Abstract
Learning ultimately relies on changes in the flow of activity within neural microcircuits. Plasticity of neural dynamics is particularly relevant for the processing of temporal information. Chronic stimulation of cultured rat cortical networks revealed ‘experience-dependent’ plasticity in neural dynamics. We observed changes in the temporal structure of activity that reflected the intervals used during training, suggesting that cortical circuits are inherently capable of temporal processing on short time scales.
Timing and temporal processing in the range of tens and hundreds of milliseconds is critical for many forms of sensory and motor processing, yet the neural mechanisms underlying the ability to discriminate or produce short intervals remains unknown1,2. Recent studies have lent support to the notion that timing is a inherent computational ability of cortical circuits and can be performed locally2. This view implies that the temporal structure of the internal dynamics of cortical networks should be shaped by the temporal patterns the network experiences. To examine this issue we studied the effects of the presentation of simple spatiotemporal stimulus patterns on cortical neural dynamics using organotypic slices. As with in vivo cortical networks, evoked stimulation in organotypic networks can elicit complex polysynaptic responses that reflect local network dynamics. Thus this preparation is well suited to study plasticity of neural dynamics.
External stimulation was presented to cortical cultures via two implanted bipolar stimulating electrodes3. We first examined whether the temporal pattern of stimulation produced any form of network plasticity – defined as changes in evoked patterns of activity. The implanted electrodes (E1 and E2) were activated with a burst of pulses presented in-phase (synchronously) or with a 100 ms interval (onset to onset), every 10 seconds for two hours (see Fig. 1a and Supplemental Fig. 3). Given the large degree of variability in the presence and structure of polysynaptic activity in naïve slices all the presented data is derived from “paired” experiments wherein “sister” slices were trained with one of two protocols and compared4. After training in the incubator for two hours, whole-cell recordings were performed from neurons near each electrode (N1 and N2 refer to neurons close to electrodes E1 and E2, respectively) and the PSP waveform in response to a single pulse from the ‘Far’ (E2→N1 or E1→N2 responses) pathways was examined. As described previously, in addition to the short-latency monosynaptic PSP, complex polysynaptic PSP waveforms were often observed5,6. Since these late PSPs provide a readout of the population activity of the neurons that synapse onto the recorded cell, we used the temporal profile of the voltage traces as a measure of the temporal pattern of network activity.
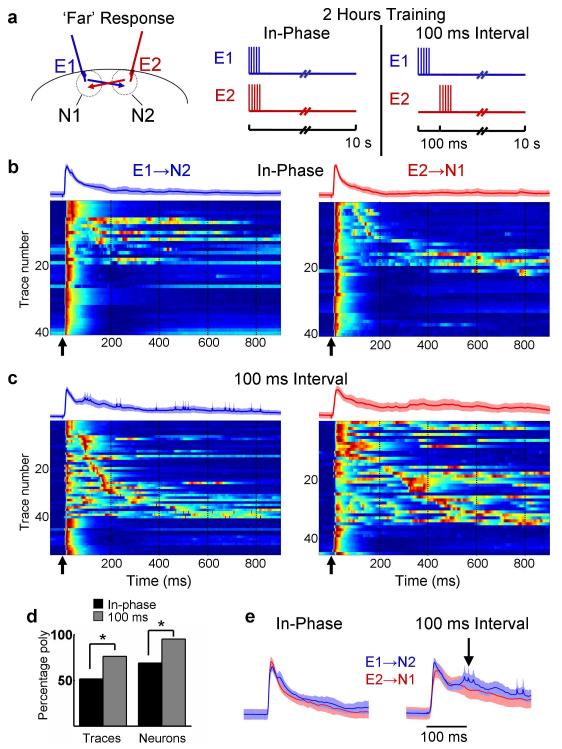
Figure 1. Network dynamics is differentially modified by training.
(a) After 2 hours of training with either an in-phase or 100 ms pattern (right panel) ‘Far’ pathway responses (E1→N2 in blue, and E2→N1 in red; left panel) were examined. (b) Voltagegrams of E1→N2 (left) and E2→N1 (right) traces in response to a single test pulse (time indicated by arrow) for slices trained with the in-phase (n = 8 neurons each pathway, 5 traces per neuron; data from 8 slices) or (c) 100 ms interval pattern (11 neurons each from a different slice for E1→N2 pathway, n = 9 neurons for E2→N1 pathway, 5 traces per neuron – N1 neurons were not recorded in two of the slices). Voltagegram traces are normalized and sorted according to latency of the first polysynaptic peak (i.e, the first peak after the monosynaptic response). Voltage is represented in color where blue is the minimum and red is the maximum. The traces above each voltagegram are the mean of all traces (shading represents the s.e.m.). Arrows represent the time of the test stimulus, and dashed lines are presented for comparison across panels. (d) After 100 ms training 76% of all test traces (‘Traces’, left bars) and 95% of tested neurons (‘Neurons’, right bars) exhibited one or more polysynaptic peaks, versus 51% of traces and 69% for in-phase group (Traces: X2 = 11.98, P < 0.001; Neurons: X2 = 4.41, P < 0.05) (e) Mean ± s.e.m. (shading) of waveform in response to ‘Far’ pathways after in-phase or 100 ms interval training. Note the secondary peak in the E1→N2 waveform after 100 ms interval training (arrow), which is lacking in the other traces.
After a 2-hour training session, examination of the PSPs evoked by a single test pulse from either electrode revealed that the 100 ms group exhibited a significantly larger number of test traces with one or more polysynaptic events (Fig. 1, x2 = 11.98, P < 0.001; analysis collapsed across pathways; see supplementary Methods online). Thus, in response to a single pulse there was a significant difference in the behavior of the network between the in-phase and 100 ms groups. In the in-phase group, as expected, the average of all E1→N2 and E2→N1 traces were very similar. However, in the 100 ms group there appeared to be a difference between the E1→N2 and E2→N1 traces; specifically, even when collapsed across all cells, the E1→N2 trace exhibited a small secondary peak at approximately 100 ms (Fig. 1e). This observation was consistent with the notion that the timing of network activity reflected the interval used during training. Although these patterns are highly variable, any preferential increase in activity around the expected time of the second pulse could be interpreted as a type of pattern completion; that is, after E1 stimulation the neurons near E2 exhibited increased activity around the interval used during training.
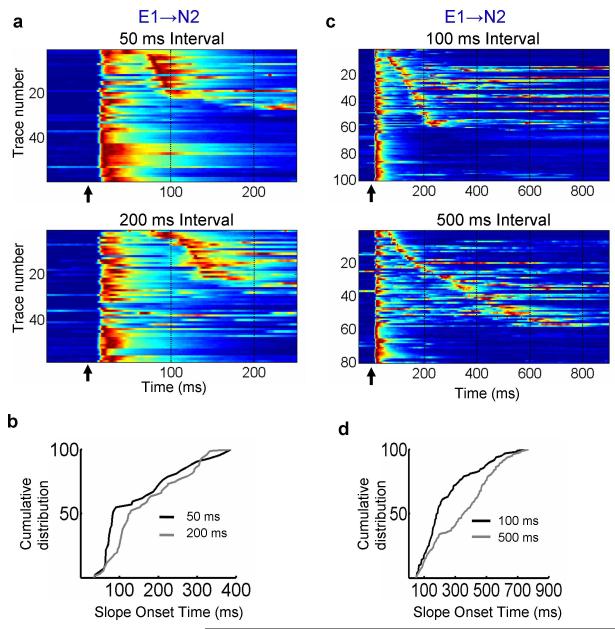
To examine the effect of the training interval on the timing of network dynamics we performed experiments in which two groups were trained for 2 hours with either a 50 ms or 200 ms interval (Fig. 2a). An analysis of the distribution of the onset times of the E1→N2 polysynaptic events revealed they were significantly shorter in the 50 compared to the 200 ms group (Fig. 2b, K-S test, P < 0.005). As shown in the ‘voltagegrams’ (Fig 2a), the polysynaptic activity tended to be clustered at earlier intervals in the 50 ms group. These results confirm that the temporal structure of neural dynamics evoked by a single stimulus is shaped in an interval-specific manner by the training stimulus.
Figure 2. Differential effects of training interval on neural dynamics.
(a) All raw data plotted as voltagegrams from the 50 (top) and 200 ms (bottom) groups (n=12 cells in each group). (b) Cumulative distribution (K-S test, P < 0.005) of polysynaptic event onset times for the E1→N2 pathway of the 50 (black) and 200 ms (grey) groups. (c) Voltagegram data from the 100 versus 500 ms experiments. Data is derived from 19 and 15 neurons (one neuron per slice) in the 100 (top) and 500 ms (bottom) groups respectively. (d) Cumulative distribution of polysynaptic event onset times for the E1→N2 pathway in experiments trained with a 100 (black) or 500 ms (grey) interval (K-S test, P < 10−4).
The above experiments were performed by training in the incubator, and testing on the electrophysiology rig. To determine the robustness of the phenomenon and sensitivity to training conditions (training in culture media versus ACSF, and 35°C incubator versus 30°C on-rig) we repeated these experiments while training for two hours on the recording rig. Again, we observed a significant difference in the timing of the E1→N2 polysynaptic events between the 50 and 200 ms groups as can be observed in the voltage traces and in the derivative of the voltage traces, which highlights the upward deflections in the voltage due to synaptic input (Supplementary Fig. 1a; K-S test, P < 0.005). To ensure that the timing effects were not somehow specific to 50 and 200 ms intervals, and to examine the range over which interval-selective effects are observed, we also trained two groups of slices with intervals of 100 and 500 ms. Again, the results revealed that the distribution of the polysynaptic events was significantly shorter in the 100 ms as compared to the 500 ms group (Fig 2c and 2d; K-S tests, P<10−4). We emphasize that while these data establish that the timing of polysynaptic activity is influenced by the interval used during training, it is not the case that all the cells in the network ‘learn’ to time at the trained interval or that the timing is highly accurate (in some experiments, but not in others, the difference in temporal structure was detectable as a secondary peak of the averaged traces). Indeed, because activity during any time window T presumably relies on activity at time window T-1, mechanistic considerations (see below) are consistent with the notion of a fairly broad distribution of timed responses, and that network plasticity is best understood as changes in the distribution of polysynaptic responses.
It has previously been reported that in “naïve” slices evoked stimulation can induce propagation of activity characterized by a time-varying pattern of neurons firing at different points in time5,6. Thus, one hypothesis is that the first pulse (E1) engages a time-varying pattern of activity, and the second pulse (E2) functions as a ‘reinforcer’ potentiating the synapses that are active at the time of the second pulse through conventional associative synaptic plasticity. Consistent with this hypothesis, the timing of the PSPs was significantly different in slices trained with a 50 ms interval in the presence or absence of the NMDA receptor antagonist APV (Fig. S2). This result suggests a role for the NMDA receptor, but the interpretation is limited by the fact that APV itself can alter neural dynamics during training or have induced homeostatic forms of plasticity. Furthermore, the amplification of preexisting responses at the time of the second pulse does not explain the qualitative changes in the distribution of responses (e.g., note the slope of the diagonal ‘band’ in Fig. 2c). We hypothesize that plasticity of neural dynamics may be an emergent property that relies on orchestrated changes at the multiple synaptic and cellular loci that ultimately govern the propagation of activity through recurrent neural networks. For example, theoretical studies have shown that some forms of homeostatic plasticity can lead to increases in the propagation of activity throughout networks7,8; these patterns might then be further shaped by associative forms of synaptic plasticity. Nevertheless, the types of synaptic and cellular changes underlying the network plasticity reported here remain to be elucidated.
It is well established that cortical function and network dynamics are sculpted by sensory experience9. Here we have demonstrated that the ‘behavior’ of cultured cortical networks is also shaped by the stimulus history of the network, in a manner that suggests that cortical networks are capable of ‘learning’ or adapting to the timing of the stimuli. Specifically, the dynamics of the network activity was altered in a manner that reflected the temporal patterns of stimuli used during training. Previous studies have reported stimulation-dependent changes in the levels of activity, or in the correlation of activity, after repeated stimulation of in vitro networks10-13. Additionally, studies in dissociated cultures14 and in the Xenopus optic tectum15 have revealed that specific temporal patterns of stimulation can alter the timing of neural responses in recurrent circuits. For example, in dissociated cultures the interval of paired-pulse stimulation resulted in the emergence (or disappearance) of polysynaptic PSPs and changes in their timing. However, the timing of these events was primarily a product of the propagation delays of the circuitry and not directly predictable from the training interval per se14. The current results extend previous studies in two ways. First, by showing that neural dynamics in a complex circuit can be modified in a computationally functional fashion as a result of ‘experience’ - i.e, these circuits would be better able to ‘tell time’ around the stimulated interval. Second, the temporal structure of neural dynamics reflects the temporal structure of the training interval. In this sense these results represent an example of stimulus-specific modifications of network dynamics, and an in-vitro analog of “learning”.
While further work is required to dissect the mechanisms, these findings support the view that, on short-time scales, cortical circuits are inherently capable of telling time, suggesting that temporal and spatial processing are inextricably linked within cortical networks, and that specialized mechanisms and circuits are not necessary for temporal processing1,2.
Supplementary Material
Acknowledgements
We thank Tiago Carvalho, Jack Feldman, Tom O’Dell, and Felix Schweizer for helpful discussion and comments on this manuscript. We thank Kayla Gurley, Janet Lee, and Tyler Lee for technical assistance. This work was supported by the NIMH (MH60163).
Footnotes
Note: Supplementary material is available on the Nature Neuroscience website.
References
- 1.Mauk MD, Buonomano DV. The Neural Basis of Temporal Processing. Annual Rev. Neuroscience. 2004;27:304–340. doi: 10.1146/annurev.neuro.27.070203.144247. [DOI] [PubMed] [Google Scholar]
- 2.Ivry RB, Schlerf JE. Dedicated and intrinsic models of time perception. Trends in Cognitive Sciences. 2008;12:273–280. doi: 10.1016/j.tics.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson HA, Buonomano DV. A method for chronic stimulation of cortical organotypic cultures using implanted electrodes. Journal of Neuroscience Methods. 2009;176:136–143. doi: 10.1016/j.jneumeth.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 4.Wagenaar DA, Pine J, Potter SM. An extremely rich repertoire of bursting patterns during the development of cortical cultures. BMC Neurosci. 2006;7:11. doi: 10.1186/1471-2202-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacLean JN, Watson BO, Aaron GB, Yuste R. Internal Dynamics Determine the Cortical Response to Thalamic Stimulation. Neuron. 2005;48:811–823. doi: 10.1016/j.neuron.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 6.Buonomano DV. Timing of Neural Responses in Cortical Organotypic Slices. Proc. Natl. Acad. Sci. USA. 2003;100:4897–4902. doi: 10.1073/pnas.0736909100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu JK, Buonomano DV. Embedding Multiple Trajectories in Simulated Recurrent Neural Networks in a Self-Organizing Manner. J. Neurosci. 2009;29:13172–13181. doi: 10.1523/JNEUROSCI.2358-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiete IR, Senn W, Wang CZH, Hahnloser RHR. Spike-Time-Dependent Plasticity and Heterosynaptic Competition Organize Networks to Produce Long Scale-Free Sequences of Neural Activity. Neuron. 2010;65:563–576. doi: 10.1016/j.neuron.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Karmarkar UR, Dan Y. Experience-dependent plasticity in adult visual cortex. Neuron. 2006;52:577–85. doi: 10.1016/j.neuron.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Wagenaar DA, Pine J, Potter SM. Searching for plasticity in dissociated cortical cultures on multi-electrode arrays. J Negat Results Biomed. 2006;5:16. doi: 10.1186/1477-5751-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jimbo Y, Tateno T, Robinson HPC. Simultaneous induction of pathway-specific potentiation and depression in networks of cortical neurons. Biophys Journal. 1999;76:670–678. doi: 10.1016/S0006-3495(99)77234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahaf G, Marom S. Learning in networks of cortical neurons. J Neurosci. 2001;21:8782–8. doi: 10.1523/JNEUROSCI.21-22-08782.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruaro ME, Bonifazi P, Torre V. Toward the neurocomputer: image processing and pattern recognition with neuronal cultures. IEEE Trans Biomed Eng. 2005;52:371–83. doi: 10.1109/TBME.2004.842975. [DOI] [PubMed] [Google Scholar]
- 14.Bi G, Poo M. Distributed synaptic modification in neural networks induced by patterned stimulation. Nature. 1999;401:792–6. doi: 10.1038/44573. [DOI] [PubMed] [Google Scholar]
- 15.Pratt KG, Dong W, Aizenman CD. Development and spike timing-dependent plasticity of recurrent excitation in the Xenopus optic tectum. Nat Neurosci. 2008;11:467–475. doi: 10.1038/nn2076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.