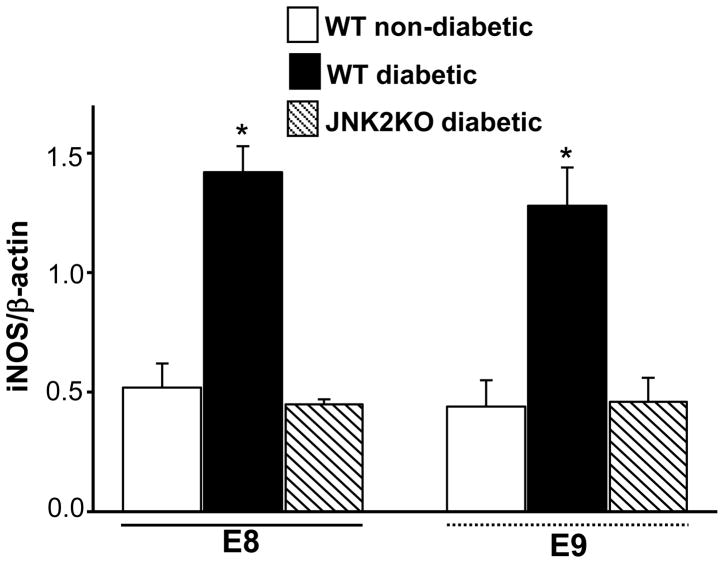
Targeted deletion of jnk2 gene abrogates hyperglycemia-increased iNOS mRNA levels
Pharmacological inhibition of JNK1/2 activation blocks hyperglycemia-induced iNOS transcription (Fig. 2B), suggesting that JNK1/2 activation plays a key role in iNOS induction by hyperglycemia. JNK2 is the predominant form of JNK that is activated by hyperglycemia 4, 5. Our previous findings demonstrate that targeted deletion of jnk2 gene significantly ameliorates hyperglycemia-induced embryonic malformations 4. Therefore, JNK2KO mice were used to determine if JNK1/2 activation plays a causative role in the induction of iNOS gene by maternal hyperglycemia. E8 or E9 conceptuses from WT diabetic mice had higher levels of iNOS mRNA compared to those in E8 or E9 conceptuses from WT non-diabetic mice (Fig. 3). Conceptuses from diabetic JNK2KO mice had significantly lower levels of iNOS mRNA than those in conceptuses from WT diabetic mice (Fig. 3). The levels of iNOS mRNA in conceptuses from WT non-diabetic mice or JNK2KO diabetic mice did not differ (Fig. 3). These results demonstrate that targeted deletion of jnk2 gene is able to block hyperglycemia-induced iNOS gene expression.
Figure 3.
Deletion of jnk2 gene abolishes hyperglycemia-induced iNOS gene expression. E8 and E9 conceptuses were harvested from WT non-diabetic, WT diabetic and JNK2KO diabetic mice. iNOS mRNA levels were determined in E8 and E9 conceptuses by RT-PCR and normalized by β-actin mRNA. Blood glucose levels in WT diabetic and JNK2KO diabetic were similar and they were above 300 mg/dl glucose. Blood glucose levels in WT non-diabetic mice were 146.1 ± 4.3 mg/dl glucose and were significantly lower than those in diabetic mice. Conceptuses from three different pregnant mice were used. Data expressed as Mean ± SE (n = 3). * denotes significant difference (p<0.05) when compared with other groups in the same embryonic stages (E8 or E9) when analyzed by one-way ANOVA and Tukey test.
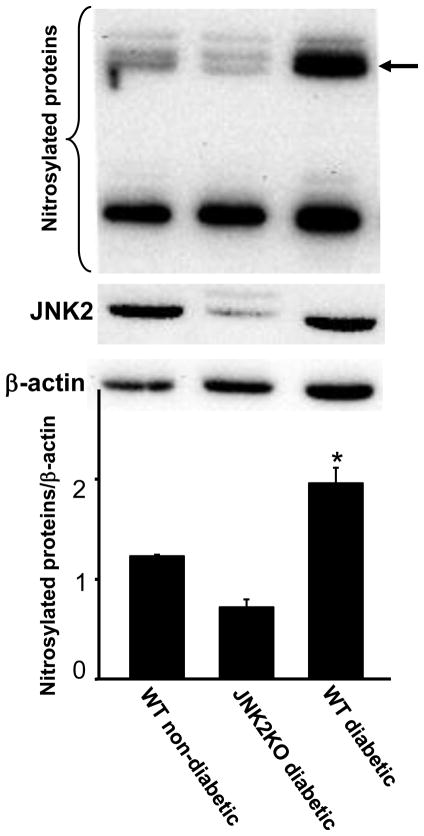
Targeted deletion of jnk2 gene abolishes hyperglycemia-induced protein nitrosylation
Hyperglycemia-induced iNOS expression leads to NO production which reacts with ROS to produce reactive nitrogen species resulting in nitrosative stress. Indeed, hyperglycemia increased levels of nitrosylated proteins in cell culture models (Fig. 2C). To determine if JNK1/2 activation was responsible for maternal hyperglycemia-induced nitrosative stress, levels of nitrosylated proteins were determined in conceptuses from WT non-diabetic, WT diabetic and JNK2KO diabetic mice. Conceptuses from WT diabetic mice had significantly higher levels of nitrosylated proteins than those in conceptuses from WT non-diabetic mice or conceptuses from JNK2KO diabetic mice (Fig. 4). Levels of nitrosylated proteins in conceptuses from WT non-diabetic mice or JNK2KO diabetic mice were comparable (Fig. 4). Thus, JNK2 activation contributes to hyperglycemia-induced nitrosative stress.
Figure 4.
Deletion of jnk2 gene abrogates hyperglycemia-induced protein nitrosylation. E9 conceptuses were harvested from WT non-diabetic, WT diabetic and JNK2KO diabetic mice and were subjected to detection of nitrosylated protein levels. Nitrosylated proteins and JNK2 were detected by Western blotting. β-actin was served as an equal protein loading control. Experiments were repeated for three times by using conceptuses from different mice. Representative images were shown in the top panel. The arrow pointed a most prominent band of nitrosylated protein elevated in conceptuses from WT diabetic mice. Densitometrical analysis of nitrosylated proteins against β-actin was shown in bottom panel. Data were expressed as mean ± SE with n = 3. * denotes significant difference (p<0.001) with other groups when analyzed by one-way ANOVA and Tukey test.
Comment
Our previous findings using pharmacological inhibitors and JNK2KO mice 4–6 demonstrate that JNK1/2 activation plays a causative role in maternal hyperglycemia-induced adverse effects on embryonic development, namely embryonic malformations and embryonic vasculopathy. However, the downstream effectors of JNK1/2 activation in mediating the adverse effects of hyperglycemia are elusive. In the present study, using pharmacological and genetic approaches, we for the first time demonstrate that iNOS gene induction and resultant nitrosative stress in diabetic embryopathy are downstream of JNK1/2 activation.
In the present study, we provide evidence that hyperglycemia increases iNOS but not eNOS expression and pharmacological inhibition of JNK1/2 blocks hyperglycemia-induced iNOS expression. Our pharmacological approach utilized SP600125, a potent inhibitor of JNK1/2 activation. Our previous dose-dependent studies 4, 6 demonstrate the effectiveness of this inhibitor to block hyperglycemia-induced embryonic malformations and embryonic vasculopathy. The 400 nM and doses of SP600125 used in the current study are the most effective doses. The in vitro whole-conceptus culture system, allows us to use pharmacological inhibitors while avoiding complicating maternal influences. The results obtained from in vitro whole-conceptus culture support the conclusion that the JNK1/2-iNOS pathway in strongly implicated in diabetes-associated adverse pregnancy outcomes.
Our genetic approach utilized JNK2KO mice to define the role of JNK1/2 activation in hyperglycemia-induced iNOS gene expression at molecular levels. Because JNK2KO mice do not have any adverse phenotypes12, they are suitable models in our studies. Based on our examination of E11 embryos from non-diabetic JNK2KO mice, JNK2KO background does not affect normal embryonic development. Therefore, the JNK2KO background does not confound our findings in the present study. Our previous studies demonstrated that JNK2 (not JNK1) is the predominant JNK isoform activated by maternal hyperglycemia because phosphorylated p54 JNK, a major product of jnk2 gene, is significantly increased in diabetic embryopathy 5. Consistent with the predominant activation of JNK2, maternal diabetes-induced embryonic anomalies are significantly reduced in the jnk2 null background 4. Indeed, targeted deletion of jnk2 gene abrogates maternal hyperglycemia-induced iNOS gene expression and consequent nitrosative stress. These results further underscore the importance of JNK2 in mediating the adverse effects of maternal diabetes on embryonic development.
Our study utilized several suitable models to establish the relationship between JNK1/2 activation and iNOS expression under hyperglycemic conditions. The in vivo diabetic embryopathy models mimic the conditions of pregnant women with preexisting diabetes. Experimental evidence demonstrates that conceptuses at period of organogenesis (E7-E10) are most vulnerable to hyperglycemic insults. The use of E8 and E9 conceptuses in the present study is most suitable to delineate the molecular events mediating the adverse effects of hyperglycemia in embryonic development. The ex-vivo whole-conceptuses culture systems faithfully mimic the conditions of conceptuses exposed to maternal hyperglycemic conditions.
Because antioxidant supplementations to diabetic animals block JNK1/2 activation in embryonic tissues 21, hyperglycemia-induced oxidative stress triggers JNK1/2 activation. The present study determines that JNK1/2 activation leads to iNOS gene expression and increased NO production. NO is generally considered as a beneficial factor to embryonic development. However, under oxidative stress conditions NO reacts with ROS resulting in over-production of reactive nitrogen species, which is detrimental to embryonic development. Deletion of jnk2 gene blocks hyperglycemia-induced iNOS expression thus abolishing the increase in nitrosylated proteins. In summary, the present study determined that hyperglycemia induced iNOS gene expression and nitrosative stress in embryonic tissues exposed to hyperglycemia. Therefore, the findings of the present study may provide new paradigms in iNOS expression and consequent nitrosative stress for the oxidative stress hypothesis in diabetic embryopathy.
Acknowledgments
This research is supported by NIH R01 DK083243 to P. Y.. Peixin Yang is a BIRCWH scholar supported by NIH K12HD043489 (Principal investigator: Dr. Patricia Langenberg). The authors are grateful to Drs. E. Albert Reece and Ronald Zielke at the University of Maryland School of Medicine for discussions and review of the manuscript.
Footnotes
Reprint Requests: Not Available
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lawrence JM, Contreras R, Chen W, Sacks DA. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005. Diabetes Care. 2008;31:899–904. doi: 10.2337/dc07-2345. [DOI] [PubMed] [Google Scholar]
- 2.Correa A, Gilboa SM, Besser LM, et al. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008;199:237, e1–9. doi: 10.1016/j.ajog.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holing EV, Beyer CS, Brown ZA, Connell FA. Why don’t women with diabetes plan their pregnancies? Diabetes Care. 1998;21:889–95. doi: 10.2337/diacare.21.6.889. [DOI] [PubMed] [Google Scholar]
- 4.Yang P, Zhao Z, Reece EA. Involvement of c-Jun N-terminal kinases activation in diabetic embryopathy. Biochem Biophys Res Commun. 2007;357:749–54. doi: 10.1016/j.bbrc.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Yang P, Zhao Z, Reece EA. Activation of oxidative stress signaling that is implicated in apoptosis with a mouse model of diabetic embryopathy. Am J Obstet Gynecol. 2008;198:130, e1–7. doi: 10.1016/j.ajog.2007.06.070. [DOI] [PubMed] [Google Scholar]
- 6.Yang P, Zhao Z, Reece EA. Blockade of c-Jun N-terminal kinase activation abrogates hyperglycemia-induced yolk sac vasculopathy in vitro. Am J Obstet Gynecol. 2008;198:321, e1–7. doi: 10.1016/j.ajog.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121:115–25. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 8.Essers MA, Weijzen S, de Vries-Smits AM, et al. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. Embo J. 2004;23:4802–12. doi: 10.1038/sj.emboj.7600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin A. Activation of the JNK signaling pathway: breaking the brake on apoptosis. Bioessays. 2003;25:17–24. doi: 10.1002/bies.10204. [DOI] [PubMed] [Google Scholar]
- 10.Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838–49. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 11.Lin A, Dibling B. The true face of JNK activation in apoptosis. Aging Cell. 2002;1:112–6. doi: 10.1046/j.1474-9728.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- 12.Kuan CY, Yang DD, Samanta Roy DR, Davis RJ, Rakic P, Flavell RA. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22:667–76. doi: 10.1016/s0896-6273(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 13.Nath AK, Enciso J, Kuniyasu M, Hao XY, Madri JA, Pinter E. Nitric oxide modulates murine yolk sac vasculogenesis and rescues glucose induced vasculopathy. Development. 2004;131:2485–96. doi: 10.1242/dev.01131. [DOI] [PubMed] [Google Scholar]
- 14.Forstermann U, Gath I, Schwarz P, Closs EI, Kleinert H. Isoforms of nitric oxide synthase. Properties, cellular distribution and expressional control. Biochem Pharmacol. 1995;50:1321–32. doi: 10.1016/0006-2952(95)00181-6. [DOI] [PubMed] [Google Scholar]
- 15.Albina JE. On the expression of nitric oxide synthase by human macrophages. Why no NO? J Leukoc Biol. 1995;58:643–9. doi: 10.1002/jlb.58.6.643. [DOI] [PubMed] [Google Scholar]
- 16.Hosogi S, Iwasaki Y, Yamada T, et al. Effect of inducible nitric oxide synthase on apoptosis in Candida-induced acute lung injury. Biomed Res. 2008;29:257–66. doi: 10.2220/biomedres.29.257. [DOI] [PubMed] [Google Scholar]
- 17.Forrester K, Ambs S, Lupold SE, et al. Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild-type p53. Proc Natl Acad Sci U S A. 1996;93:2442–7. doi: 10.1073/pnas.93.6.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett M, Macdonald K, Chan SW, Luzio JP, Simari R, Weissberg P. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science. 1998;282:290–3. doi: 10.1126/science.282.5387.290. [DOI] [PubMed] [Google Scholar]
- 19.Bossi G, Griffiths GM. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat Med. 1999;5:90–6. doi: 10.1038/4779. [DOI] [PubMed] [Google Scholar]
- 20.Yang P, Kriatchko A, Roy SK. Expression of ER-alpha and ER-beta in the hamster ovary: differential regulation by gonadotropins and ovarian steroid hormones. Endocrinology. 2002;143:2385–98. doi: 10.1210/endo.143.6.8858. [DOI] [PubMed] [Google Scholar]
- 21.Reece EA, Wu YK, Zhao Z, Dhanasekaran D. Dietary vitamin and lipid therapy rescues aberrant signaling and apoptosis and prevents hyperglycemia-induced diabetic embryopathy in rats. Am J Obstet Gynecol. 2006;194:580–5. doi: 10.1016/j.ajog.2005.08.052. [DOI] [PubMed] [Google Scholar]