Abstract
The present review updates the current knowledge on the question of whether high fructose consumption is harmful or not and details new findings which further pushes this old debate. Due to large differences in its metabolic handling when compared to glucose, fructose was indeed suggested to be beneficial for the diet of diabetic patients. However its growing industrial use as a sweetener, especially in soft drinks, has focused attention on its potential harmfulness, possibly leading to dyslipidemia, obesity, insulin resistance/metabolic syndrome and even diabetes. Many new data have been generated over the last years, confirming the lipogenic effect of fructose as well as risks of vascular dysfunction and hypertension. Fructose exerts various direct effects in the liver, affecting both hepatocytes and Kupffer cells and resulting in non-alcoholic steatotic hepatitis, a well known precursor of the metabolic syndrome. Hepatic metabolic abnormalities underlie indirect peripheral metabolic and vascular disturbances, for which uric acid is possibly the culprit.
Nevertheless major caveats exist (species, gender, source of fructose, study protocols) which are detailed in this review and presently prevent any firm conclusion. New studies taking into account these confounding factors should be undertaken in order to ascertain whether or not high fructose diet is harmful.
Keywords: Fructose, Triglycerides, Metabolic syndrome, Uric acid, Hypertension, Diet
INTRODUCTION
In recent years, there has been increasing interest in the role of dietary fructose (F) as a possible health risk. For decades, there has been a debate as to whether F, which has a lower glycemic index than glucose (G) and does not induce insulin secretion, is a good dietary alternative for diabetic patients. This important question has never been answered. More recently, it has been suggested that the worldwide burden of obesity and type 2 diabetes (T2DM) in young adults might be linked to a parallel increase in the consumption of artificially sweetened food, particularly soft drinks.1 Frequently in the United States, beverages (sodas and fruit juices) are sweetened by F, which is added as high F corn syrup (HFCS). HFCS has suddenly attracted attention regarding its responsibility in causing these metabolic disturbances. However, it must be noted here that this phenomenon particularly applies to the United States because countries in Europe and elsewhere have limited the use of HFCS. This is one reason, as will be seen later, for the ongoing discrepancies and as-yet uncertain conclusions.
The present review describes the various aspects of this important clinical question, exposes the reasons for some major discrepancies and details some reasons why we are missing firm conclusions and what must still be done to reach them.
WHAT DO FRUCTOSE PHARMACODYNAMICS TELL US?
Intestines
In normal alimentation, F is found in fruits and honey, either as pure F or as sucrose (S), which is composed of F and G in equimolar amounts. Natural F represents approximately 15% of total F intake. The higher sweetening capacity of F has led to its use as a frequent additive to liquid and solid foods. As will be seen later, this development has major consequences for answering the questions addressed in this review.
Although structurally similar, F behaves very differently from G; it is absorbed in limited amounts, and most healthy subjects exhibit signs of intolerance at a daily F intake of approximately 50 g,2 free fatty acids and some amino acids favor the absorption of F,3,4 which means that the threshold for intolerance may be even lower. F is transported via a specific glucose transporter, GLUT5, and exits enterocytes via GLUT2 transporters. It is important to note that chronic F intake upregulates GLUT5 expression.3 The lipidogenic effect of F starts in the intestines because F increases the levels of fatty acids associated with ApoB48, which are released as chylomicrons.
Hepatocytes
Once in the portal vein, F is rapidly and almost completely extracted by the liver; the hepatic first pass is close to 100%. Hepatocytes can transform F into various metabolites: glucose, glycogen, lactate, and fat. In contrast to G, F is rapidly converted into triose-P independently of insulin. F is also a good precursor for gluconeogenesis because it generates lactate. Approximately 17% of F becomes glycogen, and approximately 20–50% of an F load leaves the liver as G.5 In healthy individuals, compensatory mechanisms reduce other gluconeogenic pathways, maintaining constant total hepatic glucose production. Over a long period of time, however, F decreases the insulin inhibition of hepatic glucose production, leading to hepatic insulin resistance. The phosphorylation of F requires high levels of ATP, leading to acute states of ATP depletion in hepatocytes and possible synthesis of uric acid (discussed below).
A typical feature of F is the induction of hypertrigly-ceridemia (HTG), notably because of reduced clearance. This is considered to be the most prominent negative effect of F. In addition to its effects on TGs, F also decreases HDL cholesterol levels.
KUPFFER CELLS
In the liver, the majority of cells are non-parenchymal cells. Sinusoids are lined with a dense net of Kupffer cells (KCs) and endothelial cells. KCs make up the largest proportion of macrophages in the body; they are strongly involved in detoxification and inflammatory reactions, liberating TNFα, leukotrienes, interleukins and TGF-β.6 Interestingly, KCs have a high density of insulin receptors and extract up to 50% of a G load. Thus, KCs may play an important but hitherto unrecognized and poorly understood role in the regulation of metabolic homeostasis.7 The few data sets that are presently available appear paradoxical. One might expect that KC impairment leads to metabolic disturbances because a blockage of KCs has been shown to result in hyperinsulinemia8. Furthermore, the elimination of KCs by clodronate-encapsulated liposomes was recently shown to provoke non-alcoholic steatotic hepatitis (NASH) due to the concomitant elimination of protective IL-10.9 Paradoxically, other models using gadolinium chloride to eliminate KCs have shown that this procedure improved insulin resistance and NASH induced by high fat10 or S, one of the primary sources of F.11 TNFα is considered to be responsible for these disturbances. These data are intriguing, and it is difficult to know if this result is due to the use of gadolinium salt or to a massive overproduction of TNFα, which would then overcome the beneficial effects of these cells. Whatever the explanation, we suggest that the role of KCs requires clarification because this very important hepatic tissue might represent a key element in the problem.
WHAT DOES PATHOPHYSIOLOGY TELL US?
Metabolic disturbances
High F (HF) is suspected to be involved in a series of diseases linked to metabolic disturbances, the best known being HTG and insulin resistance. As will be discussed later, several mechanisms are responsible for these direct and indirect effects of F.
F metabolism does not seem to differ significantly between healthy and diabetic subjects. It impairs triglyceride (TG) clearance in both populations, but dyslipidemia is higher in subjects who are already resistant to insulin.12 Experimentally, HF diets have long been used to induce a metabolic state that is similar to human metabolic syndromes (MSs), namely insulin resistance and hyperlipidemia. However, this usually does not result in weight gain13 although the effects of F might vary among rat strains and some studies have reported weight gain in adolescents.14 In hamsters, fasting glycemia and weight were increased after chronic HF feeding.15 In mice, F increased adiposity and lipids in the liver, while S did not.16 This finding points to possible differences in species reactions to HF, which is important to keep in mind when interpreting different sets of data. In rats, F is usually given in pellets, but it is also efficacious when added to drinking water. According to the concentration of F used, it is possible to generate groups of animals presenting various symptoms ranging from simple insulin resistance to full diabetes. When pure F was used instead of HFCS, no postprandial HTG was observed for oral intakes up to approximately 50 g/d.17 HTG and fasting ApoB was, however, increased in overweight or obese women.18 Fasting increased levels of TG, VLDL-TAG and leptin without inducing insulin resistance or ectopic fat deposition in healthy males after four weeks of HF administration.19 More recently, however, the same group reported that just one week of HF administration increased ectopic fat deposition in the liver and skeletal muscle, with a greater effect seen in the offspring of diabetic parents than in healthy controls.20
The lipogenic activity of F can be demonstrated in vitro; adding HFCS-55 (a sweetener widely used in the United States) to hepatocytes leads to HTG and the elevation of lipogenic proteins and oxidative stress. At the same time, insulin resistance and endoplasmic reticulum stress are increased, and mitochondrial dysfunction is illustrated by cytochrome C release.21 This finding fits with the mitochondrial disruption seen in human NASH.
In rats, the effects of F appear to be stronger in adults than in young animals.22 F is usually administered in high doses to rats to rapidly produce insulin resistance, but lower, more relevant levels of F also induce G intolerance if they are administered over long periods of time.23 An HF diet in rats is considered to be a relevant model for human NASH, leading to typical hepatic lobular inflammation.24
In humans, a high intake of dietary F or sweetened fruit juice (but not whole fresh fruits) leads to impaired G tolerance in genetically susceptible individuals.25
In addition to dyslipidemia and insulin resistance/metabolic syndromes, the F in a daily regimen of two or more colas has been shown to increase the risk of gallstones26 and chronic kidney disease (stones).27
Vascular disturbances
Because of the high potential of F to glycate proteins compared to G, concern has been raised about the possible roles of F in cardiovascular complications. The reason for this is partly because the acyclic (linear) form of the sugar (which is the glycating form) is larger in F than in G.28,29 A meta-analysis showed that high F consumption increased cardiovascular risk by 24%.30 The direct effects of F on protein glycation are questionable, however, because F is almost completely metabolized by the intestines and liver, leaving little if any intact F in the blood. HF is commonly assimilated into soft drinks; however, soft drinks (particularly colas) contain many other glycating (or glycated) agents. Thus, there is possible confusion about the real role of F in in vivo glycation. Some direct glycation might occasionally occur when food and drinks come into direct contact during eating. Indeed, the initial steps of the Maillard reaction occur more rapidly with F than with G, and the glycation of food proteins decreases their digestibility.31
The glycation of albumin occurs on Lys-524 and leads to the formation of carboxymethyllysine (CML).32 Interestingly, vegetarians show higher levels of CML than normal consumers, which can be explained by their high consumption of fruits, honey and vegetables.28 F is known to be involved in cataractogenesis.33 However, this is more likely to be due to intracellular F formation resulting from the sorbitol pathway; when F synthesis increases, it can glycate lens crystalline.34
Atheromatous plaques are increased in hyper-cholesterolemic rabbits when F is added to their diet.35 F is also suspected to cause hypertension (HT); acute F intake has been shown to increase blood pressure and heart rate in humans.36,37 In rats, an HF diet does not automatically generate HT; it seems that only some rat strains develop HT, which is dependent on an animal’s age and diet protocol. This phenomenon may be due to the varying intra- and interspecies sensitivity toward F; indeed, rats, in contrast to humans, possess active uricase.38,39
Vascular reactivity is modified with chronic HF diets, as endothelial dysfunction most likely occurs due to the inhibition of NO synthesis by F.40 Our experiments showed an abnormal reactivity of small terminal arterioles in the skeletal muscles of chronically F-fed rats.41
Finally, it is important to note that F also increases PAI-1,42,43 a fibrinolytic factor that is known to be involved in the pathology of both metabolic and vascular disturbances of metabolic syndromes.44
MECHANISMS INVOLVED IN FRUCTOSE-INDUCED DISTURBANCES
Direct effects in splanchnic organs
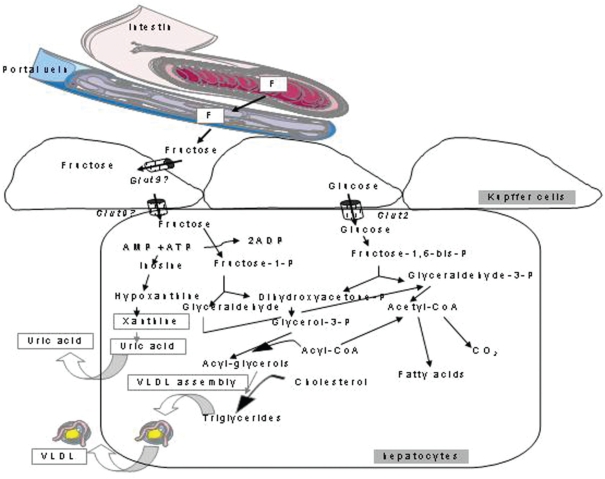
In contrast to G, F does not increase insulin secretion, which is considered to be potentially beneficial for diabetic patients. However, as described earlier, the primary negative side effect of F is its ability to increase lipid levels, especially TG. F increases both intestinal and hepatic production of atherogenic lipid particles,45 in part through chylomicron assembly and secretion.46 Because F may even reduce insulin secretion, TG clearance also decreases.47 The quantitative effect of F on lipogenesis is a topic of debate. F affects the partitioning of fatty acids toward their esterification, which has been shown in isolated perfused livers.48,49 In addition, F increases phosphofructokinase mRNA as well as G-6PDH and CHREB50 and fatty acid synthase activities.51 These effects lead to hepatic lipid loading, resulting in NASH (of which an HF diet is a relevant model in rats). The combined negative effects on liver lipid content and lactate and lipid output52 makes this organ a crucial effector of HF-induced metabolic disturbances. Independently of an MS diagnosis, patients presenting NASH were found to consume more soft drinks than healthy subjects.53 Interestingly, when moderate intake levels of various sweeteners were compared in rats during a ten-week period, both F and HFCS increased alanine aminotransferase activity, although no other differences were observed.54 These data show that the first premises of fatty liver can be seen even following moderate F consumption. Figure 1 depicts the major fate of F in the splanchnic area as well as some differences between F and G.
Figure 1.
Fructose is absorbed in the intestine by GLUT5 and exits enterocytes via GLUT2 transporters. It is taken up by Kupffer cells and hepatocytes, where it enters various metabolic pathways that largely differ from those of glucose. Fructose is partly transformed into uric acid, which may cause metabolic and vascular disturbances in peripheral tissues.
Recently, the involvement of intestinal bacterial flora in the regulation of metabolic homeostasis has been the subject of intense interest.55,56 In particular, studies have revealed that NASH is associated with intestinal bacterial overgrowth and increased intestinal permeability, leading to the endotoxin-dependent activation of KCs.55 This effect was also shown with an HF diet in rats.57 Thus, intestinal effects may add to hepatic effects as causal effectors of HF-linked metabolic abnormalities.
While F intolerance does not seem to differ between normal subjects and those suffering from irritable bowel syndrome,58,59 an improvement in F intolerance was observed in 1/3 of these patients after F restriction.60 Conversely, F and fructans may induce irritable bowel syndrome and intestinal permeability.55,61–63
Indirect metabolic effects
By increasing the liver lipid content and provoking HTG, F leads to global metabolic derangements that are typical of MSs, eventually leading to T2DM. Several studies have shown that HF induces hepatic insulin resistance, resulting in an elevated G output due to the lack of gluconeogenesis suppression. Once in the circulation, TGs may accumulate in skeletal muscle cells and induce peripheral insulin resistance.
High energy intake
It has been suggested that energy intake is higher with oral F than with oral G. The reasons for this belief are the following: 1) the thermic effect of F is higher than G because of high ATP consumption, and 2) it was observed that the satiety phenomenon normally regulating food intake did not always operate after chronic F. F lowers ghrelin and leptin levels to a lesser degree than does G, resulting in lower food intake suppression.1,3 This phenomenon applies mainly to liquid F, leading to a higher energy balance than F in solid food.64,65 When sweetened beverages were taken together with meals, a higher mean energy intake of 104 kcal was measured.66 Nevertheless, the “energy balance” hypothesis of HF is still a subject of debate.67
Is uric acid the culprit?
Uric acid (UA) is a major antioxidant, but, like most molecules of this category,68 it can also induce opposite effects if concentrations are sufficiently high.69 There are likely to be concentration thresholds delineating positive from negative effects of such substances; acute and even relatively high UA levels protect against oxidative stress70, but chronically elevated UA levels are linked to MSs, reduced adiponectin and elevated E-selectin, in parallel with positive effects such as reduced nitrotyrosine and increased total antioxidant capacity.71 Thus, high UA levels could be a compensatory mechanism that counteracts the oxidative stress related to metabolic or vascular disturbances.72
In vitro UA increases NADPH oxidase activity and oxidative stress in adipocytes, leading to increased p38 MAP kinase activity and insulin resistance.73 UA levels correlate with 24-h urinary excretion, waist circumference, insulin concentrations and the HOMA index, which are all indices of a MS.74 High insulin levels reduce renal UA excretion, suggesting that hyperuricemia results from a combination of stimulated formation and reduced elimination. In adolescents, body mass index and a number of MS components correlate with UA.75 In the San Bernardo Study, UA was a good predictor of T2DM in older adults with impaired fasting glycemia.76 Elevated UA was shown to be linked to NASH independently of body mass index.77–80 In elderly male subjects, high levels of UA were correlated with MS components and elevated levels of PAI-1.81 Thus, it seems that at any age, high levels of UA are linked to MS. Decreasing UA levels in rats with allopurinol prevents MS, as shown by reductions in insulin, TG and weight.40
On the vascular side, high levels of UA are linked to cardiovascular diseases, especially HT.82–86 If patients are already at risk, then UA represents a serious problem.87,88 Mechanisms that are responsible for the deleterious UA effects are linked to increased C reactive protein, inflammation and reduced NO production, thereby hampering vascular reactivity.38,82,84,89
HF increases UA formation as a consequence of ATP consumption and high synthesis of AMP, which is then deaminated to form UA.3,90 Several studies have shown that HF increases UA levels. However, in the first phase of the Nanes III study, MS was not linked to UA in subjects who consumed diet soft drinks91. UA is known to be responsible for gout due to the deposition of monosaccharide urate crystals; this effect is strongly related to diet.92 In a prospective cohort study over 12 years, F intake was correlated with gout regardless of whether sweetened soft drinks, fruit juices or F-rich fresh fruits were consumed.77 However, while this manuscript was in its final phase, a new publication showed that an analysis of more recent data from the Nanes III database (>9000 subjects) failed to confirm an association between F and UA. Thus, over a long time period, UA might not be as harmful in an HF diet as was initially claimed, which is in agreement with other reports.93
FRUCTOSE VS. OTHER SUGARS
A long-lasting controversy exists regarding the harmfulness of F versus other sugars because HFCS is now commonly used to sweeten drinks and food. Two questions must therefore be addressed: 1) how does “pure” F compare with S, a sugar containing one moiety of F, and 2) how does F compare with G? It is evident that such studies can easily be biased if energy equivalents are not matched in the clinical investigations. Most studies comparing F and S have shown that both sugars behave in essentially similar manners, pointing to the fact that F is actually the culprit.94–97 No differences in energy balance were found between HFCS, S and milk.98 When compared to G, HFCS and S always lead to higher TG or glucose/insulin responses over a 24-h period.99 In a study investigating the effects of 34% F in 3 different diets (F, F+G and S), a reduction in insulin sensitivity in rats was observed in every regimen.97 Several studies have compared F and G, and in a one-week study, F increased VLDL more than G, but intramuscular lipids were higher with G.100 Essentially the same qualitative difference was found in another study that extended the comparison to one month.96 Giving sweetened beverages to overweight or obese subjects for up to 10 weeks showed that F but not G increased lipid synthesis and visceral adiposity and reduced insulin sensitivity; G administration led to increased subcutaneous fat, which is relatively harmless.101 When F or G was administered with meals, obese patients showed lower insulin and leptin levels but increased postprandial TGs; HTG lasted for over 24 hours in the insulin-resistant subgroup.12
Altogether, these data show that F has roughly the same harmfulness whether it is administered as a concentrate such as HFCS or as sucrose. When the comparison is made with G, however, there are strong qualitative differences.
Therapeutic effects
Various clinical studies have shown that reducing F intake improves ones’ metabolic situation, which is expected. Thus, intestinal side effects due to F intolerance or excess F in food can be improved by a F-limited diet. Metabolic changes such as abdominal fat deposition, insulin resistance, oxidative stress and HT are improved by treatment with the antidiabetic metformin.37,102–105
Caveats and limits of the actual dataset
As is often found in nutritional studies, many factors, which can be controlled or not controlled, can bias interpretations of data. As a consequence, our present knowledge must consider and eventually investigate these factors before delivering clear-cut conclusions.
Species
As discussed above, differences in metabolic reactions to F have been described among various species: intra-species differences among rat strains, inter-species differences among mice, rats and hamsters and differences between rodents and humans. The presence of uricase in rats usually requires the utilization of high F concentrations in these animals, particularly when researchers wish to produce pathological individuals rapidly.
Type of food
It is known that the source of F partly determines its harmfulness; not only is it different among fresh fruits, fruit juices and sweetened soft drinks, but some sources, such as honey, appear to be much safer than others. Furthermore, several studies have shown that hormonal and metabolic reactions are more pronounced with liquid F intake than with solid F intake, likely because of the bypass of the satiety process with liquid F.
Another important caveat is that most clinical studies use soft drinks as the source of F. However, usually other parameters, such as daily alimentary habits or lifestyle (e.g., food type, activity levels) can affect ones’ metabolism. Focusing on F soft drinks as the sole cause of metabolic modifications limits the power of the data. The same holds true for vascular disturbances because colas and other soft drinks contain many other molecules that could interfere with vascular reactivity or induce glycation.
Protocols
a). Study Duration
One major aspect of study protocols is the duration of the study. Available data have been obtained primarily from acute (2–24 h) or mid-term (1–4 weeks) administration of F; only some data have been obtained from long-term investigations. It is clear that acute and short-term administration of moderate and even high F will cause tissues to react against what they might sense as an aggression. We know that metabolic disturbances take years to manifest as clinical symptoms due to stepwise developing compensations, metabolic adaptations and new equilibrium levels (the development of T2DM is a good example). Concerning F, the UA aspect may also illustrate how an initially positive reaction (hyperuricemia) may turn into a negative one. Acute and chronic F administration can yield different results. This has been observed for TG levels, which were increased in acute but not chronic HF diets.101 Moreover, a recent report did not observe a correlation between F and UA over long time periods.93
b). Gender/Populations
The relatively large number of studies published in recent years can be divided into various categories of subjects or patients: young, old, healthy, overweight/obese, and diabetic. It is understandable that data might look very different among these populations. Ethnicity is another possible confounder because we know that certain populations have greater risks of developing insulin resistance, obesity and/or MSs. For F, gender may play a role. Differences in most domains of physiology between males and females have become increasingly recognized in the last 5–10 years. This may also apply to HF. For example, it is known that female rats are largely protected compared to males. Insulin sensitivity decreases to a much larger extent in males106, and generally only males develop HT. The same effect holds true for humans; a careful examination of the literature shows a more severe picture in males than in females. In adolescents, weight gain under HF conditions was higher in males than in females.14 Furthermore, TG levels were higher in males than in females under HFCS or S administration conditions.99 The incidences of hyperuricemia and MS were higher in males than in females.79 Finally, in a study of Japanese subjects, UA increased the incidence of MS in males but not in females.107
c). Dosage
As discussed previously, F is only partly absorbed and rapidly leads to intestinal intolerance, which illustrates the fact that an HF condition is very different from a low F condition. However, most studies deal with high or very high F concentrations, which are not relevant to daily F consumption in humans, even if one considers ‘cola-addicted’ young people. Moreover, many studies were performed in the United States using HFCS, a sweetener that is not used or is used only in limited amounts in many other countries. Thus, extreme caution should be taken when trying to develop firm conclusions.17 In rats, moderate intake of various sweeteners (F, HFCS or agave) for 3 nights per week during a 10-week period increased serum alanine aminotransferase levels, but no other serious disturbances, such as those seen with HF, were observed.54 In humans, the deleterious effects seen with 20–25% F as an energy source were not observed when more realistic quantities of 4–12% F were investigated.96
CONCLUSIONS
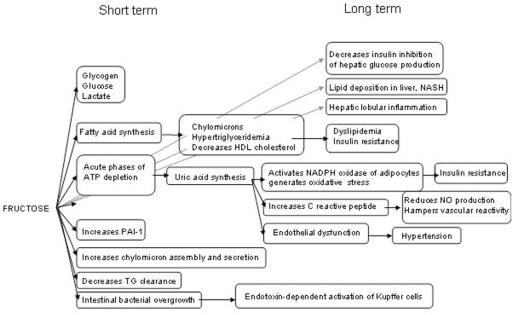
The controversy that has existed for the last 10 years regarding the potential harmfulness of excess fructose is legitimate in view of the dramatic increases in both sweetened beverage consumption and the burdens of obesity, MS and T2DM.108 There is no doubt from many preclinical and clinical studies that HF can induce negative effects on both energy metabolism and blood vessels (summarized in Figure 2).
Figure 2.
Summary of short- and long-term metabolic and vascular disturbances and the underlying mechanistic pathways induced by high fructose intake.
Unfortunately, the vast majority of the studies were performed using protocols in which the duration or dosage of F administration was not relevant to common daily usage in the general population. Nevertheless, the merit of these studies was to provide valuable information as to what can be expected from excessive F intake and the nature of the underlying mechanisms. However, many important confounding factors were ignored, which makes data interpretation too hazardous to draw conclusions about the deleterious nature of HF.
Assuming there is no F intolerance, we infer from the available dataset that F is harmless in healthy individuals, at least at levels below 50–100 g/d. However, it also appears clear that individuals at risk for metabolic syndromes, T2DM or cardiovascular diseases should be cautious because evidence shows that they are much more susceptible to HF than the general population. Thus, the controversy continues, and new protocols that take into account our present knowledge and eliminate the aforementioned confounding factors are required. From the available literature, four main aspects of F intake appear to be particularly important: a) the use of “natural” F sources (sucrose) instead of HFCS or sweetened soft drinks (which contain many additional substances), b) the use of relevant F concentrations based on existing data about common daily F consumption in the general population, c) the mixing of male and female subjects and d) the investigation of moderately elevated F over long periods of time.
REFERENCES
- 1.Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 2.Rao SS, Attaluri A, Anderson L, Stumbo P. Ability of the normal human small intestine to absorb fructose: evaluation by breath testing. Clin Gastroenterol Hepatol. 2007;5:959–63. doi: 10.1016/j.cgh.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson RJ, Perez-Pozo SE, Sautin YY, Manitius J, Sanchez-Lozada LG, Feig DI, et al. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev. 2009;30:96–116. doi: 10.1210/er.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skoog SM, Bharucha AE. Dietary fructose and gastrointestinal symptoms: a review. Am J Gastroenterol. 2004;99:2046–50. doi: 10.1111/j.1572-0241.2004.40266.x. [DOI] [PubMed] [Google Scholar]
- 5.Nuttall FQ, Khan MA, Gannon MC. Peripheral glucose appearance rate following fructose ingestion in normal subjects. Metabolism. 2000;49:1565–71. doi: 10.1053/meta.2000.18553. [DOI] [PubMed] [Google Scholar]
- 6.Kmiec Z. Cooperation of liver cells in health and disease. Adv Anat Embryol Cell Biol. 2001;161:III–XIII. 1–151. doi: 10.1007/978-3-642-56553-3. [DOI] [PubMed] [Google Scholar]
- 7.Spolarics Z, Ottlakan A, Lang CH, Spitzer JJ. Kupffer cells play a major role in insulin-mediated hepatic glucose uptake in vivo. Biochem Biophys Res Commun. 1992;186:455–60. doi: 10.1016/s0006-291x(05)80829-0. [DOI] [PubMed] [Google Scholar]
- 8.Cornell RP. Mechanisms of acute hyperinsulinemia after Kupffer cell phagocytosis. Am J Physiol. 1980;238:E276–83. doi: 10.1152/ajpendo.1980.238.3.E276. [DOI] [PubMed] [Google Scholar]
- 9.Clementi AH, Gaudy AM, van Rooijen N, Pierce RH, Mooney RA. Loss of Kupffer cells in diet-induced obesity is associated with increased hepatic steatosis, STAT3 signaling, and further decreases in insulin signaling. Biochim Biophys Acta. 2009;1792:1062–72. doi: 10.1016/j.bbadis.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neyrinck AM, Cani PD, Dewulf EM, De Backer F, Bindels LB, Delzenne NM. Critical role of Kupffer cells in the management of diet-induced diabetes and obesity. Biochem Biophys Res Commun. 2009;385:351–6. doi: 10.1016/j.bbrc.2009.05.070. [DOI] [PubMed] [Google Scholar]
- 11.Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, et al. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 2010;59:347–57. doi: 10.2337/db09-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teff KL, Grudziak J, Townsend RR, Dunn TN, Grant RW, Adams SH, et al. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. J Clin Endocrinol Metab. 2009;94:1562–9. doi: 10.1210/jc.2008-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livesey G, Taylor R. Fructose consumption and consequences for glycation, plasma triacylglycerol, and body weight: meta-analyses and meta-regression models of intervention studies. Am J Clin Nutr. 2008;88:1419–37. doi: 10.3945/ajcn.2007.25700. [DOI] [PubMed] [Google Scholar]
- 14.Berkey CS, Rockett HR, Field AE, Gillman MW, Colditz GA. Sugar-added beverages and adolescent weight change. Obes Res. 2004;12:778–88. doi: 10.1038/oby.2004.94. [DOI] [PubMed] [Google Scholar]
- 15.Barros CM, Lessa RQ, Grechi MP, Mouco TL, Souza MG, Wiernsperger N, et al. Substitution of drinking water by fructose solution induces hyperinsulinemia and hyperglycemia in hamsters. Clinics. 2007;62:327–34. doi: 10.1590/s1807-59322007000300019. [DOI] [PubMed] [Google Scholar]
- 16.Jurgens H, Haass W, Castaneda TR, Schurmann A, Koebnick C, Dombrowski F, et al. Consuming fructose-sweetened beverages increases body adiposity in mice. Obes Res. 2005;13:1146–56. doi: 10.1038/oby.2005.136. [DOI] [PubMed] [Google Scholar]
- 17.Livesey G. Fructose ingestion: dose-dependent responses in health research. J Nutr. 2009;139:1246S–52S. doi: 10.3945/jn.108.097949. [DOI] [PubMed] [Google Scholar]
- 18.Swarbrick MM, Stanhope KL, Elliott SS, Graham JL, Krauss RM, Christiansen MP, et al. Consumption of fructose-sweetened beverages for 10 weeks increases postprandial triacylglycerol and apolipoprotein-B concentrations in overweight and obese women. Br J Nutr. 2008;100:947–52. doi: 10.1017/S0007114508968252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le KA, Faeh D, Stettler R, Ith M, Kreis R, Vermathen P, et al. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am J Clin Nutr. 2006;84:1374–9. doi: 10.1093/ajcn/84.6.1374. [DOI] [PubMed] [Google Scholar]
- 20.Le KA, Ith M, Kreis R, Faeh D, Bortolotti M, Tran C, et al. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr. 2009;89:1760–5. doi: 10.3945/ajcn.2008.27336. [DOI] [PubMed] [Google Scholar]
- 21.Collison KS, Saleh SM, Bakheet RH, Al-Rabiah RK, Inglis AL, Makhoul NJ, et al. Diabetes of the liver: the link between nonalcoholic fatty liver disease and HFCS-55. Obesity (Silver Spring) 2009;17:2003–13. doi: 10.1038/oby.2009.58. [DOI] [PubMed] [Google Scholar]
- 22.de Moura RF, Ribeiro C, de Oliveira JA, Stevanato E, de Mello MA. Metabolic syndrome signs in Wistar rats submitted to different high-fructose ingestion protocols. Br J Nutr. 2009;101:1178–84. doi: 10.1017/S0007114508066774. [DOI] [PubMed] [Google Scholar]
- 23.Blakely SR, Hallfrisch J, Reiser S, Prather ES. Long-term effects of moderate fructose feeding on glucose tolerance parameters in rats. J Nutr. 1981;111:307–14. doi: 10.1093/jn/111.2.307. [DOI] [PubMed] [Google Scholar]
- 24.Kawasaki T, Igarashi K, Koeda T, Sugimoto K, Nakagawa K, Hayashi S, et al. Rats fed fructose-enriched diets have characteristics of nonalcoholic hepatic steatosis. J Nutr. 2009;139:2067–71. doi: 10.3945/jn.109.105858. [DOI] [PubMed] [Google Scholar]
- 25.Sartorelli DS, Franco LJ, Gimeno SG, Ferreira SR, Cardoso MA. Dietary fructose, fruits, fruit juices and glucose tolerance status in Japanese-Brazilians. Nutr Metab Cardiovasc Dis. 2009;19:77–83. doi: 10.1016/j.numecd.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Dietary carbohydrates and glycaemic load and the incidence of symptomatic gall stone disease in men. Gut. 2005;54:823–8. doi: 10.1136/gut.2003.031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saldana TM, Basso O, Darden R, Sandler DP. Carbonated beverages and chronic kidney disease. Epidemiology. 2007;18:501–6. doi: 10.1097/EDE.0b013e3180646338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krajcovicova-Kudlackova M, Sebekova K, Schinzel R, Klvanova J. Advanced glycation end products and nutrition. Physiol Res. 2002;5:313–6. [PubMed] [Google Scholar]
- 29.Sakai M, Oimomi M, Kasuga M. Experimental studies on the role of fructose in the development of diabetic complications. Kobe J Med Sci. 2002;48:125–36. [PubMed] [Google Scholar]
- 30.Bray GA. Soft drink consumption and obesity: it is all about fructose. Curr Opin Lipidol. 2010;21:51–7. doi: 10.1097/MOL.0b013e3283346ca2. [DOI] [PubMed] [Google Scholar]
- 31.Dills WL., Jr Protein fructosylation: fructose and the Maillard reaction. Am J Clin Nutr. 1993;58(5 Suppl):779S–87S. doi: 10.1093/ajcn/58.5.779S. [DOI] [PubMed] [Google Scholar]
- 32.Hinton DJ, Ames JM. Site specificity of glycation and carboxymethylation of bovine serum albumin by fructose. Amino Acids. 2006;30:425–34. doi: 10.1007/s00726-006-0269-2. [DOI] [PubMed] [Google Scholar]
- 33.Gul A, Rahman MA, Hasnain SN. Role of fructose concentration on cataractogenesis in senile diabetic and non-diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2009;247:809–14. doi: 10.1007/s00417-008-1027-9. [DOI] [PubMed] [Google Scholar]
- 34.Miyazawa N, Kawasaki Y, Fujii J, Theingi M, Hoshi A, Hamaoka R, et al. Immunological detection of fructated proteins in vitro and in vivo. Biochem J. 1998;336:101–7. doi: 10.1042/bj3360101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tokita Y, Hirayama Y, Sekikawa A, Kotake H, Toyota T, Miyazawa T, et al. Fructose ingestion enhances atherosclerosis and deposition of advanced glycated end-products in cholesterol-fed rabbits. J Atheroscler Thromb. 2005;12:260–7. doi: 10.5551/jat.12.260. [DOI] [PubMed] [Google Scholar]
- 36.Brown CM, Dulloo AG, Yepuri G, Montani JP. Fructose ingestion acutely elevates blood pressure in healthy young humans. Am J Physiol Regul Integr Comp Physiol. 2008;294:R730–7. doi: 10.1152/ajpregu.00680.2007. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Jia X, Chang T, Desai K, Wu L. Attenuation of hypertension development by scavenging methylglyoxal in fructose-treated rats. J Hypertens. 2008;26:765–72. doi: 10.1097/HJH.0b013e3282f4a13c. [DOI] [PubMed] [Google Scholar]
- 38.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–42. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 39.Terzuoli L, Porcelli B, Ponticelli F, Marinello E. Purine nucleotide catabolism in rat liver. Certain preliminary aspects of uricase reaction. Nucleosides Nucleotides Nucleic Acids. 2009;28:193–203. doi: 10.1080/15257770902865381. [DOI] [PubMed] [Google Scholar]
- 40.Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290:F625–31. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- 41.Wiernsperger N, Nivoit P, De Aguiar LG, Bouskela E. Microcirculation and the metabolic syndrome. Microcirculation. 2007;14:403–38. doi: 10.1080/10739680701285617. [DOI] [PubMed] [Google Scholar]
- 42.Alzamendi A, Giovambattista A, Raschia A, Madrid V, Gaillard RC, Rebolledo O, et al. Fructose-rich diet-induced abdominal adipose tissue endocrine dysfunction in normal male rats. Endocrine. 2009;35:227–32. doi: 10.1007/s12020-008-9143-1. [DOI] [PubMed] [Google Scholar]
- 43.Scelles V, Alessi MC, Raccah D, Juhan-Vague I, Vague P. Plasma plasminogen activator inhibitor activity in rats with nutritionally induced insulin resistance. Thromb Haemost. 1995;74:806–7. [PubMed] [Google Scholar]
- 44.Kosugi T, Nakamura M, Sunagawa M. Transition of pathophysiological significance of plasminogen activator inhibitor-From a chief player in antiinflammation, antifibrinolysis to that in the development of insulin resistance. Pathophysiology. 17:109–18. doi: 10.1016/j.pathophys.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Basciano H, Federico L, Adeli K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab (Lond) 2005;2:5. doi: 10.1186/1743-7075-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsieh J, Hayashi AA, Webb J, Adeli K. Postprandial dyslipidemia in insulin resistance: mechanisms and role of intestinal insulin sensitivity. Atheroscler Suppl. 2008;9:7–13. doi: 10.1016/j.atherosclerosissup.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 47.Chong MF, Fielding BA, Frayn KN. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr. 2007;85:1511–20. doi: 10.1093/ajcn/85.6.1511. [DOI] [PubMed] [Google Scholar]
- 48.Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr. 1993;58(5 Suppl):754S–65S. doi: 10.1093/ajcn/58.5.754S. [DOI] [PubMed] [Google Scholar]
- 49.Topping DL, Mayes PA. The immediate effects of insulin and fructose on the metabolism of the perfused liver. Changes in lipoprotein secretion, fatty acid oxidation and esterification, lipogenesis and carbohydrate metabolism. Biochem J. 1972;126:295–311. doi: 10.1042/bj1260295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koo HY, Wallig MA, Chung BH, Nara TY, Cho BH, Nakamura MT. Dietary fructose induces a wide range of genes with distinct shift in carbohydrate and lipid metabolism in fed and fasted rat liver. Biochim Biophys Acta. 2008;1782:341–8. doi: 10.1016/j.bbadis.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, et al. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008;48:993–9. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tobey TA, Mondon CE, Zavaroni I, Reaven GM. Mechanism of insulin resistance in fructose-fed rats. Metabolism. 1982;31:608–12. doi: 10.1016/0026-0495(82)90100-7. [DOI] [PubMed] [Google Scholar]
- 53.Abid A, Taha O, Nseir W, Farah R, Grosovski M, Assy N. Soft drink consumption is associated with fatty liver disease independent of metabolic syndrome. J Hepatol. 2009;51:918–24. doi: 10.1016/j.jhep.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 54.Figlewicz DP, Ioannou G, Bennett Jay J, Kittleson S, Savard C, Roth CL. Effect of moderate intake of sweeteners on metabolic health in the rat. Physiol Behav. 2009;98:618–24. doi: 10.1016/j.physbeh.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rapin JR, Wiernsperger N. Possible links between intestinal permeability and food processing: a therapeutic niche for glutamine? Clinics. 2010. in press. [DOI] [PMC free article] [PubMed]
- 57.Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC, Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50:1094–104. doi: 10.1002/hep.23122. [DOI] [PubMed] [Google Scholar]
- 58.Skoog SM, Bharucha AE, Zinsmeister AR. Comparison of breath testing with fructose and high fructose corn syrups in health and IBS. Neurogastroenterol Motil. 2008;20:505–11. doi: 10.1111/j.1365-2982.2007.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Corlew-Roath M, Di Palma JA. Clinical impact of identifying lactose maldigestion or fructose malabsorption in irritable bowel syndrome or other conditions. South Med J. 2009;102:1010–2. doi: 10.1097/SMJ.0b013e3181b64c7f. [DOI] [PubMed] [Google Scholar]
- 60.Choi YK, Kraft N, Zimmerman B, Jackson M, Rao SS. Fructose intolerance in IBS and utility of fructose-restricted diet. J Clin Gastroenterol. 2008;42:233–8. doi: 10.1097/MCG.0b013e31802cbc2f. [DOI] [PubMed] [Google Scholar]
- 61.Beyer PL, Caviar EM, McCallum RW. Fructose intake at current levels in the United States may cause gastrointestinal distress in normal adults. J Am Diet Assoc. 2005;105:1559–66. doi: 10.1016/j.jada.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 62.Gibson PR, Newnham E, Barrett JS, Shepherd SJ, Muir JG. Review article: fructose malabsorption and the bigger picture. Aliment Pharmacol Ther. 2007;15:25, 349–63. doi: 10.1111/j.1365-2036.2006.03186.x. [DOI] [PubMed] [Google Scholar]
- 63.Fernandez-Banares F, Esteve M, Viver JM. Fructose-sorbitol malabsorption. Curr Gastroenterol Rep. 2009;11:368–74. doi: 10.1007/s11894-009-0056-9. [DOI] [PubMed] [Google Scholar]
- 64.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab Disord. 2000;24:794–800. doi: 10.1038/sj.ijo.0801229. [DOI] [PubMed] [Google Scholar]
- 65.Van Wymelbeke V, Beridot-Therond ME, de La Gueronniere V, Fantino M. Influence of repeated consumption of beverages containing sucrose or intense sweeteners on food intake. Eur J Clin Nutr. 2004;58:154–61. doi: 10.1038/sj.ejcn.1601762. [DOI] [PubMed] [Google Scholar]
- 66.DellaValle DM, Roe LS, Rolls BJ. Does the consumption of caloric and non-caloric beverages with a meal affect energy intake? Appetite. 2005;44:187–93. doi: 10.1016/j.appet.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 67.Bantle JP. Dietary fructose and metabolic syndrome and diabetes. J Nutr. 2009;139:1263S–8S. doi: 10.3945/jn.108.098020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wiernsperger NF. Oxidative stress: the special case of diabetes. Biofactors. 2003;19:11–8. doi: 10.1002/biof.5520190103. [DOI] [PubMed] [Google Scholar]
- 69.Sanchez-Lozada LG, Mu W, Roncal C, Sautin YY, Abdelmalek M, Reungjui S, et al. Comparison of free fructose and glucose to sucrose in the ability to cause fatty liver. Eur J Nutr. 2010;49:1–9. doi: 10.1007/s00394-009-0042-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vukovic J, Modun D, Budimir D, Sutlovic D, Salamunic I, Zaja I, et al. Acute, food-induced moderate elevation of plasma uric acid protects against hyperoxia-induced oxidative stress and increase in arterial stiffness in healthy humans. Atherosclerosis. 2009;207:255–60. doi: 10.1016/j.atherosclerosis.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 71.Bo S, Gambino R, Durazzo M, Ghione F, Musso G, Gentile L, et al. Associations between serum uric acid and adipokines, markers of inflammation, and endothelial dysfunction. J Endocrinol Invest. 2008;31:499–504. doi: 10.1007/BF03346397. [DOI] [PubMed] [Google Scholar]
- 72.Nieto FJ, Iribarren C, Gross MD, Comstock GW, Cutler RG. Uric acid and serum antioxidant capacity: a reaction to atherosclerosis? Atherosclerosis. 2000;148:131–9. doi: 10.1016/s0021-9150(99)00214-2. [DOI] [PubMed] [Google Scholar]
- 73.Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293:C584–96. doi: 10.1152/ajpcell.00600.2006. [DOI] [PubMed] [Google Scholar]
- 74.Yoo HG, Lee SI, Chae HJ, Park SJ, Lee YC, Yoo WH. Prevalence of insulin resistance and metabolic syndrome in patients with gouty arthritis. Rheumatol Int. 2009;20 doi: 10.1007/s00296-009-1304-x. [DOI] [PubMed] [Google Scholar]
- 75.Croymans DM, Sanchez A, Barth JD, Roberts CK. Carotid intima-media thickness, dietary intake, and cardiovascular phenotypes in adolescents: relation to metabolic syndrome. Metabolism. 2010;59:533–9. doi: 10.1016/j.metabol.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 76.Kramer CK, von Muhlen D, Jassal SK, Barrett-Connor E. Serum uric acid levels improve prediction of incident type 2 diabetes in individuals with impaired fasting glucose: the Rancho Bernardo Study. Diabetes Care. 2009;32:1272–3. doi: 10.2337/dc09-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. Bmj. 2008;336:309–12. doi: 10.1136/bmj.39449.819271.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee K. Relationship between uric acid and hepatic steatosis among Koreans. Diabetes Metab. 2009;35:447–51. doi: 10.1016/j.diabet.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 79.Lin KP. The relationship between serum uric acid concentration and metabolic syndrome in university freshmen. J Nurs Res. 2009;17:286–92. doi: 10.1097/JNR.0b013e3181c003a7. [DOI] [PubMed] [Google Scholar]
- 80.Nakagawa T, Tuttle KR, Short RA, Johnson RJ. Hypothesis: fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Clin Pract Nephrol. 2005;1:80–6. doi: 10.1038/ncpneph0019. [DOI] [PubMed] [Google Scholar]
- 81.Chang CH, Chen YM, Chuang YW, Liao SC, Lin CS, Tang YJ, et al. Relationship between hyperuricemia (HUC) and metabolic syndrome (MS) in institutionalized elderly men. Arch Gerontol Geriatr. 2009;49(Suppl 2):S46–9. doi: 10.1016/S0167-4943(09)70013-5. [DOI] [PubMed] [Google Scholar]
- 82.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–21. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Forman JP, Choi H, Curhan GC. Uric acid and insulin sensitivity and risk of incident hypertension. Arch Intern Med. 2009;26:169, 155–62. doi: 10.1001/archinternmed.2008.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kanellis J, Kang DH. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol. 2005;25:39–42. doi: 10.1016/j.semnephrol.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 85.Sundstrom J, Sullivan L, D’Agostino RB, Levy D, Kannel WB, Vasan RS. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension. 2005;45:28–33. doi: 10.1161/01.HYP.0000150784.92944.9a. [DOI] [PubMed] [Google Scholar]
- 86.Wexler BC, Greenberg BP. Effect of increased serum urate levels on virgin rats with no arteriosclerosis versus breeder rats with preexistent arteriosclerosis. Metabolism. 1977;26:1309–20. doi: 10.1016/0026-0495(77)90027-0. [DOI] [PubMed] [Google Scholar]
- 88.Strazzullo P, Puig JG. Uric acid and oxidative stress: relative impact on cardiovascular risk? Nutr Metab Cardiovasc Dis. 2007;17:409–14. doi: 10.1016/j.numecd.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 89.Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553–62. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 90.Angelopoulos TJ, Lowndes J, Zukley L, Melanson KJ, Nguyen V, Huffman A, et al. The effect of high-fructose corn syrup consumption on triglycerides and uric acid. J Nutr. 2009;139:1242S–5S. doi: 10.3945/jn.108.098194. [DOI] [PubMed] [Google Scholar]
- 91.Choi JW, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2008;59:109–16. doi: 10.1002/art.23245. [DOI] [PubMed] [Google Scholar]
- 93.Sun SZ, Flickinger BD, Williamson-Hughes PS, Empie MW. Lack of association between dietary fructose and hyperuricemia risk in adults. Nutr Metab. (Lond) 2010;7:16. doi: 10.1186/1743-7075-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Melanson KJ, Zukley L, Lowndes J, Nguyen V, Angelopoulos TJ, Rippe JM. Effects of high-fructose corn syrup and sucrose consumption on circulating glucose, insulin, leptin, and ghrelin and on appetite in normal-weight women. Nutrition. 2007;23:103–12. doi: 10.1016/j.nut.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 95.Murphy SP. The state of the science on dietary sweeteners containing fructose: summary and issues to be resolved. J Nutr. 2009;139:1269S–70S. doi: 10.3945/jn.108.097964. [DOI] [PubMed] [Google Scholar]
- 96.Schaefer EJ, Gleason JA, Dansinger ML. Dietary fructose and glucose differentially affect lipid and glucose homeostasis. J Nutr. 2009;139:1257S–62S. doi: 10.3945/jn.108.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thresher JS, Podolin DA, Wei Y, Mazzeo RS, Pagliassotti MJ. Comparison of the effects of sucrose and fructose on insulin action and glucose tolerance. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1334–40. doi: 10.1152/ajpregu.2000.279.4.R1334. [DOI] [PubMed] [Google Scholar]
- 98.Soenen S, Westerterp-Plantenga MS. No differences in satiety or energy intake after high-fructose corn syrup, sucrose, or milk preloads. Am J Clin Nutr. 2007;86:1586–94. doi: 10.1093/ajcn/86.5.1586. [DOI] [PubMed] [Google Scholar]
- 99.Stanhope KL, Griffen SC, Bair BR, Swarbrick MM, Keim NL, Havel PJ. Twenty-four-hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals. Am J Clin Nutr. 2008;87:1194–203. doi: 10.1093/ajcn/87.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ngo Sock ET, Le KA, Ith M, Kreis R, Boesch C, Tappy L. Effects of a short-term overfeeding with fructose or glucose in healthy young males. Br J Nutr. 2009;24:1–5. doi: 10.1017/S0007114509992819. [DOI] [PubMed] [Google Scholar]
- 101.Stanhope KL, Havel PJ. Fructose consumption: considerations for future research on its effects on adipose distribution, lipid metabolism, and insulin sensitivity in humans. J Nutr. 2009;139:1236S–41S. doi: 10.3945/jn.109.106641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Anurag P, Anuradha CV. Metformin improves lipid metabolism and attenuates lipid peroxidation in high fructose-fed rats. Diabetes Obes Metab. 2002;4:36–42. doi: 10.1046/j.1463-1326.2002.00178.x. [DOI] [PubMed] [Google Scholar]
- 103.Baret G, Peyronnet J, Grassi-Kassisse D, Dalmaz Y, Wiernsperger N, Geloen A. Increased intraabdominal adipose tissue mass in fructose fed rats: correction by metformin. Exp Clin Endocrinol Diabetes. 2002;110:298–303. doi: 10.1055/s-2002-34593. [DOI] [PubMed] [Google Scholar]
- 104.Faure P, Rossini E, Wiernsperger N, Richard MJ, Favier A, Halimi S. An insulin sensitizer improves the free radical defense system potential and insulin sensitivity in high fructose-fed rats. Diabetes. 1999;48:353–7. doi: 10.2337/diabetes.48.2.353. [DOI] [PubMed] [Google Scholar]
- 105.Srividhya S, Anuradha CV. Metformin improves liver antioxidant potential in rats fed a high-fructose diet. Asia Pac J Clin Nutr. 2002;1:319–22. doi: 10.1046/j.1440-6047.2002.00306.x. [DOI] [PubMed] [Google Scholar]
- 106.Tran LT, Yuen VG, McNeill JH. The fructose-fed rat: a review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol Cell Biochem. 2009;332:145–59. doi: 10.1007/s11010-009-0184-4. [DOI] [PubMed] [Google Scholar]
- 107.Oda E, Kawai R, Sukumaran V, Watanabe K. Uric acid is positively associated with metabolic syndrome but negatively associated with diabetes in Japanese men. Intern Med. 2009;48:1785–91. doi: 10.2169/internalmedicine.48.2426. [DOI] [PubMed] [Google Scholar]
- 108.Hu FB, Malik VS. Sugar-sweetened beverages and risk of obesity and type 2 diabetes: Epidemiologic evidence. Physiol Behav. 2010;100:47–54. doi: 10.1016/j.physbeh.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]