Abstract
Our goal was to determine whether treatment of depressive symptoms with escitalopram during buprenorphine treatment for opioid dependence, would improve treatment retention compared to placebo in a 12-week, randomized, double-blind trial. Treatment drop-out was defined as missing seven consecutive buprenorphine dosing days. Participants were 76% male, 80% non-Hispanic Caucasian, and 64% heroin users. At baseline, the mean Beck Depression Inventory-II (BDI-II) score was 28.4 (±9.7). Sixty-one percent of participants completed the 12 week buprenorphine protocol. Dropout rates were 33.3% and 44.0% among those randomized to escitalopram or placebo respectively (p=.19). Relative to baseline, mean BDI-II scores were significantly lower at all follow-up assessments, but the treatment by time interaction effect was not statistically significant (p = .18). Participants randomized to escitalopram also did not have a significantly lower likelihood of testing positive for either opiates or other drugs during follow-up. Depressive symptoms often resolved with buprenorphine treatment and the immediate initiation of escitalopram does not improve treatment retention, depression outcomes, or illicit drug use. Clinicians should determine the need for antidepressant treatment later in buprenorphine care.
Keywords: Depression, Buprenorphine, Retention, Antidepressant, Opiate
1. Introduction
The disease burden of the opioid-dependent individuals is substantial, related to overdose, transmission of infectious diseases, and mental health concerns (Hulse, English, Milne, & Holman, 1999; Stein, 1999). The burden to society in terms of crime, law enforcement costs, family disruption, and lost productivity is also considerable (Mark, Woody, Juday, & Kleber, 2001). A common finding in the substance abuse treatment literature is that patients who stay in treatment longer have better outcomes (Simpson, 2004; Zhang, Friedmann, & Gerstein, 2003). Retention in opioid treatment has been associated with reduced heroin use, crime, and acquisition of HIV infection, as well as with improved functioning (Farre, Mas, Torrens, Moreno, & Cami, 2002; Tenore, 2008; Villafranca, McKellar, Trafton, & Humphreys, 2006) Based on such studies, research, policy-makers and treatment providers have focused on ways to increase patients’ treatment retention.
Buprenorphine, a long-acting partial opioid agonist, is an office-based maintenance treatment for opioid dependence (O'Connor & Fiellin, 2000). With regard to treatment effectiveness, buprenorphine maintenance compares favorably to methadone treatment (MMT), the standard for maintenance opioid dependence treatment (Fudala, et al., 2003; Johnson, et al., 2000). As with methadone treatment, treatment retention, particularly during the early months, remains problematic for buprenorphine patients. In studies of buprenorphine maintenance, retention rates three to six months after initiation range from 50-60% (Finch, Kamien, & Amass, 2007; Lee, Grossman, DiRocco, & Gourevitch, 2009; Magura, et al., 2007; Mintzer, et al., 2007; Stein, Cioe, & Friedmann, 2005).
Depression is common among opioid dependent patients and associated with a poor prognosis (Kosten & Rounsaville, 1988; Rounsaville, Kosten, Weissman, & Kleber, 1986; Rounsaville, Weissman, Crits-Christoph, Wilber, & Kleber, 1982). With lifetime prevalence rates of 20-75% and current prevalence rates of 10-30%, depression influences addiction treatment (Brooner, King, Kidorf, Schmidt Jr, & Bigelow, 1997; Croughan, Miller, Wagelin, & Whitman, 1982; Khantzian & Treece, 1985; Kosten & Rounsaville, 1988; Kranzler & Liebowitz, 1988; Rounsaville, et al., 1986). A significant challenge in the study of depression among opioid users is that opioid use may induce transient symptoms that are difficult to distinguish from endogenous mood disorders (Kadden, Kranzler, & Rounsaville, 1995). These depressive symptoms can be attributable to a drug's toxic effects, drug withdrawal or drug-use-related life crises. Alternatively, a full mood disorder may be induced by substance use. Finally, substance use may be motivated by efforts to alleviate symptoms of the primary psychiatric mood disorder. Despite research to develop diagnostic criteria and interview instruments that differentiate such opioid-related symptoms from independent mood disorders including depression (Bryant, Rounsaville, Spitzer, & Williams, 1992; Hasin, Trautman, & Endicott, 1998; Kranzler, Kadden, Babor, Tennen, & Rounsaville, 1996; Kranzler, et al., 1995; E. Nunes, et al., 1996), the task of establishing a primary/secondary distinction is especially difficult. Regardless of the etiology, however, comorbid depression confers poor outcomes, including a high risk of continued drug abuse and high relapse rates after substance abuse treatment is initiated (Kosten & Rounsaville, 1988; McLellen, Luborsky, Woody, O'Brien, & Druley, 1983; Rounsaville, et al., 1986; Rounsaville, et al., 1982; Woody, et al., 1983). Research suggests that negative intrapersonal states (dysphoria, anxiety or stress, frustration, anger, fatigue) contribute to over 70% of lapse incidents (Marlatt, 1996). The inability to cope adequately when faced with these precipitants is an important facilitator of relapse (Marlatt, 1996; W. Miller, Westerberg, Harris, & Tonigan, 1996).
Effective pharmacological treatment for opioid dependence—alleviating withdrawal, blocking reinforcement, eliminating the stress of drug-seeking, allowing the establishment of a treatment alliance, and the direct pharmacologic effects of the medication—may alleviate depressive symptoms, even without additional psychosocial intervention. Several studies have demonstrated that in the early phase of substance abuse treatment, depressive symptoms remit (Dean, Bell, Christie, & Mattick, 2004; Kosten, Morgan, & Kosten, 1990; E. Nunes & Levin, 2004; Petrakis, et al., 1998; Strain, Stitzer, & Bigelow, 1991), but this finding has not been consistent (E. Nunes, et al., 1998; Subramaniam, Lewis, Stitzer, & Fishman, 2004) and has not been studied in the primary care setting where buprenorphine is usually administered.
Though depressive symptoms are common in the early stage of recovery from opioid use, assessment and pharmacologic management of such symptoms are not standard practice in most buprenorphine maintenance programs (Fiellin, Barthwell, & Treatment, 2003; Fiellin, Pantalon, Chawarski, O'Connor, & Schottenfeld, 2006). Treating depression symptoms, however, has become more common with office-based primary care providers, due to the advent of selective serotonin reuptake inhibitors (SSRIs) (Kessler, et al., 1999; Olfson, et al., 2002; Pirraglia, Stafford, & Singer, 2003; Wells, et al., 2000). It is this group of providers that is expanding buprenorphine care as well. Demonstration that early pharmacological management of depressive symptoms can benefit addiction recovery in opioid-dependent, buprenorphine-seeking patients would provide impetus for the assessment and treatment of these comorbid symptoms by office-based primary care physicians and psychiatrists.
Because negative affective states in the first 12 weeks of office-based buprenorphine maintenance can impair the ability to cope with urges to use drugs, our primary hypothesis was that antidepressant treatment would decrease the propensity to relapse, and consequently decrease drop-out from buprenorphine maintenance. We performed a randomized, double-blind, placebo-controlled trial to determine whether escitalopram treatment of depressive symptoms prior to and concurrent with buprenorphine treatment would decrease treatment drop-out among opioid dependent patients initiating office-based buprenorphine maintenance treatment. Our secondary aim was to determine if escitalopram treatment reduced depressive symptom scores and drug use compared to the placebo condition among office-based buprenorphine treatment recipients.
2. Materials and Methods
2.1 Study Population
Participants were recruited through community advertising, physician referrals and word-of-mouth. Interested individuals called the study phone to complete a brief, anonymous 5-minute screen assessing basic demographics, current drug use, current depressive symptoms, and current use of antidepressant medications. Potentially eligible individuals were given an appointment to come to the study office, complete informed consent and be more fully screened for the study. The study was approved by the Rhode Island Hospital and Butler Hospital Institutional Review Boards.
Study inclusion criteria included age 18-65; a SCID (DSM-IV) diagnosis of opioid dependence (First, Spitzer, Williams, & Gibbon, 1995); a score on the Modified Hamilton Depression Revised Scale (MHDRS) greater than 14 (I. Miller, Bishop, Norman, & Maddever, 1985); the absence of suicidal ideation; willingness to complete a three-month buprenorphine treatment; no medical contraindications to taking escitalopram or buprenorphine such as pregnancy or the presence of a disorder that might either contribute to depression or make pharmacological treatment contraindicated; no history of severe mental illness (bipolar disorder, schizophrenia, schizo-affective, schizophreniform or paranoid disorder); no currently prescribed psychotropic medications; discontinuation of MAOIs and SSRIs for at least 4 weeks and/or depot neuroleptics for 6 months; no previous non-response to escitalopram; and the ability to complete the study assessment in English.
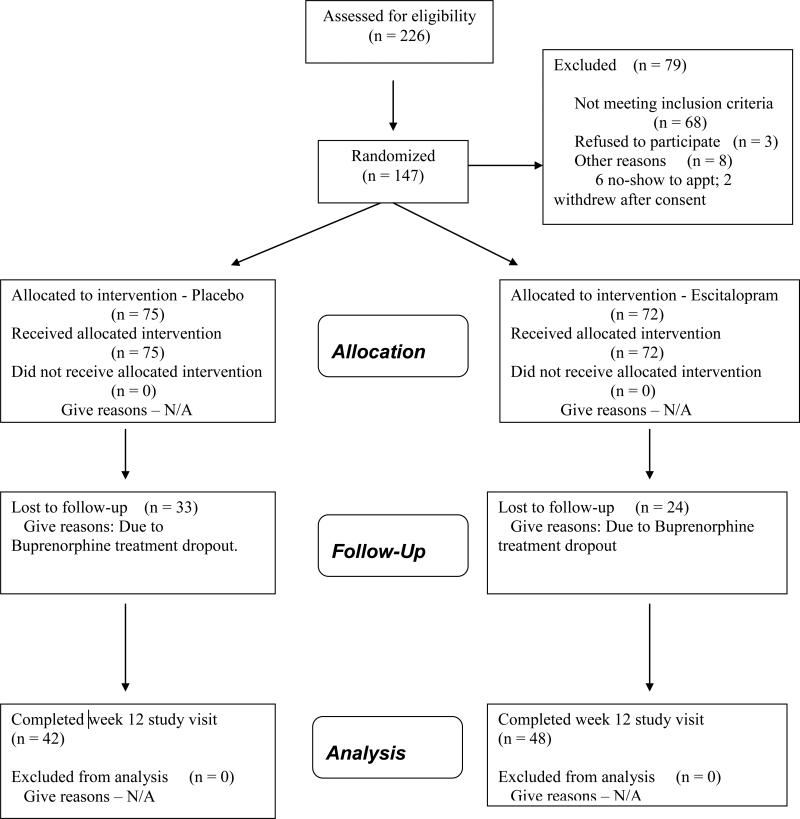
Between November 2006 and May 2009, 932 individuals completed the telephone screen. Of these, 538 were ineligible due to: a history of bipolar disorder or psychotic symptoms (n=244); not endorsing depressive symptoms (n=177); already taking an SSRI (n=85); methadone dose >30 mg/d (n=17); already taking buprenorphine (n=10); not providing enough information at the screen to determine study eligibility (n=5). The remaining 394 callers were invited for an in-person screening visit. Of the 226 who attended this visit, 79 did not enroll due to: MHDRS score < 15 (n=33); psychiatric contraindication to taking escitalopram (n=22); currently taking an SSRI (n=5), a previous non-response to escitalopram (n=2); currently taking buprenorphine (n=1); or refusal of the study procedures or medications (n=16).
Following screening, informed consent was completed and participants had a brief meeting with one of four buprenorphine providers to describe the study procedure. One hundred forty seven persons enrolled in the protocol.
2.2 Study Procedure
At a second appointment during the initial enrollment week, participants completed the 45-minute baseline interview. At the conclusion of this interview, the individual was randomized and provided study medication. Study medication was provided in identical capsule form, and consisted of either 10 milligrams of escitalopram or placebo. Double-blinding regarding the medication group was maintained throughout the study period for all research staff; the off-site compounding pharmacist kept the key to the blind.
Approximately five days after beginning the study medication, participants returned to the research office for a brief, follow-up interview and for buprenorphine (buprenorphine/naloxone) induction. Induction was performed under the treating physician's supervision, as previously described (Stein, et al., 2005). Dose adjustments were made based on previous opioid use, craving, and reported withdrawal symptoms. In general, buprenorphine doses ranging from 12-24 mg/day were required for stabilization.
At each biweekly follow-up appointment (which coincided with research interviews), participants were provided with exactly enough of the study medication (escitalopram or placebo, one pill daily) and buprenorphine to last until the next appointment.
Participants had follow-up interviews at weeks 1, 2, 4, 6, 8, 10 and 12 post-enrollment, assessing drug use, study medication use, presence of side effects and depressive symptoms. At each interview, participants were asked to provide a urine specimen to test for urine toxicology. The Screeners® Dip Drug Test with the Integrated Screeners® Autosplit® KO12B™ Test Cup was used.
The results of urine toxicology testing were provided to the treating physician during the visit and included in the medical record. Participants were not discharged for continued use of drugs, nor was their frequency of follow-up medical visits changed by positive toxicological results. Figure 1 outlines the study recruitment and follow-up.
Figure 1.
Recruitment Flowchart
To compensate participants for their time, each received $25 remuneration for the baseline assessment, in the form of a gift card to a local store, and $10 for each of the seven follow-up appointments. In addition, participants received transportation (taxis) to and from all study visits as needed, and buprenorphine at no cost during their study participation.
2.3 Physician Management (PM)
At each buprenorphine visit, providers offered PM, a brief (10-15 minutes per session), manual-guided, medically-focused advice and brief counseling consistent with the standard of care provided to buprenorphine patients (Fiellin, et al., 2006). The four study buprenorphine providers received 90 minutes of individual PM training that included detailed review of the manual prior to study initiation. PM focused on reducing illicit drug use, adhering to buprenorphine maintenance, and adhering to antidepressant treatment, but did not provide any form of psychotherapy. Patients were referred to other services in the community, including Narcotics Anonymous and Alcoholics Anonymous groups that were accepting of patients receiving opioid agonist maintenance.
2.4 Measures
At baseline, research staff, blinded to treatment group assignment, asked participants their age in years, gender, participant defined racial and ethnic origin, educational attainment, frequency of cocaine use in the 30 days prior to baseline, opioid of choice (heroin versus other opioid), and whether they were enrolled in methadone maintenance.
The Beck Depression Inventory II (Beck, Steer, Ball, & Ranieri, 1996; Hess, 2006; Steer, Iguchi, & Platt, 1992) was used to assess depression at baseline and at each of 7-follow-up assessments. BDI-II scores ≤ 14 are often interpreted as representing “minimal” depression, scores of 14-19 “mild” depression, 20-28 “moderate” depression, and scores of 29 or higher “severe” depression. Changes in mean BDI-II scores and changes in the proportion of participants with BDI-II scores less than 14 were analyzed.
Timeline Follow-Back methods were used to assess adherence to the medication protocol (Sobell & Sobell, 1992). Specifically, adherence was assessed as the proportion of days during each follow-up interval on which the participant reported taking study medication. We defined high-adherence as use on 85% or more days, which is roughly 6 of every 7 assessed days.
2.5 Treatment Outcome and Statistical Methods
Treatment drop-out was defined as missing seven consecutive buprenorphine dosing days. Time to dropout was defined as the number of days between the buprenorphine induction date and the date of their last prescribed buprenorphine dose. When analyzing time to dropout, participants who completed the protocol were censored on the day of their last assessment.
A priori, we estimated a conservative retention rate at 12-weeks of 50% in the control condition. To find an increase to 75% buprenorphine treatment retention in the intervention group, based on a Type I error rate of .05, two-tailed testing, and a power level of .80, we needed to recruit 132 participants (n=66 per arm).
Descriptive statistics are reported to describe the characteristics of the sample. T-tests and χ2 are used to compare intervention groups with respect to background characteristics, baseline depression and sample attrition.
We used the log-rank χ2-test for differences in treatment retention time between intervention groups. Bar graphs are presented to summarize changes in mean BDI-II scores and the proportion of participants with “minimal” (BDI-II < 14) levels of depression by intervention group. We present random effects regression and logistic regression models to test the effect of intervention on changes in mean BDI-II scores and the odds of having minimal depression symptoms at each follow-up (Molenberghs, et al., 2004). After selecting the best fitting growth model, the effect of intervention was estimated as the interaction of treatment by time. Because p-values for the Hausman test comparing all estimated models exceeded .10, we report the more efficient random effects estimates (Hausman, 1978). In all cases the fixed- and random-effects estimates yielded substantively and statistically consistent conclusions regarding the effects of intervention.
To determine if intervention effects were conditional on baseline level of depression severity, we also used ANCOVA models to test the treatment by baseline level of depression severity interaction effect. Separate tests were conducted using data from each of the 7 follow-up assessment using each of 4 possible moderator variables (linear effect of BDI, BDI of 20+, BDI of 29+, or SCID diagnosis of major depressive disorder).
Auxiliary analyses were conducted to describe adherence to the study protocol during follow-up. Because adherence was measured as a proportion, we used a fractional logistic regression model with standard errors adjusting for within subject clustering to test for differences in adherence to the medication protocol between intervention groups. We used random-effects regression to compare those with high adherence to escitalopram to those with low escitalopram adherence and controls (Papke & Wooldridge, 1996). Because adherence was not observed at baseline we used baseline BDI scores as a covariate when testing for differences in depression outcomes by adherence.
We used random-effect logistic regression to estimate the effect of intervention on the likelihood of testing positive for opioids or for other drugs of abuse (tetrahydrocannabinol, benzodiazepines, amphetamines, cocaine) at any follow-up assessment. Group differences in percentage of toxicological analyses that were positive (positive tests/ total tests) for opioids of other drugs of abuse were calculated.
Finally, we used bivariate logistic regression to test the effect of selected background characteristics as predictors of treatment drop-out. All models were estimated using Stata 10.1 (StatCorp, 2007).
3. Results
Participants (n=147) averaged 37.5 (±9.9) years of age, 112 (76.2%) were male, and 117 (80.1%) were non-Hispanic Caucasian (Table 1). Heroin was the opioid of choice for 93 (63.7%) participants and 17 (11.6%) participants were enrolled in methadone maintenance at the time of study entry. Forty-seven (32.0%) reported using cocaine on at least 1 day during the 30-days prior to baseline. The mean BDI-II score at baseline was 28.4 (±9.7). Eighty-two (55.8%) participants met DSM-IV criteria for major depressive disorder or substance-induced major depressive disorder. Between group differences on background characteristics, baseline depression scores, and rates of follow-up were not statistically significant (Table 1).
Table 1.
ARISE Baseline Characteristics by Intervention (n = 147).
| Total (n = 147) | Control (n = 75) | Int. (n = 72) | t (p = ) | |
|---|---|---|---|---|
| Mean Age (Yrs) | 37.5 (±9.9) | 36.8 (±9.8) | 38.3 (±9.6) | -087 (.388) |
| Mean BDI Score | 28.4 (±9.7) | 28.6 (±9.8) | 28.3 (±9.6) | 0.22 (.828) |
| Mean HAM-D Score | 25.9 (±6.0) | 26.3 (±6.3) | 25.4 (±5.7) | 0.90 (.371) |
| Mean Days Used Cocaine | 1.7 (±4.2) | 1.4 (±3.2) | 2.0 (±4.9) | 0.80 (.426) |
| χ2 (p =) | ||||
| n % Male | 112 (76.2%) | 57 (76.0%) | 55 (76.4%) | 0.02 (.897) |
| n (%) Ethnicity | ||||
| Caucasian | 117 (80.1%) | 60 (81.1%) | 57 (79.2%) | 0.19 (.979) |
| African-American | 7 (4.8%) | 3 (4.0%) | 4 (5.6%) | |
| Hispanic | 14 (9.6%) | 7 (9.5%) | 7 (9.7%) | |
| Other | 8 (5.5%) | 4 (5.4%) | 4 (5.6%) | |
| n (%) Completed HS | 111(75.5%) | 60 (80.0%) | 51 (70.8%) | 1.67 (.196) |
| n (%) Heroin User | 93 (63.7%) | 44 (59.5%) | 49 (68.1%) | 1.16 (.280) |
| n (%) Enrolled MMT | 17 (11.6%) | 9 (12.2%) | 8 (11.1%) | 0.01 (.938) |
| n (%) Any FU Assessment | 138 (93.9%) | 69 (92.0%) | 69 (95.8%) | 0.94 (.332) |
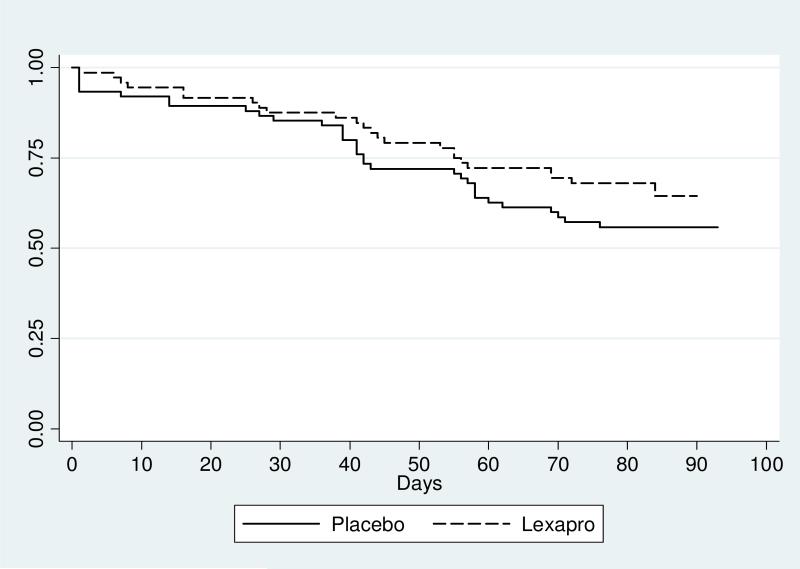
Fifty-seven (38.8%) participants missed seven consecutive buprenorphine dosing days prior to study completion. Dropout rates were 33.3% and 44.0% among those randomized to escitalopram and placebo (p=.19). In a time to event analysis (see Figure 2), the hazard ratio was .70 (95% CI .41-1.19). Mean and median survival times were 74 and 81 days for escitalopram and placebo respectively. Among those who did not remain in buprenorphine treatment, 25% of participants dropped out by week 2, 35% by week 4, 63% by week 6, and 79% by week 8.
Figure 2.
Kaplan-Meier Survival Estimates by Groups
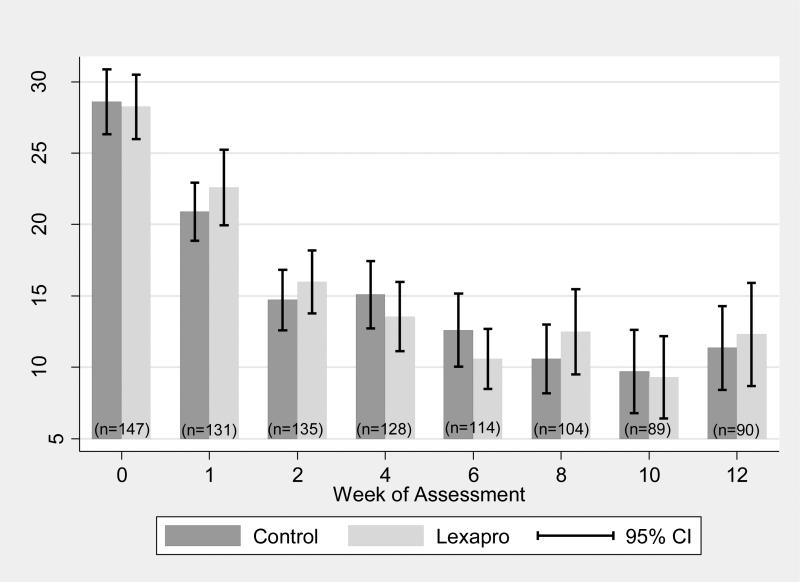
Figure 3 gives mean BDI-II scores by intervention group for each assessment. Observed between group differences in mean BDI-II scores were small at all assessments. Regardless of intervention group, observed mean BDI-II scores dropped sharply over time.
Figure 3.
Mean BDI by Group and Time
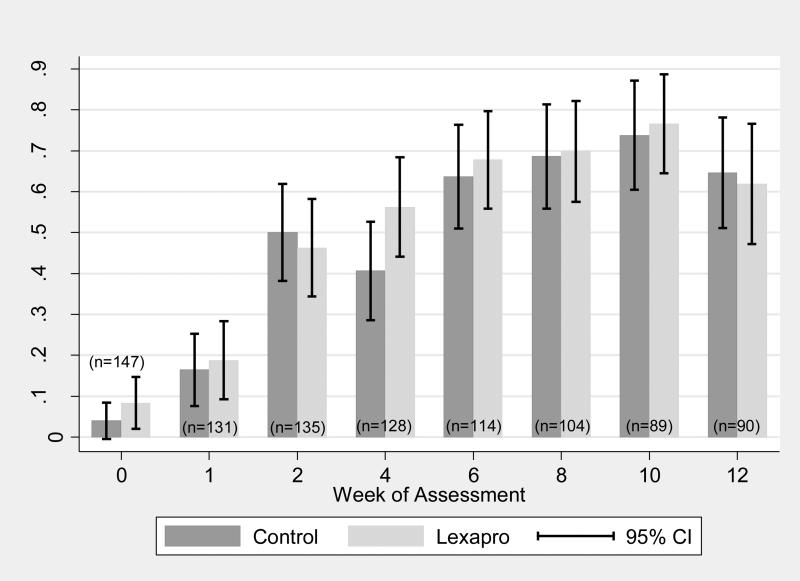
The proportion of participants having “minimal” depression symptoms (BDI-II < 14) at each assessment is shown in Figure 4. While all participants screened positive for depression on the MHRDS at baseline, nine (6.1%) had baseline BDI-II scores lower than 14. By week two, 48.2% of the participants had BDI-II scores below 14, and by week ten, 75.3% of all participants had BDI-II scores less than 14. Paralleling the observed changes in mean BDI-II scores were sharp increases in the proportion of participants reporting minimal depressive symptoms (BDI-II <14) during the follow-up period.
Figure 4.
Proportion w BDI < 14 by Group and Time
We used random-effects regression to estimate three growth models with time parameterized as linear, quadratic, and unconstrained. Likelihood ratio tests indicated that the quadratic time model fit the data significantly better than the linear time model (LR2 = 182.23, df = 1, p < .001) and the unconstrained time model fit the data significantly better than the quadratic time model (LR2 = 75.51, df = 5, p < .001). This model included only the dummy variables contrasting mean BDI-II score at each follow-up to baseline.
Relative to baseline, mean BDI-II scores were significantly lower at all follow-up assessments. At 1-week, mean BDI-II scores were 7.08 (95% CI -8.57;-5.60) points lower than at baseline. Mean BDI-II scores were 13.45 (95% CI -15.13;-11.80) points lower at week 2 and 16.45 (95% CI -18.87;-14.04) points lower at 12-weeks.
Under the hypothesis that participants randomized to escitalopram would exhibit larger reduction in BDI-II scores than controls, the effect of intervention was estimated as the first-order treatment by time interaction. The treatment by time interaction effect was not statistically significant (LR2 = 10.25, df = 7, p = .18) and none of the individual coefficients estimating the effect of treatment at each of the 7 follow-up assessments were statistically significant (p>.05)
Models describing change in the proportion of participants with BDI-II scores < 14 gave similar conclusions. While significant linear (OR = 1.65, z = 11.75, p < .001) and quadratic (OR = 0.93, z = -7.74, p < .001) change components were observed the more complex unconstrained time model provided significantly better fit to the observed data (LR2 = 30.49, df = 5, p < .001). The odds of having minimal depression at any of the follow-up assessments was significantly lower than at baseline.
We used random-effects logistic regression to estimate the effect of intervention on the odds of minimal depression at follow-up. Consistent with the analysis of mean BDI scores, the treatment by time interaction was not statistically significant (LR2 = 7.03, df = 7, p = .43).
There was no evidence that the effect of intervention was significantly moderated by baseline severity of depressive symptoms or depression diagnosis.
Self-reported adherence to study medications was high in both arms of the study; between group differences were not statistically significant (z = 0.47, p = .64). Aggregating data across all follow-up assessments, participants randomized to intervention took escitalopram on 91.4% (± 20.0) of assessment days; controls reporting taking placebo on 90.4% (± 18.7) of all days assessed during follow-up.
We used random-effects regression to determine if those with high adherence to escitalopram had significantly better outcomes with respect to mean BDI scores at follow-up. We compared those with high adherence to escitalopram, those with lower adherence, and controls. Because treatment adherence is only relevant during follow-up, baseline depression scores were entered as a covariate. Compared to controls, participants randomized to escitalopram with high adherence did not have significantly lower adjusted mean scores on depression during the follow-assessments (b = -0.69, z = -.54, p = .59). Compared to those with low adherence to escitalopram, those with high adherence had significantly lower adjusted mean depression at the follow-up assessments (b = -3.03, z = 2.51, p = .01). Those with low adherence to escitalopram did not differ significantly from controls (b = 2.34, z = 1.47, p = .14).
A random-effects model indicated that changes in the rate of testing positive for opioids decreased significantly between baseline and follow-up (Wald χ2 = 89.11, df = 7, p < .001). Participants randomized to receive escitalopram did not have a significantly lower likelihood of testing positive for either opioids (OR = 0.62, z = -0.86, p = .39) or other drugs (OR = 0.67, z = -1.21, p = .23) during follow-up. On average those randomized to escitalopram tested positive on 29.8% (± 35.2) of all follow-up opioid tests compared with an average of 31.8% (± 36.2) among controls (t = 0.34, df = 135, p = .36). The likelihood of testing positive for opioids remained relatively constant from week 2 through week 12; 23.0% of the participants tested opioid positive at 12-weeks. Of the 90 persons who remained in treatment for the entire 12 week follow-up, 48% tested negative for opioids throughout the study period. During weeks 1-12, opioid positive test results were associated with lower likelihood of having BDI-II <14 (OR .25, z = -4.77, p<.001).
Compared to baseline, the likelihood of testing positive for any other drug (tetrahydrocannabinol, benzodiazepines, amphetamines, or cocaine) was also significantly lower (Wald χ2 = 81.99, df = 7, p < .001); again differences were statistically significant (p < .01) at all follow-ups. The observed rates of positives tests for other drugs were 55.8% (week 1), 56.5% (week 2), 51.7% (week 4), 42.9% (week 6), 38.8% (week 8), 31.3% (week 10), and 37.4% (week 12).
In logistic regression analysis, the likelihood of dropout was not associated significantly with age, race, gender, educational attainment, choice of opioid (heroin vs. prescription), or baseline BDI-II or MHRDS scores. Participants who reported using any cocaine in the 30 days prior to baseline were significantly (OR 3.6, 95% CI 1.7-7.5; p = .001) more likely to dropout than those who were not recent cocaine users.
4. Discussion
Among opioid dependent persons with depressive symptoms seeking office-based buprenorphine treatment, contemporaneous initiation of antidepressant medication did not improve treatment retention compared to placebo. Sixty-one percent of study participants remained on buprenorphine for twelve weeks after induction, a rate similar to that reported in other primary care cohorts (Finch, et al., 2007; Lee, et al., 2009; Magura, et al., 2007; Mintzer, et al., 2007; Stein, et al., 2005). In addition, despite high self-reported rates of treatment adherence, escitalopram produced neither faster nor deeper reductions in depressive symptoms.
Regardless of group assignment, the decline in self-reported depressive symptoms was dramatic during the first two weeks following buprenorphine/naloxone induction. By week two, nearly half of all participants had BDI-II scores below 14, the threshold for minimal depressive symptoms. By week ten, three-quarters of those remaining in treatment had score below 14, suggesting continued improvement in symptoms. These findings suggest that the assessment of depression in this population may be more accurate if performed at least two weeks or possibly longer after treatment initiation rather than at treatment admission. Nonetheless, depressive symptoms that remain after the first 1-2 months of buprenorphine/naloxone treatment are likely to persist. Later administration of antidepressant treatment may be necessary to optimize the care of this population.
Research by Strain et al. studying persons entering a six-month methadone detoxification program reported a similar decline in depressive symptoms early in treatment (Strain, et al., 1991). Using low dose methadone (25 mg), and assessing depressive symptoms using the BDI, these authors found significant declines in symptoms after seven days. In both that study and ours, it remains uncertain whether the decline in symptoms was due to opioid substitution therapy and the resolution of opioid withdrawal symptoms or to nonspecific treatment entry effects and the relief of physiological and psychological stressors (E. V. Nunes, Sullivan, & Levin, 2004). Studies monitoring the symptoms of opioid dependent persons entering drug-free treatment would clarify this dilemma. The depression symptom response rates were lower here than described in another buprenorphine treatment study (Kosten, et al., 1990). Lower rates may be explained by our higher baseline symptom score and our exclusion of methadone maintenance transfer patients.
Others have also noted that negative outcomes of buprenorphine treatment were related to depression (Laqueille, et al., 2001), but we found that those with greater depressive symptoms at baseline did not have lower rates of treatment retention. Some studies have noted the opposite, that depressed persons have higher rates of retention in buprenorphine treatment (Gerra, et al., 2004). These authors have attributed this outcome to the partial kappa-antagonistic effect of buprenorphine on a dysfunctional endogenous kappa-opioid system in depressed persons. One report concluded that buprenorphine had an effective and rapid antidepressant effect independent of ongoing drug use or opioide withdrawal symptoms (Maremmani, Pacini, & Pani, 2006).
Escitalopram did not significantly affect participants’ ongoing use of either opioids or other drugs. While opioid-positive urine toxicologies declined over time, this effect can be attributed to buprenorphine-naloxone use. Declining opioid use likely contributed to a decrease in depressive symptoms, and conversely, with improved mood.
Traditional clinical teaching has been that the diagnosis of intrinsic psychiatric disorders should be delayed until drug users have had a period of abstinence. Our findings suggest that after several weeks of buprenorphine treatment and lessening opioid use, depressive symptoms relent for nearly half of opioid dependent persons. Still, a several month period might be necessary for depressive symptoms to minimize in another quarter of the persons treated with buprenorphine. Those whose depressive symptoms persisted after 10-12 weeks on buprenorphine were more likely to be continuing illicit opioid or other drug use. Strategies to address persistent symptoms—either through more aggressive substance abuse treatment or through antidepressant therapy-- are needed because depression predicts both short and long-term outcomes in opioid dependent persons (Kosten & Rounsaville, 1988; Rounsaville, et al., 1986). Further research should also test whether alternative treatments of depressive symptoms early after buprenorphine initiation might influence retention.
Although baseline depression severity did not predict treatment outcomes and symptoms abate in most cases, there may be exceptions to the “wait and see” approach to early antidepressant therapy. For instance, persons with a clear history of antidepressant medication responsiveness or severe depression clearly independent of substance use may warrant earlier treatment.
This study had limitations. We chose to use the BDI-II as a self-report measure of depressive symptoms because of its brevity, the need for repeated administrations, its potential applicability to office-based settings and its use in other studies with opioid-dependent persons, but other measures might have been used. Second, while baseline BDI-II score did not predict treatment retention, those who dropped-out might have had BDI-II scores different from those who remained in care. Third, self-reports of medication adherence were high, and if participants overestimated their actual medication use, the escitalopram group effect might have been mitigated. Fourth, we tested a low dose of escitalopram. Escitalopram dose escalation or use of a different antidepressant medication alone or combined with psychotherapy might produce different findings. Finally, these study participants sought substance abuse treatment. There has been little research regarding mood disorders in opioid users not seeking substance abuse treatment. In one non-treatment cohort of opioid abusers recruited from a needle exchange site, lower rates of depression were found compared to those enrolled in a methadone maintenance treatment program (Brienza, et al., 2000). Depression itself may motivate the substance abuser to seek treatment as psychiatric impairment may reduce the addict's ability to maintain a drug supply. Alternatively, drugs users with mounting depressive symptoms may seek treatment because they are suffering and finding opioids less rewarding or more unpleasant to take. Treatment-seeking reduces levels of psychological distress across treatment modalities (Darke, et al., 2009; Havard, Teesson, Darke, & Ross, 2006).
In this study, we proposed that pharmacotherapy would improve depressive symptoms commonly seen at the time of and after the initiation of buprenorphine therapy and thereby improve the stability of recovery. Our results suggest that the immediate initiation of escitalopram with buprenorphine treatment will not improve treatment retention, illicit drug use, or depressive symptoms.
Acknowledgements
This study was funded by the National Institute on Drug Abuse DA022207, Clinical Trial NCT# 00475878. Dr. Stein is a recipient of a NIDA Mid-Career Award DA 000512.
We would like to thank Reckitt-Benckiser for providing buprenorphine/naloxone for all study participants.
No author on this manuscript has any personal or financial interest that would influence the results.
Dr. Stein had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No author on this manuscript has any personal or financial interest that would influence the results
References
- Beck A, Steer R, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Brienza R, Stein M, Chen M, Gogineni A, Sobota M, Maksad J, et al. Depression among needle exchange program and methadone maintenance clients. Journal of Substance Abuse Treatment. 2000;18(4):331–337. doi: 10.1016/s0740-5472(99)00084-7. [DOI] [PubMed] [Google Scholar]
- Brooner R, King V, Kidorf M, Schmidt C, Jr, Bigelow G. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Archives of General Psychiatry. 1997;54(1):71–80. doi: 10.1001/archpsyc.1997.01830130077015. [DOI] [PubMed] [Google Scholar]
- Bryant K, Rounsaville B, Spitzer R, Williams J. Reliability of dual diagnosis: substance dependence and psychiatric disorders. Journal of Nervous and Mental Disease. 1992;180(4):251–257. doi: 10.1097/00005053-199204000-00007. [DOI] [PubMed] [Google Scholar]
- Croughan J, Miller J, Wagelin D, Whitman B. Psychiatric illness in male and female narcotic addicts. Journal of Clinical Psychiatry. 1982;43(6):225–228. [PubMed] [Google Scholar]
- Darke S, Mills K, Teesson M, Ross J, Williamson A, Havard A. Patterns of major depression and drug-related problems amongst heroin users across 36 months. Psychiatry Research. 2009;166(1):7–14. doi: 10.1016/j.psychres.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Dean A, Bell J, Christie M, Mattick R. Depressive symptoms during buprenorphine vs. methadone maintenance: findings from a randomised, controlled trial in opioid dependence. European Psychiatry. 2004;19(8):510–513. doi: 10.1016/j.eurpsy.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Farre M, Mas A, Torrens M, Moreno V, Cami J. Retention rate and illicit opioid use during methadone maintenance interventions: a meta-analysis. Drug and Alcohol Dependence. 2002;65(3):283–290. doi: 10.1016/s0376-8716(01)00171-5. [DOI] [PubMed] [Google Scholar]
- Fiellin D, Barthwell A, Treatment C. f. S. A. Guideline development for office-based pharmacotherapies for opioid dependence. Journal of Addictive Diseases. 2003;22(4):109–120. doi: 10.1300/j069v22n04_09. [DOI] [PubMed] [Google Scholar]
- Fiellin D, Pantalon M, Chawarski M, O'Connor P, Schottenfeld R. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. New England Journal of Medicine. 2006;355(4):365–374. doi: 10.1056/NEJMoa055255. [DOI] [PubMed] [Google Scholar]
- Finch J, Kamien J, Amass L. Two-year experience with buprenorphine-naloxone (Suboxone) for maintenance treatment of opioid dependence within a private practice setting. Journal of Addiction Medicine. 2007;1(2):104–110. doi: 10.1097/ADM.0b013e31809b5df2. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Williams J, Gibbon M. Structured clinical interview for DSM-IV - patient version. NY State Psychiatric Institute; New York, NY: 1995. [Google Scholar]
- Fudala P, Bridge T, Herbert S, Williford W, Chiang C, Jones K, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. New England Journal of Medicine. 2003;349(10):949–958. doi: 10.1056/NEJMoa022164. [DOI] [PubMed] [Google Scholar]
- Gerra G, Borella F, Zaimovic A, Moi G, Bussandri M, Bubici C, et al. Buprenorphine versus methadone for opioid dependence: predictor variables for treatment outcome. Drug and Alcohol Dependence. 2004;75(1):37–45. doi: 10.1016/j.drugalcdep.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Hasin D, Trautman K, Endicott J. Psychiatric research interview for substance and mental disorders: phenomenologically based diagnosis in patients who abuse alcohol or drugs. Pharmacological Bulletin. 1998;34(1):3–8. [PubMed] [Google Scholar]
- Hausman J. Specification tests in econometrics. Econometrica. 1978;46(6):1251–1271. [Google Scholar]
- Havard A, Teesson M, Darke S, Ross J. Depression among heroin users: 12-Month outcomes from the Australian Treatment Outcome Study (ATOS). Journal of Substance Abuse Treatment. 2006;30(4):355–362. doi: 10.1016/j.jsat.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Hess M. The Beck Depression Inventory in patients undergoing opiate agonist maintenance treatment. British Journal of Clinical Psychology. 2006;45(3):417–425. doi: 10.1348/014466505x68069. [DOI] [PubMed] [Google Scholar]
- Hulse G, English D, Milne E, Holman C. The quantification of mortality resulting from the regular use of illicit opiates. Addiction. 1999;94(2):221–229. doi: 10.1046/j.1360-0443.1999.9422216.x. [DOI] [PubMed] [Google Scholar]
- Johnson R, Chutuape M, Strain E, Walsh S, Stitzer M, Bigelow G. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. New England Journal of Medicine. 2000;343(18):1290–1297. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- Kadden R, Kranzler H, Rounsaville B. Validity of the distinction between “substance-induced” and “independent” depression and anxiety disorders. American Journal on Addictions. 1995;4(2):107–117. [Google Scholar]
- Kessler R, Zhao S, Katz S, Kouzis A, Frank R, Edlund M, et al. Past-year use of outpatient services for psychiatric problems in the National Comorbidity Survey. American Journal of Psychiatry. 1999;156(1):115–123. doi: 10.1176/ajp.156.1.115. [DOI] [PubMed] [Google Scholar]
- Khantzian E, Treece C. DSM-III psychiatric diagnosis of narcotic addicts: recent findings. Archives of General Psychiatry. 1985;42(11):1067–1071. doi: 10.1001/archpsyc.1985.01790340045007. [DOI] [PubMed] [Google Scholar]
- Kosten T, Morgan C, Kosten T. Depressive symptoms during buprenorphine treatment of opioid abusers. Journal of Substance Abuse Treatment. 1990;7(1):51–54. doi: 10.1016/0740-5472(90)90035-o. [DOI] [PubMed] [Google Scholar]
- Kosten T, Rounsaville B. Suicidality among opioid addicts: 2.5 year follow-up. American Journal of Drug and Alcohol Abuse. 1988;14(3):357–369. doi: 10.3109/00952998809001557. [DOI] [PubMed] [Google Scholar]
- Kranzler H, Kadden R, Babor T, Tennen H, Rounsaville B. Validity of the SCID in substance abuse patients. Addiction. 1996;91(6):859–868. [PubMed] [Google Scholar]
- Kranzler H, Kadden R, Burleson J, Babor T, Apter A, Rounsaville B. Validity of psychiatric diagnoses in patients with substance use disorders: is the interview more important than the interviewer? Comprehensive Psychiatry. 1995;36(4):278–288. doi: 10.1016/s0010-440x(95)90073-x. [DOI] [PubMed] [Google Scholar]
- Kranzler H, Liebowitz N. Anxiety and depression in substance abuse: clinical impications. The Medical Clinics of North America. 1988;72(4):867–885. doi: 10.1016/s0025-7125(16)30749-0. [DOI] [PubMed] [Google Scholar]
- Laqueille X, Poirier M, Jalfre V, Bourdel M, Willard D, Olie J. Predictive factors of response to buprenorphine in the substitutive treatment of heroin addicts. Results of a multicenter study of 73 patients. Presse Medicale. 2001;30(32):1581–1585. [PubMed] [Google Scholar]
- Lee J, Grossman E, DiRocco D, Gourevitch M. Home buprenorphine/naloxone induction in primary care. Journal of General Internal Medicine. 2009;24(2):226–232. doi: 10.1007/s11606-008-0866-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magura S, Lee S, Salsitz E, Kolodny A, Whitley S, Taubes T, et al. Outcomes of buprenorphine maintenance in office-based practice. Journal of Addictive Diseases. 2007;26(2):13–23. doi: 10.1300/J069v26n02_03. [DOI] [PubMed] [Google Scholar]
- Maremmani I, Pacini M, Pani P. Effectiveness of buprenorphine in double diagnosed patients: buprenorphine as a psychotropic drug. Heroin Addiction & Related Clinical Problems. 2006;8(1):31–48. [Google Scholar]
- Mark T, Woody G, Juday T, Kleber H. The economic costs of heroin addiction in the United States. Drug and Alcohol Dependence. 2001;61(2):195–206. doi: 10.1016/s0376-8716(00)00162-9. [DOI] [PubMed] [Google Scholar]
- Marlatt G. Taxonomy of high-risk situations for alcohol relapse: evolution and development of a cognitive-behavioral model. Addiction. 1996;91(Suppl):S37–49. [PubMed] [Google Scholar]
- McLellen A, Luborsky L, Woody G, O'Brien C, Druley K. Predicting response to alcohol and drug abuse treatment: role of psychiatric severity. Archives of General Psychiatry. 1983;40(6):620–625. doi: 10.1001/archpsyc.1983.04390010030004. [DOI] [PubMed] [Google Scholar]
- Miller I, Bishop S, Norman W, Maddever H. The modified Hamilton rating scale for depression: reliability and validity. Psychiatry Research. 1985;14(2):131–142. doi: 10.1016/0165-1781(85)90057-5. [DOI] [PubMed] [Google Scholar]
- Miller W, Westerberg V, Harris R, Tonigan J. What predicts relapse? Prospective testing of antecedent models. Addiction. 1996;91(Suppl):S155–172. [PubMed] [Google Scholar]
- Mintzer I, Eisenberg M, Terra M, MacVane C, Himmelstein D, Woolhandler S. Treating opioid addiction with buprenorphine-naloxone in community-based primary care settings. Annals of Family Medicine. 2007;5(2):146–150. doi: 10.1370/afm.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs G, Thijs H, Jansen I, Beunckens C, Kenward MG, Mallinckrodt C, et al. Analyzing incomplete longitudinal clinical trial data. Biostatistics. 2004;5(3):445–464. doi: 10.1093/biostatistics/5.3.445. [DOI] [PubMed] [Google Scholar]
- Nunes E, Goehl L, Seracini A, Deliyannides D, Donovan S, Koenig T, et al. A modification of the structured clinical interview for DSM-III-R to evaluate methadone patients test retest reliability. American Journal on Addictions. 1996;5(3):241–248. [Google Scholar]
- Nunes E, Levin F. Treatment of depression in patients with alcohol or other drug dependence: a meta-analysis. JAMA. 2004;291(15):1887–1896. doi: 10.1001/jama.291.15.1887. [DOI] [PubMed] [Google Scholar]
- Nunes E, Quitkin F, Donovan S, Deliyannides D, Ocepek-Welikson K, Koenig T, et al. Imipramine treatment of opiate dependent patients with depressive disorders: a placebo-controlled trial. Archives of General Psychiatry. 1998;55(2):153–160. doi: 10.1001/archpsyc.55.2.153. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Sullivan MA, Levin FR. Treatment of depression in patients with opiate dependence. Biol Psychiatry. 2004;56(10):793–802. doi: 10.1016/j.biopsych.2004.06.037. [DOI] [PubMed] [Google Scholar]
- O'Connor P, Fiellin D. Pharmacologic treatment of heroin-dependent patients. Annals of Internal Medicine. 2000;133(1):40–54. doi: 10.7326/0003-4819-133-1-200007040-00008. [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus S, Druss B, Ellinson L, Tanielian T, Pincus H. National trends in the outpatient treatment of depression. JAMA. 2002;287(2):203–209. doi: 10.1001/jama.287.2.203. [DOI] [PubMed] [Google Scholar]
- Papke L, Wooldridge J. Econometric methods for fractional response variables with an application to 401(k) plan participation rates. Journal of Applied Econometrics. 1996;11(6):619–632. [Google Scholar]
- Petrakis I, Carroll K, Nich C, Gordon L, Kosten T, Rounsaville B. Fluoxetine treatment of depressive disorders in methadone-maintained opioid addicts. Drug and Alcohol Dependence. 1998;50(3):221–226. doi: 10.1016/s0376-8716(98)00032-5. [DOI] [PubMed] [Google Scholar]
- Pirraglia P, Stafford R, Singer D. Trends in prescribing of selective serotonin reuptake inhibitors and other newer antidepressant agents in adult primary care. Primary Care Companion to the Journal of Clinical Psychiatry. 2003;5(4):153–157. doi: 10.4088/pcc.v05n0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounsaville B, Kosten T, Weissman M, Kleber H. Prognostic significance of psychopathology in treated opiate addicts: a 2.5 year follow-up study. Archives of General Psychiatry. 1986;43(8):739–745. doi: 10.1001/archpsyc.1986.01800080025004. [DOI] [PubMed] [Google Scholar]
- Rounsaville B, Weissman M, Crits-Christoph K, Wilber C, Kleber H. Diagnosis and symptoms of depression in opiate addicts: course and relationship to treatment outcome. Archives of General Psychiatry. 1982;39(2):151–156. doi: 10.1001/archpsyc.1982.04290020021004. [DOI] [PubMed] [Google Scholar]
- Simpson D. A conceptual framework for drug treatment process and outcomes. Journal of Substance Abuse Treatment. 2004;27(2):99–121. doi: 10.1016/j.jsat.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Sobell L, Sobell M. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Allen L, editor. Measuring Alcohol Consumption. The Humana Press; New York: 1992. pp. 41–72. [Google Scholar]
- StatCorp . Stata Statistical Software: Release 10. StatCorp LP; College Station, TX: 2007. [Google Scholar]
- Steer R, Iguchi M, Platt J. Use of the revised Beck Depression Inventory with intravenous drug users not in treatment. Psychology of Addictive Behaviors. 1992;6(4):225–232. [Google Scholar]
- Stein M. Medical consequences of substance abuse. The Psychiatric Clinics of North America. 1999;22(2):351–370. doi: 10.1016/s0193-953x(05)70081-2. [DOI] [PubMed] [Google Scholar]
- Stein M, Cioe P, Friedmann P. Buprenorphine retention in primary care. Journal of General Internal Medicine. 2005;20(11):1038–1041. doi: 10.1111/j.1525-1497.2005.0228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain E, Stitzer M, Bigelow G. Early treatment time course of depressive symptoms in opiate addicts. Journal of Nervous and Mental Disease. 1991;179(4):215–221. doi: 10.1097/00005053-199104000-00007. [DOI] [PubMed] [Google Scholar]
- Subramaniam G, Lewis L, Stitzer M, Fishman M. Depressive symptoms in adolescents during residential treatment for substance use disorders. American Journal on Addictions. 2004;13(3):256–267. doi: 10.1080/10550490490459924. [DOI] [PubMed] [Google Scholar]
- Tenore PL. Psychotherapeutic benefits of opioid agonist therapy. Journal of Addictive Diseases. 2008;27(3):49–65. doi: 10.1080/10550880802122646. [DOI] [PubMed] [Google Scholar]
- Villafranca SW, McKellar JD, Trafton JA, Humphreys K. Predictors of retention in methadone programs: a signal detection analysis. Drug and Alcohol Dependence. 2006;83(3):218–224. doi: 10.1016/j.drugalcdep.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Wells K, Sherbourne C, Schoenbaum M, Duan N, Meredith L, Unutzer J, et al. Impact of disseminating quality improvement programs for depression in managed primary care: a randomized controlled trial. JAMA. 2000;283(2):212–220. doi: 10.1001/jama.283.2.212. [DOI] [PubMed] [Google Scholar]
- Woody G, Luborsky L, McLellen A, O'Brien C, Beck A, Blaine J, et al. Psychotherapy for opiate addicts: does it help? Archives of General Psychiatry. 1983;40(6):639–645. doi: 10.1001/archpsyc.1983.04390010049006. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Friedmann P, Gerstein D. Does retention matter? Treatment duration and improvement in drug use. Addiction. 2003;98(5):673–684. doi: 10.1046/j.1360-0443.2003.00354.x. [DOI] [PubMed] [Google Scholar]