Abstract
BACKGROUND:
Early fluid and electrolyte imbalances may be associated with an increased risk of bronchopulmonary dysplasia.
OBJECTIVE:
We sought to establish an association between fluid and electrolyte balance in the first week of life and the risk of bronchopulmonary dysplasia.
METHODS:
Clinical charts of 205 neonates <32 weeks gestational age and/or <1,250 g birth weight (admitted to our NICU between 1997 and 2008) were analyzed. Clinical features, fluid and electrolyte balance were analyzed for the first 7 days of life using multivariate models of generalized estimation equations. A p value <0.05 was considered significant in all of the hypothesis tests.
RESULTS:
The prevalence of bronchopulmonary dysplasia was 22%. Lower gestational age and birth weight, male gender, less frequent use of antenatal steroids, respiratory distress syndrome, use of surfactant, patent ductus arteriosus, duration of invasive ventilation and NICU stay were significantly associated with bronchopulmonary dysplasia. The variation in serum values of potassium, phosphorus and creatinine during the first week of life also revealed an association with bronchopulmonary dysplasia. Higher mean plasma calcium values were associated with spontaneous closure of the patent ductus arteriosus. The use of indomethacin to induce patent ductus arteriosus closure was significantly higher in bronchopulmonary dysplasia patients.
CONCLUSIONS:
Differences in renal function and tubular handling of potassium and phosphorus are present during the first week of life among preterm neonates who will develop bronchopulmonary dysplasia. The higher rate of patent ductus arteriosus and indomethacin use may influence these differences. Serum levels of calcium also appear to play a role in spontaneous ductus arteriosus closure.
Keywords: Fluid, Electrolyte, Bronchopulmonary dysplasia, Preterm neonate, Phosphorus
INTRODUCTION
Low gestational age and birth weight are among the many known etiologic factors for the development of bronchopulmonary dysplasia (BPD). One of the first retrospective reviews on fluid balance1 demonstrated that compared to control infants, infants with BPD received greater quantities of total, crystalloid and colloid fluids per kilogram of body weight in the first days of life. Another retrospective analysis of a cohort of 1,382 extremely low birth weight infants performed by Oh et al.2 concluded that higher fluid intake and reduced weight loss during the first 10 days of life are associated with an increased risk of BPD. Bell and Acarregui3 examined the effect of water intake on postnatal weight loss and the risk of neonatal morbidity. They found that restricted water intake significantly increases postnatal weight loss and significantly reduces the risk of patent ductus arteriosus and necrotizing enterocolitis. They also found a trend toward a reduced risk of BPD, intracranial hemorrhage and death. Preterm neonates with delayed diuresis appear to be at a significantly greater risk of BPD.4. Costarino et al.5 demonstrated that withholding sodium supplementation during the first 3–5 days of life reduces BPD. In that study, care givers tended to prescribe daily increases in parenteral fluids for the salt-supplemented infants, perhaps because serum sodium concentrations were elevated after the first days of the study. There is also some evidence for magnesium imbalances in the pathogenesis of BPD.6,7
These findings suggest that careful attention to fluid and electrolyte balance may be an important means to reduce the incidence of BPD.
Here, we reviewed the clinical data from the first week of life of preterm neonates admitted to our center during a 12-year period establish an association between fluid and electrolyte balance and the risk of BPD.
MATERIALS AND METHODS
Preterm neonates <32 weeks of gestational age and/or <1,250 g birth weight (admitted between January 1997 and December 2008) were identified from the database of our neonatal intensive care unit (NICU), a tertiary center. Preterm neonates affected by a TORCH infection, a chromosomal or major congenital anomaly, twin-twin transfusion syndrome, asphyxia, and any inborn error of metabolism detected during the neonatal period, as well as those infants who were admitted after day 2 of life or died before 36 weeks corrected age, were excluded.
Clinical features, including gender, gestational age, birth weight, antenatal steroid pulses, mode of delivery, histological chorioamnionitis, neonatal morbidity, use of exogenous surfactant, oxygen therapy, mechanical ventilation, daily weight and urine output, fluid and electrolyte balance, hematocrit and need for blood transfusions during the first 7 days of life, were collected from hospital charts.
The study protocol was approved by the institute’s committee on human research.
Standard care
Until 2003, the antenatal steroid regimen included dexamethasone (total dose of 24 mg, divided into two doses given intramuscularly every 12 h). Thereafter, treatment consisted of betamethasone (24 mg, divided into two doses given intramuscularly 24 h apart) in pregnancies at risk of preterm labor prior to 35 weeks gestation.
All infants received standard management in the NICU, including an incubator humidity of 80–85% in the first week of life. Neutral thermal environmental temperatures in range of 34.0–35.4 ºC were maintained to keep the infant’s temperature between 36.0–37.0 ºC. Fluid and electrolyte management in the first day of life included 70–80 ml/kg 10% dextrose in water supplemented with 10% calcium gluconate (2 ml/kg/day before 2007 and 4–6 ml/kg/day after 2007). When possible, parenteral and enteral nutrition were initiated on the second day of life. Parenteral nutrition supplemented with 0.5 g/kg/day protein, with daily increments of 0.5 g/kg/day to a final level of 3 g/kg/day was used prior to 2003. In 2003, we began to use a parenteral nutrition regimen containing 1 g/kg/day of protein, with daily increments of 1 g/kg/day up to 3.5 g/kg/day. Parenteral nutrition was also supplemented with sodium (2 mEq/kg/day, as 20% sodium chloride or glycerophosphate), potassium (2 mEq/kg/day, as 7.5% potassium chloride), calcium (20–60 mg/kg/day, as 10% calcium gluconate), magnesium (0.3–0.4 mEq/kg/day, as 20% magnesium sulfate) and phosphorus (1 mmol/kg/day, as glycerophosphate). Phosphorus supplementation with sodium glycerophosphate (Glycophos®) contains 2 mEq of sodium per milliliter, which was considered as the total amount of daily sodium supplementation. When possible, lipids were infused beginning on the second day of life. Lipid concentrations started at 0.5 g/kg/day with daily increments of 0.5 g/kg/day up to 3.0 g/kg/day before 2003 and at 1.0 g/kg/day with daily increment of 1.0 g/kg/day until 3.0 g/kg/day thereafter. Enteral feeding was usually initiated on the second day of life using either mother’s milk or preterm formula, according to the infant’s clinical condition. The infant received 1 ml every 3 h, with daily increments according to enteral tolerance and clinical condition. Total fluid administration (parenteral and enteral) started at 70–80 ml/kg/day, with increments of 10–15 ml/kg/day until 150 ml/kg/day was reached by day 7 of life and maintained until 3 weeks of age. The fluid volume was increased in 10 ml/kg/day increments in infants undergoing phototherapy. Fluid and electrolyte replacement therapy were monitored by daily weight measurements, vital signs determinations, urine output and serum laboratory studies. Glucose and electrolytes were maintained within the normal range: 40–150 mg/dl glucose; 135–145 mEq/L sodium; 98–108 mEq/L chloride; 4–6 mEq/L potassium; 4.4–5.4 mg/dl calcium; 1.5–2.3 mEq/L magnesium; 30–90 mg/L phosphorus. Blood pressure should be at least the same number as gestational age in weeks, and urine output should be between 1–5 ml/kg/h.8 Plasma osmolality was considered normal when values were between 275–290 mOsm/L. The blood transfusion practice was used according to the criteria of Simon et al. 9.
Definitions
The diagnosis of BPD was made if the infant was chronically oxygen dependent at 36 weeks of corrected age and had a characteristic chest radiograph.10 Gestational age (in this study, we considered the completed weeks) was assessed by menstrual age (women with regular menstrual cycles), ultrasound examination (when a discrepancy of two or more weeks existed between the age derived by menstrual dating and the age derived sonographically, or in the absence of a menstrual date)11 or the New Ballard Score (in the absence of obstetrical indexes).12 Intrauterine growth restriction was defined as a birth weight below the 10th percentile on Fenton’s fetal growth charts.13 Histological chorioamnionitis was classified according to the method proposed by Blanc.14 All grades of chorioamnionitis were grouped together. Respiratory distress syndrome (hyaline membrane disease) was defined according to the criteria of Rudolf et al.15 Proven neonatal sepsis was defined as any systemic bacterial or fungal infection documented by a positive blood culture. Hemodynamically significant patent ductus arteriosus was diagnosed on the basis of the echocardiographic findings. The first evaluation is usually between 24 and 72 h of life, with daily evaluation until closure of the ductus. The standard treatment for patent ductus arteriosus is indomethacin. The criteria of Bell were used for the diagnosis and staging of necrotizing enterocolitis.16 Retinopathy of prematurity was staged according to the international classification.17,18 Intraventricular hemorrhage was classified according to Papile.19 Periventicular leukomalacia was classified according to de Vries and Rennie.20
Fluid and electrolyte balance evaluation
Fluid, plasma glucose, electrolyte balance (sodium, chloride, potassium, magnesium, calcium, and phosphorus), urea nitrogen, creatinine, osmolality, hematocrit, need for blood transfusions, daily urine output and weight were analyzed for the first 7 days of life. Sodium, potassium, chloride, calcium and hematocrit were evaluated daily along with acid-base status during the first week of life or while on respiratory support. Glycemia, evaluated by Dextrostix or Chemstrip-bG testing, was determined every 6–8 h, or at different time intervals according to either the previous determination or clinical condition. Plasma glucose, magnesium, phosphorus, urea nitrogen and creatinine, as well as ionogram and calcium, are routinely evaluated on the second day of life and at 1–3-day intervals thereafter during the first week of life, according to the infant’s clinical condition. For all of these parameters except glucose, an average value of two consecutive determinations was used to calculate an approximate daily balance. Plasma osmolality was estimated based on the following formula: osmolality (mOsm/L) = 2 x [plasma sodium (mEq/L)] + [plasma glucose (mg/dl)] / 18 + [blood urea nitrogen (mg/dl)] / 2.8. (Note: this estimated osmolality is usually slightly lower than the measured osmolality; we considered estimated values of 275–290 mOsm/L as normal, which is slightly lower than the normal measured values of 285–295 mOsm/L).
Fluid and electrolyte balance, daily plasma osmolality, weight, urine output, hematocrit and need for blood transfusions were compared between preterm neonates who developed BPD and those who did not develop this condition.
Statistics
The appropriate summary statistics were applied to describe the analyzed samples. Categorical variables were described using absolute and relative (%) frequencies. Continuous variables were described using either means and standard deviations or medians, percentile 25 and percentile 75, depending on their symmetrical or asymmetrical distribution, respectively. To assess the association between categorical variables, we used the chi-squared test or Fischer’s exact test. The Student’s t-test or the respective nonparametric Mann-Whitney U test was used to compare two independent samples. To assess the risk factors associated with the outcomes of BPD and patent ductus arteriosus, odds ratios (ORs) and the respective 95% confidence intervals were calculated through logistic regression. To assess renal function and the 7-day fluid and electrolyte longitudinal variation, multivariate models of generalized estimation equations (GEEs) were used.
A p value <0.05 was considered significant in all of the hypothesis tests. The analyses were done using SPSS v. 15.0 for Windows.
RESULTS
Demographic and clinical characteristics
Of 440 preterm neonates, 205 were included in this study. The prevalence of BPD was 22% (n = 45). Demographics and clinical characteristics of the studied sample are reported in Tables 1 and 2. Younger gestational age, lower birth weight, male gender and less frequent use of antenatal steroids were associated was BPD (Table 1). The association between respiratory distress syndrome, use of surfactant, duration of invasive ventilation, patent ductus arteriosus, duration of NICU stay and BPD was statistically significant after adjustments for gender, gestational age, birth weight and antenatal steroids use (Table 2). There was no difference in BPD prevalence between two time periods in which different guidelines for antenatal steroids and parenteral nutrition was used (1997–2002 and 2003–2008) (Table 3).
Table 1.
Demographics of the studied population (n=205).
| Total n=205 | BPD absent n=160 (78%) | BPD present n=45 (22%) | p | OR(1) | 95% CI | OR(2) | 95% CI | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Birth weight (g), mean (sd) | 1007 (219) | 1041 (218) | 886 (175) | <0.001‡ | 0.996 | 0.994 | 0.998 | - | - | - |
| Gestational age (weeks), mean (sd) | 29 (2) | 29 (2) | 27 (2) | <0.001‡ | 0.580 | 0.468 | 0.719 | - | - | - |
| Female, n (%) | 99 (48) | 87 (54) | 12 (27) | 0.001* | 1.000 | - | - | - | - | - |
| Male, n (%) | 106 (52) | 73 (46) | 33 (73) | - | 3.277 | 1.579 | 6.803 | - | - | - |
| IUGR, n (%) | 53 (26) | 44 (28) | 9 (20) | 0.310* | 0.659 | 0.294 | 1.480 | 0.611 | 0.147 | 2.545 |
| Antenatal steroids, n (%) | 179 (87) | 144 (90) | 35 (78) | 0.030* | 0.389 | 0.163 | 0.930 | - | - | - |
| full cycle, n (%) | 136 (66) | 111 (69) | 25 (56) | 0.083* | 0.552 | 0.280 | 1.086 | 0.939 | 0.388 | 2.275 |
| C-section, n (%) | 146 (71) | 117 (73) | 29 (64) | 0.256* | 0.666 | 0.330 | 1.346 | 1.366 | 0.541 | 3.452 |
IUGR, intrauterine growth restriction; sd, standard deviation;
Chi-squared test;
student’s t-test;
simple odds ratio (OR);
adjusted OR to the variables birth weight (g), gestational age (weeks), gender and antenatal steroids; CI, confidence interval
Table 2.
Clinical characteristics of the studied population (n=205).
| Total n=205 | BPD absent n=160 (78%) | BPD present n=45 (22%) | p | OR(1) | 95% CI | OR(2) | 95% CI | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Histological chorioamnionitis, n (%) | 75 (37) | 51 (32) | 24 (53) | 0.008* | 2.443 | 1.246 | 4.790 | 1.965 | 0.807 | 4.785 |
| RDS, n (%) | 144 (70) | 102 (64) | 42 (93) | <..001* | 7.961 | 2.362 | 26.826 | 4.214 | 1.170 | 15.181 |
| Surfactant, n (%) | 126 (61) | 85 (53) | 41 (91) | <.001* | 9.044 | 3.094 | 26.436 | 5.140 | 1.661 | 15.902 |
| Ventilation (ETT), n (%) | 167 (81) | 123 (77) | 44 (98) | 0.001* | 13.236 | 1.763 | 99.366 | 4.424 | 0.541 | 36.152 |
| Days of ventilation (ETT), median (P25-P75) | 5 (1 - 21) | 3 (1 - 11) | 40 (25- 63) | <0.001* | 1.100 | 1.069 | 1.131 | 1.093 | 1.057 | 1.129 |
| NCPAP, n (%) | 182 (89) | 142 (89) | 40 (89) | 0.979* | 1.014 | 0.354 | 2.901 | 0.307 | 0.086 | 1.098 |
| Days of NCPAP, median (P25-P75) | 18 (5 - 31) | 16 (4 - 30) | 25 (10 - 38) | 0.014§ | 1.029 | 1.010 | 1.049 | 1.010 | 0.988 | 1.033 |
| Oxygen, n (%) | 181 (88) | 136 (85) | 45 (100) | 0.006* | - | - | - | - | - | |
| Days of oxygen, median (P25-P75) | 12 (1 - 52) | 4 (1 - 27) | 84 (70 - 112) | <0.001§ | 1.273 | 1.133 | 1.430 | - | - | - |
| PDA, n (%) | 56 (27) | 31 (19) | 25 (56) | <0.001* | 5.202 | 2.566 | 10.545 | 4.302 | 1.809 | 10.231 |
| Nosocomial sepsis, n (%) | 123 (60) | 86 (54) | 37 (82) | <0.001* | 3.980 | 1.744 | 9.081 | 2.401 | 0.965 | 5.974 |
| NEC ≥ 2A, n (%) | 10 (5) | 5 (3) | 5 (11) | 0.043** | 3.875 | 1.069 | 14.041 | 2.491 | 0.569 | 10.909 |
| IVH (grades III-IV), n (%) | 17 (8) | 7 (4) | 10 (22) | 0.001** | 6.245 | 2.222 | 17.551 | 2.647 | 0.816 | 8.581 |
| PVL, n (%) | 9 (4) | 3 (2) | 6 (13) | 0.004** | 8.051 | 1.928 | 33.630 | 3.552 | 0.666 | 18.938 |
| ROP > 2, n (%) | 13 (6) | 6 (4) | 7 (16) | 0.009** | 1.076 | 0.497 | 2.329 | 0.693 | 0.283 | 1.695 |
| NICU stay (days), median (P25-P75) | 64 (51 - 80) | 60 (48 - 70) | 88 (73 - 105) | <0.001§ | 1.080 | 1.053 | 1.107 | 1.071 | 1.041 | 1.102 |
| Deceased > 36 weeks, n (%) | 6 (3) | 0 (0) | 6 (13) | <0.001** | - | - | - | - | - | |
ETT, endotracheal tube; NCPAP, nasal continuous positive airway pressure; NICU, neonatal intensive care unit; NEC, necrotizing enterocolitis; P, percentile; PDA, patent ductus arteriosus; PVL, periventricular leukomalacia; RDS, respiratory distress syndrome; ROP, retinopathy of prematurity; sd, standard deviation;
Chi-squared test;
Fisher’s exact test;
Mann-Whitney U test;
simple odds ratio (OR);
adjusted OR to the variables birth weight (g), gestational age (weeks), gender and antenatal steroids; CI, confidence interval
Table 3.
Incidence of BPD during two different treatment periods (n=205).
| 1997–2002 (n=118) (58%) | 2003–2008 (n=87) (42%) | p | |||
|---|---|---|---|---|---|
| Birth weight (g), median (P25-P75) | 990 | (875–1,123) | 1,040 | (860–1,185) | 0.247§ |
| Gestational age (weeks), mean (sd) | 28 | (2) | 29 | (2) | 0.353‡ |
| BPD | |||||
| Absent | 90 | (76) | 70 | (81) | 0.474* |
| Present | 28 | (24) | 17 | (19) | |
sd, standard deviation; P, percentile;
Chi-squared test;
Student’s t-test;
Mann-Whitney U test
Multivariate model of GEE of fluid and electrolyte longitudinal variation
The values from the first evaluation of the studied parameters are reported in Table 4. On the first or second day of life, serum glucose and phosphorus were higher in BPD patients and serum calcium, creatinine and hematocrit were lower in these patients compared to neonates without BPD. A multivariate model of GEE of the longitudinal variation (from the first or second to the seventh day of life) of total fluids, glucose, electrolytes, urea, creatinine, osmolality, diuresis and weight is reported in Tables 5 and 6. Gestational age (β2 coefficient, Table 5) had a significant effect on all parameters except total fluids, sodium and chloride. An increase of 1 week in gestational age (including all neonates in the study) gave rise to a decrease in serum values of potassium, magnesium, phosphorus, urea, creatinine, glucose, osmolality and diuresis and increased calcium serum values, hematocrit and weight. The daily variation (coefficient β3, Table 5) from the first or second day of life to the seventh day of life was significant in all of the analyzed parameters except potassium, magnesium and urea. Total fluids, serum calcium and diuresis increased from the first to the seventh day of life (including all neonates of the study). The other analyzed parameters decreased from the first or second day of life to the seventh. None of the analyzed parameters showed significant variation in patients who developed BPD (coefficient β4, Table 5). Calcium supplementation of 40–60 mg/kg/day (versus 20–30 mg/kg/day used as a reference value) gave rise to an increase in serum calcium from the first to the seventh day of life (β6, Table 6). Supplementing with phosphate (phosphorus 1–2 mmol/kg/day), however, decreased the phosphorus serum values from the second to the seventh day of life (β6, Table 6). Phosphorus presented two interactions in this GEE model: one related to the clinical intervention (supplementation with phosphate) and another related to BPD (Table 6). Both the clinical intervention with phosphorus (phosphate supplementation) and developing BPD were associated with a decrease in serum values of phosphorus from the second to the seventh day of life.
Table 4.
Clinical parameters obtained on the first or second day of life.
| Total n = 205 | BPD absent n = 160 (78%) | BPD present n = 45 (22%) | p | ||||
|---|---|---|---|---|---|---|---|
| Total fluids, day 1 (mlkg/day) median (P25-P75) | 90 | (80–100) | 90 | (80–100) | 90 | (90–100) | 0.081§ |
| Glucose, day 1 (mg/dl) median (P25-P75) | 100 | (80–120) | 95 | (80–110) | 110 | (94–121) | 0.012§ |
| Sodium, day 1 (mEq/l) mean (sd) | 138.75 | (5.71) | 139.01 | (5.96) | 137.84 | (4.66) | 0.229‡ |
| Chloride, day 1 (mEq/l) mean (sd) | 107.07 | (5.57) | 107.29 | (5.58) | 106.31 | (5.56) | 0.300‡ |
| Potassium, day 1 (mEq/l) median (P25-P75) | 4.90 | (4.40–5.50) | 4.90 | (4.30–5.50) | 4.l80 | (4.50–5.60) | 0.634§ |
| Calcium, day 2 (mg/dl) mean (sd) | 4.08 | (0.62) | 4.13 | (0.66) | 3.89 | (0.42) | 0.004‡ |
| Magnesium, day 2 (mEq/l) median (P25-P75) | 1.60 | (1.50–1.80) | 1.60 | (1.50–1.80) | 1.60 | (1.50–1.80) | 0.532§ |
| Phosphorus, day 2 (mg/l) mean (sd) | 55.96 | (13.03) | 54.99 | (12.92) | 59.40 | (12.99) | 0.045‡ |
| Urea, day 2 (g/l) median (P25-P75) | 0.36 | (0.27–0.47) | 0.36 | (0.26–0.46) | 0.39 | (0.31–0.49) | 0.145§ |
| Creatinine, day 2 (mg/l) mean (sd) | 10.04 | (2.31) | 10.21 | (2.20) | 9.45 | (2.61) | 0.052‡ |
| Osmolality, day 2 (mOsm/l) median (P25-P75) | 296 | (289–305) | 296 | (289–305) | 296 | (289–302) | 0.759§ |
| Hematocrit, day 1 (%)mean (sd) | 47.60 | (6.98) | 48.30 | (6.93) | 45.13 | (6.69) | 0.007‡ |
| Diuresis, day 1 (ml/kg/hour) median (P25-P75) | 4.30 | (3.50–5.60) | 4.30 | (3.50–5.50) | 4.30 | (3.30–6.20) | 0.685§ |
sd, standard deviation; P, percentile;
Mann-Whitney U test;
Student’s t-test.
Table 5.
Multivariate model of the generalized estimation equation (GEE) of the longitudinal variation (from the first or second day of life to the seventh) of total fluids, glucose, electrolytes, urea, creatinine, osmolality, hematocrit, diuresis and weight adjusted to gestational age, daily variation, BPD and clinical intervention (when indicated).
| Independent variables |
||||||||
|---|---|---|---|---|---|---|---|---|
| Constant | Gestational age (weeks) | Daily variation (from day 1 or 2 to day 7) | BPD present | |||||
| Dependent variable | β1 | CI 95% | β2 | CI 95% | β3 | CI 95% | β4 | CI 95% |
| Total fluids, (ml/kg/day) | 77.444 | 58.779; 96.109 | −0.310 | −0.949; 0.329 | 12.588 | 12.269; 12.907 | 1.241 | −1.700; 4.181 |
| Glucose, (mg/dl) | 185.897 | 147.841; 223.953 | −3.040 | −4.329; −1.751 | −1.652 | −2.540; −0.760 | 5.249 | −1.662; 12,160 |
| Sodium, (mEq/L) | 120.817 | 100.196; 141.437 | 0.019 | −0.187; 0.224 | −0.470 | −0.667; −0.274 | −0.366 | −1.501; 0.769 |
| Chloride, (mEq/L) | 88.994 | 66.759; 111.229 | 0.003 | −0.286; 0.291 | −0.403 | −0.602; −0.205 | −0.650 | −1.948; 0.648 |
| Potassium, (mEq/L) | 5.731 | 4.582; 6.881 | −0.034 | −0.068; 0.000 | 0.013 | −0.023; 0.049 | 0.267 | −0.138; 0.673 |
| Calcium, (mg/dl) | 1.215 | 0.378; 2.051 | 0.089 | 0.061; 0.117 | 0.132 | 0.110; 0.153 | −0.044 | −0.173; 0.084 |
| Magnesium, (mEq/L) | 2.921 | 2.105; 3.737 | −0.028 | −0.048; −0.007 | −0.040 | −0.109; 0.030 | −0.029 | −0.152; 0.095 |
| Phosphorus, (mg/L) | 112.149 | 88.130; 136.169 | −1.551 | −2.332; −0.770 | −4.520 | −7.318; −1.722 | 4.098 | −2.130; 10.325 |
| Urea, (g/L) | 1.841 | 1.553; 2.129 | −0.052 | −0.062; −0.042 | 0.007 | −0.001; 0.015 | −0.003 | −0.055; 0.048 |
| Creatinine, (mg/L) | 16.114 | 12.840; 19.388 | −0.179 | −0.290; −0.069 | −0.396 | −0.482; −0.310 | −0.993 | −2.037; 0.052 |
| Osmolality, (mOsm/L) | 359.384 | 341.403; 377.365 | −2.039 | −2.655; −1.423 | −0.687 | −1.198; −0.176 | −0.333 | −3.328; 2.663 |
| Hematocrit, (%) | 4.655 | −5.598; 14.908 | 1.588 | 1.232; 1.944 | −0.943 | −1.101; −0.784 | 0.729 | −0.811; 2.270 |
| Diuresis, (ml/kg/hour) | 7.182 | 5.581; 8.783 | −0.121 | −0.176; −0.067 | 0.183 | 0.152; 0.214 | −0.050 | −0.331; 0.231 |
| Weight, (g) | −567.33 | −906.782; −227.888 | 55.239 | 43.187; 67.292 | −18.539 | −20.312; −16.767 | −40.312 | −92.488; 11.863 |
β1, the initial value of the evaluated parameter in the GEE model; β2, the coefficient of gestational age in the GEE model; β3, the coefficient of daily variation of the analyzed parameter in the GEE model; β4, the coefficient of BPD in the GEE model; BPD, bronchopulmonary dysplasia; CI, confidence interval. Significant CIs are in bold
Table 6.
Multivariate model of the generalized estimation equation (GEE) of the longitudinal variation (from the first or second day of life to the seventh) of glucose, sodium, chloride, potassium, calcium, magnesium, phosphorus, creatinine and hematocrit adjusted to gestational age, daily variation, BPD and clinical intervention (when indicated).
| Independent variables |
||||||||
|---|---|---|---|---|---|---|---|---|
| Values on the first day of life | Clinical intervention | Interaction between days and BPD | Interaction between days and intervention | |||||
| Dependent variable | β5 | CI 95% | β6 | CI 95% | β7 | CI 95% | β8 | CI 95% |
| Glucose, (mg/dl) | - | - | 0.363 (1) | −1.248; 1.973 | - | - | - | - |
| Sodium, (mEq/l) | 0.144 | 0.000; 0.288 | −2.630 (2) | −5.933; 0.672 | - | - | - | - |
| Chloride, (mEq/l) | 0.193 | 0.021; 0.364 | −1.581 (3) | −3.568; 0.406 | - | - | - | - |
| Potassium, (mEq/l) | 0.094 | 0.015; 0.173 | −0.250 (4) | −0.606; 0.106 | −0.087(5) | −0.166; −0.008 | - | - |
| Calcium, (mg/dl) | - | - | 0.449(6) | 0.204; 0.694 | - | - | −0.0437) | −0.084; −0.001 |
| Magnesium, (mEq/l) | - | - | −0.417 (8) | −0.947; 0.112 | 0.077(9) | 0.007; 0.147 | ||
| Phosphorus, (mg/l) | - | - | −11.318(10) | −18.237; −4.399 | 1.222(11) | −2.373; −0.071 | 3.873(12) | 1.063; 6.683 |
| Creatinine, (mg/l) | - | - | - | - | 0.169(13) | 0.000; 0.339 | - | - |
| Hematocrit, (%) | - | - | −8.267(14) | −10.022; −6.513 | - | - | - | - |
β5, the coefficient of the serum value of the analyzed parameter on the first or second day of life in the GEE model; β6, the coefficient of the clinical intervention (glucose and electrolytes supplementation, and packed red blood cell transfusions) for the analyzed parameters in the GEE model; β7 and β8, the coefficients of interaction between two variables in the GEE model; CI, confidence interval;
intervention with glucose as continuous variable;
intervention with sodium (20% NaCl) [0- 2 mEq/Kg/day] (as a reference) vs. [2–8 mEq/Kg/day];
intervention with chloride (20% NaCl) [0- 2 mEq/kg/day] (as a reference) vs. [2–8 mEq/kg/day];
intervention with potassium (7.5% KCl) [0–1 mEq/kg/day] (as a reference) vs. = 2 mEq/kg/day;
interaction between BPD absent * days (as a reference) vs. BPD present * days;
intervention with calcium (10% calcium gluconate) [20–30 mg/kg/day] (as a reference) vs. [40–60 mg/kg/day];
interaction calcium < 40 mg/kg/day * days (reference) vs. calcium ≥ 40 mg/kg/day * days;
intervention with magnesium (20% magnesium sulfate) [0.0–0.3 mEq/kg/day] (as a reference) vs. [0.3–0.6 mEq/kg/day];
interaction with magnesium [0.0–0.3 ml/kg/day] * days (as a reference) vs. magnesium [0.3–0.6 ml/kg/day] * days;
intervention with phosphate [0–1 mmol/kg/day] (as a reference) vs. [1–2 mmol/kg/day];
BPD absent * days (as a reference) vs. BPD present * days;
phosphorus [0–1 mmol/kg/day] * days (as a reference) vs. phosphorus [1–2 mmol/kg/day] * days;
BPD absent * days (as a reference) vs. BPD present * days; * interaction between two variables; significant CI values are in bold;
no packed red blood cells transfusion (as a reference) vs. packed red blood cells transfusion
The variation in serum values of potassium, phosphorus and creatinine (β7, Table 6) during the first week of life revealed an association with BPD. A daily decrease in serum levels of potassium and phosphorus and a daily increase in serum creatinine were observed. Although these parameters (potassium, phosphorus and creatinine) were not associated with BPD when evaluated as one isolated day value (β4, Table 5), their serum level variation during the first 7 days of life revealed a statistically significant association with developing BPD (β7 and β8, Table 6).
The clinical practice (or intervention) of providing a transfusion of packed red blood cells during the first week of life was significant in both BPD and non-BPD neonates (β6, Table 6). The rate of transfusions in non-BPD patients was 35% (56/160) vs. 77.7% (35/45) in BPD patients. However, there was no interaction between transfusions and BPD in the GEE model. Thus, there is no association between the number of packed red blood cells transfusions and BPD.
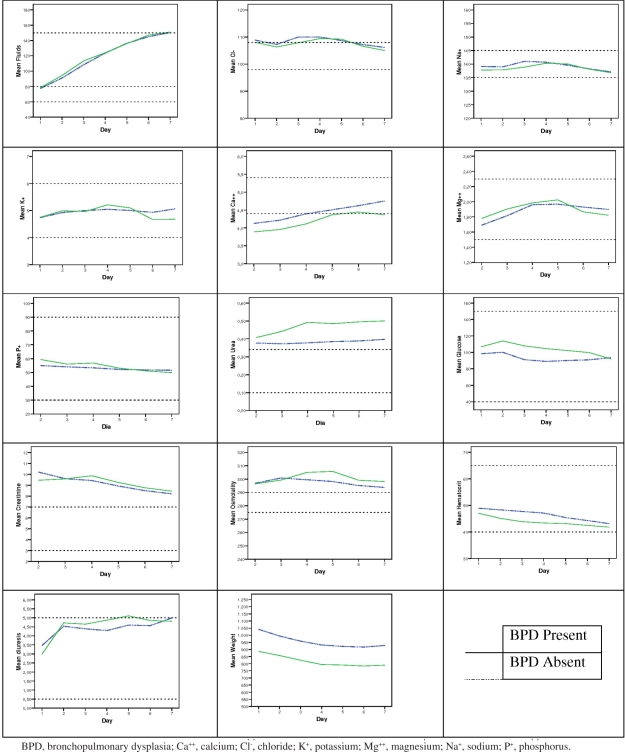
Figure 1 graphically shows the longitudinal variation (from the first to the seventh day of life) of the studied parameters in BPD and non-BPD neonates.
Figure 1.
Graphical representation of the longitudinal variation (first week of life) of the studied parameters in BPD and non-BPD neonates.
Parameters of renal function
The association between parameters of renal function on the seventh day of life and BPD is reported in Table 7. Serum creatinine values were not statistically different between BPD and non-BPD patients. Although the serum values of creatinine were not significantly different between BPD and non-BPD neonates, the GEE model demonstrated that the variation of the creatinine values from day 2 to day 7 of life was associated with the development of BPD (β7, Table 6). The serum level of urea was significantly higher and the serum level of potassium was significantly lower in BPD patients compared to non-BPD patients.
Table 7.
Comparison of renal function parameters on the seventh day of life between BPD and non-BPD patients.
| Total (n=205) | BPD absent (n=160, 78%) | BPD present (n=45, 22%) | p | ||||
|---|---|---|---|---|---|---|---|
| Creatinine, mean (sd) | 8.26 | (2.10) | 8.20 | (2.04) | 8.46 | (2.30) | 0.457‡ |
| Urea, median (P25-P75) | 0.34 | (0.19–0.60) | 0.33 | (0.18–0.57) | 0.42 | (0.30–0.72) | 0.019§ |
| Potassium, mean (sd) | 4.98 | (0.84) | 5.07 | (0.81) | 4.68 | (0.88) | 0.006‡ |
sd, standard deviation; P, percentile;
Student’s t-test;
Mann-Whitney test.
Patent ductus arteriosus
Clinically significant patent ductus arteriosus presented a significant association with BPD (Table 2). We found no association between the variation of daily total fluid intake during the first week of life and patent ductus arteriosus (p = 0.960, Table 8). The daily total fluid increase did not show a significant change with gestational age (p = 0.221). We also did not find an association between weight variation during the first week of life and patent ductus arteriosus (p = 0.831, Table 9).
Table 8.
Generalized estimation equation (GEE) model to assess the association between total fluid intake during the first week of life and patent ductus arteriosus.
| β | CI 95% | p | ||
|---|---|---|---|---|
| Patent ductus arteriosus | ||||
| Absent | - | - | ||
| Present | 0.068 | −2.548 | 2.684 | 0.960 |
| Gestational age (weeks) | −0.405 | −1.053 | 0.243 | 0.221 |
| Days | 12.588 | 12.269 | 12.907 | <0.001 |
| Constant | 80.405 | 61.346 | 99.464 | <0.001 |
CI, confidence interval; dependent variable: total fluid intake; independent variables: days, patent ductus arteriosus, gestational age (weeks) and without interactions.
Table 9.
Generalized estimation equation (GEE) model to assess the association between weight loss during the first week of life and patent ductus arteriosus.
| β | CI 95% | P | ||
|---|---|---|---|---|
| Patent ductus arteriosus | ||||
| Absent | - | - | ||
| Present | 4.881 | −39.939 | 49.702 | 0.831 |
| Gestational age (weeks) | 58.932 | 47.033 | 70.831 | <0.001 |
| Days | −18.539 | −20.312 | −16.767 | <0.001 |
| Constant | −682.870 | −1019.851 | −345.888 | <0.001 |
CI, confidence interval; dependent variable: weight; independent variables: days, patent ductus arteriosus, gestational age (weeks) and without interaction.
Higher mean plasma calcium and lower mean urea values during the first week of life were associated with the spontaneous closure of ductus arteriosus (Table 10).
Table 10.
Association between mean values of fluids, electrolytes, urea, creatinine and osmolality during the first week of life and patent ductus arteriosus.
| Total n=205 | PDA absent n=149 (73%) | PDA present n=56 (27%) | p | ||||
|---|---|---|---|---|---|---|---|
| Fluids, median (P25-P75) | 119.57 | (114.29–125.00) | 119.29 | (114.29–124,57) | 120.36 | (115.71–126.07) | 0.491§ |
| Sodium, mean (sd) | 139.08 | (2.59) | 139.19 | (2.48) | 138.81 | (2.85) | 0.351‡ |
| Potassium, mean (sd) | 4.95 | (0.42) | 4.92 | (0.43) | 5.02 | (0.39) | 0.135‡ |
| Chloride, mean (sd) | 108.12 | (3.03) | 108.23 | (3.16) | 107.83 | (2.68) | 0.407‡ |
| Phosphorus, mean (sd) | 53.33 | (11.57) | 53.06 | (12.16) | 54.07 | (9.89) | 0.579‡ |
| Calcium, mean (sd) | 4.38 | (0.46) | 4.47 | (0.46) | 4.16 | (0.39) | <0.001‡ |
| Magnesium, median (P25-P75) | 1.83 | (1.70–2.03) | 1.85 | 1.70–2.01) | 1.82 | (1.72–2.11) | 0.877 § |
| Urea, median (P25-P75) | 0.38 | (0.27–0.51) | 0.36 | (0.25–0.48) | 0.47 | (0.35–0.55) | <0.001§ |
| Creatinine, mean (sd) | 9.16 | (1.61) | 9.10 | (1.56) | 9.32 | (1.73) | 0.388 ‡ |
| Osmolality, mean (P25-P75) | 298.17 | (291.83–303.67) | 296.50 | (290.50–302.50) | 300.42 | (292.75–304.50) | 0.091§ |
P, percentile; sd, standard deviation;
Student’s t-test;
Mann-Whitney test.
Indomethacin was used significantly more frequently during the first week of life for patent ductus arteriosus closure in BPD patients than in non-BPD patients (Table 11).
Table 11.
Indomethacin use during the first week of life for patent ductus arteriosus treatment in BPD and non-BPD neonates.
| Total n=205 | BPD absent n=149 | BPD present n=56 | p | ||||
|---|---|---|---|---|---|---|---|
| Indomethacin use, n (%) | |||||||
| No | 151 | (83) | 131 | (88) | 20 | (61) | <0.001* |
| Yes | 31 | (17) | 18 | (12) | 13 | (39) | |
BPD – bronchopulmonary dysplasia;
Chi-squared test.
DISCUSSION
The first week of life is a critical transition period for very low birth weight neonates. During this time, fluid and electrolyte imbalances may be harmful to various organs and systems, including the lung, which is usually affected by a membrane hyaline disease and other noxious events related to mechanical ventilation, inflammation and a left-to-right shunt from a patent ductus arteriosus. All of these noxious events are recognized as risk factors for developing DBP. However, little is known about fluid and electrolyte imbalances as direct or indirect risk factors for BPD.
In this study, we found an association between BPD and low birth weight, young gestational age, male gender, less antenatal steroids use, respiratory distress syndrome and patent ductus arteriosus, which are already known risk factors. Additionally, the variation in plasma values of potassium, phosphorus and creatinine (β7, Table 6) during the first week of life was associated with BPD. Serum potassium presented an increasing trend during the first 4 days of life, followed by a decrease in neonates with BPD compared to non-BPD neonates, even though the clinical intervention (supplementation with potassium) was not different between the two groups. Phosphorus presented a more rapidly decreasing trend during the first week of life in BPD patients. Serum creatinine values presented a sustained increase over the first 4 days of life in BPD patients, subsequently followed by a decrease, when compared to non-BPD newborns. Creatinine and potassium showed a similar pattern, with an increase in the first 4 days of life followed by a decrease.
In the first weeks after birth, serum creatinine decreases, initially exponentially as the maternally derived creatinine load is excreted and then more gradually.21 A single measurement of serum creatinine provides no more than a crude estimation of renal function, and observing the change over time is more informative. A useful clinical indicator of renal insufficiency is the failure to observe the expected postnatal decline in serum creatinine. Therefore, the change in serum creatinine should be considered instead of the absolute level. Initially high, the neonatal serum creatinine level decreases during the first week. A sustained increase or failure to decrease is indicative of a reduction in the glomerular filtration rate. The blood urea concentration is of little value because it is influenced by numerous non-renal factors.22
The body potassium content is a reflection of the balance between its intake and output. Potassium output occurs through three primary routes: urine, the gastrointestinal tract, and skin. The kidney is the major excretory organ and is primarily responsible for regulating the external potassium balance.23 A negative potassium balance may be relatively common in neonates receiving intensive care.22 In this study, the neonates who developed BPD were those who received more intensive care with a higher rate of respiratory distress syndrome and mechanical ventilation, and this association may explain the greater renal losses of both potassium and creatinine after day 4 of life.
The kidney regulates the phosphorus balance, which is determined by intrarenal mechanisms and hormonal actions on the nephron.24 Parathormone, which is secreted in response to low plasma calcium, decreases the resorption of phosphate, thus increasing urinary phosphate. In this study, although the variation is serum calcium during the first 7 days of life was not different between BPD and non-BPD patients, the medium serum levels of calcium in BPD neonates were lower than those observed in non-BPD patients and were similar to the values in neonates who presented a patent ductus arteriosus. Although levels of parathormone were not assessed in this study, we speculate that BPD patients may have a higher production of parathormone, and there may be an association between the development of BPD, the calcium-phosphorus balance and parathormone regulation.
There are some problem areas in achieving fluid and electrolyte balance in very low and extremely low birth weight preterm newborns. One of these problems is poor epidermal barrier function. Thin, gelatinous skin promotes rapid transcutaneous evaporation, producing severe electrolyte disturbances during the first few days of life. After the first week of life, however, the risk of dehydration diminishes as the skin barrier matures. A second area of major concern is pulmonary edema. Fluid replenishment may result in increased lung water and contribute to the pathogenesis of BPD.23,25–30 Moreover, high fluid intake, which is associated with the development of clinically significant patent ductus arteriosus and congestive heart failure,28,29 may also contribute to the pathogenesis of BPD.30 Perhaps the root of this problem is the premature infant’s markedly immature renal development, with a small glomerular surface available for filtering any fluid volume or salt excess.23
The results of this study support the idea of clinically significant patent ductus arteriosus being a risk factor for BPD but do not support the idea of an association between increased fluid intake or a reduced weight loss and patent ductus arteriosus. An association between a lesser or delayed diuresis and BPD were also not supported.
Antenatal steroid treatment is associated with lower estimated insensible water loss, a decreased incidence of hypernatremia (>150 mmol/L sodium) and earlier diuresis and natriuresis in extremely low birth weight neonates. An explanation is that antenatal steroid causes these changes by enhancing epithelial cell maturation and thus improving skin barrier function. Antenatal steroid treatment may also enhance lung Na+, K+-ATPase activity, leading to an earlier postnatal reabsorption of fetal lung fluid, which increases extracellular volume expansion and helps prevent hypernatremia.31 Antenatal steroids also increase renal cell differentiation and maturation of the renal autoregulatory mechanisms, resulting in an increase in the tubular excretory capacity to handle excess solutes.32 Thus, the kidney responds to the increased extracellular space by increasing sodium excretion, leading to earlier natriuresis and lowering the extracellular fluid volume.33 Once a normal extracellular space is achieved, renal sodium excretion decreases. Maturation of this response would result in a less negative sodium balance. Conversely, an up-regulation of tubular Na+,K+-ATPase expression, which would lead to renal tubular function and increased renal sodium reabsorption, may also contribute to this less negative sodium balance.34 Here, we verified that BPD patients benefited less from antenatal steroids use. Also, they presented a slightly higher diuresis after the first 24 h of life and slightly lower serum levels of sodium during the first 4 days of life when compared to non-BPD patients. This is caused by higher renal water loss and lower renal reabsorption of sodium, which may be related to the more immature renal mechanisms that are associated with a reduced use of antenatal steroids. The slightly lower serum sodium values observed in the first 4 days of life in BPD patients, followed by a slight increase (similar to the values observed in the non-BPD patients), and the associated decrease in creatinine after day 4 of life are suggestive of a delayed maturation in the renal handling of sodium. However, according to the statistical GEE model used in this study, no significant differences were observed in the variation trend of total daily fluids, serum sodium and chloride between BPD and non-BPD neonates. Also, the decreasing weight pattern was similar in both groups.
With advancing postnatal age, significant improvement occurs in renal sodium conservation.35,36 Lorenz et al.37 identified three distinct phases of fluid and electrolyte homeostasis in low birth weight infants with and without respiratory distress syndrome during the first days of life. The low urine output of the first day (pre-diuretic phase) is followed by spontaneous diuresis and natriuresis during the second and third days, independent of fluid intake (diuretic phase). The onset, duration, and extent of diuresis appear to vary. The high rate of urine flow and sodium excretion is assumed to be the result of abrupt increases in glomerular filtration rate and fractional sodium excretion subsequent to the reabsorption of residual lung fluid and expansion of extracellular space. During the post-diuretic phase, the glomerular filtration rate remains unchanged, and urine flow and sodium excretion decrease to values between those observed in the pre-diuretic and diuretic phases and begin to vary appropriately in response to changes in fluid intake.37,38
Though not significantly associated with BPD, the variation of diuresis, serum urea, glucose and osmolality presented the same trend. Higher values of diuresis, serum urea, and glucose may explain the higher osmolality observed during the first week in BPD patients. This higher diuresis may be related to renal water loss caused by a more immature kidney.
Although with slightly lower values, the hematocrit variation of BPD patients was not associated with the development of lung disease in the mathematical GEE model used in this analysis.
Caddell6 reviewed the evidence for a role of magnesium deficiency in the pathogenesis of BPD. Magnesium deficiency increases the susceptibility of cells and tissues to peroxidation, worsens the inflammatory reaction, reduces the immune response, exaggerates catecholamine release in stress, and diminishes energy metabolism. Stigson and Kjellmer7 analyzed the absolute levels and normal variations of magnesium concentration in cord blood and during the first 3 weeks after birth for 69 infants born before 32 gestational weeks of age. Their results showed that during the first week of life, higher values of magnesium are associated to a lower rate of PDA closure, for every patient. Higher levels of serum magnesium at birth within normal variations were associated with a delayed closure of the ductus arteriosus and mild but not severe peri- and intraventricular hemorrhage. Magnesium is a natural calcium channel blocker that inhibits vasoconstriction in numerous vascular beds and may be associated with a delayed closure of the ductus arteriosus.
In this study, magnesium plasma values were in the normal range in both BPD and non-BPD neonates, as well as in neonates with and without patent ductus arteriosus. An association between magnesium levels, patent ductus arteriosus and BPD could not be established.
Possibly because of the danger of magnesium toxicity and the difficulty in studying preterm extremely low birth weight neonates, little is known about magnesium supplementation in this group.
Our results demonstrated significantly lower mean calcium plasma levels in patients with patent ductus arteriosus, suggesting that calcium may have a role in ductus closure. The role of calcium sensitization during the normoxic contraction of the ductus arteriosus has been emphasized in some studies.39, 42 A significantly higher mean plasma urea level was also observed during the first week of life in patients with patent ductus arteriosus, which is likely related to the increased rate of indomethacin use for patent ductus arteriosus closure.43
There are some limitations in this study. First, this is a retrospective study, and although the methods of fluid and electrolyte supplies were the same during the study period, the conditions were not exactly the same for all patients. A second limitation is the small number (n = 45) of patients with BPD (oxygen dependency at 36 weeks gestational age). Third, the global severity of the illness may not be entirely reflected by the multivariate models used in this study. Aside from these limitations, we believe that our results indicate that real variations in fluid and electrolyte balance during the first week of life may play a role in the pathogenesis of BPD and should therefore be considered as risk factors. The scientific contribution of this study is the fact that our results should be considered as a hypothesis for future studies in the field.
CONCLUSIONS
In this study, the variation in plasma levels of potassium, phosphorus and creatinine during the first week of life revealed an association with BPD. Although the variation in serum calcium was not different between BPD and non-BPD patients, the mean serum levels of calcium in BPD neonates were lower than those observed in non-BPD patients. We speculate that there is an association between the development of BPD, calcium-phosphorus balance and parathormone regulation during the first week of life, especially in neonates who did not benefit from the renal maturation effect of antenatal steroids.
Our results also suggest that calcium may have a role in ductus arteriosus closure, and the higher mean plasma urea values observed in patients with patent ductus arteriosus are likely related to an increased rate of indomethacin use. Indomethacin may be partially responsible for the variation in plasma values of potassium, phosphorus and creatinine observed during the first week of life in BPD patients.
All of these issues appear to be interrelated. Indeed, despite the multivariate analysis performed, the data presented on the evolution of plasma potassium and plasma phosphorus during the first week of life and the risk for BPD at 36 weeks post-menstrual age may also reflect a bias induced by the global severity of the illness, which may not be entirely reflected by the multivariate models used in the study. Thus, a causative association cannot be established. The same lack of causation may be true for the association of plasma calcium levels and patent ductus arteriosus, both conditions being widely associated with immaturity and the severity of disease in premature infants in a number of observational studies.
More studies are needed to clarify the role of glomerular and tubular function on phosphorus and potassium metabolism, the role of parathormone regulation on calcium-phosphorus equilibrium, and the effects of antenatal steroids, lower serum calcium and a patent ductus arteriosus and indomethacin on patients who will develop BPD.
REFERENCES
- 1.van Marter LJ, Levinton A, Allred EN, Pagano M, Kuban KCK. Hydration during the first days of life and the risk of bronchopulmonary dysplasia in low birth weight infants. J Pediatr. 1990;116:942–9. doi: 10.1016/s0022-3476(05)80658-4. [DOI] [PubMed] [Google Scholar]
- 2.Oh W, Poindexter BB, Perrit R, Lemons JA, Bauer CR, Ehrenkranz RA, et al. Association between fluid intake and weight loss during the first ten days of life and risk of bronchopulmonary dysplasia in extremely low birth weight infants. J Pediatr. 2005;147:786–90. doi: 10.1016/j.jpeds.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 3.Bell E, Acarregui M. Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane database Syst Rev. 2008;23:CD000503. doi: 10.1002/14651858.CD000503.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Spitzer AR, Fox WW, Delivoria-Papadopoulos M. Maximum diuresis – a factor in predicting recovery from respiratory distress syndrome and the development of bronchopulmonary dysplasia. J Pediatr. 1981;98:476–9. doi: 10.1016/s0022-3476(81)80728-7. [DOI] [PubMed] [Google Scholar]
- 5.Costarino AT, Gruskay JA, Corcoran L, Polin RA, Baumgart S. Sodium restriction versus daily maintenance replacement in very low premature neonates: a randomized blind therapeutic trial. J Pediatr. 1992;120:99–106. doi: 10.1016/s0022-3476(05)80611-0. [DOI] [PubMed] [Google Scholar]
- 6.Caddell JL. Evidence for magnesium deficiency in the pathogenesis of bronchopulmonary dysplasia (BPD) Magnes Res. 1996;9:205–16. [PubMed] [Google Scholar]
- 7.Stigson L, Kjellmer I. Serum levels of magnesium at birth related to complications of immaturity. Acta Paediatr. 1997;86:991–4. doi: 10.1111/j.1651-2227.1997.tb15185.x. [DOI] [PubMed] [Google Scholar]
- 8.Gomella TL, Cunningham MD, Eyal FG, Zenk KE. 5th edition. New York: McGraw-Hill Companies; 2004. Neonatology. Management, procedures, On-call Problems, Diseases, and Drugs. [Google Scholar]
- 9.Simon TL, Alverson DC, AuBochon J. Practice parameter for the use of red blood cell transfusions. Arch Pathol Lab Med. 1998;122:130–8. [PubMed] [Google Scholar]
- 10.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respi Crit Care Med. 2001;163:1723–9. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald H. American Academy of Pediatrics. Committee on Fetus and Newborn. Perinatal Care at the Threshold of Viability. Pediatrics. 2002;110:1024–7. doi: 10.1542/peds.110.5.1024. [DOI] [PubMed] [Google Scholar]
- 12.Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard Score, expanded to include extremely premature infants. J Pediatr. 1991;119:417–23. doi: 10.1016/s0022-3476(05)82056-6. [DOI] [PubMed] [Google Scholar]
- 13.Fenton TR. A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format. BMC Pediatr. 2003;3:13. doi: 10.1186/1471-2431-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanc WA. Pathology of the Placenta, Membranes, and Umbilical Cord in Bacterial, Fungal, and Viral Infections in Man. In: Naeye RL, Kissane JM, editors. International Academy of Pathology Monograph Perinatal Diseases by 14 authors. Baltimore: Williams and Willkins; 1981. [PubMed] [Google Scholar]
- 15.Rudolph AJ, Smith CA. Idiopathic respiratory distress syndrome of the newborn. J Pediatr. 1960;57:905–21. [Google Scholar]
- 16.Walsh MC, Kliegman RM. Necrotizing Enterocolitis: Treatment Based on Staging Criteria. Ped Clin N Am. 1986;33:179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.An International Classification of Retinopathy of Prematurity. Pediatrics. 1984;74:127–33. [PubMed] [Google Scholar]
- 18.The International Classification of Retinopathy of Prematurity revisited. International Committee for the Classification of Retinopathy of Prematurity. Arch Ophthalmol. 2005;123:991–9. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 19.Papile LA, Burstein J, Burstein R. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birthweights less than 1500g. J Pediatr. 1978;92:529–34. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 20.de Vries L, Rennie JM. Preterm brain injury. In: Rennie J M, Roberton N R C, editors. Textbok of Neonatology. 3 th edition. London: Churchill Livingstone; 1999. pp. 1252–70. [Google Scholar]
- 21.Rudd PT, Hughes EA, Placzek MM, Hodes DT. Reference ranges for plasma creatinine during the first month of life. Arch Dis Child. 1983;58:212–15. doi: 10.1136/adc.58.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Modi N. Disorders of the kidneys and urinary tract Renal function, fluid and electrolyte balance and neonatal renal disease. In: Rennie JM, Roberton NRC, editors. Textbook of Neonatology. 3rd edition. Edinburgh: Churchil Livingstone; 2000. pp. 1009–37. [Google Scholar]
- 23.Baumgart S. Acute problems of prematurity: balancing fluid volume and electrolyte replacements in very low birth weight (VLBW) and extremely low birth weight (ELBW) neonates. In: Oh W, Guignard J-P, Baumgart S, Polin RA, editors. Nephrology and Fluid/Electrolyte Physiology Neonatal Questions and Controversies. Philadelphia, Saunders: Elsevier; 2008. pp. 161–83. [Google Scholar]
- 24.Greenbaum L. The pathophysiology of body fluids and fluid therapy. In: Kliegman R, Behrman R, Jenson H, Stanton B, editors. Nelson Textbook of Pediatrics. 18th edition. Philadelphia, Saunders: Elsevier; 2007. pp. 267–309. [Google Scholar]
- 25.Palta M, Babbert D, Weinstein MR, Peters ME. Multivariate assessment of traditional risk factors for chronic lung disease in very low birth weight neonates. J Pediatr. 1991;119:285–92. doi: 10.1016/s0022-3476(05)80746-2. [DOI] [PubMed] [Google Scholar]
- 26.Van Marter LJ, Pagano M, Allred EN, Leviton A, Kuban KCK. Rate of bronchopulmonary dysplasia as a function of neonatal intensive care practices. J Pediatr. 1992;120:938–46. doi: 10.1016/s0022-3476(05)81968-7. [DOI] [PubMed] [Google Scholar]
- 27.Oh W, Poindexter BB, Perritt R, Lemons JA, Bauer CR, Ehrenkranz RA, et al. Association between fluid intake and weight loss during the first ten days of life and risk of bronchopulmonary dysplasia in extremely low birth weight infants. J Pediatr. 2005;147:786–90. doi: 10.1016/j.jpeds.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 28.Bell EF, Warburton D, Stonestreet BS, Oh W. Effect of fluid administration on the development of symptomatic patent ductus arteriosus and congestive heart failure in premature infants. N Engl J Med. 1980;302:598–604. doi: 10.1056/NEJM198003133021103. [DOI] [PubMed] [Google Scholar]
- 29.Lorenz JM, Kleinman LI, Kotagal UR, Reller MD. Water balance in very low birth weight infants: relationship to water and sodium intake and effect on outcome. J Pediatr. 1982;101:423–32. doi: 10.1016/s0022-3476(82)80078-4. [DOI] [PubMed] [Google Scholar]
- 30.Tammella OKT, Koivisto ME. Fluid restriction for preventing bronchoulmonary dysplasia ? Reduced fluid intake during the first weeks of life improves the outcome of low birth weight infants. Acta Paediatr. 1992;81:207–12. doi: 10.1111/j.1651-2227.1992.tb12205.x. [DOI] [PubMed] [Google Scholar]
- 31.Omar SA, DeCristofaro JD, Agarwal BI, La Gamma EF. Effects of prenatal steroids on water and sodium homeostasis in extremely low birth weight neonates. Pediatrics. 1999;104:482–88. doi: 10.1542/peds.104.3.482. [DOI] [PubMed] [Google Scholar]
- 32.Slotkin TA, Seidler FJ, Kavlock RJ, Gray JA. Fetal dexamethasone exposure accelerates development of renal function: relationship to dose, cell differentiation and growth inhibition. J Dev Physiol. 1992;17:55–61. [PubMed] [Google Scholar]
- 33.Shaffer SG, Bradt SK, Meade VM, hall RT. Extracellular fluid volume changes in very low birth weight infants during first two postnatal months. J Pediatr. 1987;111:124. doi: 10.1016/s0022-3476(87)80358-x. [DOI] [PubMed] [Google Scholar]
- 34.Celsi G, Zheng-Ming W, Akusjarvi G, Aperia A. Sensitive periods for glucocorticoids regulation of Na+,k+-ATPase mRNA in the developing lung and kidney. Pediatr Res. 1993;33:5–9. doi: 10.1203/00006450-199301000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Sulyok E, Varga F, Gyory E, Jobst K, Csaba IF. On the mechanism of renal sodium handling in newborn infants. Biology of the Neonate. 1980;37:75–79. doi: 10.1159/000241258. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Soriano J, Vallo A, Oliveros R, Castillo G. Renal handling of sodium in premature and full-term neonates: a study using clearance method during water diuresis. Ped Research. 1983;17:1013–16. doi: 10.1203/00006450-198312000-00017. [DOI] [PubMed] [Google Scholar]
- 37.Lorenz JM, Kleinman LI, Ahmed G, Makarian K. Phases of fluid and electrolyte homeostasis in the extremely low birth weight infants. Pediatrics. 1995;95:484–89. [PubMed] [Google Scholar]
- 38.Bidiwala KS, Lorenz JM, Kleinman LI. Renal function correlates of postnatal diuresis in preterm infants. Pediatrics. 1988;82:50–8. [PubMed] [Google Scholar]
- 39.Hong Z, Hong F, Olschewski A, Cabrera JA, Varghese A, Nelson DP, et al. Role of store-operated calcium channels and calcium sensitization in normoxic contraction of the ductus arteriosus. Circulation. 2006;114:1372–9. doi: 10.1161/CIRCULATIONAHA.106.641126. [DOI] [PubMed] [Google Scholar]
- 40.Thébaud B, Wu XC, Kajimoto H, Bonnet S, Hashimoto K, Michelakis ED, et al. Developmental absence of the O2 sensitivity of L-type calcium channels in preterm ductus arteriosus smooth muscle cells impairs O2 constriction contributing to patent ductus arteriosus. Pediatr Res. 2008;63:176–81. doi: 10.1203/PDR.0b013e31815ed059. [DOI] [PubMed] [Google Scholar]
- 41.Greyner H, Dzialowski EM. Mechanisms mediating the oxygen-induced vasoreactivity of the ductus arteriosus in the chicken embryo. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1647–59. doi: 10.1152/ajpregu.00001.2008. [DOI] [PubMed] [Google Scholar]
- 42.Weir EK, Obreztchikova M, Varghese A, Cabrera JÁ, Peterson DA, Hong Z. Mechanisms of oxygen sensing: a key to therapy of pulmonary hypertension and patent ductus arteriosus. Br J Pharmacol. 2008;155:300–7. doi: 10.1038/bjp.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adamska E, Helwich E, Rutkowska M, Zacharska E, Piotrowska A. Comparison of the efficacy of ibuprofen and indomethacin in the treatment of patent ductus arteriosus in prematurely born infants. Med Wieku Rozwoj. 2005;9:335–54. [PubMed] [Google Scholar]