Abstract
In addition to atrophy of mesial temporal lobe structures critical for memory function, white matter projections to the hippocampus may be compromised in individuals with mild Alzheimer’s disease (AD), thereby compounding the memory difficulty. In the present study, we used high-resolution structural imaging and diffusion tensor imaging techniques to examine micro-structural alterations in the parahippocampal white matter (PWM) region that includes the perforant path. Results demonstrated white matter volume loss bilaterally in the PWM in patients with mild AD. In addition, the remaining white matter had significantly lower fractional anisotropy and higher mean diffusivity values. Both increased mean diffusivity and volume reduction in the PWM were associated with memory performance and ApoE ε4 allele status. These findings indicate that, in addition to partial disconnection of the hippocampus from incoming sensory information due to volume loss in PWM, micro-structural alterations in remaining fibers may further degrade impulse transmission to the hippocampus and accentuate memory dysfunction. The results reported here also suggest that ApoE ε4 may exacerbate PWM changes.
Keywords: Entorhinal cortex, perforant path, temporal lobe, ApoE, diffusion tensor imaging
1. Introduction
High resolution, quantitative magnetic resonance imaging (MRI) is a valuable tool for evaluating alterations in brain anatomy in vivo in age-related degenerative diseases and may provide a surrogate marker for the underlying pathology. One of the early clinical hallmarks of Alzheimer’s disease (AD) is a disturbance in memory, especially characterized by difficulty in the acquisition of new declarative knowledge. The nature of this mnemonic dysfunction is similar to that seen with bilateral lesions, dysfunction or disconnection of the hippocampal formation and related structures (Squire and Zola-Morgan, 1991), thus implicating the pathophysiologic disruption of this neural system in the early stages of AD.
Post mortem pathological studies have shown the entorhinal cortex and trans-entorhinal region to be early sites of involvement in AD and in individuals with mild cognitive impairment (MCI) who are at high risk of developing AD (Braak and Braak, 1991, 1995; Gomez-Isla et al., 1996; Kordower et al., 2001). In vivo MRI investigations have also demonstrated atrophy of both the entorhinal cortex and hippocampus not only in patients with mild AD, but also in people with amnestic MCI and subjective cognitive complaints (e.g., deToledo-Morrell et al., 2004; Dickerson et al., 2001; Du et al., 2001; Jack et al., 1997, 1999; Jessen et al., 2006; Killiany et al., 2000, 2002; Saykin et al., 2006; Stoub et al., 2005).
In addition to pathology and atrophy in gray matter regions, there may be changes in white matter that could disconnect different cortical regions and accentuate cognitive dysfunction. However, such white matter changes have received less attention in investigations on the pathophysiology of AD. Entorhinal cortex neurons receive multi-modal sensory input from primary sensory and association cortices and relay this information to the hippocampus via the perforant path, a white matter tract located in the anterior medial portion of the parahippocampal gyrus (Amaral et al., 1987; van Hoesen and Pandya, 1975; van Hoesen et al., 1975). Post mortem studies have demonstrated loss of entorhinal layer II neurons in patients with mild AD (Hyman et al., 1984) and in those with MCI (Gomez-Isla et al., 1996; Kordower et al., 2001) that could results in a partial disconnection of information flow to the hippocampus. In addition, damage to the parahippocampal white matter could disrupt afferent connections to the entorhinal cortex and ultimately degrade multimodal sensory information relayed to the hippocampus.
These changes in white matter may be detectable with high resolution MRI techniques, such as diffusion tensor imaging (DTI). DTI is a newer MRI technique that allows examination of the micro-structural integrity of white matter in vivo. This emerging technique combines MR diffusion-weighted pulse sequences with tensor mathematics to measure molecular diffusion in three dimensions. In fact, recently there has been a proliferation of investigations using DTI to examine white matter changes in AD (e.g., Hanyu et al., 1998; Head et al., 2004; Kalus et al., 2006; Medina et al., 2006; Salat et al., 2008; Zhang et al., 2007). Many of these investigations explored whole brain white matter changes in patients with AD, while two studies further examined the parahippocampal white matter region that includes the perforant path (Kalus et al., 2006; Salat et al., 2008). In addition to the volume of the parahippocampal white matter region, Kalus et al. (2006) reported the coherence index (CI) that measures the similarity in diffusion between adjoining voxels as the measure of white matter integrity, rather than the commonly used measures of fractional anisotropy (FA) and mean diffusivity (MD). Salat et al. (2008) defined anatomical regions of interest (ROIs) on DTI maps (Salat et al., 2008). Defining anatomical regions using the dependent variable map (e.g., FA map) may have methodological shortcomings as it precludes the identification of abnormal tissue with low FA (Pfefferbaum and Sullivan, 2003).
In the present study, we used a high-resolution DTI protocol and high-resolution structural imaging to examine micro-structural and macro-structural alterations in parahippocampal white matter and their relation to memory function in patients with mild AD compared to elderly controls. DTI analysis was restricted to the parahippocampal white matter by applying manually traced ROIs derived from high-resolution structural images to DTI scans. FA or MD values were quantified without direct manipulation of the DTI volumes. FA is a scalar metric that describes the directionality of the diffusion tensor, while MD is a non-directional measure of free translational diffusion and provides an index of general tissue integrity. These two measures are the most commonly used and sensitive indices of micro-structural integrity of white matter. We also examined the relationship between ApoE ε4 allele status and parahippocampal white matter changes. ApoE is a group of proteins that bind reversibly with lipoprotein for transporting endogenous lipids for uptake and use in myelin repair, growth and structural integrity maintenance (Bartzokis et al., 2006). In fact, previous studies have demonstrated a reduction in ApoE concentration in brain tissue from ApoE ε4 allele carriers (Poirier, 2005). The ApoE ε4 allele is known to be a risk factor for Alzheimer’s disease (Saunders et al., 1993) and may affect white matter integrity even in healthy carriers of the ApoE ε4 allele (Persson et al., 2006).
2. Methods
2.1 Participants
Participants in the study consisted of 17 patients with mild AD (mean age 77.18 ± 5.23 years) and 26 elderly individuals with no cognitive impairment (NCI; mean age 74.92 ± 8.03 years). They were recruited from the Rush Alzheimer’s Disease Center (RADC) clinic, the community, and the Rush Memory and Aging Project (MAP). MAP is a longitudinal, clinicopathologic study of aging in older participants who have agreed to annual evaluations and brain autopsy at the time of death (Bennett et al., 2005).
All participants received the same standard clinical evaluation as previously described (Bennett et al., 2002; deToledo-Morrell et al., 2004). Briefly, the evaluation incorporated the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD; Morris et al., 1989) procedures and included a medical history, neurological examination, neuropsychological testing, and informant interview. Blood tests and clinical imaging studies were used as needed. The clinical diagnosis of probable AD followed NINCDS/ADRDA guidelines (McKhann et al., 1984); it required a history of cognitive decline and neuropsychological test evidence of impairment in at least two cognitive domains, one of which had to be memory. All participants diagnosed with mild AD in the present study had a Mini-Mental Status Examination (MMSE; Folstein et al., 1975) score ≥ 21 (mean = 24.29±1.76, range = 21–27).
Control subjects received the same clinical evaluation as patients diagnosed with AD. Selection as an elderly control participant required a normal neurological examination, normal cognition, and a MMSE score ≥ 27. The mean MMSE score for controls was 29.31 ± 0.92 (range = 27–30). It is important to note that individuals who came to the clinic for a work-up, but did not show any cognitive impairment were not recruited as controls; the latter were recruited from the community or MAP.
Exclusion criteria for entry into the study consisted of evidence of other neurological, psychiatric or systemic conditions that could cause cognitive impairment (e.g., stroke, tumor, Parkinson’s disease, alcoholism, major depression, a history of temporal lobe epilepsy), and age <65 years.
For all participants in the study, vascular risk factors were noted and summarized as the number of the following: hypertension, diabetes mellitus, hypercholesterolemia and smoking.
Informed consent was obtained from all participants as approved by the Institutional Review Board of Rush University Medical Center.
2.2 Magnetic resonance imaging
2.2.1 Acquisition parameters
All participants underwent two MRI scan sequences, a high-resolution structural scan and a high-resolution DTI scan; both were acquired with a 1.5 Tesla General Electric Signa scanner (General Electric Medical Systems, Milwaukee, WI, USA), equipped with high speed gradients (LX Horizon, Rev 10.4). The structural scan consisted of a T1 weighted high-resolution 3D spoiled gradient recalled (SPGR) pulse sequence with the following parameters: 124 contiguous images acquired in the coronal plane, 1.6 mm thick sections, matrix = 256 × 196, repetition time (TR)/echo time (TE) = 34/7 ms, signals averaged = 1, flip angle = 35°, field of view (FOV) = 22 cm.
The high-resolution DTI scans were diffusion weighted single shot spin echo, echo planar images axially acquired with the following parameters: TR / TE = 12100 / 97 ms, field of view = 25 cm, matrix = 128 × 128, 38 3mm gapless slices, 6 repetitions, in plane resolution = 1.95 mm. Two diffusion weights (b-values) were used: b = 0 and b = 800 s/mm2. The high b-value was obtained by applying diffusion encoding gradients along 24 non-collinear directions. This acquisition scheme was repeated six times for each slice, with the sign of all the gradient directions inverted for every other repetition. An additional set of inversion recovery images with cerebrospinal fluid nulling (TI ∼ 2100 ms) were acquired for each slice with b = 0 s/mm2. These images were used to un-warp the eddy current effect of the diffusion gradients (de Crespigny and Moseley, 1998). Scans were transferred to an off-line workstation (Sun Microsystems, Palo Alto, CA) for processing.
2.2.2 Processing of DTI scans
The methods for processing the DTI images are detailed elsewhere (Wang et al., 2006). Briefly, the set of CSF nulled inversion recovery images were used as the reference for un-warping eddy current effects in the diffusion weighted images (de Crespigny and Moseley, 1998). Next, the six coefficients defining the elements of the diffusion tensor were calculated (Basser et al., 1994) from the eddy-current corrected, averaged images. Eigenvectors, defining the three principle directions of diffusion for each voxel were derived from the diffusion tensor. The magnitude of diffusivity in each direction was represented by the eigenvalues for the three eigenvectors; fractional anisotropy (FA) and mean diffusivity (MD) were derived for each voxel from these eigenvalues (Basser, 1995; Basser and Pierpaoli, 1996). From this processing, three values were constructed for each voxel: FA, MD and b = 0 (0 diffusion-weighted image =T2 image). Individual participant slice images for FA, MD and T2 were concatenated into whole-brain volumes in a format acceptable for processing in SPM 2, implemented in Matlab R14, sp1 (The Math Works, Natick, Massachusetts).
To assess potential group differences in white matter hyperintensities, individual participant slice images for the zero diffusion-weighted image (T2) acquisitions were concatenated into whole-brain volumes in acceptable format. Whole-brain volumes were imported into SPM2 for analysis. To facilitate voxel-by-voxel comparison between the two groups, all images were spatially normalized to a standard template. To avoid the geometric distortions associated with diffusion weighted echo planar imaging, we used the 0 diffusion-weighted image obtained during the scanning sequence for normalization. The T2 weighted image was normalized to the standard T2 template in SPM2 using a 12 iteration affine transformation and a non-linear transformation with 7×8×7 basis functions. To limit our analysis of group differences in T2 signal in white matter, we created individual subject mask volumes. These masks were created by segmenting the normalized T2 images into CSF, gray matter, and white matter compartments. The segmentation algorithms are based on signal intensity and prior probabilities for location. The individual white matter masks were then applied to individual subject T2 volumes. A Gaussian filter was applied to these individual masked images (6mm full width at half maximum) to increase signal-to-noise ratio and meet requirements of a Gaussian distribution for general linear analyses. A filter size of 6mm was chosen to allow for interrogation of relatively small regions (e.g., parahippocampal white matter), while assuring normal distribution of data across voxels. Group differences in voxel level T2 signal were assessed using the two-sample t-test module in SPM2.
2.2.3 Regional analyses of FA and MD values
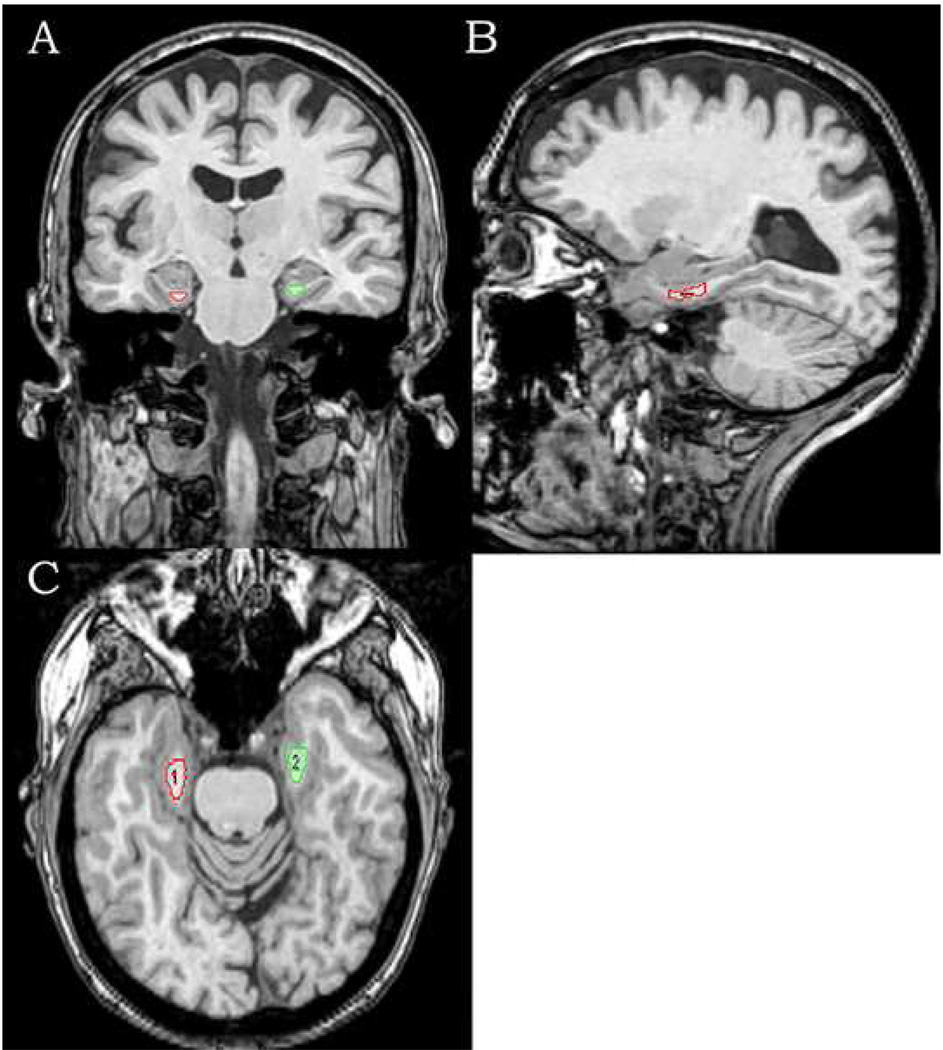
The first step in developing the regions of interest (ROI) for the measurement of FA and MD in the parahippocampal region required the co-registration of the 3D SPGR structural scan and the DTI scan for each subject. The co-registration module in SPM2 was used for this process with the 0 diffusion weighted DTI volume as the target to which the 3D SPGR structural image was registered. Co-registration of the two volumes was accomplished using a rigid-body transformation and the resultant deformation matrix was stored for later processing. ROIs of parahippocampal white matter were determined on the co-registered 3D SPGR structural images with the Analyze Software package (Mayo Clinic, Rochester, MN). Transverse, coronal, and sagittal planes of 3D SPGR structural images were used to view and manually trace parahippocampal white matter regions bilaterally, slice by slice (see Figure 1). Tracings began with the coronal slice in which the amygdala was fully visible and ended three 1.6-mm images rostral to the slice in which the lateral geniculate nucleus was first seen. This ROI covered only the anterior medial portion of parahippocampal white matter that includes the perforant path. Volumes were calculated for the ROI in each hemisphere with the Analyze software, taking all traced slices into account. Tracings were conducted while blind to diagnostic status. All tracings were performed by CW and were reviewed and corrected by LTM. The intra-rater correlation coefficient for CW based on 10 cases was 0.98 for the left hemisphere and 0.97 for the right.
Figure 1.
Transverse, coronal, and sagittal views of tracing the bilateral parahippocampal white matter in T1 3D images, manually created slice by slice, beginning with the first slice in which the amygdala fully appeared visible, and ending with three 1.6 mm slices rostral to the image in which the lateral geniculate nucleus first appeared visible.
To ensure that the location of traced ROIs and the DTI FA or MD maps were properly matched, the deformation matrix derived from the co-registration of the 3D SPGR structural images to the DTI volumes was applied to the traced ROIs. These co-registered ROIs were re-sliced to backfill the volume and then used to extract FA and MD values from parahippocampal regions.
2.3 Declarative memory testing
The declarative memory tests administered to all participants and used to define a memory deficit consisted of immediate and delayed recall of the East Boston Story (Albert et al., 1991) and of Story A from the Logical Memory of the Wechsler memory scale–Revised (Wechsler, 1987). An additional test involved the learning and retention of a 10-word list from the CERAD battery (Morris et al., 1989). The three scores for this test included Word List Memory (the total number of words immediately recalled after each of three consecutive presentations of the list),Word List Recall (the number of words recalled after a delay) and Word List Recognition (the number of words correctly recognized in a four-alternative, forced-choice format, administered after Word List Recall).
2.4 Statistical analyses
Differences between the mild AD and control groups in demographic variables, vascular risk factors and ApoE ε4 status were assessed with two-tailed t-tests for independent samples or chi-square analyses, as appropriate, using SPSS for windows, release 11 (SPSS, Inc., Chicago, IL, USA). Group differences in parahippocampal white matter volume, FA or MD were examined separately for each measure using a repeated measures analysis of variance (ANOVA), with groups as the between subject factor and hemispheres as the repeated measure. In addition, to assess the relationship between changes in parahippocampal white matter and memory performance, individual subject summary scores were calculated for performance on the seven declarative memory tests. To develop these summary scores, we used the means and standard deviations of each test from the baseline visits of the first wave of 86 participants entered into our ongoing longitudinal project to generate z-scores for the present sample on each memory test. The average z-score from the seven declarative memory tests constituted the summary memory score. Multiple regression analyses with memory summary z-scores as the dependent variable were carried out to determine if MRI measures of parahippocampal white matter could predict memory performance. A multiple regression model was used to examine the contribution of ApoE ε4 and age to parahippocampal white matter changes.
3. Results
Demographic characteristics of participants are listed in Table 1. Patients with mild AD did not differ from the controls in age [t(41) = 1.02, p = 0.31], education [t(41) = 0.54, p = 0.59], or gender distribution [χ2(1) = 0.67, p = 0.53]. In addition, these two groups did not differ in systolic or diastolic blood pressure, or in vascular risk factor summary scores. However, as expected, the two groups did differ in MMSE scores [t(41) =12.21, p<0.001] and memory z-scores [t(41) =9.39, p<0.001], with the mild AD participants having significantly lower scores for both. Moreover, the percentage of ApoE ε4 carriers (any ApoE ε4 allele) was higher among patients with mild AD and differed significantly from controls (χ2(1) = 4.37, p = 0.039). As there was no difference in age and education between the two groups, they were not entered as covariates in the following analyses.
Table 1.
Demographic and other characteristics of participants
| Mild AD | NCI | ||
|---|---|---|---|
| Number of participants | 17 | 26 | |
| Male / Female | 8/9 | 9/17 | |
| Age, years (SD) | 77.18 (±5.22) | 74.92 (±8.03) | |
| Education, years (SD) | 14.94 (±4.99) | 15.58 (±2.66) | |
| MMSE (SD) *** | 24.29 (±1.76) | 29.30 (±0.93) | |
| Memory z-score*** | −1.1623 (±0.6529) | 0.5467 (±0.5341) | |
| Systolic blood pressure | 147.6 (±23.68) | 139.2 (±20.7) | |
| Diastolic blood pressure | 81.2 (±13.62) | 75.6 (±10.86) | |
| Vascular risk factors (total) | 1.68 (±1.13) | 1.28 (±1.02) | |
| ApoE status * | |||
| 2/3 + 3/3 | 6 | 17 | |
| 3/4 + 4/4 | 10 | 7 | |
| Not available | 1 | 2 | |
p<0.001,
p<0.05
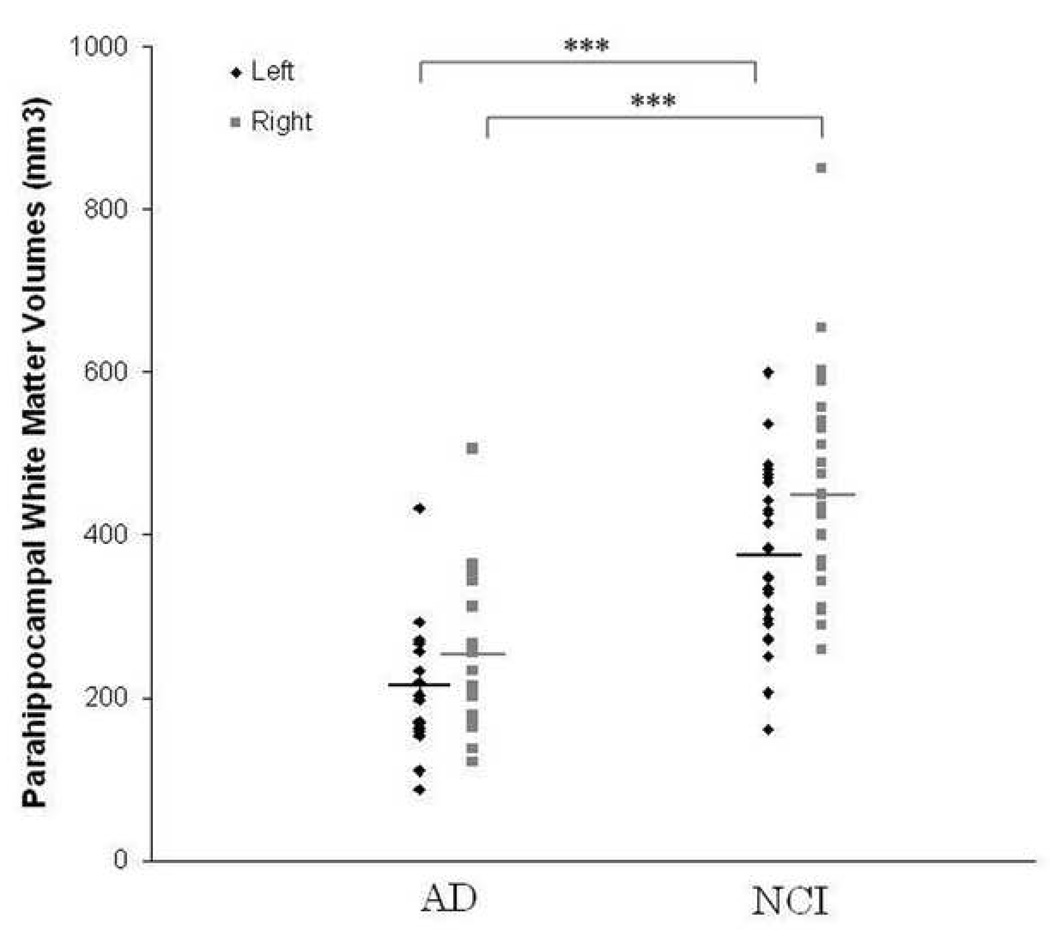
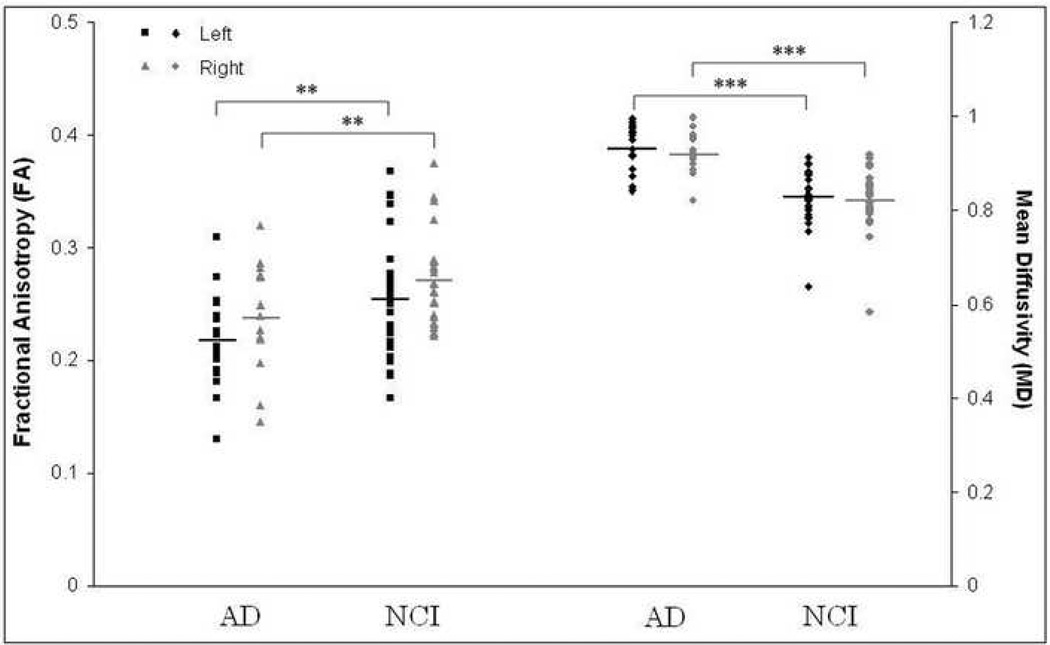
Individual data for parahippocampal white matter volumes among patients with mild AD and control subjects are shown in Figure 2, while those for FA and MD are presented in Figure 3. Repeated measures ANOVAs with groups and hemisphere as the two factors were carried out separately to assess differences in white matter volume, FA and MD. Results showed a significant effect for group with a 43.2% reduction in white matter volume (F[1,41] = 31.89, p <0.001), a 13.0% decrease in white matter FA (F[1,41] = 7.35, p = 0.01), and 12.4% increase in white matter MD (F[1,41] = 36.34, p <0.001) in patients with mild AD compared to control participants. In addition, there was a significant hemisphere effect for volume (F[1,41]=16.03, p<0.001) and FA (F[1,41]=8.70, p=0.005), with the right hemisphere white matter volume and FA being greater than the left. The difference in MD between the two hemispheres was not significant. The interaction between group and hemisphere did not reach significance for any of the three measures.
Figure 2.
Parahippocampal white matter volumes in patients with mild AD and controls (NCI), plotted for each participant as a function of hemisphere. The bars indicate the group mean. Volumes were significantly reduced (*** p < 0.001) in patients with AD compared to controls bilaterally.
Figure 3.
Parahippocampal white matter FA and MD values in patients with mild AD and controls (NCI), shown separately for each participant as a function of hemisphere. The bars indicate the group mean. MD was significantly increased in patients with mild AD bilaterally, while FA was significantly decreased (*** p<0.001, ** p=0.01). Please note the difference in scale for FA and MD.
To assess whether MRI measures of parahippocampal white matter are related to memory performance, each of the three measures (volume, FA, MD) was entered singly into a regression analysis, with memory z-scores as the dependent variable. Each of these measures was found to be a significant predictor of memory function [F(1,41)=8.25, p=0.006, R2=0.17 for FA; F(1,41)=47.70, p<0.001, R2=0.54 for MD; and F(1,41)=24.92, p<0.001, R2=0.38 for volume]. When all three measures were entered simultaneously, the regression model remained significant [F(6,36)=22.09, p<0.0001]. However, only main effects for parahippocampal white matter volume [t(36)=2.76, p=0.009] and mean diffusivity [t(36)=4.69, p<0.001] were significant predictors of memory performance; FA was not. The group×MD interaction [t(36)=4.17, p <0.001] and group×volume interaction [t(36) = −2.07, p=0.045] reached significance indicating increased MD and decreased volume in the mild AD group compared to controls. The group×FA interaction was not significant.
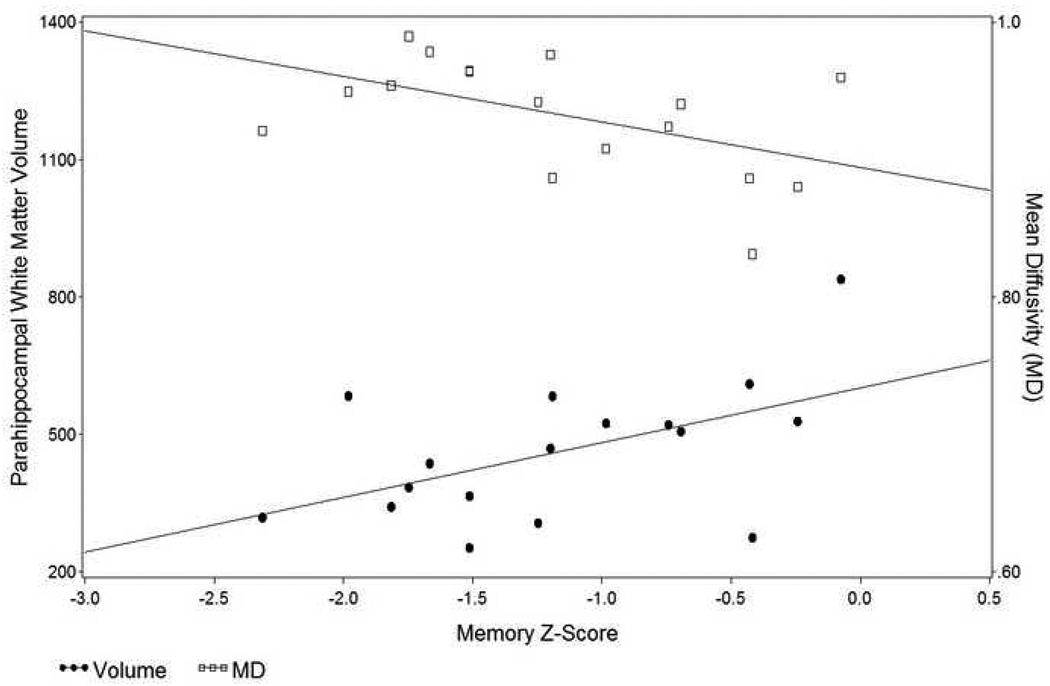
The contribution of these variables to memory performance was also analyzed for each group separately. These analyses showed that parahippocampal white matter volume and MD, but not FA, were significant predictors of memory z-scores in the mild AD group [t(13)=2.43, p = 0.03 for volume; t(13) = −2.25, p = 0.04 for MD), but not in the control group. These relationships for the mild AD group are presented in Figure 4.
Figure 4.
Scatterplot showing the relation of parahippocampal white matter MD and volume to memory z-scores in participants with mild AD.
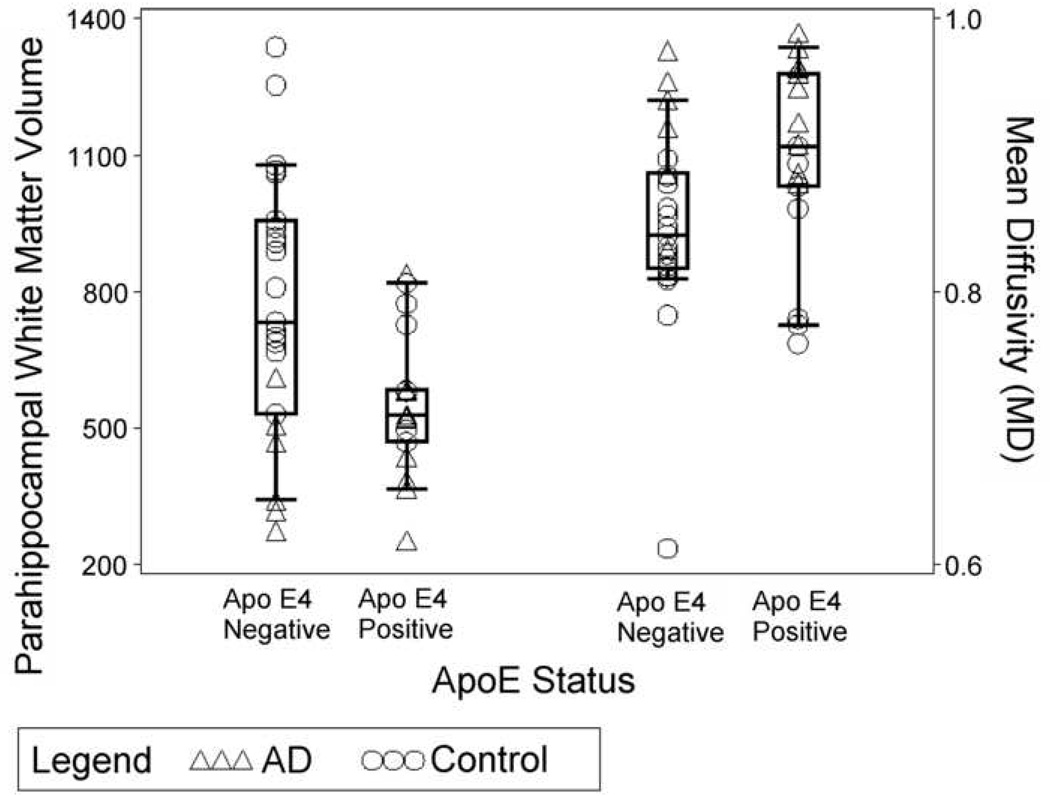
A multiple regression model was applied to assess the contribution of ApoE ε4 status and age to parahippocampal white matter volume, FA and MD, separately, with parahippocampal white matter volume, FA or MD as dependent variables. ApoE ε4 and age were found to contribute significantly to both parahippocampal white matter volume [F(2,39)=9.538, p<0.001) and MD [F(2,39)=8.112, p=0.001]. ApoE ε4 allele status and age accounted for approximately 34% of the variance for parahippocampal white matter volume and 30.5% for MD, respectively (see Figure 5). However, neither ApoE ε4 allele status nor age was found to contribute significantly to FA.
Figure 5.
Scatterplot of parahippocampal white matter MD and volume shown separately for ApoE ε4 carriers and non-carriers in the two groups of participants.
4. Discussion
The results of the present study indicate that, in addition to volume loss, there was a significant decrease in FA and an increase in MD in parahippocampal white matter in patients with mild AD, indicating that remaining normal appearing white matter in this region was not really “normal”. These white matter changes reflect not only loss of afferent and efferent fibers in the region, but also may be indicative of partial demyelination or other damage to remaining fibers. These results suggest that impulse transmission to the hippocampus may be further degraded in patients with mild AD due to microstructural alterations in remaining normal appearing white matter. Thus, it is not surprising that parahippocampal white matter changes were found to contribute significantly to memory dysfunction in the present study. Furthermore, the frequency of ApoE ε4 allele carriers in the mild AD group was significantly greater than in the control group. ApoE ε4 allele status was associated with parahippocampal white matter changes, not only replicating previous findings that ApoE ε4 is a risk factor for AD (Lindsay et al., 2002), but also further suggesting that it might influence parahippocampal white matter changes.
In the present study, volume loss and two microstructural integrity metrics, MD and FA, derived from DTI images were used to assess parahippocampal white matter changes. The parahippocampal white matter includes multiple fiber systems. Its major component, the perforant path, originates from cells in the entorhinal cortex and provides the hippocampus with its major cortical input. Numerous post mortem tissue studies on the pathophysiology of AD have demonstrated that the cells of origin of this white matter tract in the entorhinal cortex are partly lost very early in the disease process (Gomez-Isla et al., 1996; Hyman et al., 1984; Hyman et al., 1986; Kordower et al., 2001). In addition to these pathological findings, in vivo imaging studies have shown entorhinal atrophy very early in AD, probably reflecting cell loss, cell shrinkage and other pathological changes in this structure (deToledo-Morrell et al., 2004; Dickerson et al., 2001; Du et al., 2001; Killiany et al., 2000; Killiany et al., 2002; Xu et al., 2000). With new developments in imaging techniques such as DTI, it is now possible to quantify, in vivo, not only volume loss in given white matter regions, but also microstructural alterations in normal appearing white matter.
Two previous DTI studies examined microstructural changes in parahippocampal white matter in AD (Kalus et al., 2006; Salat et al., 2008). Kalus and colleagues (2006) only examined the coherence index (CI) as a measure of white matter integrity; they reported CI to be significantly reduced in parahippocampal white matter in AD and the reduction to be related to cognitive impairment. Salat et al. (2008) used FA and MD to quantify white matter integrity; they found FA to be reduced and MD to be increased in parahippocampal white matter in patients with AD. However, they defined anatomical ROIs on DTI maps. Defining anatomical regions using the FA map may introduce bias, since abnormal white matter with low FA might be excluded (Pfefferbaum and Sullivan, 2003). Furthermore, these two studies either globally manipulated FA or MD maps by resizing brain parenchyma to a template (Salat et al., 2008), or co-registering the DTI maps to high-resolution structural images (Kalus et al., 2006). These methods would change DTI map values during reslicing or coregisteration and may lead to DTI measure errors. In the present study, we defined ROIs on a high-resolution structural image. These defined ROIs were then transferred to DTI maps. FA or MD values were quantified without direct manipulation of FA or MD, thus avoiding the problems outlined above.
Alterations in white matter have been demonstrated both with histopathological and MRI methods in AD (Bronge et al., 2002; Brun and Englund, 1986a, b; Meyerhoff et al., 1994). White matter changes associated with AD may have different causes or mechanisms. First, white matter changes in the disease process may reflect anterograde Wallerian degeneration, especially in regions close to cortical areas with the greatest pathological burden. Since the entorhinal cortex is one of the earliest sites of pathological involvement in AD, cell loss in layer II of the entorhinal cortex could lead to alterations in the parahippocampal white matter region. Secondly, there may be white matter rarefaction (sometimes referred to as “white matter disease”; Englund, 1998) with axonal damage and gliosis. This type of change is diffuse and does not follow the regional extension of pathologically involved gray matter, as may be the case with Wallerian degeneration. This second type of white matter degeneration may be vascular or ischemic in origin. In fact, alterations in microvasculature are common in AD and have been observed in the majority of brains from patients with AD (Kalaria, 2002). Third, it has been recently suggested that myelin breakdown is an important component of the disease process in AD due to increased susceptibility of oligodendrocytes to free radical and other metabolic damage (Bartzokis, 2004, 2006). According to this hypothesis, damage to oligodendrocytes may be a critical first step in AD. Furthermore, since late developing oligodendrocytes are more vulnerable, late-myelinating association areas are predicted to be more susceptible to myelin breakdown, thus leading to disconnection among different cortical areas and to cognitive decline. In fact, Bartzokis (2004) does site the parahippocampal region as having a very long cycle of myelination, going into the fifth decade of life.
The mild AD and control participants in the present study did not differ from each other in cardiovascular risk factors that may have exacerbated global white matter changes. Our major emphasis in this paper was on the parahippocampal white matter region that includes the perforant path. Although this region is affected very early in the disease process of AD, recent data from our laboratory (Wang et al., 2010) indicate that vascular cognitive impairment following stroke is not associated with parahippocampal white matter changes. Thus, the microstructural changes in remaining parahippocampal white matter reported here are not likely to be due to cardiovascular risk factors between the two groups.
A previous study from our laboratory (Stoub et al., 2006) and others have demonstrated that parahippocampal white matter volume loss and intervoxel coherence were associated with declarative memory dysfunction in participants with amnestic MCI and in patients with AD (Kalus et al., 2006; Stoub et al., 2006). In the present study, we further found that changes in white matter volume and in the microstructural integrity of remaining normal appearing white matter fibers in the parahippocampal region accounted for 60.5% of the variance in memory z-scores, indicating that alterations in these remaining normal appearing fibers further degraded information flow to the hippocampus and affected memory function in mild AD. It should be noted that, of the two microstructural integrity metrics, MD was found to be more sensitive to memory dysfunction than FA. This is probably because FA is more significantly affected by heterogeneous organization or crossing fibers in given regions (Pfefferbaum and Sullivan, 2003).
Interestingly, in the present study we also found that the frequency of ApoE ε4 allele carriers in patients with mild AD was significant greater than in controls, a finding consistent with previous studies (Saunders et al., 1993). In addition, we found ApoE ε4 allele status to be associated with a decrease in parahippocampal white matter volume and an increase in mean diffusivity, suggesting that ε4 may influence the integrity of parahippocampal white matter. Consistent with these findings, two previous studies reported a relationship between ApoE ε4 status and white matter changes in AD (Bronge et al., 1999; Wen et al., 2006), but others did not find such an association (Hirono et al., 2000; Sawada et al., 2000; Schmidt et al., 1996). This inconsistency may be due to the fact that previous studies only examined global or regional white matter other than that specific to the parahippocampal gyrus. In addition, most studies reported only visual rating scores or simply the presence or absence of white matter changes (Barber et al., 1999; Doody et al., 2000; Hirono et al., 2000; Sawada et al., 2000; Schmidt et al., 1996). Such qualitative methods may reduce sensitivity. Quantitative methods used in the present study, combining volume measures with DTI metrics, may be more accurate, reliable and sensitive than simply visual rating observations.
In conclusion, the results reported here not only demonstrated that there is volume loss in the parahippocampal white matter in patients with mild AD compared to age appropriate controls, but also revealed that microstructural integrity was compromised in remaining normal appearing white matter fibers. These changes in parahippocampal white matter were associated with ApoE ε4 genotype, and contributed significantly to declarative memory impairment in mild AD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Acknowledgements: This research was supported by grants P01 AG09466, P30 AG10161 and R01 AG17917 from the National Institute on Aging, National Institutes of Health and the Illinois Department of Public Health.
References
- Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer's disease. Int. J. Neurosci. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Insausti R, Cowan WM. The entorhinal cortex of the monkey: I. Cytoarchitectonic organization. J. Comp. Neurol. 1987;264:326–355. doi: 10.1002/cne.902640305. [DOI] [PubMed] [Google Scholar]
- Barber R, Gholkar A, Scheltens P, Ballard C, McKeith IG, Morris CM, O’Brien JT. Apolipoprotein E epsilon4 allele, temporal lobe atrophy, and white matter lesions in late-life dementias. Arch. Neurol. 1999;56:961–965. doi: 10.1001/archneur.56.8.961. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer's disease. Neurobiol. Aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Geschwind DH, Edwards N, Mintz J, Cummings JL. Apolipoprotein E genotype and age-related myelin breakdown in healthy individuals: implications for cognitive decline and dementia. Arch. Gen. Psychiatry. 2006;63:63–72. doi: 10.1001/archpsyc.63.1.63. [DOI] [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol. Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Bronge L, Bogdanovic N, Wahlund LO. Postmortem MRI and histopathology of white matter changes in Alzheimer brains. A quantitative, comparative study. Dement. Geriatr. Cogn. Disord. 2002;13:205–212. doi: 10.1159/000057698. [DOI] [PubMed] [Google Scholar]
- Bronge L, Fernaeus SE, Blomberg M, Ingelson M, Lannfelt L, Isberg B, Wahlund LO. White matter lesions in Alzheimer patients are influenced by apolipoprotein E genotype. Dement. Geriatr. Cogn. Disord. 1999;10:89–96. doi: 10.1159/000017107. [DOI] [PubMed] [Google Scholar]
- Brun A, Englund E. Brain changes in dementia of Alzheimer's type relevant to new imaging diagnostic methods. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1986a;10:297–308. doi: 10.1016/0278-5846(86)90009-6. [DOI] [PubMed] [Google Scholar]
- Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type:a pathoanatomical study. Ann. Neurol. 1986b;19:253–262. doi: 10.1002/ana.410190306. [DOI] [PubMed] [Google Scholar]
- de Crespigny A, Moseley M. Eddy current induced image warping in diffusion weighted EPI; Proc. ISMRM 6th Meeting; Sydney. 1998. p. 661. [Google Scholar]
- deToledo-Morrell L, Stoub TR, Bulgakova M, Wilson RS, Bennett DA, Leurgans S, Wuu J, Turner DA. MRI-derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiol. Aging. 2004;25:1197–1203. doi: 10.1016/j.neurobiolaging.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Goncharova I, Sullivan MP, Forchetti C, Wilson RS, Bennett DA, Beckett LA, deToledo-Morrell L. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer's disease. Neurobiol. Aging. 2001;22:747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- Doody RS, Azher SN, Haykal HA, Dunn JK, Liao T, Schneider L. Does APO epsilon4 correlate with MRI changes in Alzheimer's disease? J Neurol. Neurosurg. Psychiatry. 2000;69:668–671. doi: 10.1136/jnnp.69.5.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Amend D, Laakso MP, Hsu YY, Jagust WJ, Yaffe K, Kramer JH, Reed B, Norman D, Chui HC, Weiner MW. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 2001;71:441–447. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund E. Neuropathology of white matter changes in Alzheimer's disease and vascular dementia. Dement. Geriatr. Cogn. Disord. 1998;9 Suppl 1:6–12. doi: 10.1159/000051183. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J. Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyu H, Sakurai H, Iwamoto T, Takasaki M, Shindo H, Abe K. Diffusion-weighted MR imaging of the hippocampus and temporal white matter in Alzheimer's disease. J. Neurol. Sci. 1998;156:195–200. doi: 10.1016/s0022-510x(98)00043-4. [DOI] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cereb. Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Hirono N, Kitagaki H, Kazui H, Hashimoto M, Mori E. Impact of white matter changes on clinical manifestation of Alzheimer's disease: A quantitative study. Stroke. 2000;31:2182–2188. doi: 10.1161/01.str.31.9.2182. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Kromer LJ, Damasio AR. Perforant pathway changes and the memory impairment of Alzheimer's disease. Ann. Neurol. 1986;20:472–481. doi: 10.1002/ana.410200406. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu YC, Waring SC, O’Brien PC, Tangalos EG, Smith GE, Ivnik RJ, Kokmen E. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Feyen L, Freymann K, Tepest R, Maier W, Heun R, Schild HH, Scheef L. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol. Aging. 2006;27:1751–1756. doi: 10.1016/j.neurobiolaging.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Kalaria R. Similarities between Alzheimer's disease and vascular dementia. J Neurol. Sci. 2002;203–204:29–34. doi: 10.1016/s0022-510x(02)00256-3. [DOI] [PubMed] [Google Scholar]
- Kalus P, Slotboom J, Gallinat J, Mahlberg R, Cattapan-Ludewig K, Wiest R, Nyffeler T, Buri C, Federspiel A, Kunz D, Schroth G, Kiefer C. Examining the gateway to the limbic system with diffusion tensor imaging: the perforant pathway in dementia. Neuroimage. 2006;30:713–720. doi: 10.1016/j.neuroimage.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Killiany RJ, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, Tanzi R, Jones K, Hyman BT, Albert MS. Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease. Ann. Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- Killiany RJ, Hyman BT, Gomez-Isla T, Moss MB, Kikinis R, Jolesz F, Tanzi R, Jones K, Albert MS. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002;58:1188–1196. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Stebbins GT, DeKosky ST, Cochran EJ, Bennett D, Mufson EJ. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann. Neurol. 2001;49:202–213. [PubMed] [Google Scholar]
- Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, McDowell I. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am. J Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Medina D, DeToledo-Morrell L, Urresta F, Gabrieli JD, Moseley M, Fleischman D, Bennett DA, Leurgans S, Turner DA, Stebbins GT. White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiol. Aging. 2006;27:663–672. doi: 10.1016/j.neurobiolaging.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, MacKay S, Constans JM, Norman D, Van Dyke C, Fein G, Weiner MW. Axonal injury and membrane alterations in Alzheimer's disease suggested by in vivo proton magnetic resonance spectroscopic imaging. Ann. Neurol. 1994;36:40–47. doi: 10.1002/ana.410360110. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Persson J, Lind J, Larsson A, Ingvar M, Cruts M, Van Broeckhoven C, Adolfsson R, Nilson L-G, Nyberg L. Altered brain white matter integrity in healthy carriers of the APOE ε4 allele: A risk for AD? Neurology. 2006;66:1029–1033. doi: 10.1212/01.wnl.0000204180.25361.48. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Increased brain white matter diffusivity in normal adult aging: relationship to anisotropy and partial voluming. Magn. Reson. Med. 2003;49:953–961. doi: 10.1002/mrm.10452. [DOI] [PubMed] [Google Scholar]
- Poirier J. Apolipoprotein E, cholesterol transport and synthesis in sporadic Alzheimer's disease. Neurobiol. Aging. 2005;26:355–361. doi: 10.1016/j.neurobiolaging.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, van der Kouwe AJ, Greve DN, Pappu V, Lee SY, Hevelone ND, Zaleta AK, Growdon JH, Corkin S, Fischl B, Rosas HD. White matter pathology isolates the hippocampal formation in Alzheimer's disease. Neurobiol. Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.03.013. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Sawada H, Udaka F, Izumi Y, Nishinaka K, Kawakami H, Nakamura S, Kameyama M. Cerebral white matter lesions are not associated with apoE genotype but with age and female sex in Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 2000;68:653–656. doi: 10.1136/jnnp.68.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, McHugh TL, Mamourian AC. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Schmidt R, Fazekas F, Semmler J, Kapeller P, Reinhart B, Kostner GM. Apolipoprotein E e4 allele in the normal elderly: neuropsychologic and brain MRI correlates. Clin. Genet. 1996;50:293–299. doi: 10.1111/j.1399-0004.1996.tb02377.x. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Stoub TR, Bulgakova M, Leurgans S, Bennett DA, Fleischman D, Turner DA, de Toledo-Morrell L. MRI predictors of risk of incident Alzheimer disease: a longitudinal study. Neurology. 2005;64:1520–1524. doi: 10.1212/01.WNL.0000160089.43264.1A. [DOI] [PubMed] [Google Scholar]
- Stoub TR, deToledo-Morrell L, Stebbins GT, Leurgans S, Bennett DA, Shah RC. Hippocampal disconnection contributes to memory dysfunction in individuals at risk for Alzheimer's disease. Proc. Natl. Acad. Sci. U S A. 2006;103:10041–10045. doi: 10.1073/pnas.0603414103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoesen G, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. I. Temporal lobe afferents. Brain Res. 1975;95:1–24. doi: 10.1016/0006-8993(75)90204-8. [DOI] [PubMed] [Google Scholar]
- van Hoesen G, Pandya DN, Butters N. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. II. Frontal lobe afferents. Brain Res. 1975;95:25–38. doi: 10.1016/0006-8993(75)90205-x. [DOI] [PubMed] [Google Scholar]
- Wang C, Stebbins GT, Nyenhuis DL, deToledo-Morrell L, Freels S, Gencheva E, Pedelty L, Sripathirathan K, Moseley ME, Turner DA, Gabrieli JD, Gorelick PB. Longitudinal changes in white matter following ischemic stroke: a three-year follow-up study. Neurobiol. Aging. 2006;27:1827–1833. doi: 10.1016/j.neurobiolaging.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Wang C, Stoub TR, Sripathirathan K, Stebbins GT, Nyenhuis D, Gorelick P, Shah RC, deToledo-Morrell L. Vascular cognitive impairment is not associated with parahippocampal white matter changes. 2010 (Manuscript in preparation) [Google Scholar]
- Wechsler D. Wechsler Memory Scale - Revised Manual. San Antonio: Psychological Corp; 1987. [Google Scholar]
- Wen HM, Baum L, Cheung WS, Mok V, Lam WW, Tomlinson B, Wong KS, Ng HK. Apolipoprotein E epsilon4 allele is associated with the volume of white matter changes in patients with lacunar infarcts, Eur. J. Neurol. 2006;13:1216–1220. doi: 10.1111/j.1468-1331.2006.01436.x. [DOI] [PubMed] [Google Scholar]
- Xu Y, Jack CR, Jr, O’Brien PC, Kokmen E, Smith GE, Ivnik RJ, Boeve BF, Tangalos RG, Petersen RC. Usefulness of MRI measures of entorhinal cortex versus hippocampus in AD. Neurology. 2000;54:1760–1767. doi: 10.1212/wnl.54.9.1760. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Jahng GH, Bayne W, Mori S, Schad L, Mueller S, Du AT, Kramer JH, Yaffe K, Chui H, Jagust WJ, Miller BL, Weiner MW. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007;68:13–19. doi: 10.1212/01.wnl.0000250326.77323.01. [DOI] [PMC free article] [PubMed] [Google Scholar]