Abstract
Background
Residual kidney function (RKF) is associated with improved survival in peritoneal dialysis patients but its role in hemodialysis patients is less well known. Urine output may provide an estimate of RKF. The aim of our study was to determine the association of urine output with mortality, quality of life (QOL) and inflammation in incident hemodialysis patients.
Study Design
Nationally representative prospective cohort study
Setting & Participants
734 incident hemodialysis participants treated in 81 clinics; enrollment, 1995-1998, follow-up until December 2004.
Predictor
Urine output, defined as producing at least 250 mL (1 cup) of urine daily, ascertained by questionnaires at baseline and year 1.
Outcomes & Measurements
Primary outcomes were all-cause and cardiovascular (CVD) mortality, analyzed using Cox regression adjusted for demographic, clinical and treatment characteristics. Secondary outcomes were QOL, inflammation (CRP and interleukin-6 [IL-6] levels) and erythropoietin (EPO) requirements.
Results
617/734 (84%) participants reported urine output at baseline and 163/579 (28%) at year 1. Baseline urine output was not associated with survival. Urine output at year 1, indicating preserved RKF, was independently associated with lower all-cause mortality (Hazard Ratio [HR], 0.70; 95% Confidence Interval [CI], 0.52-0.93; p=0.02) and a trend towards lower CVD mortality (HR, 0.69; 95% CI, 0.45-1.05; p=0.09). Participants with urine output at baseline reported better QOL and had lower CRP (p=0.02) and IL-6 (p=0.03) levels. Importantly, EPO dose was 12,000 units/week lower in those with urine output at year 1 compared with those without (p=0.001).
Limitations
Urine volume was measured in only a subset of patients (42%) but was in agreement with self-report (p<0.001).
Conclusions
RKF in hemodialysis patients is associated with better survival and QOL, lower inflammation and significantly less EPO use. RKF should be monitored routinely in hemodialysis patients. Development of methods to assess and preserve RKF is important and may improve dialysis care.
Keywords: End-stage Renal Disease, Hemodialysis, Residual Kidney Function, Mortality, Quality of Life, Inflammation
Residual kidney function (RKF) is an important predictor of survival in peritoneal dialysis (PD) patients but its role in hemodialysis (HD) patients is less well known.1, 2 RKF, even at the low glomerular filtration rate (GFR) levels in dialysis patients, plays a crucial role in clearance of uremic toxins, prevents volume overload and its sequelae, such as left ventricular hypertrophy (LVH) and congestive heart failure (CHF), and is associated with improved metabolic parameters.3-6 RKF is referred to as the “heart of PD” but very few studies have analyzed the relation between RKF and mortality and other important outcomes in HD patients.4, 7, 8 The 2006 National Kidney Foundation’s KDOQI (Kidney Disease Outcome Quality Initiative) Guidelines recommend “striving to preserve RKF in HD patients (grade A recommendation)” and ranked clinical outcomes research on RKF as a “Critical Research Recommendation”.9 RKF, however, is difficult to assess and is measured in <5% of HD patients, limiting outcomes research.10 Urine output may serve as a simple indicator of RKF in HD patients.
The objective of this study was to determine the association of urine output with mortality, quality of life (QOL) and inflammation in incident HD patients participating in the CHOICE (Choices for Healthy Outcomes in Caring for End-Stage Renal Disease) Study.
METHODS
Study Design
The CHOICE Study is a nationally representative prospective cohort study of incident dialysis patients.11 From October 1995 to June 1998, 1,041 participants from 19 U.S. states were enrolled, of whom 767 were on HD. CHOICE patients were followed through December 31, 2004. Eligibility criteria were new onset of long-term dialysis therapy in the preceding 3 months, ability to provide informed consent, age >18 years and ability to speak English or Spanish. The Johns Hopkins Medicine Institutional Review Board (Baltimore, Maryland) and the clinical centers’ review boards approved the study.
Data Collection
Exposure
RKF was assessed by the self-described ability to make at least 1 full cup (250 cc) of urine daily and was ascertained from study questionnaires at baseline and follow-up. Information about urine output was available for 734/767 (96%) participants at baseline. During the first year, 60 participants died and 36 underwent transplant. Of the remaining participants, 579 (91%) had information available about urine output at year 1. The characteristics of those with missing urine output information were analyzed. Measured urine volume was available for a subsample (42%) of the participants at baseline and was used to validate self-reported urine output.
Outcomes
The primary outcomes were all-cause and cardiovascular disease (CVD) mortality. Mortality information was adjudicated using information from clinic report, medical records, National Death Index, Centers for Medicare & Medicaid Services (CMS) death notification forms and Social Security records.12 Secondary outcomes were QOL, inflammation and erythropoietin (EPO) dose requirement. QOL scores were obtained from the CHOICE Health Experience Questionnaire (CHEQ), a validated patient self-report questionnaire completed at baseline and year 1.13 Presence of inflammation was assessed in a subset of participants (n=655) by measuring high-sensitivity C-reactive protein (CRP) using a colorimetric competitive enzyme-linked immunosorbent (ELISA) assay (coefficient of variation [CV], 8.9%) and interleukin-6 (IL-6), a proinflammatory cytokine measured using an ultrasensitive ELISA (CV, 7%). Average weekly EPO dose during the first six months after enrollment was calculated from United States Renal Data System (USRDS) medical evidence and claims data. EPO resistance index was calculated by dividing the average weekly EPO dose divided by hemoglobin concentration (in g/dL).
Other Variables
All participants provided information about demographics, work history, medical history and pre-dialysis care. Body mass index (BMI; weight (in kg)/[height (in meters)]2, based on the height and weight reported on the 2728 form. Late referral was defined as <4 months between first nephrologist evaluation and start of dialysis. Comorbidities were assessed using the Index of Coexistent Disease (ICED), a validated medical record-derived index that captures both presence and severity of comorbid conditions.14 15 ICED scores range from 0 to 3, with 3 as the highest severity level. Data on use of diuretics, calcium channel blockers and angiotensin converting enzyme (ACE) inhibitors was abstracted from patient charts. Laboratory data from routine patient care were available for: serum albumin, calcium, phosphorus, potassium, ferritin, transferrin saturation (TSAT) and hemoglobin. Dialysis dose (Kt/V) was calculated using the Daugirdas formula.16
Statistical Analysis
Baseline characteristics of participants were compared based on their urine output status at baseline and year 1 using Pearson’s chi-square test, Student’s t test or robust regression, as appropriate. Survival analysis techniques were used to analyze the risk of mortality based on urine output. Individuals were censored at transplant or at the end of study period (December 31, 2004). Cumulative incidence of all-cause and CVD mortality was calculated using the Kaplan-Meier product-limit estimator. Cox proportional hazards regression, stratified by dialysis clinics, was used to model the risk of death based on urine output status at baseline and year 1. Proportional hazards assumptions were checked graphically and by hypothesis-based tests. Hazard ratios (HR) were calculated to assess the risk of death in unadjusted models and after adjustment for a priori defined confounders including: (a) demographic characteristics (age, race [white or other], sex, educational status [completed high school or not], marital status [married or not], employment status [employed or not employed]); and (b) clinical and treatment factors (smoking history [ever smoked], pulse pressure, BMI, primary cause of kidney failure [diabetes, hypertension, glomerulonephritis or other], ICED score, CVD, CHF, LVH, diabetes and serum albumin. Missing data for variables were as follows: educational status (0.9%), smoking history (0.6%), BMI (6.7%), pulse pressure (4.9%), baseline albumin (1.6%) and year 1 albumin (5.9%). Missing data values were imputed with 10 data replicates using multiple imputation by chained equations method implemented by ice program in Stata 10.1 (Stata Corp., www.stata.com). Multivariate linear regression was used to model the association between urine output and QOL, inflammation (CRP and IL-6) and EPO dose. Logistic regression was used to determine the odds of preserved urine output at year 1 with use of diuretics, calcium channel blockers or ACE-inhibitors. Statistical analyses were performed using Stata software, version 10.1. Statistical significance was defined as p<0.05 using 2-tailed tests.
RESULTS
Participant Characteristics
Baseline characteristics of the participants, stratified by urine output at baseline and year 1, are described in Table 1. Individuals with urine output were more likely to be white, undergone earlier nephrologist evaluation, have higher systolic blood pressure and pulse pressure and receiving diuretics compared with those without urine output. Participants with missing urine output information at baseline (n=33 [4%]) were more likely to be older (p=0.008) and have higher comorbidities (p=0.004) including prevalent CVD (p=0.009) and history of CHF (p=0.05). At year 1, those with missing urine output information (n=60 [9%]) were more likely to have a higher pulse pressure (p=0.03) and less likely to have finished high school (p=0.01).
Table 1.
Baseline Characteristics of 734 Incident Hemodialysis Participants of the CHOICE Study by Urine Output Status
| Characteristics | Ability to make at least 250 cc (1 cup) of urine per day | |||||
|---|---|---|---|---|---|---|
| Status at Baseline (n=734) | Status at Year 1 (n=579) | |||||
| Yes (n=617) | No (n=117) | P | Yes (n=163) | No (n=416) | P | |
| Demographic | ||||||
| Age (years) | 61 (15) | 59 (15) | 0.3 | 59 (14) | 60 (15) | 0.4 |
| Sex (% Female) | 46 | 50 | 0.4 | 46 | 54 | 0.2 |
| Race (% White) | 70 | 56 | 0.003 | 67 | 52 | 0.003 |
| Education (% high school graduate) | 32 | 28 | 0.4 | 30 | 26 | 0.4 |
| Employment (% working) | 9 | 7 | 0.4 | 9 | 5 | 0.2 |
| Marital Status (% married) | 53 | 50 | 0.6 | 53 | 46 | 0.3 |
| Clinical | ||||||
| Smoking Status (% ever smoker) | 61 | 59 | 0.7 | 59 | 57 | 0.7 |
| ICED Score (%) | 0.1 | 0.4 | ||||
| ≤1 | 32 | 23 | 32 | 25 | ||
| 2 | 38 | 45 | 40 | 43 | ||
| 3 | 30 | 32 | 28 | 32 | ||
| Comorbidities (%) | ||||||
| Diabetes | 55 | 52 | 0.6 | 58 | 53 | 0.4 |
| CVD | 58 | 64 | 0.3 | 60 | 61 | 0.8 |
| CHF | 48 | 57 | 0.09 | 50 | 56 | 0.3 |
| LVH | 27 | 25 | 0.6 | 28 | 26 | 0.7 |
| BMI, kg/m2 | 27.3 (6.8) | 27.5 (8.1) | 0.3 | 27.5 (6.9) | 27.6 (6.9) | 0.7 |
| Systolic BP, mm Hg | 152 (17) | 147 (20) | 0.007 | 153 (17) | 152 (17) | 0.9 |
| Diastolic BP, mm Hg | 79 (10) | 77 (11) | 0.1 | 80 (10) | 79 (10) | 0.2 |
| Pulse Pressure mm Hg | 73 (14) | 70 (14) | 0.006 | 72 (14) | 74 (14) | 0.5 |
| ESRD Related | ||||||
| eGFR at Dialysis Initiation (ml/min/1.73 m2) a |
7.7 (3.5) | 7.2 (3.8) | 0.03 | 7.8 (3.2) | 7.6 (3.5) | 0.3 |
| Primary Cause of Kidney Failure (%) | 0.2 | 0.1 | ||||
| Diabetes | 47 | 42 | 49 | 44 | ||
| Hypertension | 19 | 26 | 19 | 27 | ||
| Glomerulonephritis | 15 | 17 | 13 | 17 | ||
| Late Referral* (%) | 31 | 48 | 0.001 | 29 | 48 | 0.001 |
| Average baseline Kt/V | 1.26 (0.30) | 1.31 (0.32) | 0.2 | 1.23 (0.28) | 1.28 (0.32) | 0.1 |
| Laboratory b | ||||||
| Serum Albumin, g/dL | 3.7 (0.3) | 3.7 (0.4) | 0.4 | 3.9 (0.3) | 3.8 (0.3) | 0.2 |
| Serum Potassium, mEq/L | 4.6 (0.6) | 4.6 (0.6) | 0.8 | -- | -- | |
| Serum Calcium, mg/dL | 9.2 (0.6) | 9.2 (0.6) | 0.4 | 9.3 (0.7) | 9.4 (0.7) | 0.3 |
| Serum Phosphorus, mg/dL | 5.4 (1.2) | 5.4 (1.4) | 0.4 | 5.5 (1.3) | 5.5 (1.3) | 0.4 |
| Hb, mg/dL | 10.9 (1.0) | 10.6 (1.1) | 0.03 | 11.2 (1.2) | 11.2 (1.0) | 0.8 |
| Ferritin, (ng/mL) | 222 (250) | 311 (284) | 0.002 | 383 (341) | 472 (426) | 0.09 |
| TSAT | 20 (10) | 23 (11) | 0.4 | 22 (8) | 23 (9) | 0.8 |
| CRP, mg/dLc | 0.58 (0.26-1.40) | 0.95 (0.36-2.37) | 0.02d | 0.63 (0.25-1.61) | 0.91 (0.35-2.22) | 0.03d |
| IL-6, pg/mLc | 5.4 (3.4-9.0) | 6.9 (3.9-12.5) | 0.03d | 4.8 (3.2-8.8) | 7.9 (3.9-12.9) | 0.5d |
| Medications | ||||||
| Diuretics (%) | 30 | 17 | 0.004 | 30 | 15 | 0.007 |
| ACE inhibitors (%) | 27 | 31 | 0.4 | 27 | 28 | 0.8 |
| Calcium Channel Blockers (%) | 62 | 55 | 0.1 | 64 | 58 | 0.3 |
| Beta-Blockers (%) | 24 | 21 | 0.4 | 24 | 22 | 0.6 |
| Phosphate Binders (%) | 89 | 92 | 0.2 | 89 | 92 | 0.4 |
| EPO Dose (units per week) e | 46,791 (24,000) | 52,676 (28,225) | 0.2d | 39,053 (19,743) | 51,012 (25,058) | 0.001d |
| EPO Resistance Index (EPO units per g/dL hb)f |
4,405 (2,543) | 5,297 (3,467) | 0.06d | 3,605 (1,950) | 4,917 (2,796) | <0.001d |
Note: Numbers presented are mean (standard deviation), percent, or median (25th-75th percentile). Conversion factors for units: albuminin g/dL to g/L, × 10; calcium in mg/dL to mmol/L, × 0.2495; phosphorus in mg/dL to mmol/L, × 0.3229; Hb in mg/dL to g/L, × 10. No conversion is necessary for potassium in mEq/L and mmol/L and ferritin in ng/mL and μg/L.
Abbreviations: BMI, Body Mass Index; BP, blood pressure; HD, hemodialysis; eGFR, estimated Glomerular Filtration Rate; CHOICE, Choices for Healthy Outcomes in Caring for End-Stage Renal Disease; CVD, Cardiovascular Disease; CHF, Congestive Heart Failure; LVH, Left Ventricular Hypertrophy; EPO, erythropoietin; ESRD, End-Stage Renal Disease; ACE, Angiotensin Converting Enzyme; TSAT, Percent Transferrin Saturation (Serum Iron/Total Iron Binding Capacity × 100); IL-6, interleukin 6; CRP, C-reative protein; Hb, hemoglobin; ICED, Index of Coexistent Disease.
eGFR at Dialysis Initiation: eGFR at the time of initiation of renal replacement therapy. Calculated using four-variable Modification of Diet in Renal Disease (MDRD) Study equation from serum creatinine reported on Form 2728.
Late referral was defined as <4 months between first nephrologist evaluation and start of dialysis.
Laboratory values are from baseline (for baseline urine output) or year 1 (for urine output at year 1).
At baseline, CRP was measured in 655/734 (89%) and IL-6 in 645/734 (88%) participants. At year 1, CRP was measured in 351/579 (61%) and IL-6 in 159/579 (28%) participants.
Linear regression adjusted for age, sex, race, body mass index and ICED score.
EPO Dose: Average weekly erythropoietin dose requirements during the first six months after enrollment.
EPO Resistance Index: Average weekly EPO dose divided by hemoglobin concentration (g/dL). Higher values indicate greater EPO resistance.
All-Cause Mortality
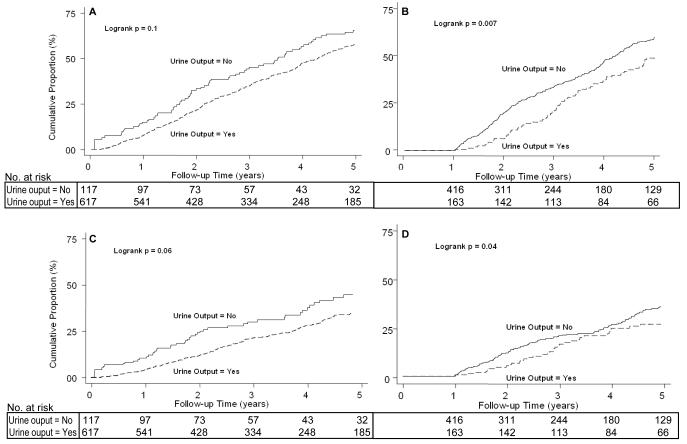
Of the 734 CHOICE participants at baseline, 481 (66%) died over 2,657 person-years of follow-up (median 3.2 years). The protective effect of urine output on mortality was most pronounced among individuals with presence of urine output at year 1 (Figure 1).
Figure 1.
A. C[ND2]umulative Incidence of All-Cause Mortality in 734 Participants of the CHOICE Study. By Urine Output at Baseline.
B. Cumulative Incidence of All-Cause Mortality in 579 Participants of the CHOICE Study. By Urine Output at Year 1.
C. Cumulative Incidence of CVD Mortality in 734 Participants of the CHOICE Study. By Urine Output at Baseline.
D. Cumulative Incidence of CVD Mortality in 579 Participants of the CHOICE Study. By Urine Output at Year 1.
The incidence rate (IR) of all-cause mortality and adjusted relative hazards are described in Table 2. Baseline urine output was not associated with improved survival. Urine output at baseline and year 1, indicating preserved RKF, was associated with a 35% lower risk of death in unadjusted models and this protective effect persisted even after extensive adjustment for putative confounders including demographic, clinical and treatment characteristics (adjusted HR, 0.70; 95% Confidence Interval [CI], 0.52-0.93).
Table 2.
Association between urine output and mortality in 734 incident hemodialysis participants of the CHOICE Study[ND1]
| Urine Output Status at Baseline (n=734) | Urine Output Status at Year 1 (n=579) | |||||
|---|---|---|---|---|---|---|
| Yes a | No | p b | Yes a | No | p b | |
| All-Cause Mortality | ||||||
| Events | ||||||
| Number of Deaths/Participants | 403/617 | 78/117 | 101/163 | 289/416 | ||
| Unadjusted Incidence Rate per 1000 person-years (95% CI) |
179 (162-197) | 192 (154-240) | 0.6 | 169 (139-206) | 227 (202-255) | 0.009 |
| Risk of Death; HR (95% CI) c | ||||||
| Unadjusted | 0.89 (0.69-1.15) | Referent | 0.4 | 0.65 (0.49-0.87) | Referent | 0.004 |
| Adjusted | ||||||
| Demographic d | 0.87 (0.67-1.14) | 0.3 | 0.70 (0.52-0.93) | 0.01 | ||
| Plus clinical factors e | 0.92 (0.70-1.21) | 0.6 | 0.70 (0.52-0.93) | 0.02 | ||
| Cardiovascular Disease Mortality | ||||||
| Events | ||||||
| Number of Deaths/Participants | 198/617 | 39/117 | 50/163 | 141/416 | ||
| Unadjusted Incidence Rate per 1000 person-years (95% CI) |
88 (77-101) | 96 (70-131) | 0.6 | 84 (63-110) | 111 (94-130) | 0.09 |
| Risk of Death; HR (95% CI) c | ||||||
| Unadjusted | 0.87 (0.61-1.25) | Referent | 0.4 | 0.66 (0.44-0.99) | Referent | 0.05 |
| Adjusted | ||||||
| Demographic c | 0.82 (0.56-1.18) | 0.3 | 0.69 (0.46-1.04) | 0.08 | ||
| Plus clinical factors e | 0.83 (0.57-1.21) | 0.3 | 0.69 (0.45-1.05) | 0.09 | ||
Abbreviations: CHOICE, Choices for Healthy Outcomes in Caring for End-Stage Renal Disease; HR, Hazard Ratio; CI, Confidence Interval.
Yes refers to the ability to make at least 250 cc (1 cup) of urine per day.
p-value for incidence rate ratio or HR; participants without urine output is the comparison group.
Cox proportional hazards regression stratified by clinic.
Demographic characteristics: age, race (white or other), gender, educational status (completed high school or not), marital status (married or not), employment status (employed or not employed).
Clinical and treatment factors in addition to demographic characteristics: smoking history (ever smoked), pulse pressure, body mass index, primary cause of kidney failure (diabetes, hypertension, glomerulonephritis or other), Index of Coexistent Disease (ICED) score (0-3), cardiovascular disease, congestive heart failure, left ventricular hypertrophy, diabetes, serum albumin (at baseline or year 1).
We also analyzed the effect of change in residual urine output over year 1 on mortality (Table 3). Preserved urine output (urine output at baseline and year 1; n=163) was associated with 31% lower risk of all-cause mortality (HR, 0.69; 95% CI, 0.51-0.93) compared with loss of RKF (urine output at baseline but not year 1; n=319). A weaker association was noted comparing preserved urine output with the group with no urine output at baseline or year 1.
Table 3.
Change in urine output status at year 1 and risk of death in 579 incident hemodialysis participants of the CHOICE Study
| All-Cause Mortality | CVD Mortality | |||
|---|---|---|---|---|
| HR (95% CI) a | P | HR (95% CI) a | P | |
| Preserved urine output (n=163)b vs no urine output (n=97)c | 0.72 (0.44-1.05) | 0.09 | 0.63 (0.37-1.10) | 0.7 |
| Preserved urine output (n=163)b vs loss of urine output (n=319)d | 0.69 (0.51-0.93) | 0.02 | 0.70 (0.45-1.09) | 0.1 |
Abbreviations: CHOICE, Choices for Healthy Outcomes in Caring for End-Stage Renal Disease; HR, Hazard Ratio; CI, Confidence Interval; CVD, Cardiovascular Disease
Cox proportional hazards regression clustered by clinic and adjusted for demographic characteristics (age, race [white or other], gender, educational status [completed high school or not], marital status [married or not], employment status [employed or not employed] and clinical and treatment factors (smoking history [ever smoked], pulse pressure, body mass index, primary cause of kidney failure [diabetes, hypertension, glomerulonephritis or other], Index of Coexistent Disease [ICED] score [0-3], cardiovascular disease, congestive heart failure, left ventricular hypertrophy, diabetes, and serum albumin [at year 1]).
Urine output at baseline and year 1.
No urine output at baseline and year 1.
Loss of urine output indicates urine output at baseline but not at year 1.
CVD Mortality
CVD deaths accounted for 48% of all deaths. Overall, the trends were similar to that seen with all-cause mortality. Preserved urine output was associated with improved survival but the results did not reach statistical significance (Figure 1 B & C; Table 2).
Quality of Life
Participants with urine output at baseline, reported overall better QOL (p=0.05) and less dietary restriction (p=0.05); analyses are provided in more detail in Item S1, available as online supplementary material associated with this article at www.ajkd.org.
Inflammation and EPO Dose Requirement
Presence of urine output was associated with lower levels of inflammatory markers (Table 1). CRP levels were lower in those with urine output at baseline compared with those without (median difference, − 0.37 mg/dL; p= 0.02). Similar differences were observed with IL-6. At year 1, the highest CRP levels were noted in those participants that did not have urine output at baseline or year 1 (median, 1.06; 25th-75th percentiles, 0.41-1.75), intermediate in those with urine output at baseline but not year 1 (median, 0.91; 25th-75th percentiles, 0.33-2.38) and the lowest in those with urine output at baseline and year 1 (median, 0.63; 25th-75th percentiles, 0.25-1.61; adjusted p-trend, 0.006).
Mean EPO dose during the first 6 months after enrollment was available for 416 (57%) participants (Table 1). Mean EPO dose was 5,885 units/week lower in those with urine output at baseline vs. those without although it was not statistically significant (adjusted p=0.2). At year 1, EPO dose requirements were the highest in those without urine output at baseline or year 1 (52,543 units/week; 95% CI, 45,526-59,558), intermediate in those with urine output at baseline but not year 1 (loss of RKF; 50,767 units/week; 95% CI, 47,586-53,949) and the lowest in those with urine output at baseline and year 1 (preserved RKF; 39,186 units/week; 95% CI, 35,396-42,975; adjusted p-trend=0.002). Similar trends were noted with EPO resistance index (Table 1). Both urine output groups had adequate iron stores, as assessed by ferritin and transferrin saturation, at baseline and year 1.
Sensitivity Analyses
Analysis without data imputation yielded results similar to the primary analysis (data not shown). Kt/V information was available for 77% of the participants at baseline. Adjusting for Kt/V did not change the results. Further adjustment for putative mediators of RKF, identified a priori, including hemoglobin, potassium, calcium and phosphate did not change the results. (HR for all-cause mortality risk with urine output at year 1, 0.73; 95% CI, 0.54-0.99; p=0.05). Among the subset of patients with urine volume measurements (n=306 [42%]), the median urine volume was 400 ml/day (25th-75th percentiles, 200-625) in those reporting <1 cup of urine per day (n=281; 92%), and 725 ml/day (25th-75th percentiles, 500-1160) in those reporting >1 cup of urine per day (p <0.001). Those on diuretics had greater urine volume (mean 228 mL/day higher; p=0.03). Analysis of the subset of the participants with urine volume measurement at baseline demonstrated results similar to primary analysis (adjusted HR of all-cause mortality per 250 ml of urine/day, 0.93 (95% CI, 0.87-1.0; p=0.04). Although diuretic use was greater in those with urine output at baseline and year 1, diuretic use at baseline was not associated with preserved urine output at year 1 (adjusted odds ratio [OR], 1.55; 95% CI, 0.75-3.23; p=0.2). ACE-inhibitors were also not associated with preserved urine output at year 1 (adjusted OR, 0.91; 95% CI, 0.57-1.47; p=0.7). There were 19 individuals that reported urine output < 1 cup at baseline and > 1 cup at year 1. These individuals were reclassified as producing > 1 cup at baseline. Analyses performed excluding these individuals demonstrated the demonstrated results similar to the primary analysis (data not shown).
DISCUSSION
To date, very few large representative studies have reported on the role of RKF in HD patients, particularly in the US. This analysis of urine output from a national prospective cohort study of incident dialysis patients demonstrates several important findings. RKF, identified by self-reported urine output, was reliable, and was independently associated with lower all-cause mortality in HD patients. The survival benefit was especially notable in participants that had preserved urine output during year 1. Urine output was also associated with a better QOL, lower inflammation as measured by CRP and IL-6 and lower EPO use.
RKF is well recognized as an important and independent predictor of survival in PD patients. In 680 PD participants of the CANUSA (Canada-USA) Study, each 1 ml/min/1.73 m2 higher eGFR was associated with a 23% lower risk of death.17 Urine volume appeared to mediate this effect with 36% lower risk of mortality per 250 ml (1 cup) urine output per day. In 413 PD participants of NECOSAD (the Netherlands Cooperative Study of Dialysis), each 1 ml/min/1.73 m2 higher eGFR was associated with 12% lower mortality.18 Similarly, in 965 participants of ADEMEX (the Adequacy of Dialysis in Mexico) Trial, each 1 ml/min/1.73 m2 higher eGFR was associated with 11% lower risk of death.19
RKF seems to play an equally important role in HD patients but is not routinely assessed or reported. In a single-center US study of 114 prevalent HD patients, presence of any urine output (> 100 ml per day) was associated with a 65% lower risk of death over the subsequent 2 year period.8 In 740 incident HD participants of NECOSAD, 56% lower mortality was noted per 1/week higher renal urea Kt/V over a median follow-up of 1.7 years.4 In another single-center study 650 incident HD patients followed at the Lister Renal Unit in UK, individuals with urea clearance ≥ 1 ml/min at 6 months had 31% lower mortality compared with those with urea clearance < 1 ml/min.20 Our study adds robustness to these previously reported studies with a national cohort representative of the US hemodialysis population,21 prospective design, long follow-up and meticulous assessment of comorbidities, laboratory data and outcomes, allowing for extensive adjustment for confounders. Moreover, it demonstrates that a simply obtained patient-reported crude measure of urine output (making “one cup of urine”) is reliable and predicts prognosis. More precise measures are often difficult to obtain and sometimes thwarted by patient non-adherence and/or obvious dialysis unit “burden”.9, 10
In the context of PD, it is well known that RKF contributes significantly to the QOL.18 To our knowledge, this is the first time that an association between urine output and QOL has been demonstrated in patients treated with HD. Patients with urine output reported greater vitality, better cognitive function and noted less dietary restrictions during year 1.
RKF in HD patients may prevent volume overload and its sequelae including LVH and uncontrolled hypertension.1, 2, 5, 22-25 RKF, even at the low levels present in dialysis patients, can provide a substantial level of clearance for solutes such as β2-microglobulin, P-cresol and indican.26, 27 In the Hemodialysis (HEMO) trial, each 1 ml/min higher renal urea clearance was associated with a 7.2 mg/L lower β2-microglobulin levels.28 Loss of RKF is also associated with higher levels of CRP, IL-6 and other inflammatory markers such as soluble vascular adhesion molecule and neopterin.29, 30 Presence of inflammation in dialysis patients is associated with all-cause and CVD mortality as well as faster decline in RKF.31-36 In our study, participants with preserved RKF (urine output at baseline and year 1) had lower levels of inflammatory markers and improved survival independent of potential confounders.
We noted decreased EPO requirements in participants with urine output and the effect was especially pronounced in the participants with preserved urine output during year 1. Both urine output groups had adequate iron stores as assessed by ferritin and transferrin saturation. Weekly EPO dose was 12,000 units per week lower in those with preserved urine output at year 1 (p=0.001). EPO resistance index was also lower in participants with urine output, suggesting lower EPO resistance or greater endogenous erythropoietin production. Similar findings were noted in the Lister Renal Unit cohort. Among individuals with urea clearance ≥ 1 ml/min compared to those with urea clearance <1 ml/min, the EPO dose was 1,993 units/week lower at 12 months and 2,238 units/week lower at 24 months (p<0.001 at each time point).20 In 2005, outpatient payments by Medicare for EPO amounted to $1.9 billion and represented approximately 25% of the total end-stage renal disease (ESRD) payments.37 Our study suggests that preservation of RKF may reduce dialysis related expenses while improving survival but this hypothesis needs to be tested in randomized clinical trials.
Blockade of the renin-angiotensin system is considered standard therapy to preserve RKF in PD patients.38 Much less is known about preservation of RKF in HD patients. In an analysis of 811 HD participants in the USRDS Dialysis Morbidity and Mortality Study (DMMS) Wave 2, use of ACE inhibitors showed a trend towards preservation of RKF (adjusted OR, 0.7; p=0.06).10 Other medications such as diuretics and calcium channel blockers had no effect on RKF. In an analysis of 16,420 HD patients from DOPPS (the Dialysis Outcomes and Practice Patterns Study), however, diuretic use was associated with a two-fold higher odds of preserved RKF at year 1 (OR, 1.93; p=0.01).39 In our study, participants with urine output were more likely to be on a diuretic, however neither diuretics nor ACE-inhibitors were associated with preserved urine output at year 1. While diuretic use may be associated with increased urine volume, it is more likely, based on data in non-ESRD and PD patients, that ACE-inhibitors may have a greater long-term protective effect on RKF. With the emerging importance of RKF in HD patients, randomized control trials are sorely needed to address this important question.
Our study has several important limitations. First, urine output was not directly measured in the study and is based on self-report. The participants were asked about their ability to make 1 cup (250 ml) of urine daily in questionnaires at baseline and year 1. We validated the self-report by measured urine volume in a subset of participants (n=306; 42%). Individuals that reported <1 cup of urine output per day (n=25) had approximately half the urine volume of individuals that reported >1 cup urine output per day (n=281). As the urine collections were part of routine clinical care at the dialysis units, it is highly likely that the individuals reporting no urine output or minimal amounts were not asked to collect urine. Based on prior studies, the presence of any urine output is a strong predictor of survival as we also demonstrated in this study.8, 20 Second, presence of RKF may be a reflection of better overall health and the survival benefit seen in those with preserved urine output in our study may represent survival due to lower comorbidities. We, however, did not notice a difference in the comorbidities, assessed using ICED score, by urine output status although patients with urine out were more likely to be white and less likely to be referred late for nephrologist care. Third, lead-time bias remains important as a random group of dialysis patients with different levels of RKF may have had differing duration of renal replacement therapy. Our study design with inclusion of only incident dialysis patients and accurate determination of dialysis start dates overcomes this concern. Individuals with urine output at baseline had a statistically significant but not clinically relevant higher eGFR prior to start of HD. This eGFR difference, however, was not observed at year 1. Fourth, information on dialysis dose (Kt/V) was available for a limited number of participants. Analyses restricted to those with available data yielded results similar to the primary analysis. Fifth, EPO dose data was determined from Medicare claims data and not chart abstraction. Finally, although we demonstrate higher inflammation in individuals without urine output at baseline or year 1, the data do not prove causality and it is not possible to deduce temporality of this association.
These limitations are balanced by the strengths of our study including prospective study design, inclusion of only incident HD patients, detailed and precise information on demographics, clinical and treatment factors and a systematic adjudication process for baseline comorbidities and outcomes. Availability of these comprehensive data allowed us to adjust extensively for potential confounding by baseline comorbidities. While the possibility of uncontrolled confounding remains, the survival benefit of preserved urine output appears to be independent of most putative confounders. The 2006 KDOQI hemodialysis adequacy group had commented that the kinetic effect of RKF in clearing solutes is so powerful that “all possible efforts should be expended to maintain RKF”, for example by avoiding the use of nephrotoxic medications (e.g., aminoglycosides, intravenous contrast) in patients on HD who have significant residual urine output.9 Our results support this opinion expressed in the KDOQI guidelines. In our study, preserved RKF was associated with improved survival compared with loss of RKF over year 1.
In conclusion, this study provides evidence for several beneficial effects of RKF in HD patients. We demonstrate a strong and independent relationship between a simply obtained urine output assessment and survival as well as improved QOL, lower inflammation and less EPO use in a national prospective cohort study of 734 incident HD patients. Assessment of RKF is currently not part of routine hemodialysis care in the US. These results provide a strong rationale for routine monitoring of RKF in HD patients. Furthermore, development of methods to assess and preserve RKF is important and may improve dialysis care.
Supplementary Material
Acknowledgement
We thank the patients, staff, laboratory, and physicians of Dialysis Clinic Inc. (DCI), New Haven CAPD and St Raphael’s Hospital for their participation in the CHOICE Study.
We thank the Cardiovascular Endpoint Committee, which in addition to authors Jaar, Coresh, Fink, Plantinga, and Powe, comprises Michael J. Choi, MD; Joseph A. Eustace, MD, MHS; Caroline Fox, MD, MPH; Melanie H. Katzman, MD, MHS; Michael J. Klag, MD, MPH; Yongmei Liu, MD, PhD; J. Craig Longenecker, MD, PhD; Michal Melamed, MD, MHS; Renuka Sothinathan, MD, MHS; Richard M. Ugarte, MD, MHS; and Gayanne Yenokian, MD.
Support: CHOICE was supported by RO1-HL-62985 (National Heart, Lung, and Blood Institute (NHLBI) from 9/00 to 6/06) and RO1-DK-59616 (National Institute of Diabetes & Digestive & Kidney Diseases (NIDDK) from 9/00 to 6/07). Dr Parekh was supported by 1UO1-DK-57304-01 and 1 R01 DK070657-01. Dr Coresh was supported in part as an American Heart Association established investigator (01-4019-7N). Dr Powe was supported in part by K24-DK-02643 (NIDDK). Dr Shafi was supported by 5T32-DK-007732 (NIDDK) and The National Kidney Foundation of Maryland – Extraordinary Professional Development Award.
Footnotes
N section: Because the Editor-in-Chief recused himself from consideration of this manuscript, the Deputy Editor (Daniel E. Weiner, MD, MS) served as Acting Editor-in-Chief. Details of the journal’s procedures for potential editor conflicts are given in the Editorial Policies section of the AJKD website.
Parts of this work were presented in an abstract form at 2009 annual meeting of the American Society of Nephrology in San Diego, California.
Financial Disclosure: The authors declare that they have no relevant financial interests.
Supplementary Materials Item S1. Urine output and quality of life in incident hemodialysis participants of the CHOICE Study.
Note: The supplementary material accompanying this article (doi ) is available at www.ajkd.org.
) is available at www.ajkd.org.
Descriptive Text for Online Delivery Hyperlink: Supplementary Item S1 (PDF)
About: Urine output and quality of life in incident hemodialysis participants of the CHOICE Study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis. 2009 Jun;53(6):1068–1081. doi: 10.1053/j.ajkd.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Wang AYM, Lai KN. The importance of residual renal function in dialysis patients. Kidney Int. 2006;69(10):1726–1732. doi: 10.1038/sj.ki.5000382. [DOI] [PubMed] [Google Scholar]
- 3.Konings CJ, Kooman JP, Schonck M, et al. Fluid status in CAPD patients is related to peritoneal transport and residual renal function: evidence from a longitudinal study. Nephrol Dial Transplant. 2003 Apr;18(4):797–803. doi: 10.1093/ndt/gfg147. [DOI] [PubMed] [Google Scholar]
- 4.Termorshuizen F, Dekker FW, van Manen JG, Korevaar JC, Boeschoten EW, Krediet RT. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol. 2004 Apr;15(4):1061–1070. doi: 10.1097/01.asn.0000117976.29592.93. [DOI] [PubMed] [Google Scholar]
- 5.Wang AY-M, Wang M, Woo J, et al. A novel association between residual renal function and left ventricular hypertrophy in peritoneal dialysis patients. Kidney Int. 2002;62(2):639–647. doi: 10.1046/j.1523-1755.2002.00471.x. [DOI] [PubMed] [Google Scholar]
- 6.Tian JP, Du FH, Cheng LT, Wang T. Residual renal function and arterial stiffness mediated the blood pressure change during interdialytic weight gain in hemodialysis patients. Hemodial Int. 2009 Oct;13(4):479–486. doi: 10.1111/j.1542-4758.2009.00374.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang AY. The “heart” of peritoneal dialysis. Perit Dial Int. 2007 Jun;27(Suppl 2):S228–232. [PubMed] [Google Scholar]
- 8.Shemin D, Bostom AG, Laliberty P, Dworkin LD. Residual renal function and mortality risk in hemodialysis patients. Am J Kidney Dis. 2001 Jul;38(1):85–90. doi: 10.1053/ajkd.2001.25198. [DOI] [PubMed] [Google Scholar]
- 9.National Kidney Foundation K/DOQI Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis. 2006 Jul;48(Suppl 1):S2–90. doi: 10.1053/j.ajkd.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 10.Moist LM, Port FK, Orzol SM, et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol. 2000 Mar;11(3):556–564. doi: 10.1681/ASN.V113556. [DOI] [PubMed] [Google Scholar]
- 11.Powe NR, Klag MJ, Sadler JH, Anderson GF, Bass EB, Briggs WA, Fink NE, Levey AS, Levin NW, Meyer KB, Rubin HR, Wu AW, for the CHOICE Study Choices for healthy outcomes in caring for end stage renal disease. Semin Dial. 1996;9:9–11. [Google Scholar]
- 12.Jaar BG, Coresh J, Plantinga LC, et al. Comparing the risk for death with peritoneal dialysis and hemodialysis in a national cohort of patients with chronic kidney disease. Ann Intern Med. 2005 Aug 2;143(3):174–183. doi: 10.7326/0003-4819-143-3-200508020-00003. [DOI] [PubMed] [Google Scholar]
- 13.Wu AW, Fink NE, Cagney KA, et al. Developing a health-related quality-of-life measure for end-stage renal disease: The CHOICE Health Experience Questionnaire. Am J Kidney Dis. 2001 Jan;37(1):11–21. doi: 10.1053/ajkd.2001.20631. [DOI] [PubMed] [Google Scholar]
- 14.Miskulin DC, Meyer KB, Athienites NV, et al. Comorbidity and other factors associated with modality selection in incident dialysis patients: the CHOICE Study. Choices for Healthy Outcomes in Caring for End-Stage Renal Disease. Am J Kidney Dis. 2002 Feb;39(2):324–336. doi: 10.1053/ajkd.2002.30552. [DOI] [PubMed] [Google Scholar]
- 15.Nicolaos VA, Dana CM, Gladys F, et al. Comorbidity Assessment in Hemodialysis and Peritoneal Dialysis Using the Index of Coexistent Disease. Seminars in Dialysis. 2000;13(5):320–326. doi: 10.1046/j.1525-139x.2000.00095.x. [DOI] [PubMed] [Google Scholar]
- 16.Daugirdas JT. Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. J Am Soc Nephrol. 1993 November 1;4(5):1205–1213. doi: 10.1681/ASN.V451205. 1993. [DOI] [PubMed] [Google Scholar]
- 17.Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001 Oct;12(10):2158–2162. doi: 10.1681/ASN.V12102158. [DOI] [PubMed] [Google Scholar]
- 18.Termorshuizen F, Korevaar JC, Dekker FW, van Manen JG, Boeschoten EW, Krediet RT. The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD )-2. Am J Kidney Dis. 2003 Jun;41(6):1293–1302. doi: 10.1016/s0272-6386(03)00362-7. [DOI] [PubMed] [Google Scholar]
- 19.Paniagua R, Amato D, Vonesh E, et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002 May;13(5):1307–1320. doi: 10.1681/ASN.V1351307. [DOI] [PubMed] [Google Scholar]
- 20.Vilar E, Wellsted D, Chandna SM, Greenwood RN, Farrington K. Residual renal function improves outcome in incremental haemodialysis despite reduced dialysis dose. Nephrol. Dial. Transplant. 2009 February 24;:gfp071. doi: 10.1093/ndt/gfp071. 2009. [DOI] [PubMed] [Google Scholar]
- 21.Longenecker JC, Coresh J, Powe NR, et al. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: the CHOICE Study. J Am Soc Nephrol. 2002 Jul;13(7):1918–1927. doi: 10.1097/01.asn.0000019641.41496.1e. [DOI] [PubMed] [Google Scholar]
- 22.Foley RN, Parfrey PS, Harnett JD, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995 Jan;47(1):186–192. doi: 10.1038/ki.1995.22. [DOI] [PubMed] [Google Scholar]
- 23.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol. 1995 Jun;5(12):2024–2031. doi: 10.1681/ASN.V5122024. [DOI] [PubMed] [Google Scholar]
- 24.Ates K, Nergizoglu G, Keven K, et al. Effect of fluid and sodium removal on mortality in peritoneal dialysis patients. Kidney Int. 2001 Aug;60(2):767–776. doi: 10.1046/j.1523-1755.2001.060002767.x. [DOI] [PubMed] [Google Scholar]
- 25.Kalantar-Zadeh K, Regidor DL, Kovesdy CP, et al. Fluid Retention Is Associated With Cardiovascular Mortality in Patients Undergoing Long-Term Hemodialysis. Circulation. 2009 Jan 26; doi: 10.1161/CIRCULATIONAHA.108.807362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pham NM, Recht NS, Hostetter TH, Meyer TW. Removal of the Protein-Bound Solutes Indican and P-Cresol Sulfate by Peritoneal Dialysis. Clin J Am Soc Nephrol. 2008 January 1;3(1):85–90. doi: 10.2215/CJN.02570607. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bammens B, Evenepoel P, Verbeke K, Vanrenterghem Y. Removal of middle molecules and protein-bound solutes by peritoneal dialysis and relation with uremic symptoms. Kidney Int. 2003;64(6):2238–2243. doi: 10.1046/j.1523-1755.2003.00310.x. [DOI] [PubMed] [Google Scholar]
- 28.Cheung AK, Rocco MV, Yan G, et al. Serum beta-2 microglobulin levels predict mortality in dialysis patients: results of the HEMO study. J Am Soc Nephrol. 2006 Feb;17(2):546–555. doi: 10.1681/ASN.2005020132. [DOI] [PubMed] [Google Scholar]
- 29.Pecoits-Filho R, Heimburger O, Barany P, et al. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis. 2003 Jun;41(6):1212–1218. doi: 10.1016/s0272-6386(03)00353-6. [DOI] [PubMed] [Google Scholar]
- 30.Wang AY, Lam CW, Wang M, et al. Circulating soluble vascular cell adhesion molecule 1: relationships with residual renal function, cardiac hypertrophy, and outcome of peritoneal dialysis patients. Am J Kidney Dis. 2005 Apr;45(4):715–729. doi: 10.1053/j.ajkd.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Arici M, Walls J. End-stage renal disease, atherosclerosis, and cardiovascular mortality: is C-reactive protein the missing link? Kidney Int. 2001 Feb;59(2):407–414. doi: 10.1046/j.1523-1755.2001.059002407.x. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Coresh J, Eustace JA, et al. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. Jama. 2004 Jan 28;291(4):451–459. doi: 10.1001/jama.291.4.451. [DOI] [PubMed] [Google Scholar]
- 33.Schiffl H, Lang SM, Fischer R. Ultrapure dialysis fluid slows loss of residual renal function in new dialysis patients. Nephrol Dial Transplant. 2002 Oct;17(10):1814–1818. doi: 10.1093/ndt/17.10.1814. [DOI] [PubMed] [Google Scholar]
- 34.Panichi V, Maggiore U, Taccola D, et al. Interleukin-6 is a stronger predictor of total and cardiovascular mortality than C-reactive protein in haemodialysis patients. Nephrol Dial Transplant. 2004 May;19(5):1154–1160. doi: 10.1093/ndt/gfh052. [DOI] [PubMed] [Google Scholar]
- 35.Panichi V, Rizza GM, Paoletti S, et al. Chronic inflammation and mortality in haemodialysis: effect of different renal replacement therapies. Results from the RISCAVID study. Nephrol Dial Transplant. 2008 Jul;23(7):2337–2343. doi: 10.1093/ndt/gfm951. [DOI] [PubMed] [Google Scholar]
- 36.Wetmore JB, Lovett DH, Hung AM, et al. Associations of interleukin-6, C-reactive protein and serum amyloid A with mortality in haemodialysis patients. Nephrology (Carlton) 2008 Oct;13(7):593–600. doi: 10.1111/j.1440-1797.2008.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leavitt MO. [Accessed July 30, 2009];Secretary of Health and Human Services Report to Congress: A Design for a Bundled End-Stage Renal Disease Prospective Payment System. 2008 http://www.cms.hhs.gov/ESRDGeneralInformation/Downloads/ESRDReportToCongress.pdf.
- 38.National Kidney Foundation K/DOQI Clinical Practice Guidelines for Peritoneal Dialysis Adequacy. American Journal of Kidney Diseases. 2006;48(Supplement 1):S98–S129. doi: 10.1053/j.ajkd.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Bragg-Gresham JL, Fissell RB, Mason NA, et al. Diuretic use, residual renal function, and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Pattern Study (DOPPS) Am J Kidney Dis. 2007 Mar;49(3):426–431. doi: 10.1053/j.ajkd.2006.12.012. 2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.